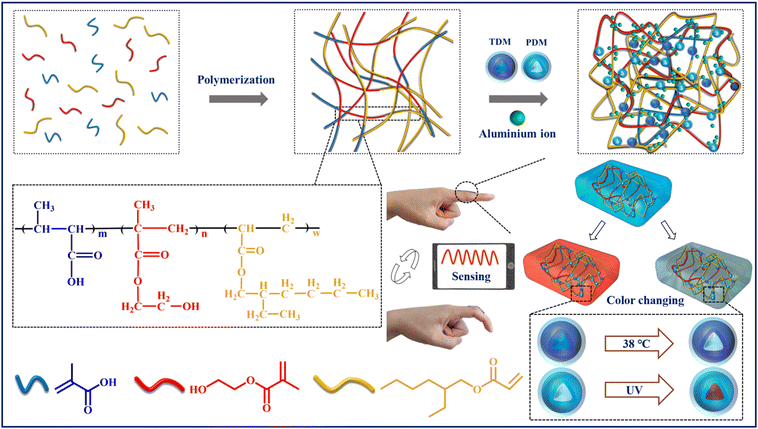
Bio-inspired color-changing and self-healing hybrid hydrogels for wearable sensors and adaptive camouflage†
Han
Liu
,
Long
Yu
,
Bingqian
Zhao
,
Yezhou
Ni
,
Peng
Gu
 *,
Hua
Qiu
,
Wan
Zhang
and
Kunlin
Chen
*,
Hua
Qiu
,
Wan
Zhang
and
Kunlin
Chen
 *
*
Key Laboratory of Eco-Textile, Ministry of Education, School of Textile Science and Engineering, Jiangnan University, Wuxi 214122, China. E-mail: chenkunlin@jiangnan.edu.cn; Tommy861007@hotmail.com
First published on 25th November 2022
Abstract
Hydrogels have been extensively investigated for their unique mechanical and ionic conductive properties. Currently, conventional hydrogels are not sufficiently durable for use and do not respond sensitively to environmental stimuli. Accordingly, the development of a hybrid hydrogel exhibiting self-healing ability and environmental responsiveness is of critical significance in broadening its application in smart wearable devices. For the formation of a color-changing and self-healing hybrid hydrogel, thermochromic dye microcapsules and photochromic dye microcapsules were mixed with a multi-branched polyacrylate and zinc sulphate in this study. This hybrid hydrogel exhibited an excellent sensing property, and can be applied to wearable device for monitoring the actions of human joints or faces. Even though the hybrid hydrogel was damaged during its application, the damaged parts of the hydrogel were self-repaired, which was dependent on the powerful ionic bonds and multi-hydrogen bonds among the polyacrylate, zinc ions, as well as microcapsules. Furthermore, under the synergistic effect of microcapsules and polyacrylate, the rapid change of color and current under thermal or UV stimulus facilitates monitoring human physiological health, as well as achieving adaptive camouflage. This smart hybrid hydrogel exhibiting self-healing, conductive, and color-changing properties has promising applications in human body monitoring, safety warning, adaptive camouflage, and artificial intelligence for wearable electronic devices.
1. Introduction
Electronic devices exhibiting bio-flexible and stretchable functions have been advancing, which is expediting the advancement towards intelligent human life.1–5 Rapid progress has been achieved in the fabrication of a wide variety of electronic devices (e.g., wearable sensors, optoelectronic sensing devices, electronic skin, and flexible energy storage devices6–10). Among them wearable sensors indispensably contribute to several aspects e.g., information collection, security warning, and intelligent human life.11–16 However, the durability or persistence of electronic devices (in particular wearable sensors) is a critical consideration for their application. Inspired by the special abilities of creatures in nature, the introduction of new design strategies promises to solve these problems and give new meaning to smart wearables. For instance, the skin of a chameleon is highly interesting and sensitive and capable of changing color for adaptation, self-protection, or safety warning by sensing external stimuli (e.g., temperature, sunlight, and pressure).17–24 Moreover, the skin of a chameleon has a self-healing ability even after being injured. Accordingly, chameleon skin provides inspiration for effective fabrication of durable wearable sensors. Thus, a suitable polymer matrix should be selected for wearable sensors.25–28 Wearable sensors that are formed based on the freely designed polymeric structures of the conductive polymers are highly desirable compared with conventional wearable sensors that are not resistant to stretching.29–33 To solve the problems regarding the widespread and sustainable application of wearable sensors, self-healing hydrogels with excellent ionic conductivity are an irreplaceable candidate.34–38 Flexible hydrogels exhibiting high ionic conductivity are one of the irreplaceable candidates for wearable sensing materials, as compared with conventional wearable sensing materials that are not tensile resistant.39–41Hydrogels refer to a type of polymer material with a three-dimensional network structure, and exhibit similar properties to biological tissues, so that they have been extensively employed in intelligent wearable devices.42–45 Conventional hydrogels are poorly flexible, prone to breakage, and not adapted to complex external stimulus responses.46–48 Accordingly, numerous studies have been conducted to develop hybrid hydrogels by introducing organic and inorganic hybrid networks. Lin et al.49 pre-stretched chemically cross-linked poly(acrylamide-co-acrylic acid) and froze the pre-stretched state by loading Fe3+. Thus, maximum chelation and molecular conformation were achieved between the carboxylic acid anion and Fe3+, so that the mechanical strength increased significantly. Moreover, with titanium oxide polydopamine-perfluorosilicon carbon dot-conjugated chitosan-polyvinyl alcohol-loaded tannic acid as the raw material, Ryplida et al.50 prepared a thin-film hydrogel with controlled hydrophobic–hydrophilic transition under UV-Vis irradiation. The formed intermolecular hydrogen bonds and electrostatic interactions in the hydrogel increased its rigidity and elasticity, thus improving its mechanical property. Although hybridization or recombination is capable of enhancing the mechanical properties of the hybrid hydrogels, durability and stability are confirmed as the critical factors for hydrogels, especially for wearable applications. For instance, Wei et al.51 chelated polyacrylic acid (PAA) with a high molecular weight with calcium ions (Ca2+). Subsequently, PAA was physically cross-linked with modified amorphous calcium phosphate to synthesize a Ca-PAA-ACP mineral hydrogel. To be specific, the prepared hydrogen and ionic bonds endowed the hydrogels with excellent durability and stability, so that they can be useful in the smart wearable electronics field. Moreover, although hydrogel materials have been widely used, numerous hydrogels do not possess specific functional properties, such as environmental adaptability. Thus, the design and preparation of intelligent responsive hydrogels is of great significance.52–56
In this study, a color-changing and self-healing TDM/PDM/polyacrylate hybrid hydrogel was designed and prepared through the bionic process. As depicted in Fig. 1, thermochromic dye microcapsules and photochromic dye microcapsules were first synthesized, and then these microcapsules were added to the multi-branched polyacrylate solution to prepare a hybrid hydrogel under the complexation of zinc sulphate. The prepared hybrid hydrogel exhibited excellent wearable tensile sensing properties, and it can change its color at different temperatures and under sunlight conditions. The hydrogel had promising self-healing ability due to the strong coordinate bond and multi-hydrogen bonds between the polyacrylate, zinc ions and color-changing microcapsules, thus maintaining electrical conductivity and mechanical and color changing properties even after being damaged. Moreover, this hybrid hydrogel can change its color and electric current quickly under thermal or UV stimuli because of the synergistic effect of color-changing microcapsules and the polyacrylate. As a result, it can timely warn human physiological health through visualization and data sensing, and can be applied for adaptive camouflage.
2. Experimental section
2.1 Materials
2-Ethylhexyl acrylate (EHA, purity ≥99%), methacrylic acid (MAA), hydroxyethyl methylacrylate (HEMA), zinc sulphate hepta (purity ≥99.5%), butyl methacrylate (BMA, purity ≥99%), sodium dodecyl benzene sulfonate (SDBS), dichloromethane (purity ≥99.5%) and ethyl acetate (purity ≥99.5%) were purchased from Sinopharm Chemical Reagent Co. Ltd. Temperature dye (TD) microcapsules were provided by Shenzhen Qianse New Material Technology Co., Ltd. Photochromic dye (PD) microcapsules were purchased from Shanghai Jingyan Chemical Co., Ltd. Azodiisobutylcyanide (AIBN) was purchased from Hubei Chengfeng Chemical Co. Furthermore, other reagents were employed without further purification.2.2 Synthesis of flexible polyacrylate resin
First, a uniform mixture was obtained by dissolving 1.1 g of AIBN, 25 g of AA, 15 g of HEMA and 5 g of EHA into 60 mL of anhydrous ethanol. Afterward, the mixture was heated to 75 °C by magnetically stirring at 300 rpm. Subsequently, the solution was reacted for 8 h to prepare the polyacrylate. The detailed chemical equation is expressed in Fig. S1 (ESI†).2.3 Preparation of color-changing microcapsules
First, 5 g AA, 10 g HEMA, and 15 g BMA were dissolved in 50 mL of anhydrous ethanol, and then 0.9 g of AIBN was added. Next, the mixture was heated to 75 °C, and the reaction was continued for 8 h by magnetic stirring at 400 rpm to prepare hydroxyl polyacrylate.1.5 g of hydroxyl polyacrylate resin was dissolved in 10 g of dichloromethane and 3 g of ethyl acetate, and then 0.6 g of TD was added to the polyacrylate resin solution. Afterwards, 200 g of deionized water containing 0.75 g of SDBS was added to the above solution to form the uniform emulsion under ultrasonic conditions. The emulsion in an open flask was magnetically stirred (300 rpm) for 3 h to obtain TD microcapsules (TDMs). The preparation of PD microcapsules (PDMs) is the same as the above preparation method of TDMs.
2.4 Preparation of the TDM/PDM/polyacrylate hybrid hydrogel
20 g of zinc sulfate was mixed with 80 mL of deionized water by ultrasound treatment for 15 min to obtain a homogeneous solution. After that, 0.3 g of TDMs and 0.3 g of PDMs were added to the previous aqueous solution of zinc sulfate to obtain a zinc sulfate/TDM/PDM mixture. Finally, 30 mL of zinc sulfate/TDM/PDM solutions was mixed with 30 g of prepared polyacrylate by mechanical stirring for 10 min to obtain a TDM/PDM/polyacrylate hybrid hydrogel. In addition, 20 g of zinc sulfate was mixed with 80 mL of deionized water by ultrasound treatment for 15 min to obtain a homogeneous solution. Subsequently, 30 mL of zinc sulfate solution was mixed with 30 g of prepared polyacrylate by mechanically stirring for 10 min to obtain the control hybrid hydrogel.2.5 Characterization
Scanning electron microscopy (SEM, Regulus810) was used to observe the surface morphology of TD, PD, TDM/PDM and the hybrid hydrogel. An energy dispersive spectrometer (EDS) was used to analyze the surface elements of the hybrid hydrogel. The chemical structures of the TD, PD, polyacrylate and TDM/PDM were characterized using a Fourier transform infrared (FT-IR) spectrometer (Nicolet Nexus 470, ThermoFisher Corp. USA) in the wavenumber range from 500 to 3800 cm−1. The self-healing process of the TDM/PDM/polyacrylate hybrid hydrogel sample with a size of 10 mm × 10 mm × 2 mm was examined using an optical microscope (KEYENCE, VHX-7000). A precision digital multimeter (Keithley 2400, SMU) was employed to examine the resistance of the TDM/PDM/polyacrylate hybrid hydrogel. The I–T curves at different voltages were measured using an electrochemical workstation (GAMRY, Interface 1010B). The stress–strain curve of the TDM/PDM/polyacrylate hybrid hydrogel with a sample size of 30 mm × 10 mm × 2 mm was measured using an electronic universal testing machine (EMS303, Mark-10). The temperature of the hybrid hydrogel was measured using an infrared thermal camera (FLIRT440). The sample size is 30 mm × 20 mm × 2 mm, and the stretching rate is 100 mm min−1. The rheological properties of the hydrogels were examined using a rotational rheometer (Physica MCR 301, Anton Paar), and a gap of 2.5 mm was maintained. The pore size of the hybrid hydrogel was analyzed using a fully automated piezometric pore size analyzer (MicroActive AutoPore V 9600 Version, Micromeritics Instrument Corporation). The particle size of TDMs and PDMs was tested using a wet and dry laser particle size meter (Winner2309, Jinan Micro-Nano Particle Instrument Co.).3. Results and discussion
3.1 Preparation and characterization of colored self-healing hybrid hydrogels
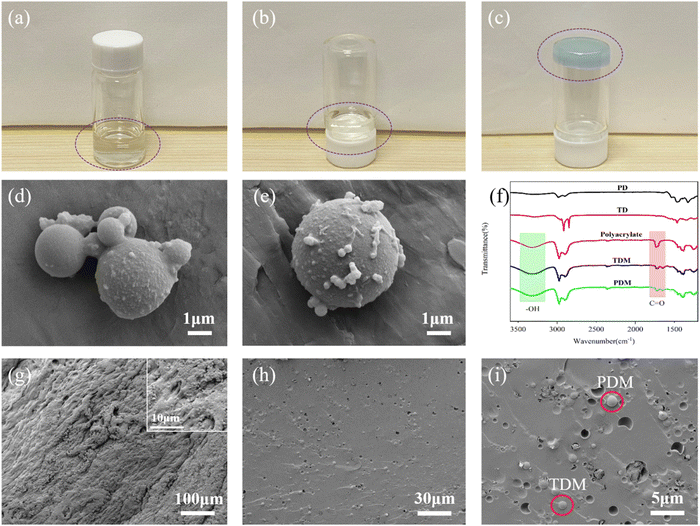
The prepared polyacrylate resin was transparent and capable of flowing (Fig. 2a and b). Subsequently, the prepared TDMs and PDMs and zinc sulphate were introduced into the polyacrylate resin to prepare colored hybrid hydrogels (Fig. 2c). In addition, rheological tests were performed on the fully gelatinized hydrogel (TDM/PDM/polyacrylate hybrid hydrogel) and the incompletely gelatinized hydrogel (TDMs/PDMs, zinc ions, and polyacrylate not fully crosslinked), respectively. As depicted in Fig. S2 (ESI†), the energy storage modulus and loss modulus in both hydrogel systems increased with the increase of frequency. The increase in the fully gelatinized hydrogel was nearly linear, and the energy storage modulus was constantly higher than the loss modulus, thus suggesting the better stability and solid state behavior of the hydrogel. However, the energy storage modulus and loss modulus of incompletely gelatinized hydrogel were significantly smaller than those of the fully gelatinized hydrogel. No significant difference was identified between the values of energy storage modulus and loss modulus, and the loss modulus was kept to be higher larger than the energy storage modulus. The above result suggested that the incompletely gelatinized hydrogel was not fully cross-linked, and it was in a semi-solid state. As depicted in Fig. 2d and e, the TD and PD microcapsules were all round in shape with a size of 2–10 μm, and their surfaces were covered with a layer of polymers. In addition, as indicated by the result of the particle size distribution test, the particle size of the PDM primarily ranged from 1 to 10 μm (Fig. S3a, ESI†), consistent with the particle size of TDMs (Fig. S3b, ESI†). Besides, the particle size distribution of both was consistent with that in the SEM images. Fig. 2f presents the FT-IR spectra of the dyes, polyacrylates, TDMs, and PDMs. A significant absorption peak appearing at around 3400 cm−1 is attributed to the –OH group, which originated from the polyacrylate. At 1720 cm−1, a new peak was identified, attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group, thus further confirming the surface coating of TD and PD microcapsules with polyacrylate. As depicted in Fig. 2g through Fig. 2i, the prepared hybrid hydrogel had a rough surface; notably, there were many holes or humps in the cross-section of the hybrid hydrogel (Fig. 2h and i), due to the addition of microcapsules. As depicted in Fig. S4a (ESI†), the process of mercury pressure and mercury withdrawal of the hydrogel after swelling indicated the different sizes of pore structures covered in the hydrogel. Fig. S4b (ESI†) presents the distribution of the pore structure of the hybrid hydrogel, with the peak of the respective stage as the maximum amount of mercury feeding the pore size. The pore size of the hydrogel macromolecular network chain after swelling and drying was primarily distributed at 10
O group, thus further confirming the surface coating of TD and PD microcapsules with polyacrylate. As depicted in Fig. 2g through Fig. 2i, the prepared hybrid hydrogel had a rough surface; notably, there were many holes or humps in the cross-section of the hybrid hydrogel (Fig. 2h and i), due to the addition of microcapsules. As depicted in Fig. S4a (ESI†), the process of mercury pressure and mercury withdrawal of the hydrogel after swelling indicated the different sizes of pore structures covered in the hydrogel. Fig. S4b (ESI†) presents the distribution of the pore structure of the hybrid hydrogel, with the peak of the respective stage as the maximum amount of mercury feeding the pore size. The pore size of the hydrogel macromolecular network chain after swelling and drying was primarily distributed at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 nm, consistent with the pore size identified using the SEM several times before swelling. Furthermore, based on the EDS mapping of the hybrid hydrogel surface elements (Fig. S5, ESI†), it comprised C, O and Zn elements, thus suggesting that zinc ions were hybridized into the polyacrylate hydrogel.
000 nm, consistent with the pore size identified using the SEM several times before swelling. Furthermore, based on the EDS mapping of the hybrid hydrogel surface elements (Fig. S5, ESI†), it comprised C, O and Zn elements, thus suggesting that zinc ions were hybridized into the polyacrylate hydrogel.
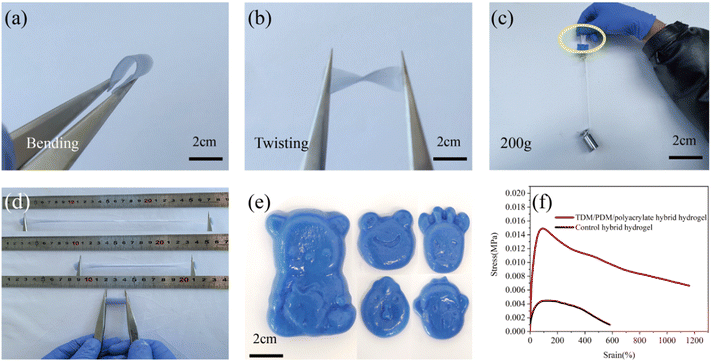
The prepared TDM/PDM/polyacrylate hybrid hydrogel was easy to bend and twist without leaving any marks (Fig. 3a and b), thus illustrating an excellent flexible property. Even under 200 g of weight load, the hybrid hydrogel was not pulled off (Fig. 3c), and the TDM/PDM/polyacrylate hybrid hydrogel stretched from 20 mm to 2380 mm (Fig. 3d). As depicted in Fig. 3e, the hybrid hydrogel easily formed a considerable number of interesting shapes through the different moulds, thus having a promising application in the personalization based on people's requirements. Next, the mechanical property of the hybrid hydrogel was investigated. As depicted in Fig. 3f, the tensile property of the control hydrogel without the TDM/PDM was significantly poorer than that of the TDM/PDM/polyacrylate hybrid hydrogel. The maximum stress of the TDM/PDM/polyacrylate hybrid hydrogel reached 0.015 MPa, significantly higher than that of the control hybrid hydrogel (0.0042 MPa), and the corresponding strain of the TDM/PDM/polyacrylate hybrid hydrogel with 1180% was twice as much as that of the control hybrid hydrogel (590%). Notably, the enhanced mechanical properties of the hydrogel formed after cross-linking were primarily dependent on the coordination bonding formed by the zinc ion and the polyacrylate and the multiple hydrogen bonding formed by the TDM and the PDM with the polyacrylate. Furthermore, the mechanical properties of some of the hydrogels that were reported were compared (Table S1, ESI†). The stress and strain of the hydrogels were closely correlated with the sample size, and the hydrogel prepared in this study exhibited a large strain at a smaller fracture stress, thus suggesting that the hydrogel exhibited better flexibility.
3.2 Self-healing property of the TDM/PDM/polyacrylate hybrid hydrogel
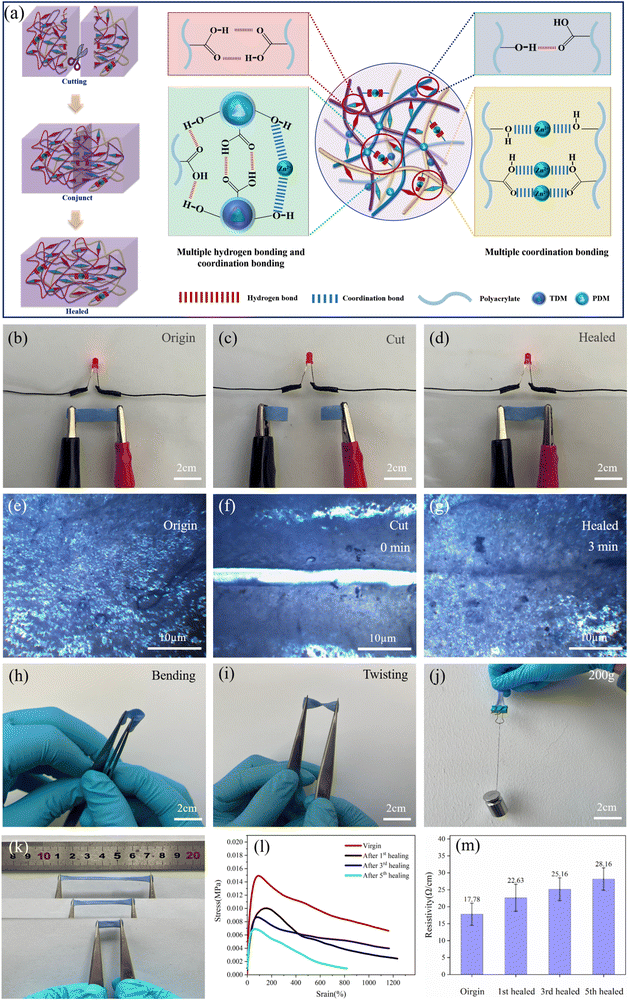
Fig. 4a illustrates the mechanism of self-healing of the TDM/PDM/polyacrylate hybrid hydrogel. The cut hybrid hydrogel was repaired in 3 min. The major reason for this result is that the hydroxyl and carboxyl groups of the polyacrylate were prone to form multi-hydrogen bonds with TDMs and PDMs. Besides, the hydroxyl or carboxyl groups of polyacrylates and TDMs/PDMs formed coordination bonds with zinc ions. It is noteworthy that the rapid formation of multiple hydrogen bonds and coordination bonds led to the self-healing property of the TDM/PDM/polyacrylate hybrid hydrogel. The effect of different proportions of zinc ions on the self-healing rate of the TDM/PDM/polyacrylate hybrid hydrogel was further characterized. The self-healing efficiency is written as follows: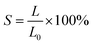 | (1) |
3.3 Sensing ability of the TDM/PDM/polyacrylate hybrid hydrogel
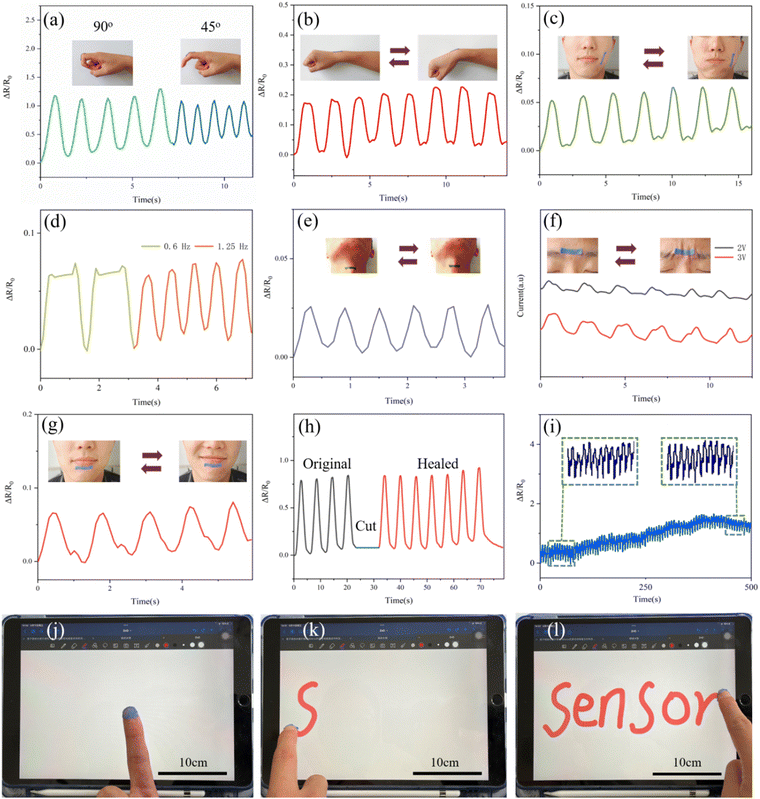
The TDM/PDM/polyacrylate hybrid hydrogel may have promising applications in sensing for its superior conductivity and flexibility. Based on this, the human activity sensing of the hydrogel was investigated (ΔR/R0, where R0 denotes the original resistance value of the hydrogel; ΔR represents the absolute value of the change in resistance produced). The sensing performance of the hybrid hydrogel was first examined under the bending of 45° and 90°, respectively. Notably, the ΔR/R0 values increased with the increase of the angle of bending, and the hybrid hydrogel rapidly changed with the changing angle (Fig. 5a). When this hybrid hydrogel was attached to the wrist of a wearer with repeated bending, the current signal was easily detected by the expression of ΔR/R0 (Fig. 5b). Moreover, the ΔR/R0 values of the hybrid hydrogel attached to the cheek of a wearer was changing with the blowing movement of the mouth of the wearer (Fig. 5c); its ΔR/R0 values changed at different frequencies (Fig. 5d), such that the facial movement can be quickly and accurately detected. Furthermore, the hybrid hydrogel attached to the larynx of a wearer even detected the change in resistance as swallowing food (Fig. 5e). The facial micro-movement of the wearer was further examined. As depicted in Fig. 5f and g, the hybrid hydrogel attached to the wearer's glabellum or jaw expressed the change in signal accurately when smiling (Fig. 5g). All the above results suggest that the TDM/PDM/polyacrylate hybrid hydrogel can be applied to the throat or face to collect the change sensing signal even under minor changes. The transient response of the damaged hybrid hydrogel was recovered again immediately after the repair though the transient response corresponding to the cut TDM/PDM/polyacrylate hybrid hydrogel disappeared when the hybrid hydrogel was stretched (Fig. 5h). Besides, the TDM/PDM/polyacrylate hybrid hydrogel maintained the high response state even after 500 bending/recovering cycles (Fig. 5i). In addition, this TDM/PDM/polyacrylate hybrid hydrogel attached to a wearer's finger wrote a word “Sensor” on the screen, and it was sufficiently responsive and accurate to serve as an electronic skin (Fig. 5j–l). Accordingly, the above beneficial effects reveal that the TDM/PDM/polyacrylate hybrid hydrogel has promising sensing applications in movement monitoring, body information capture, smart screen writing, and so forth.3.4 Bio-inspired color-changing and self-healing properties of the TDM/PDM/polyacrylate hybrid hydrogel
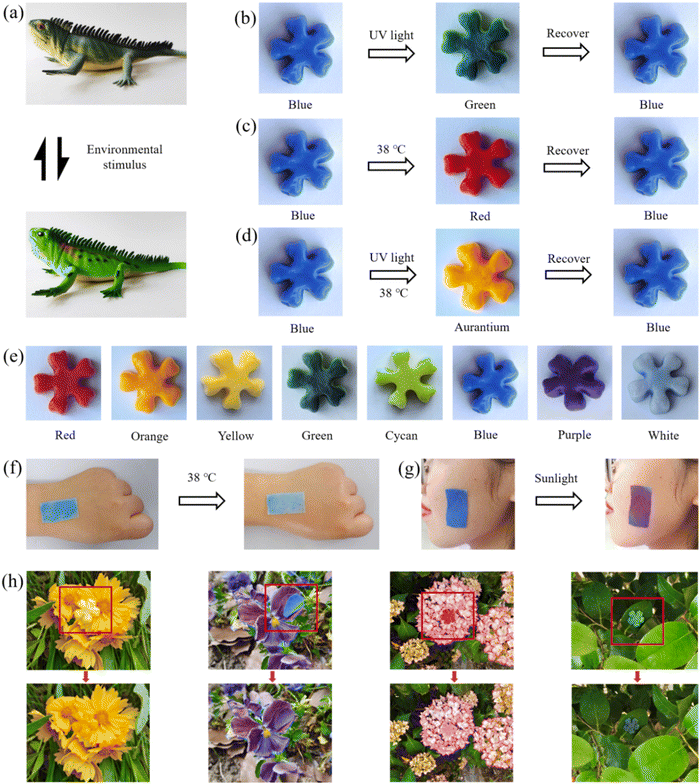
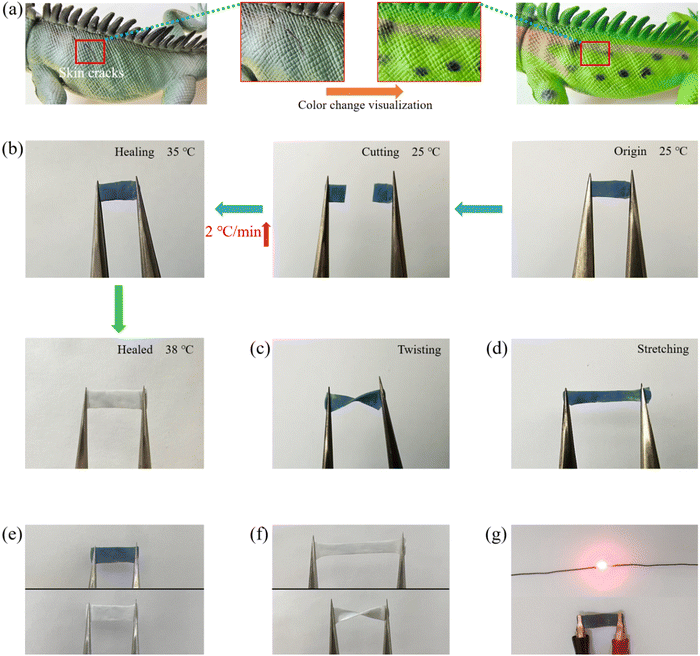
The body surface of a chameleon is colorful, thus changes its color under different environmental conditions (Fig. 6a) (e.g., sunlight and temperature). The TDM and the PDM were introduced into the self-healing hydrogel to inspire its color changing property (e.g., chameleon organisms). The TD was formed by coalescing and curing the wall material through physical changes and encapsulating the core material, which was prepared from an electron transfer organic compound system. Besides, triaryl and phthalides were employed as the electron donors to provide the color changing substrate, and phenol was adopted as the electron acceptor to provide the color shade. The relative change in the redox potential between the electron donor and the electron acceptor with similar initial potentials under thermal stimulation at 38 °C was different, thus resulting in electron transfer between the electron donor and the electron acceptor. As a result, the molecular structure of the electron donor and the color changed.57–59 The PD was reversible for color change under UV radiation, which could activate the diaryl ethylene isomer of the thermodynamically stable organic photochromic compound in it, allowing it to undergo reversible opened-loop and closed-loop transitions and to repeat the color change several times.60–63 The polyacrylate coating of TD and PD microcapsules did not affect their discoloration properties. The color of TDM aqueous solution could be changed from blue to white when heated to 38 °C (Fig. S7, ESI†), while the color of the PDM aqueous solutions changed from white to deep red under UV light irradiation (Fig. S8, ESI†). After TDM and PDM were added into the polyacrylate, respectively, the formed TDM/polyacrylate hybrid hydrogel and PDM/hybrid hydrogel could also change their color under heating or UV light irradiation (Fig. S9 and S10, ESI†). From this, it is possible to combine different TD and PD microcapsules, thus enabling multiple colors to be switched freely. Accordingly, the color changing property of the TDM/PDM/polyacrylate hybrid hydrogel was further investigated under different environmental conditions. As depicted in Fig. 6b, the blue TDM/PDM/polyacrylate hybrid hydrogel turned green under UV light irradiation. When the UV light was removed, the hybrid hydrogel could return to its original color blue again. Analogously, TDM/PDM/polyacrylate hybrid hydrogel could change its blue to deep red at 38 °C, and its original color blue was restored when the environmental temperature was below 38 °C (Fig. 6c). Interestingly, the color of the TDM/PDM/polyacrylate hybrid hydrogel changed from blue to aurantium under the action of combined heating and UV light irradiation, and the color of it was reverted to its original color after removing the combine action (Fig. 6d). It is noteworthy that this TDM/PDM/polyacrylate hybrid hydrogel can show a wide range of color changing like a chameleon (e.g., deep red, yellow, green, blue, and white) under different external stimuli (e.g., UV light and temperature) (Fig. 5e). Moreover, the dark or light color of the TDM/PDM/polyacrylate hybrid hydrogel was shown by adjusting the amount of TDMs/PDMs in the hydrogel, such that the TDM/PDM/polyacrylate hybrid hydrogel changed to different colors in complex environments. When this TDM/PDM/polyacrylate hybrid hydrogel was placed on the skin of a wearer, its color dramatically changed from blue to white (Fig. 6f), reminding that her or his body temperature may be over 38 °C. Moreover, the TDM/PDM/polyacrylate hybrid hydrogel attached to the cheek of a wearer changed its color rapidly from blue to violet under strong sunlight (Fig. 6g), which indicates that UV light from sunlight at this time is strong and may damage her or his skin. Furthermore, the above color-changing hybrid hydrogels on the surfaces of flowers and leaves changed their color to the corresponding color of flowers or leaves (Fig. 6h). This excellent color changing property (e.g., a chameleon) will provide a potential application in the outdoor stealth garment.When the skin of a chameleon was injured, the chameleon could repair its skin and the color changing property was also recovered (Fig. 7a). In addition, self-healing of the TDM/PDM/polyacrylate hybrid hydrogel was visualized. The two parts of cut TDM/PDM/polyacrylate hybrid hydrogel came into contact with each other under heating from 25 °C at a rate of 2 °C min−1, and the hybrid hydrogel healed (Fig. 7b). With the rise of the temperature to 38 °C at 2 °C min−1, the TDM/PDM/polyacrylate hybrid hydrogel changed its color from blue to white, while this damaged hybrid hydrogel achieved the self-healing in 3 min. Notably, the occurrence of the discoloration can indicate that the hydrogel has achieves its self-healing. The above visual healing process was even more significant than microscopic microscopy and fluorescent labelling to monitor the self-healing process. After the hybrid hydrogel was placed back in the 25 °C environment, the color of the repaired hybrid hydrogel turned to blue, and the hydrogel sustained the twisting and stretching action without any cracks (Fig. 7c and d). Furthermore, the repaired TDM/PDM/polyacrylate hybrid hydrogel could change color rapidly (Fig. 7e). The discolored TDM/PDM/polyacrylate hybrid hydrogel also exhibited excellent stretching and twisting properties (Fig. 7f). The LED lamp could be lighted again by the repaired hybrid hydrogel (Fig. 7g), suggesting that its electrical conductivity was also recovered.
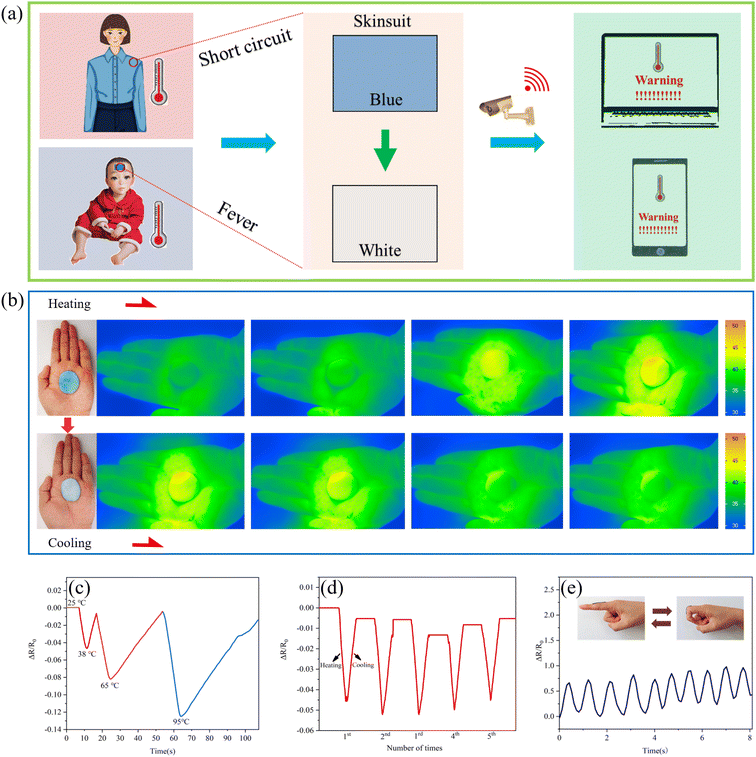
Due to the color changing property and conductivity of the TDM/PDM/polyacrylate hybrid hydrogel, it can be applied to the monitoring of body temperature change based on color or resistance changes. As depicted in Fig. 8a, when a short circuit occurred in the TDM/PDM/polyacrylate hybrid hydrogel attached to the body, the monitored signal was immediately transmitted to a computer or mobile phone for early warning, so that the problem could be solved in time. Moreover, when a child had a fever, the TDM/PDM/polyacrylate hybrid hydrogel attached to the forehead was able to show a rapid color change, warning that the child had a temperature well above normal. To prove the above results, the TDM/PDM/polyacrylate hybrid hydrogel was placed on the palm of a hand to detect surface temperature change using a thermal camera, thus suggesting that the TDM/PDM/polyacrylate hybrid hydrogel was more sensitive to temperature responses at around 38 °C (Fig. 8b). Furthermore, the TDM/PDM/polyacrylate hybrid hydrogel exhibited various resistance change ratio (ΔR/R0) when placed in different temperature environments (e.g., 25 °C, 38 °C, 65 °C, and 95 °C) (Fig. 8c). After 5 heating/cooling cycles between 25 °C and 38 °C, the resistance change ratio (ΔR/R0) of the TCP/PCP/polyacrylate hybrid hydrogel still varied significantly (Fig. 8d). However, its resistance change ratio (ΔR/R0) changed slightly at 25 °C. Furthermore, at 38 °C, the hybrid hydrogel still exhibited a sensing property quickly responding to the angle of a finger (Fig. 8e).
4. Conclusion
In brief, a color-changing and self-healing TDM/PDM/polyacrylate hybrid hydrogel was well prepared by mixing the thermochromic dye microcapsules and photochromic dye microcapsules with the multi-branched polyacrylate. The prepared hybrid hydrogel exhibiting high conductivity and good tensile properties can serve as a sensor to monitor the actions of human joints or faces. Due to the robust ionic and multiple hydrogen bonding among the polyacrylate, zinc ions and TDM/PDM, the hybrid hydrogel exhibited an excellent self-healing capability, and it restores its mechanical, conductive and sensing properties after the self-healing process. Furthermore, the combination of TDM/PDM and polyacrylate synergistically enables the hybrid hydrogel to change color rapidly under heating or UV irradiation along with the variation of electrical resistance, which can be applied to the monitoring of human physiological health and for adaptive camouflage. The TDM/PDM/polyacrylate hybrid hydrogel prepared in this study exhibited a unique combination of self-healing, conductivity and color changing in a balanced way. The prepared self-healing and color-changing TDM/PDM/polyacrylate hybrid hydrogel will have extensive applications in human life activity monitoring, health monitoring, and safety warning in the future.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202206), and the Fundamental Research Funds for the Central Universities (JUSRP11918).References
- Q. Gao, B. A. F. Kopera, J. Zhu, X. Liao, C. Gao, M. Retsch, S. Agarwal and A. Greiner, Breathable and flexible polymer membranes with mechanoresponsive electric resistance, Adv. Funct. Mater., 2020, 30, 1907555 CrossRef CAS.
- Y. Qiao, Y. Wang, H. Tian, M. Li, J. Jian, Y. Wei, Y. Tian, D. Y. Wang, Y. Pang, X. Geng, X. Wang, Y. Zhao, H. Wang, N. Deng, M. Jian, Y. Zhang, R. Liang, Y. Yang and T. L. Ren, Multilayer graphene epidermal electronic skin, ACS Nano, 2018, 12, 8839–8846 CrossRef CAS.
- M. Yang, Y. Cheng, Y. Yue, Y. Chen, H. Gao, L. Li, B. Cai, W. Liu, Z. Wang, H. Guo, N. Liu and Y. Gao, High-performance flexible pressure sensor with a self-healing function for tactile feedback, Adv. Sci., 2022, e2200507 CrossRef.
- X. Liang, M. Zhu, H. Li, J. Dou, M. Jian, K. Xia, S. Li and Y. Zhang, Hydrophilic, breathable, and washable graphene decorated textile assisted by silk sericin for integrated multimodal smart wearables, Adv. Funct. Mater., 2022, 2200162 CrossRef CAS.
- Z. Wei, T. Liu, L. Zhang and J. Yu, Sulfide-based nickel-plated fabrics for foldable quasi-solid-state supercapacitors, Energy & Environ. Mater., 2022, 5, 883–893 Search PubMed.
- H. Wu, Z. Su, M. Shi, L. Miao, Y. Song, H. Chen, M. Han and H. Zhang, Self-powered noncontact electronic skin for motion sensing, Adv. Funct. Mater., 2017, 28, 1704641 CrossRef.
- X. Wu, J. Zhu, J. W. Evans, C. Lu and A. C. Arias, A potentiometric electronic skin for thermosensation and mechanosensation, Adv. Funct. Mater., 2021, 31, 2010824 CrossRef CAS.
- Y. Wang, H. Mao, Y. Wang, P. Zhu, C. Liu and Y. Deng, 3D geometrically structured PANI/CNT-decorated polydimethylsiloxane active pressure and temperature dual-parameter sensors for man–machine interaction applications, J. Mater. Chem. A, 2020, 8, 15167–15176 RSC.
- Y. Gao, F. Jia and G. Gao, Transparent and conductive amino acid-tackified hydrogels as wearable strain sensors, Chem. Eng. J., 2019, 375, 121915 CrossRef CAS.
- J. Zeng, L. Dong, L. Sun, W. Wang, Y. Zhou, L. Wei and X. Guo, Printable zinc-ion hybrid micro-capacitors for flexible self-powered integrated units, Nano-Micro Lett., 2020, 13, 19 CrossRef.
- X. Huang, W. Guo, S. Liu, Y. Li, Y. Qiu, H. Fang, G. Yang, K. Zhu, Z. Yin, Z. Li and H. Wu, Flexible mechanical metamaterials enabled electronic skin for real-time detection of unstable grasping in robotic manipulation, Adv. Funct. Mater., 2022, 2109109 CrossRef CAS.
- H. Yuan, T. Lei, Y. Qin and R. Yang, Flexible electronic skins based on piezoelectric nanogenerators and piezotronics, Nano Energy, 2019, 59, 84–90 CrossRef CAS.
- S. Zhao and R. Zhu, Electronic skin with multifunction sensors based on thermosensation, Adv. Mater., 2017, 29, 1606151 CrossRef.
- M. Vosgueritchian, J. B. H. Tok and Z. Bao, Light-emitting electronic skin, Nat. Photonics, 2013, 7, 769–771 CrossRef CAS.
- W. Cao, Z. Wang, X. Liu, Z. Zhou, Y. Zhang, S. He, D. Cui and F. Chen, Bioinspired MXene-based user-interactive electronic skin for digital and visual dual-channel sensing, Nanomicro. Lett., 2022, 14, 119 CAS.
- Y. Feng, H. Liu, W. Zhu, L. Guan, X. Yang, A. V. Zvyagin, Y. Zhao, C. Shen, B. Yang and Q. Lin, Muscle-inspired MXene conductive hydrogels with anisotropy and low-temperature tolerance for wearable flexible sensors and arrays, Adv. Funct. Mater., 2021, 31, 2105264 CrossRef CAS.
- H. Chen, Y. Song, X. Cheng and H. Zhang, Self-powered electronic skin based on the triboelectric generator, Nano Energy, 2019, 56, 252–268 CrossRef CAS.
- M. Zhu, J. Li, J. Yu, Z. Li and B. Ding, Superstable and intrinsically self-healing fibrous membrane with bionic confined protective structure for breathable electronic skin, Angew. Chem., Int. Ed., 2022, 61, e202200226 CAS.
- X. Tang, C. Wu, L. Gan, T. Zhang, T. Zhou, J. Huang, H. Wang, C. Xie and D. Zeng, Multilevel microstructured flexible pressure sensors with ultrahigh sensitivity and ultrawide pressure range for versatile electronic skins, Small, 2019, 15, e1804559 CrossRef.
- K. Hu, R. Xiong, H. Guo, R. Ma, S. Zhang, Z. L. Wang and V. V. Tsukruk, Self-powered electronic skin with biotactile selectivity, Adv. Mater., 2016, 28, 3549–3556 CrossRef CAS.
- S. Xiang, D. Liu, C. Jiang, W. Zhou, D. Ling, W. Zheng, X. Sun, X. Li, Y. Mao and C. Shan, Liquid-metal-based dynamic thermoregulating and self-powered electronic skin, Adv. Funct. Mater., 2021, 31, 2100940 CrossRef CAS.
- C. H. Lu, W. Guo, Y. Hu, X. J. Qi and I. Willner, Multitriggered shape-memory acrylamide-DNA hydrogels, J. Am. Chem. Soc., 2015, 137, 15723–15731 CrossRef CAS PubMed.
- X. Han, X. Chen, X. Tang, Y. Chen, J. Liu and Q. D. Shen, Flexible polymer transducers for dynamic recognizing physiological signals, Adv. Funct. Mater., 2016, 26, 3640–3648 CrossRef CAS.
- H. Liu, Y. Ni, J. Hu, Y. Jin, P. Gu, H. Qiu and K. Chen, Self-healing and antibacterial essential oil-loaded mesoporous silica/polyacrylate hybrid hydrogel for high-performance wearable body-strain sensing, ACS Appl. Mater. Interfaces, 2022, 14, 21509–21520 CrossRef CAS.
- Z. Chen, D. Zhao, B. Liu, G. Nian, X. Li, J. Yin, S. Qu and W. Yang, 3D printing of multifunctional hydrogels, Adv. Funct. Mater., 2019, 29, 1900971 CrossRef.
- J. Park, S. Pramanick, D. Park, J. Yeo, J. Lee, H. Lee and W. J. Kim, Therapeutic-gas-responsive hydrogel, Adv. Mater., 2017, 29, 1702859 CrossRef PubMed.
- F. Guan, B. Yang, M. Han, C. Ling, H. Yin, P. Yang, X. Zhao and H. Yu, Sustainable cellulose-nanofiber-based hydrogels, ACS Nano, 2021, 15, 7889–7898 CrossRef PubMed.
- X. Liu, C. Steiger, S. Lin, G. A. Parada, J. Liu, H. F. Chan, H. Yuk, N. V. Phan, J. Collins, S. Tamang, G. Traverso and X. Zhao, Ingestible hydrogel device, Nat. Commun., 2019, 10, 493 CrossRef CAS.
- X. Liu, J. Liu, S. Lin and X. Zhao, Hydrogel machines, Mater. Today, 2020, 36, 102–124 CrossRef CAS.
- C. Calvino, A. Guha, C. Weder and S. Schrettl, Self-calibrating mechanochromic fluorescent polymers based on encapsulated excimer-forming dyes, Adv. Mater., 2018, 30, e1704603 CrossRef.
- J. Cai, W. Luo, J. Pan, G. Li, Y. Pu, L. Si, G. Shi, Y. Shao, H. Ma and J. Guan, Glucose-sensing photonic nanochain probes with color change in seconds, Adv. Sci., 2022, 9, e2105239 CrossRef.
- G. Lee, M. Kong, D. Park, J. Park and U. Jeong, Electro-photoluminescence color change for deformable visual encryption, Adv. Mater., 2020, 32, e1907477 CrossRef.
- C. B. Highley, C. B. Rodell and J. A. Burdick, Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels, Adv. Mater., 2015, 27, 5075 CrossRef CAS PubMed.
- A. Naranjo, C. Martín, A. López-Díaz, A. Martín-Pacheco, A. M. Rodríguez, F. J. Patiño, M. A. Herrero, A. S. Vázquez and E. Vázquez, Autonomous self-healing hydrogel with anti-drying properties and applications in soft robotics, Appl. Mater. Today, 2020, 21, 100806 CrossRef.
- C. Xu, Z. Chen, C. Wang and K. Chen, Fabrication of dual self-healing multifunctional coating based on multicompartment microcapsules, ACS Appl. Mater. Interfaces, 2021, 13, 59298–59309 CrossRef CAS.
- X. Zhu, W. Zheng, H. Zhao and L. Wang, Non-covalent assembly of a super-tough, highly stretchable and environmentally adaptable self-healing material inspired by nacre, J. Mater. Chem. A, 2021, 9, 20737–20747 RSC.
- X. Xu, V. V. Jerca and R. Hoogenboom, Bio-inspired hydrogels as multi-task anti-icing hydrogel coatings, Chem., 2020, 6, 820–822 CAS.
- V. Venkatesh, N. K. Mishra, I. Romero-Canelon, R. R. Vernooij, H. Shi, J. P. C. Coverdale, A. Habtemariam, S. Verma and P. J. Sadler, Supramolecular photoactivatable anticancer hydrogels, J. Am. Chem. Soc., 2017, 139, 5656–5659 CrossRef CAS.
- X. Long, X. Xu, D. Sun, Y. Hong, C. Wen, Y. Xie, B. Yan, H. Zhang, Q. Ge, W. Li, L. Duan, H. Ouyang and D. Wang, Biomimetic macroporous hydrogel with a triple-network structure for full-thickness skin regeneration, Appl. Mater. Today, 2022, 27, 101442 CrossRef.
- J. Wang, J. He, L. Ma, Y. Zhang, L. Shen, S. Xiong, K. Li and M. Qu, Multifunctional conductive cellulose fabric with flexibility, superamphiphobicity and flame-retardancy for all-weather wearable smart electronic textiles and high-temperature warning device, Chem. Eng. J., 2020, 390, 124508 CrossRef.
- Y. Hu, W. Guo, J. Kahn, M. Aleman-Garcia and I. Willner, A shape-memory DNA-based hydrogel exhibiting two internal memories, Angew. Chem., Int. Ed., 2016, 55, 42104 Search PubMed.
- L. Li, Y. Bai, L. Li, S. Wang and T. Zhang, A superhydrophobic smart coating for flexible and wearable sensing electronics, Adv. Mater., 2017, 29, 1702517 CrossRef PubMed.
- L. Chen, Y. Zhang, H. Ye, G. Duan, H. Duan, Q. Ge and Z. Wang, Color-changeable four-dimensional printing enabled with utraviolet-curable and thermochromic shape memory polymers, ACS Appl. Mater. Interfaces, 2021, 13, 18120–18127 CrossRef CAS.
- B. Liu, A. Rasines Mazo, P. A. Gurr and G. G. Qiao, Reversible nontoxic thermochromic microcapsules, ACS Appl. Mater. Interfaces, 2020, 12, 9782–9789 CrossRef CAS.
- G. Isapour and M. Lattuada, Bioinspired stimuli-responsive color-changing systems, Adv. Mater., 2018, 30, e1707069 CrossRef.
- Q. Wang, T. Ruan, Q. Xu, B. Yang and J. Liu, Wearable multifunctional piezoelectric MEMS device for motion monitoring, health warning, and earphone, Nano Energy, 2021, 89, 106324 CrossRef CAS.
- D. K. Macharia, S. Ahmed, B. Zhu, Z. Liu, Z. Wang, J. I. Mwasiagi, Z. Chen and M. Zhu, UV/NIR-light-triggered rapid and reversible color switching for rewritable smart fabrics, ACS Appl. Mater. Interfaces, 2019, 11, 13370–13379 CrossRef CAS.
- Z. Xu, L. Li, L. Du, L. Wang, L. Shi, K. Yang and Y. Wang, Multiscale shape-memory effects in a dynamic polymer network for synchronous changes in color and shape, Appl. Mater. Today, 2022, 26, 101276 CrossRef.
- P. Lin, T. Zhang, X. Wang, B. Yu and F. Zhou, Freezing molecular orientation under stretch for high mechanical strength but anisotropic hydrogels, Small, 2016, 12, 4386–4392 CrossRef CAS.
- B. Ryplida, K. D. Lee, I. In and S. Y. Park, Light-induced swelling-responsive conductive, adhesive, and stretchable wireless film hydrogel as electronic artificial skin, Adv. Funct. Mater., 2019, 29, 1903209 CrossRef.
- J. Wei, F. Wan, P. Zhang, Z. Zeng, H. Ping, J. Xie, Z. Zou, W. Wang, H. Xie, Z. Shen, L. Lei and Z. Fu, Bioprocess-inspired synthesis of printable, self-healing mineral hydrogels for rapidly responsive, wearable ionic skin, Chem. Eng. J., 2021, 424, 130549 CrossRef CAS.
- J. Sun, J. Wang, M. Chen, X. Pu, G. Wang, L. Li, G. Chen, Y. Cai, X. Gu and B. Z. Tang, Fluorescence turn-on visualization of microscopic processes for self-healing gels by aIEgens and anticounterfeiting application, Chem. Mater., 2019, 31, 5683–5690 CrossRef CAS.
- J. Wu, Z. Wu, X. Lu, S. Han, B. R. Yang, X. Gui, K. Tao, J. Miao and C. Liu, Ultrastretchable and stable strain sensors based on antifreezing and self-healing ionic organohydrogels for human motion monitoring, ACS Appl. Mater. Interfaces, 2019, 119, 9405–9414 CrossRef PubMed.
- J. Zhou, H. Liu, Y. Sun, C. Wang and K. Chen, Self-healing titanium dioxide nanocapsules-graphene/multi-branched polyurethane hybrid flexible film with multifunctional properties toward wearable electronics, Adv. Funct. Mater., 2021, 31, 2011133 CrossRef CAS.
- S. A. Odom, A. C. Jackson, A. M. Prokup, S. Chayanupatkul, N. R. Sottos, S. R. White and J. S. Moore, Autonomous indication of mechanical damage in polymeric coatings, Adv. Mater., 2016, 28, 2189–2194 CrossRef PubMed.
- Q. Ding, Z. Wu, K. Tao, Y. Wei, W. Wang, B. R. Yang, X. Xie and J. Wu, Environment tolerant, adaptable and stretchable organohydrogels: preparation, optimization, and applications, Mater. Horiz., 2022, 95, 1356–1386 RSC.
- S. Gupta and M. Milton, Y-shaped novel AIEE active push-pull quinoxaline derivatives displaying acidochromism and use towards white light emission by controlled protonation, Dyes Pigm., 2021, 195, 109690 CrossRef CAS.
- G. Turkoglu, M. Cinar and T. Ozturk, Synthesis and photophysical and anion-sensing properties of triarylborane-substituted cross-conjugated and conjugated thienothiophenes, Eur. J. Org. Chem., 2017, 4552–4561 CrossRef CAS.
- E. Odella, M. Secor, E. Cruz, W. Guerra, M. Urrutia, P. Liddell, T. Moore, G. Moore, S. Schiffer and A. Moore, Managing the redox potential of PCET in grotthuss-type proton wires, J. Am. Chem. Soc., 2022, 144, 15672–15679 CrossRef CAS.
- H. Liu, S. Pu, G. Liu and B. Chrn, Photochromism of asymmetrical diarylethenes with a pyrimidine unit: synthesis and substituent effects, Dyes Pigm., 2014, 102, 159–168 CrossRef CAS.
- C. Fan, S. Pu and G. Liu, Effects of solvents on the growth of an asymmetrical photochromic diarylethene crystal, Dyes Pigm., 2015, 113, 61–69 CrossRef CAS.
- H. Liu, H. Gao, S. Wang, S. Yao, F. Wu, Y. Zhao, K. Chan and Z. Shen, Regulation of an ambient-light-induced photocyclization pathway (norrish-yang versus 6π) by Substituent Choice, Chem. – Eur. J., 2020, 26, 12418–12430 CrossRef CAS.
- M. Irie, H. T. Fukaminato, K. Matsuda and S. Kobatake, Photochromism of diarylethene molecules and crystals: memories, switches, and actuators, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2tc03102g |
| This journal is © The Royal Society of Chemistry 2023 |