Open Access Article
Open Access ArticleInvestigating the urinary concentrations and distribution of phthalate metabolites in cow urine distillate in India†
Sachin B.
Jorvekar
 ,
Jaya Ajay
Singh
,
Manthan
Sharma
,
Gayatri
Narkhede
,
Rahul
Moriya
,
Dhanashri
Pimpare
and
Roshan M.
Borkar
,
Jaya Ajay
Singh
,
Manthan
Sharma
,
Gayatri
Narkhede
,
Rahul
Moriya
,
Dhanashri
Pimpare
and
Roshan M.
Borkar
 *
*
Department of Pharmaceutical Analysis, National Institute of Pharmaceutical Education and Research (NIPER), Guwahati, Changsari, 781101, India. E-mail: roshanudps@gmail.com; roshan.borkar@niperguwahati.ac.in; roshan@niperguwahati.in
First published on 27th April 2023
Abstract
The exposure of cows to a variety of endocrine-disrupting chemicals has been linked to the development of diseases and poses a significant concern. In India, distillates of cow urine are utilized in numerous products such as food, medicine, and cosmetics. The safety of these products is being questioned due to the potential presence of endocrine-disrupting chemicals as a cow is exposed to these chemicals through multiple pathways. Hence, we determine phthalate metabolites in cow urine distillate products by LC-MS/MS. The calibration curves for all phthalate metabolites ranged from 1 ng mL−1 to 200 ng mL−1. The accuracy and precision of phthalate metabolites at all concentrations examined were well within acceptable limits at inter-day and intra-day levels. Almost all the cow urine distillate samples showed a higher proportion of low molecular weight phthalates. The mean concentrations of phthalate metabolites were 407.6 ng mL−1 (mono-methyl phthalate, MMP), 35.6 ng mL−1 (mono-isobutyl phthalate, MiBP) and 22.25 ng mL−1 (mono-ethyl phthalate, MEP). MMP, MiBP and MEP were predominant in cow urine distillate accounting for 86.1%, 7.5% and 4.7%, respectively. Investigations are required to identify the source and scale of endocrine-disrupting chemicals in cow urine and the risk of contamination in its products. This information can guide the development of strategies to minimize endocrine-disrupting chemical exposure and ensure the safety of animal-derived products. Additionally, it can provide insight into the impact on cow health.
Environmental significancePhthalate metabolites were found to be more dominant in cow urine distillate products. Investigations are required to identify the source and scale of endocrine-disrupting chemicals in cow urine and the risk of contamination in its products. This information can guide the development of strategies to minimize endocrine-disrupting chemical exposure and ensure the safety of animal-derived products. |
1. Introduction
Phthalates are a type of ester derived from phthalic acid and are categorized into high- and low-molecular-weight varieties. High-molecular-weight phthalates, such as di(2-ethylhexyl) phthalate (DEHP), di-isononyl phthalate (DiNP), butyl benzyl phthalate (BBzP), and dioctyl phthalate (DOP), are commonly used as plasticizers in the production of products such as PVC building materials, flooring, food packaging, and medical devices. Low-molecular-weight phthalates, including di-n-butyl phthalate (DnBP), dimethyl phthalate (DMP), and di-isobutyl phthalate (DiBP), are frequently utilized in personal care products. Given the widespread presence of phthalates in everyday items, it is difficult to avoid exposure to them. Unfortunately, some of these substances disrupt the endocrine system, potentially impacting human reproductive health. Due to the lack of chemical bonding between phthalates and the polymer matrix, they can migrate out of phthalate-containing plastics and enter the environment, resulting in widespread human exposure.1 Studies have also shown a link between phthalates found in the environment and human exposure.2–6 Phthalate toxicity is believed to be greatly influenced by nuclear receptors, hormone sensitizers, and additional transcription factors connected to the endocrine system. Due to their high octanol–water and octanol–air partition coefficients, the phthalates DEHP, DiNP, and DiDP are more prone to bioaccumulation in lipid-rich tissues. Studies have indicated a potential connection between exposure to phthalates and developing allergies and asthma. A recent study revealed that even when only four phthalates were considered, one out of every five children was exposed to an amount of phthalates that could potentially cause antiandrogenic effects. Children in areas with a high concentration of factories are especially prone to developing health issues due to phthalate exposure. Studies have indicated a connection between oxidative stress and obesity with phthalates, as well as bisphenol exposure and its associative effects on oxidant stress, insulin resistance, and endothelial dysfunction in children and adolescents.7,8 Higher levels of phthalate exposure have been associated with serious illnesses such as diabetes, thyroid disease, kidney damage, endometriosis, and osteoporosis.9–14 High concentrations of DEHP metabolites have been strongly linked to autism.15The medicinal properties of cow urine have been long recognized in Ayurvedic scriptures, such as the Charaka Samhita and Sushruta Samhita. Cow urine has been found to contain anti-infective, anti-cancer, antioxidant, antibiotic, and nutrient elements, and as a result, a US patent has been granted for its medicinal properties.16–18 It has been used to create several products, such as soap, shampoo, face wash, natural fragrance, and oil, which have gained widespread popularity in the consumer market. The presence of allantoin, which has multiple effects on human skin, such as keratolytic, wound healing, anti-irritant, and synthesis of the extracellular matrix, has made cow urine distillate a highly sought-after ingredient in India's cosmetic products. In addition to its medicinal properties, cow urine is also considered holy due to its spiritual therapeutic properties and the presence of the antioxidants uric acid and allantoin.19
Cow urine distillate is very popular in India, and over the years, it has built up a strong customer base. It has traditionally been sold through informal channels such as word of mouth. Ingesting items made with cow urine distillate posed a significant threat to health; animal diseases can be spread to humans. Cow urine products contain harmful strains of Mycobacterium paratuberculosis and E. coli, as identified by Washington State University in 2016. Furthermore, the Centers for Disease Control and Prevention have acknowledged the presence of mucoromycetes in animal feces.20 To ensure the safety and stability of cow urine distillate, the amount of preservative used should be kept to a minimum, and the containers used to store it should be of the highest quality. This will reduce the risk of adverse health effects and guarantee the product's safety.
Cow urine distillates are used in various products, such as food, medicine, and cosmetics. However, due to the potential presence of endocrine-disrupting chemicals (EDCs), the safety of these products is in question. Indeed, there are multiple exposure pathways for cows to EDCs, including soil and surface water. Consequently, it is essential to investigate the magnitude of exposure to EDCs in cow urine distillate to guarantee consumer safety. Additionally, strategies to reduce the risk of exposure to humans must be developed. Unfortunately, no data are currently available on the exposure of phthalate through cow urine distillate in India. Thus, in the present study, we investigated the occurrence and profiles of phthalates in marketed cow urine distillates in India.
2. Materials and methods
2.1 Standards and reagents
Nine targeted compounds, including mono-(3-carboxypropyl) phthalate (MCPP), mono-methyl phthalate (MMP), mono-ethyl phthalate (MEP), mono-(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono-(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono-isononyl phthalate (MiNP), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono-isobutyl phthalate (MiBP), and mono-benzyl phthalate (MBzP) were purchased from Sigma-Aldrich. The isotopically labeled internal standard (IS) 13C2 – di(2-ethylhexyl) phthalate (DEHP) was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). Liquid Chromatography-Mass Spectrometry (LC-MS) grade solvent acetonitrile was purchased from J.T. Baker (Center Valley, PA, USA)). Ammonium acetate, formic acid, acetic acid, sodium phosphate monobasic monohydrate and sodium phosphate dibasic monohydrate were purchased from Sigma-Aldrich (St. Louis, MO). Ultrapure water was generated through a Milli-Q water purification system.2.2 Preparation of stock solution and standard solution
Stock solutions of all 9 phthalate metabolites were prepared independently at 1 mg mL−1. Stock solutions were serially diluted (100 μL, 1 mg mL−1) with acetonitrile to generate a 100 ng mL−1 concentration of each compound. A 1 mg mL−1 stock solution of 13C2 DEHP (IS) in acetonitrile was prepared and then diluted to a total volume of 1 mL, resulting in a working concentration of 100 ng mL−1.2.3 Sample collection and sample extraction
Seventeen cow urine distillate products, purchased from local markets and online retail sites across India, were stored at room temperature. A solid phase extraction (SPE) method was used for the extraction of phthalate metabolites from cow urine distillate. Briefly, 500 μL of cow urine distillate was buffered with 500 μL of 1 M ammonium acetate (pH 5) containing 2 units per μL of β-glucuronidase and 0.5 mL Milli-Q water and was incubated at 37 °C for 14 h for deconjugation. ABS Elut-NEXUS SPE cartridges (60 mg, 3 mL, Agilent, Santa Clara, CA) were used to extract urine samples. 1.0 mL of acetonitrile and phosphate buffer (1.0 mL, pH 2) were used to precondition the cartridges. The urine sample was loaded and diluted with phosphate buffer (1 mL, pH 2). Next, 0.1 M formic acid (2 mL) and water (1.0 mL) were used to wash the cartridges. Targeted metabolites were eluted with acetonitrile (900 μL) followed by ethyl acetate (900 μL). Combined eluents were evaporated to dryness in a vacuum concentrator and reconstituted with 180 μL of acetonitrile/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 v/v) and transferred into glass inserts. Further analysis was performed using LC-MS/MS.
9 v/v) and transferred into glass inserts. Further analysis was performed using LC-MS/MS.
2.4 Instrumental analysis
An Agilent 1290 Infinity II HPLC system (Agilent Technologies, Santa Clara, California, USA) coupled with a 6495C triple quadrupole (Agilent Technologies, Santa Clara, CA, USA) equipped with an Agilent Jet Stream electrospray ionization source was used for the determination of 9 phthalate metabolites in cow urine distillates. Data were acquired using the Mass Hunter Workstation software program (version 10.1). Chromatographic separation of phthalate metabolites was achieved using a Zorbax Eclipse plus C18 column (2.10 × 100 mm, 1.80 μm) (Agilent Technologies, Santa Clara, CA, USA) with gradient elution. The sample injection volume was set to 10 μL, and the auto-sampler temperature was maintained at 15 °C during the analysis to minimize storage errors. The column temperature was maintained at 45 °C. The mobile phase was composed of (A) 0.1% acetic acid in water and (B) 0.1% acetic acid in acetonitrile with a flow rate of 0.25 mL min−1. The gradient profile started with a 98% A and held constant for 0.20 minutes and then linearly decreased to 5% A over 5 minutes and held up to 12 minutes. At 13 minutes it increased to 98% A and was kept for equilibration for 5 minutes. The total run time was 18 minutes. Standard phthalate metabolites and IS solutions were injected into the mass spectrometer to optimize each analyte’s MS/MS parameters. A negative mode of the AJS-ESI interface and multiple reaction monitoring (MRM) mode were used for the detection. The operating source condition for determination of phthalate metabolites in negative MRM is optimized as follows: gas temperature 290 °C; gas flow 11 L min−1; nebulizer pressure 25 psi; sheath gas temperature 375 °C; sheath gas flow 11 L min−1; capillary and nozzle voltages set were 4500 V and 1500 V, respectively. MRM transition and standard chromatograms of phthalate metabolites are summarized in Table S1 and Fig. S1,† respectively.2.5 Method validation
The developed LC-MS/MS method to determine the concentration of phthalate metabolites in cow urine distillates was validated with respect to linearity, accuracy, precision, recovery, and matrix effect. The limit of determination and the limit of quantification of the method were determined. For each batch of 5 samples, two solvent blanks, two procedural blanks, and two zero samples (matrix processed with IS) were processed. The validation batch consisted of a solvent blank, a procedural blank, a zero sample, calibration standards, and four sets of quality control samples. Using standard samples, calibration curves were generated by plotting peak area ratios between the analyte and IS versus actual concentrations. The slope, intercept, and correlation coefficient were calculated using linear regression analysis and a weighting factor of 1/x. Each back-calculation standard concentration should be within 20% of its nominal value to be considered acceptable. The calibration curve range plotted for phthalate metabolite standards was 1–200 ng mL−1. Each analyte's LOQ value was close to that of the calibration standard with acceptable accuracy and precision. The calculated LOQ for MCPP, MMP, MECPP, MEHHP, MiNP, MEOHP, MiBP, and MBzP was 1 ng mL−1, and for MEP it was 5 ng mL−1. QC samples were used to quantify accuracy and precision at four concentration levels (1 ng mL−1, 3 ng mL−1, 100 ng mL−1, and 150 ng mL−1) in two analytical batches on the same day (intra-day) and two on consecutive days (inter-day) and are summarised in Table S2.† The accuracy and precision of intra- and interday measurements are analyzed using freshly prepared calibration curves. The values are expressed as percent nominal (% nominal) and percent relative standard deviation (% RSD, which should be 20%). In order to estimate the percentage recovery of phthalate metabolites spiked in the matrix it is estimated by comparing the difference between spiked and non-spiked concentrations at four different concentrations (1 ng mL−1, 3 ng mL−1, 100 ng mL−1, and 150 ng mL−1). The percent recovery rate for the phthalate metabolites was in the range of 80–97% and the internal standard recovery rate was 94%. The values are summarised in Table S2.† The ideal range for percent recovery is around 80% to ≤120%. A recovery outside this range is permitted as long as it is consistent (RSD ≤ 20%) and the reason for the variation is well-established. Nonetheless, the mean recovery should not be less than 30% or greater than 140%.2.6 Data analysis
The statistical analysis was performed using SPSS 20.0 and Origin 2021b.3. Results and discussion
In India, cow urine has been a medicine since ancient time and is also used in cosmetics. Due to its varied medical qualities, cow urine distillate is now commercially available in the Indian market. Preservatives are commonly added to cow urine distillate products to increase their shelf life and prevent the growth of microorganisms that can spoil the product or cause foodborne illnesses. Soil and surface water are potential sources of exposure for cows to EDCs. Furthermore, the presence of phthalates in cow urine distillate products can also be attributed to animal feedstuffs. Phthalates are commonly used as plasticizers in packaging materials and can leach into feedstuffs during storage or transportation. As a result, cows that consume contaminated feedstuffs can excrete phthalates in their urine, which can end up in the final product. It is, therefore, worthwhile to investigate the extent of exposure to EDCs in cow urine distillates. We can ensure their safety by better understanding how EDCs are introduced into cow urine distillates. Developing strategies to mitigate human exposure will be critical to ensuring the safety of these products. In India, there is no information on human exposure to phthalates through cow urine distillate. Thus, we have evaluated the concentrations and profiles of phthalate metabolites in cow urine distillate products to determine whether they are potential sources of EDC exposure. Optimisation of HPLC-MS/MS conditions, extraction procedures and validation of the method is discussed in more detail in the ESI file.†3.1 Concentration profiles and detection frequencies of phthalate metabolites in cow urine distillate
Phthalates are classified into high molecular weight phthalates (HMWPs) and low molecular weight phthalates (LMWPs). The sum of the concentrations of all HMWPs and LMWPs, along with their individual and total percentiles in each cow urine distillate sample, is shown in Table 1. Almost all the cow urine distillate samples showed a higher proportion of LMWPs. HMWPs composed 1.70% of total phthalate metabolites, whereas LMWPs composed 98.30% of the total phthalate metabolites (Fig. 1A). The composition profile data show more than 80% of LMWPs in each cow urine distillate sample (Fig. 2A).| Name | HMWPs (ng mL−1) | LMWPs (ng mL−1) | ∑HMWPs | ∑LMWPs | ∑Phthalates | Percentile of HMWs (%) | Percentile of LMWs (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MCPP | MEOHP | MECPP | MEHHP | MiNP | MBzP | MEP | MiBP | MMP | ||||||
| CU_1 | 0.70 | 4.21 | 0.70 | 0.70 | 0.70 | 2.76 | 42.17 | 28.88 | 767.14 | 9.77 | 838.19 | 847.96 | 1.15 | 98.85 |
| CU_2 | 0.70 | 4.41 | 0.70 | 0.70 | 0.70 | 1.56 | 84.63 | 330.63 | 357.45 | 8.77 | 772.71 | 781.48 | 1.12 | 98.88 |
| CU_3 | 0.70 | 0.19 | 0.70 | 0.70 | 0.70 | 1.54 | 6.03 | 18.36 | 987.45 | 4.53 | 1011.84 | 1016.38 | 0.45 | 99.55 |
| CU_4 | 0.70 | 1.32 | 0.70 | 0.70 | 4.62 | 0.80 | 11.46 | 13.20 | 182.91 | 8.84 | 207.58 | 216.42 | 4.09 | 95.91 |
| CU_5 | 0.70 | 0.37 | 0.70 | 0.70 | 0.70 | 1.34 | 31.81 | 6.19 | 555.47 | 4.52 | 593.47 | 597.99 | 0.76 | 99.24 |
| CU_6 | 0.70 | 0.37 | 0.70 | 0.70 | 0.70 | 0.70 | 20.75 | 4.68 | 49.14 | 3.87 | 74.57 | 78.44 | 4.94 | 95.06 |
| CU_7 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 5.14 | 4.68 | 428.01 | 4.20 | 437.83 | 442.03 | 0.95 | 99.05 |
| CU_8 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 0.70 | 5.49 | 1.65 | 1122.97 | 4.20 | 1127.12 | 1131.32 | 0.37 | 99.63 |
| CU_9 | 0.70 | 0.17 | 0.70 | 0.70 | 0.70 | 0.70 | 8.44 | 3.65 | 39.29 | 3.67 | 51.39 | 55.06 | 6.67 | 93.33 |
| CU_10 | 0.70 | 0.70 | 0.70 | 1.32 | 0.70 | 3.07 | 24.60 | 90.53 | 369.05 | 7.19 | 484.18 | 491.37 | 1.46 | 98.54 |
| CU_11 | 0.70 | 0.20 | 0.70 | 0.70 | 0.70 | 0.70 | 26.59 | 6.65 | 969.06 | 3.70 | 1002.29 | 1005.99 | 0.37 | 99.63 |
| CU_12 | 0.70 | 0.45 | 0.70 | 0.70 | 0.70 | 0.33 | 22.47 | 7.59 | 1.10 | 3.58 | 31.15 | 34.73 | 10.31 | 89.69 |
| CU_13 | 0.70 | 1.68 | 0.70 | 0.70 | 0.70 | 0.70 | 9.24 | 7.00 | 17.59 | 5.18 | 33.83 | 39.01 | 13.29 | 86.71 |
| CU_14 | 0.70 | 28.70 | 0.70 | 0.70 | 0.70 | 0.70 | 18.62 | 33.67 | 633.18 | 32.20 | 685.47 | 717.67 | 4.49 | 95.51 |
| CU_15 | 0.70 | 0.03 | 0.70 | 0.70 | 1.67 | 13.50 | 7.16 | 19.75 | 95.62 | 17.30 | 122.54 | 139.83 | 12.37 | 87.63 |
| CU_16 | 0.70 | 0.70 | 3.75 | 1.55 | 1.83 | 0.70 | 27.06 | 16.42 | 3.37 | 9.23 | 46.85 | 56.09 | 16.46 | 83.54 |
| CU_17 | 0.70 | 0.45 | 0.70 | 0.70 | 0.70 | 0.10 | 29.63 | 11.76 | 350.71 | 3.36 | 392.10 | 395.45 | 0.85 | 99.15 |
| Total conc (ng mL−1) | 11.90 | 45.36 | 14.95 | 13.37 | 17.93 | 30.61 | 381.31 | 605.28 | 6929.52 | 134.12 | 7913.12 | 8047.23 | ||
| Percentile (%) | 0.15 | 0.56 | 0.19 | 0.17 | 0.22 | 0.38 | 4.74 | 7.52 | 86.08 | 1.67 | 98.33 | 100.00 | ||
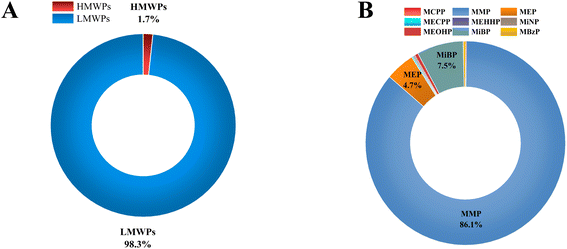 | ||
| Fig. 1 (A) Distribution profiles of LMWPs and HMWPs in cow urine distillate. (B) Distribution profiles of 9 phthalate metabolites in cow urine distillate. | ||
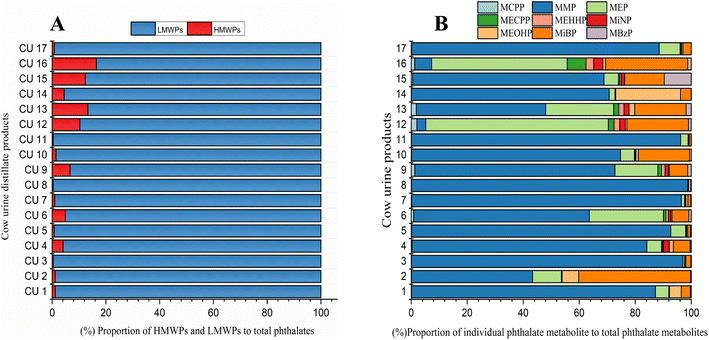 | ||
| Fig. 2 (A) Composition profiles of LMWPs and HMWPs in cow urine distillate. (B) Composition profiles of phthalate metabolites in cow urine distillate. | ||
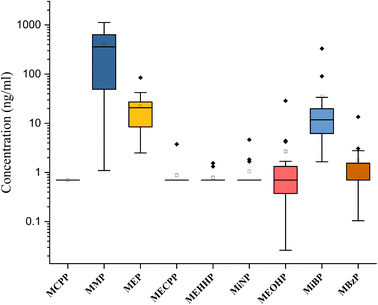
The concentration of the nine phthalate metabolites and the sum of their concentrations (∑Phthalates) in cow urine distillate is shown in Table 1. More than one phthalate metabolite was found among the tested phthalates in the cow urine distillate samples, whereas MCPP was not detected in any of the cow urine distillate samples used in this study. Most of the samples contained a detectable concentration of phthalates. ∑Phthalates mean concentrations in cow urine distillate were 473.33 ng mL−1 with a median value of 394.21 ng mL−1. In this study, LMWPs MMP, MEP, and MiBP were found with the highest detection frequency of 100%, followed by MBzP (52.94%), MiNP (17.65%), MEOHP (29.41%), MEHHP (11.76%) and MECPP (5.88%) which are categorized as HMWPs (Table 2). MMP, MEP, and MiBP had detection frequencies greater than 80%, indicating that these phthalates were commonly used in most products. The mean concentrations of phthalate metabolites in cow urine distillate were 407.60 ng mL−1 (MMP), 22.25 ng mL−1 (MEP), 0.88 ng mL−1 (MECPP), 0.79 ng mL−1 (MEHHP), 1.06 ng mL−1 (MiNP), 2.67 ng mL−1 (MEOHP), 35.60 ng mL−1 (MiBP), and 1.80 ng mL−1 (MBzP). The composition profile of phthalate metabolites in each cow urine distillate sample is shown in Fig. 2B. The composition proportion of cow urine distillate data revealed more than 85% of MMP, MEP, and MiBP in the majority of the urine distillate. A few samples also showed the presence of MECPP, MEHHP, MiNP, MEOHP, and MBzP. MMP, MEP, and MiBP were abundant and ranged from 1.09 to 1123 ng mL−1, 2.49 to 84.63 ng mL−1, and 1.65 to 330.60 ng mL−1, respectively (Fig. 3). The distribution profile of each phthalate metabolite in the cow urine distillate products is shown in Fig. 1B.
| Phthalate metabolites | MCPP | MMP | MEP | MECPP | MEHHP | MiNP | MEOHP | MiBP | MBzP |
|---|---|---|---|---|---|---|---|---|---|
| Number of values | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 | 17.00 |
| Minimum | 0.70 | 1.10 | 2.50 | 0.70 | 0.70 | 0.70 | 0.03 | 1.65 | 0.10 |
| 25% percentile | 0.70 | 44.22 | 7.80 | 0.70 | 0.70 | 0.70 | 0.29 | 5.44 | 0.70 |
| Median | 0.70 | 357.50 | 20.75 | 0.70 | 0.70 | 0.70 | 0.70 | 11.76 | 0.70 |
| 75% percentile | 0.70 | 700.20 | 28.35 | 0.70 | 0.70 | 0.70 | 1.50 | 24.32 | 1.55 |
| Maximum | 0.70 | 1123.00 | 84.63 | 3.75 | 1.55 | 4.62 | 28.70 | 330.60 | 13.50 |
| Range | 0.00 | 1122.00 | 82.14 | 3.05 | 0.85 | 3.92 | 28.67 | 329.00 | 13.39 |
| 5% percentile | 0.70 | 1.10 | 2.50 | 0.70 | 0.70 | 0.70 | 0.03 | 1.65 | 0.10 |
| 95% percentile | 0.70 | 1123.00 | 84.63 | 3.75 | 1.55 | 4.62 | 28.70 | 330.60 | 13.50 |
| Mean | 0.70 | 407.60 | 22.25 | 0.88 | 0.79 | 1.06 | 2.67 | 35.60 | 1.80 |
| Std. deviation | 0.00 | 376.30 | 19.60 | 0.74 | 0.25 | 0.98 | 6.83 | 78.85 | 3.12 |
| Std. error of mean | 0.00 | 91.27 | 4.75 | 0.18 | 0.06 | 0.24 | 1.66 | 19.12 | 0.76 |
| Geometric mean | 0.70 | 152.20 | 15.87 | 0.77 | 0.76 | 0.87 | 0.68 | 12.92 | 0.97 |
| Geometric SD factor | 1.00 | 7.79 | 2.43 | 1.50 | 1.27 | 1.69 | 4.75 | 3.56 | 2.80 |
| Detection frequency (DF) | 0.00 | 17.00 | 17.00 | 1.00 | 2.00 | 3.00 | 5.00 | 17.00 | 9.00 |
| DF (%) | 0.00 | 100.00 | 100.00 | 5.88 | 11.76 | 17.65 | 29.41 | 100.00 | 52.94 |
We found that MMP (407.60 ng mL−1) was the dominant metabolite in cow urine distillate, followed by MiBP (35.60 ng mL−1) and MEP (22.25 ng mL−1). Pet urine (dogs and cats) contained the highest levels of MEP,21 followed by MiBP and MMP. Similar trends were also found in our study, where the concentration of MMP was significantly higher than that of some other metabolites. A major metabolite of cow urine distillate is MMP, derived from DMP (parent). The most common use for DMP is in manufacturing products such as bug-repellents and plastics, which may be the source of exposure for cows. Inhabiting most cities in India, unfettered cattle widely consume garbage such as old paper and other city garbage, which can cause a surge in their exposure to phthalates. The use of sewage sludge as a fertilizer is common on cattle farms that can contaminate soil and crops. Plasticulture's growing popularity in India leads to increased cattle exposure to phthalates. Comparing phthalate exposure between different animal species, such as pet cats, cows, and dogs, may provide insights into the sources and patterns of phthalate exposure in different environments and diets. Cats and dogs have different dietary habits, and it is possible that these differences could lead to differences in phthalate exposure. For example, cats are obligate carnivores and may be exposed to higher levels of phthalates through the consumption of meat. Dogs, on the other hand, are omnivores and may have a different pattern of exposure. Cows are herbivores and may have different exposures to phthalates compared to cats and dogs. Comparing phthalate exposure in different animal species may also help identify potential sources of contamination and inform strategies for reducing exposure. For example, if a particular source of phthalate contamination is identified in cows, measures could be taken to reduce exposure for both cows and humans who consume dairy products. Cats, cows and dogs may have different exposure pathways to phthalates, depending on their behaviour and lifestyle. Cows are exposed to phthalates through their feed, bedding, and water sources, whereas cats and dogs may be exposed to phthalates through their toys, grooming products, and food packaging. Phthalate exposure is possible through the urine of cows, cats and dogs, although the extent of exposure may vary depending on specific circumstances.
Additional research is necessary to ascertain just how prevalent phthalates are within cattle populations and what effects they may have. Moreover, during distillation, cow urine is more likely to get contaminated. Throughout the distillation process of cow urine, several steps are taken, including filtration, distillation, condensation, packaging, and storage. However, it's worth noting that the distillation equipment employed may carry impurities from prior batches if not cleaned and sanitized thoroughly.
3.2 Effect of low and high molecular weight phthalates
Phthalates are a group of chemicals commonly used in the manufacturing of plastics, and they can be divided into two categories based on their molecular weight: high molecular weight phthalates (HMWPs) and low molecular weight phthalates (LMWPs). The observation that low molecular weight phthalates (LMWPs) have a higher proportion and component distribution in cow urine distillates than high molecular weight phthalates (HMWPs) may be due to several factors. One possible factor is the physicochemical properties of the phthalates. HMWPs are larger molecules with higher molecular weights, typically between 250 and 1000 g mol−1. They are less volatile and less soluble in water compared to LMWPs. HMWPs are commonly used in industrial applications, such as in the production of PVC pipes, cables, and wire insulation. LMWPs, on the other hand, are smaller molecules with lower molecular weights, typically between 200 and 250 g mol−1. They are more volatile and more soluble in water compared to HMWPs.22 Due to the structural and physicochemical properties of phthalates, metabolism will be affected.HMWPs are larger molecules and are less readily absorbed,23 resulting in slower metabolism and elimination. Once absorbed, HMWPs are metabolized by hydrolysis of their ester bonds to form their primary metabolites, which are monoesters and diesters and then, after enzymatic oxidation of the alkyl chain, to more hydrophilic, oxidative metabolites.24 These primary metabolites are then further metabolized in the liver to form secondary metabolites, which are conjugated with glucuronic acid and excreted in the urine. The slow metabolism and elimination of HMWPs can result in accumulation in the body and prolonged exposure, which may increase the risk of adverse health effects.23 In contrast, LMWPs are smaller molecules and are more readily absorbed, resulting in faster metabolism and elimination. LMWPs are rapidly metabolized by hydrolysis of their ester bonds to form their primary metabolites, which are monoesters. These primary metabolites are then further metabolized in the liver to form secondary metabolites, which are conjugated with glucuronic acid and excreted in the urine. The faster metabolism and elimination of LMWPs may reduce the risk of accumulation and prolonged exposure. Additionally, the metabolic processes in cows may also affect the proportion and distribution of HMWPs and LMWPs in their urine. Cows have different metabolic processes compared to humans and may metabolize phthalates differently, leading to variations in the proportion and distribution of HMWPs and LMWPs in their urine.
Another factor that may influence the proportion and distribution of HMWPs and LMWPs in cow urine distillates is the exposure pathway. Low molecular weight phthalates are widespread and widely dispersed in the environment due to the enormous volume of manufacture of phthalates and the vast range of their commercial usage. LMWPs are commonly used in consumer products, such as cosmetics, fragrances, and personal care items. They are also used as plasticizers in PVC products such as toys and food packaging and may have higher exposure levels in the general population.25 As a result, domestic animals may be exposed to LMWPs through various pathways. In our investigation, phthalate metabolites were discovered in cow urine distillates, indicating that they may induce toxicity in domestic animals and health issues for the Indian people. Therefore, tough limitations should be enforced on cow urine distillates and products manufactured from cow urine without compromising safety or quality.
3.3 Limitations
Our research has limitations due to the number of factors contributing to phthalate exposure, such as cow feed, water, and dust from sheds and distillation procedures. More research is needed to determine the origins of phthalates in cow urine distillate products. Despite the abundance and variety of cow urine distillates available on the Indian market, we only tested seventeen in this study. This was because this was a pilot study designed to determine whether or not hazardous substances were present in the products. In addition, several other factors may contribute to exposure to phthalates. For example, in India, free-ranging cattle often graze on paper and other garbage found in urban areas, potentially exposing them to high levels of phthalates. Additionally, plasticulture, which is becoming increasingly popular in India, may result in plastic particles being ingested or chewed by cattle, exposing them to phthalates. The combined effect of other endocrine-disrupting chemicals, such as bisphenols, parabens, etc., must be studied. Currently, the determination of phthalates in raw cow urine samples, feeds, and milk has not been thoroughly explored, making it necessary to conduct more research with large sample sizes from various parts of India.The Food Safety and Standards Authority of India has included phthalates in their international guidelines for food packaging to ensure the safety and quality of food products and packaging materials. Common phthalate exposure sources include packaged foods, medical devices, pharmaceuticals, herbal preparation and nutritional supplements, toys, house dust and indoor air, clothing, cosmetics, automobiles, lubricants, insecticides and building materials.26 These guidelines provide regulations on phthalate use in food packaging and set limits on their presence to minimize consumer exposure. Enforcing strict limitations on phthalate presence in cow urine distillates and products made from cow urine is crucial for consumer health and safety. These limitations should prioritize safety and quality while also minimizing the potential risks associated with phthalate exposure. As phthalates are prevalent in various cow urine products, it is essential to monitor and regulate their use through rigorous testing protocols and guidelines. Overall, strict limitations on phthalate presence in cow urine distillates and products made from cow urine will safeguard consumer health and safety and ensure that these products meet the highest quality standards.
4. Conclusion
To the best of our knowledge, this is the first report on the occurrence of phthalate metabolites in cow urine distillate products from India. For this, an LC-MS/MS method has been developed and validated for the simultaneous estimation of phthalate metabolites. The calibration curves for all the phthalate metabolites were in the range of 1 ng mL−1 to 200 ng mL−1. The accuracy and precision of phthalate metabolites at all concentrations examined were well within acceptable limits at inter-day and intra-day levels. Following validation of the analytical method, it was subsequently used to detect phthalate metabolites in cow urine distillate products. MMP (407.60 ng mL−1) was the dominant metabolite in cow urine distillate, followed by MiBP (35.60 ng mL−1) and MEP (22.25 ng mL−1), whereas MCPP was not detected in any of the cow urine distillate samples used in this study. Among the total phthalate metabolites, HMWPs accounted for 1.70%, while LMWPs accounted for 98.30%. This study suggests that future research should be conducted to provide more data on the prevalence of endocrine disruptors, such as phthalate metabolites in various cow urine distillate products and other forms of exposure to cattle. The presence of phthalate metabolites in cow urine distillate products indicates that they may pose health risks to domestic animals and the Indian population through products that have cow urine. In addition, stringent rules must be implemented for producing cow urine distillates and goods derived from cow urine. Future research is required to examine the sources of exposure in cows, the sources of contamination in the distillate product made from cow urine, and the effects of exposure on health.Author contributions
SBJ and RMB conceived and designed the study; SBJ, JAS, MS, GN, RM, and DP performed LC-MS/MS analysis; SBJ, JAS, MS, GN, RM, and DP analyzed the data; SBJ, JAS, MS, GN, RM, DP, and RMB wrote the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors sincerely acknowledge the Department of Pharmaceuticals under the Ministry of Chemicals and Fertilizers, Government of India, and Dr USN Murty, Director, NIPER-G for facilities and their cooperation.References
- E. Soo Kwak, A. Just, R. Whyatt and R. L. Miller, Open Allergy J., 2009, 2, 45–50 CrossRef PubMed.
- B. Zhang, T. Zhang, Y. Duan, Z. Zhao, X. Huang, X. Bai, L. Xie, Y. He, J. Ouyang, Y. Yang, Y. Wu and H. Sun, Environ. Int., 2019, 124, 1–9 CrossRef CAS PubMed.
- T. M. James-Todd, J. D. Meeker, T. Huang, R. Hauser, K. K. Ferguson, J. W. Rich-Edwards, T. F. McElrath and E. W. Seely, Environ. Int., 2016, 96, 118–126 CrossRef CAS PubMed.
- A. Jankowska, K. Polańska, H. M. Koch, C. Pälmke, M. Waszkowska, A. Stańczak, E. Wesołowska, W. Hanke, S. Bose-O'Reilly, G. Calamandrei and M. Garí, Environ. Res., 2019, 179, 108829 CrossRef CAS PubMed.
- L. López-Carrillo, R. U. Hernández-Ramírez, A. M. Calafat, L. Torres-Sánchez, M. Galván-Portillo, L. L. Needham, R. Ruiz-Ramos and M. E. Cebrián, Environ. Health Perspect., 2010, 118, 539–544 CrossRef PubMed.
- X. Han, Z. Cui, N. Zhou, M. Ma, L. Li, Y. Li, H. Lin, L. Ao, W. Shu, J. Liu and J. Cao, Int. J. Hyg. Environ. Health, 2014, 217, 271–278 CrossRef CAS PubMed.
- B. Xia, Q. Zhu, Y. Zhao, W. Ge, Y. Zhao, Q. Song, Y. Zhou, H. Shi and Y. Zhang, Environ. Int., 2018, 121, 159–168 CrossRef CAS PubMed.
- B. A. Rocha, A. G. Asimakopoulos, F. Barbosa and K. Kannan, Sci. Total Environ., 2017, 586, 152–162 CrossRef CAS PubMed.
- Z. Wang, Y. Sun, L. Gu, T. Zhang, S. Liu, S. Wang and Z. Wang, Ecotoxicol. Environ. Saf., 2022, 242(113930) Search PubMed.
- J. Malits, T. M. Attina, R. Karthikraj, K. Kannan, M. Naidu, S. Furth, B. A. Warady, S. Vento, H. Trachtman and L. Trasande, Environ. Res., 2018, 167, 575–582 CrossRef CAS PubMed.
- A. J. Li, M. P. Martinez-Moral, A. L. Al-Malki, M. A. Al-Ghamdi, M. M. Al-Bazi, T. A. Kumosani and K. Kannan, Environ. Int., 2019, 126, 153–161 CrossRef CAS PubMed.
- T. L. M. Hectors, C. Vanparys, K. Van Der Ven, G. A. Martens, P. G. Jorens, L. F. Van Gaal, A. Covaci, W. De Coen and R. Blust, Diabetologia, 2011, 54, 1273–1290 CrossRef CAS PubMed.
- K. B. Mi and J. Y. Min, J. Clin. Endocrinol. Metab., 2014, 99, E1997–E2003 CrossRef PubMed.
- P. C. Huang, C. H. Tsai, W. Y. Liang, S. S. Li, H. Bin Huang and P. L. Kuo, PLoS One, 2016, 11(7), e0159398 CrossRef PubMed.
- C. Testa, F. Nuti, J. Hayek, C. de Felice, M. Chelli, P. Rovero, I. G. Latini and A. M. Papini, ASN Neuro, 2012, 4, 223–229 CrossRef CAS PubMed.
- S. Khanuja, A. Kumar, S. Arya, P. Darokar, M. Singh, P. Sinha, S. Awasthi, S. Gupta, V. Gupta, M. Gupta, R. Verma, S. Agrawal, S. Mansinghka and S. Dawale, Pharmaceutical Composition Containing Cow Urine Distillate and an Antibiotic, US Pat., US6410059B1, 2002 Search PubMed.
- S. Khanuja, A. Kumar, S. Arya, P. Darokar, M. Singh, P. Sinha, S. Awasthi, S. Gupta, V. Gupta, M. Gupta, R. Verma, S. Agrawal, S. Mansinghka, S. Dawle, Use of Bioactive Fraction from Cow Urine Distillate (‘Go-Mutra’) as a Bio-Enhancer of Anti-Infective, Anti-Cancer Agents and Nutrients, US Pat., US6896907B2, 2005 Search PubMed.
- T. Chakrabarti, S. Sivanesan, K. Kannan, D. Dutta, R. Singh, S. Mansinghka and S. Dawle, Composition (RCUD) for Protecting and/or Repairing DNA from Oxidative Damages and a Method Thereof, US Pat., US7718360B2, 2003 Search PubMed.
- G. K. Randhawa, J. Ayurveda Integr. Med., 2010, 1, 240 CrossRef PubMed.
- F. I. Rahman, M. R. Islam and M. A. Bhuiyan, J. Med. Virol., 2021, 93, 6447–6448 CrossRef CAS PubMed.
- R. Karthikraj, S. Lee and K. Kannan, Sci. Total Environ., 2019, 690, 70–75 CrossRef CAS PubMed.
- J. L. Lyche, Reprod. Dev. Toxicol., 2011, 637–655 CAS.
- Overview of Phthalates Toxicity, https://www.cpsc.gov/s3fs-public/phthalover.pdf Search PubMed.
- R. Hauser and A. M. Calafat, Occup. Environ. Med., 2005, 62, 806–818 CrossRef CAS PubMed.
- Y. Wang and H. Qian, Healthc, 2021, 9, 1–9 CAS.
- T. Schettler, N. E. Skakkebæk, D. De Kretser and H. Leffers, Int. J. Androl., 2006, 29, 134–139 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3va00026e |
| This journal is © The Royal Society of Chemistry 2023 |