Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tuning conductivity while maintaining mechanical properties in perylene bisimide hydrogels at physiological pH†
Juan Antonio Mena
Jimenez
ab,
Jacquelyn
Egan
 a,
Rebecca I.
Randle
a,
Rebecca I.
Randle
 a,
Amina Omelbanine
Rezig
ac,
Benjamin O.
Orimolade
a,
Amina Omelbanine
Rezig
ac,
Benjamin O.
Orimolade
 a,
Rebecca E.
Ginesi
a,
Rebecca E.
Ginesi
 a,
Ralf
Schweins
a,
Ralf
Schweins
 d,
Mathis O.
Riehle
d,
Mathis O.
Riehle
 c and
Emily R.
Draper
c and
Emily R.
Draper
 *a
*a
aSchool of Chemistry, Joseph Black Building, University of Glasgow, Glasgow, UK. E-mail: emily.draper@glasgow.ac.uk
bDepartment of Chemical Engineering, Faculty of Sciences, University of Granada, 18071, Spain
cSchool of Molecular Biosciences, University of Glasgow, Glasgow, UK
dInstitut Laue-Langevin, Large Scale Structures Group, 71 Avenue des Martyrs, CS 20156, F-38042, Grenoble, Cedex 9, France
First published on 7th February 2024
Abstract
By using different salts as a method to achieve gelation of two different amino-acid-functionalised perylene bisimides, we were able to tune reduction potentials while maintaining the mechanical and optical properties of the system all at pH 7.4.
The complex networks formed by gelators make them ideal candidates for mimicking the extracellular matrix (ECM), i.e., the environment surrounding cells in our body. With a careful choice of gelator, these artificial ECMs can be prepared at a physiological pH and temperature and containing the media needed for cell survival.1–3 With the high demand for biosensors, implants and alternate cell scaffold materials, the need for these artificial ECMs is high.4 Moreover, the requirement for specialised functionalised gel materials such as responsive gels, biocidal gels or conductive gels will need to have extra functionality as well as maintaining the appropriate mechanical properties, which can be difficult to achieve.1,4–7 For example, when growing nerve cells, an appropriate mechanical stiffness is required and an electric field is desired.8,9 There are many options for achieving conductive materials from the assembly of low-molecular-weight gelators that can be functionalised for specific applications.10–12 The problem lies in the method of gelation, as gelation triggers being often unsuitable for biological applications or resulting in gels with unsuitable properties for physiological conditions. Gels triggered to form at low pH or by applying a solvent switching method that uses toxic solvents would be unsuitable for this application.13 It is therefore important to keep biological conditions in the forefront when designing these systems, with them being water based and at pH 7.4 constituting a minimum set of requirements. There are examples of divalent salts being used to gel systems, and these salts are present in biological systems and so could be a viable alternative.14 This method works by either ‘salting out’ charged molecules to make them less soluble and form physical gels, or in the case of divalent salts basically cross-linking different aggregates together and forming a network. Often this process depends on the salt used and concentration of salt, which in turn often influences final mechanical properties.15 In the case of dipeptides, the mechanical properties will depend on the apparent pKa of the system and whether they form suitable structures before gelation occurs. For example, worm-like micelles with COO-groups have been shown to be effective for making salt-bridged networks (Fig. 1(a)). Many peptide-based hydrogels have been used for biological applications such as cell culture with examples of these hydrogels including those made from Fmoc-RGD, Fmoc-FF and FEFKFEpYK, which have been used as cell scaffold materials due to their biocompatibility and thixotropic properties.16 Often, to introduce a different functionality into these gels, another component is added in. This addition can often lead to a change in the rheological behaviour or network type, in particular to a change that is undesirable for the application.14
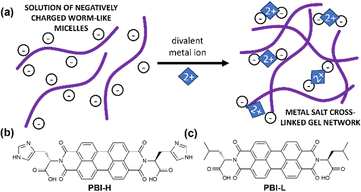 | ||
| Fig. 1 (a) Cartoon of a salt-bridged gel network. (b) and (c) chemical structures of (b) PBI-H and (c) PBI-L used in this study. | ||
Perylene bisimides (PBIs) are very interesting small molecules due to their gelation, conductivity, and thermal and optical properties.17,18 They have been used to form gels useful for applications such as sensors,19 H2 evolution20 and chromic materials21 to name a few. They provide an interesting example of an active gel network that provides mechanical and electronic properties without the need for another material present. They have been shown to have biological applications, mostly for imaging applications rather than as functional ECM materials.22 Our study has looked at the conductive and optical properties of these materials as xerogels and at their mechanical properties as hydrogels.17,23,24
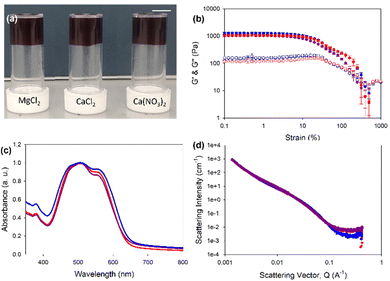
Herein, we describe the gelation behaviours of two different amino-acid-functionalised PBI molecules with three different divalent metal salts. They were chosen as they form the worm-like micelles needed for metal salt gelation at pH 7.23 We investigated the effects of changes in pH, gelator concentration and salt concentration on their gelation ability with different salts. We then focused specifically on pH 7.4 with different salts to assess the effects of these salts on the mechanical properties, structural properties, absorption properties and reduction potentials of resulting gels. PBI-L and PBI-H (Fig. 1(b) and (c)) were synthesised as previously reported.25 Solutions were prepared at desired concentrations using one equivalent of NaOH and deionised water. After the material had dissolved, the pH was then adjusted to the desired value using HCl or NaOH at a concentration of 0.1 M. To test gelation, a set amount of CaCl2, Ca(NO3)2, or MgCl2 prepared at a concentration of 200 mg mL−1 in water was pipetted onto the top of the gelator solution. The resulting system in each case was then left for 16 hours to gel. Successful gelation was first tested using a vial inversion test (Fig. 2(a) and 3(a)). Those passing this test were then prepared in triplicate, and their rheological properties were characterized to confirm that gelation had indeed occurred. By varying the concentration of gelator and salt, we were able to ascertain the minimum gelation concentration and minimum amount of salt needed for each gelators and salts. pH and salt were tested in gelation studies.
In general, PBI-L gelled in many more conditions tested, whereas the gelation of PBI-H was more dependent on these conditions. The minimum gelation concentration (mgc) for PBI-H in each of the salts was about 3 mg mL−1 of gelator, and PBI-H tended only to gel at pH values of 5, 7 and 8 (Tables S1–S4, ESI†). This result was due to PBI-H being soluble at other pHs, in turn due to ionisable histidine groups (COOH at higher pH and the imidazole group at low pH). Due to the solubility, PBI-H did not form the worm-like micelles associated with salt gelation at lower pH levels.14,24PBI-L had a mgc of 2 mg mL−1 and was gelled at pH values of 6–10 with all tested salts (Tables S5–S8, ESI†). PBI-L, due to it not having this lower-pH ionisable group, maintained fibrous structures at these pHs.23 With our interest in these materials for use in biological settings, we focused on the physiological pH 7.4, using a gelator concentration of 5 mg mL−1 and a salt concentration of 50 μL mL−1, as this set of conditions worked for gelators and salts under these conditions.
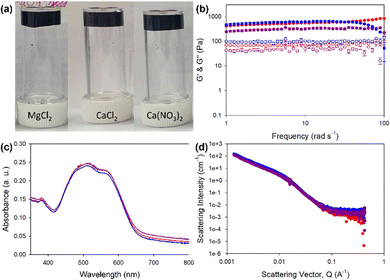
Rheology of PBI-L gels showed that at pH 7.4 and 5 mg mL−1 gelator, all gels, regardless of the salt used, showed very similar properties with a G′ of 2000 Pa and G′′ of 250 Pa (Fig. 2(a) and (b) and Fig. S2, ESI†). The gels also all had the same yield point of 6%, but those formed with MgCl2 showed a strain profile after the yield point different from those formed with CaCl2 and Ca(CO3)2, which appeared to have another yield point at around 70%. The similarity between the gels also carried through to the UV-vis absorption data, which indicated very similar absorptions and again showing the salts not affecting the local packing (Fig. 2(c)). Small-angle neutron scattering (SANS) revealed structures to indeed be essentially the same, all fitting an elliptical cylinder model (Table S9 and Fig. 2(d) and Fig. S3–S6, ESI†). These data indicated the presence of flat fibres in solutions. From solution to gel, the radius stayed about the same (at around 3 nm) with an axis ratio of around 3. However, the gel formed with MgCl2 differed slightly, with a larger radius of 3.5 nm and a larger axis ratio of 4.7, suggesting that these gel fibres were flatter. The fibres in solution had an average length of around 25 nm. When gelled, however, this value roughly doubled to 54 nm for CaCl2 and Ca(CO3)2 but only to 46 nm for MgCl2. Overall, the fibres elongated upon gelation, but with a morphology for the gelled fibres that depended on the salt used, with this dependence perhaps explaining different strain dependence behaviours shown in rheology analyses.
PBI-H gels (Fig. 3(a)) showed more variability in the stiffness values, with MgCl2 yielding the lowest G′ of 300 Pa and G′′ of 10 Pa, compared to G′ of 500 Pa and G′′ of 170 Pa for Ca(CO3)2-containing gels and G′ of 700 Pa and G′′ of 110 Pa for the CaCl2-containing gels (Fig. 3(b) and Fig. S7, ESI†). Despite differences in stiffness, the yield and flow points for all the gels were found to be similar at 5% and 25% strains, respectively. The data for the UV-vis absorption of the gels indicated almost identical local packings of the molecules into the fibres (Fig. 2(c)), meaning that differences in rheology results did not come from local packing, but more likely the network arising from using different salts.
SANS data for the PBI-H solution fit a cylinder with a power law with a fibre radius of 4 nm (Fig. S8 and Table S10, ESI†), indicative of the presence of round fibres. When PBI-H was gelled with the salts, the data then fit an elliptical cylinder (flattened fibre) with a larger radius, with the gels containing, respectively, CaCl2 and Ca(CO3)2 now showing a fibre radius of 9 nm and that containing MgCl2 showing a value of 9 nm. This result for PBI-H suggested that upon its gelation, a lateral association of two fibres caused by salt crosslinking fibres together occurred, in contrast to the case for PBI-L whose fibre width remained essentially the same upon gelation but whose fibre length increased upon addition of the metal salt. The fibre lengths for the PBI-H solution and gels were out of range suitable for this model, apart from that containing Ca(CO3)2 which showed a fibre length of 59 nm (Fig. 3(d) and Fig. S8–S11 and Table S10, ESI†). Far more variability in scattering fits were found for the PBI-H gels than for the PBI-L gels, with this difference reflected in the rheology results. Our results showed PBI-L being much less affected than PBI-H by the presence of the different salts, perhaps due to the presence of the histidine imidazole group in PBI-H, which could interact with the salts and other fibres, in contrast to the PBI-L leucine side chain, which would have limited interaction with the salts.
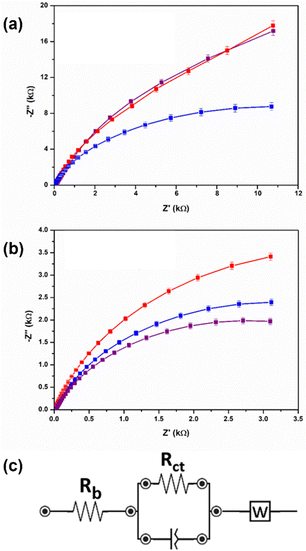
To assess the reduction potentials and conductivity of the gels, cyclic voltammetry (CV) and electrical impedance spectroscopy (EIS) were carried out. In general, perylene bisimides have two reduction potentials, one to make the radical anion and one to make the dianion.19 The ability to form these anions allow for perylene bisimide gels to pass a current and therefore gives added functionality to the gels when used as cell culture scaffolds.10 In the present study, with the PBI-H gels, only one of these reduction potentials was seen (Fig. S12, S13 and Table S11, ESI†), but for the PBI-L gels both reduction peaks were seen (Fig. S14 and Table S11, ESI†). However, in both perylene gels, the reduction potential values were different depending on the salt used. This difference was most noticeable for PBI-L with the first reduction potential, but the values for the second reduction potential were essentially the same. For PBI-H, there was less of a change in the potential difference. From EIS analyses, the PBI-H gels showed higher ionic conductivity values than their counterpart PBI-L gels (Fig. 4 and Table S12, ESI†). PBI-L gel values were more variable depending on the salt used. Herein, with PBI-L, the size of the salt molecules perhaps influenced the conductivity values—with MgCl2 having the smallest molecular size, yielding the highest conductivity. The EIS data showed an approximately 30% difference between the systems over a range of 50 to 1 Hz, and although this difference may be considered small, it could be also deemed to be significant due to the reproducibility of both the gels and of the data shown throughout. Accordingly, this difference of 30% was initially unexpected due to the lack of change in the perylene bisimide structure and due to the very similar mechanical and optical properties of the gels. This difference hence demonstrated that the metal salt used to be very influential in reduction potential and current required in the gels. Next, we carried out tests to ensure the suitability of the developed system for cell studies. First, we characterized the stability levels of the gels. This test was carried out by aging the gels for three weeks at room temperature. All gels remained invertible for at least 6 months (Fig. S15, ESI†), but rheological tests were carried out after 3 weeks (Fig. S16–S19, ESI†). These tests showed the gels having remained stable throughout this time period with the rheological stiffness of aged samples remaining very similar to those samples freshly made. This stability indicated that they would be suitable for cell differentiation.
Next, to assess the biocompatibility of PBI-L and PBI-H, tests with C2C12 cells were conducted. Fig. S20 (ESI†) shows the cytotoxic profile of gels and solutions of both PBIs when incubated with these cells for 20 hours. For gels, PBI-L showed cytotoxic behaviour with poor cellular viabilities of 38%, 23%, and 27% when gelled with CaCl2, MgCl2, and Ca(NO3)2, respectively. However, PBI-H showed a safe profile with viability levels of over 84% for all three inorganic salts. To assess the cytotoxic behaviour of PBI monomers, MTT assay was performed for PBIs in the solution state at various concentrations. For PBI-L, cell viability increased with decreasing concentration of the material, while PBI-H solutions displayed biosafety in all tested concentrations. However, as MTT is a colorimetric assay, some wells had residual red pigment, due to the nature of the PBI dye, indicating a possible limitation of the test. To overcome this issue, a Live/Dead assay was performed for each sample under same conditions used in the MTT assay to confirm biocompatibility (Fig. S21 and S22, ESI†). In agreement with MTT assay results, the overall population of viable cells determined using the Live/Dead assay was low for PBI-L, irrespective of the gelation. This was the same as seen in highly concentrated solutions. For PBI-H, no cytotoxic behaviour was found in any of its gels and solution forms. Taking the above results together, a correlation was found between the cytotoxic features of tested PBIs and the state of the perylene core—with free monomers inducing cytotoxicity and self-aggregation manifesting biosafety.
In conclusion, we have demonstrated how low-molecular-weight PBI gels can be made at physiological pH using a salt trigger. The mechanical properties and mgc of PBI-L were found to be not affected by the salt used, whilst PBI-H was found to be affected. Therefore, PBI-L showed a tolerance of the conditions that can be used, whereas for PBI-H, there would be an opportunity for tuning of properties, with no cytotoxicity found for any of the concentrations and salts tested. Most interestingly, despite PBI-L and PBI-H showing similar molecular packings, rheological properties and scattering, quite different reduction potentials for PBI-L were found, again providing the opportunity to change the potentials needed for an application, whilst maintaining mechanical, optical and network properties. However, PBI-L showed cytotoxic behaviour despite similarities to PBI-H, so further investigation of PBI-L would be needed in order to make it safe for biological systems.
For the purpose of open access, the authors have applied a Creative Commons Attribution (CC BY) licence to any Author Accepted Manuscript version arising from this submission. We would like to thank the UKRI (MR/V021087/1) and the EPSRC (EP/X5257161/1) for funding and for funding. We would like to thank Prof. Adams for the help in collecting and analysing the SANS data. We acknowledge beamtime allocation on D11 at the ILL, Grenoble. Experiment numbers 9–11–1964 and 9-12-598. This work benefitted from the SasView software, originally developed by the DANSE project under NSF award DMR-0520547.
Conflicts of interest
There are no conflicts to declare.Notes and references
- M. Rajasekar and M. Lavanya, RSC Adv., 2022, 12, 1592515949 Search PubMed.
- W. T. Truong, Y. Su, J. T. Meijer, P. Thordarson and F. Braet, Chem. – Asian J., 2011, 6, 30–42 CrossRef CAS PubMed.
- M. P. Lutolf and J. Hubbell, Nat. Biotechnol., 2005, 23, 47–55 CrossRef CAS PubMed.
- A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002–8018 CrossRef CAS PubMed.
- C.-W. Chu and C. A. Schalley, Org. Mater., 2021, 3, 025–040 CrossRef CAS.
- A. P. McCloskey, E. R. Draper, B. F. Gilmore and G. Laverty, J. Pept. Sci., 2017, 23, 131–140 CrossRef CAS PubMed.
- E. R. Draper and D. J. Adams, Responsive Materials by the Self-assembly of Low Molecular Weight Gelators, Royal Society of Chemistry, 2015 Search PubMed.
- Z. Álvarez, J. A. Ortega, K. Sato, I. R. Sasselli, A. N. Kolberg-Edelbrock, R. Qiu, K. A. Marshall, T. P. Nguyen, C. S. Smith and K. A. Quinlan, Cell Stem Cell, 2023, 30(219–238), e214 Search PubMed.
- P. Sharma and S. Roy, Nanoscale, 2023, 15, 7537–7558 RSC.
- T. Zhu, Y. Ni, G. M. Biesold, Y. Cheng, M. Ge, H. Li, J. Huang, Z. Lin and Y. Lai, Chem. Soc. Rev., 2023, 52, 473–509 RSC.
- C. Gao, S. Song, Y. Lv, J. Huang and Z. Zhang, Macromol. Biosci., 2022, 22, 2200051 CrossRef CAS PubMed.
- S. Au-Yong, M. Firlak, E. R. Draper, S. Municoy, M. D. Ashton, G. R. Akien, N. R. Halcovitch, S. J. Baldock, P. Martin-Hirsch and M. F. Desimone, Polymers, 2022, 14, 4953 CrossRef CAS PubMed.
- E. R. Draper and D. J. Adams, Supramol. Nanotechnol., 2023, 2, 619–639 Search PubMed.
- L. Chen, T. O. McDonald and D. J. Adams, RSC Adv., 2013, 3, 8714–8720 RSC.
- S. Liu, S. C. Joshi and Y. Lam, J. Appl. Polym. Sci., 2008, 109, 363–372 CrossRef CAS.
- D. K. Smith, Mol. Gels, 2018, 300–371 CAS.
- E. R. Draper, J. J. Walsh, T. O. McDonald, M. A. Zwijnenburg, P. J. Cameron, A. J. Cowan and D. J. Adams, J. Mater. Chem. C, 2014, 2, 5570–5575 RSC.
- L. Thomson, R. E. Ginesi, D. D. Osborne, E. R. Draper and D. J. Adams, Chem. – Eur. J., 2023, 29, e202300663 CrossRef CAS PubMed.
- Q. Liu, T. Liu and Y. Fang, Langmuir, 2020, 36, 2155–2169 CrossRef CAS PubMed.
- D. McDowall, B. J. Greeves, R. Clowes, K. McAulay, A. M. Fuentes-Caparrós, L. Thomson, N. Khunti, N. Cowieson, M. C. Nolan, M. Wallace, A. I. Cooper, E. R. Draper, A. J. Cowan and D. J. Adams, Adv. Energy Mater., 2020, 10, 2002469 CrossRef CAS.
- Y. Feng, Z. Ma, S. Zhong, C. Wang and X. Chen, Chem. – Eur. J., 2023, 29, e202301074 CrossRef CAS PubMed.
- M. Sun, K. Müllen and M. Yin, Chem. Soc. Rev., 2016, 45, 1513–1528 RSC.
- J. G. Egan, G. Brodie, D. McDowall, A. J. Smith, C. J. Edwards-Gayle and E. R. Draper, Mater. Adv., 2021, 2, 5248–5253 RSC.
- V. Adams, J. Cameron, M. Wallace and E. R. Draper, Chem. – Eur. J., 2020, 26, 9879–9882 CrossRef CAS PubMed.
- E. R. Draper, L. J. Archibald, M. C. Nolan, R. Schweins, M. A. Zwijnenburg, S. Sproules and D. J. Adams, Chem. – Eur. J., 2018, 24, 4006–4010 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Expriemental procedures, Uv-vis absorption spectra, rheology, SANS and electrochemical data. See DOI: https://doi.org/10.1039/d3cc04557a |
| This journal is © The Royal Society of Chemistry 2024 |