All-in-one fabrication of a ratiometric electrochemical aptasensor with tetrahedral DNA nanostructure for fumonisin B1 detection†
Na
Dong
a,
Shuda
Liu
a,
Yuye
Li
a,
Shuyun
Meng
a,
Yifan
Liu
a,
Xia
Li
 c,
Dong
Liu
c,
Dong
Liu
 *a and
Tianyan
You
*a and
Tianyan
You
 *ab
*ab
aKey Laboratory of Modern Agricultural Equipment and Technology (Jiangsu University), Ministry of Education, School of Agricultural Engineering, Jiangsu University, Zhenjiang, Jiangsu 212013, China. E-mail: dongliu@ujs.edu.cn; youty@ujs.edu.cn
bCollege of Agricultural Equipment Engineering, Henan University of Science and Technology, Luoyang, Henan 471003, China
cDepartment of Chemistry, Liaocheng University, Liaocheng, Shandong 252059, China
First published on 23rd November 2023
Abstract
Here, we develop an all-in-one strategy for efficient assembly of an electrochemical aptasensor. A multifunctional structure based on a tetrahedral DNA nanostructure (TDN) was synthesized via a one-step annealing process, providing DNA fixation, target recognition, signal amplification and space regulation. Based on the integration of this multifunctional structure, the sensing interface was assembled in one step. A ratiometric aptasensor was constructed by anchoring methylene blue (MB) to the TDN and ferrocene (Fc) on the cDNA. Using the ratio of the currents obtained from Fc and MB as a measure, the developed aptasensor shows excellent analytical performance for fumonisin B1 detection. This strategy is universal and could simplify the fabrication of aptasensors.
Due to the specific recognition properties of aptamers (Apts) and the ability to design specific DNA chains, electrochemical aptasensors have significant advantages and have become an ideal strategy for various quantifying analyses.1–4 In conventional electrochemical sensing strategies, aptasensors can be functionalized to improve analytical performance by introducing functional units into their self-assembled layers.5–10 In these strategies, the assembly of complicated DNA structures requires sophisticated techniques,11,12 such as layer-by-layer assembly methods. The layer-by-layer method commonly suffers from several potential problems, including the crowding effect at the sensing interface, cross-interference from the introduction of new interfaces, interfacial consistency issues, and the introduction of contaminants resulting from the multi-step assembly process. Ultimately, these limitations restrict the potential applications of aptasensors and must be overcome. In previous studies, researchers ingeniously designed a palindromic molecular beacon to integrate multifunctional elements, which can reduce the complexity of the sensing system.13,14
Motivated by the integrated model concept, we envisioned the possibility of directly modifying the sensing interface on a substrate electrode through the design of a multifunctional structure, enabling the all-in-one fabrication of an aptasensor without compromising the sensing performance. In this work, we synthesized a novel multifunctional structure, including complementary DNA (cDNA), Apt, and a tetrahedral DNA nanostructure (TDN) through a one-step annealing process (Scheme 1). Each of the three components played a different function. Specifically, the cDNA functionalized with sulfhydryl groups could immobilize the multifunctional structure at the sensor interface.15 The Apt enabled specific recognition of the target through its specific structural and chemical properties,16 and the TDN was used for signal amplification, space regulation, and programmable design. The abundant binding sites of the tetrahedron could enhance the payloads of the signaling molecules to improve sensing performance.17–19 The tetrahedron could also preserve spatial separation between the units for structural stability and mechanical rigidity, thus avoiding unnecessary interactions.20–22 Additionally, different functional units can be assembled in the TDN based on rational design of complementary sequences to facilitate the integration of functions. According to the integration of multifunctional structures, an aptasensor was constructed by attaching it directly to the electrode surface and closing the non-specific binding site at the sensing interface with 6-mercapto-1-hexanol (MCH). This strategy addressed potential problems in the process of layer-by-layer assembly of the functional components.
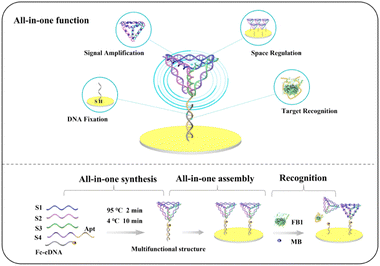 | ||
| Scheme 1 Scheme showing all-in-one fabrication process, including the all-in-one synthesis, all-in-one function, and all-in-one assembly of the ratiometric electrochemical aptasensor. | ||
Methylene blue (MB) adsorption on the TDN and the functionalization of ferrocene (Fc) on the cDNA created the output currents of MB (IMB) and Fc (IFc) which served as reference and response signals, used as a ratiometric strategy to improve accuracy. Fumonisin B1 (FB1) was selected as the model analyte to validate the applicability of the strategy. In the presence of FB1, the hybridization of Apt with FB1 induced the release of the TDN, which led to reduced steric hindrance and decreased the number of MB units present. Thus, IFc increased while IMB decreased in the presence of FB1, which in turn enhanced the IFc/IMB ratio. This strategy was not only simple in design, but also highly accurate due to the ratiometric strategy, and demonstrated a promising electrochemical detection capacity.
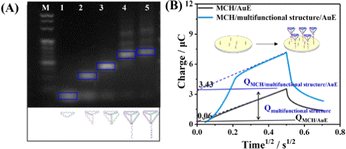
First, the designed multifunctional structure composed of cDNA, Apt, and TDN was prepared via a one-step annealing process, and the synthesis of the multifunctional structure was assessed using agarose gel electrophoresis. The leftmost lane served as the marker, while lanes 1, 2, 3, 4, and 5 corresponded to the S1, S1 + S2, S1 + S2 + S3, TDN, and multifunctional structures, respectively (Fig. 1A). Compared to other lanes, lane 5 indicated the lowest velocity migration, which was due to the large molecular weight.23 Next, atomic force microscopy (AFM) was employed to characterize the multifunctional structure. As shown in Fig. S1 (ESI†), the AFM diagram reveals that the multifunctional structures have a stereo structure with a height of 7.1–8.3 nm and have no obvious aggregates.
Next, the multifunctional structure was directly fixed on the electrode surface, and the density of the immobilized multifunctional structure was estimated from chrono-coulometric (CC) measurements using RuHex as the cation redox indicator. Increased CC charge was observed after assembling the multifunctional structure on the gold electrode (AuE) (Fig. 1B). The assembly density of the probes was deduced from the Cottrell equation and the quantitative relationship between the ruthenium ion and phosphate backbone.
According to the Cottrell equation (eqn (1)), the quantity of ruthenium ion adsorption at the AuE surface can be calculated as follows:24
| Q = 2nFAC(D/Π)t1/2 + Qc + nFAΓRu, | (1) |
According to eqn (2), the assembly density of DNA at the AuE can be converted based on the quantity of ruthenium ion adsorption as follows:24
| ΓDNA = ΓRu(z/m)NA, | (2) |
The assembled density of the multifunctional structure was calculated as 3.22 × 1012 cm−2. The presence of the TDN could enable the modulation of the probe density, improving the detection sensitivity of the aptasensor. The active electrode area after modification was estimated from cyclic voltammetry measurements at different scan rates (Fig. S2, ESI†).25 The active electrode area was calculated to be 9 mm2 using the Randles–Sevcik equation.
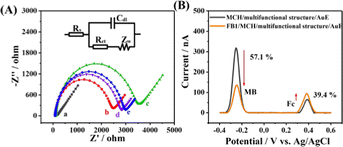
Fabrication of the electrochemical aptasensor was monitored using electrochemical impedance spectroscopy (EIS) measurements,26,27 and a Randles equivalent circuit was used to fit the experimental data, as shown in Fig. 2A. Here, Rs represents the solution resistance, Cdl represents the double-layer capacitance, Ret indicates the electron-transfer resistance, and Zw represents the Warburg impedance. The diameter of the semicircle indicates the Ret. As shown in Fig. 2A, the Ret of the AuE was small (curve a, Ret = 97.84 Ω) due to the high conductivity of the AuE. With the modification of the multifunctional structure, Ret (curve b, Ret = 2286 Ω) increased due to the blocking effect of DNA. Furthermore, after connecting with MCH, Ret increased to 3250 Ω (curve c), due to a further increase in the hindrance of electron transfer. After incubating FB1 on the modified electrode, a decreased Ret (curve d, Ret = 2640 Ω) was observed due to the stripping of the TDN. Next, a slight increase was observed in Ret after the adsorption of MB (curve e, 2803 Ω). The EIS results confirmed that the electrochemical aptasensor was successfully fabricated.
Different modified electrodes were investigated using square wave voltammetry (SWV) (Fig. 2B) to verify the feasibility of the electrochemical aptasensor for FB1 detection. Without FB1, IMB was 325 nA due to the adsorption of MB on the TDN, and IFc was 55 nA because of the steric effect of the TDN. IMB decreased by 57.1% and IFc increased by 39.4% in the presence of FB1, which was attributed to the stripping of the TDN. The above results verified that the ratiometric electrochemical aptasensor was usable for the detection of FB1.
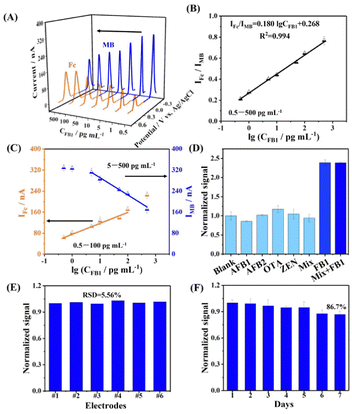
A multifunctional structure concentration of 2 μM, a multifunctional structure incubation time of 12 h, and a FB1 binding time of 40 min were used for the following experiments. The analytical performance of the electrochemical aptasensor for the detection of FB1 was then investigated. As shown in Fig. 3A, when the FB1 concentration increased from 0.5 to 500 pg mL−1, IFc increased while IMB decreased. As shown in Fig. 3B, the variation in IFc/IMB displayed a good linear relationship with the logarithmic value of FB1 concentration (lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFB1), in the range from 0.5 to 500 pg mL−1, with a fitted equation of IFc/IMB = 0.180 lg
CFB1), in the range from 0.5 to 500 pg mL−1, with a fitted equation of IFc/IMB = 0.180 lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CFB1 + 0.268 (R2 = 0.994). Ratiometric measurements provided a broader linear range than the single-signal measurements (Fig. 3C). Compared with the reported methods, the developed method exhibited a lower LoD than that of liquid chromatography-tandem mass spectrometry, surface enhanced Raman scattering, or colorimetry (Table S1, ESI†). Meanwhile, this aptasensor shows a comparable LoD to analytical methods based on electrochemiluminescence or electrochemistry, but the dual-signal output mode and one-step assembly could allow for better reliability.
CFB1 + 0.268 (R2 = 0.994). Ratiometric measurements provided a broader linear range than the single-signal measurements (Fig. 3C). Compared with the reported methods, the developed method exhibited a lower LoD than that of liquid chromatography-tandem mass spectrometry, surface enhanced Raman scattering, or colorimetry (Table S1, ESI†). Meanwhile, this aptasensor shows a comparable LoD to analytical methods based on electrochemiluminescence or electrochemistry, but the dual-signal output mode and one-step assembly could allow for better reliability.
To assess the selectivity of the electrochemical aptasensor, the effects of common interfering substances, including aflatoxin B2 (AFB2), aflatoxin B1 (AFB1), zearalenone (ZEN), and ochratoxin (OTA), on the sensing performance were investigated. Fig. 3D shows that the developed aptasensor had a significant response to FB1 or a mixture containing FB1. The developed aptasensor could only identify FB1 due to the specificity of Apt to FB1, indicating the selectivity of the aptasensor.
The reproducibility of the electrochemical aptasensor for the detection of FB1 was evaluated, indicating that the relative standard deviation (RSD) of six parallel electrodes for the detection of 100 pg mL−1 FB1 was 5.56%. This demonstrated that the developed aptasensor exhibited good reproducibility (Fig. 3E).
Moreover, the stability of the aptasensor was evaluated by recording the electrochemical response to 100 pg mL−1 of FB1 for a week. As shown in Fig. 3F, the IFc/IMB value of the aptasensor remained at 86.7% of the initial response after one week, indicating the stability of the aptasensor.
To investigate the applicability of the electrochemical aptasensor, an FB1 standard was added to obtain sample solutions of 100, 500, and 1000 ng mL−1. As shown in Table S2 (ESI†), the sample solution diluted to 1/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 was assessed 3 times, and the recoveries were in the range of 91.0–99.7% with a RSD of less than 5.9%. In addition, as a control, UPLC-MS/MS was used to determine the FB1 quantity in the samples. The results of the two methods were consistent, indicating that the aptasensor had great potential for detecting FB1 in real samples.
000 was assessed 3 times, and the recoveries were in the range of 91.0–99.7% with a RSD of less than 5.9%. In addition, as a control, UPLC-MS/MS was used to determine the FB1 quantity in the samples. The results of the two methods were consistent, indicating that the aptasensor had great potential for detecting FB1 in real samples.
In summary, we designed a multifunctional structure and developed an all-in-one fabrication strategy for an electrochemical aptasensor. The different functions, including DNA fixation, FB1 recognition, signal amplification, and space regulation, were integrated into a single multifunctional structure. The good performance of the aptasensor was attributed to the designed multifunctional structure, which favors efficient FB1 recognition and signal amplification. The all-in-one strategy could simplify the fabrication of the aptasensor, and aid the sensor portability. The dual-signal output mode improved the accuracy of the aptasensor. This approach may be universal and could be applied to the detection of other targets by changing the aptamer or adjusting the size of the TDN.
Na Dong: investigation, data curation, writing – original draft. Shuda Liu: resources, software. Yuye Li: resources, software. Shuyun Meng: resources, software. Yifan Liu: resources. Xia Li: resources. Dong Liu: supervision, methodology, conceptualization, formal analysis, writing – review & editing. Tianyan You: supervision, conceptualization, writing – review & editing.
This work was supported by the National Natural Science Foundation of China (No. 22074055, and 22374061), Natural Science Foundation of Jiangsu Province (No. BK20200104), Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX23_3663).
Conflicts of interest
There are no conflicts to declare.Notes and references
- N. Dong, D. Liu and S. Y. Meng, et al. , Sens. Actuators, B, 2022, 354, 130984 CrossRef CAS.
- M. Wei, L. K. Xin and S. Feng, et al. , Mikrochim. Acta, 2020, 187, 1–7 CrossRef.
- M. Wei, F. Zhao and S. Feng, et al. , BMC Chem., 2019, 13, 129–135 CrossRef.
- Y. Sun, L. T. Mo and X. X. Hu, et al. , ACS Nano, 2022, 16, 21129–21138 CrossRef CAS.
- P. Miao, B. D. Wang and F. Y. Meng, et al. , Bioconjugate Chem., 2015, 26, 602–607 CrossRef CAS.
- J. L. Tang, Y. L. Lei and X. X. He, et al. , Anal. Chem., 2020, 92, 10839–10846 CrossRef CAS.
- F. Jia, D. Liu and N. Dong, et al. , Biosens. Bioelectron., 2021, 182, 113169 CrossRef CAS.
- C. X. Zhu, D. Liu and Y. Y. Li, et al. , Biosens. Bioelectron., 2020, 150, 111814 CrossRef CAS PubMed.
- Y. Li, D. Liu and C. Zhu, et al. , J. Hazard. Mater., 2020, 387, 122001 CrossRef CAS.
- C. X. Zhu, D. Liu and Y. Y. Li, et al. , Food Chem., 2022, 373, 131443 CrossRef CAS.
- Z. L. Qiu, J. Shu and J. F. Liu, et al. , Anal. Chem., 2019, 91, 1260–1268 CrossRef CAS PubMed.
- J. H. Xu, J. X. Zhang and R. J. Zeng, et al. , Sens. Actuators, B, 2022, 368, 132141 CrossRef CAS.
- R. J. Zeng, L. J. Zhang and Z. B. Luo, et al. , Anal. Chem., 2019, 91, 7835–7841 CrossRef CAS.
- R. J. Zeng, Z. B. Luo and L. S. Su, et al. , Anal. Chem., 2019, 91, 2447–2454 CrossRef CAS PubMed.
- L. Martínez, L. G. Carrascosa and Y. Huttel, et al. , Phys. Chem. Chem. Phys., 2010, 12, 3301–3308 RSC.
- C. W. S. Lo, C. K. W. Chan and J. Q. Yu, et al. , Nanotheranostics, 2022, 6, 161–174 CrossRef.
- D. D. Zeng, H. Zhang and D. Zhu, et al. , Biosens. Bioelectron., 2015, 71, 434–438 CrossRef CAS.
- M. Wei and W. Y. Zhang, et al. , Sens. Actuators, B, 2018, 276, 1–7 CrossRef CAS.
- N. F. Zhu, X. S. Li and Y. Liu, et al. , Sci. Total Environ., 2021, 784, 147212 CrossRef CAS PubMed.
- M. H. Lin, J. J. Wang and G. B. Zhou, et al. , Angew. Chem., Int. Ed., 2015, 54, 2151–2155 CrossRef CAS PubMed.
- X. Chen, J. Huang and S. Zhang, et al. , ACS Appl. Mater. Interfaces, 2019, 11, 3745–3752 CrossRef CAS.
- J. Lu, J. Wang and X. L. Hu, et al. , Anal. Chem., 2019, 91, 7353–7359 CrossRef CAS PubMed.
- D. Mathur, A. Samanta and E. Oh, et al. , Chem. Mater., 2017, 29, 5762–5766 CrossRef CAS.
- J. Zhu, H. Y. Gan and J. Wu, et al. , Anal. Chem., 2018, 90, 5503–5508 CrossRef CAS.
- Y. Wang, Y. Y. Li and C. Liu, et al. , Biosens. Bioelectron., 2023, 239, 115610 CrossRef CAS.
- Z. L. Qiu, D. P. Tang and J. Shu, et al. , Biosens. Bioelectron., 2016, 75, 108–115 CrossRef CAS.
- M. D. Xu, Z. Q. Gao and Q. H. Wei, et al. , Biosens. Bioelectron., 2015, 74, 1–7 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and supplementary figures. See DOI: https://doi.org/10.1039/d3cc04991d |
| This journal is © The Royal Society of Chemistry 2024 |