A highly selective ratio-metric fluorescent sensor for visualizing nitroreductase in hypoxic cells†
Yumei
Xu
 *a,
Bing
Hu
a,
Yanjun
Cui
a,
Li
Li
a,
Fang
Nian
a,
Zhixia
Zhang
a and
Wenting
Wang
b
*a,
Bing
Hu
a,
Yanjun
Cui
a,
Li
Li
a,
Fang
Nian
a,
Zhixia
Zhang
a and
Wenting
Wang
b
aInstitute of Agricultural Resources Chemistry and Application, College of Science, Gansu Agriculture University, No. 1 Yingmen village, Anning District, Lanzhou, Gansu 730070, China. E-mail: xuym@gsau.edu.cn
bCollege of Life and Health, Wuhan Vocational College of Software and Engineering, Wuhan 430205, China
First published on 22nd November 2023
Abstract
Herein, we have developed a novel single-molecular probe (NORP) for selective and accurate determination of NTR in living cells. It was discovered that up-regulation of endogenous NTR occurred in response to hypoxic stimulation, and there was a dependence between the NTR levels and the degree of hypoxia.
Inadequate supply of oxygen to cells can lead to hypoxia, which can cause a series of diseases, such as stroke, local ischaemia and inflammation in the heart.1–4 However, hypoxia triggers the accumulation of intracellular bio-reduction reactions; for example, quinone reductase, azoreductase, and nitroreductase (NTR) are overexpressed. Typically, NTR is used as an important indicator of different levels of hypoxia in living cells.5–9 Quantitative analysis of intracellular NTR levels helps to accurately monitor the degree of hypoxia in biological systems. Despite the fact that numerous fluorescent probes with the ability to visualize NTR have been reported in recent years,10–13 these probes primarily rely on the fluorescence conductance mechanism of NTR activity to a single fluorophore, and the intensity of their emission may be dependent on both the activity of the enzyme and the local concentration of the probe. Therefore, there is an urgent need to develop a highly selective ratio-metric fluorescent probe for real-time imaging and quantification of NTR in living cells.14–17
Based on this work, a novel single-molecular platform combining an intramolecular charge transfer (ICT) and fluorescence resonance energy transfer (FRET) dual mechanism was developed as demonstrated in Scheme 1.18–20 On the one hand, a coumarin derivative was selected as the energy donor due to its good photostability, large molar absorptivity, and high fluorescence quantum yield. On the other hand, a nitro-naphthimide conjugate was selected as a potential energy acceptor as well as an enzymatic recognition site that could selectively respond to NTR, during which the electron-absorbing nitro group was exchanged for an electron-delivering amino group to activate the FRET system between the coumarin and the newly-formed amino-naphthimide group, and the latter generated ICT emission. We rationally developed a ratio-metric fluorescent probe, NORP, which displayed excellent response and good selectivity in the detection ranges of 0.5–6.0 μg mL−1 to NTR, employing this ICT-FRET response mechanism. In addition, the probe was successfully used to observe fluctuations in NTR levels in tumour cells due to its photo stability, pH-stability and low cytotoxicity. Up-regulation of endogenous NTR levels was observed in response to hypoxic stimulation, and there was a dependence between the NTR level and the degree of hypoxia.
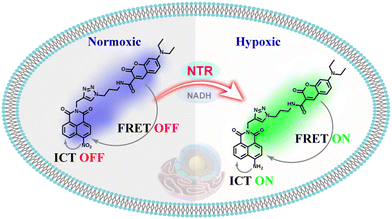 | ||
| Scheme 1 Illustration of the working principle of the developed probe NORP for the detection of NTR. | ||
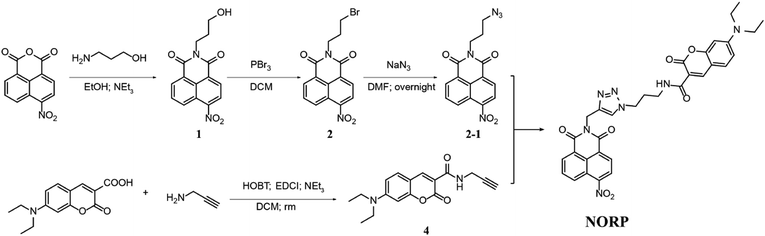
The ICT-FRET-type fluorescent probe NORP was mainly realized in two functional regions. Firstly, considering the sufficient overlap between the absorption spectrum of diethylamino coumarin and the emission spectrum of naphthylimide, diethylamino coumarin was introduced as a fluorescence donor and a naphthylimide fluorophore as a fluorescence acceptor. Secondly, the nitro group at the end of the naphthylimide served as the recognition unit of NTR. The synthesis procedures of NORP are shown in Fig. 1, and the molecular characterizations including 1H NMR, 13C NMR, and HR-MS spectra are shown in Fig. S1–S9 (ESI†).
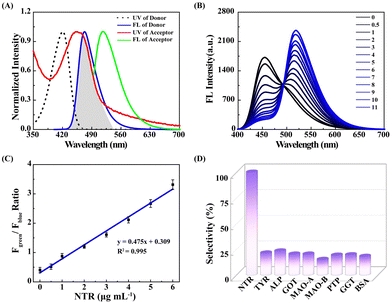
Utilizing NORP, we first assessed the NTR in vitro sensing capability in aqueous buffer (10 mM PBS, pH = 7.4). The ultraviolet-visible (UV-vis) absorption spectra and fluorescence spectrum of NORP were determined, as seen in Fig. 2A, and the fluorescence emission spectrum of the fluorescent donor exhibited a distinct spectral overlap with the absorption of the fluorescent acceptor, indicating the necessary conditions for the FRET mechanism in the optimized fluorescent system. Fluorescence titrations were performed using 405 nm excitation to quantitatively investigate the interactions between NORP and NTR. When the concentrations of NTR rose, the fluorescence intensity at 455 nm (Fblue) decreased noticeably while that at 520 nm (Fgreen) increased sharply as illustrated in Fig. 2B. With the quantity of NTR changing from 0.5 to 11.0 μg mL−1, the Fgreen/Fblue ratio of NORP exhibited an acceptable linear correlation (R2 = 0.995) when the NTR concentration varied from 0.5 to 6.0 μg mL−1 (Fig. 2C). The limit of detection (LOD) for NTR was 30 ng mL−1, while the regression equation was estimated as Fgreen/Fblue = 0.309 + 0.475 [NTR] μg mL−1. Meanwhile, the probe NORP exhibited enduring resistance to photodegradation and maintained stability within a pH range of 4 to 10 (Fig. S10, ESI†). An investigation into the selectivity of NTR was also performed. As seen in Fig. 2D, many proteins that have the potential to interfere, including tyrosinase (TYR), alkaline phosphatase (ALP), aspartic aminotransferase (GOT), monoamine oxidase-A (MAO-A), monoamine oxidase-B (MAO-B), protein tyrosine phosphatase (PTP), γ-glutamyl transferase (GCT), and bovine albumin (BAS) (100 μg mL−1 for each), yielded negligible fluorescence responses (3.5%). Additionally, a number of competition studies were carried out, which demonstrated that the determination of NTR was little impacted by the presence of possible interferents (Fig. S11, ESI†). These results provided evidence that the probe NORP was highly selective for NTR. This selectivity was ascribed to the meticulous design and engineering of the molecular structure of NORP.
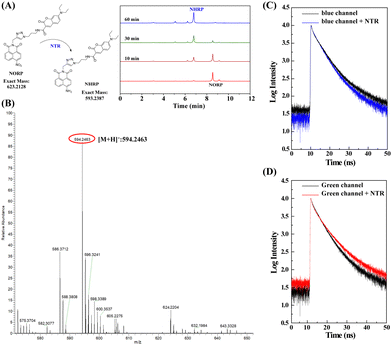
The next stage was to perform mechanistic studies to understand more about the chemical interactions between NORP and NTR. High performance liquid chromatography (HPLC) was used to better understand the molecular interaction mechanism between NORP and NTR. Two retention time peaks of 6.75 and 8.31 min could be seen in the HPLC profile for the reaction combination of NORP and NTR; the former steadily rose while the latter dropped with time, showing that the nitro was converted to amino to create the product NHRP (Fig. 3A). High resolution mass spectrometry (HR-MS), which yielded a mass to charge value (m/z) of 594.2463 belonging to [NHRP + H]+ (Fig. 3B), was used to further confirm the product's structure. Then, the response mechanism of the NORP toward NTR was further investigated by fluorescence lifetime. As shown in Fig. 3C and D, from the decay curves of the fluorescent donor (blue channel), fluorescent acceptor (green channel), and probe NORP interacting with NTR, we found that the fluorescence lifetime of the blue channel decreased from 4.8 ns to 2.7 ns and the fluorescence lifetime of the green channel increased from 3.2 ns to 5.6 ns. These results indicated that there was an energy transfer process between the fluorescent donor and acceptor after the interaction of the probe NORP with NTR, as the energy transfer from the donor to the acceptor results in an increase in the fluorescent acceptor lifetime.
Before applying the developed probe NORP for the biosensing of NTR in living cells, the cytotoxicity and biocompatibility of NORP were estimated by using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) analysis. The cell viability was higher than 91% even after the cells were incubated with NORP (30 μM) for 48 h (Fig. S12, ESI†). This result proved the low cytotoxicity of the developed probe NORP in HeLa cells. This implied that the probe NORP could be used for the detection and real-time monitoring of NTR activity in living cells without cytotoxic effects.
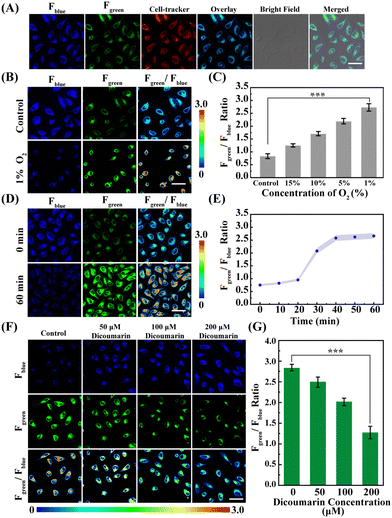
Subsequently, the fluorescence signals of the Fblue and Fgreen channels ascribed to the NORP and Cell-Tracker Red overlapped well, and the Pearson's correlation coefficient of 0.96 was obtained from the Fgreen channel and Cell-Tracker Red channel (Fig. 4A), indicating that the NORP exhibited a good ability to target the cytoplasm as expected. It is well known that cellular hypoxia can lead to the accumulation of biological reduction reactions, resulting in the overexpression of a large number of NTR. Thus, NORP was used to study the changes of NTR in neurons under hypoxic stimulation.
Next, HeLa cells cultured under different oxygen contents (20%, 10%, 5%, and 1%) were imaged with the probe NORP. The results are shown in Fig. 4B, C and Fig. S13 (ESI†). As the oxygen concentration decreases, the fluorescence signal of Fblue gradually decreases, and the fluorescence signal of Fgreen gradually enhances. Moreover, the level of NTR under different oxygen concentrations was quantitatively analysed; when the oxygen content was decreased from normoxia to 1%, then the level of NTR increased from 0.92 ± 0.03 to 5.03 ± 0.25 μg mL−1, and the level of NTR under hypoxia was almost 5.46 times higher than that under normoxia. These results demonstrate that the probe NORP could detect NTR in hypoxic cancer cells, and that there was a dependence between the level of NTR and the degree of hypoxia. Consequently, we intended to monitor the secretion process of endogenous NTR in living cells to reveal the corresponding dynamics under hypoxic (1% O2) stimulation. Experiments related to hypoxic stimulation of HeLa cells for different durations (0, 10, 20, 30, 40, 50, and 60 min) were carried out, as shown in Fig. 4D, E and Fig. S14 (ESI†), and we observed that when the duration of hypoxic stimulation was prolonged up to 20 min, a significant increase in the level of NTR was observed and the level of NTR was close to stabilising within 10 min. In order to verify that the increase in NTR level was indeed caused by the reduction reaction between NORP and NTR, we investigated the imaging analysis of HeLa cells under hypoxic conditions after adding different concentrations of dicoumarin (a competitive inhibitor of NTR).21–23 As shown in Fig. 4F and G, when the concentration of dicoumarin increased from 0 to 200 μM, the level of NTR decreased from 5.15 ± 0.25 to 1.03 ± 0.05 μg mL−1. The imaging of dicoumarin inhibition directly confirms that the probe NORP was also selective for NTR at the cellular level.
In summary, we have developed a novel ratio-metric fluorescent probe, NORP, using a unique ICT-FRET dual-mechanism integrated single-molecule platform for highly selective and precise quantitative response to NTR, which is suitable for observing NTR activity in living cells thanks to the probe's good photostability and biocompatibility. The changes of NTR level in cells under different hypoxic states were analysed, and it was convincingly demonstrated that the activity of NTR in cells is closely related to the hypoxic state. Thus, the developed probe NORP achieves precise quantitative visualization of NTR activity, and will be a valuable tool in the fields of diagnostics, drug discovery and cancer therapy, as well as providing a novel strategy for the development of novel fluorescent probes incorporating the ICT-FRET mechanism.
This work was supported by NSFC (No. 22365003).
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. H. Cho, A. L. Raybuck, K. Stengel, M. Wei, T. C. Beck, E. Volanakis, J. W. Thomas, S. Hiebert, V. H. Haase and M. R. Boothby, Nature, 2016, 537, 234–238 CrossRef CAS.
- P. Lee, N. S. Chandel and M. C. Simon, Nat. Rev. Mol. Cell Biol., 2020, 21, 268–283 CrossRef CAS.
- L. D’Ignazio and S. Rocha, Cells, 2016, 5, 10 CrossRef PubMed.
- S. P. Colgan, E. L. Campbell and D. J. Kominsky, Ann. Rev. Paleopathol., 2016, 11, 77–100 CrossRef CAS.
- E. Akiva, J. N. Copp, N. Tokuriki and P. C. Babbitt, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E9549–E9558 CrossRef CAS.
- Y.-L. Qi, L. Guo, L.-L. Chen, H. Li, Y.-S. Yang, A.-Q. Jiang and H.-L. Zhu, Coord. Chem. Rev., 2020, 421, 213460 CrossRef CAS.
- D. H. Palmer, V. Mautner, D. Hull, J. Ellis, A. Mountain, P. Searle, L. S. Young, W. Gerritsen, N. D. James and D. J. Kerr, J. Clin. Oncol., 2005, 23, 3157 Search PubMed.
- A. Negri, P. Javidnia, R. Mu, X. Zhang, J. Vendome, B. Gold, J. Roberts, D. Barman, T. Ioerger, J. C. Sacchettini, X. Jiang, K. Burns-Huang, T. Warrier, Y. Ling, J. D. Warren, D. A. Oren, T. Beuming, H. Wang, J. Wu, H. Li, K. Y. Rhee, C. F. Nathan, G. Liu and S. Somersan-Karakaya, ACS Infect. Dis., 2018, 4, 771–787 CrossRef CAS.
- N. Hannink, S. J. Rosser, C. E. French, A. Basran, J. A. H. Murray, S. Nicklin and N. C. Bruce, Nat. Biotechnol., 2001, 19, 1168–1172 CrossRef CAS PubMed.
- J. Zheng, Y. Liu, F. Song, L. Jiao, Y. Wu and X. Peng, Chem. Commun., 2020, 56, 5819–5822 RSC.
- Y. Li, Y. Sun, J. Li, Q. Su, W. Yuan, Y. Dai, C. Han, Q. Wang, W. Feng and F. Li, J. Am. Chem. Soc., 2015, 137, 6407–6416 CrossRef CAS PubMed.
- Y. Fang, W. Shi, Y. Hu, X. Li and H. Ma, Chem. Commun., 2018, 54, 5454–5457 RSC.
- Z. Mi, L. Liu, Y. Zhao and J. Guan, ACS Appl. Bio Mater., 2020, 164, 932–938 CAS.
- S. Sarkar, H. Lee, H. G. Ryu, S. Singha, Y. M. Lee, Y. J. Reo, Y. W. Jun, K. H. Kim, W. J. Kim and K. H. Ahn, ACS Sens., 2020, 6, 148–155 CrossRef.
- S. J. Kim, J. W. Yoon, S. A. Yoon and M. H. Lee, Molecules, 2021, 26, 1088 CrossRef CAS.
- D. Zhu, L. Xue, G. Li and H. Jiang, Sens. Actuators, B, 2016, 222, 419–424 CrossRef CAS.
- W. Bai, Y. Li, L. Zhao, R. Li, J. Geng, Y. Lu, Y. Zhao and J. Wang, Spectrochim. Acta, Part A, 2023, 302, 123032 CrossRef CAS.
- A. T. Aron, M. O. Loehr, J. Bogena and C. J. Chang, J. Am. Chem. Soc., 2016, 138, 14338–14346 CrossRef CAS.
- W. Zhang, F. Huo, F. Cheng and C. Yin, J. Am. Chem. Soc., 2020, 142, 6324–6331 CrossRef CAS.
- Y. Mei, Z. Liu, M. Liu, J. Gong, X. He, Q.-W. Zhang and Y. Tian, Chem. Commun., 2022, 58, 6657–6660 RSC.
- Z. Li, X. Li, X. Gao, Y. Zhang, W. Shi and H. Ma, Anal. Chem., 2013, 85, 3926–3932 CrossRef CAS.
- S. Chen, L. Xiao, Y. Li, M. Qiu, Y. Yuan, R. Zhou, C. Li, L. Zhang, Z. X. Jiang, M. Liu and X. Zhou, Angew. Chem., Int. Ed., 2022, 61, e202213495 CrossRef CAS PubMed.
- Z. Li, X. He, Z. Wang, R. Yang, W. Shi and H. Ma, Biosens. Bioelectron., 2015, 63, 112–116 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and MS spectra of NORP; photophysical properties and biocompatibility. See DOI: https://doi.org/10.1039/d3cc05063g |
| This journal is © The Royal Society of Chemistry 2024 |