[RbSr3X][(BS3)2] (X = Cl, Br): two salt-inclusion thioborates with large birefringence and structure transformation from centrosymmetric to asymmetric†
Yihan
Yun
ab,
Xueling
Hou
 ab,
Zhihua
Yang
ab,
Zhihua
Yang
 ab,
Guangmao
Li
ab,
Guangmao
Li
 *ab and
Shilie
Pan
*ab and
Shilie
Pan
 *ab
*ab
aResearch Center for Crystal Materials, CAS Key Laboratory of Functional Materials and Devices for Special Environments, Xinjiang Technical Institute of Physics & Chemistry, CAS, 40-1 South Beijing Road, Urumqi 830011, China. E-mail: slpan@ms.xjb.ac.cn; ligm@ms.xjb.ac.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China
First published on 18th November 2023
Abstract
[RbSr3X][(BS3)2] (X = Cl, Br), two salt-inclusion chalcogenides with planar [BS3] as anionic units, were obtained. Structure analysis indicates that the size effect of halogens may adjust the arrangement between the [BS3] units and further lead to the CS-to-NCS structure transformation. Experimental characterizations reveal that they have wide bandgaps (3.64–3.70 eV), large birefringence (0.136–0.144) and high LIDT (12–14 × AgGaS2). This work indicates that the thioborate family is a rich source to explore structure chemistry and promising infrared functional materials.
Laser technology has diverse applications in scientific research, industry, transportation, national defense, and healthcare.1 Optoelectronic functional crystals play a pivotal role as fundamental optical devices in laser systems. Among these, birefringent materials could generate polarized light, and further act as polarized light converters, phase compensators and polarizers.2 Currently, commercial birefringent materials like YVO4, CaCO3, LiNbO3, and α-BaB2O4 (BBO) crystals can fulfill the demand from the ultraviolet (UV) to near-infrared (IR) wavelength range.3 Meanwhile, in the longer (>5 μm) wavelength range, there is an urgent need for suitable birefringent materials. As another kind of optoelectronic functional crystal, the nonlinear optical (NLO) crystals in laser systems can broaden available coherent light sources through implementing sum-frequency generation and difference-frequency generation etc.4 At present, in the UV to near-IR spectral regions, a series of mature NLO crystals, such as KH2PO4 (KDP), KTiOPO4 (KTP), β-BBO and LiB3O5 (LBO) have been developed and applied.5 Meanwhile, in the mid to far IR region, there is a shortage of applicable NLO materials, and only commercial AgGaS2 (AGS), AgGaSe2 (AGSe), and ZnGeP2 (ZGP) crystals are relatively mature. However, owing to the drawbacks like low laser-induced damage threshold (LIDT) and harmful two-photon absorption for commercial crystals, they could not fulfill the demand in this field, and exploring IR NLO material candidates with excellent performance is of great importance.
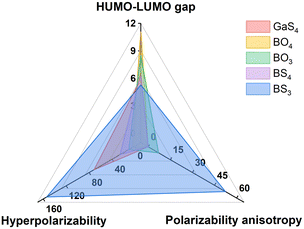
Thioborate6 system possesses the structural diversity of borates7 and the suitable transmission range of chalcogenides,8 making it a promising system for potential IR functional materials. In thioborates, [BS3] and [BS4] are the basic units, which can derive many polyfunctional groups like [B2S4], [B4S10], [B10S20], etc. Among them, [BS3] has the largest polarizability anisotropy and hyperpolarizability (Fig. 1), and also achieves good balance among HOMO–LUMO gap, polarizability anisotropy and hyperpolarizability. Recently, structures and optical properties research on thioborates has been reported, such as LaBS3 reported by Mao and zur Loye, and Ca2Ln(BS3)(SiS4) (Ln = La, Ce, and Gd) reported by Mao.9–11 Our group has also conducted a series of research studies and discovered some excellent IR functional materials, such as BaB2S4, NaBaBS3 and NaSrBS3.12–14 Nevertheless, non-centrosymmetric thioborates are still rare, and their excellent properties like balanced bandgap and SHG response are expected.
Recent research shows that introducing halide salts into chalcogenides is an efficient strategy to obtain wide bandgap and large SHG response, such as [ABa3Cl2][Ga5Se10] (A = K, Rb and Cs) reported by Chen,15 [ABa2Cl][Ga4S8] (A = Rb, Cs) reported by Guo,16 [NaBa4Cl][Ge3S10] reported by Yao17 and so on.18 Besides, the significant difference in electronegativity between halogens and S atoms may help the formation of non-centrosymmetric structures.
Under the above consideration, we attempted to synthesize new thioborate halides. Fortunately, two new salt-inclusion thioborates [RbSr3X][(BS3)2] (X = Cl, Br) were synthesized successfully. To the best of our knowledge, they are the first salt-inclusion chalcogenides with [BS3] as the fundamental building units. Besides, they show a wide bandgap (3.64–3.70 eV), large birefringence (0.136–0.144 at 1064 nm), and high LIDT (12–14 times that of AGS). Moreover, Cl–Br substitution realizes the CS-to-NCS structural transformation. Structure–property relationship analysis shows that [BS3] is the origin of the wide bandgap and large birefringence, while the head-to-head arrangement of [BS3] causes the small SHG response (0.2 times that of AGS).
Congruent melting compounds, [RbSr3X][(BS3)2] (X = Cl, Br), were obtained by a spontaneous crystallization method with the highest temperature of 800 °C in a yield of ≈90% without manual separation and purification. Herein, [RbSr3Cl][(BS3)2] crystallizes in the Pbca space group (no. 61), and the Br-based compound has the space group of Cmc21 (no. 36). The crystal data and structure refinement details are listed in Table S1 (ESI†). The atomic coordinates, equivalent isotropic displacement parameters, BVS, bond lengths and angles are provided in Tables S2–S7 and Fig. S1 (ESI†). In the asymmetric unit of [RbSr3Cl][(BS3)2], the Rb, Sr, B, S, and Cl atoms respectively occupy one, three, two, six, and one unique positions. The Cl− anion is five-coordinated with three Sr2+ and two Rb+ cations to form a [ClRb2Sr3] group. The B atoms are three-coordinated to form the planar triangle [BS3] with three S locating in the vertex angle positions. In the structure, the [BS3] units are isolated with each other, as shown in Fig. 2a. The [ClRb2Sr3] groups connect through Rb+ to form [RbSr3Cl] chains (Fig. 2b). These chains compose with [BS3] anionic groups through Rb–S and Sr–S bonds to form a three-dimensional network (Fig. 2b).
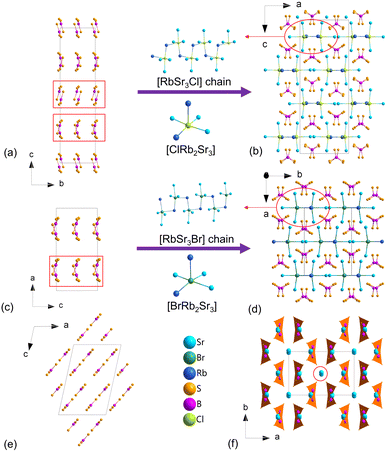 | ||
| Fig. 2 Structure comparison between [RbSr3X][(BS3)2] (X = Cl, Br) and Sr3B2S6. (a), (c) and (e) The [BS3] anionic framework; (b), (d) and (f) crystal structures. | ||
Similar to [RbSr3Cl][(BS3)2], [RbSr3Br][(BS3)2] is formed by [RbSr3Br] chains and [BS3] anion groups (Fig. 2c and d). The differences between their structures are mainly the number of atoms in the unit cell, the different [BS3] arrangements and furthermore the whole structure symmetry. Cell parameters of [RbSr3X][(BS3)2] (X = Cl, Br) ([RbSr3Cl][(BS3)2]: a = 11.0820(4) Å, b = 7.9064(3) Å, c = 29.1239(14) Å. [RbSr3Br][(BS3)2]: a = 15.0484(7) Å, b = 10.9720(5) Å, c = 7.8645(3) Å) show that the a and b values of [RbSr3Cl][(BS3)2] are almost equal to the b and c values of [RbSr3Br][(BS3)2]. Meanwhile, the c value of [RbSr3Cl][(BS3)2] is about twice the a value of [RbSr3Br][(BS3)2]. Accordingly, in the unit cells of the structures, the atom number in [RbSr3Br][(BS3)2] is half that of [RbSr3Cl][(BS3)2], as shown in Fig. 2a and c. The symmetric operation changes shown in Fig. S2 (ESI†) clearly display the evolution of symmetry breaking from [RbSr3Cl][(BS3)2] to [RbSr3Br][(BS3)2], namely the loss of the different glide planes and the inversion centre from CS to NCS. The CS-to-NCS structural transformation may originate from the size effect of the halogen (Cl and Br), which causes different bond lengths and diverse [BS3] arrangements.
From another perspective, the title compounds could be regarded as the combination of Sr3B2S6 and RbX (X = Cl, Br) (Fig. S3, ESI†). Herein, Sr3B2S6 (space group: C2/c, No. 15) was discovered in 2003 by Krebs.19 In the structure of Sr3B2S6, the [BS3] units are also isolated and parallel with each other (Fig. 2e). The title compounds have similar structures to Sr3B2S6, except that the vacancies in the title compounds are filled with RbX (X = Cl, Br) zigzag chains (Fig. 2b, d and f). Besides, the insertion of RbX results in a different arrangement of [BS3] units and derives two new structures. The results indicate that the thioborate family has large structural diversity, which may adjust the optical properties.
Energy dispersive X-ray spectroscopy (EDS) and powder X-ray diffraction (XRD) verified the reliability of the resolved structures. Rapid temperature rise and fall experiments proved that the title compounds melt congruently. As shown in Fig. S3a and S4b (ESI†), the results of EDS prove the existence of Rb, Sr, S and X atoms in [RbSr3X][(BS3)2] (X = Cl, Br). The IR and Raman absorption spectra indicate the [BS3] units, as shown in Fig. S4c–f in the ESI.† Compared with Na3BS3, Ba7(BS3)4S, and LiBaBS3, the strong absorption peaks in the Raman spectra around 450 cm−1 could be attributed to the A1′ symmetrical stretching modes of the [BS3] units. And the absorption bands at approximately 800–900 cm−1 in the IR absorption spectra are related to the E asymmetrical B–S stretching modes.20 Besides, the polycrystalline phases for both compounds were synthesized at 800 °C, and rapid temperature rise and fall experiments were conducted at 900 °C. Their experimental XRD patterns match well with the theoretical results derived from their cif files (Fig. 3a and b), which further confirms that the samples are the targeted phases, and the title compounds melt congruently.
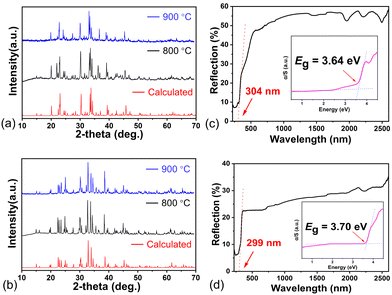 | ||
| Fig. 3 The experimental measurements of [RbSr3X][(BS3)2] (X = Cl, Br). (a) and (b) Experimental and theoretical X-ray diffraction patterns; (c) and (d) UV-vis-NIR diffuse reflection spectra. | ||
The UV-vis-NIR diffuse reflection spectra were used to estimate the bandgaps of [RbSr3X][(BS3)2] (X = Cl, Br).21 As shown in Fig. 3c and d, the bandgaps of [RbSr3X][(BS3)2] (X = Cl, Br) are 3.64 and 3.70 eV, wider than the famous IR NLO materials AGS, LiGaS2, LiInS2 and most of the salt-inclusion chalcogenides like [NaBa4Cl][Ge3S10] (3.49 eV), [ABa2Cl][Ga4S8] (A = Rb, Cs) (3.30 and 3.35 eV), and Ba4Ge3S9Cl2 (2.91 eV). Large bandgap always results in large LIDT. Thus, single pulse measurements were adopted to evaluate the powder LIDT of [RbSr3X][(BS3)2] (X = Cl, Br). Under the same conditions, the AGS sample has visible damage when the laser energy is 4 mJ, and the damage energy was 48.2 mJ and 56 mJ for [RbSr3Cl][(BS3)2] and [RbSr3Br][(BS3)2], which means the LIDTs of [RbSr3X][(BS3)2] (X = Cl, Br) are about 12–14 times that of AGS. Moreover, these LIDTs are larger than most of the promising IR NLO candidates and comparable with those of Pb17O8Cl18, β-BaGa2Se4 and so on, which implies that [RbSr3X][(BS3)2] (X = Cl, Br) has the potential to be applied in high energy laser systems.
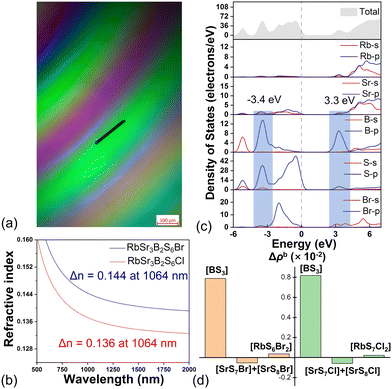
Birefringence properties are important for both birefringent crystals and NLO crystals. In order to investigate the birefringence of [RbSr3X][(BS3)2] (X = Cl, Br), a Carl Zeiss Axioscope 5 polarizing microscope was applied (Fig. 4a and Fig. S5, ESI†).22 The thicknesses of the flat crystals of [RbSr3Cl][(BS3)2] and [RbSr3Br][(BS3)2] are 0.026 and 0.030 mm. The optical path differences were measured to be 2680.2 and 3140.2 nm. The results of the improved polarization interference method show that the refractive index difference (RID) is 0.103 for [RbSr3Cl][(BS3)2] and 0.105 for [RbSr3Br][(BS3)2] at 546 nm, which indicates that the birefringence of [RbSr3X][(BS3)2] (X = Cl, Br) is at least 0.103 and 0.105, respectively. The Kurtz and Perry method was used to measure the SHG response of [RbSr3Br][(BS3)2].23 At the maximum particle size, the SHG response of [RbSr3Br][(BS3)2] is 0.2 × AGS (Fig. S6, ESI†). The NLO-active property of [RbSr3Br][(BS3)2] further confirms the CS to NCS structure transformation.
For the birefringence, calculations based on density functional theory (DFT) show that the birefringence of [RbSr3Cl][(BS3)2] is 0.155 and 0.166 for [RbSr3Br][(BS3)2] at the wavelength of 532 nm, which is in agreement with the experimental values (Fig. 4b). Besides, it could be discovered that the birefringence at 1064 nm is also larger than those of AGS, AGSe, ZGP, and the commercial birefringent crystals, α-BBO and LiNbO3.24–26 Compared with the birefringence of Sr3B2S6 (0.197 at 1064 nm), the birefringence of [RbSr3X][(BS3)2] (X = Cl, Br) is smaller (Table S8, ESI†). After structure comparison, it could be found that this decrease may be caused by the decreased density of [BS3] units, together with the cross-laying arrangements in the structure.
To analyze the structure–property relationship, we calculated the band structures and the orbital projected density of states (DOS) using DFT. The band structures indicate that [RbSr3Cl][(BS3)2] is an indirect bandgap semiconductor, while [RbSr3Br][(BS3)2] is a direct bandgap semiconductor (Fig. S7a and b, ESI†). In addition, [RbSr3X][(BS3)2] (X = Cl, Br) exhibit the similar distribution of DOS. As shown in Fig. S7c (ESI†) and Fig. 4c, the top of the valence band (VB) near the Fermi surface is mainly composed of S-3p orbitals, slightly away from the Fermi surface are the sharp peaks of the corresponding p orbitals of the halogen atoms, and the bottom of the conduction bands (CB) is mainly composed of B-2p orbitals. Therefore, the [BS3] unit plays a crucial role in the electronic structures and optical properties of these two compounds. Interestingly, compared with the bandgap of Sr3B2S6 (2.30 eV in GGA), the bandgap of [RbSr3X][(BS3)2] (X = Cl, Br) becomes wider (Fig. S8a and Table S8, ESI†). The DOS of [RbSr3Br][(BS3)2] indicates that the bonding orbital energy of B–S is about −3.4 eV, and the anti-bonding orbital energy is about 3.3 eV. In Sr3B2S6, the bonding orbital energy of B-S is about −4.1 eV, and the anti-bonding orbital energy is about 2.7 eV. Compared with Sr3B2S6 (Fig. S8b, ESI†), the introduction of ionic halide salts shifts bonding and anti-bonding orbitals to higher energy while the non-bonding S-3p orbitals still stay close to the Fermi level, so the bandgap is enlarged. Besides, it could be expected that, if the dangling non-bonding orbitals of [BS3] are eliminated, larger bandgap will be obtained. In order to investigate the origin of the birefringence, the response electron distribution anisotropy (REDA) analysis was adopted. The results reveal that the [BS3] units contribute the most to the large birefringence of [RbSr3X][(BS3)2] (X = Cl, Br) (Fig. 4d). The second-order susceptibility χ(2) tensors were also calculated, and the largest SHG coefficient in the effective SHG formula is 1.77 pm V−1, which is close to the experimental value (Table S8, ESI†). The SHG response is not large, which may be because the cancellation of second-order susceptibility derived from the inconsistent arrangement (head to head) of [BS3].
In summary, we successfully synthesized [RbSr3X][(BS3)2] (X = Cl, Br), two salt-inclusion chalcogenides with [BS3] as asymmetric building units. They have wide bandgaps (3.64–3.70 eV), large birefringence (0.136–0.144 at 1064 nm), high LIDT (12–14 times that of AGS) and SHG response (0.2 times that of AGS). Structure analysis indicates that the halogen-substitution could realize CS-to-NCS structural transformation. Theoretical calculations show that [BS3] unit is the main contributor of the optical properties. The introduction of ionic halide salts decreases the density of [BS3] units, thus reduces birefringence. Further works should be done to explore the structure diversity of the thioborate family. Moreover, legitimately regulating the arrangement of [BS3] anionic groups is a problem that needs to be further addressed to obtain high-performance thioborates.
This work is supported by the National Natural Science Foundation of China (22305264, 22335007, 61835014), the Xinjiang Major Science and Technology Project (2021A01001), the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01A333), Key Laboratory Opening Foundation of the Xinjiang Uygur Autonomous Region (2022D04013), and China Postdoctoral Science Foundation (2023T100679).
Conflicts of interest
The authors declare no conflicts of interest.Notes and references
- J. Hecht, Opt. Eng., 2010, 49(9), 091002 CrossRef.
- (a) M. Mutailipu, K. R. Poeppelmeier and S. Pan, Chem. Rev., 2021, 121, 1130–1202 CrossRef CAS PubMed; (b) H. Wu, S. Pan, K. R. Poeppelmeier, H. Li, D. Jia, Z. Chen, X. Fan, Y. Yang, J. M. Rondinelli and H. Luo, J. Am. Chem. Soc., 2011, 133, 7786–7790 CrossRef CAS; (c) H. Wu, H. Yu, Z. Yang, X. Hou, X. Su, S. Pan, K. R. Poeppelmeier and J. M. Rondinelli, J. Am. Chem. Soc., 2013, 135, 4215–4218 CrossRef CAS PubMed.
- (a) Y. Wang, B. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2018, 57, 2150–2154 CrossRef CAS; (b) X. Chen, B. Zhang, F. Zhang, Y. Wang, M. Zhang, Z. Yang, K. R. Poeppelmeier and S. Pan, J. Am. Chem. Soc., 2018, 140, 16311–16319 CrossRef CAS; (c) Z. Zhang, Y. Wang, B. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2018, 57, 6577–6581 CrossRef CAS PubMed.
- (a) B. Zhang, G. Shi, Z. Yang, F. Zhang and S. Pan, Angew. Chem., Int. Ed., 2017, 56, 3916–3919 CrossRef CAS; (b) X. Wang, Y. Wang, B. Zhang, F. Zhang, Z. Yang and S. Pan, Angew. Chem., Int. Ed., 2017, 56, 14119–14123 CrossRef CAS; (c) X. Chen, Q. Jing and K. M. Ok, Angew. Chem., Int. Ed., 2020, 59, 20323 CrossRef CAS PubMed; (d) H. Yu, M. L. Nisbet and K. R. Poeppelmeier, J. Am. Chem. Soc., 2018, 140, 8868–8876 CrossRef CAS.
- (a) J. J. De Yoreo, A. K. Burnham and P. K. Whitman, Int. Mater. Rev., 2002, 47, 113–152 CrossRef CAS; (b) C. Chen, Y. Wang, B. Wu, K. Wu, W. Zeng and L. Yu, Nature, 1995, 373, 322–324 CrossRef CAS; (c) C. Chen, G. Wang, X. Wang and Z. Xu, Appl. Phys. B: Lasers Opt., 2009, 97, 9–25 CrossRef CAS.
- Y. Lian, L. Wu and L. Chen, Dalton Trans., 2017, 46, 4134–4147 RSC.
- (a) A. Tudi, S. Han, Z. Yang and S. Pan, Coord. Chem. Rev., 2022, 459, 214380 CrossRef CAS; (b) G. Shi, Y. Wang, F. Zhang, B. Zhang, Z. Yang, X. Hou, S. Pan and K. R. Poeppelmeier, J. Am. Chem. Soc., 2017, 139, 10645–10648 CrossRef CAS.
- (a) L. Kang, M. Zhou, J. Yao, Z. Lin, Y. Wu and C. Chen, J. Am. Chem. Soc., 2015, 137, 13049–13059 CrossRef CAS PubMed; (b) Y. Chu, H. Wang, T. Abutukadi, Z. Li, M. Mutailipu, X. Su, Z. Yang, J. Li and S. Pan, Small, 2023, 2305074 CrossRef CAS; (c) G. Li, Z. Yang, X. Hou and S. Pan, Angew. Chem., Int. Ed., 2023, 62, e202303711 CrossRef CAS; (d) W. Xie, F. Li, J. Chen, Z. Yang, G. Li and S. Pan, Angew. Chem., Int. Ed., 2023, 62, e202307895 CrossRef CAS PubMed; (e) G. Li, Z. Yang and S. Pan, Sci. China Mater., 2023, 66, 1189–1196 CrossRef CAS.
- Y. Han, C. Hu, Z. Fang, Q. Chen, B. Li, Y. Lin and J. Mao, J. Mater. Chem. C, 2022, 10, 12556–12559 RSC.
- L. S. Breton, G. Morrison, M. R. Lacroix, P. S. Halasyamani and H.-C. zur Loye, Chem. Commun., 2022, 58, 7992–7995 RSC.
- Y. Han, C. Hu and J. Mao, Small, 2023, 2305828 CrossRef PubMed.
- H. Li, G. Li, K. Wu, B. Zhang, Z. Yang and S. Pan, Chem. Mater., 2018, 30, 7428–7432 CrossRef CAS.
- Y. Yun, W. Xie, Y. Huang, Z. Yang, K. Wu, G. Li and S. Pan, Chem. Mater., 2022, 34, 5215–5223 CrossRef CAS.
- Y. Yun, M. Wu, C. Xie, Z. Yang, G. Li and S. Pan, Adv. Opt. Mater., 2023, 11, 2300256 CrossRef CAS.
- P. Yu, L. Zhou and L. Chen, J. Am. Chem. Soc., 2012, 134, 2227–2235 CrossRef CAS PubMed.
- (a) B. Liu, X. Jiang, H. Zeng and G. Guo, J. Am. Chem. Soc., 2020, 142, 10641–10645 CrossRef CAS; (b) B. Liu, H. Zeng, X. Jiang, G. Wang, S. Li, L. Xu and G. Guo, Chem. Sci., 2016, 7, 6273–6277 RSC.
- K. Feng, L. Kang, Z. Lin, J. Yao and Y. Wu, J. Mater. Chem. C, 2014, 2, 4590–4596 RSC.
- P. Liu, Y. Li, Y. Zheng, J. Yu, R. Duan, H. Chen, H. Lin, L. Chen and L. Wu, Dalton Trans., 2017, 46, 2715–2721 RSC.
- A. Hammerschmidt, M. Döch, C. Püttmann and B. Krebs, Z. Anorg. Allg. Chem., 2003, 629, 551–555 CrossRef CAS.
- (a) M. Royle, J. Cho and S. W. Martin, J. Non-Cryst. Solids, 2001, 279, 97–109 CrossRef CAS; (b) Y. Kim and S. W. Martin, Inorg. Chem., 2004, 43, 2773–2775 CrossRef CAS.
- G. Kortüm, Reflectance spectroscopy: principles, methods, applications, Springer Science & Business Media, 2012 Search PubMed.
- L. Cao, G. Peng, W. Liao, T. Yan, X. Long and N. Ye, CrystEngComm, 2020, 22, 1956–1961 RSC.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- K. Kato and H. Shirahata, Jpn. J. Appl. Phys., 1996, 35, 4645–4648 CrossRef CAS.
- J. D. Beasley, Appl. Opt., 1994, 33, 1000–1003 CrossRef CAS PubMed.
- G. Zhou, J. Xu, X. Chen, H. Zhong, S. Wang, K. Xu, P. Deng and F. Gan, J. Cryst. Growth, 1998, 191, 517–519 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and calculation details, related figures, tables and crystal data. CCDC 2299855 for [RbSr3Cl][(BS3)2] and 2299856 for [RbSr3Br][(BS3)2]. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cc05205b |
| This journal is © The Royal Society of Chemistry 2024 |