Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Probing fragment ion reactivity towards functional groups on coordination polymer surfaces†
Markus
Rohdenburg
 *a,
Sebastian
Kawa
*a,
Sebastian
Kawa
 a,
Maegan
Ha-Shan
a,
Manuela
Reichelt
a,
Harald
Knorke
a,
Maegan
Ha-Shan
a,
Manuela
Reichelt
a,
Harald
Knorke
 a,
Reinhard
Denecke
a,
Reinhard
Denecke
 a and
Jonas
Warneke
a and
Jonas
Warneke
 *ab
*ab
aWilhelm-Ostwald-Institut für Physikalische und Theoretische Chemie, Universität Leipzig, Linnéstr. 2, 04103 Leipzig, Germany. E-mail: markus.rohdenburg@uni-leipzig.de; jonas.warneke@uni-leipzig.de
bLeibniz Institute of Surface Engineering (IOM), Permoserstraße 15, 04318 Leipzig, Germany
First published on 29th July 2024
Abstract
Functionalization of surface-grown coordination polymer layers by ion soft-landing of highly reactive molecular fragment ions is demonstrated. The ions form covalent bonds to terminal functional groups of the polymer at the vacuum interface, opening new perspectives for controlled bond formation using reactive ions.
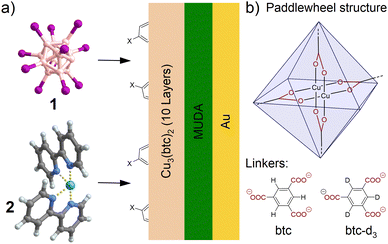
Complex reactive ions with exceptional structure and reactivity can be generated in the gas phase inside mass spectrometers, for example, by collision induced dissociation (CID), but their value is usually limited to analytical applications. Recently, new developments in electrospray ionization (ESI)-coupled mass-selective ion soft-landing (ISL)1–4 enabled using fragment ions as building blocks for new compounds deposited in thin layers on surfaces.4–7 However, when directly deposited on a surface, fragment ions often react in unexpected and seemingly uncontrolled ways. For instance, ISL of the dodecaborate fragment ion [B12Br11]− on a surface covered by a fluorinated self-assembled monolayer (FSAM) led to the covalent binding of the fragment ion to a phthalate molecule, as evidenced by ESI-MS analysis of the formed layer.8 Phthalates are common plasticizers found in vacuum chambers. Although [B12Br11]− is an anion, the vacant boron atom is highly electrophilic as may be rationalized by the high electronic stability of its dianionic precursor [B12Br12]2−.8–12 Accumulation of phthalates and similar organic molecules in layers of soft-landed dodecaborate anions was confirmed using different ISL instruments operated in different locations.6,13 MSn experiments of the doubly charged phthalate adduct allowed the conclusion that [B12Br11]− substituted a proton in one of the alkyl chains of the phthalate ([B12Br11]− + H3C–R → [B12Br11–CH2–R]2− + H+, for mechanism, see ref. 8 and 12), although binding to the more nucleophilic carbonyl groups of the phthalate is thermochemically preferred according to DFT calculations.8 These results indicate that the hydrophobic alkyl chains of the phthalate are oriented towards the vacuum interface during the ISL experiment. Apparently, the highly reactive fragment ion immediately reacts with the alkyl groups at the interface and does not reach the functional groups within the deposited layer. In follow-up experiments, we found that selective binding of non-polar hydrophobic groups is a general phenomenon observed during ISL of highly reactive ions of type [B12X11]− (X = halogen). For the present study, we confirmed this reactivity towards deliberately co-deposited reagents: ISL of [B12I11]− (1, see Fig. 1(a)) into a layer of a pre-deposited organic sulfonic acid was performed and MS2 experiments of the resulting product indicate binding via substitution of an alkyl/aryl proton although binding to the acid group would have been thermochemically preferred (see ESI1†).
The native orientation of molecules at the layer-vacuum interface apparently hinders bond formation between highly reactive fragment ions and polar functional groups. However, we aim at predicting and controlling fragment ion reactions at the surface upon ISL. In order to probe the reactivity of fragment ions towards different functional groups, molecular units containing these groups (referred to as “X” in Fig. 1(a)) must be anchored to the surface, ensuring that the targeted functional group is oriented towards the vacuum. One major challenge of this approach is characterising the binding motif between the soft-landed ion and the anchored molecule on the surface. This is because only a (sub-)monolayer of adduct can form at the interface. Nano-ESI MS is a highly sensitive analytical tool for ionic species. It can also help to elucidate structural information using MSn experiments. However, for efficient separation of the adduct from byproducts formed by deposited fragment ions that did not covalently link to surface-anchored molecules, it is necessary for the adduct to be soluble – ideally very selectively soluble. In ESI mass spectra, the byproducts can appear in high abundance, particularly when the amount of deposited fragment ions exceeds monolayer coverage. This can strongly suppress signals of the targeted products. Therefore, we aim for a procedure involving (i) dissolution of all byproducts without dissolving the surface-bound products, followed by (ii) dissolution of the targeted ionic product for sensitive nano-ESI MS analysis.
Polymer modification with highly reactive radical fragment ions previously resulted in the formation of covalent adducts with low regioselectivity.14 Herein, we employ a surface-grown Cu3(btc)2 coordination polymer as deposition substrate (btc = benzenetricarboxylate). For the preparation of Cu3(btc)2 films, a surface coated with a carboxylic acid-terminated self-assembled monolayer (COOH-SAM) was sequentially immersed in ethanolic solutions containing (i) copper(II) acetate, and (ii) trimesic acid (H3btc). The immersion sequence was repeated 10 times with intermittent rinsing with pure EtOH, accounting for a successive growth of the Cu3(btc)2 coordination polymer consisting of Cu(II) paddlewheel nodes connected by btc linkers (see Fig. 1(b)) in a layer-by-layer (LbL) fashion (for details, see ESI2†).15,16 Growth of the coordination polymer was confirmed by Reflection–Absorption Infrared Spectroscopy (RAIRS, see ESI3†). Cu3(btc)2 is (partially) soluble in solvent mixtures containing water17 but highly resistant towards EtOH, as confirmed by subjecting 10 layers of Cu3(btc)2 to liquid extraction surface analysis (LESA) using either pure EtOH or an aqueous solvent mixture (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 4
H2O 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) (see ESI4†). The coordination polymer was dissolved only by the aqueous solvent mixture, resulting in a highly abundant signal assigned to H2btc− in ESI-MS. The use of Cu3(btc)2 as deposition substrate thus allows for a characterization of covalently bound fragment ion adducts with linker molecules. EtOH can be employed to dissolve any soluble byproduct not bound to the polymer.
1 v/v) (see ESI4†). The coordination polymer was dissolved only by the aqueous solvent mixture, resulting in a highly abundant signal assigned to H2btc− in ESI-MS. The use of Cu3(btc)2 as deposition substrate thus allows for a characterization of covalently bound fragment ion adducts with linker molecules. EtOH can be employed to dissolve any soluble byproduct not bound to the polymer.
After LbL preparation, the coordination polymer substrates were mounted into the deposition chamber of a recently introduced ion soft-landing instrument optimized for fragment ion deposition.4,7 The instrument consists of two ESI sources, a dual ion funnel system, a collision cell, a 90° bent ion guide, a quadrupole mass filter and a sample holder (for details, see ESI2.3†). We deposited the fragment ions 1 (previously only bound to alkyl chains upon ISL) and [Ru(bpy)2]2+ (2, bpy = bipyridine (C10H8N2), see Fig. 1(a)) that efficiently reacts with carboxylate groups in ISL experiments.4,18 Both 1 and 2 are strong electrophiles8–12 and B and Ru have a strong affinity towards oxygen.19,20 All fragment ions were generated in the ISL instrument by CID (see ESI5†) in the gas phase from stable precursor ions. 1 and 2 were soft-landed with kinetic energies below 10 eV z−1 (see ESI2†) onto 10 layers of Cu3(btc)2. After ISL, the substrates were rinsed with EtOH to remove any soluble products not bound to Cu3(btc)2. ESI-MS analysis of the EtOH rinsing solutions can be found in ESI6.† RAIRS showed no changes neither after deposition of 1 and 2 nor after EtOH rinsing due to the dominating carboxylate bands (see ESI7 and 8†). X-Ray photoelectron spectroscopy (XPS) was thus used as a surface-sensitive tool to investigate whether the presence of 1 and 2 can be confirmed spectroscopically after dissolving all unbound products and showed the presence of I and Ru, respectively (see Fig. 2(a) and (b)). Low energy Ar+ sputtering of the sample treated with 1 revealed that the I 3d signal intensity began to decrease before the Cu 2p and C 1s signals decreased (see ESI9†), consistent with the assumption of surface-bound I-containing species resulting from 1 reacting immediately at the layer-vacuum interface. Quantification of the I 3d and Ru 3p signals relative to the Cu 2p signal indicates that more of 2 than 1 was bound to the coordination polymer surface under the chosen deposition conditions (see ESI9.1†). Post-rinsing LESA-MS of freshly prepared samples was performed with MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4
H2O (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as solvent to dissolve the Cu3(btc)2 coordination polymer. Fig. 2(c) and (d) show excerpts from respective ESI mass spectra. Note that none of the signals detected in LESA-MS from the surface (A1 or B1) were found in MS analysis of the rinsing solution, further supporting the assumption that the respective species were covalently bound to the coordination polymer surface.
1 v/v) as solvent to dissolve the Cu3(btc)2 coordination polymer. Fig. 2(c) and (d) show excerpts from respective ESI mass spectra. Note that none of the signals detected in LESA-MS from the surface (A1 or B1) were found in MS analysis of the rinsing solution, further supporting the assumption that the respective species were covalently bound to the coordination polymer surface.
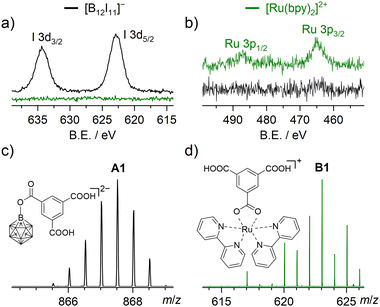 | ||
Fig. 2 Excerpts of XP core level spectra acquired after deposition of 1 (black) and 2 (green) on Cu3(btc)2 samples and subsequent rinsing with EtOH in the (a) I 3d region and (b) Ru 3p region. Excerpts of LESA mass spectra (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 4 H2O 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) acquired after deposition of (c) 1 (−ESI) and (d) 2 (+ESI) on a Cu3(btc)2 sample and subsequent rinsing with EtOH (for larger range mass spectra, see ESI10†). In (c), the I substituents of 1 were omitted. 1 v/v) acquired after deposition of (c) 1 (−ESI) and (d) 2 (+ESI) on a Cu3(btc)2 sample and subsequent rinsing with EtOH (for larger range mass spectra, see ESI10†). In (c), the I substituents of 1 were omitted. | ||
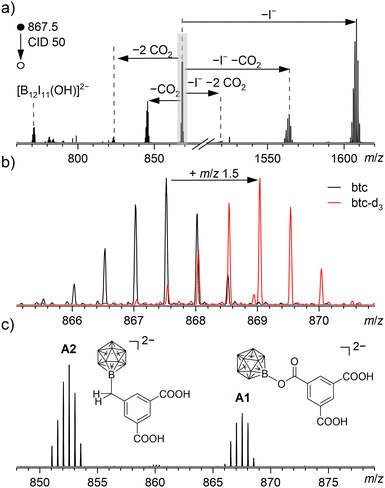
In the case of 1, only one doubly charged product of the fragment ion with btc was observed at m/z 867.5, corresponding to an ion with the molecular formula [B12I11(C9H5O6)]2− (A1), i.e., a proton within trimesic acid is substituted by 1. From the determined molecular formula of A1, it remains unclear whether 1 binds to btc via the COOH group by substitution of the oxygen-bound proton, or at the benzene ring by substitution of a carbon-bound proton (as evident from previous cases, e.g., [B12Br11]− binding to tetraphenylphosphonium cations).7 We thus decided to further explore the structure of A1via MS2 experiments, see Fig. 3(a). Upon CID of A1, elimination of up to two molecules CO2 was observed both from A1 and from a singly charged fragment of A1 that forms through I− elimination. We note that a third elimination of CO2 was not observed. The same observation was made upon CID of B1 (m/z 623.1, assigned to [(C10H8N2)2Ru(C9H5O6)]+, see ESI11†), indicating that the third carboxylate group in btc cannot be cleaved off easily from the formed adducts (see Fig. 2(c) and (d)). Further inspection of the MS2 spectrum of A1 revealed that [B12I11(OH)]2− was the fragment ion with smallest m/z value, hinting towards the presence of a B–O bond in A1 that persists upon fragmentation. Additionally, we performed ISL experiments with 1 on partially deuterated Cu3(btc-d3)2, including a derivative of btc with aromatic C–D bonds (btc-d3, see Fig. 1(b)), see Fig. 3(b).
Here, we observed the product ion signal A1 in LESA-MS at m/z 869.0, i.e., shifted by m/z 1.5 (≙3 u for a doubly charged ion). Since in btc-d3 all three H atoms on the aromatic moiety are substituted by D atoms, we deduce that A1 must also contain all three aromatic D atoms, evidencing that binding of 1 did not occur via D+ substitution but rather by H+ substitution in a COOH group. This clarifies that binding of 1 to the Cu3(btc)2 surface indeed proceeds via bond formation between the electrophilic B atom of 1 and a nucleophilic O atom of a carboxylate in a terminating linker and not – as described previously7,8,12 – by H+ substitution in C–H bonds. Binding of 2 to Cu3(btc)2 also proceeds via its electrophilic site (see ESI11–13† for CID of B1 and deuteration experiments).
The kinetic preference for H+ substitution in unfunctionalized hydrocarbons that was previously observed6,8 for 1 can thus be overcome by providing a carboxylate-terminated surface at the layer-vacuum interface through termination of a surface-grown coordination polymer with polydentate carboxylate linkers. For 2, we expect binding of a single carboxylate group of btc as a bidentate ligand to the Ru centre similar to COOH-SAM surfaces functionalized by 2.18 Further evidence can be obtained from the MS2 spectrum of B1, in which [Ru(bpy)(C10H7N2)(O2)]+ is the most abundant product ion. Upon CID, the two O atoms of the binding carboxylate group remained at the Ru centre (see ESI11†), which agrees with the high affinity of Ru towards O.19,20
In the next step, we explored whether the Cu3(btc)2 coordination polymer can serve as a templated surface providing different functional groups at the vacuum interface during ISL. A facile substitution of the btc linker with 5-functionalized isophthalic acids in the topmost layer of Cu3(btc)2 layers was already successfully demonstrated.21,22 Therefore, we substituted H3btc in the 10th preparation cycle of the surface-grown Cu3(btc)2 coordination polymer by 5-methylisophthalic acid (H2MeIp) and performed ISL of 1 on this substrate. After rinsing with EtOH, the sample was subjected to LESA-MS with the MeOH:H2O solvent mixture. Fig. 3(c) shows the relevant excerpt from the mass spectrum. Indeed, adduct formation with the terminating MeIp linker was predominantly observed (A2 at m/z 852.6, assigned to [B12I11(C9H7O4)]2−). MS2 analysis of A2 showed up to two eliminations of CO2 upon CID, indicating that 1 did not bind via addition to the carboxylate groups (which were used to anchor the MeIp linker to the Cu3(btc)2 surface), but rather by reaction with the methyl group provided at the vacuum interface (see ESI14†). However, we also observed A1 in this experiment in abundance of approximately 50% as compared to A2. This result indicates that either only an incomplete termination of the coordination polymer with MeIp linkers can be achieved by the LbL preparation method or that bond formation between reactive fragment ions and linker molecules can indeed occur beyond the mere layer-vacuum interface, likely due to the porous structure of this type of coordination polymer.23 The experiment was repeated with 2 and no evidence for bond formation between 2 and the CH3 group of a MeIp ligand was found. Note that in contrast to 1, 2 cannot bind to alkyl groups. Rather, aside from the presence of B1 in LESA mass spectra, only a carboxylate-bound adduct between 2 and MeIp was observed (see ESI15†), most likely due to defectively coordinated MeIp linkers with accessible COOH groups and in line with the affinity of 2 towards carboxylates.4,18
In summary, we introduced a method for bond formation between reactive ions available from mass-selective ISL experiments and defined surface functional groups. A Cu3(btc)2 coordination polymer ensures a defined orientation of the reagent anchored to the polymer for bonding with the reactive ions. This overcomes the issue of the native reagent orientation, which can turn polar functional groups away from the vacuum interface. In addition, this approach benefits from the solubility contrast provided by Cu3(btc)2 that allows to dissolve unbound products prior to analysing formed adducts using ESI-MS. Surface-grown Cu3(btc)2 can be terminated by different 5-functionalized isophthalic acids, providing access to a wide range of functional groups for studies on fragment ion reactivity on surfaces. The method potentially opens novel post-synthesis interface modification with reactive ions. The Cu3(btc)2 coordination polymer is the building unit of the surface-mounted metal–organic framework (SurMOF) HKUST-1.24 Functionalization of SurMOFs is essential for, e.g., adjusting entrance barriers into these porous structures.24 The functionalization with dodecaborate derivatives was shown to enhance the gas sensing and separation capabilities of HKUST-1 SurMOFs.25,26 Defined functionalization of SurMOFs has thus the potential to become a promising application of reactive ion deposition.
Conceptualization: M. Ro., J. W.; data curation: M. Ro.; formal analysis: M. Ro. (lead), M. Re., R. D., J. W.; investigation: M. Ro. (lead), S. K., M. H.-S.; validation: M. Ro.; visualization: M. Ro., S. K., M. Re., H. K.; writing – original draft: M. Ro.; writing – review & editing: M. Ro., S. K., R. D., J. W.; resources: H. K., R. D., J. W.; funding acquisition: J. W.
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) SFB TRR 102 (Polymers under multiple constraints: restricted and controlled molecular order and mobility). JW acknowledges a Freigeist Fellowship from the Volkswagen Foundation. The authors are grateful to Mrs A. Giermann, Mrs K. Papagrigoriou and Mr R. Schiewe (all Leipzig University) for their help with sample preparation, and to the Jenne group (University of Wuppertal) for providing precursor salts. Computations for this work were done using resources of Leipzig University Computing Center.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- S. A. Miller, H. Luo, S. J. Pachuta and R. G. Cooks, Science, 1997, 275, 1447–1450 CrossRef CAS.
- J. Laskin, G. E. Johnson, J. Warneke and V. Prabhakaran, Angew. Chem., Int. Ed., 2018, 57, 16270–16284 CrossRef CAS PubMed.
- S. Rauschenbach, M. Ternes, L. Harnau and K. Kern, Annu. Rev. Anal. Chem., 2016, 9, 473–498 CrossRef CAS PubMed.
- H. Y. Samayoa-Oviedo, K.-A. Behrend, S. Kawa, H. Knorke, P. Su, M. E. Belov, G. Anderson, J. Warneke and J. Laskin, Anal. Chem., 2021, 93, 14489–14496 CrossRef CAS PubMed.
- H. Gholipour-Ranjbar, H. Y. Samayoa-Oviedo and J. Laskin, ACS Nano, 2023, 17, 17427–17435 CrossRef CAS PubMed.
- F. Yang, K. A. Behrend, H. Knorke, M. Rohdenburg, A. Charvat, C. Jenne, B. Abel and J. Warneke, Angew. Chem., Int. Ed., 2021, 60, 24910–24914 CrossRef CAS PubMed.
- M. Rohdenburg, Z. Warneke, H. Knorke, M. Icker and J. Warneke, Angew. Chem., Int. Ed., 2023, 62, e202308600 CrossRef CAS PubMed.
- J. Warneke, M. Mayer, M. Rohdenburg, X. Ma, J. K. Y. Liu, M. Grellmann, S. Debnath, V. A. Azov, E. Apra, R. P. Young, C. Jenne, G. E. Johnson, H. I. Kenttämaa, K. R. Asmis and J. Laskin, Proc. Nat. Acad. Sci. U. S. A., 2020, 117, 23374–23379 CrossRef CAS PubMed.
- M. Rohdenburg, M. Mayer, M. Grellmann, C. Jenne, T. Borrmann, F. Kleemiss, V. A. Azov, K. R. Asmis, S. Grabowsky and J. Warneke, Angew. Chem., Int. Ed., 2017, 56, 7980–7985 CrossRef CAS PubMed.
- M. Mayer, V. van Lessen, M. Rohdenburg, G.-L. Hou, Z. Yang, R. M. Exner, E. Aprà, V. A. Azov, S. Grabowsky, S. S. Xantheas, K. R. Asmis, X.-B. Wang, C. Jenne and J. Warneke, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 8167–8172 CrossRef CAS PubMed.
- M. Mayer, M. Rohdenburg, V. van Lessen, M. C. Nierstenhöfer, E. Aprà, S. Grabowsky, K. R. Asmis, C. Jenne and J. Warneke, Chem. Commun., 2020, 56, 4591–4594 RSC.
- X. Ma, M. Rohdenburg, H. Knorke, S. Kawa, J. K.-Y. Liu, E. Aprà, K. R. Asmis, V. A. Azov, J. Laskin, C. Jenne, H. I. Kenttämaa and J. Warneke, Phys. Chem. Chem. Phys., 2022, 24, 21759–21772 RSC.
- J. Warneke, M. E. McBriarty, S. L. Riechers, S. China, M. H. Engelhard, E. Aprà, R. P. Young, N. M. Washton, C. Jenne, G. E. Johnson and J. Laskin, Nat. Commun., 2018, 9, 1889 CrossRef PubMed.
- L. Hanley and S. B. Sinnott, Surf. Sci., 2002, 500, 500–522 CrossRef CAS.
- O. Shekhah, H. Wang, S. Kowarik, F. Schreiber, M. Paulus, M. Tolan, C. Sternemann, F. Evers, D. Zacher, R. A. Fischer and C. Wöll, J. Am. Chem. Soc., 2007, 129, 15118–15119 CrossRef CAS PubMed.
- O. Shekhah, J. Liu, R. A. Fischer and C. Wöll, Chem. Soc. Rev., 2011, 40, 1081–1106 RSC.
- T. Hashem, E. P. V. Sanchez, E. Bogdanova, A. Ugodchikova, A. Mohamed, M. Schwotzer, M. H. Alkordi and C. Wöll, Membranes, 2021, 11(3), 207 CrossRef CAS PubMed.
- G. E. Johnson and J. Laskin, Chem. – Eur. J., 2010, 16, 14433–14438 CrossRef CAS PubMed.
- M. Rohdenburg, R. Winkler, D. Kuhness, H. Plank and P. Swiderek, ACS Appl. Nano Mater., 2020, 3(8), 8352–8364 CrossRef CAS.
- T. E. Madey, N. S. Faradzhev, B. V. Yakshinskiy and N. V. Edwards, Appl. Surf. Sci., 2006, 253, 1691–1708 CrossRef CAS.
- Z. Wang, S. Wannapaiboon, K. Rodewald, M. Tu, B. Rieger and R. A. Fischer, J. Mater. Chem. A, 2018, 6, 21295–21303 RSC.
- Z. Wang, S. Henke, M. Paulus, A. Welle, Z. Fan, K. Rodewald, B. Rieger and R. A. Fischer, ACS Appl. Mater. Interfaces, 2020, 12, 2655–2661 CrossRef CAS PubMed.
- S. S. Chui, S. M. Lo, J. P. Charmant, A. G. Orpen and I. D. Williams, Science, 1999, 283, 1148–1150 CrossRef CAS PubMed.
- A. L. Semrau, Z. Zhou, S. Mukherjee, M. Tu, W. Li and R. A. Fischer, Langmuir, 2021, 37, 6847–6863 CrossRef CAS PubMed.
- Y. Zhang, L. Yang, L. Wang, S. Duttwyler and H. Xing, Angew. Chem., Int. Ed., 2019, 58, 8145–8150 CrossRef CAS PubMed.
- C. Chen, Z. Chen, M. Zhang, S. Zheng, W. Zhang, S. Li and F. Pan, ChemSusChem, 2023, 16, e202300434 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: ESI1–15: additional MS, XPS and RAIRS spectra, results of DFT calculations. See DOI: https://doi.org/10.1039/d4cc00767k |
| This journal is © The Royal Society of Chemistry 2024 |