Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
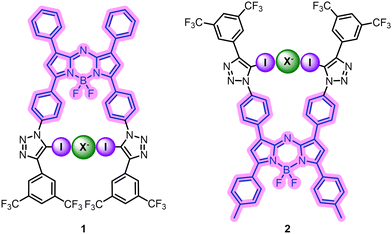
Halogen bonding aza-BODIPYs for anion sensing and anion binding-modulated singlet oxygen generation†
Andrew J.
Taylor
 and
Paul D.
Beer
and
Paul D.
Beer
 *
*
Department of Chemistry, Chemistry Research Laboratory, University of Oxford, Mansfield Road, Oxford OX1 3TA, UK. E-mail: paul.beer@chem.ox.ac.uk
First published on 21st June 2024
Abstract
Two novel aza-BODIPY based anion sensors, decorated with halogen bonding recognition sites, are capable of detecting halide anions at biologically-relevant near-IR wavelengths. With potential application for improving the selectivity of photodynamic therapy agents, unprecedented supramolecular host–guest anion binding-modulated singlet oxygen generation is demonstrated.
Following their first report in 2002,1 aza-boron-dipyrromethene (aza-BODIPY) compounds have attracted much interest in recent years due to their long-wavelength absorption and emission features and photosensitizing properties,2,3 which renders them promising photodynamic therapy (PDT) and cellular imaging agents.4–7
The combination of favourable optical properties and synthetic flexibility means that aza-BODIPY compounds are propitious candidates for supramolecular sensing materials. Indeed, aza-BODIPY compounds have found application as sensors for alkali metal,8 mercury,9 and copper cations,10 as well as pH sensors.11–13 Aza-BODIPY based chemodosimeters for anions including NO2−,14 F−,15 CN−,16 and ClO−![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 have also been reported, however these compounds do not allow for reversible, supramolecular sensing – which remains exceedingly rare for aza-BODIPY based probes.
17 have also been reported, however these compounds do not allow for reversible, supramolecular sensing – which remains exceedingly rare for aza-BODIPY based probes.
Over recent years, halogen bonding (XB) interactions have been exploited for anion recognition due to their advantages in affinity, directionality and selectivity over traditionally employed interactions, such as hydrogen bonding (HB).18,19 Within the context of optical sensing, XB interactions have additionally shown enhanced signal transduction,20 leading to the development of an array of optical sensors for anions and other analytes.21–25 However, optical sensors employing XB interactions for anion recognition which operate at biologically-relevant wavelengths remain scarce, and the use of aza-BODIPY compounds within this context is unprecedented.
The use of aza-BODIPY compounds for PDT exploits their ability to generate reactive oxygen species (ROS), in particular singlet oxygen (1O2). The generation of 1O2 is mediated by the triplet state of the aza-BODIPY fluorophore, which is itself generated by intersystem crossing (ISC) (Fig. 1).26 The use of heavy halogen atom substituents has been shown to improve the efficiency of ISC and hence 1O2 generation.4,26 Therefore, the judicious combination of iodine-based XB donor motifs with an aza-BODIPY fluorophore could conceivably facilitate both fluorescent anion sensing and also efficient 1O2 generation. Methods to reversibly modulate the generation of 1O2 are highly sought after, because they allow the selectivity of PDT techniques to be improved.27 Existing methods have focused on activatable photosensitisers which respond, often irreversibly, to stimuli including pH,28 H2O229 and thiols.30 Anion activated photosensitisers are very rare, and previous reports have focused on irreversible activation methods.31,32
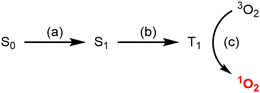 | ||
| Fig. 1 Photophysical pathway for the generation of 1O2. (a) Excitation, (b) intersystem-crossing, (c) energy transfer. | ||
Herein, we report the synthesis of two novel, XB aza-BODIPY based anion sensors 1 and 2 and investigate the relationship between the structure of the sensor, its anion binding response, and anion affinity. Through optical fluorescence titration studies, we demonstrate that the aza-BODIPY fluorophore displays strong anion binding responses in the near-IR (NIR) wavelength window, which is promising for biological applications. Furthermore, we show that one of the XB aza-BODIPY sensors displays photosensitising ability and demonstrate, in a proof-of-concept, unprecedented reversible anion binding-modulated 1O2 generation, highlighting a potential novel supramolecular host–guest approach for tuning and improving PDT selectivity.
The target anion sensors 1 and 2 feature two iodotriazole XB donor moieties appended to a planar aza-BODIPY core, allowing for anion binding in a convergent configuration (Fig. 2). It was anticipated that anion binding in close proximity to the aza-BODIPY core would induce changes in the fluorophore's emission spectrum, enabling optical sensing. Electron-withdrawing aromatic motifs, bearing –CF3 groups, were used to polarise the iodotriazole XB donors to improve anion binding affinity.33
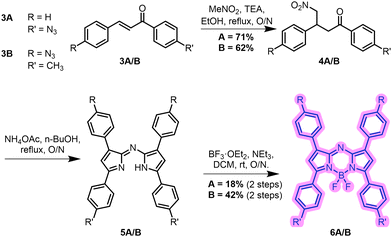
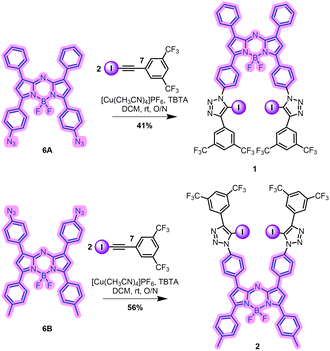
The synthetic route undertaken to prepare the two XB aza-BODIPY receptors initially targeted the parent aza-BODIPY azides, 6A and 6B, beginning from the aldol condensation products 3A and 3B (Scheme 1).34 Michael addition of nitromethane afforded intermediates 4A and 4B in 71% and 62% yields respectively.354A and 4B were refluxed in n-BuOH with NH4OAc to afford intermediates 5A and 5B. These were used without further purification and the chelation of the BF2 unit was accomplished via treatment of 5A and 5B with BF3·OEt2, giving 6A and 6B in 18% and 42% yields over two steps respectively.35 The target XB aza-BODIPY anion receptors 1 and 2 were synthesised in 41% and 56% yield respectively via CuAAC methodology, combining 6A and 6B with two equivalents of the electron-withdrawing iodo-alkyne 7 (Scheme 2).36 Novel compounds were characterised by, 1H, 13C, 19F and 11B NMR, UV-vis spectroscopy and HR-MS (see ESI,† Section S2).‡
Both receptors displayed characteristic aza-BODIPY optical properties,26 with absorption and emission features in the NIR biologically-relevant wavelength range and typical Stokes shifts (Table 1 and Fig. S28, S29, ESI†).
| Compound | 1 | 2 |
|---|---|---|
| λ max,abs (nm) | 656 | 670 |
| ε (M−1 cm−1) | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| λ max,em (nm) | 684 | 701 |
| Stokes shift (nm) | 28 | 31 |
The anion sensing properties of the receptors were investigated via fluorescence studies in acetone, by addition of aliquots of a variety of tetrabutylammonium salts (TBAX, X = Cl, Br, I, HSO4, OAc, H2PO4). Upon addition of halide anions, both receptors exhibited a significant turn-off quenching response with negligible changes in either absorbance or emission wavelength maxima (Fig. 3 and Fig. S30, ESI†), whereas no response was observed upon addition of TBAHSO4 or TBAH2PO4,§ in line with the frequently observed preference of XB sensors for halide anions.37
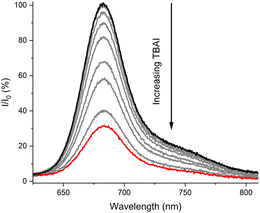 | ||
| Fig. 3 Normalised fluorescence emission response of 1 μM sensor 1 upon addition of increasing concentrations of I− (up to 2.8 mM) in acetone at 298 K. | ||
Receptor 1 displayed a relatively larger turn-off response to all halide anions. Invariably, I− produced the strongest quenching, which was ascribed to a heavy atom effect enhancing ISC, with receptor 1 showing up to a 70% diminishment in fluorescence intensity in the presence of 2.8 mM TBAI (Fig. 4). Cl− and Br− produced smaller, but still significant quenching effects.¶ Pleasingly, the receptors remained sensitive to Cl− in the presence of water in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetone/water (v/v) mixture (the halide anion with the highest hydration enthalpy tested; see Fig. S31 and S32, ESI†).
1 acetone/water (v/v) mixture (the halide anion with the highest hydration enthalpy tested; see Fig. S31 and S32, ESI†).
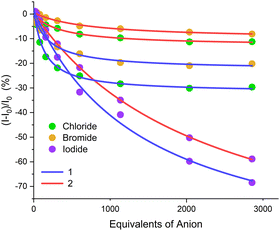 | ||
| Fig. 4 Representative fluorescence isotherms for the titration of halide anions with receptors 1 (blue) and 2 (red) (1 μM, acetone, 298 K). | ||
Global fitting of the fluorescence isotherms to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric binding model determined the association constant values shown in Table 2. It is important to note that the magnitude of the fluorescence turn-off response is not correlated with the strength of anion binding. Indeed, the binding constants indicate that the halide anions are bound with affinities correlating to their charge-density, as expected in organic solvent media.38 It is noteworthy that receptor 1 exhibited stronger halide binding, which may be due to the convergent orientation of the iodo-triazole motifs of the XB donor binding cavity being relatively closer together and of a more complementary geometry in receptor 1 compared to receptor 2, determined from a computational study of similar compounds.39
1 stoichiometric binding model determined the association constant values shown in Table 2. It is important to note that the magnitude of the fluorescence turn-off response is not correlated with the strength of anion binding. Indeed, the binding constants indicate that the halide anions are bound with affinities correlating to their charge-density, as expected in organic solvent media.38 It is noteworthy that receptor 1 exhibited stronger halide binding, which may be due to the convergent orientation of the iodo-triazole motifs of the XB donor binding cavity being relatively closer together and of a more complementary geometry in receptor 1 compared to receptor 2, determined from a computational study of similar compounds.39
| Anion | 1 | 2 |
|---|---|---|
Association constants (M−1) in acetone determined by global fitting to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model. All errors <10%. Acetone, 298 K. 1 binding model. All errors <10%. Acetone, 298 K. |
||
| Cl− | 7100 | 2900 |
| Br− | 4100 | 1200 |
| I− | 730 | 440 |
Having established the halide anion sensing properties of both receptors, the photosensitising abilities of receptor 1 were investigated, as it exhibits a relatively greater magnitude of fluorescence response. 1O2 generation studies in acetone were conducted with receptor 1, using diphenylisobenzofuran (DPBF), a dye known to be a trap for 1O2 and other ROS.40,41 DPBF has a characteristic absorbance at 410 nm that diminishes when it reacts with 1O2, forming non-absorbing products. The rate at which the absorbance of DPBF decreases is therefore correlated to the 1O2 quantum yield of the photosensitising compound under investigation.42
In a typical experiment, an acetone solution of receptor 1 (20 μM) and DPBF (30 μM) was irradiated at 625 nm and the absorbance at 410 nm monitored. Receptor 1 proved capable of generating 1O2, shown by the decrease in DPBF absorbance (Fig. 5). No changes in the aza-BODIPY absorbance were observed, indicating the photostability of receptor 1.
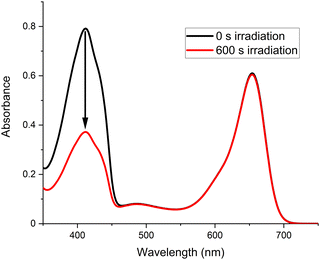 | ||
| Fig. 5 Absorbance of a solution initially containing DPBF (30 μM) and receptor 1 (20 μM), before and after 600 s irradiation at 625 nm (acetone, 298 K). | ||
Attention then turned to determining whether anion binding could modulate 1O2 generation efficiency. Cl− was used, because the halide had shown a large magnitude of fluorescence anion sensing response.|| In these experiments, an acetone solution of receptor 1 (20 μM), DPBF (30 μM) and TBACl (2 mM) was irradiated and the rate of diminishment in absorbance of the DPBF chromophore was monitored (Fig. 6). Relative to receptor 1 in the absence of any anion, the presence of Cl− decreased 1O2 quantum yield by 37%. Notably, control experiments showed the non-binding precursor azide 6A did not demonstrate anion binding-modulated 1O2 generation and the 1O2 generation of receptor 1 was not affected by the presence of the non-coordinating PF6− anion, ruling out any non-specific effects (Fig. S33–S35, ESI†).
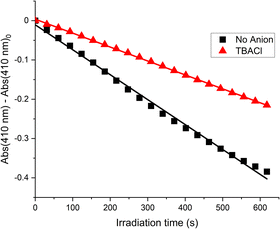 | ||
| Fig. 6 Plot of DPBF absorbance against irradiation time, in the presence and absence of TBACl (receptor 1, acetone, 298 K). | ||
To gain insight into the mechanism of host–guest anion binding-modulated 1O2 generation, measurements of the fluorescence lifetime of receptor 1 in the presence and absence of 2 mM TBACl were conducted (Table 3). The lifetime was not significantly affected by the presence of TBACl, despite the emission intensity decreasing, which indicates the rate of non-radiative decay processes from the excited S1 state is increased in the presence of TBACl (Section S5, ESI†).23 Conceivably therefore, these non-radiative decay processes may increasingly compete with ISC upon anion binding, reducing the triplet quantum yield and hence the 1O2 quantum yield, which have been shown to be highly correlated.26
| Anion | τ F |
|---|---|
| Determined in acetone at 298 K. Errors <5%. | |
| No anion | 1.13 |
| Cl− | 1.07 |
In summary, we report the synthesis of two novel XB aza-BODIPY fluorescent anion sensors 1 and 2, both of which demonstrate significant turn-off quenching responses to halide anion binding, extending the optical range of XB fluorescent sensors to the biologically-relevant NIR window. Notably receptor 1 displayed both larger magnitude fluorescence changes and stronger anion binding, which will inform the design of future aza-BODIPY based anion sensors. Receptor 1 was also shown to act as a photosensitiser to generate 1O2. Importantly, in a proof-of-concept, combining the anion sensing and photosensitising capabilities of receptor 1, we demonstrate unprecedented supramolecular host–guest anion binding-modulated 1O2 generation, opening a new avenue for improving the selectivity of PDT agents.
This work was supported by the EPSRC (studentship EP/T517811/1).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- J. Killoran, L. Allen, J. F. Gallagher, W. M. Gallagher and D. F. O′Shea, Chem. Commun., 2002, 1862–1863 RSC.
- Y. Ge and D. F. O'Shea, Chem. Soc. Rev., 2016, 45, 3846–3864 RSC.
- Z. Shi, X. Han, W. Hu, H. Bai, B. Peng, L. Ji, Q. Fan, L. Li and W. Huang, Chem. Soc. Rev., 2020, 49, 7533–7567 RSC.
- N. Adarsh, R. R. Avirah and D. Ramaiah, Org. Lett., 2010, 12, 5720–5723 CrossRef CAS PubMed.
- H. Niu, J. Liu, H. M. O’Connor, T. Gunnlaugsson, T. D. James and H. Zhang, Chem. Soc. Rev., 2023, 52, 2322–2357 RSC.
- C. Caulfield, D. Wu, M. Garre and D. F. O'Shea, RSC Adv., 2023, 13, 14963–14973 RSC.
- T. D. Ashton, K. A. Jolliffe and F. M. Pfeffer, Chem. Soc. Rev., 2015, 44, 4547–4595 RSC.
- L. Li, P. Li, J. Fang, Q. Li, H. Xiao, H. Zhou and B. Tang, Anal. Chem., 2015, 87, 6057–6063 CrossRef CAS PubMed.
- X.-D. Jiang, J. Zhao, Q. Li, C.-L. Sun, J. Guan, G.-T. Sun and L.-J. Xiao, Dyes Pigm., 2016, 125, 136–141 CrossRef CAS.
- J. Zuo, H. Pan, Y. Zhang, Y. Chen, H. Wang, X.-K. Ren and Z. Chen, Dyes Pigm., 2020, 183, 108714 CrossRef CAS.
- S. O. McDonnell and D. F. O'Shea, Org. Lett., 2006, 8, 3493–3496 CrossRef CAS PubMed.
- C. Staudinger, J. Breininger, I. Klimant and S. M. Borisov, Analyst, 2019, 144, 2393–2402 RSC.
- M. M. Salim, E. A. Owens, T. Gao, J. H. Lee, H. Hyun, H. S. Choi and M. Henary, Analyst, 2014, 139, 4862–4873 RSC.
- N. Adarsh, M. Shanmugasundaram and D. Ramaiah, Anal. Chem., 2013, 85, 10008–10012 CrossRef CAS PubMed.
- B. Zou, H. Liu, J. Mack, S. Wang, J. Tian, H. Lu, Z. Li and Z. Shen, RSC Adv., 2014, 4, 53864–53869 RSC.
- F. Wu, H. Liu, C. Zhong and L. Zhu, Tetrahedron Lett., 2016, 57, 5120–5123 CrossRef CAS.
- Y. Gao, Y. Pan, Y. He, H. Chen and V. N. Nemykin, Sens. Actuators, B, 2018, 269, 151–157 CrossRef CAS.
- J. Y. C. Lim and P. D. Beer, Chem, 2018, 4, 731–783 CAS.
- J. T. Wilmore and P. D. Beer, Adv. Mater., 2024, 2309098 CrossRef CAS PubMed.
- R. Hein and P. D. Beer, Chem. Sci., 2022, 13, 7098–7125 RSC.
- S. Mondal, A. Rashid and P. Ghosh, J. Organomet. Chem., 2021, 952, 122027 CrossRef CAS.
- R. Kampes, R. Tepper, H. Görls, P. Bellstedt, M. Jäger and U. S. Schubert, Chem. – Eur. J., 2020, 26, 14679–14687 CrossRef CAS PubMed.
- A. J. Taylor, R. Hein, S. C. Patrick, J. J. Davis and P. D. Beer, Angew. Chem., Int. Ed., 2024, 63, e202315959 CrossRef CAS PubMed.
- A. K. A. Jaini, L. B. Hughes, M. M. Kitimet, K. J. Ulep, M. C. Leopold and C. A. Parish, ACS Sens., 2019, 4, 389–397 CrossRef CAS PubMed.
- A. Ravi, A. S. Oshchepkov, K. E. German, G. A. Kirakosyan, A. V. Safonov, V. N. Khrustalev and E. A. Kataev, Chem. Commun., 2018, 54, 4826–4829 RSC.
- N. Adarsh, M. Shanmugasundaram, R. R. Avirah and D. Ramaiah, Chem. – Eur. J., 2012, 18, 12655–12662 CrossRef CAS PubMed.
- T. C. Pham, V.-N. Nguyen, Y. Choi, S. Lee and J. Yoon, Chem. Rev., 2021, 121, 13454–13619 CrossRef CAS PubMed.
- Y. Tang, L. Xue, Q. Yu, D. Chen, Z. Cheng, W. Wang, J. Shao and X. Dong, ACS Appl. Bio Mater., 2019, 2, 5888–5897 CrossRef CAS PubMed.
- Q. Zeng, R. Zhang, T. Zhang and D. Xing, Biomaterials, 2019, 207, 39–48 CrossRef CAS PubMed.
- Z. Li, Y. Liu, L. Chen, X. Hu and Z. Xie, J. Mater. Chem. B, 2017, 5, 4239–4245 RSC.
- P. Wei, F. Xue, Y. Shi, R. Strand, H. Chen and T. Yi, Chem. Commun., 2018, 54, 13115–13118 RSC.
- T. Huang, H. Ji, S. Yan, Y. Zuo, J. Li, J. W. Y. Lam, C. Han and B. Z. Tang, Biomaterials, 2023, 297, 122108 CrossRef CAS PubMed.
- A. Docker, C. H. Guthrie, H. Kuhn and P. D. Beer, Angew. Chem., Int. Ed., 2021, 60, 21973–21978 CrossRef CAS PubMed.
- A. Zarghi, T. Zebardast, F. Hakimion, F. H. Shirazi, P. N. Praveen Rao and E. E. Knaus, Bioorg. Med. Chem., 2006, 14, 7044–7050 CrossRef CAS PubMed.
- A. Gorman, J. Killoran, C. O'Shea, T. Kenna, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2004, 126, 10619–10631 CrossRef CAS PubMed.
- M. Kaasik, S. Kaabel, K. Kriis, I. Järving, R. Aav, K. Rissanen and T. Kanger, Chem. – Eur. J., 2017, 23, 7337–7344 CrossRef CAS PubMed.
- H. Min Tay, T. G. Johnson, A. Docker, M. J. Langton and P. D. Beer, Angew. Chem. Int. Ed., 2023, 62, e202312745 CrossRef CAS PubMed.
- S. C. Patrick, P. D. Beer and J. J. Davis, Nat. Rev. Chem., 2024, 8, 256–276 CrossRef PubMed.
- J. K. G. Karlsson and A. Harriman, J. Phys. Chem. A, 2016, 120, 2537–2546 CrossRef CAS PubMed.
- F. Mitzel, S. Fitzgerald, A. Beeby and R. Faust, Chem. Commun., 2001, 2596–2597 RSC.
- P. Carloni, E. Damiani, L. Greci, P. Stipa, F. Tanfani, E. Tartaglini and M. Wozniak, Res. Chem. Intermed., 1993, 19, 395–405 CrossRef CAS.
- S. O. McDonnell, M. J. Hall, L. T. Allen, A. Byrne, W. M. Gallagher and D. F. O'Shea, J. Am. Chem. Soc., 2005, 127, 16360–16361 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc02330g |
| ‡ Synthesis of hydrogen bonding analogues of receptors 1 and 2 was attempted, but the products were too insoluble to isolate. |
| § Addition of TBAOAc caused decomposition of the receptors. |
| ¶ A dynamic quenching mechanism is most likely, as the absorbance spectrum was unchanged in the presence of anions. |
| || ESI,† Section S5 for discussion of other anions. |
| This journal is © The Royal Society of Chemistry 2024 |