Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrochemical reduction of nitrate to hydroxylamine on gold electrode†
Yangshan
Xie
a,
Michiel
De Ras
 b,
Jiwu
Zhao
cd,
Tianxi
Liu
e,
Feili
Lai
b,
Jiwu
Zhao
cd,
Tianxi
Liu
e,
Feili
Lai
 e,
Johan
Hofkens
e,
Johan
Hofkens
 bf and
Maarten B. J.
Roeffaers
bf and
Maarten B. J.
Roeffaers
 *a
*a
aCentre for Membrane Separations, Adsorption, Catalysis, and Spectroscopy for Sustainable Solutions, Department of Microbial and Molecular Systems, KU Leuven Celestijnenlaan 200F, 3001 Leuven, Belgium. E-mail: maarten.roeffaers@kuleuven.be
bMolecular Imaging and Photonics, Department of Chemistry, KU Leuven, Celestijnenlaan 200F, Leuven, 3001, Belgium
cState Key Laboratory of Photocatalysis on Energy and Environment, College of Chemistry, Fuzhou University, Fuzhou, 350116, China
dDivision of Physical Sciences and Engineering, King Abdullah University of Science and Technology (KAUST), Thuwal, 23955–6900, Saudi Arabia
eThe Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, International Joint Research Laboratory for Nano Energy Composites, Jiangnan University, Wuxi, China
fMax Plank Institute for Polymer Research, Mainz, D-55128, Germany
First published on 30th August 2024
Abstract
In this study, we explore the efficacy of gold (Au) as a selective electrocatalyst for the reduction of nitrate to hydroxylamine, a valuable nitrogen-based chemical, while also evaluating the by-product formation of ammonia. We systematically optimized various experimental parameters including nitrate concentration, pH, and applied potential. We found that at an applied potential of −0.7 V vs. RHE in 0.1 M HNO3, Au achieves a 230.1 ± 19 μmol NH2OH h−1 cm−2 yield, with a 34.2 ± 2.8% faradaic efficiency. This study underscores the potential of Au as an efficient and selective electrocatalyst for generating value-added nitrogen products through an electrochemical pathway.
Nitrogen, an element essential to all life, manifests in numerous forms, from its stable diatomic gas in the atmosphere to a myriad of nitrogen-containing molecules such as ammonia, nitrate, and organic compounds. Human activities, from industrial manufacturing to agriculture, transform these nitrogen states in ways that significantly impact the global nitrogen cycle. Such transformations—either as unintended byproducts in chemical and combustion processes or through deliberate production of fertilizers and chemicals—distort natural pathways.1,2 Nitrate, the most oxidized form of nitrogen, is a well know pollutant in water bodies, which in excess threatens the biodiversity of ecosystems and has proven to have negative health effects.3,4 Additionally, excesses are eventually removed by natural denitrification processes, performed by microbial organisms. Due to poor selectivity, this also leads to the production of significant amounts of nitrous oxide (N2O), a potent greenhouse gas.5 Converting waste nitrates into valuable chemicals represents a key aspect of sustainable development and poses a significant challenge that engages engineering and research communities worldwide.
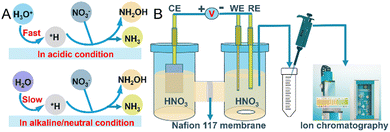
The electrochemical reduction of nitrate (NO3RR) is gaining prominence as a method that is not only energy-efficient in producing valuable products but also environmentally beneficial for treating water pollution.4 This process can yield a variety of nitrogen products with different oxidation states; however, research has predominantly focused on producing ammonia,6 often overlooking the potential for selective reduction to hydroxylamine (NH2OH). Hydroxylamine deserves further investigation due to its critical role as a precursor in the synthesis of ε-caprolactam for Nylon-6 production, which consumes more than 95% of the world's estimated production of 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year.7,8 Additionally, the potential of NH2OH as a renewable energy carrier amplifies its significance.9,10 Traditional methods for producing NH2OH, such as the Raschig process and catalytic hydrogenation of nitric oxide, face challenges including the use of toxic reagents such as SO2 or NO, harsh conditions, complex processes, low conversion rates and environmental hazards.11 Selective electrochemical NO3RR to NH2OH conversion presents a greener, more sustainable, cost-effective, and operationally simpler alternative. Concentrating on electrochemical routes, especially for the selective production of hydroxylamine, could drastically lower the carbon footprint as well as environmental impact of the synthesis of this key industrial chemical. This underscores the importance of targeted research into optimizing NO3RR selectivity and efficiency. Recent studies have identified mercury as an effective electrode material for the electrochemical NO3RR to NH2OH.12 However, its utility is considerably restricted due to toxicity concerns and the challenges of handling it in its liquid form. Compared to standard NO3RR reactions performed in neutral conditions, which use nitrate salts and lead to the generation of stoichiometric alkali metal hydroxide byproducts, exploring the reaction in acidic nitrate conditions becomes interesting.13–15 The increased acidity, compared to alkaline and neutral conditions, provides ample protons for sustained NO3− hydrogenation reactions, resulting in an enhanced conversion rate of NO3− and more energy-efficient NH2OH and NH3 generation (Fig. 1A).15 The hydrogen evolution reaction (HER) inevitably competes with NO3RR in acidic media. Such conditions necessitate careful electrode material selection to mitigate corrosion. Among noble metal-based electrodes, palladium generally favors NH3 production,16 while platinum is known for its efficacy in the hydrogen evolution reaction. Given these considerations, gold emerges as a particularly suitable material, noted for its durability and exceptional resistance to corrosion, even in strongly acidic conditions. This prompted our investigation into the capabilities of Au as an electrode for NO3RR, particularly focusing on its selectivity for NH2OH production. We rigorously tested the effects of applied potential, NO3− concentration, and the pH of the electrolyte on the selectivity and electrochemical activity of Au towards NH2OH. Our experiments revealed a significant peak faradaic efficiency (FE) of 34.2 ± 2.8% for NH2OH at an applied potential of −0.7 V versus RHE in a 0.1 M HNO3 solution. Notably, the NO3− concentration was found to have a profound impact on both the yield and selectivity towards NH2OH, with yields diminishing from 230.1 ± 19 μmol h−1 cm−2 in 0.1 M NO3− to zero at 1 M KNO3. Furthermore, the optimum yield of NH2OH, 333 μmol h−1 cm−2, was achieved at a pH of 0.3 in H2SO4 with a 0.1 M NO3− concentration. These findings underline the potential of Au electrodes to serve as both an efficient and selective catalyst for the electrochemical reduction of NO3− to NH2OH, offering an environmentally friendly alternative to conventional production methods and mitigating associated environmental impacts.
000 tons per year.7,8 Additionally, the potential of NH2OH as a renewable energy carrier amplifies its significance.9,10 Traditional methods for producing NH2OH, such as the Raschig process and catalytic hydrogenation of nitric oxide, face challenges including the use of toxic reagents such as SO2 or NO, harsh conditions, complex processes, low conversion rates and environmental hazards.11 Selective electrochemical NO3RR to NH2OH conversion presents a greener, more sustainable, cost-effective, and operationally simpler alternative. Concentrating on electrochemical routes, especially for the selective production of hydroxylamine, could drastically lower the carbon footprint as well as environmental impact of the synthesis of this key industrial chemical. This underscores the importance of targeted research into optimizing NO3RR selectivity and efficiency. Recent studies have identified mercury as an effective electrode material for the electrochemical NO3RR to NH2OH.12 However, its utility is considerably restricted due to toxicity concerns and the challenges of handling it in its liquid form. Compared to standard NO3RR reactions performed in neutral conditions, which use nitrate salts and lead to the generation of stoichiometric alkali metal hydroxide byproducts, exploring the reaction in acidic nitrate conditions becomes interesting.13–15 The increased acidity, compared to alkaline and neutral conditions, provides ample protons for sustained NO3− hydrogenation reactions, resulting in an enhanced conversion rate of NO3− and more energy-efficient NH2OH and NH3 generation (Fig. 1A).15 The hydrogen evolution reaction (HER) inevitably competes with NO3RR in acidic media. Such conditions necessitate careful electrode material selection to mitigate corrosion. Among noble metal-based electrodes, palladium generally favors NH3 production,16 while platinum is known for its efficacy in the hydrogen evolution reaction. Given these considerations, gold emerges as a particularly suitable material, noted for its durability and exceptional resistance to corrosion, even in strongly acidic conditions. This prompted our investigation into the capabilities of Au as an electrode for NO3RR, particularly focusing on its selectivity for NH2OH production. We rigorously tested the effects of applied potential, NO3− concentration, and the pH of the electrolyte on the selectivity and electrochemical activity of Au towards NH2OH. Our experiments revealed a significant peak faradaic efficiency (FE) of 34.2 ± 2.8% for NH2OH at an applied potential of −0.7 V versus RHE in a 0.1 M HNO3 solution. Notably, the NO3− concentration was found to have a profound impact on both the yield and selectivity towards NH2OH, with yields diminishing from 230.1 ± 19 μmol h−1 cm−2 in 0.1 M NO3− to zero at 1 M KNO3. Furthermore, the optimum yield of NH2OH, 333 μmol h−1 cm−2, was achieved at a pH of 0.3 in H2SO4 with a 0.1 M NO3− concentration. These findings underline the potential of Au electrodes to serve as both an efficient and selective catalyst for the electrochemical reduction of NO3− to NH2OH, offering an environmentally friendly alternative to conventional production methods and mitigating associated environmental impacts.
The nitrate reduction is a complicated multi-electron, multi-proton transfer process. The electroreduction of NO3− to NH2OH involves the transfer of eight protons and six electrons. Conversely, the production of NH3 from NO3− requires ten protons and eight electrons for electroreduction. Consequently, the HER inevitably emerges as a competing reaction for NO3RR in acidic media. The reactions in this work are summarized in eqn (1)–(3), where E0 is relative to the standard hydrogen electrode (SHE):
| NO3− + 8H+ + 6e− → NH3OH+ + 2H2O E0 = 0.73 V vs. SHE | (1) |
| NO3− + 10H+ + 8e− → NH4+ + 3H2O E0 = 0.88 V vs. SHE | (2) |
| 2H+ + 2e− → H2 E0 = 0 V vs. SHE | (3) |
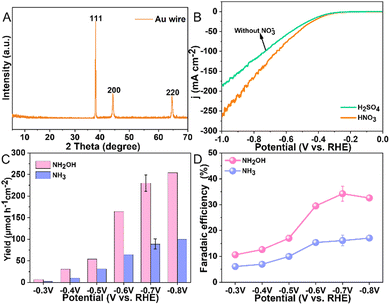
The morphology of the Au wire (diam. 0.5 mm, 99.999% pure) electrode was analyzed using scanning electron microscopy, revealing an irregular surface texture as illustrated in Fig. S1, (ESI†). Compositional analysis through energy-dispersive X-ray spectroscopy confirmed that this electrode solely exists of Au, with the detailed spectrum shown in Fig. S2, (ESI†). To ascertain the crystallinity of the electrode, X-ray diffraction was employed, with the results displayed in Fig. 2A. The recorded XRD pattern clearly exhibits the characteristic peaks of pure gold at 38.2°, 44.4°, and 65.58°. These peaks correspond to the (111), (200), and (220) crystallographic planes of Au, respectively. These results are in agreement with standard data (JCPDS 04-0784), confirming the purity of the Au wire.
We commenced our study by evaluating the electrocatalytic behavior of the Au electrode in 0.1 M HNO3 and H2SO4 solutions, respectively, employing linear sweep voltammetry (LSV) for this characterization. This approach allowed us to assess the electrode's activity across a range of potentials from 0 V to −1 V vs. RHE (Fig. 2B). A notable increase in current density was observed beyond −0.3 V vs. RHE in 0.1 M HNO3, peaking at −266 mA cm−2 at −1 V vs. RHE. The elevated current density in HNO3, compared to that in H2SO4 (without NO3−), underscores the Au electrode's specific activity towards NO3− reduction. Subsequent, our focus shifted to identifying the reaction products produced in an H-shaped three-electrode quartz cell, which was partitioned by a Nafion 117 membrane and contained 0.1 M HNO3 as the electrolyte (Fig. 1B). The products of the NO3RR were monitored using ion chromatography (IC), which revealed only the presence of NH3 and NH2OH (Fig. S3, ESI†). Subsequent experiments aimed to determine the impact of various applied potentials on the production rates and selectivity of these identified products. By experimenting with potentials ranging from −0.3 V to −0.8 V vs. RHE (Fig. S4, ESI†), we observed that the yield of NH2OH increased with more negative potentials, increasing from 6.7 μmol h−1 cm−2 at −0.3 V vs. RHE to 254.3 μmol h−1 cm−2 at −0.8 V vs. RHE (Fig. 2C). Additionally, the FE for NH2OH production demonstrated a progressive increase from 10.6% at −0.3 V vs. RHE, peaking at 34.2 ± 2.8% at −0.7 V vs. RHE, and then slightly declining to 32.6% at −0.8 V vs. RHE (Fig. 2D), possibly due to the enhanced competition from the HER at these more negative potentials. The yield and FE of H2 were determined to be 482.7 ± 40.3 μmol h−1 cm−2 and 27.1 ± 0.9% at −0.7 V vs. RHE, respectively (Fig. S5, ESI†). The NH3 yield measured from UV-vis method (81 ± 10.1 μmol h−1 cm−2) was close to IC method (89 ± 11.9 μmol h−1 cm−2) at −0.7 V vs. RHE, indicative of the reliability of the determined experimental data (Fig. S6, ESI†). Notably, throughout these experiments, the yields and FE for NH3 were consistently lower than those for NH2OH, highlighting a pronounced selectivity towards NH2OH production.
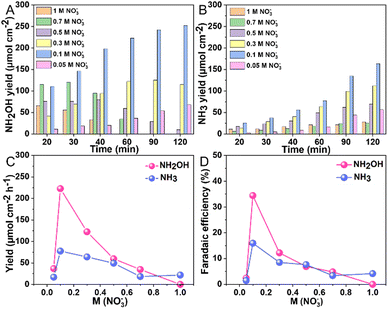
To examine the effect of nitrate concentration on product selectivity while maintaining a constant pH of 1, experiments were conducted at an applied potential of −0.7 V vs. RHE using 0.1 M HNO3 as the electrolyte and varying concentrations of KNO3; for the most dilute nitrate solution, a 0.05 M KNO3 solution was used with its pH adjusted to 1 using H2SO4 (Fig. S7, ESI†). Fig. 3 illustrated the trends in product yield and FE with nitrate concentration at pH 1, demonstrating that the highest yield and FE of NH2OH were achieved at a nitrate concentration of 0.1 M. At a reduced nitrate concentration of 0.05 M, the diminished adsorption of nitrate anions on the Au surface, likely slowed the NO3RR reaction. Also at increased nitrate concentrations, a marked decline in NH2OH yield and FE were observed. Note that, the detected hydroxylamine concentration consistently decreases over time, likely due to its oxidation by HNO2 in acidic medium (NH2OH + HNO2 → N2O + 2H2O). This was confirmed by a control experiment where KNO2 was added to a 0.1 M HNO3 solution containing NH2OH. As shown in Fig. S8, (ESI†), there was a sharp drop in NH2OH concentration following the addition. The reduced yields for both NH2OH and NH3 at higher nitrate concentrations are likely due to additional nitrate adsorbates obstructing the Au surface. It is assumed that adsorbed hydrogen or water is necessary for nitrate to undergo NH3 conversion. Further analysis involved measuring the pH of the electrolyte solution post-reaction (Table S1, ESI†). Clearly, higher starting nitrate concentrations resulted in increased pH levels. Specifically, the pH increments post-reaction were ordered as follows: 0.1 M (pH = 1.30) < 0.3 M (pH = 1.73) < 0.5 M (pH = 1.96) < 0.7 M (pH = 2.20) < 1 M (pH = 2.74). The increased pH was attributed to the consumption of eight protons to produce NH3OH+ and ten protons to produce NH4+, respectively.
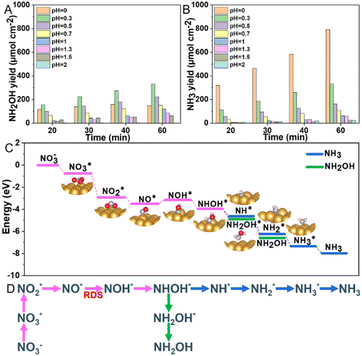
To investigate the effect of pH on the selectivity and yield of electrocatalytic NO3RR over the Au electrode, we modulated the electrolyte's pH, adjusted H2SO4 while keeping 0.1 M KNO3 constant, and applied a potential of −0.7 V vs. RHE for 1 hour (Fig. S9, ESI†). Fig. 4A and B and Fig. S10 (ESI†) illustrated that the selectivity and yield of NH2OH and NH3 significantly depended on the acidity of the solution, particularly at pH values below 0.5. The yield of NH2OH notably increased from 63.5 μmol h−1 cm−2 at pH 2 to 333.1 μmol h−1 cm−2 at pH 0.3, but then decreased to 146.6 μmol h−1 cm−2 at pH 0. Meanwhile, the yield of NH3 consistently rose at lower electrolyte pH levels, with a significant uptick observed within the pH range of 0 to 0.5, culminating in a yield of 792.5 μmol h−1 cm−2 at pH 0. These data highlight the substantial impact of acidity on the performance and selectivity of the Au electrode in the reduction of nitrate. At more acidic conditions, there is an increased proton availability facilitating the reaction outlined in eqn (1) and (2) for NH2OH and NH3 production. However, this also favors the competing HER.
To evaluate the stability of the Au wire, five consecutive cycles were performed in 0.1 M HNO3 at −0.7 V vs. RHE, with each cycle lasting 1 hour. The consistent NH2OH yield rate and FE observed indicate the favorable stability of the Au electrode under these conditions (Fig. S11, ESI†). SEM analysis of the Au wire after reaction showed preserved surface microstructure, with no apparent damage observed (Fig. S12, ESI†). Furthermore, also the XRD patterns remained unchanged (Fig. S13, ESI†). The current response results from LSV remained similar except for a slight increase (Fig. S14, ESI†), which may be attributed to the improved activation of the catalyst. Elemental analysis via inductively coupled plasma mass spectrometry (ICP-MS) analysis evidenced that there was no detectable Au3+ leaching in the electrolyte after reaction (Table S2, ESI†). These results demonstrated that Au wire serves as a robust catalyst under acidic conditions for the NO3RR conversion to NH2OH and NH3.
To elucidate the mechanism of nitrate reduction on Au wire, density functional theory (DFT) calculations on Au (111) facet were performed. Fig. 4C details these simulations, which begins with the spontaneous adsorption of nitrate onto the Au electrode surface, marked by a favorable Gibbs free energy change of −0.75 eV. The reduction sequence involves adsorbed nitrate 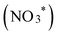 being reduced to adsorbed nitrite
being reduced to adsorbed nitrite 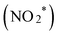 which is then converted to NO*. The subsequent step involves the reduction of this adsorbed nitric oxide to hydroxylamine oxime (NOH*), identified as the rate-determining step (RDS) in this pathway, requiring an energy of 0.35 eV. NOH* is then further reduced to NHOH*, NH2OH* and finally NH2OH desorbes. Conversely, the formation of NH3 from NHOH* involves multiple steps beginning with NHOH* first converting to NH*, followed by
which is then converted to NO*. The subsequent step involves the reduction of this adsorbed nitric oxide to hydroxylamine oxime (NOH*), identified as the rate-determining step (RDS) in this pathway, requiring an energy of 0.35 eV. NOH* is then further reduced to NHOH*, NH2OH* and finally NH2OH desorbes. Conversely, the formation of NH3 from NHOH* involves multiple steps beginning with NHOH* first converting to NH*, followed by 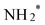 , then to
, then to 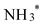 before
before 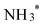 desorbs to produce NH3 (Fig. 4D and Fig. S15, ESI†). The lower Gibbs free energies of the formation and the desorption of NH2OH* indicate it occurs spontaneously, inherently favoring the formation of NH2OH (Table S3, ESI†). The relatively simplicity of the NH2OH pathway, compared to the multi-step process required for NH3 formation, predominantly drives the selective formation of NH2OH. The energy barrier of the RDS from Au (0.35 eV) is lower than that of other reported catalysts, such as Ni (0.37 eV),17 Rh (0.39 eV),17 Pd (0.72 eV),17 PdFe (0.74 eV),18 Cu/Cu2O (0.84 eV)19 and Fe3C–Cu3 (1.28 eV)20 (Table S4, ESI†).
desorbs to produce NH3 (Fig. 4D and Fig. S15, ESI†). The lower Gibbs free energies of the formation and the desorption of NH2OH* indicate it occurs spontaneously, inherently favoring the formation of NH2OH (Table S3, ESI†). The relatively simplicity of the NH2OH pathway, compared to the multi-step process required for NH3 formation, predominantly drives the selective formation of NH2OH. The energy barrier of the RDS from Au (0.35 eV) is lower than that of other reported catalysts, such as Ni (0.37 eV),17 Rh (0.39 eV),17 Pd (0.72 eV),17 PdFe (0.74 eV),18 Cu/Cu2O (0.84 eV)19 and Fe3C–Cu3 (1.28 eV)20 (Table S4, ESI†).
In summary, this study establishes Au as a highly effective electrocatalyst for the selective NO3− reduction to NH2OH. Through meticulous experimentation involving electrolyte composition, nitrate concentration, pH and applied potential, we achieved an NH2OH yield of 230.1 ± 19 μmol h−1 cm−2 under optimal conditions. This performance is notably exceeding the yields commonly reported in the literature and achieves a faradaic efficiency of 34.2 ± 2.8% at −0.7 V vs. RHE, setting a new benchmark in electrochemical nitrate reduction. This study provides an understanding of the electrolyte engineering of nitrate solutions and may motivate further studies into gold or other materials to selectively generate NH2OH and other products from waste nitrates.
This work is supported by Internal Funds KU Leuven (C14/23/090), the National Natural Science Foundation of China (No. 52303151, No. 52161135302, No. 52211530489), the Research Foundation of Flanders (FWO, No. G0F2322N, No. VS06523N, No. 1298323N, No. 1SA3321N). J. H acknowledges support of the MPI as a fellow.
Data availability
The data supporting this article have been included as part of ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- G. Qing, R. Ghazfar, S. T. Jackowski, F. Habibzadeh, M. M. Ashtiani, C. P. Chen, M. R. Smith, 3rd and T. W. Hamann, Chem. Rev., 2020, 120, 5437–5516 CrossRef CAS PubMed.
- V. Rosca, M. Duca, M. T. de Groot and M. T. M. Koper, Chem. Rev., 2009, 109, 2209–2244 CrossRef CAS PubMed.
- S. Garcia-Segura, M. Lanzarini-Lopes, K. Hristovski and P. Westerhoff, Appl. Catal., B, 2018, 236, 546–568 CrossRef CAS.
- H. Xu, Y. Ma, J. Chen, W.-X. Zhang and J. Yang, Chem. Soc. Rev., 2022, 51, 2710–2758 RSC.
- Y. Zeng, C. Priest, G. Wang and G. Wu, Small Methods, 2020, 4, 2000672 CrossRef CAS.
- A. Adalder, S. Paul, N. Barman, A. Bera, S. Sarkar, N. Mukherjee, R. Thapa and U. K. Ghorai, ACS Catal., 2023, 13, 13516–13527 CrossRef CAS.
- W. Lewdorowicz, W. Tokarz, P. Piela and P. K. Wrona, J. New Mater. Electrochem. Syst., 2006, 9, 339–343 CAS.
- J. Ritz, H. Fuchs and H. G. Perryman, Ulmann’s Encyclopedia of Industrial Chemistry, 2000 DOI:10.1002/14356007.a13_527.
- S. Jia, L. Wu, X. Tan, J. Feng, X. Ma, L. Zhang, X. Song, L. Xu, Q. Zhu, X. Kang, X. Sun and B. Han, J. Am. Chem. Soc., 2024, 146, 10934–10942 CrossRef CAS PubMed.
- D. H. Kim, S. Ringe, H. Kim, S. Kim, B. Kim, G. Bae, H.-S. Oh, F. Jaouen, W. Kim, H. Kim and C. H. Choi, Nat. Commun., 2021, 12, 1856 CrossRef CAS PubMed.
- X. Kong, J. Ni, Z. Song, Z. Yang, J. Zheng, Z. Xu, L. Qin, H. Li, Z. Geng and J. Zeng, Nat. Sustainability, 2024, 7, 652–660 CrossRef.
- I. Taniguchi, N. Nakashima, K. Matsushita and K. Yasukouchi, J. Electroanal. Chem. Interfac. Electrochem., 1987, 224, 199–209 CrossRef CAS.
- J. Shen, Y. Y. Birdja and M. T. M. Koper, Langmuir, 2015, 31, 8495–8501 CrossRef CAS PubMed.
- Y. Lv, S.-W. Ke, Y. Gu, B. Tian, L. Tang, P. Ran, Y. Zhao, J. Ma, J.-L. Zuo and M. Ding, Angew. Chem., Int. Ed., 2023, 62, e202305246 CrossRef CAS PubMed.
- R. Zhang, C. Li, H. Cui, Y. Wang, S. Zhang, P. Li, Y. Hou, Y. Guo, G. Liang, Z. Huang, C. Peng and C. Zhi, Nat. Commun., 2023, 14, 8036 CrossRef CAS PubMed.
- J. Lim, C.-Y. Liu, J. Park, Y.-H. Liu, T. P. Senftle, S. W. Lee and M. C. Hatzell, ACS Catal., 2021, 11, 7568–7577 CrossRef CAS.
- M. Karamad, T. J. Goncalves, S. Jimenez-Villegas, I. D. Gates and S. Siahrostami, Faraday Discuss., 2023, 243, 502–519 RSC.
- Y. Zhou, L. Zhang, Z. Zhu, M. Wang, N. Li, T. Qian, C. Yan and J. Lu, Angew. Chem., Int. Ed., 2024, 63, e202319029 CrossRef CAS PubMed.
- N. Zhou, Z. Wang, N. Zhang, D. Bao, H. Zhong and X. Zhang, ACS Catal., 2023, 13, 7529–7537 CrossRef CAS.
- Y. Hua, N. Song, Z. Wu, Y. Lan, H. Luo, Q. Song and J. Yang, Adv. Funct. Mater., 2024, 34, 2314461 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and experimental details. See DOI: https://doi.org/10.1039/d4cc03620d |
| This journal is © The Royal Society of Chemistry 2024 |