 Open Access Article
Open Access ArticleUnsaturated chiral-only-at-metal rhodium(III) complexes bearing SiN-type ligands†
Unai
Prieto-Pascual
a,
Itxaso
Bustos
 a,
Pablo
Salcedo-Abraira
a,
Pablo
Salcedo-Abraira
 b,
Iñigo J.
Vitorica-Yrezabal
b,
Iñigo J.
Vitorica-Yrezabal
 b,
Aitor
Landa
b,
Aitor
Landa
 a,
Zoraida
Freixa
a,
Zoraida
Freixa
 *ac and
Miguel A.
Huertos
*ac and
Miguel A.
Huertos
 *ac
*ac
aFacultad de Química de San Sebastián, Universidad del País Vasco (UPV/EHU), Apartado 1072, 20080, San Sebastián, Spain. E-mail: zoraida_freixa@ehu.eus; miguelangel.huertos@ehu.eus
bDepartamento de Química Inorgánica, Universidad de Granada, Avenida Fuentenueva s/n, 18071 Granada, Spain
cIKERBASQUE, Basque Fundation for Science, 48011, Bilbao, Spain
First published on 16th September 2024
Abstract
Enantiopure chiral-at-metal rhodium(III) unsaturated 16e complexes have been obtained from racemic [Rh(SiN)2Cl] (SiN= 8-(dimethylsilyl)quinoline) using a readily accessible chiral spiroborate as chiral resolution agent. This strategy allows an easy access to enantiopure neutral Δ/Λ-Rh(SiN)2Cl and cationic Δ/Λ-Rh(SiN)2[BAr4F] unsaturated complexes, wherein rhodium(III) is coordinated to two inert silylquinoline ligands in a propeller-like arrangement.
In the field of asymmetric catalysis, transition metal catalysts are commonly used due to their remarkable efficiency.1 The predominant methodology in transition metal asymmetric catalysis involves the use of chiral ligands. However, there is a growing interest in a less studied method involving chiral-at-metal complexes formed by non-chiral ligands, mainly due to the seminal work of E. Meggers.2 This approach consists of a metal centre coordinated by two bidentate ligands in a propeller-type fashion. High configurational stability at the stereogenic metal centre is the main requirement for chiral metal catalysts. In addition, for the substrate to interact with the metal centre of the catalyst, the presence of labile auxiliary ligands, such as acetonitrile, is required.
Two advantages of using chiral-at-metal complexes as asymmetric catalysts should be noted. First, the non-chiral ligands are easier to prepare than their chiral counterparts, thus offering a wider variety. Secondly, in chiral-at-metal catalysts, the metal centre, which is the reaction centre for catalysis, is also the stereogenic centre responsible for the overall enantioselectivity.
Most of the chiral-at-metal complexes used as asymmetric catalysts reported to date are octahedral complexes with d6 transition metals.3–6 Cationic complexes of iridium(III)3 and rhodium(III)4 with two bidentate anionic ligands (CN ligands; Fig. 1a left) and two labile acetonitrile ligands have been widely used chiral-at-metal catalysts. More recently, ruthenium(II)5 and iron(II)6 di-cationic complexes bearing bidentate neutral ligands (CN ligands; Fig. 1a right) and also two labile acetonitrile ligands have also been studied.
Here, we describe a spiroborate-mediated methodology for the resolution of chiral-at-metal neutral rhodium(III) complexes bearing two silicon-based achiral ligands. Further functionalization of the isolated enantiomers rendered the cationic derivatives with retention of the enantiopurity. This work demonstrates the possibility of accessing unsaturated chiral-at-metal rhodium(III) complexes containing silicon-based ligands.
For this study, it was selected the 16e unsaturated neutral complex rac-Rh(SiN)2Cl (HSiN= 8-(dimethylsilyl)quinoline) which was recently reported by our research group.7 It can be readily obtained by reacting [Rh(coe)2Cl]2 (coe = cyclooctene) with 4 equivalents of the HSiN ligand (Scheme 1).
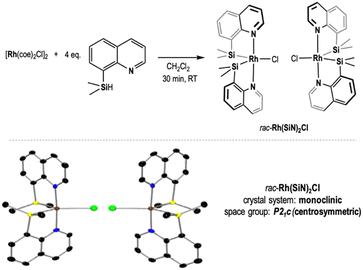 | ||
| Scheme 1 Synthesis and molecular structure of rac-Rh(SiN)2Cl complex.7 | ||
The resolution of rac-Rh(SiN)2Cl was attempted by reacting it with one equivalent of the enantiopure sodium bis-(mandelate)borate, Na[S-B(R-Man)2] in CH2Cl2/MeOH 3/1 for 30 minutes (Scheme 2). After removal of the solvent and NaCl, the reaction residue was analysed by 1H NMR. Encouragingly, two sets of signals of equal relative intensities, attributable to two new C2-symmetric compounds, were observed in the 1H NMR spectra (Fig. S3, ESI†). Initially, this spectroscopic pattern was temptatively ascribed to the diastereoisomeric mixture of the [Λ-Rh(SiN)2][S-B(R-Man)2] and [Δ-Rh(SiN)2][S-B(R-Man)2] salts, and the different NMR spectra attributed to these salts forming close-contact ions in solution. Surprisingly, the two singlets assigned to the benzylic hydrogens of the bis-(mandelate)borate anion were observed at rather different chemical shifts: δ = 4.47 and δ = 5.20, respectively. In other octahedral complexes reported in the literature containing bis-(mandelate)borate as counterion this proton resonates at 5.22 ppm, which is just slightly shifted compared to the corresponding sodium salt (5.13 ppm).8 The strongly upfield shifted signal observed at 4.47 ppm seems to point to a rather different chemical environment of the chiral anion in one of the formed diastereomers. Crystallization of the mixture results in the separation of the diastereomer characterized by the benzylic proton at 4.47 ppm in the 1H NMR spectra, as pale yellow crystals (34% yield). Accordingly, the 1H NMR of the mother liquor displayed the same two sets of signals but now in an approximate 15/85 ratio, being the main species present the one characterized by a singlet at 5.20 ppm. Crystals suitable for X-ray diffraction allowed the identification of the precipitated compound as a neutral rhodium(III) complex with the chiral bis-(mandelate)borate anion coordinated to the metal center through two oxygen atoms. The two quinoline nitrogen atoms complete the pseudo-octahedral coordination sphere in a Λ-configured propeller arrangement. Therefore, this compound should be better referred as Λ-Rh(SiN)2(S-B(R-Man)2) (Fig. 2A). The coordination of the mandelate ion to a transition metal was unexpected, as it is unprecedented, and it is consistent with the high-field shift observed for the benzylic proton of the mandelate in the 1H NMR spectra of this species.
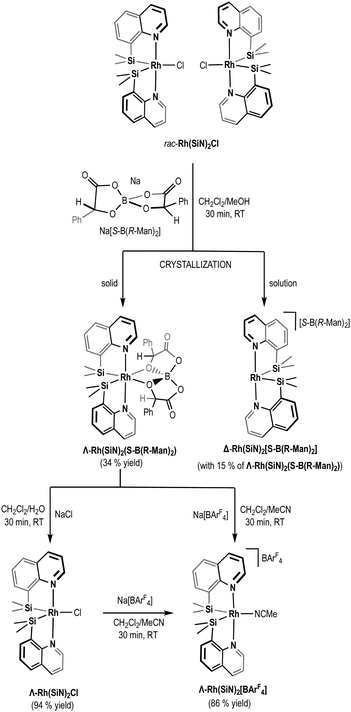 | ||
| Scheme 2 Spiroborate-mediated resolution of enantiomerically pure chiral-at-metal 16e rhodium(III) complexes. | ||
Based on the chemical shift of the benzylic hydrogen, the main species in solution was assigned to the salt [Δ-Rh(SiN)2][S-B(R-Man)2]. This structural hypothesis is supported by a qualitative analysis of the shape of the C2-symmetric chiral pocket in the cation [Λ-Rh(SiN)2]+ and that of the C2-symmetric [S-B(R-Man)2]− anion (see Fig. 2B). A face-to-face superposition of the calculated topographic maps,9 used to estimate attractive and repulsive interaction upon coordination, reveals that they are perfectly complementary surfaces (matching combination). In contraposition, and based on the same analysis, important steric repulsion is expected to impede the coordination of [S-B(R-Man)2]− to a Δ-configured ion [Δ-Rh(SiN)2]+. Therefore, we assume that the neutral compound Δ-Rh(SiN)2(S-B(R-Man)2) is not viable. Obviously, Δ-Rh(SiN)2(R-B(S-Man)2) was the solid isolated by crystallization when the same resolution procedure was followed using the enantiomeric Na[R-B(S-Man)2] salt.
Alternatively, the mother liquor (a diastereomeric mixture enriched in the presumed [Δ-Rh(SiN)2][S-B(R-Man)2] salt) was converted back to Rh(SiN)2Cl by dissolving it in dichloromethane and repeated washes with brine. This Δ-enriched mixture was reacted with Na[R-B(S-Man)2] to obtain, after crystallization, the enantiopure Δ-Rh(SiN)2(R-B(S-Man)2) in 27% yield (Scheme S2 in ESI†). Crystals suitable for X-ray diffraction were obtained by slow diffusion of pentane on a CH2Cl2 solution of Δ-Rh(SiN)2(R-B(S-Man)2), which confirms the structure and chiral assignment of the compound (Fig. 2A). Noticeably, this methodology permits the isolation of both enantiomeric compounds from the same starting batch of rac-Rh(SiN)2Cl, increasing the maximum theoretical yield of the resolution process to 100% (experimental yield 61%).
The isolated Λ-Rh(SiN)2(S-B(R-Man)2) and Δ-Rh(SiN)2(R-B(S-Man)2) enantiomers, containing the coordinated chiral spiroborate, were converted back to the enantiopure neutral complexes Λ-Rh(SiN)2Cl and Δ-Rh(SiN)2Cl, respectively, by simply dissolving them in dichlorometane and washing them repeatedly with brine (94% and 95% yield, respectively). Alternatively, if Λ-Rh(SiN)2(S-B(R-Man)2) or Δ-Rh(SiN)2(R-B(S-Man)2) were reacted with Na[BAr4F] in the presence of acetonitrile, the corresponding cationic complexes containing the non-coordinating anion [BAr4F]− (Λ-Rh(SiN)2[BAr4F] and Δ-Rh(SiN)2[BAr4F]) were formed (yield = 86% and 78%, respectively). The formation of these compounds was confirmed by NMR and MS spectroscopies (see ESI†), and their enantiopurity was evaluated using electronic circular dichroism (ECD) (Fig. 2C).
It should be noted that Λ/Δ-Rh(SiN)2Cl and Λ/Δ-Rh(SiN)2[BAr4F] are 16-electron rhodium(III) compounds, and represent the first examples of unsaturated chiral-at-rhodium complexes.
The catalytic properties of the new chiral-at-rhodium complexes were evaluated in a model reaction. Initially, compounds rac-Rh(SiN)2Cl and rac-Rh(SiN)2[BAr4F] were tested as catalysts in the Diels–Alder reaction between pyridine-2-carbaldehyde (1) and Danishefsky's diene (2) (Table 1). The initial screening, aiming to check the catalytic activity, was performed using 1 mol% of catalyst loading in CH2Cl2 at room temperature after 16 hours of reaction. The results obtained showed that the neutral rac-Rh(SiN)2Cl is not catalytically active (Table 1, entry 1). In contrast, when rac-Rh(SiN)2[BAr4F] was used as a catalyst the reaction resulted in the formation of the desired product (3) with nearly full conversion (77% isolated yield) (Table 1, entry 2). Based on these results, enantiopure Λ-Rh(SiN)2[BAr4F] and Δ-Rh(SiN)2[BAr4F] were tested under identical conditions to evaluate their enantioselectivity. Using these catalysts, 3 was obtained with a moderate enantiomeric excess (Table 1, entries 3 and 4). As expected, the yield is similar to that obtained with the racemic catalyst, and enantiomeric catalysts produced opposite major products. To demonstrate their sterochemical stability, complex Δ-Rh(SiN)2[BAr4F] was synthesized and stored under air conditions for one month before being used as a catalyst, producing results identical to those of freshly prepared samples (Table 1, entry 5).
| Entry | Catalyst [Rh] | Conv.b (%) | Yieldc (%) | e.e.d (%) |
|---|---|---|---|---|
| a Reaction conditions: aldehyde (0.2 mmol), diene (0.4 mmol), with 1 mol% of [Rh] in 2 mL of CH2Cl2 at room temperature. b Conversions determined by 1H NMR. c Isolated Yield. d e.e. determined by HPLC using chiral AD-H column.10 e Catalyst was prepared one month prior to use and stored under air. | ||||
| 1 | rac-Rh(SiN)2Cl | — | — | — |
| 2 | rac-Rh(SiN)2[BArF4] | >99 | 77 | 0 |
| 3 | Λ-Rh(SiN)2[BArF4] | >99 | 88 | 70 (S) |
| 4 | Δ-Rh(SiN)2[BArF4] | >99 | 80 | 78 (R) |
| 5e | Δ-Rh(SiN)2[BArF4] | >99 | 85 | 76 (R) |
In conclusion, the first unsaturated chiral-at-metal rhodium(III) complexes and the first chiral-at-metal rhodium(III) complexes with bidentate silyl-type ligands have been reported. These compounds have been isolated through a chiral spiroborate-mediated resolution from racemic 16-electron unsaturated precusors. The cationic complexes (Δ/Λ-Rh(SiN)2[BAr4F]) were found to be effective catalysts for hetero-Diels–Alder reactions. Although the enantiomeric ratios are not noticeable, these compounds represent the first examples of a new family of asymmetric catalysts.
We thank the financial support of the UPV/EHU (EHU-G23/03), Basque Government (IT1741-22 and IT1583-22) and MCIN/AEI/10.13039/501100011033 and FEDER A way of making Europe through projects PID2019-111281GB-I00, PID2022-139760NB-I00 and PID2022-137153NB-C21.
Data availability
The data supporting this article has been included in ESI.† Crystallographic data has been deposited at the CCDC under 2341546 and 2341547.†Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) T. Akiyama and I. Ojima, Catalytic Asymmetric Synthesis, 4th edn, John Wiley & Sons, 2022 CrossRef; (b) E. N. Platz and H. Yamamote, Comprehensive Asymmetric Catalysis, 1st edn, Springer-Verlag, 1999 Search PubMed; (c) E. M. Carreira and H. Yamamoto, Comprehensive Chirality, 1st edn, Elsevier Science, 2012 Search PubMed.
- (a) E. B. Bauer, Chem. Soc. Rev., 2012, 41, 3153–3167 RSC; (b) Z.-Y. Cao, W. D. G. Brittain, J. S. Fossey and F. Zhou, Catal. Sci. Technol., 2015, 5, 3441–3451 RSC; (c) L. Zhang and E. Meggers, Chem. – Asian J., 2017, 12, 2335–2342 CrossRef CAS; (d) P. Dey, P. Rai and B. Maji, ACS Org. Inorg. Au, 2022, 2, 99–125 CrossRef CAS PubMed.
- For chiral-at-iridium catalysts examples: (a) H. Huo, C. Fu, K. Harms and E. Meggers, J. Am. Chem. Soc., 2014, 136, 2990–2993 CrossRef CAS PubMed; (b) X. Shen, H. Huo, C. Wang, B. Zhang, K. Harms and E. Meggers, Chem. – Eur. J., 2015, 21, 9720–9726 CrossRef CAS; (c) H. Huo, C. Wang, K. Harms and E. Meggers, J. Am. Chem. Soc., 2015, 137, 9551–9556 CrossRef CAS PubMed; (d) Y. Li, M. Lei, W. Yuang and E. Meggers, Chem. Commun., 2017, 53, 8089–8092 RSC; (e) J. Ma, X. Zhang, X. Huang, S. Luo and E. Meggers, Nat. Protoc., 2018, 13, 605–632 CrossRef CAS PubMed; (f) Y. Tan, K. Harms and E. Meggers, Eur. J. Inorg. Chem., 2018, 2500–2504 CrossRef CAS; (g) J. Qin, V. A. Larinov, K. Harms and E. Meggers, ChemSusChem, 2019, 12, 320–325 CrossRef CAS.
- For chiral-at-rhodium catalysts examples: (a) C. Wang, L.-A. Chen, H. Huo, X. Shen, K. Harms, L. Gong and E. Meggers, Chem. Sci., 2015, 6, 1094–1100 RSC; (b) Y. Tan, W. Yuan, L. Gong and E. Meggers, Angew. Chem., Int. Ed., 2015, 54, 13045–13048 CrossRef CAS PubMed; (c) H. Huo, K. Harms and E. Meggers, J. Am. Chem. Soc., 2016, 138, 6936–6939 CrossRef CAS; (d) J. Ma, X. Shen, K. Harms and E. Meggers, Dalton Trans., 2016, 45, 8320–8323 RSC; (e) X. Shen, K. Harms, M. Marsch and E. Meggers, Chem. – Eur. J., 2016, 22, 9102–9105 CrossRef CAS; (f) S. Chen, X. Huang, E. Meggers and K. N. Houk, J. Am. Chem. Soc., 2017, 139, 17902–17907 CrossRef CAS; (g) Y. Grell, Y. Hong, X. Huang, T. Mochizuki, X. Xie, K. Harms and E. Meggers, Organometallics, 2019, 38, 3948–3954 CrossRef CAS.
- For chiral-at-ruthenium catalysts examples: (a) Y. Zheng, Y. Tan, K. Harms, M. Marsch, R. Riedel, L. Zhang and E. Meggers, J. Am. Chem. Soc., 2017, 139, 4322–4325 CrossRef CAS; (b) S. Chen, Y. Zheng, T. Cui and E. Meggers, J. Am. Chem. Soc., 2018, 140, 5146–5152 CrossRef CAS; (c) T. Cui, J. Qin, K. Harms and E. Meggers, Eur. J. Inorg. Chem., 2019, 195–198 CrossRef CAS; (d) E. Winterling, S. Ivlev and E. Meggers, Organometallics, 2021, 40, 1148–1155 CrossRef CAS.
- For chiral-at-iron catalysts examples: (a) Y. Hong, K. Harms and E. Meggers, J. Am. Chem. Soc., 2019, 141, 4569–4572 CrossRef CAS; (b) Y. Hong, T. Cui, S. Ivlev and E. Meggers, Chem. – Eur. J., 2021, 27, 8557–8563 CrossRef CAS; (c) N. Demirel, J. Haber, S. Ivlev and E. Meggers, Organometallics, 2022, 41, 3852–3860 CrossRef CAS.
- U. Prieto-Pascual, I. V. Alli, I. Bustos, I. J. Vitorica-Yrezabal, J. M. Matxain, Z. Freixa and M. A. Huertos, Organometallics, 2023, 42, 2991–2998 CrossRef CAS.
- L. W.-Y. Wong, J. W.-H. Kan, T. Nguyen, H. H.-Y. Sung, D. Li, A. S.-F. Au-Yeung, R. Sharma, Z. Lin and I. D. Williams, Chem. Commun., 2015, 51, 15760–15763 RSC.
- L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano and L. Cavallo, Nat. Chem., 2019, 11, 872–879 CrossRef CAS.
- B. Wang, X. Feng, Y. Huang, H. Liu, X. Cui and Y. Jiang, J. Org. Chem., 2002, 67, 2175–2182 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General synthetic experimental details, NMR, ESI-MS, ECD and X-Ray. CCDC 2341546 and 2341547. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4cc03665d |
| This journal is © The Royal Society of Chemistry 2024 |



