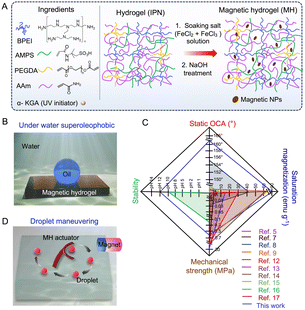
Underwater superoleophobic and magnetic hydrogel for cascade chemical reactions†
Hrisikesh
Sarma‡
a,
Subhankar
Mandal‡
b,
Saurav
Kumar
a and
Uttam
Manna
 *abc
*abc
aDepartment of Chemistry, Indian Institute of Technology Guwahati, Kamrup, Assam 781039, India. E-mail: umanna@iitg.ac.in
bCentre for Nanotechnology, Indian Institute of Technology Guwahati, Kamrup, Assam 781039, India
cSchool of Health science & Technology, Indian Institute of Technology Guwahati, Kamrup, Assam 781039, India
First published on 17th October 2024
Abstract
Magnetic hydrogels often suffer from low saturation magnetization and poor chemical and mechanical tolerance. Herein, we report magnetic nanoparticles (i.e. Fe3O4) grown in situ in an interpenetrating network containing both physical and covalent crosslinkages, which allowed the development of a high-water-content (∼95 wt%) and chemically (e.g. stable at extreme pH values of 1 and 12) and mechanically (Young's modulus of 550 kPa) stable magnetic hydrogel with high saturation magnetization (85 emu g−1). Moreover, the inherent high water content endowed the magnetic hydrogel with underwater superoleophobicity (OCA 160°), which enabled no-loss transport and mixing of liquid droplets as well as a cascade droplet (microliters) chemical reaction underwater through on-demand application of external magnetic field.
On-demand actuation of soft materials in the presence of an appropriate stimulus, including heat, light, solvents, vapor and electric or magnetic field, is an emerging research area because of its various prospective applications.1,2 In this regard, the design of a magnetically active hydrogel provides a simple basis for noncontact, nondestructive, and remotely controlled actuation in response to an external magnetic field.3 In general, magnetic hydrogels are made up of a polymer matrix embedded with a magnetic nanoparticles (MNPs, e.g. γ-Fe2O3 and Fe3O4), following different fabrication methods, including blending, in situ precipitation, and grafting.4 Even with significant progress in the past, most of the existing magnetic hydrogels suffer from weak mechanical strength, poor tolerance to alkaline conditions, and low saturation magnetization (Ms). For example, Zou et al. designed a magnetic hydrogel by uniformly dispersing MNPs into a silk fibroin hydrogel precursor, but the low loading of MNPs resulted in poor Ms (6.5 emu g−1).5 Moreover, a mechanically strong alginate/polyacrylamide hydrogel with embedded iron oxide MNPs has also been shown to suffer from a low Ms of 15 emu g−1 and uneven distribution of MNPs.6 Similarly, magnetically active cellulose nanocomposites were embedded into a zwitterionic hydrogel, which resulted in a tensile strength of 125 kPa but exhibited low Ms (0.05 emu g−1).7 For better dispersion of MNPs, silane-coated Fe3O4 MNPs were incorporated into a polyacrylamide hydrogel in another work to achieve a considerable Ms value of 42 emu g−1 with a mechanical strength of 25 kPa.8 Amine-functionalized Fe3O4 nanoparticles embedded in a gelatin/polyvinylalcohol network have also shown improved mechanical properties (Young's modulus up to 600 kPa) with a relatively high Ms of 75 emu g−1; however, their stability in different chemical environments has not been experimentally validated.9 Meanwhile, an in situ growth method has provided a tough and bilayer hydrogel with a moderate Ms of 32 emu g−1.10 Even, in situ-grown Fe3O4 MNPs embedded in a chitosan hydrogel network displayed a low Ms of ∼10 emu g−1.11 Hence, better designs of mechanically tough hydrogels with (i) high Ms and (ii) the ability to tolerate exposure to different and harsh chemical environments, including extreme pH, is essential for their realistic application. Moreover, the strategic association of such materials with bio-inspired underwater oil wettability would likely to provide an efficient approach for no-loss oil/oily droplet maneuvering underwater.
Herein, we introduce a highly water-rich hydrogel with interpenetrating networks made of a selected polymer (branched polyethyleneimine (BPEI)) and monomers (i.e. 2-acrylamido-2-methyl-1-propanesulfonic acid (AMPS) and acrylamide (AAm)) through successive UV-light-assisted polymerization, during which the available functional groups (e.g. acrylate, amine, sulphonic acid etc.) enabled physical and covalent cross-linkages in the prepared hydrogel (Fig. 1A). Thereafter, the in situ deposition of Fe3O4 nanoparticles allowed the fabrication of a magnetically active hydrogel (Fig. 1A). In the current strategy, the MNPs grown in situ without any additional surface modifications are anticipated to be involved in various physical interactions with the functional groups of the prepared hydrogel. The resulting hydrogel displayed extreme oil repellency underwater (Fig. 1B), where the high water content (≈95 wt%) in the prepared hydrogel led to heterogeneous oil wettability underwater. The covalent and non-covalent interactions present in the hydrogel matrix and the association of magnetic nanoparticles endow the material with high mechanical strength and ensure stability in harsh chemical environments. Eventually, the prepared hydrogel displayed superior performance to recently reported magnetic hydrogels (Fig. 1C and Table S1, ESI†).5–9,12–17 Because of the association of extreme oil repellence, adequate mechanical strength and high magnetic response, the prepared magnetic hydrogel (MH) was applied to demonstrate no-loss transport and mixing of oil droplets (Fig. 1D). These features enable precise droplet manipulation and cascade microdroplet chemical reaction underwater.
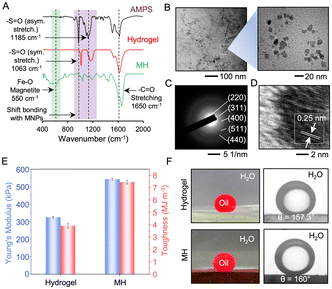
Firstly, a pre-gel solution consisting of a polymer (BPEI), monomer (AMPS), crosslinker (poly (ethyleneglycol) diacrylate (PEGDA)) and photoinitiator (α-keto glutaric acid) was photopolymerized prior to soaking with another monomer, i.e. acrylamide (AAm) and its subsequent photopolymerization to form a hydrogel with an interpenetrated network (IPN). Thereafter, it was soaked in an aqueous solution of mixed iron salts (Fe3+/Fe2+) prior to alkaline treatment for the in situ formation of MNPs in the interpenetrated polymer network. The 1,4-conjugate addition reaction between the available amine groups of BPEI and acrylate of PEGDA yielded β-amino ester-type covalent cross-linkages, as evident from the Fourier transform infrared (FTIR) spectroscopic analysis presented in Fig. 2A. A depletion of the characteristic IR signature at 1410 cm−1 corresponding to the vinylic C–H stretching of the acrylate moiety was observed, suggesting a mutual reaction between the amine and acrylate groups (Fig. S1, ESI†).18 Additionally, the shifting of the IR peak (symmetric vibrational stretching of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) from 1063 to 1070 cm−1 and the broadening of another characteristic IR signature at 1185 cm−1 (assigned to the asymmetric vibrational stretching of S
O) from 1063 to 1070 cm−1 and the broadening of another characteristic IR signature at 1185 cm−1 (assigned to the asymmetric vibrational stretching of S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) suggest the formation of ionic linkages (–NH3+⋯–SO3−–) due to the interaction between the sulfonic acid moiety of AMPS and amines of BPEI (Fig. 2A, red and black spectra).18,19 Other non-covalent interactions (e.g., H-bonding and coordination bond with MNPs) are likely to co-exist in the prepared hydrogel. An additional FTIR peak appeared at 550 cm−1 due to the stretching of the Fe–O bond in the magnetite nanoparticles. Moreover, the shifting of the IR peaks at 1063 cm−1 (–S
O) suggest the formation of ionic linkages (–NH3+⋯–SO3−–) due to the interaction between the sulfonic acid moiety of AMPS and amines of BPEI (Fig. 2A, red and black spectra).18,19 Other non-covalent interactions (e.g., H-bonding and coordination bond with MNPs) are likely to co-exist in the prepared hydrogel. An additional FTIR peak appeared at 550 cm−1 due to the stretching of the Fe–O bond in the magnetite nanoparticles. Moreover, the shifting of the IR peaks at 1063 cm−1 (–S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1650 cm−1 (–C
O) and 1650 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) suggests the existence of physical interactions between the MNPs and the interpenetrated polymeric network (Fig. 2A, green spectrum).
O) suggests the existence of physical interactions between the MNPs and the interpenetrated polymeric network (Fig. 2A, green spectrum).
The scanning electron microscopic (SEM) images revealed a change in topography because of the in situ deposition of MNPs, and apparently more globular domains were observed in the MH (Fig. S2, ESI†). On the other hand, the high-resolution transmission electron microscopic (HR-TEM) images revealed a nearly uniform dispersion of MNPs with an average diameter of ∼10 nm in MH (Fig. 2B). The selected area diffraction study (SAED) of MH revealed distinct diffraction rings for the 220, 311, 400, 511, and 440 planes, resembling the lattice parameters of the cubic spinel structure of magnetite (Fe3O4) NPs (Fig. 2C and Table S2, ESI†).20 Further, both the HR-TEM image (Fig. 2D) of a single NP and the inverse fast Fourier transition (IFFT; Fig. S3, ESI†) micrograph derived from the HR-TEM image indicated a specific lattice pattern with a lattice distance of 0.25 nm, corresponding to the (311) plane of the cubic spinel structure. Further, X-ray diffraction (XRD) peaks of the 220, 311, 400, 422, 511, and 440 planes were observed, suggesting the formation of magnetite Fe3O4 (JCPDS no. 19-0629) (Fig. S4, ESI†). Additionally, a broad peak at 2θ = 23° attributed to the amorphous polymeric network was found.21 Nevertheless, the peak-broadening could be ascribed to the amorphous polymer network around the crystalline MNPs.22,23 These findings confirm the in situ formation of crystalline cubic spinel Fe3O4 MNPs, which are known for their superparamagnetic behavior with a high Ms.
Thereafter, the mechanical properties, chemical tolerance and underwater superoleophobicity of the prepared MH were analyzed in detail. While the interpenetrated network provided the hydrogel with Young's modulus of 330 kPa, the in situ deposition of MNPs improved its mechanical properties, resulting in a high Young's modulus (550 kPa) and toughness of 7.5 M Jm−3 (Fig. 2E and Fig. S5, ESI†). The favourable interaction of the crystalline nanoparticles with the available functional groups of the interpenetrated polymeric network (Fig. 2A), provided additional physical cross-linkages to improve the mechanical properties of the MH. Aggregation-free physical reinforcement of nanoparticles is known to improve their mechanical properties.24 In addition, the resulting MH withstood different common mechanical deformations like stretching, twisting, folding and punching, as shown in Fig. S6 (ESI†). In addition to the mechanical properties, the MH displayed high chemical stability even after prolonged (7 days) exposure to extremes of pH (1 and 12), seawater and river water, as evident from the low swelling ratios of the treated MH. Such high tolerance can be attributed to covalent crosslinking chemistry.18 In the control study, the same MH lacking the β-amino ester-type covalent linkage suffered from a relatively high swelling ratio in different and complex aqueous phases (Fig. S7, ESI†). However, the hydrogel prepared by the direct mixing of all selected components in a single step suffered from high (89.7%) swelling and disintegration in a basic medium during the in situ growth of the MNPs (Fig. S8, ESI†). On the other hand, the content of water in the prepared hydrogel containing the interpenetrated network was estimated to be high at ∼ 95%, which makes it an appropriate underwater superoleophobicity biomimetic with an OCA > 150°; in the hydrogel, the entrapped water acts as the external third phase as per the Cassie–Baxter wettability model (eqn (1)).25
Cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θCB = ϕ θCB = ϕ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θY + ϕ − 1 θY + ϕ − 1 | (1) |
The deposition of MNPs did not affect this oil-wettability, rather it continued to display extreme oil repellence with an OCA of ∼160° (Fig. 2F). Moreover, the MH could efficiently repel various oils and organic solvents with different surface tensions (Fig. S9, ESI†).
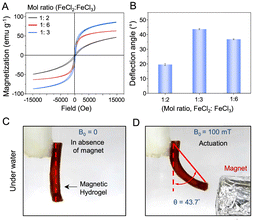
The in situ deposition of iron oxide nanoparticles (Fe3O4) makes the prepared hydrogel magnetic with small magnetic hysteresis. Alteration in the composition of the ferric and ferrous salts, improved the Ms from 50 to 85 emu g−1, as shown in Fig. 3A. The maximum Ms was noticed for the magnetic hydrogel prepared a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 molar ratio of the iron salts (FeCl2
3 molar ratio of the iron salts (FeCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FeCl3). In the past, it was observed that the Ms varies depending on the size, shape and crystalline lattice of the MNPs.26–28 The XRD data of the magnetic hydrogel prepared by altering the molar ratio of iron salts displayed differences in diffraction patterns and crystallite sizes (calculated using the standard Scherrer equation, eqn (2)) (Table S3, ESI†).29
FeCl3). In the past, it was observed that the Ms varies depending on the size, shape and crystalline lattice of the MNPs.26–28 The XRD data of the magnetic hydrogel prepared by altering the molar ratio of iron salts displayed differences in diffraction patterns and crystallite sizes (calculated using the standard Scherrer equation, eqn (2)) (Table S3, ESI†).29
D = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (2) |
Thus, the prepared hydrogel acts as a soft magnetic material and efficiently actuates in response to an external magnetic field. A cantilever bending beam actuator model was set up to demonstrate its actuation behaviour underwater when exposed to an external magnetic field; the MH beam was suspended by attaching its one end to a support underwater. When a permanent magnet (neodymium alloy) was placed at a distance of 2 cm, the MH deflected by an angle of 43.7° (Fig. 3B–D). However, the deflection of other MHs prepared by varying the molar ratio of the iron salts remained relatively less under identical experimental conditions because of their low Ms (Fig. 3B and Fig. S10, ESI†)
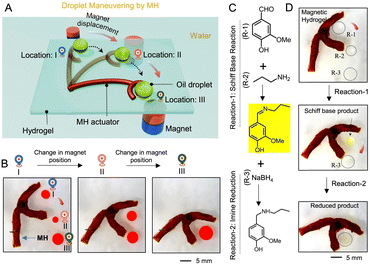
Finally, given the excellent mechanical properties, magnetic properties and underwater superoleophobicity of the prepared MH, we developed a lab-made prototype to demonstrate no-loss liquid droplet transport and coalescence and on-demand cascade microdroplet reactions. The as-prepared MH was given a specific shape with a tail and an arched head, as shown in Fig. 4A and B. The end of the tail was attached to a bed of the extremely oil-repellent hydrogel with the interpenetrated network but lacking deposited MNPs. A model oil droplet was placed on this hydrogel bed near the arch of the MH. Under an external magnetic field, the arch of the MH pushed the beaded oil droplet, displacing it in a 360° angular direction without causing any adhesion loss of the liquid (Fig. S11, ESI†). This concept was further extended to mix multiple oil droplets remotely by actuating the MH adequately. Fig. 4A shows that the MH arch actuator could push an oil droplet from location: I in a desired direction and cause coalescence with another oil droplet beaded at a different location (location: II, Fig. 4A and B) by placing an external magnet at a distance of ∼ 3 cm from the MH. Thereafter, the subsequent coalescence of other oil droplets was demonstrated similarly without any adhesion loss of the beaded oil droplet (Movie S1, ESI†). Next, a cascade chemical reaction was performed utilizing the same MH (Fig. 4C). In this regard, a Schiff base reaction between selected reactants (vanillin (R-1) and propylamine (R-2)) followed by the reduction of the synthesized Schiff base using a reducing agent (NaBH4; R-3) are demonstrated (Fig. 4C) via the sequential coalescence of strategically placed droplets of selected reagents (R1 and R-2) and the reducing agent (R-3) using MH and a permanent magnet (Fig. 4D). The on-demand mixing of droplets containing R-1 and R-2 initiated a mutual reaction between the aldehyde and amine groups to form an imine bond. As a result, the merged droplet turned yellow (Movie S2, ESI†). The formation of the imine bond was revealed by the FTIR analysis. The depletion of the aldehyde peak at 1670 cm−1 and the appearance of a new peak at 1623 cm−1 (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) confirmed the formation of an imine-based product in the first step (Fig. S12, ESI†). Further, the displacement of this droplet to another droplet with R-3 resulted in coalescence and decoloration (Movie S2, ESI†) because of the reduction of the imine bond, as suggested by the disappearance of the characteristic IR signature of the imine bond at 1623 cm−1 (Fig. S12, ESI†). Thus, the prepared MH successfully demonstrates no-loss transport and cascade microdroplet reactions.
N–) confirmed the formation of an imine-based product in the first step (Fig. S12, ESI†). Further, the displacement of this droplet to another droplet with R-3 resulted in coalescence and decoloration (Movie S2, ESI†) because of the reduction of the imine bond, as suggested by the disappearance of the characteristic IR signature of the imine bond at 1623 cm−1 (Fig. S12, ESI†). Thus, the prepared MH successfully demonstrates no-loss transport and cascade microdroplet reactions.
Thus, the current approach provides a facile method for on-demand, contact-less and no-loss oil droplet manipulation without any sophisticated devices, micropumps and microelectronics. The developed magnetic hydrogel (MH) is likely to be useful in various other applications, including clinical magnetic navigation devices, soft robotics systems, and lab-on-chip devices.
We thank Science and Engineering Research Board (CRG/2022/000710), DBT(BT/PR45283/NER/95/1919/2022), and Ministry of Electronics and Information Technology (no. 5(1)/2022-NANO) for generous financial support. The authors are thankful to CIF, CFN, SHST, and Department of Chemistry, Indian Institute of Technology-Guwahati.
Data availability
The data that support the findings of this study are included in ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Guo, X. Yang, C. Zhou and C. Li, Nat. Commun., 2024, 15, 1694 CrossRef PubMed.
- Q. L. Zhu, W. Liu, O. Khoruzhenko, J. Breu, H. Bai, W. Hong, Q. Zheng and Z. L. Wu, Adv. Mater., 2024, 36, 2314152 CrossRef CAS PubMed.
- M. Li, A. Pal, A. Aghakhani, A. Pena-Francesch and M. Sitti, Nat. Rev. Mater., 2022, 7, 235–249 CrossRef PubMed.
- Y. Li, G. Huang, X. Zhang, B. Li, Y. Chen, T. Lu, T. J. Lu and F. Xu, Adv. Funct. Mater., 2013, 23, 660–672 CrossRef CAS.
- Y. P. Zou, H. F. Liang, B. Wang, Q. C. Zhang, D. H. Su, S. Y. Lu, Q. Y. Zhang, T. Wu, L. Xiao, Y. Xiao, J. Dong, L. B. Jiang and X. L. Li, Adv. Funct. Mater., 2023, 33, 2302442 CrossRef CAS.
- H. Haider, C. H. Yang, W. J. Zheng, J. H. Yang, M. X. Wang, S. Yang, M. Zrínyi, Y. Osada, Z. Suo, Q. Zhang, J. Zhou and Y. M. Chen, Soft Matter, 2015, 11, 8253–8261 RSC.
- R. Nasseri, N. Bouzari, J. Huang, H. Golzar, S. Jankhani, X. Tang, T. H. Mekonnen, A. Aghakhani and H. Shahsavan, Nat. Commun., 2023, 14, 6108 CrossRef CAS PubMed.
- X. Hu, G. Nian, X. Liang, L. Wu, T. Yin, H. Lu, S. Qu and W. Yang, ACS Appl. Mater. Interfaces, 2019, 11, 10292–10300 CrossRef CAS PubMed.
- F. Gao, H. Jiang, D. Wang, S. Wang and W. Song, Adv. Mater., 2024, 36, 2401645 CrossRef CAS PubMed.
- J. Tang, Z. Tong, Y. Xia, M. Liu, Z. Lv, Y. Gao, T. Lu, S. Xie, Y. Pei, D. Fang and T. J. Wang, J. Mater. Chem. B, 2018, 6, 2713–2722 RSC.
- Y. Wang, B. Li, F. Xu, Z. Han, D. Wei, D. Jia and Y. Zhou, Biomacromolecules, 2018, 19, 3351–3360 CrossRef CAS PubMed.
- Z. Wang, H. Zhu, H. Li, Z. Wang, M. Sun, B. Yang, Y. Wang, L. Wang and L. Xu, ACS Nano, 2023, 17, 9622–9632 CrossRef CAS PubMed.
- Y. Lee, F. Koehler, T. Dillon, G. Loke, Y. Kim, J. Marion, M. J. Antonini, I. C. Garwood, A. Sahasrabudhe, K. Nagao, X. Zhao, Y. Fink, E. T. Roche and P. Anikeeva, Adv. Mater., 2023, 35, 2301916 CrossRef CAS PubMed.
- M. Saadli, D. L. Braunmiller, A. Mourran and J. J. Crassous, Small, 2023, 19, 2207035 CrossRef CAS PubMed.
- S. R. Goudu, I. C. Yasa, X. Hu, H. Ceylan, W. Hu and M. Sitti, Adv. Funct. Mater., 2020, 30, 2004975 CrossRef CAS.
- M. C. Mendes, J. A. Pereira, A. S. Silva and J. F. Mano, Adv. Mater., 2024, 2402988 CrossRef CAS PubMed.
- B. Sun, M. Sun, Z. Zhang, Y. Jiang, B. Hao, X. Wang, Y. Cao, T. K. Chan and L. Zhang, Adv. Intell. Syst., 2024, 6, 2300092 CrossRef.
- H. Sarma, S. Mandal, A. Borbora, A. Phukan, S. Kumar, M. Dhar, D. Sarkar and U. Manna, Adv. Funct. Mater., 2024, 2403607 CrossRef CAS.
- S. Aleid, M. Wu, R. Li, W. Wang, C. Zhang, L. Zhang and P. Wang, ACS Mater. Lett., 2022, 4, 511 CrossRef CAS.
- L. Qiao, Z. Fu, J. Li, J. Ghosen, M. Zeng, J. Stebbins, P. N. Prasad and M. T. Swihart, ACS Nano, 2017, 11, 6370–6381 CrossRef CAS PubMed.
- L. Pu, Z. Yuan, Y. Cai, X. Li, Z. Xue, Y. Niu, Y. Li, S. Ma and W. Xu, ACS Appl. Mater. Interfaces, 2024, 16, 32762 CrossRef CAS PubMed.
- T. Ahn, J. H. Kim, H. M. Yang, J. W. Lee and J. D. Kim, J. Phys. Chem. C, 2012, 116, 6069–6076 CrossRef CAS.
- A. H. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339 CrossRef CAS PubMed.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546 RSC.
- S. Xuan, Y. X. Wang, J. C. Yu and K. C. Leung, Chem. Mater., 2009, 21, 5079–5087 CrossRef CAS.
- H. C. Roth, S. P. Schwaminger, M. Schindler, F. E. Wagner and S. Berensmeier, J. Magn. Magn. Mater., 2015, 377, 81–89 CrossRef CAS.
- D. Wen, T. Ralph, J. Han, S. Bradley, M. J. Giansiracusa, V. Mitchell, C. Boskovic and N. Kirkwood, J. Phys. Chem. C, 2023, 127, 9164–9172 CrossRef CAS.
- C. F. Holder and R. E. Schaak, ACS Nano, 2019, 13, 7359–7365 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc04432k |
| ‡ HS and SM contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |