Recent progress of porphyrin metal–organic frameworks for combined photodynamic therapy and hypoxia-activated chemotherapy
Qiuyun
Zhang
a,
Xiaohui
Wang
b,
Jiayi
Chen
a,
Junjie
Wu
a,
Mengjiao
Zhou
a,
Rui
Xia
*b,
Weiqi
Wang
 *a,
Xiaohua
Zheng
*a,
Xiaohua
Zheng
 *a and
Zhigang
Xie
*a and
Zhigang
Xie
 c
c
aSchool of Pharmacy, Nantong University, Nantong, Jiangsu Province 226001, China. E-mail: wwq1990@ntu.edu.cn; xiaohuaz@ntu.edu.cn
bSchool of Public Health, Nantong University, Nantong, Jiangsu Province 226001, China. E-mail: xra@ntu.edu.cn
cState Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, 5625 Renmin Street, Changchun, Jilin 130022, P. R. China
First published on 31st October 2024
Abstract
Nanoscale metal–organic frameworks integrated with porphyrins (Por-nMOFs) have emerged as efficient nanoplatforms for photodynamic therapy (PDT), which relies on the conversion of molecular oxygen into cytotoxic singlet oxygen. However, the hypoxic microenvironment within tumors significantly limits the efficacy of PDT. To address this challenge, researchers have explored various strategies to either alter or exploit the hypoxic conditions in tumors. One such strategy involves leveraging the porous structure of Por-nMOFs to load hypoxia-activated prodrugs (HAPs) like tirapazamine (TPZ), thereby utilizing the tumor's intrinsic hypoxic environment to trigger a chemotherapeutic effect that synergizes with PDT. Advances in nanoscience have enabled the development of porphyrin-based nMOFs capable of simultaneously loading both porphyrin photosensitizers and TPZ, ensuring effective release within cancer cells under high-phosphate conditions. The subsequent activation of co-loaded TPZ, by the tumor's own hypoxic microenvironment, and that created during PDT, facilitates a combined PDT and chemotherapy approach. This method not only enhances the suppression of cancer cell proliferation but also improves control over tumor metastasis while mitigating the negative impact of hypoxia on singular Por-nMOFs in PDT. This review summarizes recent advances in Por-nMOFs research, focusing on the design strategies for enhancing water dispersibility, circulatory stability, and targeting specificity through post-synthetic modifications. Additionally, this review highlights the bioapplication of Por-nMOFs by integrating TPZ chemotherapy and other therapeutic modalities to combat hypoxic and metastatic malignancies. We anticipate that this review will inspire further research into Por-nMOFs and advance their application in biomedicine.
1. Introduction
According to the 2022 global cancer statistics released by the International Agency for Research on Cancer (IARC), cancer remains a highly lethal disease. With evolving demographic trends, the IARC projects that by 2050, the number of new cancer cases could reach 35 million.1 These statistics underscore the profound impact of cancer on human health, underscoring the critical need for innovative therapeutic strategies. Among the diverse array of treatment modalities, including chemotherapy,2–5 radiotherapy,6–9 immunotherapy,10–13 and PDT,14–16 PDT has garnered significant attention due to its non-invasive nature, minimal side effects, and cost-effectiveness.17–20 PDT is based on the triplet excited state of a photosensitizer, which upon light activation can generate reactive oxygen species (ROS), primarily singlet oxygen (1O2), through a type II mechanism.21–23 Additionally, PSs can undergo a type I mechanism, where they directly interact with cellular substrates to produce ROS such as hydrogen peroxide (H2O2), superoxide (O2˙−), and hydroxyl radicals (˙OH).24 It has been observed that most photosensitizers primarily operate via the type II mechanism, which relies on the presence of oxygen.25–31 However, the hypoxic microenvironment resulting from the uncontrolled proliferation of malignant tumors represents a significant barrier to the efficacy of PDT.17,32–38 Therefore, the development of novel strategies to overcome the limitations imposed by hypoxia on PDT holds great promise for advancing cancer therapy.39–46To overcome the limitations posed by tumor hypoxia, several strategies have been proposed.45 These include direct oxygen delivery via carriers such as perfluorocarbons47–54 or hemoglobin,46,55,56 generation of oxygen through Fenton reactions involving manganese or iron(II) compounds,19,57–60 or the use of type I PDT mechanisms that do not require oxygen.24,61–69 Other approaches involve the inhibition of mitochondrial respiration70–78 or the combination of PDT with other therapies, such as photothermal therapy (PTT),79–86 ferroptosis induction,59,69,87–90 and immunotherapy.91–97 One particularly promising approach involves the utilization of hypoxia-activated prodrugs in conjunction with PDT.46,98–104 Hypoxia-activated prodrugs, such as TPZ, are inactive under normoxic conditions but become activated in hypoxic environments.105–108 Upon reduction by intracellular reductases, TPZ generates cytotoxic metabolites that can damage DNA and induce apoptosis.107 The synergy between PDT and TPZ chemotherapy offers a potent strategy for overcoming tumor hypoxia.46
Metal–organic frameworks, with their biocompatibility, tunable porosity, and ease of surface modification, have been widely utilized in the biomedical and biosensor field.109–111 They are particularly promising as drug delivery systems for cancer therapy. These characteristics make them a versatile platform for enhancing treatment efficacy and targeting capabilities.112 Owing to the intrinsic photosensitivity, Por-nMOFs have shown particular promise in PDT.113–120 Although the porphyrin core tends to aggregate in aqueous media, it benefits from the ordered structure of nMOFs, which prevents aggregation. The ordered structure of nMOFs also enhances the quantum yield of singlet oxygen production.121–125 Moreover, the porous architecture of nMOFs facilitates the loading of TPZ, ensuring its controlled release in response to the high-phosphate environment within cancer cells.126,127
This review aims to summarize recent advancements in the use of porphyrin-integrated nMOFs for the combined PDT and TPZ chemotherapy of cancer (Fig. 1). This review discusses the design principles and surface modifications required to ensure effective tumor targeting and controlled release of TPZ. Additionally, this review explores the synergistic mechanisms underlying the enhancement of PDT efficacy by TPZ under hypoxic conditions, as well as the integration of additional therapeutic modalities into multifunctional nMOFs platforms. Through these insights, we hope to inspire further research and development in the field of porphyrin-based nMOFs for the effective treatment of cancer.
2. Polymer coated por-nMOFs for combined PDT and TPZ-mediated chemotherapy
The porous structure of porphyrin-based nMOFs can be utilized to load the hypoxia-activated prodrug TPZ. The consumption of O2 during PDT further exacerbates the hypoxic environment within the tumor. Therefore, the O2-consuming PDT mechanism is highly conducive to enhancing the chemotherapeutic efficacy of TPZ. By rationally modifying the surface of nMOFs with polymers to improve their dispersibility and circulatory stability, such a designed nanoplatform may achieve a synergistically enhanced therapeutic effect. Notably, the coating or modification with polymers, particularly polyethylene glycol (PEG), has been shown to effectively enhance the therapeutic efficacy of nanomedicines.128–130For example, Liu et al. developed a multifunctional hafnium-porphyrin nMOFs platform (Hf-TCPP).127 The ordered coordination array effectively prevents the self-quenching of porphyrin molecules. This structure property can ensure enhanced ROS generation for efficient PDT. The synthesized Hf-TCPP NPs contain a high concentration of meso-tetra(4-carboxyphenyl)porphine (TCPP) photosensitizer and utilize a large BET surface area to load a high amount of hypoxia-activated prodrug (TPZ) (Fig. 2A). Surface modification with dopamine-derived polymer (DOPA-PIMA-mPEG) significantly improved dispersibility and structural stability, enabling combined PDT and TPZ chemotherapy (Fig. 2B). Transmission electron microscopy (TEM) results confirmed that the prepared TPZ/Hf-TCPP/PEG particles were spherical with an average diameter of approximately 55 nm (Fig. 2C). Powder X-ray diffraction (PXRD) tests indicated that the synthesized Hf-TCPP NPs were highly crystalline, and the high molecular weight surface modification did not affect their crystallinity (Fig. 2D). The introduction of PEG improved the dispersion stability of MOFs in physiological solutions. UV-vis spectra testing verified the presence of porphyrin photosensitizer and TPZ chemotherapeutic (absorption peak at 265 nm) molecules (Fig. 2E). UV-vis spectroscopy testing confirmed that TPZ was rapidly released (∼60%) within 10 h in PBS solution, followed by a sustained slow release. The PEG coating slowed down the release rate and achieved a controlled release effect (Fig. 2F). The 1,3-diphenylisobenzofuran (DPBF) probe confirmed the singlet oxygen generation capability of TPZ/Hf-TCPP/PEG under illumination. At 635 nm light exposure, the absorption of DPBF at 410 nm significantly decreased, indicating excellent singlet oxygen generation without noticeable interference from the loaded TPZ (Fig. 2G). The effective intracellular generation of singlet oxygen and the hypoxic environment also indicate the feasibility of this system for cancer cell inhibition (Fig. 2H).
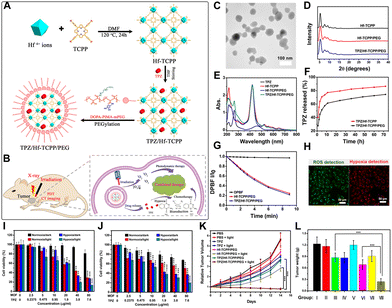 | ||
| Fig. 2 (A) Schematic illustrating the synthesis of TPZ/Hf/TCPP/PEG. (B) In vivo synergistic photodynamic and hypoxia-activated therapy using TPZ/Hf/TCPP/PEG. (C) TEM image showing the morphology of Hf/TCPP. (D) PXRD patterns. (E) UV-vis absorbance spectra. (F) TPZ release kinetics from TPZ/Hf/TCPP and TPZ/Hf/TCPP/PEG in PBS. (G) Singlet oxygen generation detected by DPBF. (H) CLSM images detecting ROS and hypoxia in HeLa cells treated with TPZ/Hf/TCPP/PEG and light. (I) and (J) In vitro cytotoxicity against HeLa and 4T1 cells under hypoxic and normoxic conditions with or without light exposure. (K) Tumor growth curves of different groups. (L) Weights of excised tumors. Groups: (I) PBS, (II) PBS + light, (III) TPZ, (IV) TPZ + light, (V) Hf/TCPP/PEG, (VI) Hf/TCPP/PEG + light, (VII) TPZ/Hf/TCPP/PEG, (VIII) TPZ/Hf/TCPP/PEG + light. Reprinted with permission from ref. 127. Copyright 2018 American Chemical Society. | ||
To verify the synergistic enhancement of PDT and TPZ chemotherapy, the authors conducted cytotoxicity experiments on HeLa and breast cancer (4T1) cells, as well as animal studies. Cell survival assays showed that under hypoxic conditions (2% O2), Hf-TCPP/PEG exhibited lower cell survival rates compared to normoxic conditions (20% O2) due to the reduced efficacy of PDT. However, TPZ/Hf-TCPP/PEG demonstrated enhanced cell survival inhibition under hypoxic conditions, directly proving the combined effect of PDT and TPZ (Fig. 2I and J). In vivo studies revealed that TPZ/Hf-TCPP/PEG treated mice with 635 nm light illumination achieved significant tumor suppression (Fig. 2K and L). This system leverages nMOFs as carrier materials to achieve the combined therapeutic effects of porphyrin-mediated PDT and TPZ-mediated chemotherapy. This multifunctional nMOFs reverses the disadvantages of PDT, enhancing antitumor capabilities through the synergistic action of photodynamic and hypoxia-responsive therapies. This work also highlights the significant potential of porous materials in drug delivery and the treatment of hypoxic tumors.
3. HSA coated nMOFs for enhanced PDT and hypoxia-activated chemotherapy
For the chemotherapeutic agent TPZ to be effective, it must be released from the carrier material.107 Traditional nMOFs require the presence of phosphate ions in PBS or physiological solutions to degrade and release TPZ, which limits its effective release.127 To investigate the release mechanism of TPZ when carried by nMOFs and to achieve higher release ratios, new responsiveness mechanisms need to be introduced, such as azo-bond degradation triggered by hypoxia.131–136For example, Zhao et al. fabricated a hypoxia-responsive nanoscale metal–organic framework (UiO-AZB) containing azo groups in the organic linkers and post-modified it with Ce6-conjugated human serum albumin (HSA) to obtain the UiO-AZB/HC composite.137 TPZ molecules were then loaded into the framework to form UiO-AZB/HC-TPZ. Experiments confirmed that the UiO-AZB/HC-TPZ nanosystem efficiently generates singlet oxygen upon 660 nm light irradiation, leading to severe hypoxia in tumors. This process triggers the degradation of the framework and the controlled release of TPZ. Consequently, it enhances antitumor therapy by combining PDT with hypoxia-activated chemotherapy (Fig. 3A). PXRD results confirmed the successful synthesis of nMOFs, with UiO-AZB successfully synthesized at varying formic acid concentrations (0.16 to 2.28 M). Particles prepared with 0.16 M formic acid had diameters less than 200 nm, favorable for good blood circulation, and were used in subsequent in vitro and in vivo experiments (Fig. 3B). Results also indicated no morphological changes after TPZ loading (Fig. 3C). UV-vis spectra testing confirmed the presence of Ce6 photosensitizer and TPZ chemotherapeutic molecules within the nanosystem (Fig. 3D). DPBF probes detected ROS generation under light exposure (Fig. 3E), validating the material's photoactivity. To verify the hypoxia sensitivity of the azo bonds, the hypoxia-responsive degradation of UiO-AZB/HC-TPZ was investigated. Incubation with rat liver microsomes/NADPH and argon bubbling or light exposure for 1 h led to significant structural changes and degradation within approximately 48 h (Fig. 3F). In contrast, untreated samples remained unchanged after 48 h. These experiments demonstrated that ROS generated by UiO-AZB/HC-TPZ consumes oxygen, creating a hypoxic environment that causes azo bond cleavage and subsequent degradation. To monitor TPZ release under normoxic and hypoxic conditions, real-time measurements were performed in PBS solution. Under normoxic conditions, only 23.7% of TPZ was released, whereas this increased to 78.7% and 54.2% under hypoxic (argon-treated) and light-exposed conditions, respectively. This result indicates that hypoxia or PDT-induced hypoxia facilitates nMOFs structural breakdown, proving that introducing azo bonds enhances drug release due to their hypoxia sensitivity (Fig. 3G).
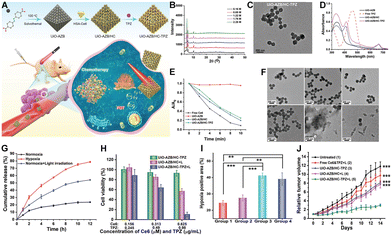 | ||
| Fig. 3 (A) Schematic depicting the synthesis of UiO-AZB/HC-TPZ and its light-activated, hypoxia-responsive drug delivery mechanism for tumor therapy. (B) PXRD patterns of UiO-AZB with varying sizes. (C) TEM images of UiO-AZB/HC-TPZ. (D) UV-vis spectra. (E) DPBF absorption changes in different samples. (F) TEM images showing morphological changes in UiO-AZB/HC-TPZ under different treatments over time. (G) Percentage of TPZ released from UiO-AZB/HC-TPZ over time under various conditions. (H) Viability of 4T1 cells treated with UiO-AZB/HC-TPZ in the dark, with light irradiation alone, and with light irradiation post-incubation. (I) Quantification of tumor hypoxia in different treatment groups. (J) Time-dependent tumor growth curves in mice following different treatments. Groups: (1) PBS; (2) UiO-AZB/HC-TPZ; (3) UiO-AZB/HC-TPZ + light (2 h); (4) UiO-AZB/HC-TPZ + light (24 h). Reprinted with permission from ref. 137. Copyright 2023 John Wiley & Sons, Ltd. | ||
To validate the anticancer efficacy of the material, experiments showed that the combined treatment of UiO-AZB/HC-TPZ under light exposure provided the highest cytotoxicity against tumor cells. These results indicate that UiO-AZB/HC-TPZ is effective for both PDT and hypoxia-activated chemotherapy, enhancing overall therapeutic performance (Fig. 3H). A 4T1 tumor-bearing nude mouse model was established to study the in vivo efficacy of UiO-AZB/HC-TPZ. Prior to tumor suppression experiments, hypoxia immunofluorescence staining using an imidazole probe was conducted. Results showed that hypoxic areas in tumors were 24% and 27% for PBS and UiO-AZB/HC-TPB alone, respectively. However, these percentages increased significantly to 41% and 39% about 2 and 24 h post-PDT treatment, respectively (Fig. 3I). This further confirms that PDT induces severe hypoxia in tumors, beneficial for the hypoxia-sensitive release of TPZ, thereby achieving combined PDT and TPZ chemotherapy (Fig. 3I). Indeed, the animal study results demonstrated that the UiO-AZB/HC-TPZ + light group (Group 5) showed the highest antitumor efficacy, with the smallest average tumor volume (Fig. 3J). This confirms the nanosystem's ability to achieve combined therapy (PDT + hypoxia-activated chemotherapy), demonstrating the most effective tumor suppression. The successful design of this system demonstrates the effectiveness of incorporating appropriate responsiveness mechanisms, such as hypoxia-responsive azo bonds. These mechanisms are well-aligned with the hypoxic tumor microenvironment and the additional hypoxia induced by PDT. This alignment enhances the release of TPZ and activates its chemotherapeutic effects, leading to significantly improved outcomes in combined PDT and chemotherapy.
4. Super stable por-nMOFs for combined PDT and hypoxia-activated chemotherapy
Although TPZ itself is non-toxic in non-hypoxic environments, premature release of TPZ from nMOFs due to phosphate ions in the bloodstream can reduce the total drug dose delivered to the target site.138 Surface modifications of nMOFs with polymers alone are insufficient to prevent early TPZ release. Therefore, enhancing the stability of TPZ-loaded nMOFs is an essential research topic.138 Improving the stability of TPZ-loaded nMOFs can prevent unnecessary side effects and reduce the actual dosage required.Addressing this, Zhu et al. developed a hybrid material by integrating porphyrin-based nMOFs with gold nanoparticles (AuNPs) and loading TPZ chemotherapeutic drugs.138 To promote dispersion, PEG polymers were used to modify the surface, yielding the PAMNPs@TPZ nanosystem (Fig. 4A). The addition of AuNPs enhanced the stability of nMOFs in phosphate solutions. Upon reaching the tumor site, the high concentration of phosphate ions degrades the nMOFs structure, releasing the TPZ chemotherapy drugs. TEM images show that the MOFNPs have a monodisperse spherical morphology with a diameter of approximately 50 nm (Fig. 4B). TEM also reveals that approximately 6 nm AuNPs are anchored on the surface of MOFNPs, which confirmed the successful preparation of AuMOFNPs (Fig. 4C). Furthermore, the surface modification with PEG significantly improved the water dispersibility of the PAMNPs nanocomposites (Fig. 4D). Subsequent drug release studies demonstrated that the nanosystem remains stable in water, saline, and PBS (2 mM) solutions (Fig. 4E). Within 24 h, only a small fraction (<25%) of TPZ was released from PAMNPs@TPZ in water, saline, and PBS (2 mM) (Fig. 4F). However, in PBS (10 mM), there was accelerated release, with almost complete release (98.4%) observed within the same incubation period (Fig. 4G). This suggests that the presence of AuNPs and PEG surface modification effectively prevented phosphate ions from attacking the Zr clusters within the MOF, ensuring structural integrity and reducing drug leakage during circulation. As a photosensitizer, the singlet oxygen generation capability is crucial. The enhanced fluorescence changes of SOSG probes confirmed that PAMNPs@TPZ effectively produces singlet oxygen under light exposure, indicating its efficacy for PDT (Fig. 4H).
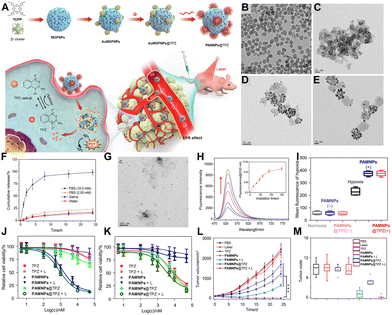 | ||
| Fig. 4 (A) Schematic illustration of the preparation of PAMNPs@TPZ and its synergistic PDT and hypoxia-activated chemotherapy mechanism. (B)–(D) TEM images of MOFNPs, AuMOFNPs, and PAMNPs. (E) TEM image of PAMNPs@TPZ in PBS (2 mM). (F) TPZ release profiles from PAMNPs@TPZ in saline and PBS. (G) TEM image of PAMNPs@TPZ after 4 h incubation in PBS (10 mM). (H) Fluorescence changes of SOSG in PAMNPs@TPZ solutions upon irradiation for different times. (I) Fluorescence intensity changes of BioTracker 520 Green Hypoxia Dye in 4T1 cells after different treatments. (J-K) Cytotoxicity of nanoformulations towards 4T1 cells under (J) normoxic and (K) hypoxic conditions. (L) Tumor growth curves of mice treated with various formulations. (M) Number of lung tumor nodules in mice treated with different formulations. Reprinted with permission from ref. 138. Copyright 2023 Elsevier. | ||
To verify the formation of a hypoxic microenvironment during oxygen-consuming PDT, the authors used an oxygen-sensitive probe. Stronger green fluorescence indicates lower oxygen concentration and higher hypoxia levels. Fluorescence intensity measurements showed that PAMNPs@TPZ treated and illuminated samples exhibited strong green fluorescence (Fig. 4I), indicating the occurrence of hypoxia during PDT. This result sets the foundation for the subsequent conversion of TPZ into reactive radicals capable of irreversibly damaging DNA, leading to tumor cell apoptosis under hypoxic conditions. Cell viability assays demonstrated that PAMNPs@TPZ with light exposure effectively inhibited cell survival under both normoxic and hypoxic conditions (Fig. 4J and K). Notably, the IC50 value of PAMNPs under hypoxic conditions was significantly increased (Fig. 4K), indicating the synergistic effect of PDT and TPZ chemotherapy in a hypoxic environment. In vivo antitumor and anti-metastasis experiments were also conducted. Compared to control groups, PAMNPs@TPZ with light exposure showed the best inhibition of primary breast cancer (Fig. 4L). Post-treatment analysis of lung metastatic nodules in mice revealed that PAMNPs@TPZ + L treatment resulted in the fewest nodules per lung (Fig. 4M). This indicates that the nanosystem not only effectively inhibits breast cancer proliferation but also possesses anti-metastatic properties, providing new design ideas for the treatment of primary breast cancer with nanomedicines.
5. Cancer cell membrane-coated por-nMOFs for combined PDT and TPZ-mediated chemotherapy
Surface modification of nMOFs with polymers139 or cell membranes140–143 enhances the stability of the framework materials in physiological solutions and improves the stability of the nanosystems in the bloodstream. Particularly, nanomaterials coated with cell membranes have become a current research hotspot.142 Studies have shown that encapsulating nanomaterials with cancer cell membranes aids immune escape and homologous targeting, thereby enhancing delivery efficiency to the tumor site.141 Encapsulating porphyrin-based nMOFs with cancer cell membranes and loading them with TPZ chemotherapeutic agents could potentially improve the inhibition of 4T1 orthotopic tumors.126For example, Zhang et al. utilized nMOFs (PCN-224) loaded with porphyrin molecules and TPZ chemotherapy drugs, then coated these with cancer cell membranes to create the TPZ@PCN@Mem nanoplatform (Fig. 5A).126 Owing to the cancer cell membrane coating, results indicated that TPZ@PCN@Mem exhibited efficient immune escape and homologous targeting characteristics. This enabled selective accumulation and prolonged retention in tumor tissues. Under light exposure, PCN-224 mediated the production of toxic ROS for photodynamic therapy. This action further created a hypoxic microenvironment that accelerated TPZ activation, thereby enhancing the chemotherapeutic effect against 4T1 orthotopic tumors (Fig. 5A). TEM results confirmed the spherical morphology of the nMOFs and the core–shell structure, indicating successful encapsulation of the cell membrane (Fig. 5B). The immune evasion and homologous targeting capabilities of cancer cells are primarily attributed to multiple antigens present on their cell membranes. To evaluate the retained membrane proteins on TPZ@PCN@Mem, the authors used western blotting to analyze the protein components and a series of biomarkers. Results showed that the membrane markers present on TPZ@PCN@Mem indicate that membrane protein functionality was selectively preserved (Fig. 5C). PXRD patterns showed similarity between the nanosystem and PCN-224 (Fig. 5D). Additionally, TPZ showed more robust release in acetate buffer solution (ABS, pH 5.5) compared to physiological solutions (Fig. 5E). This release mechanism suggests that the nanosystem releases more easily in the acidic environment of tumors, avoiding certain side effects associated with delivery. DCFH-DA is a probe used for detecting ROS within cells; stronger green fluorescence indicates higher ROS production.144 Flow cytometry analysis showed that the TPZ@PCN@Mem group had the strongest fluorescence (DCF derived from DCFH-DA), likely due to the homologous targeting ability-induced higher cellular uptake (Fig. 5F). This indicates that TPZ@PCN@Mem has significant potential for effective PDT. Notably, the efficient conversion of oxygen to ROS during PDT exacerbates hypoxia in tumor tissues, creating conditions favorable for subsequent bio-reductive therapy.
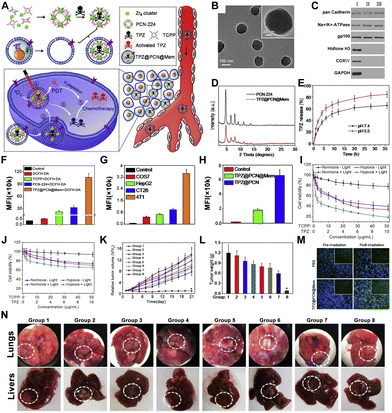 | ||
| Fig. 5 (A) Preparation of TPZ@PCN@Mem for synergistic PDT and TPZ-mediated chemotherapy. (B) TEM images of TPZ@PCN@Mem. (C) Western blot analysis of cell lysate (I), cell membrane (II), and TPZ@PCN@Mem (III) for membrane and intracellular protein markers. (D) PXRD patterns. (E) TPZ release profiles at different pH values. (F) Flow cytometry analysis of ROS generation and mean fluorescence intensity values. (G) Corresponding MFI values of flow cytometry analysis after cellular uptake. (H) Corresponding MFI values after cellular uptake. (I) and (J) Cytotoxicity of TPZ@PCN@Mem against 4T1 and COS7 cells. (K) Relative tumor volume changes. (L) Tumor weights changes. (M) Evaluation of tumor hypoxia after PBS and TPZ@PCN@Mem administrations. (Nuclei: DAPI (blue); hypoxic sites: HIF-1α antibody (green)). (N) Photographs of Lung and liver metastases. Reprinted with permission from ref. 126. Copyright 2017 Elsevier. | ||
To further validate the homologous targeting endocytosis ability conferred by cancer cell membranes, the authors performed endocytosis experiments using four different cell types. The results showed that 4T1 cells internalized the highest amount of TPZ@PCN@Mem (Fig. 5G). Under the same conditions, TPZ@PCN@Mem was also more efficiently internalized than TPZ@PCN (Fig. 5H). These results demonstrate that nMOFs coated with cancer cell membranes exhibit pronounced homologous targeting, promoting internalization by corresponding cancer cells. Given the efficient ROS generation and 4T1 cell-specific targeting capabilities of TPZ@PCN@Mem, the authors conducted MTT cytotoxicity assays.145–149 Results showed that TPZ@PCN@Mem exhibited better suppression of 4T1 cells under both normoxic (21%) and hypoxic (2%) conditions (Fig. 5I and J). Under hypoxic conditions, the IC50 value of the TPZ@PCN@Mem plus light group was lower, indicating enhanced PDT and hypoxia-enhanced TPZ chemotherapy effects.
Subsequently, animal experiments were conducted to assess the therapeutic efficacy of TPZ@PCN@Mem in 4T1 tumor-bearing mice. Results showed that TPZ@PCN@Mem with light exposure (Group 8) displayed the strongest antitumor activity (Fig. 5K and L). These results confirm that TPZ@PCN@Mem, due to the synergistic effect of PCN-224-mediated PDT and hypoxia-activated TPZ chemotherapy, achieves the strongest inhibition of tumor growth. To explore the underlying mechanisms of this synergistic effect, the authors performed hypoxia-inducible factor (HIF-1α) staining. As shown in Fig. 5M, the TPZ@PCN@Mem group with light exposure exhibited stronger green fluorescence signals and higher HIF-1α expression, indicating increased hypoxia levels within the tumor post-PDT. Thus, the superior antitumor effects of TPZ@PCN@Mem arise from the combination of porphyrin-mediated PDT, the hypoxic microenvironment, and PDT-enhanced hypoxia-induced TPZ chemotherapy (Fig. 5M). Animal experimental results also showed that TPZ@PCN@Mem successfully inhibited the metastasis of primary breast cancer to the lungs and liver (Fig. 5N). The novel bionically engineered nanoplatform (TPZ@PCN@Mem) demonstrates excellent cell-specific targeting and immune evasion properties, ultimately conferring PDT and hypoxia-activated chemotherapy effects for inhibiting primary tumor proliferation or metastasis. This multifunctional theranostic nanoplatform exhibits significant advantages and potential clinical applications for the precise diagnosis and treatment of metastatic tumors and hypoxia-related diseases.
6. Cell membrane-coated por-nMOFs for combined PDT, chemotherapy and ferroptosis
Given the challenges in treating breast cancer, current therapeutic approaches are shifting from monotherapy to multimodal combined therapies.150,151 Renowned for their exceptional biocompatibility, nMOFs emerge as promising vectors for advanced drug delivery systems.112 They are especially distinguished for their applications in the targeted treatment of breast cancer.59 The structural diversity of nMOFs allows for the integration of multiple therapeutic modalities.152 For example, coordination of Fe3+ with TCPP and the encapsulation of the chemotherapeutic drug TPZ can enable a combination of porphyrin-mediated PDT, iron-mediated ferroptosis, and TPZ-mediated chemotherapy. This multifunctional combination may offer a new nanomedicine platform for breast cancer treatment.59 For example, Pan et al. developed the PFT nMOFs by combining Fe3+ and TCPP.59 Upon loading with TPZ, the material became PFTT. Further surface modification with cancer cell membranes produced the PFTT@CM nanosystem, enabling a combination therapy of PDT, ferroptosis, and TPZ against breast cancer cells (Fig. 6A). TEM image revealed the fusiform shape characteristic of PFT nMOFs (Fig. 6B), with a clear core–shell structure indicating successful cell membrane encapsulation (Fig. 6C). Particle size increased progressively from PFT to PFTT@CM, as expected (Fig. 6D). Fluorescent labeling of the cell membrane confirmed the formation of the core–shell structure (Fig. 6E). Since ferroptosis relies on iron ion concentration, the researchers measured the release of Fe3+ ions from the nanosystem under acidic conditions. Results demonstrated that the nanosystem released Fe3+ ions efficiently (Fig. 6F), which could interact with intracellular glutathione (GSH) to form Fe2+. The process induced the GSH depletion and thus inducing ferroptosis (Fig. 6G). Moreover, it was shown that Fe3+/Fe2+ ions catalyzed the decomposition of hydrogen peroxide (H2O2) within tumor cells (Fig. 6H), increasing oxygen levels (Fig. 6I) and enhancing PDT efficacy. The ability of PFTT@CM to elevate singlet oxygen concentrations through catalytic decomposition of H2O2 was validated using the singlet oxygen sensor green (SOSG) probe (Fig. 6J). To verify the combination effect, the authors performed the cytotoxicity test. The stronger cytotoxicity of PFTT@CM compared to PFTT was obtained due to the greater accumulation of PFTT@CM in homologous breast cancer cells (Fig. 6K). In vivo animal studies showed that the PFTT@CM treatment group achieved the best inhibition effect (Fig. 6L). This result further corroborated the effectiveness of the combinatorial mechanism. This study provides new insights into the design of porphyrin-based nMOFs for enhanced PDT and chemotherapy.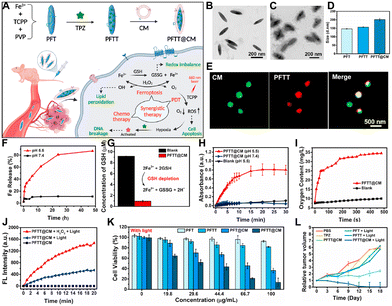 | ||
| Fig. 6 (A) Schematic illustration of PFTT@CM for multimodal synergistic breast cancer therapy. TEM images of (B) PFT and (C) PFTT@CM. (D) Hydrodynamic size distributions of PFT, PFTT, and PFTT@CM. (E) Images of PFTT@CM stained with membrane dye PKH67 (green). (F) Fe3+ release from PFTT@CM under different conditions. (G) GSH depletion mechanism. (H) Continuous H2O2 catalyzing ability of PFTT@CM monitored by TMB assay over 30 min. (I) Time-dependent oxygen generation ability of PFTT@CM in H2O2 solution. (J) SOSG fluorescence changes. (K) Cytotoxicity of different groups. (L) Tumor volume growth curves for different treatment groups. Reprinted with permission from ref. 59. Copyright 2022 Elsevier. | ||
7. UCNPs-supported por-nMOFs for combined PDT, chemotherapy and immunotherapy
PDT relies on three key components: excitation light source, oxygen, and photosensitizer.14 The maximum absorption peak of porphyrins around 650 nm limits the in vivo applications due to the limited skin penetration depth of the excitation wavelength.16 To address this limitation, researchers have employed strategies such as two-photon153–155 or upconversion nanoparticles (UCNPs)156–158 combined with porphyrin photosensitizers. This approach offers an effective alternative, providing new insights into addressing the penetration depth issue of excitation light sources for in vivo porphyrin-based PDT.For instance, Li et al. synthesized lanthanide-doped UCNPs and surface-modified them with citric acid (CA) or polyvinylpyrrolidone (PVP) to control the growth of Zr4+ ions and TCPP on their surfaces, creating a core–shell structure (UCS).159 The chemotherapeutic drug TPZ was then loaded into the pores of the nMOFs to obtain TPZ@UCSs NPs. Under 980 nm laser irradiation, TPZ@UCSs were found to generate singlet oxygen effectively, enabling a combination therapy of porphyrin-mediated PDT and TPZ-mediated chemotherapy. When combined with PD-L1 checkpoint inhibitors, this strategy enabled near-infrared light-activated PDT/ferroptosis/immunotherapy (Fig. 7A). TEM images confirmed the core–shell structure of UCNPs as the core and Zr-TCPP as the shell (Fig. 7B, C). PXRD analysis also indicated the presence of both Zr-TCPP and UCNPs within the UCSs (Fig. 7D). UV-vis spectroscopy showed that the fluorescence emitted by UCNPs under 980 nm laser excitation matched the absorption spectrum of TCPP, suggesting energy resonance transfer between the two (Fig. 7E). A significant decrease in upconversion luminescence (UCL) intensity of UCSs compared to UCNPs alone indicated efficient energy transfer within these heterostructures (Fig. 7F). SOSG probes confirmed that 980 nm laser-irradiated UCNPs effectively activated the porphyrin molecules, generating singlet oxygen, which is crucial for subsequent PDT and activation of TPZ under hypoxic conditions (Fig. 7G). The authors also demonstrated that TPZ release was enhanced under mildly acidic conditions (Fig. 7H).
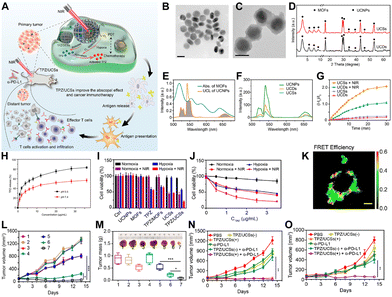 | ||
| Fig. 7 (A) Diagram of TPZ/UCSs structure and its use in tumor therapy combining NIR-light activated PDT, hypoxia-activated chemotherapy, and immunotherapy. (B) TEM images of UCNPs. Scale bar: 50 nm. (C) TEM images of UCSs. Scale bar: 50 nm. (D) Powder XRD patterns for UCSs. (E) UV-vis spectrum of MOFs and UCL spectrum of UCNPs. (F) UCL spectra for UCNPs, UCDs, and UCSs. (G) 1O2 production by UCDs or UCSs under NIR light, measured by SOSG assay. (H) TPZ release profiles from TPZ/UCSs in buffers at varying pH levels over time. (I) Viability of CT26 cells after different treatments. (J) Viability of CT26 cells treated with TPZ/UCSs under various conditions. (K) FRET efficiency of UCSs in CT26 cells, excited at 980 nm. Scale bar: 10 μm. (L) Tumor volume changes in mice following different treatments. (M) Weights and photos of excised tumors on the final day: (1) PBS, (2) PBS(+), (3) TPZ, (4) UCSs(−), (5) TPZ/UCSs(−), (6) UCSs(+), (7) TPZ/UCSs(+). Data: mean ± SD; n = 5. *P < 0.05, **P < 0.001. (N) and (O) Volume changes from (N) primary and (O) distant tumors. Reprinted with permission from ref. 159. Copyright 2020 American Chemical Society. | ||
After confirming the photophysical properties of UCSs, the cytotoxicity of the materials was evaluated (Fig. 7I and J). Results showed that TPZ/UCSs had a stronger inhibitory effect on cell viability under hypoxic conditions compared to normoxic conditions. This is because PDT mediated by porphyrin photosensitizers generates singlet oxygen, leading to a hypoxic cellular environment, which activates TPZ for chemotherapy (Fig. 7I and J). Cell experiments also demonstrated the effective combination therapy of PDT and TPZ under laser irradiation. FRET efficiency within UCSs was assessed at the cellular level using fluorescence lifetime imaging microscopy to quantify the changes in UCL lifetimes of Er3+ emissions in CT26 cells (Fig. 7K).
These results provided direct evidence of effective FRET processes occurring within live cells.
The authors then investigated the near-infrared light-triggered therapeutic effects of TPZ/UCSs in mice bearing CT26 tumors. Results showed that the TPZ/UCSs plus light group had the best tumor suppression effect (Fig. 7L and M). It is well-known that PDT can induce immunogenic cell death (ICD), leading to an antitumor immune response. Based on this, the authors evaluated whether the TPZ/UCSs platform could synergize with PD-L1 checkpoint blockade. They tested the bystander effect in a bilateral CT26 tumor model, where the left tumor (primary tumor) was treated with TPZ/UCSs(+) and the right tumor (distant tumor) remained untreated (Fig. 7N and O). PD-L1 blockade was administered on days 1, 4, 7, and 10 after near-infrared (NIR) light triggering. The results showed complete suppression of the primary tumor in groups treated with TPZ/UCSs(+) or TPZ/UCSs(+) plus α-PD-L1 (Fig. 7N). Importantly, TPZ/UCSs(+) plus PD-L1 blockade effectively inhibited the growth of the untreated distant tumor (Fig. 7O), indicating that TPZ/UCSs(+) treatment enhanced immunotherapy by eliciting a consistent bystander effect. In this system, the authors skillfully controlled the growth of MOFs on carboxylic acid-modified UCNPs to synthesize UCNP@MOFs core–shell structures. The UCNPs absorb tissue-penetrating 980 nm NIR light and transfer energy to the porphyrin within the MOF shell, enabling efficient NIR-triggered singlet oxygen generation. This work provides a novel approach for the biomedical application of porphyrin-based nMOFs in phototherapy.
8. Silk fibroin-capped por-nMOFs for combined PDT, chemotherapy, CDT and immunotherapy
Repeated administration of PD-L1 checkpoint blockade therapy can achieve immunotherapeutic effects, but multiple dosing often complicates the process.160–162 Utilizing nMOFs as carriers for Resiquimod163,164 as an adjuvant or combining them with proteins165 that elicit therapeutic effects may provide a more streamlined nanosystem for combinatorial therapy. For example, Xu et al. utilized Fe3+ coordination with TCPP to create NMOF nanoparticles, which were then surface-modified with silk fibroin (SF) through chemical reactions, resulting in NMOF@SF (NS) nanoparticles.165 This nanoplatform serves as a suitable carrier for TPZ delivery and NS/TPZ (NST) NPs is formed (Fig. 8A). Mechanistic studies confirmed that Fe3+ ions are released and interact with intracellular GSH, reducing the cellular redox balance and generating Fe2+ ions (Fig. 8B). Under acidic conditions, Fe2+ ions react with endogenous H2O2 to produce highly reactive hydroxyl radicals (˙OH), achieving chemodynamic therapy (CDT). Upon 660 nm laser irradiation, NST materials efficiently generate singlet oxygen for PDT (Fig. 8C). Moreover, nMOFs release TPZ under the weakly acidic conditions within tumor cells. In the hypoxic microenvironment of tumors, exacerbated by PDT-induced hypoxia, TPZ is activated through reductive products and exerts its chemotherapeutic effects (Fig. 8D). Importantly, SF has been shown to effectively activate the antitumor immune system by polarizing tumor-associated macrophages (TAMs) into the M1 phenotype. Engineering SF into multifunctional nanocomposites, especially in conjunction with porphyrin-based nMOFs, enhances the immunotherapeutic efficacy. Surface modification with SF successfully introduces immunotherapeutic effects, ultimately achieving an integrated combinatorial therapy of PDT, TPZ chemotherapy, CDT, and immunotherapy (Fig. 8E). The design of this system highlights the significant potential of porphyrin-based nMOFs in biomedical applications. The structural diversity and modifiable nature of nMOFs enable the integration of various therapeutic modalities in a simple manner, achieving an all-in-one therapeutic effect.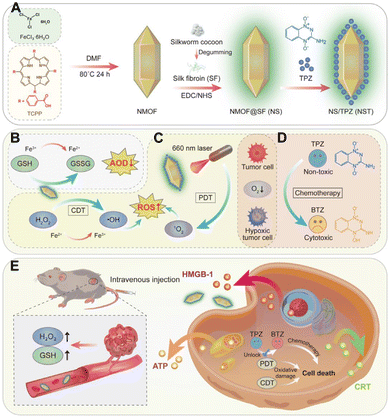 | ||
| Fig. 8 (A) Schematic of the NST NP synthesis process. (B)–(E) Mechanism of NST NPs inducing tumor-specific redox imbalance, enhanced by deoxygenation-driven chemotherapy and accompanied by immune response stimulation. Reprinted with permission from ref. 165. Copyright 2022 Elsevier. | ||
9. HA-coated por-nMOFs for combined PDT and TPZ chemotherapy of rheumatoid arthritis
It is well established that polarizing TAMs to the M1 phenotype can effectively activate antitumor immunity, making it a common strategy for enhancing immunotherapy.166 However, the inflammatory cytokines produced by activated M1 macrophages exacerbate the progression of rheumatoid arthritis (RA).167 As a result, eliminating M1 macrophages using nanomedicines has become a critical target for inhibiting the progression of RA.167 Porphyrin-based light-responsive materials, such as those with nMOFs, can load TPZ molecules within their pores.138 Upon exposure to light, these nanosystems can generate highly reactive singlet oxygen and create a hypoxic microenvironment, activating TPZ and thereby facilitating its chemotherapeutic effects. These dual mechanisms complement each other, inhibiting cancer cell proliferation or metastasis.Building on this concept, researchers hypothesized that these light-responsive materials might also be effective against M1 macrophages, which could help alleviate RA. Liu et al. developed PCN-224, a crystalline nMOFs formed by coordinating TCPP with Zr4+ ions.167 They loaded TPZ into the porous structure of the nMOFs and modified the surface with hyaluronic acid (HA) to create the TPNPs-HA nanosystem (Fig. 9A). Under light exposure, this nanosystem generates singlet oxygen and creates a hypoxic environment, activating the TPZ molecules. The synergistic effect of photodynamic therapy and TPZ-mediated chemotherapy significantly reduces the number of activated M1 macrophages, demonstrating the effectiveness of the nanosystem in suppressing the progression of RA (Fig. 9B). This innovative application broadens the scope of biological applications for porphyrin-based nMOFs loaded with TPZ chemotherapeutic agents. It underscores the remarkable potential of this photosensitive nanodelivery system in providing substantial relief from RA. Furthermore, these findings open up new possibilities for developing precise and accurate strategies for treating RA.
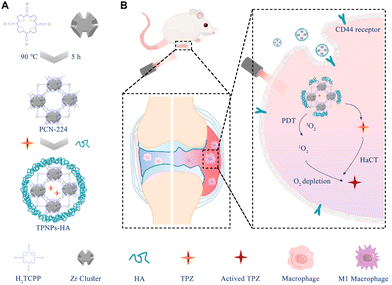 | ||
| Fig. 9 (A) Schematic of the TPNPs-HA synthesis steps. (B) Mechanism of targeting activated macrophages for synergistic rheumatoid arthritis (RA) inhibition via PDT and hypoxia-activated chemotherapy. Reprinted with permission from ref. 167. Copyright 2024 Elsevier. | ||
10. Summary and outlook
In recent years, porphyrin-based nMOFs have seen widespread application in phototherapies.27 However, the combination of porphyrin-based nMOFs with hypoxia-sensitive prodrugs for combined therapy remains relatively unexplored. Through a comprehensive review of existing work, we find that the limitations of porphyrin-based nMOFs-mediated PDT due to the hypoxic microenvironment can be overcome by combining them with hypoxia-sensitive prodrugs like TPZ.104 Even under hypoxic conditions, these combinations can effectively inhibit cancer cell proliferation or metastasis.46 Moreover, the porous structure of nMOFs enables the loading of relatively high proportions of TPZ molecules, which can be efficiently released in the high phosphate concentration (10 mM) typical of cancer cells, with release rates exceeding 60%.137 This sets the stage for combined PDT and TPZ-mediated chemotherapy. Additionally, the facile surface modification of nMOFs with polymers like PEG,127 albumin,137 or cancer cell membranes126 enhances their dispersion stability in aqueous solutions, promotes circulation stability, and facilitates accumulation at disease sites, laying the groundwork for targeted PDT and TPZ chemotherapy.However, the preclinical translation of porphyrin-based nMOFs loaded with TPZ for combined PDT and chemotherapy faces significant challenges.117 Collaborative efforts across multiple disciplines are needed to fully understand the structure–activity relationships of nMOFs in biological systems, as well as their absorption, distribution, metabolism, and excretion following intravenous or intraperitoneal administration.168 Extensive pharmacological and toxicological studies are required to elucidate the mechanisms of action of nMOFs. Other issues warrant attention, including: (1) investigating the use of other activation methods, such as sonodynamic therapy169 or X-rays,170 to generate ROS from porphyrins could facilitate deeper tumor penetration and enhance the synergistic effect of ROS generation and TPZ chemotherapy. (2) Developing gentler synthesis strategies for nMOFs and controlling their size and shape would facilitate large-scale production and potential clinical applications. (3) While effective for localized tumors, the treatment of metastatic tumors requires further investigation, including the exploration of combined therapies with immunotherapy to provide effective treatment options for metastatic cancers. (4) Transitioning from small to large animal models, such as pigs or monkeys, can more accurately assess the pharmacokinetics and toxicology of nMOFs in vivo.171 (5) Integrating diagnostic imaging capabilities, such as computed tomography imaging or magnetic resonance imaging with porphyrin-coordinated metals, would enable real-time monitoring of nMOFs distribution and inform optimal timing for light exposure.172 (6) Exploring alternatives to porphyrin molecules, such as chlorins or bacteriochlorins, which possess enhanced photochemical properties, could reduce the required dosages and minimize adverse effects.173 Addressing these challenges will pave the way for the successful clinical translation of porphyrin-based nMOFs for combined PDT and hypoxia-activated chemotherapy, offering promising therapeutic strategies for cancer treatment.
Abbreviations
| PDT | Photodynamic therapy |
| HAPs | Hypoxia-activated prodrugs |
| TPZ | Tirapazamin |
| PTT | Photothermal therapy |
| nMOFs | Nanoscale metal–organic frameworks |
| Por-nMOFs | Porphyrin-based nanoscale metal–organic frameworks |
| IARC | International Agency for Research on Cancer |
| ROS | Reactive oxygen species |
| PEG | Polyethylene glycol |
| TCPP | meso-tetra(4-Carboxyphenyl)porphine |
| TEM | Transmission electron microscopy |
| PXRD | Powder X-ray diffraction |
| DPBF | 1,3-Diphenylisobenzofuran |
| HSA | Human serum albumin |
| GSH | Glutathione |
| CDT | Chemodynamic therapy |
| SOSG | Singlet oxygen sensor green |
| PVP | Polyvinylpyrrolidone |
| CA | Citric acid |
| UCNPs | Upconversion nanoparticles |
| TAMs | Tumor-associated macrophages |
| RA | Rheumatoid arthritis |
| HA | Hyaluronic acid |
Author contributions
QZ: writing – original draft, writing – review and editing. XW: writing – original draft. JC: writing – original draft, writing – review and editing. JW: writing – original draft, writing – review and editing. MZ: writing – original draft. RX: writing – original draft. WW: writing – review and editing. XZ: funding acquisition, writing – original draft, writing – review and editing. ZX: writing – review and editing.Data availability
Not applicable to the current paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52103169).Notes and references
- (a) F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263 CrossRef PubMed; (b) F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 74, 229–263 CrossRef PubMed.
- K. Shitara, J. A. Ajani, M. Moehler, M. Garrido, C. Gallardo, L. Shen, K. Yamaguchi, L. Wyrwicz, T. Skoczylas, A. C. Bragagnoli, T. Liu, M. Tehfe, E. Elimova, R. Bruges, T. Zander, S. de Azevedo, R. Kowalyszyn, R. Pazo-Cid, M. Schenker, J. M. Cleary, P. Yanez, K. Feeney, M. V. Karamouzis, V. Poulart, M. Lei, H. Xiao, K. Kondo, M. Li and Y. Y. Janjigian, Nature, 2022, 603, 942–948 CrossRef PubMed.
- N. M. Kuderer, A. Desai, M. B. Lustberg and G. H. Lyman, Nat. Rev. Clin. Oncol., 2022, 19, 681–697 CrossRef PubMed.
- D. Kotani, E. Oki, Y. Nakamura, H. Yukami, S. Mishima, H. Bando, H. Shirasu, K. Yamazaki, J. Watanabe, M. Kotaka, K. Hirata, N. Akazawa, K. Kataoka, S. Sharma, V. N. Aushev, A. Aleshin, T. Misumi, H. Taniguchi, I. Takemasa, T. Kato, M. Mori and T. Yoshino, Nat. Med., 2023, 29, 127–134 CrossRef PubMed.
- U. Anand, A. Dey, A. K. S. Chandel, R. Sanyal, A. Mishra, D. K. Pandey, V. De Falco, A. Upadhyay, R. Kandimalla, A. Chaudhary, J. K. Dhanjal, S. Dewanjee, J. Vallamkondu and J. M. Pérez de la Lastra, Genes Dis., 2023, 10, 1367–1401 CrossRef PubMed.
- P. J. Keall, C. Brighi, C. Glide-Hurst, G. Liney, P. Z. Y. Liu, S. Lydiard, C. Paganelli, T. Pham, S. Shan, A. C. Tree, U. A. van der Heide, D. E. J. Waddington and B. Whelan, Nat. Rev. Clin. Oncol., 2022, 19, 458–470 CrossRef PubMed.
- G. Petroni, L. C. Cantley, L. Santambrogio, S. C. Formenti and L. Galluzzi, Nat. Rev. Clin. Oncol., 2022, 19, 114–131 CrossRef CAS PubMed.
- M.-C. Vozenin, J. Bourhis and M. Durante, Nat. Rev. Clin. Oncol., 2022, 19, 791–803 CrossRef PubMed.
- J. M. Price, A. Prabhakaran and C. M. L. West, Nat. Rev. Clin. Oncol., 2023, 20, 83–98 CrossRef CAS PubMed.
- L. Kraehenbuehl, C.-H. Weng, S. Eghbali, J. D. Wolchok and T. Merghoub, Nat. Rev. Clin. Oncol., 2022, 19, 37–50 CrossRef CAS PubMed.
- G. Oliveira and C. J. Wu, Nat. Rev. Cancer, 2023, 23, 295–316 CrossRef CAS PubMed.
- S. R. Durham and M. H. Shamji, Nat. Rev. Immunol., 2023, 23, 317–328 CrossRef CAS PubMed.
- L. H. Butterfield and Y. G. Najjar, Nat. Rev. Immunol., 2024, 24, 399–416 CrossRef PubMed.
- J. Karges, Angew. Chem., Int. Ed., 2022, 61, e202112236 CrossRef PubMed.
- M. Li, Y. Xu, X. Peng and J. S. Kim, Acc. Chem. Res., 2022, 55, 3253–3264 CrossRef PubMed.
- B. Sun, J. N. Bte Rahmat and Y. Zhang, Biomaterials, 2022, 291, 121875 CrossRef PubMed.
- J. An, S. Tang, G. Hong, W. Chen, M. Chen, J. Song, Z. Li, X. Peng, F. Song and W.-H. Zheng, Nat. Commun., 2022, 13, 2225 CrossRef PubMed.
- L. He, X. Yu and W. Li, ACS Nano, 2022, 16, 19691–19721 CrossRef PubMed.
- D. Lee, S. Kwon, S.-Y. Jang, E. Park, Y. Lee and H. Koo, Bioact. Mater., 2022, 8, 20–34 Search PubMed.
- M. Li, Y. Xu, Z. Pu, T. Xiong, H. Huang, S. Long, S. Son, L. Yu, N. Singh, Y. Tong, J. L. Sessler, X. Peng and J. S. Kim, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2210504119 CrossRef CAS PubMed.
- S. Moghassemi, A. Dadashzadeh, R. B. Azevedo and C. A. Amorim, J. Controlled Release, 2022, 351, 164–173 CrossRef PubMed.
- J. Wang, Q. Gong, L. Jiao and E. Hao, Coord. Chem. Rev., 2023, 496, 215367 CrossRef.
- Y. Wu, S. Li, Y. Chen, W. He and Z. Guo, Chem. Sci., 2022, 13, 5085–5106 RSC.
- J. Tian, B. Li, F. Zhang, Z. Yao, W. Song, Y. Tang, Y. Ping and B. Liu, Angew. Chem., Int. Ed., 2023, 62, e202307288 CrossRef PubMed.
- M. Ethirajan, Y. Chen, P. Joshi and R. K. Pandey, Chem. Soc. Rev., 2011, 40, 340–362 RSC.
- X. Liu, W. Zhan, G. Gao, Q. Jiang, X. Zhang, H. Zhang, X. Sun, W. Han, F.-G. Wu and G. Liang, J. Am. Chem. Soc., 2023, 145, 7918–7930 CrossRef PubMed.
- N. Rabiee, M. T. Yaraki, S. M. Garakani, S. M. Garakani, S. Ahmadi, A. Lajevardi, M. Bagherzadeh, M. Rabiee, L. Tayebi, M. Tahriri and M. R. Hamblin, Biomaterials, 2020, 232, 119707 CrossRef PubMed.
- J. Tian, B. Huang, M. H. Nawaz and W. Zhang, Coord. Chem. Rev., 2020, 420, 213410 CrossRef.
- Z. Wang, Q. Sun, B. Liu, Y. Kuang, A. Gulzar, F. He, S. Gai, P. Yang and J. Lin, Coord. Chem. Rev., 2021, 439, 213945 CrossRef.
- D. Xu, Q. Duan, H. Yu and W. Dong, J. Mater. Chem. B, 2023, 11, 5976–5989 RSC.
- F. Yang, M. Xu, X. Chen and Y. Luo, Biomed. Pharmacother., 2023, 164, 114933 CrossRef PubMed.
- J. Dang, H. He, D. Chen and L. Yin, Biomater. Sci., 2017, 5, 1500–1511 RSC.
- H. S. Jung, J. Han, H. Shi, S. Koo, H. Singh, H.-J. Kim, J. L. Sessler, J. Y. Lee, J.-H. Kim and J. S. Kim, J. Am. Chem. Soc., 2017, 139, 7595–7602 CrossRef PubMed.
- X. Li, N. Kwon, T. Guo, Z. Liu and J. Yoon, Angew. Chem., Int. Ed., 2018, 57, 11522–11531 CrossRef PubMed.
- W.-L. Liu, T. Liu, M.-Z. Zou, W.-Y. Yu, C.-X. Li, Z.-Y. He, M.-K. Zhang, M.-D. Liu, Z.-H. Li, J. Feng and X.-Z. Zhang, Adv. Mater., 2018, 30, 1802006 CrossRef PubMed.
- L. Huang, S. Zhao, J. Wu, L. Yu, N. Singh, K. Yang, M. Lan, P. Wang and J. S. Kim, Coord. Chem. Rev., 2021, 438, 213888 CrossRef.
- J. Du, T. Shi, S. Long, P. Chen, W. Sun, J. Fan and X. Peng, Coord. Chem. Rev., 2021, 427, 213604 CrossRef.
- M. Dirak, C. M. Yenici and S. Kolemen, Coord. Chem. Rev., 2024, 506, 215710 CrossRef.
- Y. Liu, Y. Liu, W. Bu, C. Cheng, C. Zuo, Q. Xiao, Y. Sun, D. Ni, C. Zhang, J. Liu and J. Shi, Angew. Chem., Int. Ed., 2015, 54, 8105–8109 CrossRef PubMed.
- W. Lv, Z. Zhang, K. Y. Zhang, H. Yang, S. Liu, A. Xu, S. Guo, Q. Zhao and W. Huang, Angew. Chem., Int. Ed., 2016, 55, 9947–9951 CrossRef PubMed.
- W. Zhu, Z. Dong, T. Fu, J. Liu, Q. Chen, Y. Li, R. Zhu, L. Xu and Z. Liu, Adv. Funct. Mater., 2016, 26, 5490–5498 CrossRef.
- W. Piao, K. Hanaoka, T. Fujisawa, S. Takeuchi, T. Komatsu, T. Ueno, T. Terai, T. Tahara, T. Nagano and Y. Urano, J. Am. Chem. Soc., 2017, 139, 13713–13719 CrossRef PubMed.
- Y. Sun, D. Zhao, G. Wang, Y. Wang, L. Cao, J. Sun, Q. Jiang and Z. He, Acta Pharm. Sin. B, 2020, 10, 1382–1396 CrossRef PubMed.
- C. Zhang, W.-J. Qin, X.-F. Bai and X.-Z. Zhang, Nano Today, 2020, 35, 100960 CrossRef.
- Y. Wan, L.-H. Fu, C. Li, J. Lin and P. Huang, Adv. Mater., 2021, 33, 2103978 CrossRef PubMed.
- C. Zhang, X. Hu, L. Jin, L. Lin, H. Lin, Z. Yang and W. Huang, Adv. Healthcare Mater., 2023, 12, 2300530 CrossRef PubMed.
- Y. Cheng, H. Cheng, C. Jiang, X. Qiu, K. Wang, W. Huan, A. Yuan, J. Wu and Y. Hu, Nat. Commun., 2015, 6, 8785 CrossRef PubMed.
- H. Ren, J. Liu, F. Su, S. Ge, A. Yuan, W. Dai, J. Wu and Y. Hu, ACS Appl. Mater. Interfaces, 2017, 9, 3463–3473 CrossRef PubMed.
- D. Hu, L. Zhong, M. Wang, H. Li, Y. Qu, Q. Liu, R. Han, L. Yuan, K. Shi, J. Peng and Z. Qian, Adv. Funct. Mater., 2019, 29, 1806199 CrossRef.
- H. Hu, X. Yan, H. Wang, J. Tanaka, M. Wang, W. You and Z. Li, J. Mater. Chem. B, 2019, 7, 1116–1123 RSC.
- M. Wu, C. Chen, Z. Liu, J. Tian and W. Zhang, Acta Biomater., 2022, 142, 242–252 CrossRef PubMed.
- Z. Yang, D. Tao, W. Zhong, Z. Liu, L. Feng and M. Chen, Biomaterials, 2022, 280, 121250 CrossRef PubMed.
- K. Wei, Y. Wu, X. Zheng, G. Ma, C. Ji and M. Yin, Adv. Funct. Mater., 2023, 33, 2305187 CrossRef.
- H. Ren, M. Hao, G. Liu, J. Li, Z. Jiang, W. Meng and Y. Zhang, ACS Appl. Bio Mater., 2024, 7, 3306–3315 CrossRef PubMed.
- L. Bai, E. Shi, Y. Li, M. Yang, C. Li, C. Li, Y. Wang and Y. Wang, ACS Biomater. Sci. Eng., 2023, 9, 485–497 CrossRef PubMed.
- H. S. Lee, S.-Y. Yoo, S. M. Lee, N.-W. Kang, S. K. Kim, G. Y. Song, D.-D. Kim and J.-Y. Lee, Chem. Eng. J., 2023, 457, 141224 CrossRef.
- Y. Bai, M. Liu, X. Wang, K. Liu, X. Liu and X. Duan, ACS Appl. Mater. Interfaces, 2023, 15, 55379–55391 CrossRef PubMed.
- F. Liu, T. He, S. Gong, M. Shen, S. Ma, X. Huang, L. Li, L. Wang, Q. Wu and C. Gong, Acta Biomater., 2022, 154, 510–522 CrossRef PubMed.
- W.-L. Pan, Y. Tan, W. Meng, N.-H. Huang, Y.-B. Zhao, Z.-Q. Yu, Z. Huang, W.-H. Zhang, B. Sun and J.-X. Chen, Biomaterials, 2022, 283, 121449 CrossRef PubMed.
- J. Qi, G. Jiang, Y. Wan, J. Liu and F. Pi, Chem. Eng. J., 2023, 466, 142960 CrossRef.
- B. Hao, J. Wang, C. Wang, K. Xue, M. Xiao, S. Lv and C. Zhu, Chem. Sci., 2022, 13, 4139–4149 RSC.
- B. Lu, Z. Zhang, Y. Huang, Y. Zhang, J. Wang, Y. Ding, Y. Wang and Y. Yao, Chem. Commun., 2022, 58, 10353–10356 RSC.
- D. Zhu, T. Zhang, Y. Li, C. Huang, M. Suo, L. Xia, Y. Xu, G. Li and B. Z. Tang, Biomaterials, 2022, 283, 121462 CrossRef PubMed.
- W. Chen, Z. Wang, M. Tian, G. Hong, Y. Wu, M. Sui, M. Chen, J. An, F. Song and X. Peng, J. Am. Chem. Soc., 2023, 145, 8130–8140 CrossRef PubMed.
- L. Liu, C. Li, J. Gong, Y. Zhang, W. Ji, L. Feng, G. Jiang, J. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2023, 62, e202307776 CrossRef PubMed.
- B. Lu, L. Wang, H. Tang and D. Cao, J. Mater. Chem. B, 2023, 11, 4600–4618 RSC.
- H. Wang, T. Qin, W. Wang, X. Zhou, F. Lin, G. Liang, Z. Yang, Z. Chi and B. Z. Tang, Adv. Sci., 2023, 10, 2301902 CrossRef PubMed.
- Y. Zhang, Q. Jia, F. Nan, J. Wang, K. Liang, J. Li, X. Xue, H. Ren, W. Liu, J. Ge and P. Wang, Biomaterials, 2023, 293, 121953 CrossRef PubMed.
- J. Zhuang, B. Wang, H. Chen, K. Zhang, N. Li, N. Zhao and B. Z. Tang, ACS Nano, 2023, 17, 9110–9125 CrossRef PubMed.
- J.-Q. Huang, L.-P. Zhao, X. Zhou, L.-S. Liu, R.-R. Zheng, F.-A. Deng, Y.-B. Liu, X.-Y. Yu, S.-Y. Li and H. Cheng, Small, 2022, 18, 2107467 CrossRef PubMed.
- G. Luo, Z. Li, M. Chen, J. Zheng, X. Deng, G. Xu, M. Cheng, X. Li and Y. Duo, Chem. Eng. J., 2022, 442, 136169 CrossRef.
- J. Wang, H. Wu, Q. Zhao, Y. Zou, D. Ding, H. Yin and H. Xu, ACS Appl. Mater. Interfaces, 2022, 14, 29613–29625 CrossRef PubMed.
- H. Zhang, X. Yan, Y. Zhang, C. Bao and C. Li, J. Mater. Chem. B, 2022, 10, 4623–4631 RSC.
- R. Zhou, X. Zeng, H. Zhao, Q. Chen and P. Wu, Coord. Chem. Rev., 2022, 452, 214306 CrossRef.
- Y. Cen, X. Chen, Y. Liu, B. Yu, M. Yan, N. Yang, R. Kong, S. Li, H. Ti and H. Cheng, J. Controlled Release, 2023, 358, 654–666 CrossRef PubMed.
- M. Tian, W. Chen, G. Hong, M. Chen, F. Song, W.-H. Zheng and X. Peng, ACS Mater. Lett., 2023, 5, 1922–1928 CrossRef.
- J. Yu, Q. Li, Z. Wei, G. Fan, F. Wan and L. Tian, Acta Biomater., 2023, 170, 330–343 CrossRef PubMed.
- G. Lin, L. Tillman, T. Luo, X. Jiang, Y. Fan, G. Liu and W. Lin, Angew. Chem., Int. Ed., 2024, e202410241 Search PubMed.
- H. Hu, H. Wang, Y. Yang, J.-F. Xu and X. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202200799 CrossRef CAS PubMed.
- X. Hu, J. Li, Y. Chen, Q. Long, Y. Bai, R. Li, K. Wang, M. Jiang, C. Chen, J. Mao, Y. Zheng and Z. Gao, ACS Biomater. Sci. Eng., 2022, 8, 4535–4546 CrossRef CAS PubMed.
- X. Sun, Y. Xu, Q. Guo, N. Wang, B. Wu, C. Zhu, W. Zhao, W. Qiang and M. Zheng, Langmuir, 2022, 38, 1360–1367 CrossRef CAS PubMed.
- G. Xu, C. Li, C. Chi, L. Wu, Y. Sun, J. Zhao, X.-H. Xia and S. Gou, Nat. Commun., 2022, 13, 3064 CrossRef PubMed.
- J. Yue, P. Miao, L. Li, R. Yan, W.-F. Dong and Q. Mei, ACS Appl. Mater. Interfaces, 2022, 14, 49582–49591 CrossRef PubMed.
- H. Luo and S. Gao, J. Controlled Release, 2023, 362, 425–445 CrossRef PubMed.
- M. Overchuk, R. A. Weersink, B. C. Wilson and G. Zheng, ACS Nano, 2023, 17, 7979–8003 CrossRef PubMed.
- X. Li, X. Li, S. Park, S. Wu, Y. Guo, K. T. Nam, N. Kwon, J. Yoon and Q. Hu, Coord. Chem. Rev., 2024, 520, 216142 CrossRef.
- F. Wei, J. Karges, J. Shen, L. Xie, K. Xiong, X. Zhang, L. Ji and H. Chao, Nano Today, 2022, 44, 101509 CrossRef.
- J. Zheng, J. Du, H. Ge, N. Xu, Q. Yao, S. Long, J. Fan and X. Peng, Chem. Eng. J., 2022, 449, 136565 CrossRef.
- P. Zou, R. Lin, Z. Fang, J. Chen, H. Guan, J. Yin, Z. Chang, L. Xing, J. Lang, X. Xue and M. Chen, Adv. Sci., 2023, 10, 2302731 CrossRef PubMed.
- J. Chen, Z. Duan, L. Deng, L. Li, Q. Li, J. Qu, X. Li and R. Liu, Adv. Healthcare Mater., 2024, 13, 2304436 CrossRef CAS PubMed.
- H. Huang, W. Xie, D. Hu, X. He, R. Li, X. Zhang and Y. Wei, Chem. Eng. J., 2023, 451, 138617 CrossRef CAS.
- X. Liu, J. Zhang, X. Guo, J. Huang, Z. Lou, X. Zhao, Q. Lin, X. Li, J. You and L. Luo, J. Controlled Release, 2023, 364, 343–356 CrossRef CAS.
- C. Qu, H. Yuan, M. Tian, X. Zhang, P. Xia, G. Shi, R. Hou, J. Li, H. Jiang, Z. Yang, T. Chen, Z. Li, J. Wang and Y. Yuan, ACS Nano, 2024, 18, 4019–4037 CrossRef CAS PubMed.
- H. Song, Z. Cai, J. Li, H. Xiao, R. Qi and M. Zheng, J. Nanobiotechnol., 2022, 20, 329 CrossRef PubMed.
- M. Warszyńska, P. Repetowski and J. M. Dąbrowski, Coord. Chem. Rev., 2023, 495, 215350 CrossRef.
- S. Zeng, C. Chen, L. Zhang, X. Liu, M. Qian, H. Cui, J. Wang, Q. Chen and X. Peng, Bioact. Mater., 2023, 25, 580–593 Search PubMed.
- H. Zhou, D. Tang, Y. Yu, L. Zhang, B. Wang, J. Karges and H. Xiao, Nat. Commun., 2023, 14, 5350 CrossRef PubMed.
- H. He, L. Du, H. Xue, J. Wu and X. Shuai, Acta Biomater., 2022, 149, 297–306 CrossRef PubMed.
- M. Jiang, Y. Liu, Y. Dong, K. Wang and Y. Yuan, Biomaterials, 2022, 284, 121480 CrossRef PubMed.
- H.-H. Han, H.-M. Wang, P. Jangili, M. Li, L. Wu, Y. Zang, A. C. Sedgwick, J. Li, X.-P. He, T. D. James and J. S. Kim, Chem. Soc. Rev., 2023, 52, 879–920 RSC.
- M. Wang, M. He, M. Zhang, S. Xue, T. Xu, Y. Zhao, D. Li, F. Zhi and D. Ding, Biomaterials, 2023, 301, 122257 CrossRef CAS PubMed.
- J. Xiong, X. Wang, J. Kim, J. Gong, Z. Mao, J. S. Kim and Z. Liu, Adv. Funct. Mater., 2024, 34, 2312590 CrossRef CAS.
- J. Yuan, Q.-H. Zhou, S. Xu, Q.-P. Zuo, W. Li, X.-X. Zhang, T.-B. Ren, L. Yuan and X.-B. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202206169 CrossRef CAS PubMed.
- H. Zhang, C. Shi, F. Han, M. Li, H. Ma, R. Sui, S. Long, W. Sun, J. Du, J. Fan, H. Piao and X. Peng, Biomaterials, 2022, 289, 121770 CrossRef CAS PubMed.
- B. Zhang, R. Xue, J. Lyu, A. Gao and C. Sun, J. Mater. Chem. B, 2022, 10, 3849–3860 RSC.
- M. Sun, H. Jiang, T. Liu, X. Tan, Q. Jiang, B. Sun, Y. Zheng, G. Wang, Y. Wang, M. Cheng, Z. He and J. Sun, Acta Pharm. Sin. B, 2022, 12, 952–966 CrossRef CAS PubMed.
- X.-X. Xu, S.-Y. Chen, N.-B. Yi, X. Li, S.-L. Chen, Z. Lei, D.-B. Cheng and T. Sun, J. Controlled Release, 2022, 350, 829–840 CrossRef CAS PubMed.
- J. Lv, S. Wang, D. Qiao, Y. Lin, S. Hu and M. Li, J. Nanobiotechnol., 2022, 20, 42 CrossRef CAS PubMed.
- Q.-Y. Xu, Z. Tan, X.-W. Liao and C. Wang, Chin. Chem. Lett., 2022, 33, 22–32 CrossRef CAS.
- W.-C. Hu, X.-P. Zhao, J. Wang, C. Wang and X.-H. Xia, TrAC, Trends Anal. Chem., 2024, 178, 117857 CrossRef CAS.
- Y. Liu, P. Lei, X. Liao and C. Wang, J. Nanostruct. Chem., 2024, 14, 1–19 CrossRef CAS.
- X. Ge, R. Wong, A. Anisa and S. Ma, Biomaterials, 2022, 281, 121322 CrossRef CAS PubMed.
- J. Jiao, J. He, M. Li, J. Yang, H. Yang, X. Wang and S. Yang, Nanoscale, 2022, 14, 6373–6383 RSC.
- Q. Xiang, W. Li, Y. Tan, J. Shi, M. Dong, J. Cheng, J. Huang, W. Zhang, Y. Gong, Q. Yang, L. Yang, H. Dong and X. Zhang, Chem. Eng. J., 2022, 444, 136706 CrossRef CAS.
- H. Zhang and X.-B. Yin, ACS Appl. Mater. Interfaces, 2022, 14, 26528–26535 CrossRef PubMed.
- M. Liu, L. Wang, X. Zheng and Z. Xie, ACS Appl. Mater. Interfaces, 2017, 9, 41512–41520 CrossRef CAS PubMed.
- L. Wang, M. Zheng and Z. Xie, J. Mater. Chem. B, 2018, 6, 707–717 RSC.
- X. Zheng, L. Wang, M. Liu, P. Lei, F. Liu and Z. Xie, Chem. Mater., 2018, 30, 6867–6876 CrossRef CAS.
- S.-Z. Ren, X.-H. Zhu, B. Wang, M. Liu, S.-K. Li, Y.-S. Yang, H. An and H.-L. Zhu, J. Mater. Chem. B, 2021, 9, 4678–4689 RSC.
- J. Zhou, Y. Li, L. Wang and Z. Xie, J. Mater. Chem. B, 2021, 9, 7760–7770 RSC.
- Y. Li, J. Zhou, Y. Chen, Q. Pei, Y. Li, L. Wang and Z. Xie, Chem. Eng. J., 2022, 437, 135370 CrossRef CAS.
- X. Li, Z. Ma, H. Wang, Q. Shi, Z. Xie and J. Yu, Coord. Chem. Rev., 2024, 514, 215943 CrossRef CAS.
- T. Luo, Y. Fan, J. Mao, X. Jiang, L. Albano, E. Yuan, T. Germanas and W. Lin, Angew. Chem., Int. Ed., 2023, 62, e202301910 CrossRef PubMed.
- Y. Yu, B.-R. Xie, X.-H. Liu, J.-J. Ye, Z. Zhong and X.-Z. Zhang, ACS Nano, 2023, 17, 12471–12482 CrossRef PubMed.
- J. Zhang, L. Chang, R. Hao, G. Zhang, T. Liu, Z. Li, T. Wang and L. Zeng, Chem. Eng. J., 2023, 474, 145485 CrossRef.
- S.-Y. Li, H. Cheng, W.-X. Qiu, L. Zhang, S.-S. Wan, J.-Y. Zeng and X.-Z. Zhang, Biomaterials, 2017, 142, 149–161 CrossRef PubMed.
- M. Liu, L. Wang, X. Zheng, S. Liu and Z. Xie, ACS Appl. Mater. Interfaces, 2018, 10, 24638–24647 CrossRef PubMed.
- G. Su, D. Miao, Y. Yu, M. Zhou, P. Jiao, X. Cao, B. Yan and H. Zhu, J. Drug Targeting, 2019, 27, 201–210 CrossRef PubMed.
- Y. Yu, M. Zhou, W. Zhang, L. Huang, D. Miao, H. Zhu and G. Su, Mol. Pharmaceutics, 2019, 16, 1929–1938 CrossRef PubMed.
- Y. Zhao, G. Chen, Z. Meng, G. Gong, W. Zhao, K. Wang and T. Liu, Drug Delivery, 2019, 26, 717–723 CrossRef PubMed.
- J.-N. Liu, W. Bu and J. Shi, Chem. Rev., 2017, 117, 6160–6224 CrossRef CAS PubMed.
- R. Kumari, D. Sunil and R. S. Ningthoujam, J. Controlled Release, 2020, 319, 135–156 CrossRef CAS PubMed.
- S. Zhou, X. Hu, R. Xia, S. Liu, Q. Pei, G. Chen, Z. Xie and X. Jing, Angew. Chem., Int. Ed., 2020, 59, 23198–23205 CrossRef CAS PubMed.
- F. Zhuang, Q. Ma, C. Dong, H. Xiang, Y. Shen, P. Sun, C. Li, Y. Chen, B. Lu, Y. Chen and B. Huang, ACS Nano, 2022, 16, 5439–5453 CrossRef CAS PubMed.
- Y. Pan, L. Liu, X. Mou and Y. Cai, ACS Nano, 2023, 17, 20875–20924 CrossRef PubMed.
- J. George Joy, G. Sharma and J.-C. Kim, Chem. Eng. J., 2024, 496, 153978 CrossRef CAS.
- G. Yang, A. K. Bindra, S. Z. F. Phua, J. Liu, H. Wu, D. Wang, C. Qian, G. Liu and Y. Zhao, Adv. Opt. Mater., 2023, 11, 2201043 CrossRef CAS.
- Z. Jia, Y. Gao, J. Ni, X. Wu, Z. Mao, G. Sheng and Y. Zhu, J. Colloid Interface Sci., 2023, 629, 379–390 CrossRef CAS PubMed.
- D.-Y. Fu, X. Liu, X. Zheng, M. Zhou, W. Wang, G. Su, T. Liu, L. Wang and Z. Xie, Coord. Chem. Rev., 2022, 456, 214393 CrossRef CAS.
- D. Zhang, Z. Ye, L. Wei, H. Luo and L. Xiao, ACS Appl. Mater. Interfaces, 2019, 11, 39594–39602 CrossRef CAS PubMed.
- J. Zhuang, Y. Duan, Q. Zhang, W. Gao, S. Li, R. H. Fang and L. Zhang, Nano Lett., 2020, 20, 4051–4058 CrossRef CAS PubMed.
- W. Liu, Q. Yan, C. Xia, X. Wang, A. Kumar, Y. Wang, Y. Liu, Y. Pan and J. Liu, J. Mater. Chem. B, 2021, 9, 4459–4474 RSC.
- S. Wang, M. Kai, Y. Duan, Z. Zhou, R. H. Fang, W. Gao and L. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202203115 CrossRef CAS PubMed.
- W. Shen, G. Han, L. Yu, S. Yang, X. Li, W. Zhang and P. Pei, Int. J. Nanomed., 2022, 17, 1397–1408 CrossRef PubMed.
- J.-J. Wu, X.-M. Yuan, C. Huang, G.-Y. An, Z.-L. Liao, G.-A. Liu and R.-X. Chen, Int. Immunopharmacol., 2019, 68, 218–225 CrossRef CAS PubMed.
- G. Chen, Y. Zhao, Y. Xu, C. Zhu, T. Liu and K. Wang, Int. J. Pharm., 2020, 589, 119763 CrossRef CAS PubMed.
- M. Hu, W. Zhou, Y. Wang, D. Yao, T. Ye, Y. Yao, B. Chen, G. Liu, X. Yang, W. Wang and Y. Xie, Acta Pharm. Sin. B, 2020, 10, 1943–1953 CrossRef CAS PubMed.
- S. Yang, G. Han, Q. Chen, L. Yu, P. Wang, Q. Zhang, J. Dong, W. Zhang and J. Huang, Int. J. Nanomed., 2021, 16, 239–248 CrossRef PubMed.
- Y. Zhao, Y. Zheng, Y. Zhu, Y. Zhang, H. Zhu and T. Liu, Pharmaceutics, 2021, 13, 1493 CrossRef CAS PubMed.
- W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed.
- W. Sang, Z. Zhang, Y. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 3771–3810 RSC.
- G. Lan, K. Ni, Z. Xu, S. S. Veroneau, Y. Song and W. Lin, J. Am. Chem. Soc., 2018, 140, 5670–5673 CrossRef CAS PubMed.
- M. Balaz, H. A. Collins, E. Dahlstedt and H. L. Anderson, Org. Biomol. Chem., 2009, 7, 874–888 RSC.
- J. Schmitt, V. Heitz, A. Sour, F. Bolze, H. Ftouni, J.-F. Nicoud, L. Flamigni and B. Ventura, Angew. Chem., Int. Ed., 2015, 54, 169–173 CrossRef CAS PubMed.
- Y. Shen, A. J. Shuhendler, D. Ye, J.-J. Xu and H.-Y. Chen, Chem. Soc. Rev., 2016, 45, 6725–6741 RSC.
- J. Shan, S. J. Budijono, G. Hu, N. Yao, Y. Kang, Y. Ju and R. K. Prud'homme, Adv. Funct. Mater., 2011, 21, 2488–2495 CrossRef CAS.
- M. Guan, H. Dong, J. Ge, D. Chen, L. Sun, S. Li, C. Wang, C. Yan, P. Wang and C. Shu, NPG Asia Mater., 2015, 7, e205–e205 CrossRef CAS.
- Y. Chen, Y. Yang, S. Du, J. Ren, H. Jiang, L. Zhang and J. Zhu, ACS Appl. Mater. Interfaces, 2023, 15, 35884–35894 CrossRef CAS PubMed.
- Y. Shao, B. Liu, Z. Di, G. Zhang, L.-D. Sun, L. Li and C.-H. Yan, J. Am. Chem. Soc., 2020, 142, 3939–3946 CrossRef CAS PubMed.
- C. He, X. Duan, N. Guo, C. Chan, C. Poon, R. R. Weichselbaum and W. Lin, Nat. Commun., 2016, 7, 12499 CrossRef CAS PubMed.
- K. Ni, G. Lan, C. Chan, B. Quigley, K. Lu, T. Aung, N. Guo, P. La Riviere, R. R. Weichselbaum and W. Lin, Nat. Commun., 2018, 9, 2351 CrossRef PubMed.
- X. Duan, C. Chan, W. Han, N. Guo, R. R. Weichselbaum and W. Lin, Nat. Commun., 2019, 10, 1899 CrossRef PubMed.
- X. Duan, C. Chan and W. Lin, Angew. Chem., Int. Ed., 2019, 58, 670–680 CrossRef CAS PubMed.
- X. Jiang, J. Liu, M. J. Lee, C. Peng, T. Luo, L. Tillman, R. R. Weichselbaum and W. Lin, Biomaterials, 2023, 302, 122334 CrossRef CAS PubMed.
- H. Yu, Y. Li, Z. Zhang, J. Ren, L. Zhang, Z. Xu, Y. Kang and P. Xue, Acta Biomater., 2022, 138, 545–560 CrossRef CAS PubMed.
- Q. Yang, N. Guo, Y. Zhou, J. Chen, Q. Wei and M. Han, Acta Pharm. Sin. B, 2020, 10, 2156–2170 CrossRef CAS PubMed.
- S. Zhang, M. Zhang, J. Zhang, G. Li, X. Lu, F. Sun and W. Liu, Colloids Surf., B, 2024, 234, 113707 CrossRef CAS PubMed.
- Y. Song, L. Wang and Z. Xie, Biotechnol. J., 2021, 16, 1900382 CrossRef CAS PubMed.
- J. Chen, Q. Zhou and W. Cao, Adv. Funct. Mater., 2024, 2405844 CrossRef CAS.
- J. Chen, Y. Zhu and S. Kaskel, Angew. Chem., Int. Ed., 2021, 60, 5010–5035 CrossRef CAS PubMed.
- W. Fan, P. Huang and X. Chen, Chem. Soc. Rev., 2016, 45, 6488–6519 RSC.
- X. Zheng, L. Wang, Y. Guan, Q. Pei, J. Jiang and Z. Xie, Biomaterials, 2020, 235, 119792 CrossRef CAS PubMed.
- T. Luo, K. Ni, A. Culbert, G. Lan, Z. Li, X. Jiang, M. Kaufmann and W. Lin, J. Am. Chem. Soc., 2020, 142, 7334–7339 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |