Constructing perfect cubic Ag–Cu alloyed nanoclusters through selective elimination of phosphine ligands†
Li
Tang‡
a,
Qikai
Han‡
a,
Bin
Wang
a,
Zhonghua
Yang
b,
Chunyuan
Song
a,
Guanyu
Feng
a and
Shuxin
Wang
 *a
*a
aCollege of Materials Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, Shandong, P. R. China. E-mail: shuxin_wang@qust.edu.cn
bCollege of Chemistry and Molecular Engineering, Qingdao University of Science and Technology, Qingdao 266042, Shandong, P. R. China
First published on 12th December 2023
Abstract
The aspiration of chemists has always been to design and achieve control over nanoparticle morphology at the atomic level. Here, we report a synthesis strategy and crystal structure of a perfect cubic Ag–Cu alloyed nanocluster, [Ag55Cu8I12(S-C6H32,4(CH3)2)24][(PPh4)] (Ag55Cu8I12 for short). The structure of this cluster was determined by single-crystal X-ray diffraction (SCXRD) and further validated by X-ray photoelectron spectroscopy (XPS), inductively coupled plasma (ICP), Energy-dispersive X-ray spectroscopy (EDX), thermogravimetric analysis (TGA), and 1H and 31P nuclear magnetic resonance (NMR). The surface deviation of the cube was measured to be 0.291 Å, making it the flattest known cube to date.
Regulating nanoparticle morphology has always been a prominent topic in current research, as the physical and chemical properties of nanoparticles are closely tied to their shape and structure.1–5 For instance, spherical nanoparticles and rod-shaped nanoparticles exhibit distinct behaviours in terms of optical properties, electron transport, and catalytic reactions.6–8 With advancements in synthesis technology, researchers can now tune nanoparticle morphology using various approaches.9–13 For instance, Mirkin's group reported the photoinduced transformation of Ag nanospheres into triangular nanoprisms.12 Yang's group achieved cubic Ag nanoparticles through the use of mercaptan as a directing agent.13 However, studying ordinary nanoparticles at the atomic level directly presents challenges due to their larger size and complex structure. Fortunately, metal nanoclusters have emerged as a swiftly advancing category of nanomaterials characterised by their atomically precise structures and diverse applications.14–29 Furthermore, the morphology of metal nanoclusters, such as metal–metal bonds and metal–ligand interactions, can be precisely controlled through ligand exchange,30–35 metal doping,36–40 and other techniques.41,42 Most importantly, single-crystal X-ray diffraction enables us to obtain atomic-level interface information, including the arrangement of doped metals within nanoclusters and the bonding modes between peripheral ligands and metals.
The synthesis of metal nanoparticles with polyhedral shapes, such as nanocubes, has always posed challenges compared to thermodynamically stable nanospheres. We have observed that Zheng's group recently synthesized an almost perfect silver nanocube.43 The main reason for the deviation from a perfect cube is the displacement of metal atoms at the eight vertices from their ideal positions within the cube. Structural analysis suggests that this deviation is most likely caused by the coordination of triphenylphosphine ligands with the vertex Ag atoms. If the dissociation of the phosphine ligands can be achieved, it is theoretically possible to bring the displaced Ag atoms back to the vicinity of their ideal positions, thus realizing the construction of a perfect cube. Notably, introducing copper atoms into Ag29(SSR)12(PPh3)4 nanoclusters not only enhances their stability but also modifies their luminescent properties and catalytic activities.44,45 By fine-tuning the doping methods of copper atoms, precise control over the size and composition of copper-based nanoclusters can be achieved.46–49 In essence, copper atom doping imparts novel properties and functionalities to metal nanoclusters, thereby broadening their application scope and contributing to the advancement of nanotechnology.
In this study, we introduced Cu atoms during the synthesis of 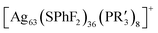 (R′ = nBu or Ph, Ag63 for short) nanoclusters, where the Cu atoms occupied the vertices of the cube due to the vertex effect.50 By utilizing the difference in bonding energies between Cu–P and Ag–P bonds,51 we achieved the dissociation of the ligands at the vertex positions, leading to the synthesis of the [Ag55Cu8I12(S-C6H32,4(CH3)2)24][(PPh4)] nanocluster (abbreviated as Ag55Cu8I12). The surface deviation of this cluster has been measured to be 0.291 Å, making it the metal nanocube with the highest level of smoothness achieved to date.
(R′ = nBu or Ph, Ag63 for short) nanoclusters, where the Cu atoms occupied the vertices of the cube due to the vertex effect.50 By utilizing the difference in bonding energies between Cu–P and Ag–P bonds,51 we achieved the dissociation of the ligands at the vertex positions, leading to the synthesis of the [Ag55Cu8I12(S-C6H32,4(CH3)2)24][(PPh4)] nanocluster (abbreviated as Ag55Cu8I12). The surface deviation of this cluster has been measured to be 0.291 Å, making it the metal nanocube with the highest level of smoothness achieved to date.
The Ag55Cu8I12 nanocluster was prepared via the “One-pot method”. Details of synthesis are provided in the ESI.† Briefly, the Ag55Cu8I12 nanocluster was synthesized by the reduction of a mixture of AgNO3, PPh3, PPh4Br, HS-C6H32,4(CH3)2, and CuI in the mixed solvent of CH3OH and CH2Cl2 at room temperature. NaBH4 was used as the primary reducing agent, while triethylamine was added to reduce the reactivity of NaBH4. The reaction was conducted at room temperature and lasted for 12 hours. After completion, the crude product was washed with an excess of methanol and subsequently subjected to crystallization in a mixture of dichloromethane and n-hexane. Ag55Cu8I12 crystallizes in a monoclinic system with C12/m1 group. SCXRD analysis of Ag55Cu8I12 showed one PPh4+ counterion in the system, indicating that the NC bears a −1 charge. The details of crystal data and refinement are provided in Table S1 (ESI†). It should be noted that due to its high symmetry, the complete PPh4+ molecule was not resolved. Further confirmation of PPh4+ was attained through the utilization of nuclear magnetic resonance (NMR) analyses, X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), and energy-dispersive X-ray spectrum (EDX).
The optical properties of Ag55Cu8I12 and Ag63 were compared. As shown in Fig. 1a and b, Ag55Cu8I12 showed three main absorption peaks located at 353, 413, 503, and 827 nm, which are distinct from those of Ag63 (located at 325, 415, 470, 580 and 840 nm). X-ray photoelectron spectroscopy (XPS) and energy-dispersive X-ray spectrum (EDX) further verify the existence of Ag, Cu, S, P, C and I atoms (Fig. 1d and Fig. S2 and S3, ESI†). The Cu 2p XPS spectrum shows that the binding energies of Cu 2p3/2 and Cu 2p1/2 are at 932.8 and 952.6 eV (Fig. S2b, ESI†), respectively. The Auger Cu LMM spectrum was further utilized to verify that the copper atoms in Ag55Cu8I12 exhibit a Cu(I) oxidation state (Fig. S2c, ESI†).52 Furthermore, the Ag 3d5/2 binding energy can be deconvoluted into two distinct peaks, as depicted in Fig. S2d and e (ESI), suggesting the simultaneous presence of Ag(i) and Ag(0) within the Ag55Cu8I12 nanocluster. These peaks, at approximately 368.89 eV and 367.95 eV, correspond to Ag(I) and Ag(0),53 respectively, and are represented by a blue curve and a turquoise curve. The ratio of these peaks is found to be 2.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Furthermore, XPS, EDX and inductively coupled plasma (ICP) measurements were performed to validate the ratio of Ag/Cu in the bi-metallic Ag55Cu8I12 nanoclusters (Table S2, ESI†), and the results perfectly matched the theoretical value (55/8 of Ag/Cu), which is close to the ratio of 55/8 obtained from the crystal analysis results. TGA result of the product shows a weight loss of about 33.83%. The theoretical weight loss for the cluster is 30.36% (excluding solvent), with the additional loss attributed to the solvent. The results from SQUEEZE indicate the removal of approximately 388 electrons. Considering the varying electron counts at different positions and the solvent used in crystallization, we deduced the presence of about 2.4 dichloromethane molecules and 1.88 n-hexane molecules. The theoretical weight loss calculated from the SQUEEZE results is approximately 33.42%, which is in good agreement with the TGA findings (Fig. S4, ESI†). In order to further confirm the formula and valence state of Ag55Cu8I12, we performed nuclear magnetic resonance (NMR) analysis (Fig. 1c and Fig. S5–S7, ESI†). 31P NMR spectra of Ag55Cu8I12 were also recorded, further verifying the existence of a phosphine ligand in the structure (Fig. 1c and Fig. S5, ESI†). In the 1H NMR spectrum of Ag55Cu8I12 (Fig. S6 and S7, ESI†), the peaks at 6.70–7.90 ppm (92 H) are assigned to –C6H5 in Ph4P+ and –C6H3 in –S-C6H32,4(CH3)2 the peaks at 1.65–1.80 ppm (144 H) are assigned to –CH3 in the –S-C6H32,4(CH3)2 ligands. According to the proton ratio (C6H5 + C6H3:CH3 = 92
1. Furthermore, XPS, EDX and inductively coupled plasma (ICP) measurements were performed to validate the ratio of Ag/Cu in the bi-metallic Ag55Cu8I12 nanoclusters (Table S2, ESI†), and the results perfectly matched the theoretical value (55/8 of Ag/Cu), which is close to the ratio of 55/8 obtained from the crystal analysis results. TGA result of the product shows a weight loss of about 33.83%. The theoretical weight loss for the cluster is 30.36% (excluding solvent), with the additional loss attributed to the solvent. The results from SQUEEZE indicate the removal of approximately 388 electrons. Considering the varying electron counts at different positions and the solvent used in crystallization, we deduced the presence of about 2.4 dichloromethane molecules and 1.88 n-hexane molecules. The theoretical weight loss calculated from the SQUEEZE results is approximately 33.42%, which is in good agreement with the TGA findings (Fig. S4, ESI†). In order to further confirm the formula and valence state of Ag55Cu8I12, we performed nuclear magnetic resonance (NMR) analysis (Fig. 1c and Fig. S5–S7, ESI†). 31P NMR spectra of Ag55Cu8I12 were also recorded, further verifying the existence of a phosphine ligand in the structure (Fig. 1c and Fig. S5, ESI†). In the 1H NMR spectrum of Ag55Cu8I12 (Fig. S6 and S7, ESI†), the peaks at 6.70–7.90 ppm (92 H) are assigned to –C6H5 in Ph4P+ and –C6H3 in –S-C6H32,4(CH3)2 the peaks at 1.65–1.80 ppm (144 H) are assigned to –CH3 in the –S-C6H32,4(CH3)2 ligands. According to the proton ratio (C6H5 + C6H3:CH3 = 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 144), we determined that the ratio of [Ag55Cu8I12(SC8H9)24]− to PPh4+ is 1
144), we determined that the ratio of [Ag55Cu8I12(SC8H9)24]− to PPh4+ is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. All the aforementioned results indicate that the precise formula of obtained nanocluster is [Ag55Cu8I12(S-C6H32,4(CH3)2)24][(PPh4)]. In contrast to Ag63, the distinguishing factor between the two lies in their oxidation states and the consequent variation in the number of free electrons. Ag63 exhibits 26 free electrons, whereas Ag55Cu8I12 possesses 28 free electrons. As demonstrated in Fig. S8 (ESI†), the energy scale spectra of Ag55Cu8I12 and Ag63 have been analyzed, revealing that the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) in Ag55Cu8I12 (Eg = 1.18 eV) is smaller compared to that in Ag63 (Eg = 1.24 eV). This reduction in the energy gap may be attributed to the presence of two additional electrons in Ag55Cu8I12.
1. All the aforementioned results indicate that the precise formula of obtained nanocluster is [Ag55Cu8I12(S-C6H32,4(CH3)2)24][(PPh4)]. In contrast to Ag63, the distinguishing factor between the two lies in their oxidation states and the consequent variation in the number of free electrons. Ag63 exhibits 26 free electrons, whereas Ag55Cu8I12 possesses 28 free electrons. As demonstrated in Fig. S8 (ESI†), the energy scale spectra of Ag55Cu8I12 and Ag63 have been analyzed, revealing that the energy gap between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) in Ag55Cu8I12 (Eg = 1.18 eV) is smaller compared to that in Ag63 (Eg = 1.24 eV). This reduction in the energy gap may be attributed to the presence of two additional electrons in Ag55Cu8I12.
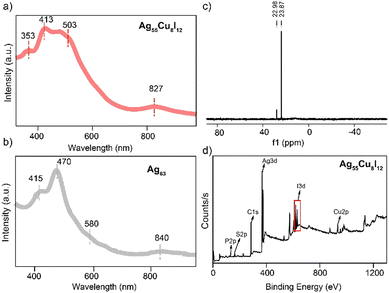 | ||
| Fig. 1 The UV-vis spectra of Ag55Cu8I12 (a) and Ag63 (b); (c) 31P-NMR spectrum of Ag55Cu8I12 and (d) XPS result of Ag55Cu8I12. | ||
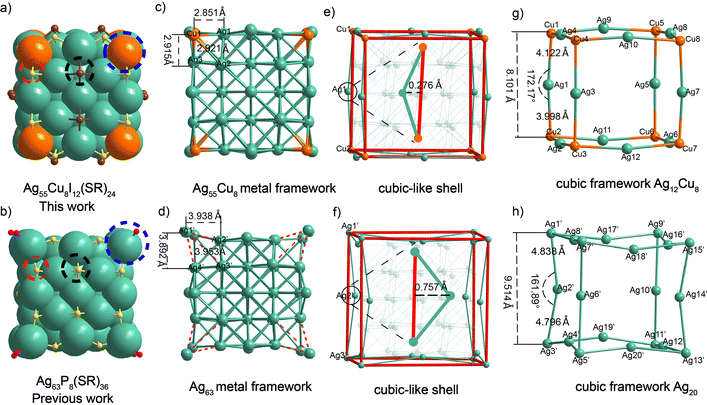
The crystal structure is composed of an Ag55Cu8 metal kernel, 24 μ3-S ligands, and 12 μ4-I ligands, with one PPh4+ group as counterions (Fig. 2a and Fig. S9, ESI†). Specifically, for Ag55Cu8I12, eight Cu atoms were substituted for the vertex site of the eight Ag atoms coordinated to the phosphine ligand in the mentioned Ag63 nanocluster (Fig. 2a and b, blue dotted line). Additionally, in comparison to the Ag63 nanocluster, the two sulfur ligands located at the center of the (100) facet of the nanocube were replaced by two iodine atoms, while maintaining a similar coordination mode (Fig. 2a and b, indicated by black dotted lines). The remaining 24 thiolate ligands are present on the edges of the cube, as depicted by the red dotted lines in Fig. 2a and b.
The results of the single-crystal analysis clearly demonstrate that the Ag55Cu8I12 cluster exhibits a closer approximation to a perfect cube compared to the Ag63 nanocluster (Fig. 2a–h). All the data regarding bond lengths in the clusters can be found in Table S3 (ESI†). In the main text, we only discuss the key data that determine whether the cluster exhibits a perfect cubic structure. In the Ag55Cu8I12, the bond lengths between the vertex Cu atom and the three adjacent Ag atoms are 2.851 Å, 2.921 Å, and 2.915 Å, respectively (Fig. 2c). In Ag63, the bond lengths between the vertex silver atom and the neighboring silver atoms are 3.938 Å, 3.953 Å, and 3.892 Å, respectively (Fig. 2d). These values are significantly larger than the Ag–Ag (2.880 Å) van der Waals radius. This indicates closer proximity and stronger bonding compared to Ag63, where the vertex silver atom protrudes outward due to phosphine ligand coordination. The analysis of the deviation of two clustered cube structures compared to the standard cube is depicted in Fig. 2e and f. In the case of Ag55Cu8I12, the distance between Cu1–Ag1–Cu2, representing one edge of the cubic-like structure, is 0.276 Å from the corresponding edge of the standard cube, while for Ag63, it is 0.757 Å. Similarly, the other edges were also evaluated (Fig. S10, ESI†). The results indicate that the Ag55Cu8I12 cube exhibits a surface deviation closer to that of the standard cube compared to Ag63 (0.291 Å vs. 0.723 Å). As shown in Fig. 2g, for Ag55Cu8I12, the cubic framework is constructed by 12 Ag and 8 Cu. Every edge of the cube is formed by connecting two Cu atoms and one Ag atom. Taking the Cu1–Ag1–Cu2 unit as an example, the bond lengths of Cu1–Ag1 and Ag1–Cu2 are 4.122 Å and 3.998 Å, respectively.
Notably, the sum of the Cu1–Ag1 and Ag1–Cu2 bond lengths (8.120 Å) in the case of Ag55Cu8I12 is almost equivalent to the Cu1–Cu2 distance (8.101 Å) with a difference of 0.019 Å (Fig. 2g and Table S4, ESI†). The bond angle of Cu1–Ag1–Cu2 is 172.17°, indicating that Cu1, Ag1, and Cu2 are almost in a straight line, deviating by only 4.35% from 180° (Fig. 2g and Table S5, ESI†). In contrast, for Ag63, the Ag1′–Ag3′ bond length is 9.514 Å, which is significantly shorter than the sum of the bond lengths Ag1′-Ag2′ (4.838 Å) and Ag2′–Ag3′ (4.796 Å), with a difference of 0.12 Å (Fig. 2h and Table S4, ESI†). Furthermore, the Ag1′–Ag2′–Ag3′ bond angle is 161.89°, deviating by 10.06% from 180° (Fig. 2h and Table S5, ESI†). This difference indicates that Cu1, Ag1, and Cu2 in Ag55Cu8I12 are more nearly in a straight line compared to Ag1′-Ag2′-Ag3′. The comparison was extended to the remaining edges of the cubic metal kernel, leading to the conclusion that Ag55Cu8I12 is closer to the standard cubic structure (Tables S4 and S5, ESI†). This exciting result demonstrates the possibility of customizing nanocluster morphology through metal doping. Furthermore, we assessed the stability of the two nanoclusters and found that the shape-modified Ag55Cu8I12 exhibited higher stability (Fig. S11, ESI†).43 Meanwhile, the packing modes of these two nanoclusters exhibit distinct differences in their crystal lattices, as shown in Fig. S12 (ESI†).
In summary, our work focuses on the synthesis and precise structural characterization of the flattest nanocube achieved to date. The formula of this nanocluster, [Ag55Cu8I12(S-C6H32,4(CH3)2)24][(PPh4)], was determined by single-crystal X-ray diffraction (SC-XRD) and further validated through UV-Vis, XPS, ICP, EDX, 31P NMR, and 1H NMR analyses. By combining the vertex effect of metal doping and the weak Cu-P interaction, we successfully achieved directional modulation of the Ag63 nanocluster morphology. Notably, the morphologically modified clusters exhibited significant differences in terms of UV-vis absorption and stability compared to their pre-modulation counterparts. The directed control of metal nanoparticle cluster morphology holds great potential in enhancing the performance and expanding the applications of nanoclusters, while deepening our understanding of the structure-performance relationship in nanomaterials.
S.W. designed the project, analyzed the data, and wrote the manuscript. L. T. and Q. H. carried out experiments, analyzed the data and revised the manuscript. B. W., Z. Y., C. S. and G. F. assisted in the synthesis of samples.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. W. acknowledges the financial support provided by the National Natural Science Foundation of China (22171156 and 21803001), the Taishan Scholar Foundation of Shandong Province, and Startup Foundation of Qingdao University of Science and Technology.Notes and references
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- T. Song, F. Gao, S. Guo, Y. Zhang, S. Li, H. You and Y. Du, Nanoscale, 2021, 13, 3895–3910 RSC.
- T. K. Sau and A. L. Rogach, Adv. Mater., 2010, 22, 1781–1804 CrossRef CAS PubMed.
- J. Li, Z. Zhu, F. Liu, B. Zhu, Y. Ma, J. Yan, B. Lin, G. Ke, R. Liu, L. Zhou, S. Tu and C. Yang, Small, 2016, 12, 5449–5487 CrossRef CAS PubMed.
- C. Ma, J. Zhang, T. Zhang, H. Sun, J. Wu, J. Shi and Z. Xie, Front. Chem., 2019, 7, 765 CrossRef CAS PubMed.
- R. Bhole, D. Gonsalves, G. Murugesan, M. K. Narasimhan, N. R. Srinivasan, N. Dave, T. Varadavenkatesan, R. Vinayagam, M. Govarthanan and R. Selvaraj, Appl. Nanosci., 2022, 13, 6003–6014 CrossRef.
- X. Yuan, L. Ge, H. Zhou and J. Tang, Spectrochim. Acta, Part A, 2023, 287, 122082 CrossRef CAS PubMed.
- M. Kang and Y. Kim, J. Ind. Eng. Chem., 2020, 86, 61–72 CrossRef CAS.
- S. Ali, A. S. Sharma, W. Ahmad, M. Zareef, M. M. Hassan, A. Viswadevarayalu, T. Jiao, H. Li and Q. Chen, Crit. Rev. Anal. Chem., 2021, 51, 454–481 CAS.
- M. L. Personick, M. R. Langille, J. Zhang and C. A. Mirkin, Nano Lett., 2011, 11, 3394–3398 CrossRef CAS PubMed.
- M. Ueji, M. Harada and Y. Kimura, J. Colloid Interface Sci., 2008, 322, 358–363 CrossRef CAS PubMed.
- R. Jin, Y. Cao, C. A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zheng, Science, 2001, 294, 1901–1903 CrossRef CAS PubMed.
- D. G. Li, S. H. Chen, S. Y. Zhao, X. M. Hou, H. Y. Ma and X. G. Yang, Thin Solid Films, 2004, 460, 78–82 CrossRef CAS.
- R. Jin, C. Zeng, M. Zhou and Y. Chen, Chem. Rev., 2016, 116, 10346–10413 CrossRef CAS PubMed.
- L. Tang, A. Ma, C. Zhang, X. Liu, R. Jin and S. Wang, Angew. Chem., Int. Ed., 2021, 60, 17969–17973 CrossRef CAS PubMed.
- J.-Q. Fan, Y. Yang, C.-B. Tao and M.-B. Li, Angew. Chem., Int. Ed., 2023, 62, e202215741 CrossRef CAS PubMed.
- X. Wei, H. Li, H. Li, Z. Zuo, F. Song, X. Kang and M. Zhu, J. Am. Chem. Soc., 2023, 145, 13750–13757 CrossRef CAS PubMed.
- Z. H. Gao, K. Wei, T. Wu, J. Dong, D. E. Jiang, S. Sun and L. S. Wang, J. Am. Chem. Soc., 2022, 144, 5258–5262 CrossRef CAS PubMed.
- T. Thomas, H. Kuttoth, R. V. Nair and N. Sandhyarani, Langmuir, 2023, 39, 10011–10020 CrossRef CAS PubMed.
- J. Sun, X. Yan, L. Wang, Z. Xie, G. Tian, L. Wang, A. He, S. Li, Q. Guo, L. Chao, J. He and H. Shen, Inorg. Chem., 2023, 62, 9005–9013 CrossRef CAS PubMed.
- T. Jia, Z. J. Guan, C. Zhang, X. Z. Zhu, Y. X. Chen, Q. Zhang, Y. Yang and D. Sun, J. Am. Chem. Soc., 2023, 145, 10355–10363 CrossRef CAS PubMed.
- X. H. Ma, Y. Si, L. L. Luo, Z. Y. Wang, S. Q. Zang and T. C. W. Mak, ACS Nano, 2022, 16, 5507–5514 CrossRef CAS PubMed.
- J. J. Li, Z. Liu, Z. J. Guan, X. S. Han, W. Q. Shi and Q. M. Wang, J. Am. Chem. Soc., 2022, 144, 690–694 CrossRef CAS PubMed.
- A. Jana, M. Jash, A. K. Poonia, G. Paramasivam, M. R. Islam, P. Chakraborty, S. Antharjanam, J. Machacek, S. Ghosh, K. Adarsh, T. Base and T. Pradeep, ACS Nano, 2021, 15, 15781–15793 CrossRef CAS PubMed.
- F. Xiao, Y. Chen, J. Qi, Q. Yao, J. Xie and X. Jiang, Adv. Mater., 2023, 35, e2210412 CrossRef PubMed.
- H. Hirai, S. Takano, T. Nakashima, T. Iwasa, T. Taketsugu and T. Tsukuda, Angew. Chem., Int. Ed., 2022, 61, e202207290 CrossRef CAS PubMed.
- W. Fan, Y. Yang, Q. You, J. Li, H. Deng, N. Yan and Z. Wu, J. Phys. Chem. C, 2023, 127, 816–823 CrossRef CAS.
- S. Li, A. V. Nagarajan, X. Du, Y. Li, Z. Liu, D. R. Kauffman, G. Mpourmpakis and R. Jin, Angew. Chem., Int. Ed., 2022, 61, e202211771 CrossRef CAS PubMed.
- J. Xu, L. Xiong, X. Cai, S. Tang, A. Tang, X. Liu, Y. Pei and Y. Zhu, Chem. Sci., 2022, 13, 2778–2782 RSC.
- L. Tang, T. Duan, Y. Pei and S. Wang, ACS Nano, 2023, 17, 4279–4286 CrossRef CAS PubMed.
- T.-A. D. Nguyen, Z. R. Jones, D. F. Leto, G. Wu, S. L. Scott and T. W. Hayton, Chem. Mater., 2016, 28, 8385–8390 CrossRef CAS.
- L. Tang, B. Wang, R. Wang and S. Wang, Nanoscale, 2023, 15, 1602–1608 RSC.
- X. Kang and M. Zhu, Chem. Mater., 2019, 31, 9939–9969 CrossRef CAS.
- J. Zhao, A. Ziarati, A. Rosspeintner, Y. Wang and T. Bürgi, Chem. Sci., 2023, 14, 7665–7674 RSC.
- S. K. Eswaramoorthy, N. A. Sakthivel and A. Dass, J. Phys. Chem. C, 2019, 123, 9634–9639 CrossRef CAS.
- A. Ghosh, O. F. Mohammed and O. M. Bakr, Acc. Chem. Res., 2018, 51, 3094–3103 CrossRef CAS PubMed.
- M. S. Bootharaju, H. Chang, G. Deng, S. Malola, W. Baek, H. Häkkinen, N. Zheng and T. Hyeon, J. Am. Chem. Soc., 2019, 141, 8422–8425 CrossRef CAS PubMed.
- Y. Zhang, J. Zhang, Z. Li, Z. Qin, S. Sharma and G. Li, Commun. Chem., 2023, 6, 24 CrossRef CAS PubMed.
- H. Yi, S. M. Han, S. Song, M. Kim, E. Sim and D. Lee, Angew. Chem., Int. Ed., 2021, 60, 22293–22300 CrossRef CAS PubMed.
- H. Xiang, H. Yan, J. Liu, R. Cheng, C. Q. Xu, J. Li and C. Yao, J. Am. Chem. Soc., 2022, 144, 14248–14257 CrossRef CAS PubMed.
- L. Tang, X. Kang, S. Wang and M. Zhu, Langmuir, 2019, 35, 12350–12355 CrossRef CAS PubMed.
- Z. Gan, N. Xia, N. Yan, S. Zhuang, J. Dong, Y. Zhao, S. Jiang, Q. Tao and Z. Wu, Angew. Chem., Int. Ed., 2021, 60, 12253–12257 CrossRef CAS PubMed.
- H. Yang, J. Yan, Y. Wang, H. Su, L. Gell, X. Zhao, C. Xu, B. K. Teo, H. Hakkinen and N. Zheng, J. Am. Chem. Soc., 2017, 139, 31–34 CrossRef CAS PubMed.
- X. Kang, H. AIoshan, S. Wang and M. Zhu, Inorg. Chem., 2019, 58, 11000–11009 CrossRef CAS PubMed.
- X. Kang, X. Wei, S. Jin, Q. Yuan, X. Luan, Y. Pei, S. Wang, M. Zhu and R. Jin, PANS, 2019, 116, 18834–18840 CrossRef CAS PubMed.
- H. Shen, Y. Z. Han, Q. Wu, J. Peng, B. K. Teo and N. Zheng, Small Methods, 2020, 5, 2000603 CrossRef PubMed.
- A. Baksi, E. K. Schneider, P. Weis, I. Chakraborty, O. Fuhr, S. Lebedkin, W. J. Parak and M. M. Kappes, ACS Nano, 2020, 14, 15064–15070 CrossRef CAS PubMed.
- X. H. Ma, Y. Si, L. L. Luo, Z. Y. Wang, S. Q. Zang and T. C. W. Mak, ACS Nano, 2022, 16, 5507–5514 CrossRef CAS PubMed.
- S. Mukherjee, A. Das, A. K. Das, A. Sheriff, K. Sunny, A. S. Nair, S. Bhandary, R. Bhowal, D. Chopra, B. Pathak, S. Yamazoe and S. Mandal, Chem. Mater., 2023, 35, 1659–1666 CrossRef CAS.
- S. Wang, L. Xiong, G. Sun, L. Tang, J. Zhang, Y. Pei and M. Zhu, Nanoscale Adv., 2020, 2, 664–668 RSC.
- H. Li, C. Zhou, E. Wang, X. Kang, W. W. Xu and M. Zhu, Chem. Commun., 2022, 58, 5092–5095 RSC.
- P. Liu and E. J. Hensen, J. Am. Chem. Soc., 2013, 135, 14032–14035 CrossRef CAS PubMed.
- R. S. Dhayal, J.-H. Liao, Y.-C. Liu, M.-H. Chiang, S. Kahlal, J.-Y. Saillard and C. W. Liu, Angew. Chem., Int. Ed., 2015, 54, 3702 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S9 and Tables S1–S5 for the crystal structure, XPS, EDX, NMR and UV-vis results of nanoclusters. CCDC 2283567. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3cp04224c |
| ‡ These authors contributed equally to this work. |
| This journal is © the Owner Societies 2024 |