Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multiple hydrogen-bonded dimers: are only the frontier atoms relevant?†
Celine
Nieuwland
 a,
David
Almacellas
b,
Mac M.
Veldhuizen
a,
Lucas
de Azevedo Santos
a,
David
Almacellas
b,
Mac M.
Veldhuizen
a,
Lucas
de Azevedo Santos
 a,
Jordi
Poater
a,
Jordi
Poater
 bc and
Célia
Fonseca Guerra
bc and
Célia
Fonseca Guerra
 *a
*a
aDepartment of Chemistry and Pharmaceutical Sciences, Amsterdam Institute for Molecular and Life Sciences (AIMMS), Vrije Universiteit Amsterdam, De Boelelaan 1108, Amsterdam 1081 HZ, The Netherlands. E-mail: c.fonsecaguerra@vu.nl; Web: https://www.theochem.nl/
bDepartament de Química Inorgànica i Orgànica & Institut de Química Teòrica i Computacional, Universitat de Barcelona, Martí i Franquès 1-11, Barcelona 08028, Catalonia, Spain
cICREA, Pg. Lluís Companys 23, Barcelona 08010, Spain
First published on 30th November 2023
Abstract
Non-frontier atom exchanges in hydrogen-bonded aromatic dimers can induce significant interaction energy changes (up to 6.5 kcal mol−1). Our quantum-chemical analyses reveal that the relative hydrogen-bond strengths of N-edited guanine–cytosine base pair isosteres, which cannot be explained from the frontier atoms, follow from the charge accumulation in the monomers.
Introduction
Intermolecular hydrogen bonding is one of the central interactions underlying self-assembly and molecular recognition in biochemistry, such as in protein folding and DNA duplex formation by constituting the hydrogen bonds (H-bonds) between the complementary DNA base pairs.1 The ability to self-assemble has inspired the field of supramolecular chemistry to incorporate intermolecular H-bonding in the design of novel catalysts,2 (macro)molecules,3 and materials.4 However, in order to rationally design new and improved molecules and materials, a profound understanding of the mechanism of intermolecular H-bonding and the prediction of the interaction strength is required.The strength of an H-bond is often explained from the properties of the interacting frontier atoms, that is, the partially positively charged H-bond donor (i.e., H(–O) or H(–N)) and the partially negatively charged H-bond acceptor (i.e., O or N) groups.5–7 An example of this is the secondary electrostatic interaction (SEI) model6 from Jorgensen and Pranata which is widely used to predict and explain trends in intermolecular H-bond strengths.7 This model is based on the assumption that the H-bond strength between monomers can be predicted from the position and charge of the frontier atoms. However, the SEI model is physically incorrect due to the oversimplification of the H-bonding mechanism by regarding the H-bond donor and acceptor groups as interacting point charges. Popelier and Joubert8 showed that the consideration of only frontier-atom electrostatic interactions, like in the SEI model, is elusive because also the electrostatic interactions between distant atoms contribute to the stability of H-bonded pairs. More importantly, it is nowadays well-established that H-bonds are not purely electrostatic in nature but also contain a significant covalent character,9 which arises from the donation of electronic density from the filled σ-lone pair orbital of the H-bond acceptor into the empty antibonding σ* orbital on the H-bond donor. This additional stabilizing orbital-interaction component is in fact essential to overcome the destabilizing Pauli repulsion associated with H-bond formation (see ref. 9a for an overview of all the relevant interaction components of H-bonds). Our previous work into the predictive nature of the SEI model revealed that the relative stability of H-bonded pairs follows from measuring the charge accumulation in the monomers rather than from the secondary interactions between H-bond frontier atoms.10,11
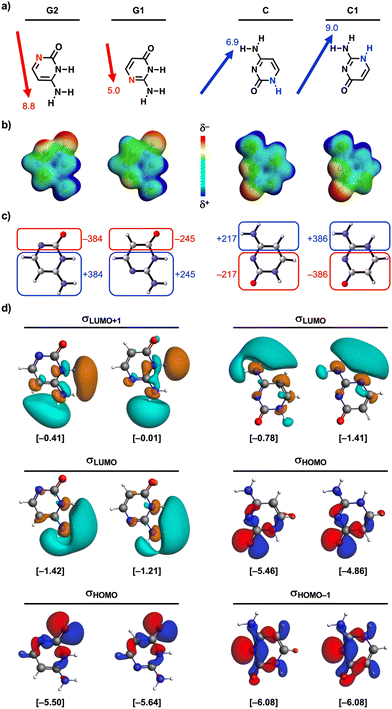
In this work, we challenge the concept of explaining H-bond strengths by the frontier atoms further. We show that the binding strength of H-bonded pairs with identical H-bond frontier atoms can be adjusted by minimal non-frontier atom exchanges. Our dispersion-corrected density functional theory (DFT-D) based analyses at the ZORA12-BLYP13-D3(BJ)14/TZ2P15 level using the Amsterdam Modeling Suite16 (AMS2022.101) (see ESI,† Method S1 for the full computational details) show that the stability of mimics of the guanine–cytosine (GC) DNA base pair can be systematically tuned by varying the position of the non-frontier N and NH groups (see Fig. 1a). We demonstrate that these minimal non-frontier modifications have significant implications on the H-bond strength of the corresponding base pairs although the charges on the frontier atoms are barely affected in these so-called N-edited G and C isosteres (Fig. 1b). We find that the relative binding strengths can be rather explained and predicted by measuring the amount of charge accumulation in the monomers. This allows for control of the H-bond strength of these N-edited nucleobases, which can be applied in the design of innovative (bio)supramolecular building blocks.17
Results and discussion
Based on the similar charges on the H-bond frontier atoms, shown in Fig. 1b, one would expect no significant change in the H-bond strength of the GC base pair upon changing the position of the non-frontier heteroatoms in the G and C monomers.18 However, we find that the interaction energy (ΔEint) of the GC H-bonded base pair analogues, presented in Fig. 2a, becomes more stabilizing from G1C (blue) < G2C (orange) < G1C1 (pink) < G2C1 (green), associated with a maximum stabilization of the H-bond interaction energy by ca. 6.5 kcal mol−1. So, the H-bond interaction is enhanced upon (i) changing the G1 isostere to G2, thereby grouping the partially negative N and O atoms on one side of the molecule, and (ii) upon changing C to C1, in which the partially positive NH(2) groups are in closer proximity. Note that we discuss here the interaction energies because they dictate the trend in the relative H-bond strengths (see ESI,† Data S1 and see Method S2 for computational details).To understand what causes this stabilization, ΔEint was partitioned into four physically meaningful terms using a quantitative energy decomposition analysis (EDA):19 (i) the classical electrostatic interaction (ΔVelstat), (ii) the steric Pauli repulsion (ΔEPauli) arising from the repulsion between overlapping closed-shell orbitals on the interacting monomers, (iii) the orbital interaction (ΔEoi) which accounts for charge transfer (i.e., covalency) in the σ-electronic system and polarization of the π-electronic system, and (iv) the dispersion energy (ΔEdisp) (see ESI,† Method S2 for a theoretical overview of this method).
The EDA can be performed on the equilibrium dimers (see ESI,† Data S1). However, as the H-bond distances vary for the different GC base-pair isosteres (see Fig. 2a), a more insightful picture can be obtained when performed at similar H-bond distances. This allows us to differentiate between interaction terms that are intrinsically more stabilizing, from the interaction terms that are simply enhanced by shortened H-bond distances. To this end, the EDA was performed as a function of the middle H-bond distance rN(H)⋯N using the PyFrag 2019 software,20 in which the two monomers in the geometry of the equilibrium dimer approach each other as two frozen blocks (ESI,† Method S2 for details). The results of this analysis are presented in Fig. 2b and show that the stabilization of ΔEint from G1C (blue) < G2C (orange) < G1C1 (pink) < G2C1 (green) is preserved along the entire H-bond distance range. Furthermore, it shows that ΔEint becomes more stabilizing upon interchanging G1 to G2 and C to C1 because both the electrostatic (ΔVelstat) and orbital interactions (ΔEoi) become more stabilizing along this trend. For ΔEoi, in particular, the σ-charge transfer orbital interactions (ΔEσoi) are enhanced, while the π-orbital interactions (ΔEπoi) do not really vary for the different isosteres. This means that the π-resonance assistance in the H-bonds, that is π-polarization, is not affected by the non-frontier modifications. Note that ΔEPauli does not dictate the trend, as this interaction component becomes more destabilizing from G1 to G2 and from C to C1 (ΔEdisp stays constant for all isosteres, see ESI,† Data S2).
While the similar frontier atomic charges of the isosteres cannot explain the enhancement of ΔVelstat and ΔEσoi (vide supra), we find that the origin of this effect lies in the amount of charge accumulation in the N-edited monomers, as is presented in Fig. 3. Upon going from G1 to G2 and from C to C1, the partially negative (O and N) and partially positive (NH and NH2) functional groups, respectively, are grouped on one side of the molecule, which induces an increase of the molecular dipole moment (Fig. 3a). This grouping of the polar functional groups is associated with an increase of charge accumulation on both sides of the molecule, as is visualized by the electrostatic potential surfaces in Fig. 3b and the Voronoi deformation density (VDD)21 charges in Fig. 3c (see ESI,† Method S3 for details about the VDD method and Data S3 for the complete VDD charge analysis). Although the charge on the H-bond frontier atoms does not significantly change (Fig. 1b), the grouping of all polar groups on one side of the molecule by going from G1 to G2 and from C to C1, enhances the molecular charge accumulation, that is, the interacting monomers become more pronounced ‘mirrored dipoles’ of each other (see Fig. 3c), which enhances the intermolecular electrostatic interactions. This is a manifestation of the fact that the total electrostatic interaction is not exclusively determined by the frontier atoms but also by long-range electrostatic interactions between distant atoms.
Besides ΔVelstat, the σ-orbital interactions ΔEσoi are also enhanced upon grouping the polar functional groups on one side of the nucleobases thereby increasing the molecular charge accumulation. A larger accumulation of negative charge destabilizes occupied orbitals (i.e., they become better electron donors), while a larger accumulation of positive charge stabilizes unoccupied orbitals (i.e., they become better electron acceptors) (see Fig. 4).10 Important to note here is that molecular orbitals (MOs) involved in the H-bonds are delocalized over larger parts of the molecule and are not exclusively located on the frontier groups (Fig. 3d). So even if the charge at the frontier atoms does not change, a change in charge on other parts of the molecule causes shifts in the energies of the MOs [as long as the MO is asymmetric with respect to the molecule (see below)]. As such, we observe that upon changing from G1 to G2 and from C to C1, the larger accumulation of negative charge on the side of the H-bond acceptor group(s), that is, where the highest-occupied molecular orbitals (σHOMOs) involved in the intermolecular H-bonds have their coefficients, destabilizes the σHOMOs (see Fig. 3d). At the same time, the larger accumulation of positive charge on the side of the H-bond donor group(s) stabilizes the lowest-unoccupied molecular orbitals (σLUMOs) as these MOs have a coefficient on this side. Note that the σHOMO−1 of the cytosine isosteres is not affected upon changing C to C1 because this MO is relatively symmetric and therefore there is no significant net effect of the change in molecular charge accumulation. Nevertheless, both the destabilization of the other σHOMOs and the stabilization of the σLUMOs reduces the energy gap (Δε) between the interacting orbitals and, therefore, results in better σ-orbital interactions in the case of enhanced charge accumulation (Fig. 4), that is, upon changing from G1 to G2 and from C to C1.22
Conclusions
The binding strength of H-bonded aromatic dimers can be systematically tuned by varying the position of heteroatoms on the backside of the monomers while keeping the H-bond frontier atoms unchanged. These minimal non-frontier modifications can induce large interaction energy changes (ΔΔEint up to 6.5 kcal mol−1) although the charge of the H-bond frontier atoms is almost unaffected, as appears from our quantum-chemical analyses. Nevertheless, the grouping of the polar functional groups on one side of the monomer induces a larger accumulation of positive and negative charge in the molecule. This enhances the intermolecular electrostatic interactions, as well as the σ-orbital interactions by reducing the energy gaps between the interacting molecular orbitals. This is a manifestation of two effects: (i) electrostatic interactions are not only between frontier atoms but also between distant atoms (i.e., long-range); (ii) molecular orbitals are delocalized over the molecule and their energies are therefore influenced by charge changes in non-frontier parts of the molecule. Our findings challenge the concept of explaining H-bond strengths by the frontier atoms, revealing that the strength of the electrostatic interactions, as well as the orbital interactions, are determined by the whole backbone of the H-bond donor and acceptor fragments.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Dutch Research Council (NWO), the Spanish MINECO (PID-2019-106830GB-I00, PID2022-138861NB-I00, and CEX2021-001202-M), and the Generalitat de Catalunya (2021SGR442) for financial support. This work was carried out on the Dutch national e-infrastructure with the support of SURF Cooperative.Notes and references
- (a) J. M. Berg, J. L. Tymoczko and L. Stryer, Biochemistry, W. H. Freeman and Company, New York, 5th edn, 2002 Search PubMed; (b) G. A. Jeffrey and W. Saenger, H-bonding in Biological Structures, Springer, Berlin, 1991 CrossRef.
- (a) S. Vera, A. García-Urricelqui, A. Mielgo and M. Oiarbide, Eur. J. Org. Chem., 2023, 26, e202201254 CrossRef CAS; (b) G. Su, M. Formica, K. Yamazaki, T. A. Hamlin and D. J. Dixon, J. Am. Chem. Soc., 2023, 145, 12771 CrossRef CAS PubMed.
- (a) S. M. Wales, D. T. J. Morris and J. Clayden, J. Am. Chem. Soc., 2022, 144, 2841 CrossRef CAS PubMed; (b) L. N. J. de Windt, Z. Fernández, M. Fernández-Míguez, F. Freire and A. R. A. Palmans, Chem. – Eur. J., 2022, 28, e202103691 CrossRef CAS PubMed; (c) K. Aratsu, R. Takeya, B. R. Pauw, M. J. Hollamby, Y. Kitamoto, N. Shimizu, H. Takagi, R. Haruki, S. I. Adachi and S. Yagai, Nat. Commun., 2020, 11, 1623 CrossRef CAS PubMed.
- (a) R. B. Lin, Y. He, P. Li, H. Wang, W. Zhou and B. Chen, Chem. Soc. Rev., 2019, 48, 1362 RSC; (b) R. Sarkar, S. Majumdar, S. Kuil, J. Mallens, J. J. B. van der Tol, R. P. Sijbesma, J. P. A. Heuts and A. R. A. Palmans, J. Polym. Sci., 2023, 61, 1335 CrossRef CAS.
- (a) E. Arunan, G. R. Desiraju, R. A. Klein, J. Sadlej, S. Scheiner, I. Alkorta, D. C. Clary, R. H. Crabtree, J. J. Dannenberg, P. Hobza, H. G. Kjaergaard, A. C. Legon, B. Mennucci and D. J. Nesbitt, Pure Appl. Chem., 2011, 83, 1619 CrossRef CAS; (b) IUPAC, Compendium of Chemical Terminology, ed. A. D. McNaught, A. Wilkinson, Blackwell Scientific Publications, Oxford, 2nd edn (the “GoldBook”), 1997 Search PubMed.
- (a) W. L. Jorgensen and J. Pranata, J. Am. Chem. Soc., 1990, 112, 2008 CrossRef CAS; (b) J. Pranata, S. G. Wierschke and W. L. Jorgensen, J. Am. Chem. Soc., 1991, 113, 2810 CrossRef CAS.
- (a) M. Papmeyer, C. A. Vuilleumier, G. M. Pavan, K. O. Zhurov and K. Severin, Angew. Chem., Int. Ed., 2016, 55, 1685 ( Angew. Chem. , 2016 , 128 , 1717 ) CrossRef CAS PubMed; (b) G. Bella, A. Santoro, F. Nicolò, G. Bruno and M. Cordaro, ChemPhysChem, 2021, 22, 593 CrossRef CAS PubMed.
- P. L. A. Popelier and L. Joubert, J. Am. Chem. Soc., 2002, 124, 8725 CrossRef CAS PubMed.
- (a) S. C. C. van der Lubbe and C. Fonseca Guerra, Chem. – Asian J., 2019, 14, 2760 CrossRef CAS PubMed; (b) S. C. C. van der Lubbe and C. Fonseca Guerra, Chem. – Eur. J., 2017, 23, 10249 CrossRef CAS PubMed; (c) L. de Azevedo Santos, T. C. Ramalho, T. A. Hamlin and F. M. Bickelhaupt, Chem. – Eur. J., 2022, 29, e202203791 CrossRef PubMed; (d) C. Nieuwland and C. Fonseca Guerra, Chem. – Eur. J., 2022, 28, e202200755 CrossRef CAS PubMed; (e) C. Fonseca Guerra, F. M. Bickelhaupt, J. G. Snijders and E. J. Baerends, Chem. – Eur. J., 1999, 5, 3581 CrossRef; (f) C. Nieuwland, S. J. Lekanne Deprez, C. de Vries and C. Fonseca Guerra, Chem. – Eur. J., 2023, 29, e202300850 CrossRef CAS PubMed; (g) C. Nieuwland, T. A. Hamlin, C. Fonseca Guerra, G. Barone and F. M. Bickelhaupt, ChemistryOpen, 2022, 11, e202100231 CrossRef CAS PubMed; (h) F. Weinhold and R. A. Klein, Angew. Chem., Int. Ed., 2014, 53, 11214 CrossRef CAS PubMed; (i) S. J. Grabowski, Chem. Rev., 2011, 111, 2597 CrossRef CAS PubMed.
- S. C. C. van der Lubbe, F. Zaccaria, X. Sun and C. Fonseca Guerra, J. Am. Chem. Soc., 2019, 141, 4878 CrossRef CAS PubMed.
- S. C. C. van der Lubbe, A. Haim, T. van Heesch and C. Fonseca Guerra, J. Phys. Chem. A, 2020, 124, 9451 CrossRef CAS PubMed.
- E. van Lenthe, A. Ehlers and E. J. Baerends, J. Chem. Phys., 1999, 110, 8943 CrossRef CAS.
- (a) A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS PubMed; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS PubMed; (c) Q. Wu and W. Yang, J. Chem. Phys., 2002, 116, 515 CrossRef CAS.
- (a) S. Grimme, J. Comput. Chem., 2004, 25, 1463 CrossRef CAS PubMed; (b) S. Grimme, J. Comput. Chem., 2006, 27, 1787 CrossRef CAS PubMed; (c) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed; (d) S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456 CrossRef CAS PubMed.
- E. van Lenthe and E. J. Baerends, J. Comput. Chem., 2003, 24, 1142 CrossRef CAS PubMed.
- (a) R. Rüger, M. Franchini, T. Trnka, A. Yakovlev, E. van Lenthe, P. Philipsen, T. van Vuren, B. Klumpers and T. Soini, AMS2022.101, SCM, Theoretical Chemistry, Vrije Universiteit Amsterdam, Amsterdam, 2022, https://www.scm.com Search PubMed; (b) C. Fonseca Guerra, J. G. Snijders, G. te Velde and E. J. Baerends, Theor. Chem. Acc., 1998, 99, 391 Search PubMed; (c) G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef CAS.
- X. Li, M. Diguele Romero, S. Tcaturian, K. Kurpiewska and A. Dömling, J. Org. Chem., 2023, 88, 9823 CrossRef CAS PubMed.
- We focus in our interaction energy analysis on the smaller isostere of guanine, that is G1, which is obtained by removal of the 5-membered ring in guanine. However, this structural alteration does not change the H-bond strength of the corresponding GC base pair (see ESI† Data S4) and yields very similar frontier atomic charges (ESI† Data S3: Fig. S2). Also, there is a third possible guanine isostere (G3) which yields an average effect of G1 and G2 (see ESI† Data S1).
- (a) F. M. Bickelhaupt and E. J. Baerends, in Reviews in Computational Chemistry, ed. K. B. Lipkowitz and D. B. Boyd, Wiley-VCH, New York, 2000, vol. 15, pp. 1–86 Search PubMed; (b) T. A. Hamlin, P. Vermeeren, C. Fonseca Guerra and F. M. Bickelhaupt, in Complementary Bonding Analysis, ed. S. Grabowsky, De Gruyter, Berlin, 2021, vol. 8, pp. 199–212 Search PubMed.
- (a) X. Sun, T. M. Soini, J. Poater, T. A. Hamlin and F. M. Bickelhaupt, J. Comput. Chem., 2019, 40, 2227 CrossRef CAS PubMed; (b) X. Sun, T. Soini, L. P. Wolters, W.-J. van Zeist, C. Fonseca Guerra, T. A. Hamlin and F. M. Bickelhaupt, PyFrag 2019, Vrije Universiteit, Amsterdam, 2019 Search PubMed.
- (a) C. Nieuwland, P. Vermeeren, F. M. Bickelhaupt and C. Fonseca Guerra, J. Comput. Chem., 2023, 44, 2108 CrossRef CAS PubMed; (b) C. Fonseca Guerra, J. W. Handgraaf, E. J. Baerends and F. M. Bickelhaupt, J. Comput. Chem., 2004, 25, 189 CrossRef PubMed.
- For the different GC base-pair isosteres the orbital overlap differences are small and in line with the relative H-bond distances (see ESI† Data S3: Table S4). Therefore, for the trend in the σ-orbital interactions the relative σHOMO–σLUMO energy gaps are more relevant than the overlap.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp05244c |
| This journal is © the Owner Societies 2024 |