Fourier-transform microwave spectroscopy of the ClSO radical†
Ching-Hua
Chang
,
Cheng-Han
Tsai
,
Yi-Ting
Liu
and
Yasuki
Endo
 *
*
Department of Applied Chemistry, Science Building II, National Yang Ming Chiao Tung University, 1001 Ta-Hsueh Rd., Hsinchu 300093, Taiwan. E-mail: endo@nycu.edu.tw
First published on 15th January 2024
Abstract
Pure rotational transitions of the ClSO radical have been observed by Fourier-transform microwave spectroscopy. a-type and b-type transitions, for both 35Cl and 37Cl isotopologues, were detected, and the observed very complicated fine and hyperfine components were assigned well. The intensities of the observed spectra of the two isotopologues correspond to the ratio of the isotope abundances of 35Cl and 37Cl. A total of 21 molecular constants were determined precisely for both 35ClSO and 37ClSO, including the rotational constants, centrifugal distortion constants, electronic spin-rotation constants, nuclear spin-rotation constants, magnetic hyperfine constants, and quadrupole coupling constants of chlorine. The molecular constants show ClSO to have the 2A′′ electronic ground state with an out-of-plane unpaired electron. The spin density of the chlorine atom is about 10.6%, which is similar to that of the fluorine atom for FSO, about 8%. Results of the ClSO radical are compared with those of other triatomic radicals with similar structures, the XSS, XSO, and XOO radicals with X = H, F, and Cl, leading to a conclusion that the ClSO radical is more like FSO, but fairly different from the FOO and ClOO radicals.
Introduction
The sulfinyl chloride radical, ClSO, has been investigated widely as it can be produced by UV absorption of thionyl chloride, Cl2SO,1 which is a common source of Cl atoms in organic syntheses. Cl2SO plays an important role in chemical reactions of chlorine cycles with sulfide and other molecules in the atmosphere. It can dissociate to Cl, ClSO, Cl2 and SO through different UV dissociation pathways,1–3 involving:| Cl2SO → 2Cl + SO three-body dissociation, | (1) |
| Cl2SO → ClSO + Cl Cl elimination, | (2) |
| Cl2SO → Cl2 + SO molecular elimination. | (3) |
Cl2SO photolysis was investigated using step-scan Fourier-transform infrared spectroscopy by Chu et al.4 A mixture of Cl2SO and Ar was photolyzed using a KrF excimer laser at 248 nm, and production of ClSO was confirmed. The S–O stretching (ν1) mode was detected in the region 1120–1200 cm−1 with a resolution of 0.3 cm−1. The rotational structure was revealed for the Q branch at 1162.9 cm−1 in the IR absorption spectrum.
More recently, an absorption spectrum in the region of 260–320 nm was detected by Chao et al.5 They assigned a ClSO band around 300 nm to the 12A′ ← X2A′′ transition. The kinetics of the ClSO + Cl → Cl2SO reaction was also studied using strong UV absorption at pressures in the range of 10–90 torr at 292 K.5 They found strong pressure dependence for kCl+ClSO, and it was not strongly affected by the buffer gas. They also detected the absorption spectrum in the region of 350–480 nm.6 The 12A′ ← X2A′′ and 22A′ ← X2A′′ transitions with a peak at 385 nm were observed. The conical intersection between the 12A′ and 22A′ excited states was calculated, and the geometry of the minimum-energy conical intersection was found to be similar to the ground-state geometry. They found that the two 2A′ excited states mix due to the strong vibronic coupling.
The HSO radical, a member of a series of molecules XSO, has just been observed by Marcelino et al.7 in the interstellar medium (ISM), where the fine and hyperfine components of rotational transitions of HSO were detected toward TMC-1 and the cold clouds L183, L483, L1495B, L1527, and Lupus-1A using Yebes 40 m and IRAM 30 m telescopes. This detection shows that HSO is widespread in cold dense cores. ClSO, which is a chlorine substituted species of HSO, may be one of the candidates to be detected in the ISM, although the Cl abundance is not so high in the ISM.
The pure rotational spectrum of ClSO was studied by Saito et al. around 60 GHz using microwave spectroscopy in a glow discharge of a gas mixture of O2 and S2Cl2 diluted in Ar.8 The rotational constants, the centrifugal distortion constant ΔN, the spin-rotation constants εaa, εbb, and εcc, and the hyperfine constants were determined in their research as given in Table 1. As the instruments being improved recent years, we could investigate this radical in more detail with Fourier transform microwave (FTMW) spectroscopy. It is also possible to study the isotopologues 35ClSO and 37ClSO. The FSO and HSO radicals, which also belong to the XSO molecules, have been studied using microwave and mm-wave spectroscopy,9,10 where their fine and hyperfine constants have been determined precisely. The XSS and XOO radicals are analogous species to the XSO radicals, and that they all have the A′′ ground state, and they also have been studied by rotational spectroscopy.11–18 It is thus interesting to compare the molecular constants with these radicals to see similarities and differences.
| Previous worka | Theoryb | |
|---|---|---|
| a From ref. 8. b Present study. The rotational constants and dipole moments were calculated at RCCSD(T)-F12/cc-pCVTZ, correlating all electrons. Other parameters were calculated at B3LYP/aug-cc-pVTZ. | ||
| A/MHz | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 819.3 819.3 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703.9 703.9 |
| B/MHz | 4553.2 | 4582.5 |
| C/MHz | 3992.4 | 4019.3 |
| |μa|/Debye | 0.59 | |
| |μb|/Debye | 1.83 | |
| |μc|/Debye | 0.00 | |
| ε aa /MHz | −787.8 | −818.1 |
| ε bb /MHz | −78.8 | −88.3 |
| ε cc /MHz | 4.4 | 5.2 |
| a F(Cl)/MHz | 26.4 | 3.6 |
| T aa (Cl)/MHz | −19.0 | −17.8 |
| T bb (Cl)/MHz | −10.9 | −12.7 |
| χ aa (Cl)/MHz | −7.8 | −51.5 |
| χ bb (Cl)/MHz | 3.3 | 22.9 |
Quantum chemical calculations
The molecular structure of 35ClSO was optimized using the open-shell coupled cluster theory with perturbative triples corrections (RCCSD(T)) with explicitly correlated approximation (F12A) using the basis set of cc-pCVTZ-F12,19,20 where all electrons were correlated. Calculations were performed using the MOLPRO 2022.3 program.21 The rotational constants of the RCCSD(T)-F12A/cc-pCVTZ-F12 optimized structure are Ae = 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703.8 MHz, Be = 4582.5 MHz, and Ce = 4019.3 MHz. The values agree with those reported in ref. 8 as shown in Table 1. The calculated dipole moment component, μb, is much larger than μa. The fine and hyperfine constants were calculated using the DFT method with the B3LYP/aug-cc-pVTZ level of theory performed using the Gaussian 16 computational chemistry software package.22 Although the DFT calculation reproduces well the spin-rotation constants and the dipolar hyperfine coupling constants, the calculated Fermi contact coupling constant and the quadrupole coupling constants are different from those reported in ref. 8.
703.8 MHz, Be = 4582.5 MHz, and Ce = 4019.3 MHz. The values agree with those reported in ref. 8 as shown in Table 1. The calculated dipole moment component, μb, is much larger than μa. The fine and hyperfine constants were calculated using the DFT method with the B3LYP/aug-cc-pVTZ level of theory performed using the Gaussian 16 computational chemistry software package.22 Although the DFT calculation reproduces well the spin-rotation constants and the dipolar hyperfine coupling constants, the calculated Fermi contact coupling constant and the quadrupole coupling constants are different from those reported in ref. 8.
The molecular constants of 37ClSO were calculated using the same methods as those of 35ClSO. Then, the rotational constants and the spin-rotation constants were scaled with the calculated values and experimentally determined values of 35ClSO. The magnetic hyperfine constants and the quadrupole constants of 37ClSO were calculated from the experimental values of 35ClSO, which were scaled by the ratios of the magnetic moments and the quadrupole moments of 35Cl and 37Cl, respectively.23
Experimental
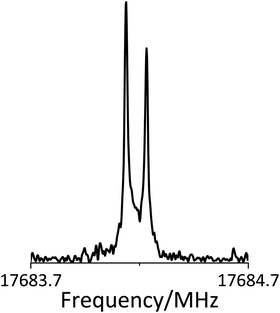
The pure rotational transitions of the ClSO radical were observed using a Balle-Flygare type Fourier-transform microwave (FTMW) spectrometer and a double resonance system, which have been described in detail previously.24 In brief, the ClSO radical was generated in a supersonic jet in a pulsed-electronic discharge of a gas mixture, ca. 0.3% of Cl2SO diluted in Ar or Ne. The mixture, at a stagnation pressure of typically 3 atm, was transferred to the vacuum chamber through a pulsed-solenoid valve located at the center of one of the mirrors for the Fabry–Pérot cavity. The radicals were produced by an electric discharge in the electrodes attached in front of the nozzle of the pulsed valve applying a pulsed voltage of 1.3 kV. The rotational temperature inside the cavity was below 2.5 K. Since the ClSO radical is an open-shell species, 3 Helmholtz coils surrounding the cavity were set in this study to compensate the Earth's magnetic field. The microwave pulse was emitted from an antenna located at the center of the other mirror of the cavity to polarize the molecules. Since the microwave pulse propagates parallel to the jet from the pulse nozzle, the spectral lines show Doppler doublings as seen in Fig. 1.The FTMW spectrometer operates in the frequency range of 4–40 GHz to measure low-N pure rotational transitions. Transitions above 40 GHz, including the 322–413, 321–414, and 221–312 transitions in the present study, were observed with an FTMW-MW double-resonance technique.25 A microwave signal was monitored using the FTMW spectrometer, and another microwave radiation was irradiated perpendicular to the monitored microwave in the cavity as a pump microwave to measure the double-resonance signals. If a rotational transition, whose upper or lower level is shared by the monitored transition, is induced by the pump microwave, the double resonance signal is observed as a decrease of the monitor signal due to the destruction of coherence. Estimated experimental accuracies of the frequency measurements for normal FTMW experiments are about 3 kHz or less, while those of the double resonance experiments are about 10 kHz or a little bit more for weak lines when we have to put more power to observe the double resonance signals.
Results
First, b-type transitions were searched since the calculated dipole moment component μb is much larger than μa. The 303–312 transition of 35ClSO was expected in the region of 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110–30
110–30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 MHz. Four strong paramagnetic lines accompanied by weak lines were observed around 30
210 MHz. Four strong paramagnetic lines accompanied by weak lines were observed around 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 140 MHz, which is very close to the frequencies predicted by the ab initio calculations and the results in ref. 8 shown in Table 1. Then, we scanned the region from 30
140 MHz, which is very close to the frequencies predicted by the ab initio calculations and the results in ref. 8 shown in Table 1. Then, we scanned the region from 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 350 to 30
350 to 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 to observe the other spin component. In this way, many paramagnetic lines were observed near the predicted frequencies. The observed fine and hyperfine patterns were reproduced well by the calculated fine and hyperfine constants. An example of the observed spectral pattern of the 35ClSO radical is shown in Fig. 2. The upper spectrum shows the spectral lines with the Earth's magnetic field compensation, and the inverted one shows those without the compensation. The spectral lines from open-shell species disappeared or split without the compensation, showing the paramagnetic behavior. After observing b-type transitions with Ka = 0 and 1, we tried to observe a-type transitions. Although the dipole moment μa is small in the calculations, the spectral lines of 101–000, 202–101, and 303–202 transitions were expected to be sufficiently strong because of the low rotational temperature inside the cavity. Later, we changed the buffer gas from Ar to Ne, since Ne is expected to have a less effective cooling effect and makes the rotational temperature higher. We tried to observe the a-type transitions with Ka = 2 with a sample gas, 0.3% of Cl2SO diluted in Ne. The intensity of the 322–221 transition was expected to be 11 times weaker than that of the 303–202 transition when the rotational temperature is assumed to be 2.5 K. However, the actual ratio was about 30 times weaker. The rotational temperature inside the cavity may be much lower than 2.5 K, even when Ne was used as a diluent gas. We also used the mixture diluted by Ne to detect the 624–717 and 716–707 transitions because the energy levels of these states are relatively high.
500 to observe the other spin component. In this way, many paramagnetic lines were observed near the predicted frequencies. The observed fine and hyperfine patterns were reproduced well by the calculated fine and hyperfine constants. An example of the observed spectral pattern of the 35ClSO radical is shown in Fig. 2. The upper spectrum shows the spectral lines with the Earth's magnetic field compensation, and the inverted one shows those without the compensation. The spectral lines from open-shell species disappeared or split without the compensation, showing the paramagnetic behavior. After observing b-type transitions with Ka = 0 and 1, we tried to observe a-type transitions. Although the dipole moment μa is small in the calculations, the spectral lines of 101–000, 202–101, and 303–202 transitions were expected to be sufficiently strong because of the low rotational temperature inside the cavity. Later, we changed the buffer gas from Ar to Ne, since Ne is expected to have a less effective cooling effect and makes the rotational temperature higher. We tried to observe the a-type transitions with Ka = 2 with a sample gas, 0.3% of Cl2SO diluted in Ne. The intensity of the 322–221 transition was expected to be 11 times weaker than that of the 303–202 transition when the rotational temperature is assumed to be 2.5 K. However, the actual ratio was about 30 times weaker. The rotational temperature inside the cavity may be much lower than 2.5 K, even when Ne was used as a diluent gas. We also used the mixture diluted by Ne to detect the 624–717 and 716–707 transitions because the energy levels of these states are relatively high.
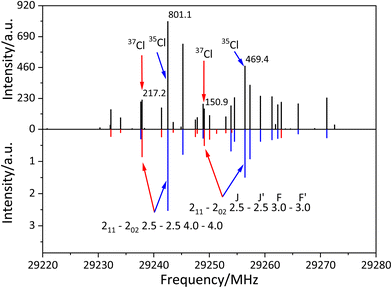
After assigning the fine and hyperfine components of 35ClSO, we tried to detect 37ClSO using the scaled constants as shown in Table 3. Since the patterns of the 211–202 transitions of 35ClSO and 37ClSO were quite similar, transitions of 37ClSO were easily assigned as shown in Fig. 3. The spectral lines of 37ClSO were three times weaker than those of 35ClSO as expected by the population ratio of 35Cl and 37Cl. We used this fact to distinguish the lines of 37ClSO in the complicated spectrum.
The FTMW-MW double resonance technique was applied to observe the spectral lines with transition frequencies above 40 GHz, after the fine and hyperfine components of the transitions below 40 GHz were observed. Only a small region was required to scan for observing each line since the prediction was already very accurate.
Finally, a total of 15 b-type transitions and 8 a-type transitions of 35ClSO with N up to 7 and Ka = 0, 1, and 2 were observed for 35ClSO. The observed transition frequencies are shown in Table S1 of the ESI.† In addition, a total of 9 b-type transitions and 6 a-type transitions with N up to 7 and Ka = 0, 1, and 2 were observed for 37ClSO. The observed transition frequencies are given in Table S2 of the ESI.†
The observed transition frequencies were analyzed with the Hamiltonian,
| H = Hrot + Hcd + Hsr + Hnsr + Hhf + HQ |
| J = N + S, |
| F = J + I(Cl). |
For the 35ClSO radical, the transition frequencies of the observed 244 lines were subjected to the least squares analysis using our proprietary program, where smaller weights were given for the lines measured by the double resonance method. A total of 21 molecular constants were determined with the standard deviation of the fit to be 2.3 kHz. Among the five centrifugal distortion constants, δK was fixed to the value obtained by the quantum chemical calculations, since it was not determinable in the fit. Similarly, for the 37ClSO radical, a total of 186 spectral lines were observed, and 21 molecular constants were determined with the standard deviation of the fit to be 1.9 kHz. The determined molecular constants of the two isotopologues are given in Tables 2 and 3.
| Exp. | Theoryc | |
|---|---|---|
| a Values in parentheses are 1σ errors applied to the last digits of the parameters. b Standard deviation of the fit is 2.3 kHz. c The rotational constants were calculated at RCCSD(T)-F12/cc-pCVTZ, correlating all electrons. Other parameters were calculated at B3LYP/aug-cc-pVTZ. d The value without uncertainty is kept fixed to that obtained theoretically. | ||
| A | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823.64409(37) 823.64409(37) |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 703.9 703.9 |
| B | 4553.94358(11) | 4582.5 |
| C | 3992.35645(10) | 4019.3 |
| Δ N/10−3 | 2.6054(20) | 2.6 |
| Δ NK/10−3 | −21.832(23) | −21.2 |
| Δ K/10−3 | 599.554(93) | 573.0 |
| δ N/10−3 | 0.47257(93) | 0.45 |
| δ K/10−3 | 14.0d | 14.0 |
| ε aa | −779.83736(79) | −818.1 |
| ε bb | −85.56951(29) | −88.3 |
| ε cc | 3.54021(34) | 5.2 |
| ε ab | −36.0827(93) | −26.8 |
| a F(Cl) | 3.23324(37) | 3.6 |
| T aa (Cl) | −17.33846(63) | −17.8 |
| T bb (Cl) | −11.74443(58) | −12.7 |
| T ab (Cl) | 3.060(29) | 1.25 |
| χ aa (Cl) | −51.9147(15) | −51.5 |
| χ bb (Cl) | 22.3736(16) | 22.9 |
| χ ab (Cl) | 26.02(16) | 25.6 |
| C aa (Cl) | 0.00670(36) | 0.0070 |
| C bb (Cl) | 0.00229(12) | 0.0025 |
| C ab (Cl) | 0.00262(14) | 0.0026 |
| Exp. | Scaledc | |
|---|---|---|
| a Values in parentheses are 1σ errors applied to the last digits of the parameters. b Standard deviation of the fit is 1.9 kHz. c The parameters were calculated using the same methods for 35ClSO, and they were scaled with the experimental values for 35ClSO. d The scaled hyperfine constants and the quadrupole coupling constants are calculated using the ratios of the magnetic moments and quadrupole moments of the two isotopes, respectively. e The value without uncertainty is kept fixed to that obtained theoretically. | ||
| A | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 713.93290(48) 713.93290(48) |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676.9 676.9 |
| B | 4422.36640(16) | 4420.1 |
| C | 3889.73021(21) | 3887.3 |
| Δ N/10−3 | 2.4706(45) | 2.4 |
| Δ NK/10−3 | −20.944(53) | −20.9 |
| Δ K/10−3 | 593.00(13) | 593.0 |
| δ N/10−3 | 0.4366(47) | 0.4 |
| δ K/10−3 | 13.5e | 13.5 |
| ε aa | −777.00024(71) | −773.0 |
| ε bb | −83.24599(28) | −83.6 |
| ε cc | 3.44907(42) | 3.5 |
| ε ab | −35.2860(93) | −36.0 |
| a F(Cl)d | 2.69324(39) | 2.7 |
| T aa (Cl) | −14.44729(59) | −14.4 |
| T bb (Cl) | −9.76197(62) | −9.8 |
| T ab (Cl) | 2.515(25) | 2.6 |
| χ aa (Cl)e | −41.0592(14) | −40.9 |
| χ bb (Cl) | 17.7810(15) | 17.6 |
| χ ab (Cl) | 20.38(15) | 20.6 |
| C aa (Cl) | 0.00610(36) | 0.0058 |
| C bb (Cl) | 0.00160(13) | 0.0020 |
| C ab (Cl) | 0.00217(15) | 0.0021 |
As seen in Table 2, the experimental results agree very well with those by the quantum chemical calculations. Compared to the constants given in ref. 8, the three rotational constants agree with their results with differences of 0.01%. In addition, our experimental values of the spin-rotation constants and the magnetic dipolar coupling constants agree with their values with differences of about 10% or even less. However, the experimental results for the Fermi contact coupling constant and the quadrupole coupling constants are quite different from their values.
Discussion
The molecular constants of ClSO show characteristic features of the radical, especially for the unpaired electron. The negative value of the electron spin-rotation constant εaa indicates that the unpaired electron occupies the π-orbital perpendicular to the molecular plane, or the radical has the 2A′′ ground electronic state. Table 4 compares the rotational constants and the spin-rotation constants for related radicals, XSO, XSS, and XOO, where X = H, F, and Cl. All of them listed here have negative εaa, and are considered to have the 2A′′ ground electronic state. Most of the radicals in Table 4 have very small εcc values, except for FOO and ClOO, which are also the characteristic for triatomic radicals with the 2A′′ ground states.| A | B | C | ε aa | ε bb | ε cc | ε ab | |
|---|---|---|---|---|---|---|---|
| a Present study. b From ref. 10. c From ref. 9. d From ref. 17. e From ref. 12. f From ref. 11. g From ref. 15. h From ref. 16. i From ref. 14. | |||||||
| ClSOa | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823.64 823.64 |
4553.94 | 3992.36 | −779.84 | −85.57 | 3.54 | −36.08 |
| HSOb | 299![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 484.63 484.63 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 504.56 504.56 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133.93 133.93 |
−10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365.99 365.99 |
−426.66 | 0.23 | 378.0 |
| FSOc | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 698.18 698.18 |
9340.81 | 7505.06 | −339.54 | 34.90 | 1.86 | 207.95 |
| HSSd | 296![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 978.96 978.96 |
7996.37 | 7776.74 | −45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 926.76 926.76 |
−424.36 | 10.07 | 234.70 |
| FSSe | 26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563.82 563.82 |
4864.68 | 4103.89 | −742.18 | −148.29 | 2.70 | 260.56 |
| ClSSf | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319.80 319.80 |
2827.55 | 2445.93 | −1329.1 | −121.29 | 1.73 | 17.9 |
| HOOg | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0273.22 0273.22 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 517.82 517.82 |
31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667.65 667.65 |
−49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571.41 571.41 |
−422.76 | 8.61 | 0.39 |
| FOOh | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 430.14 430.14 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 013.94 013.94 |
8855.25 | −887.60 | −47.96 | 20.85 | 69.31 |
| ClOOi | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 541.99 541.99 |
4936.73 | 4621.17 | −1325.69 | 17.31 | 113.37 | −38.2 |
The positive value of aF is mainly attributed to the spin polarization effect of the Cl–S σ-bonding orbital due to the unpaired π-electron on the chlorine atom. Moreover, since the F–S bond is shorter than the Cl–S bond and the magnetic moment of the F atom is larger than that of the Cl atom, aF of FSO has a much larger value. On the other hand, for the XOO radicals (X = F or Cl), the unpaired electron orbital retains the π* nature of the O2 molecule, leading to much longer and weaker X–O bonds than those of other species, and the Fermi contact constants aF of the XOO radicals have negative values. In the case of the HSO, HSS and HOO radicals, there is no spin density on the hydrogen atoms, and the negative values of aF are due to the spin-polarization of the H–S or H–O σ-bond due to the unpaired electron density on the neighboring S/O atom.
The calculated unpaired electron orbital of ClSO is shown in Fig. 4 along with its principal axes. Since Taa + Tbb + Tcc = 0, Tcc is calculated to be 29.1 MHz for 35ClSO. From this value, the spin density on the chlorine pc orbital is estimated to be about 10.6%,27 which is slightly larger than the spin density on the fluorine pc orbital of FSO, about 8%.9 In principle, the relationship, Tcc ≅ −2 Taa ≅ −2 Tbb, is satisfied for the magnetic dipolar constants for species with an unpaired electron in an out-of-plane pc orbital of the nucleus. The dipolar coupling constants of the related radicals are given in Table 5. Similar to FSO, the ClSO radical roughly follows this rule, while it is not satisfied for XOO radicals since the unpaired electron on the XOO radical is almost confined to the O2 part.
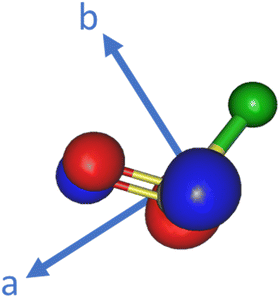 | ||
| Fig. 4 Figure of the ClSO radical showing the principal axes (a and b) and the unpaired electron orbital, where the green ball is the chlorine atom. | ||
| a F | T aa | T bb | T cc | |Tab|i | |
|---|---|---|---|---|---|
| a Present study. b From ref. 10. c From ref. 9. d From ref. 17. e From ref. 12. f From ref. 13. g From ref. 16. h From ref. 14. i The relative signs correspond to εab. | |||||
| ClSOa | 3.23 | −17.33 | −11.74 | 29.07 | 3.06 |
| HSOb | −36.37 | −11.96 | 10.44 | 1.52 | −7.8 |
| FSOc | 67.23 | −118.16 | −117.06 | 235.12 | 10.29 |
| HSSd | −22.42 | −5.87 | 5.84 | 0.03 | −4.20 |
| FSSe | 44.17 | −75.14 | −74.50 | 149.64 | 16.61 |
| HOOf | −27.48 | −8.34 | 19.68 | −11.34 | −18.3 |
| FOOg | −33.87 | −212.86 | 9.31 | 203.55 | 139.18 |
| ClOOh | −11.17 | −38.87 | 11.51 | 27.36 | 16.62 |
The rotation angle from the a-axis to the x-axis is about 23.8°, as shown in Table 6, where x, y, and z are the principal axes of the magnetic dipolar interaction tensor, where the z-axis is identical to the c-axis. It is larger than the angle between the Cl–S bond and the a-axis which is calculated to be 17.8°. It is difficult to explain the difference, since the unpaired electron on the oxygen atom also contributes to the tensor. The principal axis values of the quadrupole coupling tensor are also given in Table 6. In this case, the rotation angle from the a-axis to the x-axis is 17.5°, which clearly shows that the principal axis x of the quadrupole coupling tensor is almost parallel to the Cl–S bond and their components are axially symmetric. The value of the quadrupole coupling constant χxx along the Cl–S axis, −60.1 MHz, indicates that the Cl–S bond has a character between the ionic and the covalent characters.26
| 35ClSO | 37ClSO | |
|---|---|---|
| a The rotation angle from the a-axis to the x-axis, in degree. b The rotation angle from the a-axis to the x-axis, in degree. | ||
| a F(Cl) | 3.23 | 2.69 |
| θ T /deg. | 23.8 | 24.3 |
| T xx (Cl) | −18.69 | −15.54 |
| T yy (Cl) | −10.40 | −8.67 |
| T zz (Cl) | 29.09 | 24.21 |
| θ χ /deg. | 17.5 | 17.6 |
| χ xx (Cl) | −60.12 | −47.43 |
| χ yy (Cl) | 30.58 | 24.15 |
| χ zz (Cl) | 29.54 | 23.28 |
The r0 structure of ClSO was determined from the moments of inertia for the two isotopologues. The determined structural parameters are compared with those of related molecules in Table 7. The S–O bond lengths of FSO and ClSO are shorter than that of SO.28 For the XSS radicals, they still follow this tendency.11,12 Furthermore, the S–S bond lengths of the FSS and ClSS radicals are between those of the S2 and S3 molecules.29,30 On the other hand, the O–O bond lengths of the XOO (X = F or Cl) radicals are almost the same as that of the O2 molecule.31 This fact indicates that the O2 part of the XOO radicals retain the character of the O2 molecule.
| r(X–S or X–O)/Å | r(S–S, S–O or O–O)/Å | ∠(XSS, XSO or XOO)/deg. | |
|---|---|---|---|
| a Present study. b From ref. 9. c From ref. 28. d From ref. 32. e From ref. 33. f From ref. 17. g From ref. 12. h From ref. 11. i From ref. 29. j From ref. 30. k From ref. 15. l From ref. 16. m From ref. 18. n From ref. 31. o From ref. 34. | |||
| ClSOa | 2.052 | 1.463 | 109.86 |
| FSOb | 1.602 | 1.452 | 108.32 |
| SOc | 1.481 | ||
| SO2d | 1.431 | ||
| SFe | 1.587 | ||
| HSSf | 1.364 | 1.965 | 101.7 |
| FSSg | 1.595 | 1.915 | 107.8 |
| ClSSh | 2.071 | 1.906 | 110.3 |
| S2i | 1.889 | ||
| S3j | 1.917 | ||
| HOOk | 0.972 | 1.329 | 104.5 |
| FOOl | 1.649 | 1.200 | 111.2 |
| ClOOm | 2.084 | 1.207 | 115.4 |
| O2n | 1.208 | ||
| ClOo | 1.570 | ||
Conclusions
Pure rotational transitions of both the 35Cl and 37Cl isotopologues were observed by FTMW spectroscopy, and the observed fine and hyperfine components for each rotational transition were assigned well. The molecular constants including the fine and hyperfine coupling constants are determined precisely in this study. We compared the determined constants of ClSO to those of a number of triatomic radicals with same symmetry and similar structures. ClSO is found to be similar to FSO, but fairly different from FOO and ClOO. Since HSO has just been detected by Marcelino et al. in interstellar space7 and the formation of the ClSO radical has been studied extensively, the present data could be facilitated for the detection of atmospheric or interstellar ClSO.Author contributions
Ching-Hua Chang: data curation, formal analysis, investigation, and writing – review and editing. Cheng-Hua Tsai: formal analysis and investigation. Yi-Ting Liu: formal analysis and investigation. Yasuki Endo: conceptualization, data curation, formal analysis, funding acquisition, methodology, software, and writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Ministry of Science and Technology, Taiwan (grants MOST108-2113-M-009-025 and MOST109-2113-M-009-024).References
- A. P. Uthman, P. J. Demlein, T. D. Allston, M. C. Withiam, M. J. McClements and G. A. Takacs, Photoabsorption spectra of gaseous methyl bromide, ethylene dibromide, nitrosyl bromide, thionyl chloride, and sulfuryl chloride, J. Phys. Chem., 1978, 82, 2252–2257 CrossRef CAS
.
- H. Wang, X. Chen and B. R. Weiner, Laser photodissociation dynamics of thionyl chloride: concerted and stepwise cleavage of S-Cl bonds, J. Phys. Chem., 1993, 97, 12260–12268 CrossRef CAS
.
- G. Baum, C. Effenhauser, P. Felder and J. Huber, Photofragmentation of thionyl chloride: Competition between radical, molecular, and three-body dissociations, J. Phys. Chem., 1992, 96, 756–764 CrossRef CAS
.
- L.-K. Chu, Y.-P. Lee and E. Y. Jiang, Detection of ClSO with time-resolved Fourier-transform infrared absorption spectroscopy, J. Chem. Phys., 2004, 120, 3179–3184 CrossRef CAS PubMed
.
- W. Chao, G. H. Jones, M. Okumura, C. J. Percival and F. A. F. Winiberg, Spectroscopic and Kinetic Studies of the ClSO Radical from Cl2SO Photolysis, J. Am. Chem. Soc., 2022, 144, 20323–20331 CrossRef CAS PubMed
.
- W. Chao, G. H. Jones, M. Okumura, C. J. Percival and F. A. F. Winiberg, A-Band Absorption Spectrum of the ClSO Radical: Electronic Structure of the Sulfinyl Group, J. Phys. Chem. A, 2023, 127, 8374–8382 CrossRef CAS PubMed
.
- N. Marcelino, C. Puzzarini, M. Agúndez, R. Fuentetaja, B. Tercero, P. de Vicente and J. Cernicharo, First detection of the HSO radical in space, Astron. Astrophys., 2023, 674, L13 CrossRef CAS
.
- S. Saito, Y. Endo and E. Hirota, presented in part at the General Discussion on Molecular Structure, Fukuoka, 1980.
- Y. Endo, S. Saito and E. Hirota, Microwave spectrum, spin–rotation, and hyperfine interaction constants, dipole moment, molecular structure, and harmonic force constants of the FSO radical, J. Chem. Phys., 1981, 74, 1568–1579 CrossRef CAS
.
- Y. Endo, S. Saito and E. Hirota, Microwave spectra of the HSO and DSO radicals, J. Chem. Phys., 1981, 75, 4379–4384 CrossRef CAS
.
- M. Fujitake and E. Hirota, The microwave spectrum of the ClS2 free radical, Can. J. Phys., 1994, 72, 1043–1050 CrossRef CAS
.
- J. Tang and S. Saito, Microwave spectrum of the FS2 radical in the ground electronic
![[X with combining tilde]](https://www.rsc.org/images/entities/char_0058_0303.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2A′′ state, J. Chem. Phys., 1996, 104, 7437–7443 CrossRef CAS
2A′′ state, J. Chem. Phys., 1996, 104, 7437–7443 CrossRef CAS .
- C. E. Barnes, J. M. Brown, A. Carrington, J. Pinkstone, T. J. Sears and P. J. Thistlethwaite, The EPR spectrum of the HO2 radical and determination of ground state parameters, J. Mol. Spectrosc., 1978, 72, 86–101 CrossRef CAS
.
- K. Suma, Y. Sumiyoshi and Y. Endo, Fourier transform microwave spectroscopy and Fourier transform microwave–millimeter wave double resonance spectroscopy of the ClOO radical, J. Chem. Phys., 2004, 121, 8351–8359 CrossRef CAS PubMed
.
- A. Charo and F. C. De Lucia, The millimeter and submillimeter spectrum of HO2: The effects of unpaired electronic spin in a light asymmetric rotor, J. Mol. Spectrosc., 1982, 94, 426–436 CrossRef CAS
.
- M. Bogey, P. B. Davies, C. Demuynck, J. L. Destombes and T. J. Sears, The microwave spectrum of the FO2 radical, Mol. Phys., 1989, 67, 1033–1051 CrossRef CAS
.
- M. Tanimoto, T. Klaus, H. S. P. Müller and G. Winnewisser, Rotational Spectra of the Thiosulfeno Radical, HSS and DSS, between 0.3 and 0.9 THz, J. Mol. Spectrosc., 2000, 199, 73–80 CrossRef CAS PubMed
.
- H. Uehara, K. Kawaguchi and E. Hirota, Diode laser spectroscopy of the ν3 and ν2 fundamental bands of DO2, J. Chem. Phys., 1985, 83, 5479–5485 CrossRef CAS
.
- G. Knizia, T. B. Adler and H. J. Werner, Simplified CCSD(T)-F12 methods: theory and benchmarks, J. Chem. Phys., 2009, 130, 054104 CrossRef PubMed
.
- J. G. Hill, S. Mazumder and K. A. Peterson, Correlation consistent basis sets for molecular core-valence effects with explicitly correlated wave functions: The atoms B–Ne and Al–Ar, J. Chem. Phys., 2010, 132, 054108 CrossRef PubMed
.
-
H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby, M. Schütz, P. Celani, W. Györffy, D. Kats, T. Korona, R. Lindh, A. Mitrushenkov, G. Rauhut, K. R. Shamasundar, T. B. Adler, R. D. Amos, S. J. Bennie, A. Bernhardsson, A. Berning, D. L. Cooper, M. J. O. Deegan, A. J. Dobbyn, F. Eckert, E. Goll, C. Hampel, A. Hesselmann, G. Hetzer, T. Hrenar, G. Jansen, C. Köppl, S. J. R. Lee, Y. Liu, A. W. Lloyd, Q. Ma, R. A. Mata, A. J. May, S. J. McNicholas, W. Meyer, T. F. Miller III, M. E. Mura, A. Nicklass, D. P. O’Neill, P. Palmieri, D. Peng, K. Pflüger, R. Pitzer, M. Reiher, T. Shiozaki, H. Stoll, A. J. Stone, R. Tarroni, T. Thorsteinsson, M. Wang and M. Welborn, MOLPRO, version 2022.1, a package of ab initio programs, 2022, see https://www.molpro.net Search PubMed
.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, CT2016, Gaussian, Wallingford Search PubMed.
- N. J. Stone, Table of nuclear magnetic dipole and electric quadrupole moments, At. Data Nucl. Data Tables, 2005, 90, 75–176 CrossRef CAS
.
- C. Cabezas, J.-C. Guillemin and Y. Endo, Fourier-transform microwave spectroscopy of a halogen substituted Criegee intermediate ClCHOO, J. Chem. Phys., 2016, 145, 184304 CrossRef PubMed
.
- Y. Sumiyoshi, H. Katsunuma, K. Suma and Y. Endo, Spectroscopy of Ar–SH and Ar–SD. I. Observation of rotation-vibration transitions of a van der Waals mode by double-resonance spectroscopy, J. Chem. Phys., 2005, 123, 054324 CrossRef PubMed
.
-
W. Gordy, in Microwave Molecular Spectra, ed. R. L. Cook, Wiley, New York, 3rd edn, 1984 Search PubMed
.
- J. R. Morton, Electron Spin Resonance Spectra of Oriented Radicals, Chem. Rev., 1964, 64, 453–471 CrossRef CAS
.
- K. Kawaguchi, C. Yamada and E. Hirota, Laser magnetic resonance spectroscopy of SO in the X
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3Σ− state with a CO2 laser as a source, J. Chem. Phys., 1979, 71, 3338–3345 CrossRef CAS
3Σ− state with a CO2 laser as a source, J. Chem. Phys., 1979, 71, 3338–3345 CrossRef CAS .
- E. H. Fink, H. Kruse and D. A. Ramsay, The high-resolution emission spectrum of S2 in the near infrared: The b1Σg + -X3Σg− system, J. Mol. Spectrosc., 1986, 119, 377–387 CrossRef CAS
.
- M. C. McCarthy, S. Thorwirth, C. A. Gottlieb and P. Thaddeus, The Rotational Spectrum and Geometrical Structure of Thiozone, S3, J. Am. Chem. Soc., 2004, 126, 4096–4097 CrossRef CAS PubMed
.
-
K. P. Huber and G. Herzberg, Molecular Spectra and Molecular Structure IV. Constants of Diatomic Molecules, Van Nostrand Reinhold, New York, 1979 Search PubMed
.
- S. Saito, Microwave spectrum of sulfur dioxide in doubly excited vibrational states and determination of the γ constants, J. Mol. Spectrosc., 1969, 30, 1–16 CrossRef CAS
.
- T. Amano and E. Hirota, Microwave spectrum of the SF radical, J. Mol. Spectrosc., 1973, 45, 417–419 CrossRef CAS
.
- T. Amano, S. Saito, E. Hirota, Y. Morino, D. R. Johnson and F. X. Powell, Microwave spectrum of the ClO radical, J. Mol. Spectrosc., 1969, 30, 275–289 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available: Measured transition frequencies. See DOI: https://doi.org/10.1039/d3cp06185j |
| This journal is © the Owner Societies 2024 |