Effect of electric fields on tungsten distribution in Na2WO4–WO3 molten salt
Yuliang
Guo
a,
Xiaobo
Sun
ab,
Handong
Jiao
 c,
Liwen
Zhang
ab,
Wenxuan
Qin
d,
Xiaoli
Xi
c,
Liwen
Zhang
ab,
Wenxuan
Qin
d,
Xiaoli
Xi
 *ae and
Zuoren
Nie
*abe
*ae and
Zuoren
Nie
*abe
aCollaborative Innovation Center of Capital Resource-Recycling Material Technology, College of Materials Science and Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: xixiaoli@bjut.edu.cn
bNational Engineering Laboratory for Industrial Big-data Application Technology, Beijing University of Technology, Beijing 100124, China
cInstitute of Advanced Structure Technology, Beijing Institute of Technology, Beijing 100081, China
dSchool of Chemistry and Materials Engineering, Xinxiang University, Xinxiang, Henan 453003, China
eKey Laboratory of Advanced Functional Materials, Ministry of Education, Beijing University of Technology, Beijing 100124, China
First published on 31st January 2024
Abstract
Tungsten coatings have unique properties such as high melting points and hardness and are widely used in the nuclear fusion and aviation fields. In experiments, compared to pure Na2WO4 molten salt, electrolysis with Na2WO4–WO3 molten salt results in a lower deposition voltage. Herein, an investigation combining experimental and computational approaches was conducted, involving molecular dynamics simulations with deep learning, high-temperature in situ Raman spectroscopy and activation strain model analysis. The results indicated that the molten salt system's behaviour, influenced by migration and polarization effects, led to increased formation of Na2W2O7 in the Na2WO4–WO3 molten salt, which has a lower decomposition voltage and subsequently accelerated the cathodic deposition of tungsten. We analyzed the mechanism of the effect of the electric field on the Na2W2O7 structure based on the bond strength and electron density. This research provides crucial theoretical support for the effect of electric field on tungsten in molten salt and demonstrates the feasibility of using machine learning-based DPMD methods in simulating tungsten-containing molten salt systems.
Introduction
Tungsten is a rare refractory metal with exceptional physicochemical properties, including high melting point and great hardness, making it promising for applications in many fields like fusion devices and aerospace.1–3 The Na2WO4–WO3 molten salt system is predominantly utilized for tungsten coating since WO3 is stable and relatively easy to prepare.4–6 Electrodeposition of tungsten with molten salts as a medium is an effective approach in the production of tungsten coatings7–12 and has garnered considerable attention.13–18 In terms of electrode selection, studies have suggested that copper electrodes in Na2WO4–WO3 molten salt can produce dense tungsten coatings.19,20 Nevertheless, few theoretical research studies were conducted to reveal the mechanism in the process of tungsten coating.21–23 First-principles molecular dynamics simulation is useful for a deep understanding of the mechanisms, although accurate to study statistical properties of the molten salt system, such as radial distribution functions (RDF); however, it requires substantial computational resources. Electric fields may have an impact on reactions in solutions,24 and the research on this effect in molten salts is of great significance for understanding the principles of electrodeposition.The development of machine learning methods brings more possibilities in mechanism research.25,26 There have been reports on the application of deep potential molecular dynamics (DPMD),27 a machine learning based molecular dynamics simulation method with first-principles accuracy. DPMD has been used to study molten salts with thermodynamics.28–30 The deposition potential of the system is obtained by calculating the Gibbs free energy of the system,31 and the effect of temperature on the molten salt system is studied by calculating parameters such as RDF and viscosity.32 As far as we know, there are no research studies on molten salt systems containing tungsten using machine learning methods like DPMD. The expansion of DPMD under similar systems is also of great importance. This work also conducts simulation and experimental analysis on systems different from the training data set. This innovative application demonstrates good accuracy while saving significant computing resources.
In this work, we studied the composition and electrochemical properties of Na2WO4 and Na2WO4–WO3 molten salts through the electrolysis, which indicate that, compared to Na2WO4 molten salt, the existence of WO3 species lead to a lower deposition voltage. We then employed the DPMD method to acquire a potential energy function from a database generated by CP2K.33 Subsequently, we applied this potential energy function in LAMMPS34 to perform larger-scale molecular dynamics simulations with first-principles precision. The analysis of simulation results under various temperature and voltage conditions not only verifies the accuracy of the simulation outcomes but also provides further insights into why tungsten deposits more readily in Na2WO4–WO3 molten salt and the specific mechanism with the bond strength and electron density. This work provides essential theoretical guides for the development of tungsten coating processes.
Method
This work includes the following three parts of experimental content. First, the deposition conditions of tungsten in different molten salt systems were analysed in electrochemical experiments. Next, molecular dynamics simulations with deep potential were performed to analyse the effects of temperature and electric field on tungsten deposition in the Na2WO4–WO3 molten salt system. Finally, high-temperature in situ Raman tests were conducted on Na2WO4–WO3 molten salt under different voltages to verify the results of molecular dynamics simulations.Electrochemical experiments
In the electrodeposition experiments and electrochemical tests, the molten salts of Na2WO4 and Na2WO4–WO3 (molar ratio: 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were heated to 1173 K in a tube furnace with argon atmosphere and kept for 3 hours to melt fully. The electrodeposition process and electrochemical measurements are controlled by the electrochemical workstation VersaSTAT4 (AMETEK-Drexelbrook). A three-electrode system was used in the electrochemical test, in which a W rod was the auxiliary electrode, Pt wire was the reference electrode, and Pt wire or Cu wire was the working electrode. A two-electrode system was used in the electrodeposition experiment, with the anode being a W rod and the cathode being a Cu sheet, and the electrodeposition experiment was conducted at 40 mA cm−2 for 5 h. Electrodeposition and electrochemical testing are performed by inserting electrodes into molten salt. XRD (XRD-7000) was used for composition analysis of Na2WO4 and Na2WO4–WO3 molten salt supernatants.
1) were heated to 1173 K in a tube furnace with argon atmosphere and kept for 3 hours to melt fully. The electrodeposition process and electrochemical measurements are controlled by the electrochemical workstation VersaSTAT4 (AMETEK-Drexelbrook). A three-electrode system was used in the electrochemical test, in which a W rod was the auxiliary electrode, Pt wire was the reference electrode, and Pt wire or Cu wire was the working electrode. A two-electrode system was used in the electrodeposition experiment, with the anode being a W rod and the cathode being a Cu sheet, and the electrodeposition experiment was conducted at 40 mA cm−2 for 5 h. Electrodeposition and electrochemical testing are performed by inserting electrodes into molten salt. XRD (XRD-7000) was used for composition analysis of Na2WO4 and Na2WO4–WO3 molten salt supernatants.
High-temperature in situ Raman data
The high-temperature in situ Raman data were obtained by a multipurpose operando instrument for high-temperature electrochemistry,35 as illustrated in Fig. 1a. This instrument integrates essential components, including Raman spectroscopy, optical microscopy, and a highly precise electrical heating stage. The detachable electrode equipment and the gas path design in the high-temperature pool make it possible to conduct in situ Raman tests in an argon atmosphere to observe the effect of the electric field on the microstructure of the molten salt at high temperatures. Anhydrous Na2WO4 and WO3 with a molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was heated to 1173 K with a constant speed in a tube furnace and kept for 3 hours to completely melt, then cooled for later use. The in situ Raman test was performed in an argon atmosphere at 973 K. Fig. 1b shows the high-temperature in situ Raman test process, under the voltage conditions of 0.4 V, 0.8 V, 1.2 V and 1.4 V. Each voltage is maintained for two minutes and then the Raman signal is collected for three minutes. After the acquisition is completed, the voltage returns to zero.
1 was heated to 1173 K with a constant speed in a tube furnace and kept for 3 hours to completely melt, then cooled for later use. The in situ Raman test was performed in an argon atmosphere at 973 K. Fig. 1b shows the high-temperature in situ Raman test process, under the voltage conditions of 0.4 V, 0.8 V, 1.2 V and 1.4 V. Each voltage is maintained for two minutes and then the Raman signal is collected for three minutes. After the acquisition is completed, the voltage returns to zero.
 | ||
| Fig. 1 (a) High-temperature in situ Raman instrumentation;35 and (b) voltage conditions during Raman measurement. | ||
Molecular dynamic simulations with DPMD
In this experiment, as illustrated in Fig. 2, AIMD calculation was implemented using the DeepMD-kit software package (v2.1.1),36,37 Initially, ab initio molecular dynamics simulations were conducted using CP2K (version 2023.1)33,38 to generate the dataset to train the DPMD potential function. The interatomic potential was calculated at the density functional theory level, using the van der Waals modified PBE density functional with reference to the AIMD simulation of the halogen molten salt system. The MOLOPT basis with double-zeta valence polarized quality was selected for the elements (Na, W, and O), a plane wave cutoff of 1400 Ry adopted for the expansion of the electron density in reciprocal space.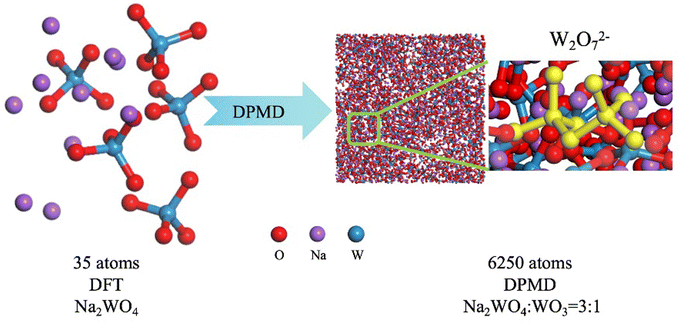 | ||
| Fig. 2 Training DPMD in the AIMD dataset and enabling large-scale molecular dynamics with first-principles precision. | ||
An initial simulation box of Na2WO4 was constructed, containing 10Na+ ions and 5WO42− ions. The calculations were performed at temperatures of 1173 K, and molecular dynamics simulations utilized the NVT ensemble with the Nose–Hoover thermostat. A timestep of 1 fs was selected, the simulations were run for a total of 25 ps to gather an extensive dataset for training. Multiwfn software (version 3.8)39 was used for preparing the input files for CP2K.
For molten salts composed of elements with smaller atomic numbers, such as NaCl and MgCl2, the initial structure typically includes 50–150 atoms.29,31,32 However, for systems with larger atomic numbers like tungsten, excessively large systems may require computationally prohibitive resources. Efficient computational methods are thus significant for these types of systems. Therefore, in this work, we constructed a system containing 35 atoms and validated our simulation results with experimental methods. This approach helps explore the minimum number of atoms required in the original system for accurate simulation results using deep potential training. A dataset of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 frames has already been proven sufficient for the convergence of neural network training in related molten salt studies.31,32
000 frames has already been proven sufficient for the convergence of neural network training in related molten salt studies.31,32
The DeepMD-Kit software package, a program for neural network training and evaluation of atomic energies and forces with machine learning, is utilized to create deep potentials for molten Na2WO4. This software employs deep neural networks for modeling interatomic interaction potentials, enabling high precision and efficiency in molecular dynamics simulations. The software converts the structural information of atoms into numerical representations that can be processed by neural networks. DeepMD-kit consists of two parts, first obtaining the descriptor of the atomic environment, and then mapping the descriptor to a fitting network of energy and atomic forces. Regarding the descriptor part, the software uses an algorithm called Deep Potential Smooth Edition (DeepPot – SE), which can automatically obtain all angle and radial information in the atomic system. The construction of descriptors is provided as follows: the cutoff radius (rcut) for this process was determined through a combination of training cost and computational accuracy considerations, ultimately settling on a value of 6.0. The selected number (sel) of atoms in rcut is {15, 35, 20}, automatically calculated by the DeepMD-kit, which represents the maximum number of W, O, and Na in rcut. In order to set up a residual network structure,40 the embedding net includes three hidden layers [25, 50, 100], and the outer layer always has twice the number of layers. In addition, the learning rate of the system is based on the official recommended value, which is starting from 0.001 and decays to 3.51 × 10−8 every 5000 steps in an exp manner.
The trained model was employed for molecular dynamics simulations using LAMMPS software (version 23Jun2022). The Na2WO4–WO3 box consisted of 250 WO3 units, 750 WO42− ions and 1500 Na+ ions. Simulations were conducted under the NVT ensemble at temperatures of 1173 K, 1200 K, 1250 K, and 1300 K to investigate the influence of temperature on the system's structure. An electric voltage was applied in the x-direction to study the impact of an electric field on the system's microstructure.
Activation strain model analysis
In order to elaborate on the bonding mechanism of the formation of W–O bond (O4W2−–WO3) and how this bond strength changes under different electric fields, the activation strain model analysis was performed using the Amsterdam Density Functional (ADF 2023)41 using relativistic density functional theory at the ZORA-PBE/TZ2P level. All computations were carried out in the gas phase and through vibrational analysis, and all energy minima were confirmed to be zero imaginary frequencies. The activation strain model of chemical reactivity42,43 is a fragment-based approach to understand the energy profile of a chemical process and to explain it in terms of the original reactants. In this work, we only focus on the stationary point of the product. This allows an easy assessment of the influence of geometrical deformation and electronic structure of the catalyst and substrate. The bonding energy ΔE is decomposed into the strain energy ΔEstrain that is associated with the geometrical deformation of the individual reactants as the process takes place, plus the actual interaction energy ΔEint between the deformed reactants. Furthermore, the strain energy ΔEstrain can be split into contributions from the deformation of the substrate and that of the catalyst (see eqn (1)).| ΔE = ΔEstrain + ΔEint | (1) |
| ΔEint = ΔVelstat + ΔEPauli + ΔEoi | (2) |
Results and discussion
Electrolysis of Na2WO4–WO3 molten salt
Fig. 3a and b show the XRD patterns of the quenched Na2WO4 and Na2WO4–WO3 molten salt supernatant. The physical phase in the Na2WO4 molten salt system is still the unit Na2WO4, which suggests the hexavalent tungsten ions are reduced at the cathode in the form of WO42−. In the Na2WO4–WO3 molten salt system, there are Na2WO4, Na2W2O7 and WO3, among which the characteristic peak intensity of WO3 is smaller, indicating that the molten salt contains less WO3. As above, the forms of hexavalent tungsten in the molten salt system include WO3, WO42− and W2O72−. We finally inferred that WO3 reacts with Na2WO4 in the molten salt to generate Na2W2O7. Therefore, the possible decomposition reactions that may occur in molten salt include Na2WO4 = Na2O + W + 3/2O2(g), Na2W2O7 = W + Na2WO4 + 3/2O2 (g), and WO3 = W + 3/2O2(g). Based on ΔGθT = −nFEθT, we calculated the theoretical decomposition voltage for each reaction. At 1173 K, the theoretical decomposition voltages of Na2WO4, WO3 and Na2W2O7 which may be used as tungsten sources in Na2WO4–WO3 molten salt system containing the Na2W2O7 phase are 1.483 V, 0.9392 V and 0.9240 V, respectively. It can be found that the theoretical decomposition voltage of Na2W2O7 is the lowest among the three tungsten compounds. Therefore, it is considered that the hexavalent tungsten ion in the form of W2O72− will produce an electroreduction reaction on the surface of the copper cathode to realize the electrochemical deposition of tungsten coating on the cathode sheet.In order to elucidate the reduction process of tungsten ions, as the Fig. 3c and d, electrochemical impedance spectroscopy tests were conducted on the Na2WO4 and Na2WO4–WO3 molten salt systems under the conditions of an applied potential of −0.6 V on the Cu electrode. The electrochemical impedance spectrum of pure Na2WO4 molten salt presents a semicircle at high frequencies and a straight line at low frequencies, indicating that the electrodeposition process of tungsten ions on the cathode is controlled by a mixture of charge transfer and ion diffusion. The electrochemical impedance spectrum of Na2WO4–WO3 molten salt only shows semicircular features at high frequencies. At this time, the electrochemical deposition process in the molten salt system is mainly controlled by charge transfer. Among the components of the equivalent circuit, Rs is the resistance generated by the ion conductor (molten salt) and the electronic conductor (electrode material), which is the liquid phase resistance; CPE is the constant phase angle component, which can be used to describe the interface electric double layer capacitance of the Cu electrode and molten salt; Rct is the charge transfer resistance of the reaction process; W is the Warburg impedance element. From the impedance value obtained by fitting in Fig. 3c, the liquid phase resistance Rs on the cathode in Na2WO4 molten salt is 0.205 Ω cm2, and the charge transfer resistance Rct is 0.790 Ω cm2. For the Na2WO4–WO3 molten salt system, the impedance value obtained in Fig. 3d shows that the liquid phase resistance Rs on the Cu cathode is 0.152 Ω cm2, and the charge transfer resistance Rct is 0.009 Ω cm2. Compared with the impedance in Na2WO4 molten salt, the charge transfer resistance decreased by 2 orders of magnitude, indicating that the reduction of tungsten ions is easier in the Na2WO4–WO3 molten salt system.
In the electrodeposition experiment, the electrolytic cell voltage in the Na2WO4 molten salt rises rapidly in the early stage of electrolysis, gradually stabilizes, and finally stays between 1.1–1.2 V, as the Fig. 3e. In Fig. 3f, the electrolytic cell voltage in the Na2WO4–WO3 molten salt system finally stabilizes at around −0.22 V after a long period of electrolysis. Compared with the electrolytic cell voltage in the Na2WO4 system, the cell voltage in the Na2WO4–WO3 system dropped by about 1 V.
DPMD simulation and verification
We employed deep potential molecular dynamics (DPMD) for training on the Na2WO4 system at 1173 K, without the influence of an electric field, as depicted in Fig. 4. Fig. 4a and b illustrate the root mean square error (RMSE) of energy and forces during the DPMD training process for this system. A descending trend observed in the scatter plot indicates that the system began converging from 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 steps onward. The top-right portion of the figure elucidates the RMSE over the final 5000 steps of the training process, where the RMSE for energy is less than 0.2%, and slightly above 0.8% for forces. The training phase reached completion, establishing a mostly continuous and stable molten salt system. To evaluate the accuracy of the model, 100 sets of energy and forces data were predicted using this model, as shown in Fig. 4c and d. The agreement between density functional theory (DFT) and DPMD demonstrates the accuracy of DPMD in simulating the molten salt behaviour of this system.
000 steps onward. The top-right portion of the figure elucidates the RMSE over the final 5000 steps of the training process, where the RMSE for energy is less than 0.2%, and slightly above 0.8% for forces. The training phase reached completion, establishing a mostly continuous and stable molten salt system. To evaluate the accuracy of the model, 100 sets of energy and forces data were predicted using this model, as shown in Fig. 4c and d. The agreement between density functional theory (DFT) and DPMD demonstrates the accuracy of DPMD in simulating the molten salt behaviour of this system.
Effect of temperature on the microstructure of the molten salt
The model trained by DPMD conduct molecular dynamics simulations of the Na2WO4–WO3 molten salt system at different temperature with no electric field, and the coordination number and RDF of the W–O bond was analysed, as depicted in Fig. 5. | ||
| Fig. 5 (a) RDF of W–O of Na2WO4–WO3 molten salt at different temperatures; (b) coordination number of W–O of Na2WO4–WO3 molten salt at different temperatures. | ||
Fig. 5a and b shows the RDF and coordination number of W–O bonds at different temperatures without adding an electric field. Because the molecular dynamics simulation sets periodic boundary conditions, the coordination number hardly changes as the temperature rises. The peak of RDF appears at 1.782 Å, and then gradually decreases as temperature rises, which indicates that the rise of temperature increases the chaos of the system. The work of Tingrui Xu proved the accuracy of this conclusion. They performed DPMD simulations on MgCl2–NaCl and MgCl2–KCl molten salts. As the temperature increased, the peak value of the RDF also declined as mentioned above. Besides, there was no correlation between temperature and tungsten distribution in molten salt systems.
Effect of electric field on the microstructure of the molten salt
Furthermore, molecular dynamic simulation with deep potential was conducted for the Na2WO4–WO3 molten salt system at 1173 K under voltage conditions of 0 V, 0.4 V, 0.8 V and 1.2 V. As shown in Fig. 6, we analyzed the RDF and coordination number of W–O in the simulation results. Migration in the electric field cannot be simulated under periodic boundary conditions, RDF indicates the structural rearrangement of the ionic groups with the polarization caused by the electric field, which makes the system more inclined to bond at 1.782 Å. Materials studio 2020 was performed for the structural optimization of WO4−, WO22−, WO3−, WO42−, and W2O72−. The results of structural optimization are shown in Table 2, and only the bond with a length of 1.782 Å exists in the W2O72− structure. The polarization effect with the electric field in Na2WO4–WO3 molten salt will increase the tendency of the system to generate W2O72−. | ||
| Fig. 6 (a) RDF of W–O of Na2WO4–WO3 molten salt at different voltages; (b) coordination number of W–O of Na2WO4–WO3 molten salt at different voltages. | ||
In particular, molecular dynamics simulation with DPMD was performed on the system under voltage conditions of 0–1.2 V, and the results are shown in Fig. 7. Fig. 7a shows the RDF with a W–O bond length of 1.782 Å in the DPMD simulation results under different voltage conditions. As the voltage increases, the RDF of 1.782 Å gradually increases, which indicates that the increase in voltage increases the distribution probability of W–O bonds here. The number of W–O bonds with a length of 1.782 Å (±0.005) in the system was calculated, as shown in Fig. 7b. The red part in the figure is the WO bond with a length of 1.782 Å, and the blue part is other WO bonds less than 1.95 Å. Corresponding to Fig. 7a, as the voltage increases, the total number of W–O bonds in the system does not change significantly, but the number of W–O bonds with a length of 1.782 Å gradually increases. Combined with the information in Table 1, the more Na2W2O7 is generated in the system under the electric field.
| Length/Å | Angle/° | |
|---|---|---|
| WO4− | 1.678 | — |
| WO22− | 1.810 | 113.351 |
| WO3− | 1.820 | 102.994 |
| WO42− | 1.811 | 109.471 |
| W2O72− | 1.782/1.930 | 108.456/110.662 |
In order to verify this simulation, we conducted high-temperature in situ Raman tests on the Na2WO4–WO3 molten salt system. Fig. 8a shows the test results of high-temperature in situ Raman, and Fig. 8b is a partial enlargement of the wavelength range of 900–1000 cm−1.
 | ||
| Fig. 8 (a) High-temperature in situ Raman data of Na2WO4–WO3 molten salt; (b) enlarged image of Raman signal in the wavelength range of 900–1000 cm−1 (the yellow part represents the vibration bond). | ||
Referring to the Raman tests of related systems in the literature,44,45 the structure vibrating in the wavelength range of 321–486 cm−1 is mainly caused by the W–O bond vibration in Na2WO4, and 832–932 cm−1 corresponds to the W–O bond in Na2W2O7. As the voltage increases, the Raman signal intensity of the W2O72− structure decreases significantly. Considering the migration of ionic groups with the electric field, it suggests the main transferred group in this molten salt system is W2O72−. Compared with WO42−, the W2O72− anion is prone to react at the anode, whereas the tungsten element tends to deposit at the cathode. Specifically, from a microscopic perspective, the W2O72− makes the oxygen ions reach the anode and react with a lower voltage. In Fig. 8b, the concentration of the W2O72− structure shows a trend of first decreasing and then increasing as the voltage increases from 0 V to 0.8 V, which is caused by the polarization of the ionic groups in the molten salt with the electric field. This electric field is more inclined to generate the W2O72− structure, which confirms the previous DPMD simulation results.
In order to understand how and why the bond strength changes in the presence of different electric fields, we perform the activation strain model analysis on the O4W2−–WO3 bond in W2O72− without the electric field (F = 0) and under electric fields (F = 0.1, 0.4, 1.0 V Å−1). All data are collected in Table 2 and to make the trends clearer, the related plots of different energy terms are shown in Fig. 9. As the electric field becomes stronger, the bonding energy ΔE values increase significantly, from −154.1 to −162.6, −191.9 and −265.3 kcal mol−1 which agree with our conclusion from experiments and AIMD simulation that the electric field can promote the formation of W2O72−. The bonding energy can be further decomposed into the sum of ΔEint and ΔEstrain, both of which contribute to the stabilization of the W–O bond. The deformation of the reactant (ΔEstrain), especially the geometric change of WO42−, which goes from a tetrahedral structure to a triangular pyramid, as shown in Fig. 9d, causes a larger dipole moment and subsequently leads to a larger strain energy change. In addition, as expected, the electrostatic attraction (Velstat) corresponding to the classical Coulomb interaction increases as the electric field becomes strong which is in line with studies on the effect of the oriented external electric fields on Diels–Alder (DA) reactions.46,47 The electric field can cause the separation of the charge density,43 as illustrated by Fig. 9, which strengthens the electrostatic attraction between two fragments.
| ASM (in kcal mol−1) | 0 | 0.1 | 0.4 | 1.0 |
|---|---|---|---|---|
| a Computed at ZORA-PBE/TZ2P. | ||||
| ΔEstrain[“WO42−”] | 5.7 | −2.6 | −27.5 | −77.4 |
| ΔEstrain[“WO3”] | 2.7 | 2.6 | 2.3 | 1.9 |
| ΔEstrain | 8.4 | 0 | −25.2 | −75.5 |
| ΔVelstat | −203.6 | −204.4 | −210.9 | −246.0 |
| ΔEPauli | 163.6 | 161.8 | 157.4 | 151.7 |
| ΔEoi | −122.5 | −120.0 | −113.2 | −102.5 |
| ΔEint | −162.5 | −162.6 | −166.7 | −189.8 |
| ΔE | −154.1 | −162.6 | −191.9 | −265.3 |
As shown in Fig. 10, the electron density difference diagrams of W2O72−, WO42−, and WO3 with and without electric field were calculated (isosurface: 0.001). After optimizing the geometry of the system, CP2K was used to calculate the electron density of W2O72−, WO42− and WO3 under 0 V and 1.2 V electric fields, respectively. The difference processing of electron density and electron transfer number are calculated based on Multiwfn.39 The green part in the figure presents an increase in charge, and the blue part indicates the decrease, relatively. The increasing direction of the abscissa is the direction of the electric field, and the ordinate is the electron transfer number. The electrons in the WO42− and WO3 systems migrate in the opposite direction of the electric field. Taking the green part as the charge center, the O3 of WO42− in Fig. 10a is readly attracted to the charge center of W1 of WO3 in Fig. 10b to promote the formation of Na2W2O7. Na2W2O7 with a symmetrical structure has the smallest number of electron transfers in an electric field. This is somewhat related to the conclusion of the response of a centrally symmetric structure to an electric field proposed by Nardo et al.48 This structure has the smallest degree of polarization.
 | ||
| Fig. 10 Density difference plots of WO42− (a), WO3 (b) and W2O72− (c) (the green part presents an increase in charge, and the blue part indicates the decrease). | ||
To sum up, in the electrochemical impedance test and electrochemical deposition experiment, we proved that the addition of WO3 allowed the tungsten in the Na2WO4 molten salt to be deposited at a lower voltage, which is mainly because the Na2W2O7 generated by the reaction of Na2WO4 and WO3 has a lower decomposition voltage. We used DPMD to train the data of Na2WO4's AIMD with CP2K to obtain deep potential. With the molecular dynamics simulations and high-temperature in situ Raman tests, we proved that the polarization effect of the ionic groups in the molten salt caused more Na2W2O7 to be generated. The simulation of the electron density difference plot also proves the promoting effect of the electric field on the generation of Na2W2O7.
Conclusions
This work employs both experiments and machine learning-based molecular dynamics simulations to analyse the effect of electric field on the distribution of tungsten in Na2WO4–WO3 molten salt. Extended deep potential-based molecular dynamics simulation under different temperature and electric field conditions, using only systems similar to the training data, was demonstrated accuracy in high-temperature in situ Raman tests on molten salt. First, the experiment shows that after adding WO3, the deposition voltage of tungsten in the Na2WO4–WO3 system is lower than that of pure Na2WO4 molten salt. Next, Combining the results of molecular dynamics simulations based on deep potential and high-temperature in-site Raman testing, we found that Na2WO4 and WO3 react to generate Na2W2O7, which has a lower decomposition voltage than Na2WO4, allowing the deposition of tungsten to be performed under lower voltage conditions. Furthermore, the polarization effect exerted by the electric field on the ionic groups in the system increases the tendency of Na2W2O7 to be generated in the molten salt, which is also conducive to the deposition of tungsten in the system. We calculated the W–O bond length of 1.782 Å in the simulation results, which is the unique W–O bond length of Na2W2O7. When the voltage increased from 0 V to 1.2 V, the W–O bond content in the system increased from 6.7% to 21.5%. The calculation of electron density and bond strength provides a more specific explanation for the generation of more Na2W2O7 in the system with the electric field. This work demonstrates the feasibility of molecular dynamics based on deep potential in tungsten molten salts and analyzes the effect of electric field on the microstructure of molten salts. This provides an important theoretical basis for understanding the behavior of ions in molten salt electrodeposition.Author contributions
Yuliang Guo: conceptualization, validation, visualization, writing – original draft. Xiaobo Sun: investigation, writing – review & editing. Handong Jiao: Raman testing assistance, Wenxuan Qin: electrochemical experiments assistance. Xiaoli Xi: methodology, supervision, writing – review & editing. Zuoren Nie: supervision, project administration, resources.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2023YFB3811800) and the National Natural Science Foundation of China for Distinguished Young Scholar (No. 52025042).Notes and references
- W. Qin, X. Xi, L. Zhang, M. Wang and Z. Nie, Surf. Coat. Technol., 2022, 440, 128497 CrossRef CAS.
- Z. Chen, M. Qin, J. Yang, L. Zhang, B. Jia and X. Qu, J. Mater. Sci. Technol., 2020, 58, 24–33 CrossRef CAS.
- M. Feng, X. Xi, L. Zhang and Z. Nie, J. Phys. Chem. C, 2022, 126, 15528–15537 CrossRef CAS.
- W. Qin, X. Xi, Q. Zhang, L. Zhang, L. Ma and Z. Nie, Int. J. Electrochem. Sci., 2019, 14, 10420–10430 CrossRef CAS.
- N.-b Sun, Y.-c Zhang, F. Jiang, S.-t Lang and M. Xia, Fusion Eng. Des., 2014, 89, 2529–2533 CrossRef CAS.
- N. Sun, Y. Zhang, S. Lang, L. Wang and L. Wang, J. Fusion Energy, 2015, 34, 1417–1422 CrossRef CAS.
- F. Jiang, Y. Zhang, N. Sun and J. Leng, Appl. Surf. Sci., 2015, 327, 432–436 CrossRef CAS.
- H. Lu, J. Qin, C. Di, Y. Yang and R. Huo, Mater. Trans., 2018, 59, 1943–1948 CrossRef CAS.
- K. Nitta, T. Nohira, R. Hagiwara, M. Majima and S. Inazawa, Electrochim. Acta, 2010, 55, 1278–1281 CrossRef CAS.
- T. Nohira, T. Ide, X. Meng, Y. Norikawa and K. Yasuda, J. Electrochem. Soc., 2021, 168, 046505 CrossRef CAS.
- Y. Tian, L. Ma, X. Xi and Z. Nie, J. Electroanal. Chem., 2022, 921, 116700 CrossRef CAS.
- Y. Qi, Y. Tang, B. Wang, M. Zhang, X. Ren, Y. Li and Y. Ma, Int. J. Refract. Met. Hard Mater., 2019, 81, 183–188 CrossRef CAS.
- H. Nakajima, T. Nohira and R. Hagiwara, Electrochem. Solid-State Lett., 2005, 8, C91–C94 CrossRef CAS.
- D. Jihong, L. Zhengxiang, L. Gaojian, Z. Hui and H. Chunliang, Surf. Coat. Technol., 2005, 198, 169–172 CrossRef.
- A. Kobayashi, S. Sharafat and N. M. Ghoniem, Surf. Coat. Technol., 2006, 200, 4630–4635 CrossRef CAS.
- V. A. Pavlovskii, Prot. Met., 2006, 42, 170–173 CrossRef CAS.
- Z. Lian, X. Fang, W. Han, J. Yu, Z. Wang, Y. Zhang and K. Zhu, Fusion Eng. Des., 2016, 112, 136–142 CrossRef CAS.
- F. Jiang, Appl. Surf. Sci., 2016, 363, 389–394 CrossRef CAS.
- F. Jiang and Y. Zhang, J. Fusion Energy, 2018, 37, 255–260 CrossRef CAS.
- Y. H. Liu, Y. C. Zhang, Q. Z. Liu, X. L. Li and F. Jiang, Fusion Eng. Des., 2012, 87, 1861–1865 CrossRef CAS.
- F. Jiang, S. Shao and H. Wu, Fusion Eng. Des., 2016, 106, 71–76 CrossRef CAS.
- M. Feng, X. Xi, L. Zhang and Z. Nie, J. Mol. Liq., 2022, 355, 118944 CrossRef CAS.
- M. Feng, X. L. Xi, L. W. Zhang and Z. R. Nie, Phys. Chem. Chem. Phys., 2022, 24, 20130–20137 RSC.
- G. Cassone, J. Sponer and F. Saija, Top. Catal., 2021, 65, 40–58 CrossRef.
- D. Lu, H. Wang, M. Chen, L. Lin, R. Car, W. E, W. Jia and L. Zhang, Comput. Phys. Commun., 2021, 259, 107624 CrossRef CAS.
- Y. Shi, S. T. Lam and T. L. Beck, Chem. Sci., 2022, 13, 8265–8273 RSC.
- L. Zhang, J. Han, H. Wang, R. Car and W. E, Phys. Rev. Lett., 2018, 120, 143001 CrossRef CAS PubMed.
- W. Liang, G. Lu and J. Yu, ACS Appl. Mater. Interfaces, 2021, 13, 4034–4042 CrossRef CAS PubMed.
- M. Bu, T. Feng and G. Lu, J. Mol. Liq., 2023, 375, 120689 CrossRef CAS.
- M. Bu, W. Liang, G. Lu and J. Yu, Sol. Energy Mater. Sol. Cells, 2021, 232, 111346 CrossRef CAS.
- J. Zhao, Y. Wang and G. Lu, J. Electrochem. Soc., 2022, 169, 056517 CrossRef CAS.
- T. Xu, X. Li, Y. Wang and Z. Tang, ACS Appl. Mater. Interfaces, 2023, 15, 14184–14195 CAS.
- T. D. Kuhne, M. Iannuzzi, M. Del Ben, V. V. Rybkin, P. Seewald, F. Stein, T. Laino, R. Z. Khaliullin, O. Schutt, F. Schiffmann, D. Golze, J. Wilhelm, S. Chulkov, M. H. Bani-Hashemian, V. Weber, U. Borstnik, M. Taillefumier, A. S. Jakobovits, A. Lazzaro, H. Pabst, T. Muller, R. Schade, M. Guidon, S. Andermatt, N. Holmberg, G. K. Schenter, A. Hehn, A. Bussy, F. Belleflamme, G. Tabacchi, A. Gloss, M. Lass, I. Bethune, C. J. Mundy, C. Plessl, M. Watkins, J. VandeVondele, M. Krack and J. Hutter, J. Chem. Phys., 2020, 152, 194103 CrossRef PubMed.
- A. P. Thompson, H. M. Aktulga, R. Berger, D. S. Bolintineanu, W. M. Brown, P. S. Crozier, P. J. in ’t Veld, A. Kohlmeyer, S. G. Moore, T. D. Nguyen, R. Shan, M. J. Stevens, J. Tranchida, C. Trott and S. J. Plimpton, Comput. Phys. Commun., 2022, 271, 108171 CrossRef CAS.
- H. Jiao, J. An, Y. Jia, Q. Liu, Z. Wang, Y. Gao, M. Wang, D. Fang, H. Zhu and S. Jiao, Proc. Natl. Acad. Sci. U. S. A., 2023, 120, e2301780120 CrossRef CAS PubMed.
- H. Wang, L. Zhang, J. Han and W. E, Comput. Phys. Commun., 2018, 228, 178–184 CrossRef CAS.
- D. Lu, W. Jiang, Y. Chen, L. Zhang, W. Jia, H. Wang and M. Chen, J. Chem. Theory Comput., 2022, 18, 5559–5567 CrossRef CAS PubMed.
- J. Hutter, M. Iannuzzi, F. Schiffmann and J. VandeVondele, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 15–25 CAS.
- T. Lu and F. Chen, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- K. He, X. Zhang, S. Ren and J. Sun, presented in part at the 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), 2016.
- S. ADF, Theoretical Chemistry, Vrije Universiteit, (https://www.scm.com).
- K. N. H. F. M. Bickelhaupt, Angew. Chem., Int. Ed., 2017, 56, 10070–10086 CrossRef PubMed.
- T. M. S. X. Sun, J. Poater, T. A. Hamlin and F. M. Bickelhaupt, J. Comput. Chem., 2019, 40, 2227–2233 CrossRef PubMed.
- J. Wang, J. L. You, A. A. Sobol, L. M. Lu, M. Wang, J. Wu, X. M. Lv and S. M. Wan, J. Raman Spectrosc., 2017, 48, 298–304 CrossRef CAS.
- J. Wang, J. You, M. Wang, L. Lu, S. Wan and A. A. Sobol, Spectrochim. Acta, Part A, 2017, 185, 188–196 CrossRef CAS PubMed.
- D. M. S. Shaik and R. Ramanan, Nat. Chem., 2016, 8, 1091–1098 CrossRef PubMed.
- P. V. S. Yu, T. A. Hamlin and F. M. Bickelhaupt, Chem. – Eur. J., 2021, 27, 5683–5693 CrossRef PubMed.
- V. Mollica Nardo, G. Cassone, R. C. Ponterio, F. Saija, J. Sponer, M. Tommasini and S. Trusso, J. Phys. Chem. A, 2020, 124, 10856–10869 CrossRef CAS PubMed.
| This journal is © the Owner Societies 2024 |




