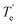Open Access Article
Open Access ArticleOn the ground and excited electronic states of LaCO and AcCO†
Isuru R.
Ariyarathna

Physics and Chemistry of Materials (T-1), Los Alamos National Laboratory, Los Alamos, NM 87545, USA. E-mail: isuru@lanl.gov
First published on 25th October 2024
Abstract
High-level ab initio electronic structure analysis of correlated lanthanide- and actinide-based species is laborious to perform and consequently limited in the literature. In the present work, the ground and electronically excited states of LaCO and AcCO molecules were explored utilizing the multireference configuration interaction (MRCI), Davidson corrected MRCI (MRCI+Q), and coupled cluster singles doubles and perturbative triples [CCSD(T)] quantum chemical tools conjoined with correlation consistent triple-ζ and quadruple-ζ quality all-electron Douglas–Kroll (DK) basis sets. The full potential energy curves (PECs), dissociation energies (Des), excitation energies (Tes), bond lengths (res), harmonic vibrational frequencies (ωes), and chemical bonding patterns of low-lying electronic states of LaCO and AcCO are introduced. The ground electronic state of LaCO is a 4Σ− (1σ11π2) which is a product of the reaction between excited La(4F) versus CO(X1Σ+), whereas the ground state of AcCO is a 12Π (1σ21π1) deriving from ground state fragments Ac(2D) + CO(X1Σ+). The spin–orbit ground states of LaCO (14Σ−3/2) and AcCO (12Π1/2) bear ∼13 and 5 kcal mol−1D0 values, respectively. At the MRCI level, the spin–orbit curves, the spin–orbit mixing, and the Tes of spin–orbit states of LaCO and AcCO were also analyzed. Lastly, the density functional theory (DFT) calculations were performed applying 16 exchange–correlation functionals that span three rungs of “Jacob's ladder” of density functional approximations (DFAs) to assess DFT errors associated on the De and ionization energy (IE) of LaCO.
I. Introduction
Electronic structure studies provide an essential avenue for analyzing and gaining insight into chemicophysical properties of highly correlated molecular species. Especially, precise theoretical representations of ground and excited states are vital for making accurate predictions of fundamental chemical reactivities, thermodynamics, and spectroscopic characteristics of highly complicated lanthanide- and actinide-based molecules. The properties of such systems are greatly challenging to calculate under electronic structure theory where the treatment of higher order electron correlation, relativistic effects, and spin–orbit effects are critical to reach higher accuracy. The multireference tools are ideal for the investigation of lanthanide- and actinide-based species where the partially occupied valence orbitals give rise to a large number of closely arranged single-reference and multireference states that are highly correlated and coupled together.The ab initio theoretical investigation of lanthanide- and actinide-based small molecules is an area of high-interest at present owing to their importance in nuclear energy.1,2 Especially the heavy primordial Th and U-based actinide species are the prime focus in this topic of research.3–13 On the other hand, early lanthanide La- and actinide Ac-based species are relatively less studied. For example, we were able to find 5 experimental and theoretical studies in total on La–CO system.14–18 To the best of our knowledge, experimental or theoretical studies are unavailable for Ac–CO. This could be due to the low abundance of Ac in the earth's crust (5.5 × 10−10 ppm)19 which makes it expensive, and its similarity in chemicophysical properties to the more abundant La.20 Furthermore, the comparatively low interest on such species could also be due to the transition-metal-like nature of the low-lying states of La and Ac atoms. For example, the La and Ac populate their corresponding valence 4f and 5f orbitals at 12th and 16th excited states respectively, hence the molecules that they form tend to carry transition-metal-like properties rather than the characteristics of typical f-orbital populated lanthanide and actinide species.21
CO is the strongest ligand in the spectrochemical series which tends to form substantially strong chemical bonds with many metals in the periodic table and therefore the lack of data on the interaction between CO versus La and Ac is rather surprising. Heavy metals are known to activate C–O bond and hence the investigation of the potential of CO activation by La and Ac is beneficial for the field of catalysis.8,22,23 Furthermore, gas-phase studies of model metal–CO species can be used to gain insight on the chemisorption process of CO on metal surfaces.24 More importantly, the investigations of La + CO and Ac + CO reactions by themselves hold a spectroscopic significance. This motivated us to carry out the present quantum chemical investigation of the low-lying electronic and spin–orbit states of LaCO and AcCO molecules which is expected to serve as a guide and motivation for their future experimental and theoretical investigations.
The first study of LaCO dates back to Hong et al.'s work in 1997 which reported several energy related properties and structural patterns of linear LaCO, linear LaOC, and side-bonded La(η2-CO) isomers at the DFT/LDA+QR+GRD (local density approximation with the gradient correction and relativistic effects).14 They found that the linear LaCO is more stable compared to the linear LaOC and La(η2-CO) by ∼27 and 14 kcal mol−1, respectively. This work further reported a quartet-spin ground state and a doublet-spin first excited state for the linear LaCO with 37.36 and 30.90 kcal mol−1Des, respectively. Their reported optimized La–C and C–O bond distances and the corresponding C–O stretching frequency of LaCO of the ground state are 2.392 Å, 1.177 Å, and 1778 cm−1. Nine years after Hong et al.'s DFT work, the first experimental study of LaCO was reported by Xu, Jiang, and Zou.15 They studied the reaction of laser-ablated La with CO in a solid argon matrix at 7 K and reported a 1772.7 cm−1 C–O stretching frequency which is in harmony with the value reported by Hong et al. Furthermore, they have performed DFT/BPW91 calculations and predicted a 4Σ− ground state for LaCO which agrees with the findings of Hong et al. The De of LaCO reported in the Xu et al.'s work is (41.24 kcal mol−1) which is ∼4 kcal mol−1 higher than the value reported by Hong et al.14 In 2007 Jiang and Xu reported another matrix infrared spectroscopic study for LaCO and assigned a 1814 cm−1 frequency for the C–O stretch which agreed reasonably well with the DFT/BPW91 frequency calculated in the same work (i.e., 1841.7 cm−1).16 At the same level of theory, the obtained La–C and C–O bond distances of LaCO(4Σ−) are 2.404 and 1.186 Å respectively, which are slightly longer than the lengths reported by Hong et al.14 In the same year, Zhang et al., reported La–C and C–O distances and frequencies for the 4Σ− (2.504 Å, 1.166 Å, 1779 cm−1) and 2Π (2.495 Å, 1.160 Å, 1918 cm−1) states of LaCO at the DFT/BP86.17 Furthermore, they reported an excited 2Π state for LaCO that lies 12.4 and 6.5 kcal mol−1 above the 4Σ− at the DFT/BP86 and CCSD(T) levels of theory, respectively. The most recent work on LaCO is reported in 2012 by Xu et al., who performed DFT/PBE calculations and reported La–C and C–O bond lengths of 2.299 and 1.181 Å respectively.18 Overall, the literature DFT analyses highlight that the predictions of LaCO are significantly dependent on the density functional approach being utilized.
In the present work, we have employed high-level MRCI, MRCI+Q, and CCSD(T) quantum chemical tools to investigate the interaction of La and Ac with CO. We report full PECs, equilibrium electronic configurations, energetics, and spin–orbit effects on LaCO and AcCO. The side-bonded La(η2-CO) and Ac(η2-CO) and the linear-to-bent transition energies were also investigated. Furthermore, DFT calculations were performed to calculate De and the IE of LaCO probing a set of exchange correlation functionals that span three rungs of Jacob's ladder of DFA; GGA (BP86,25,26 BLYP,27,28 PBE29), MGGA (TPSS,30 MN15-L31), and hybrid [global GGA hybrid (B3LYP,32,33 B3P86,25,32 B3PW91,32 PBE034,35), MGGA hybrid (TPSSh,30 M06,36 M06-2X,36 MN1537), and RSH (LRC-ωPBE,38 CAM-B3LYP,39 ωB97X40)] to assess the associated DFT errors of these properties.
II. Computational details
The MOLPRO 2023.241–43 code was utilized for all wave function theory (WFT) internally contracted MRCI44–46 (or MRCISD) and CCSD(T)47 calculations. In all cases, default MOLPRO convergence criteria was applied. For the calculations of LaCO and AcCO, the C2v Abelian sub point group of their true C∞v non-Abelian symmetry was used. First, the full PECs resulting from the La(2D) + CO(X1Σ+), La(4F) + CO(X1Σ+), La(2F) + CO(X1Σ+), La(4P) + CO(X1Σ+), and La(2D) + CO(X1Σ+) asymptotes were produced at the MRCI theory as a function of La⋯C distance while keeping the C–O bond length fixed to 1.168 Å, which is the CCSD(T) optimized C–O length of the ground state of LaCO. Similarly, at the MRCI method, PECs arising from the Ac(2D) + CO(X1Σ+), Ac(2Po) + CO(X1Σ+), Ac(4F) + CO(X1Σ+), and Ac(2Do) + CO(X1Σ+) asymptotes were considered to investigate the low-lying states of AcCO. Here the C–O length was kept constant to 1.159 Å, which is the C–O length of the CCSD(T) optimized AcCO(12Π). For these calculations, cc-pVTZ-DK348 of La and Ac and cc-pVTZ-DK49,50 of C and O basis set (hereafter TZ) was used with the third-order Douglas–Kroll–Hess (DKH) Hamiltonian. The complete active space self-consistent field (CASSCF)51–54 reference wave functions (3 electrons in 9 orbitals or CAS[3,9]) were provided for MRCI calculations of both LaCO and AcCO. At the dissociation limit, the 9 orbitals are pure nd, (n + 1)s, and (n + 1)p atomic orbitals of La/Ac and at the implemented C2v point group, they are 4a1 [(n + 1)s, ndz2, ndx2−y2, and (n + 1)pz], 2b1 [ndxz and (n + 1)px], 2b2 [ndyz and (n + 1)py], and 1a2 [ndxy] in symmetry (n = 5 for La and n = 6 for Ac). At the MRCI level, the single and double electron promotions were permitted to the virtual orbitals. Using the same active space and the basis set, full geometry optimizations were performed for 7 and 2 lowest energy electronic states of LaCO and AcCO at the MRCI level to obtain re, Te, and ωe values. Moreover, the geometries were optimized at the MRCI+Q55 level (Davidson relaxed approach) to evaluate the approximate quadruple substitution-like effect on the aforementioned properties.The spin–orbit coupling curves resulting from 14Σ−, 12Σ−, 12Π, 12Δ, and 14Φ of LaCO were produced at the MRCI level as a function of La⋯C distance. The 12Π, 4Σ−, 4Φ, 2Σ−, 2Δ, 4Π, 2Σ+, 2Π states of AcCO were used in the spin–orbit matrix to study the low energy spin–orbit curves of AcCO (see main text of the paper for more information regarding the spin–orbit analysis). For spin–orbit calculations, TZ basis set was used with the Breit–Pauli Hamiltonian as implemented in MOLPRO.
The geometries of low-lying single-reference electronic states of LaCO and AcCO and the ground state of LaCO+ were also optimized at the CCSD(T) level of theory that was built on top of Hartree–Fock (HF) wave functions. For each case, two sets of CCSD(T) calculations were performed with cc-pVTZ-DK3 (La) cc-pVTZ-DK (C and O) [hereafter TZ-CCSD(T)] and cc-pVQZ-DK3 (La) cc-pVQZ-DK (C and O) [hereafter QZ-CCSD(T)] basis sets using the third-order DKH Hamiltonian. The ground states of the side-bonded La(η2-CO) and Ac(η2-CO) molecules were also studied at the TZ-CCSD(T) level. Single-point CCSD(T) calculations were also performed for the La(2D), La(4F), and CO(X1Σ+) to calculate CCSD(T) Des. The experimental bond distance of CO(X1Σ+) (1.128 Å)21 was used for the CCSD(T) calculation.
Single-point DFT calculations were performed for the ground states of LaCO(4Σ−), LaCO+(3Σ−), and CO(X1Σ+) and excited La(4F) to calculate the DFT ionization energy (IE) of LaCO(4Σ−) and DFT De of LaCO(4Σ−) with respect to the La(4F) + CO(X1Σ+) fragments using the Gaussian 1656 package. For these calculations, the TZ-CCSD(T) optimized geometries of LaCO(4Σ−) and LaCO+(3Σ−) and the experimental bond distance of CO(X1Σ+) (1.128 Å)21 were provided. The DFT calculations were performed using a set of exchange correlation functionals that span three rungs of Jacob's ladder; semi-local generalized gradient approximation (GGA: BP86,25,26 BLYP,27,28 PBE29), meta-GGA (MGGA: TPSS,30 MN15-L31), and hybrid [global GGA hybrid (B3LYP,32,33 B3P86,25,32 B3PW91,32 PBE034,35), MGGA hybrid (TPSSh,30 M06,36 M06-2X,36 MN1537), and range-separated hybrid (RSH: LRC-ωPBE,38 CAM-B3LYP,39 ωB97X40)]. For La, the Stuttgart relativistic small-core (RSC) 1997 basis set with a 28-electron effective core potential (ECP) was used.57,58 For C and O, the cc-pVQZ basis set was used.49 In each case, the wave functions were optimized implementing the stable = opt keyword of Gaussian 16.56 The default self-consistent-field convergence thresholds, grids, frozen core settings available in Gaussian 16 were provided for all DFT calculations.56
III. Results and discussion
IIIA. LaCO
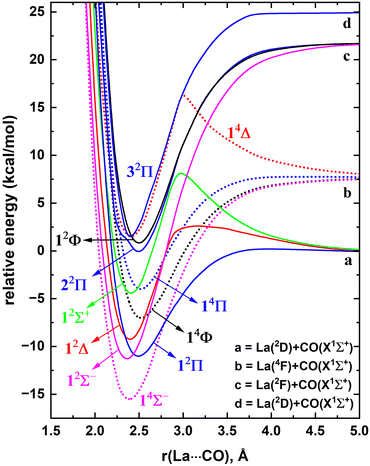
The ground electronic state of La is a 2D with a 5d16s2 valence electron configuration. An electron promotion from 6s to 5d shells produces its first six excited states with 5d26s1 configuration which extend over 7.6–28.5 kcal mol−1. The spin–orbit splitting of La is substantial. For example, the J = 5/2 and 3/2 splits of 2D state are separated by 3.0 kcal mol−1, and the spin–orbit products of the first excited state of La(4F) span over 4.2 kcal mol−1. Generally, the spin–orbit coupling effects of isolated atoms are substantially large compared to the spin–orbit coupling effects of the molecules that they form. However, the spin–orbit coupling effects of lanthanide-based molecular species are clearly non-negligible. Hence in the present work, spin–orbit effect disregarded low-lying electronic states of LaCO as well as their spin–orbit products were investigated.A full potential energy profile can provide us with useful information such as relative stabilities of the states, locations of energy minima and their origins, and avoided crossings. Hence, we have produced the full PECs of LaCO as a function of La⋯C distance while keeping the CO bond distance fixed to the C–O length of the optimized geometry of the ground state of LaCO. Specifically, here La(2D) + CO(X1Σ+), La(4F) + CO(X1Σ+), La(2F) + CO(X1Σ+), La(4P) + CO(X1Σ+), and La(2D) + CO(X1Σ+) reactions were considered to produce CASSCF PECs of LaCO. These reactions produce 2[Σ+, Π, Δ], 4[Σ−, Π, Δ, Φ], 2[Σ−, Π, Δ, Φ], 4[Σ−, Π], and 2[Σ+, Π, Δ], molecular states, respectively. Note that the first excited state of CO(a3Π) lies significantly high in energy (i.e., 139.2 kcal mol−1) and hence its reactions with low-lying electronic states of La were not studied. At the MRCI+Q level, the most stable 11 electronic states of LaCO were investigated and are illustrated in Fig. 1. The PECs originating from the La(4P) + CO(X1Σ+) are not part of the most stable 11 electronic states of LaCO and hence are not plotted in Fig. 1.
At the dissociation limit, the relative energies of the fragments correspond to the excitation energies of La atom (Fig. 1). Specifically, the first, second, and fourth excitation energies of La atom according to the MRCI+Q potential energy profile (Fig. 1) are 7.6, 21.6, and 24.9 kcal mol−1 which are in good agreement with the corresponding experimental values for the lowest energy J states of La (i.e., 7.6, 20.0, 24.1 kcal mol−1, respectively).21 This somewhat demonstrates the validity of the MRCI+Q level of theory for the investigation of a highly correlated system such as LaCO.
The ground state of LaCO is a 4Σ− derived from the La(4F) + CO(X1Σ+) fragments (Fig. 1). The 2D(5d16s2) to 4F(5d26s1) promotion of La minimizes the σ electron repulsion between La versus CO which ameliorates the σ electron donation from CO to La. The same is true for the previously reported ScCO (which is isovalent to LaCO), where its X4Σ− ground state is a result of the reaction between CO and excited Sc(4F).59,60 Similar to 14Σ−, the 14Φ and 14Π of LaCO originating from the same fragments are substantially attractive in nature except for the 14Δ which is initially strongly repulsive but turn attractive at ∼2.9 Å due to an avoided crossing. Note that an avoided crossing is a result of the interaction of two PECs with identical spins and symmetries. The initial repulsion of the 14Δ PEC arises due to the electron–electron repulsion of the (5dz2)1 (ML = 2 component of La) versus the two sigma dative electrons at the CO.
The first excited state of LaCO(12Σ−) lies ∼4 kcal mol−1 above 14Σ− and dissociates to the La(2F) + CO(X1Σ+). The next excited state of LaCO(12Π) lies energetically very close (less than 1 kcal mol−1) to the 12Σ−. The 12Π is indeed the lowest energy electronic state that is produced by the ground state fragments [i.e., La(2D) + CO(X1Σ+)]. Similar to the 14Δ, the 12Δ and 12Σ+ states of La(2D) + CO(X1Σ+) are first repulsive but turn attractive due to avoided crossings and become the third and fifth excited states of LaCO (Fig. 1). Overall, at the MRCI+Q level, the seven lowest energy electronic states of LaCO are stable with respect to the ground state fragments and they are congested within 12 kcal mol−1 which clearly captures the complexity of the electronic spectrum of the system.
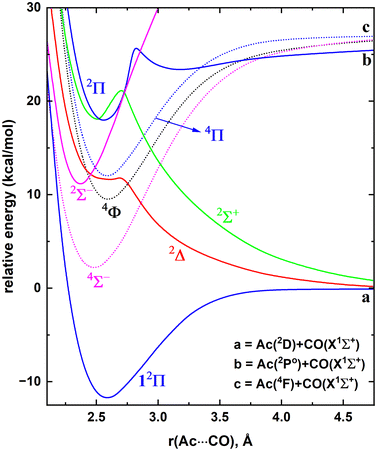
The electronic configurations of the 11 studied electronic states of LaCO and the select occupied molecular orbitals are given in Table 1 and Fig. 2, respectively. The 1σ is dominantly the polarized 6s of La (85.41%) with small contributions from 6pz (6.22%) and 5dz2 (8.37%) of La. The polarization of the valence s orbital of a metal to the opposite direction of the CO aids the chemical bond formation.8,24,61 The 1πx molecular orbital demonstrates the 5dxz(La) → π*(CO) back-donation of LaCO. The 1πx is composed of 5dxz of La (65.87%) + 2px of C (18.78%) − 2px of O (15.35%). Similarly, the antibonding 2πx is made of the same atomic orbitals as in the case of 1πx but with 5dxz(La) – 2px(C) – 2px(O) combination. Due to the symmetry effects, the non-bonding 1δx2−y2 of LaCO is a merely the 5dx2−y2 of La. Note that the contours of 1πy, 2πy, and 1δxy are identical to the shapes of 1πx, 2πx, and 1δx2−y2 orbitals and hence are not depicted in Fig. 2.
| Statea | Coefficientb | 1σ | 1πx | 1πy | 2πy | 1δx2−y2 | 1δxy |
|---|---|---|---|---|---|---|---|
| a Only A1 components of Δ and B1 components of Π and Φ states at C2v symmetry are listed. b Only the configuration interaction coefficients that are larger than 0.25 of corresponding natural orbital representations are listed. | |||||||
| 14Σ− | 0.98 | α | α | α | 0 | 0 | 0 |
| 12Σ− | 0.80 | β | α | α | 0 | 0 | 0 |
| −0.40 | α | β | α | 0 | 0 | 0 | |
| −0.40 | α | α | β | 0 | 0 | 0 | |
| 12Π | 0.91 | 2 | α | 0 | 0 | 0 | 0 |
| 12Δ | −0.66 | α | 0 | 2 | 0 | 0 | 0 |
| 0.66 | α | 2 | 0 | 0 | 0 | 0 | |
| 14Φ | −0.69 | α | 0 | α | 0 | 0 | α |
| 0.69 | α | α | 0 | 0 | α | 0 | |
| 14Π | 0.69 | α | 0 | α | 0 | 0 | α |
| 0.69 | α | α | 0 | 0 | α | 0 | |
| 12Σ+ | 0.63 | α | 0 | 2 | 0 | 0 | 0 |
| 0.63 | α | 2 | 0 | 0 | 0 | 0 | |
| 22Π | −0.55 | α | 0 | β | 0 | 0 | α |
| 0.58 | α | β | 0 | 0 | α | 0 | |
| −0.33 | 2 | α | 0 | 0 | 0 | 0 | |
| 12Φ | 0.62 | α | 0 | β | 0 | 0 | α |
| 0.62 | α | β | 0 | 0 | α | 0 | |
| 32Π | 0.62 | 0 | α | 2 | 0 | 0 | 0 |
| 0.26 | α | α | 0 | 0 | β | 0 | |
| −0.36 | 0 | α | α | β | 0 | 0 | |
| 14Δ | 0.96 | 0 | α | α | 0 | 0 | α |
 | ||
| Fig. 2 Select state average CASSCF molecular orbitals of LaCO. La (left most atom), C (central atom), and O (right most atom) are shown in magenta, gray, and red, respectively. A threshold of 80% was applied to produce orbital plots. The negative and positive phases of each orbital is shown in blue and red, respectively. The 90° rotation of 1πx and 2πx orbitals along the z-axis respectively produces 1πy and 2πy orbitals, whereas the 45° rotation of 1δx2−y2 yields the 1δxy. IboView software was used to plot molecular orbitals.62 | ||
The ground state of LaCO(14Σ−) carries the single-reference 1σ11π2 electron configuration (Table 1) which translates to the 5d26s1 valence electron configuration of the La(4F). The higher stability of this high spin electronic state is a typical instance of the Hund's rule. According to our QZ-HF Mullikan population analysis, LaCO(14Σ−) has La+0.26[CO]−0.26 charge localization. Our findings are in harmony with the DFT/PBE Mullikan charge distribution reported by Xu et al., for LaCO in 2012 (i.e., La+0.23[CO]−0.23).18
The spin-pairing of the 1σ and the two 1π electrons produces the first excited state of LaCO(12Σ−) which is multireference in nature. An electron promotion from 1π to 1σ creates the electron configuration of the single-reference second excited state of LaCO (i.e., 12Π; 1σ21π1). Next seven states of LaCO are dominantly multireference in character (i.e., 12Δ, 14Φ, 14Π, 12Σ+, 22Π, 12Φ, 32Π). The 14Φ is the first electronic state of LaCO that populates 1δ orbitals. Specifically, an electron transfer from 1π to empty 1δ from the ground electron configuration of LaCO(14Σ−) gives rise to the configuration of 14Φ (i.e., 1σ11π11δ1). The same electron configuration holds for the 14Π state of LaCO but with positive combinations of the two dominant components (Table 1). The 22Π and 12Φ are the corresponding doublet spin states of the 1σ11π11δ1 configuration (note: only two dominant configurations of 22Π considered). Among the studied states, 32Π is the only state to populate the 2π antibonding molecular orbital with a significant contribution. The highest energy state that was studied here (i.e., 14Δ) is single-reference in character and the first state to carry an unoccupied 1σ orbital. Based on the equilibrium electron arrangements, the valence-bond-Lewis (vbL) diagrams of the studied electronic states of LaCO are introduced (Fig. 3).
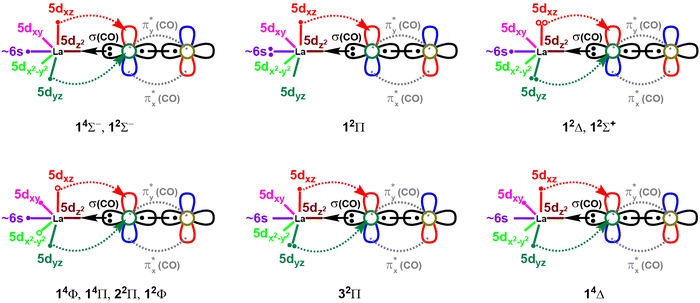 | ||
| Fig. 3 Proposed valence-bond-Lewis (vbL) diagrams for the studied 11 electronic states of LaCO. For clarity the πx and πy bonds of CO are excluded. The multireference (1πx)2 ± (1πy)2 components of the 12Δ and 12Σ+ states are shown by electron pairs with solid and open circles. Similarly, the multireference π1δ1 electron combinations of 14Φ, 14Π, 22Π, and 12Φ are depicted in open and solid circles. Only two and one dominant configurations of 22Π and 32Π states respectively are shown. See Table 1 for their exact electronic configurations. | ||
The MRCI+Q De of the LaCO(14Σ−) with respect to the La(2D) + CO(X1Σ+) asymptote is 18.99 kcal mol−1 which is only 0.73 kcal mol−1 higher than the MRCI De (Table 2). The CCSD(T) De obtained at the same basis set [TZ-CCSD(T)] of the ground state is ∼1 kcal mol−1 larger than the MRCI+Q De (19.93 kcal mol−1). The better agreement of Des between the MRCI+Q and CCSD(T) is a common observation in the literature.63–65 Generally, CCSD(T) calculations are less expensive compared to the MRCI and hence here we were able to perform QZ-CCSD(T) calculations for LaCO as well (Table 2). Moving from TZ-CCSD(T) to QZ-CCSD(T), the De of LaCO(14Σ−) increased by 1.51 kcal mol−1. All our high-level ab initio Des are significantly lower than the previously reported DFT Des of LaCO. Specifically, the De reported by Hong et al., by LDA+QR+GRD is 37.36 kcal mol−1 (ref. 14) whereas the DFT/BPW91 De of LaCO by Xu et al., is 41.24 kcal mol−1.15
| State | Level of theorya | D e | r e | T e | ω e | |
|---|---|---|---|---|---|---|
| La–C | C–O | |||||
| a Davidson corrected MRCI is denoted by MRCI+Q. For all MRCI, MRCI+Q, and TZ-CCSD(T) calculations cc-pVTZ-DK3 of La and cc-pVTZ-DK of C and O basis set was applied. The cc-pVQZ-DK3 of La and cc-pVQZ-DK of C and O basis set was used for QZ-CCSD(T) calculations. b C–O stretching mode. | ||||||
| 14Σ− | QZ-CCSD(T) | 21.44 | 2.346 | 1.165 | 0 | 281, 284, 322, 1860b |
| TZ-CCSD(T) | 19.93 | 2.352 | 1.168 | 0 | 276, 277, 317, 1857b | |
| MRCI+Q | 18.99 | 2.362 | 1.160 | 0 | ||
| MRCI | 18.26 | 2.379 | 1.149 | 0 | ||
| BPW9116 | 2.404 | 1.186 | 1841.7b | |||
| BPW9115 | 41.24 | 2.298 | 1.185 | 1790.8b | ||
| B3LYP16 | 1901.3b | |||||
| PBE18 | 2.299 | 1.181 | ||||
| BP8617 | 2.504 | 1.166 | 1779b | |||
| LDA+QR+GRD14 | 37.36 | 2.392 | 1.177 | 1778b | ||
| Experiment | 1814.0b,16 1772.7b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
|||||
| 12Σ− | MRCI+Q | 2.343 | 1.164 | 6.25 | ||
| MRCI | 2.358 | 1.153 | 7.08 | |||
| 12Π | QZ-CCSD(T) | 13.37 | 2.488 | 1.147 | 8.07 | 213, 255, 274, 1971b |
| TZ-CCSD(T) | 12.80 | 2.492 | 1.151 | 7.14 | 209, 250, 267, 1963b | |
| MRCI+Q | 2.517 | 1.140 | 7.09 | |||
| MRCI | 2.552 | 1.125 | 7.73 | |||
| CCSD(T)17 | 6.5 | |||||
| BP8617 | 2.495 | 1.160 | 12.4 | 1918b | ||
| 12Δ | MRCI+Q | 2.369 | 1.164 | 9.68 | ||
| MRCI | 2.385 | 1.153 | 10.89 | |||
| 14Φ | MRCI+Q | 2.530 | 1.148 | 11.51 | ||
| MRCI | 2.550 | 1.136 | 11.66 | |||
| 14Π | MRCI+Q | 2.520 | 1.151 | 14.05 | ||
| MRCI | 2.535 | 1.140 | 14.78 | |||
| 12Σ+ | MRCI+Q | 2.383 | 1.166 | 14.82 | ||
| MRCI | 2.404 | 1.154 | 16.03 | |||
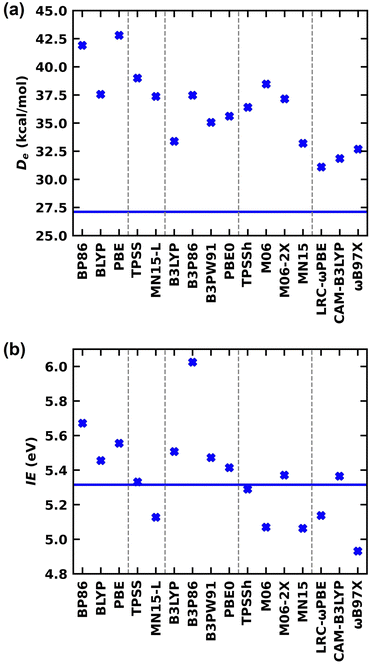
Due to the large deviations of the literature DFT Des compared to our ab initio values, as the next step we evaluated the DFT errors on the De of LaCO performing DFT calculations using a series of exchange correlation functionals that span three rungs of Jacob's ladder of DFA; GGA (BP86,25,26 BLYP,27,28 PBE29), MGGA (TPSS,30 MN15-L31), and a set of hybrid families with different complexity [global GGA hybrid (B3LYP,32,33 B3P86,25,32 B3PW91,32 PBE034,35), MGGA hybrid (TPSSh,30 M06,36 M06-2X,36 MN1537), and RSH (LRC-ωPBE,38 CAM-B3LYP,39 ωB97X40)]. The DFT De of LaCO(14Σ−) were calculated with respect to La(4F) + CO(X1Σ+) fragments and compared with the QZ-CCSD(T) De (i.e., 27.12 kcal mol−1) obtained with respect to the same fragments. The DFT Des are plotted in the Fig. 4a and numerical values and the % DFT errors with respect to the QZ-CCSD(T) are listed in the ESI,† Table S1. Generally, we expect better accuracy from DFT as we shift to more complicated and expensive functionals at the higher rungs of the Jacob's ladder of DFA. As expected, the largest DFT errors with respect to CCSD(T) were provided by the less expensive GGA family. Specifically, the errors of the BP86 and PBE predictions are larger than 50% (ESI,† Table S1). Among GGAs, BLYP carries the least error for De (∼38%). The DFT errors of the two utilized MGGAs (i.e., TPSS and MN15-L) are also high (44% and 38% respectively). The best functional among the global GGA hybrids are B3LYP with 23% errors where other functionals of the same family predicted errors of 29–39%. The performance of the MGGA hybrid MN15 is slightly better than the GGA hybrid B3LYP, which is the best performing functional of the MGGA hybrid. The DFT errors of the expensive RSHs are the lowest among all utilized DFAs. Specifically, the RSH De errors are less than 21%, and the comparatively better performance of RSHs was indeed our expectation. Among all, the best performing DFA for De of LaCO is the LRC-ωPBE with 14.7% errors compared to our CCSD(T) value (ESI,† Table S1 and Fig. 4a). Overall, all DFAs overestimated the De of LaCO. Based on our data, it is rather clear that the DFT De of LaCO is highly sensitive to the DFA utilized and hence it is reasonable to expect errors in DFA predictions on other properties of LaCO as well. To further quantify DFT errors on this system, we have calculated the DFT IE of LaCO and the findings are discussed later in the paper.
For the ground state, re(La–C) increased in the order of TZ-CCSD(T) < MRCI+Q < MRCI while the trend is the opposite for the re(C–O) (Table 2). The same pattern was observed for the excited 12Π as well. The QZ-CCSD(T) La–C and C–O re values of both 14Σ− and 12Π states are shorter compared to the TZ-CCSD(T) res. We are familiar with the observation of shorter bonds predicted by larger basis sets.65–67 The re values of LaCO have been reported by a series of DFT studies before and clearly the values are greatly dependent on the adopted DFT approach (Table 2). The 14Σ− carries two electrons in the 1π orbitals whereas only one electron occupies the 1π of 12Π and hence we can expect longer La–C and a shorter C–O for the latter compared to the former. Indeed, this is correct where the La–C and C–O of 12Π are ∼0.15 Å longer and ∼0.02 Å shorter compared to the corresponding bonds of 14Σ− under all utilized levels. Similarly, each 12Π, 14Φ, and 14Π state hosts only one electron in their 1π orbitals which correlates to their longer La–C and shorter C–O bond distances compared to the other states of LaCO (Table 2). Overall, in all cases the MRCI predicted La–C re values are longer compared to the MRCI+Q res (by ∼0.01–0.03 Å) and the MRCI predicted C–O res are shorter compared to the MRCI+Q res (by ∼0.01 Å).
The quartet-spin ground state has been proposed for the side-bonded La(η2-CO) by Hong et al.14 In the present work, the side-bonded La(η2-CO) isomer was also investigated at the TZ-CCSD(T) level to compare its relative stability with the linear LaCO. At the TZ-CCSD(T) level, the ground state 4A′′ of La(η2-CO) lies 14.21 kcal mol−1 above the LaCO(14Σ−). This value is in very good agreement with the corresponding DFT value reported by Hong et al., which is 14.07 kcal mol−1.14 The TZ-CCSD(T) optimized La–C, La-O, and C–O geometrical parameters of the La(η2-CO) are 2.485, 2.423, and 1.217 Å, respectively. The singly-occupied molecular orbitals of La(η2-CO) (4A′′) are given in ESI,† Fig. S1. According to the Mullikan population analysis performed under QZ-HF level, we observed an enhanced ionic character for the La(η2-CO) (i.e., La+0.44C−0.03O−0.41) compared to the linear LaCO (i.e., La+0.26–[CO]−0.26). The transition state barrier corresponding to the LaCO → La(η2-CO) conversion lies 19.35 kcal mol−1 above the LaCO at the TZ-CCSD(T). Note that the transition state was optimized under DFT/B3LYP using the Stuttgart RSC 1997 basis set with 28-electron ECP of La and cc-pVQZ for C and O. Then the DFT geometry was used to perform a single-point TZ-CCSD(T) calculation.
The Te values of the first six excited states of LaCO are listed in Table 2. Notice that all these states are lower in energy than the 4A2 of La(η2-CO). The exact ordering of the states predicted by both MRCI and MRCI+Q methods is 14Σ−, 12Σ−, 12Π, 12Δ, 14Φ, 14Π, and 12Σ+. This is consistent with the MRCI+Q ordering of the states of Fig. 1, except for the switching of the 12Σ+ and 14Π. In line with the Fig. 1 the 12Σ− and 12Π lie very close with less than 1 kcal mol−1 energy difference. In 2007 Zhang et al., reported DFT/BP86 and CCSD(T) 14Σ− → 12Π transition energies to be 12.4 and 6.5 kcal mol−1, respectively.17 Their CCSD(T) value is in reasonable agreement with our MRCI and CCSD(T) values (Table 2). For all computed states in the present work, the MRCI Te values are 0.1–1.3 kcal mol−1 larger than the MRCI+Q values.
The two experimentally observed C–O stretching modes of LaCO are 1772.715 and 1814.0 cm−1 (ref. 16). Our ωe values of LaCO(14Σ−) predicted by TZ-CCSD(T) and QZ-CCSD(T) are 1857 and 1860 cm−1 respectively.
Going a step further, the ground state of the LaCO+(3Σ−) was also studied at the CCSD(T) level. The optimized La–C and C–O distances of LaCO+(3Σ−) are 2.396 and 1.149 Å at the TZ-CCSD(T) and 2.390 and 1.145 Å at the QZ-CCSD(T) levels, respectively. The DFT/BPW91 La–C and C–O distances reported by Jiang and Xu16 are 2.407 and 1.169 Å respectively and are in reasonable agreement with our CCSD(T) values. The experimental C–O ωe reported by Jiang and Xu16 for LaCO+(3Σ−) is 1903.2 cm−1 and ∼90 cm−1 smaller compared to our QZ-CCSD(T) ωe value (i.e., 1994 cm−1). Regarding the electronic structure of the LaCO+, the removal of the 1σ1 (∼6s of La) electron from the LaCO(14Σ−) produces the single-reference ground state of LaCO+(3Σ−). At the TZ-CCSD(T) and QZ-CCSD(T) levels, the IE of this process is 5.290 and 5.316 eV, respectively. The DFT IEs of this process was also evaluated using the same set of functionals that was used in our DFT De error analysis of LaCO. Our DFT IEs of LaCO are shown in the Fig. 4b and the corresponding numerical values and % errors with respect to QZ-CCSD(T) IE (i.e., 5.316 eV) are given in the ESI,† Table S2. GGAs overestimated the IE of LaCO by 0.14–0.36 eV. Among all utilized DFAs, MGGA TPSS predicted almost identical IE to the QZ-CCSD(T) IE (i.e. 5.332 vs. 5.316 eV). The IE predicted by the MN15-L of the same class is lower by ∼0.19 eV compared to the CCSD(T). All global GGA hybrids overestimated the IE of LaCO compared to the QZ-CCSD(T) with B3P86 being the one to carry the largest error (∼13.3%) among all utilized functionals (Fig. 4b and ESI,† Table S2). Both TPSSh and M06-2X of MGGA hybrids represent the IE of LaCO well with only 0.03 and 0.06 eV deviations from QZ-CCSD(T) respectively. Even though the RSH family performed De of LaCO comparatively well compared to the others, the RSH LRC-ωPBE and ωB97X are not among the best representations of IE of LaCO. However, the RSH CAM-B3LYP IE deviates only by 0.05 eV with respect to QZ-CCSD(T) which is the third best among all the selected DFAs in this work. Overall, among all DFAs, CAM-B3LYP is the clear choice that represents both De and IE of LaCO with minimal errors.
In the present work we have performed spin–orbit calculations for both La and LaCO species at the MRCI level. The MRCI spin–orbit calculations of La atom were performed under three different types of active spaces, i.e., 1. CAS(3,6) where the active orbitals are 5d and 6s of La, 2. CAS(3,9) where the active orbitals are 5d, 6s, and 6p of La, 3. CAS(3,16) where the active orbitals are 5d, 6s, 6p, and 4f of La. In all three cases, spin–orbit matrices were constructed including the 2D, 4F, 2F, 4P, 2D, 2P, and 2G states of La. The MRCI/cc-pVTZ-DK3 excitation energies calculated under these three approaches and the corresponding experimental literature values are listed in the Table 3. The MRCI excitation energies obtained under the smallest active space [i.e., MRCI/CAS(3,6)] carry the largest deviations compared to the experimental excitation energies (Table 3). The MRCI/CAS(3,9) provided excitation energies are in reasonable agreement with the experimental values. For example, the discrepancy between MRCI/CAS(3,9) versus experimental excitation energies are within 10–400 cm−1 (Table 3). Interestingly, MRCI/CAS(3,9) provided a better harmony with experiment compared to the excitation energies predicted by MRCI/CAS(3,16) approach. Specifically, the discrepancy between MRCI/CAS(3,16) versus the experiment are in the range of 180–490 cm−1.
| Statea | MRCI/CAS(3,6) | MRCI/CAS(3,9) | MRCI/CAS(3,16) | Experimentb |
|---|---|---|---|---|
| a The 2D3/2 and 2D5/2 states carry 5d16s2 valence electron configuration, whereas others bear 5d26s1 configuration. b Experimental values were obtained from the ref. 21. | ||||
| 2D3/2 | 0 | 0 | 0 | 0.0 |
| 2D5/2 | 810 | 890 | 864 | 1053.2 |
| 4F3/2 | 1408 | 2682 | 2389 | 2668.2 |
| 4F5/2 | 1717 | 2988 | 2685 | 3010.0 |
| 4F7/2 | 2117 | 3415 | 3099 | 3494.5 |
| 4F9/2 | 2674 | 3971 | 3639 | 4121.6 |
| 2F5/2 | 7181 | 7270 | 6830 | 7011.9 |
| 2F7/2 | 7996 | 8202 | 7746 | 8052.2 |
| 4P1/2 | 7024 | 7620 | 7029 | 7231.4 |
| 4P3/2 | 7030 | 7798 | 7199 | 7490.5 |
| 4P5/2 | 6767 | 8005 | 7410 | 7679.9 |
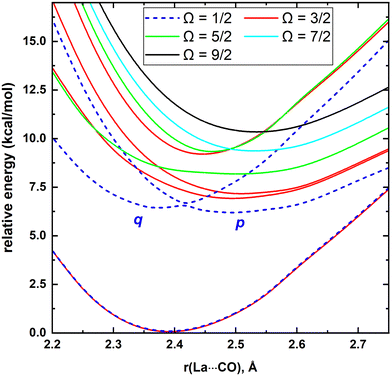
MRCI/CAS(3,9) approach was used for the spin–orbit calculations of LaCO. To evaluate its spin–orbit coupling effects, the spin–orbit states of 14Σ−, 12Σ−, 12Π, 12Δ, and 14Φ electronic states were considered. The spin–orbit coupling produces the Ω = 3/2, 1/2 (14Σ−), Ω = 1/2 (12Σ−), Ω = 3/2, 1/2 (12Π), Ω = 5/2, 3/2 (12Δ), and Ω = 9/2, 7/2, 5/2, 3/2 (14Φ). The spin–orbit coupling curves of these states with respect to La⋯C distance is shown in Fig. 5. The C–O length was kept fixed to the 1.168 Å at each scan. The Ω = 3/2 and 1/2 of 14Σ− are almost identical in energy and displayed very minimal impact from high energy spin–orbit components (Table 4). The Ω = 1/2 of 12Σ− and the Ω = 1/2 of 12Π creates an avoided crossing around 2.42 Å which yields an Ω = 1/2 spin–orbit curve with a double minima at 2.374 Å (q) and 2.493 Å (p) (Fig. 5 and Table 4). The q and p minima dominantly correlate to the parent 12Σ− and 12Π, respectively (Table 4). The p and q minima lie 6.13 and 6.42 kcal mol−1 above the ground Ω = 3/2 state of LaCO. The Ω = 1/2 avoided crossing intersection has ∼80% (12Σ−) and ∼19% (12Π) character. The next five Ω states are highly mixed as shown in the Table 4. Since the spin–orbit matrix only has one of each Ω = 7/2 and 9/2, they solely correlate to the 14Φ.
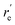 , Å), excitation energy (
, Å), excitation energy (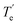 , kcal mol−1), and % compositions of several low-lying spin–orbit states of LaCO at the MRCI/TZ level
, kcal mol−1), and % compositions of several low-lying spin–orbit states of LaCO at the MRCI/TZ level
| Ω State | Composition | ||
|---|---|---|---|
a
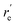 values were obtained from the minima of the spin–orbit coupling curves shown in Fig. 5. values were obtained from the minima of the spin–orbit coupling curves shown in Fig. 5.
|
|||
| 3/2 | 2.389 | 0 | 99.498% 14Σ− + 0.502% 12Π |
| 1/2 | 2.389 | 0.02 | 99.788% 14Σ− + 0.212% 12Π |
| 1/2 (p) | 2.493 | 6.13 | 99.432% 12Π + 0.366% 14Σ− + 0.202% 12Σ− |
| 1/2 (q) | 2.374 | 6.42 | 99.947% 12Σ− + 0.054% 12Π |
| 1/2 | 2.419 | 6.62 | 80.458% 12Σ− + 19.498% 12Π + 0.046% 14Σ− |
| 3/2 | 2.491 | 6.86 | 60.461% 12Π + 29.500% 14Φ + 9.494% 12Δ + 0.544% 14Σ− |
| 3/2 | 2.502 | 7.13 | 57.560% 14Φ + 37.358% 12Π + 4.771% 12Δ + 0.312% 14Σ− |
| 5/2 | 2.504 | 8.17 | 84.764% 14Φ + 15.237% 12Δ |
| 3/2 | 2.442 | 9.04 | 71.164% 12Δ + 26.940% 14Φ + 1.890% 12Π |
| 5/2 | 2.457 | 9.16 | 57.285% 12Δ + 42.698% 14Φ |
| 7/2 | 2.522 | 9.34 | 100% 14Φ |
| 9/2 | 2.534 | 10.29 | 100% 14Φ |
To obtain a more accurate spin–orbit mixing accounted De for La–CO, a single point spin–orbit calculation was performed using the same spin–orbit matrix and optimized MRCI bond lengths of LaCO(14Σ−). The resulted in De of the Ω = 3/2 ground state of LaCO is 16.53 kcal mol−1. With the addition of the zero-point energy obtained at the TZ-CCSD(T), the estimated D0 of the Ω = 3/2 of LaCO decreased to 12.63 kcal mol−1.
IIIB. AcCO
Similar to La, the ground state of the Ac atom is a 2D with the valence nd1(n + 1)s2 electronic configuration (n = 5 and n = 6 for La and Ac, respectively). The first excited state of Ac is a 2Po with the 7s27p1 valence configuration which lies 21.38 kcal mol−1 (2Po1/2) and 35.10 kcal mol−1 (2Po3/2) above.21 The next electronically excited state of Ac is a 4F (6d27s1; 26.35–34.53 kcal mol−1), whereas the analogous 4F (5d26s1) state of La is its first excited state.21 Recall that the ground state of LaCO (14Σ−) is a result of the reaction between La(4F; 5d26s1) versus CO(X1Σ+) and with respect to these fragments it is bound by ∼23 kcal mol−1. Since the 2D(5d16s2) → 4F(5d26s1) excitation of La (i.e., 7.63–11.78 kcal mol−1) is considerably low in energy compared to the 2D(6d17s2) → 4F(6d27s1) transition of Ac, the ground state of AcCO is expected to be less stable (in case it is originating from 4F of Ac) or derived from a lower energy state of Ac.21 To portray a better picture of the origination of this system, the full MRCI+Q potential energy profile was produced for AcCO as a function of Ac⋯C length where C–O distance was kept constant to 1.159 Å which is the C–O length of the TZ-CCSD(T) optimized geometry of AcCO(12Π) (Fig. 6). The 4Σ− state originating from the Ac(4F) + CO(X1Σ+) is bound by ∼25 kcal mol−1 with respect to the corresponding fragments and lies ∼14 kcal mol−1 above the ground state (12Π) of AcCO. This is indeed in line with our expectation. The ground state of AcCO(12Π) is originating from the Ac(2D) + CO(X1Σ+) fragments and carries an ∼12 kcal mol−1De. The 2Δ and 2Σ+ states deriving from the same fragments are strongly repulsive at first and become slightly attractive around ∼2.5 Å (Fig. 6). Similarly, the 2Δ and 2Σ+ PECs of the La(2D) + CO(X1Σ+) are initially repulsive but conversely form substantially stable minima that are bound with respect to the ground state fragments (Fig. 1).The shapes of the orbitals of AcCO are similar to the orbitals of LaCO (Fig. 2). The Des, res, Tes, and ωes of optimized 12Π and 4Σ− states of AcCO under MRCI, MRCI+Q, and CCSD(T) levels are given in the Table 5. The 12Π and 4Σ− of AcCO have analogous single-reference 1σ21π1 and 1σ11π2 electron configurations as 12Π and 14Σ− of LaCO, respectively. Similar to the LaCO case, the high electron density of the π* orbitals cause a shorter Ac–C and longer C–O lengths for 4Σ− of AcCO compared to the corresponding lengths of 12Π. At the TZ-CCSD(T) level, the AcCO(12Π) carries 13.42 kcal mol−1De which is larger than both MRCI+Q and MRCI Des (Table 5). As expected, the more expensive QZ-CCSD(T) predicted slightly higher De than the TZ-CCSD(T) (by 0.63 kcal mol−1). The zero-point energy correction decreased the QZ-CCSD(T) D0 of AcCO(12Π) to 10.37 kcal mol−1. The TZ-CCSD(T), QZ-CCSD(T), and MRCI+Q levels predicted a bound 4Σ− state with respect to Ac(2D) + CO(X1Σ+) asymptote although it is not bound at the MRCI level. However, after inclusion of the zero-point energy, it is only bound at the QZ-CCSD(T) level by 1.61 kcal mol−1. The side-bonded Ac(η2-CO) was also studied at the TZ-CCSD(T) level and its ground 2A′ state is found to lie 6.04 kcal mol−1 high in energy than the 12Π of linear AcCO. The TZ-CCSD(T) optimized Ac–C, Ac–O, and C–O lengths of Ac(η2-CO) (2A′) are 2.685, 2.531, and 1.214 Å, respectively. Select occupying molecular orbitals of Ac(η2-CO) (2A′) are given in ESI,† Fig. S2. At the TZ-CCSD(T) level the calculated vibrational frequencies of Ac(η2-CO) (2A′) are 271, 341, and 1616 cm−1. Similar to the La case, the side-bonded Ac(η2-CO) showed higher ionic character (i.e., Ac+0.51C−0.10O−0.41) compared to the linear AcCO (Ac+0.29–[CO]−0.29). A transition state of 14.45 kcal mol−1 was observed for the AcCO → Ac(η2-CO) transformation at the TZ-CCSD(T) level. Similar to the La case, the energy barrier of this reaction was obtained by first optimizing the transition state at the DFT/B3LYP under the Stuttgart RSC 1997 basis set with 60-electron ECP of Ac and cc-pVQZ of C and O and then performing a single-point TZ-CCSD(T) calculation to the DFT geometry.
| State | Level of theorya | D e | r e | T e | ω e | |
|---|---|---|---|---|---|---|
| Ac–C | C–O | |||||
| a Davidson corrected MRCI is denoted by MRCI+Q. For all MRCI, MRCI+Q, and TZ-CCSD(T) calculations cc-pVTZ-DK3 of Ac and cc-pVTZ-DK of C and O basis set was applied. The cc-pVQZ-DK3 of Ac and cc-pVQZ-DK of C and O basis set was used for QZ-CCSD(T) calculations. | ||||||
| 12Π | QZ-CCSD(T) | 14.05 | 2.584 | 1.156 | 0 | 168, 233, 258, 1915 |
| TZ-CCSD(T) | 13.42 | 2.589 | 1.159 | 0 | 192, 251, 257, 1910 | |
| MRCI+Q | 11.32 | 2.591 | 1.151 | 0 | ||
| MRCI | 9.81 | 2.601 | 1.141 | 0 | ||
| 4Σ− | QZ-CCSD(T) | 5.47 | 2.437 | 1.171 | 8.58 | 274, 286, 313, 1825 |
| TZ-CCSD(T) | 3.74 | 2.444 | 1.174 | 9.68 | 275, 281, 307, 1823 | |
| MRCI+Q | 2.451 | 1.166 | 9.40 | |||
| MRCI | 2.466 | 1.155 | 11.05 | |||
We expect the spin–orbit coupling effects to be significant for actinide species and therefore in order to calculate more accurate energy related properties of AcCO, the spin–orbit effects must be accounted. To perform MRCI spin–orbit calculations, all the electronic states of AcCO given in the Fig. 6 were included in the spin–orbit matrix. A few low energy spin–orbit PECs of AcCO are plotted and given in ESI,† Fig. S3. The spin–orbit coupling splits 12Π into Ω = 1/2 and Ω = 3/2 and at the equilibrium distances they are predominantly 12Π in character (>99%). At the MRCI level 12Π3/2 lies 3.20 kcal mol−1 above the 12Π1/2 making 12Π1/2 the ground state of AcCO. The 12Π1/2 and 12Π3/2 are 1.65 and 1.55 kcal mol−1 more and less stable compared to the parent 12Π, respectively. The next lowest energy spin–orbit minimum (i.e., 4Σ−1/2) is 16.62 kcal mol−1 high in energy than the 12Π1/2 and not bound with respect to the lowest energy fragments (ESI,† Fig. S3). Spin–orbit effect accounted De of AcCO(12Π1/2) is 8.94 kcal mol−1. We estimated a final D0 of 5.19 kcal mol−1 for AcCO(12Π1/2) by applying the zero-point energy of AcCO(12Π) at TZ-CCSD(T) level. The relatively less D0 of AcCO could be a major reason for the absence of experimental reports on AcCO so far.
IV. Conclusions
The present work reports full PECs, Des, res, Tes, and ωes of low-lying electronic states of LaCO and AcCO complexes by means of ab initio MRCI+Q, MRCI, and CCSD(T) levels of theory. The ground state of LaCO is a single-reference 14Σ− (1σ11π2) formed via OC → La σ dative bond and La → CO π-back bonds. The LaCO(14Σ−) is a product of the reaction between excited La(4F) versus CO(X1Σ+). The spin–orbit effect accounted zero-point energy corrected D0 of the 14Σ−3/2 ground state of LaCO is ∼13 kcal mol−1. The ground 4A′′ of La(η2-CO) was found to be ∼14 kcal mol−1 above the LaCO(14Σ−). The first six excited electronic states of LaCO are 12Σ−, 12Π, 12Δ, 14Φ, 14Π, and 12Σ+ in order under both MRCI and MRCI+Q levels of theory. The ground state of the LaCO+ is a 3Σ− formed by the ionization of the 1σ1 electron from the LaCO(14Σ−; 1σ11π2) and the corresponding IE of LaCO is 5.316 eV. We have evaluated the DFT errors associated with the De and IE of LaCO using 16 exchange correlation functionals that span three rungs of Jacob's ladder of DFA (GGA, MGGA, and hybrid) and compared with the QZ-CCSD(T) values. The expensive RSH provided the closest match with the QZ-CCSD(T) for De of LaCO (with less than 21% error). The MGGA TPSS, MGGA hybrid TPSSh and M06-2X, and RSH CAM-B3LYP predicted IEs with minimal errors with respect to CCSD(T) IE. Overall, among the chosen DFAs, the RSH CAM-B3LYP predicted De and IE of LaCO with relatively less errors.Even though the ground state of LaCO is originating from the excited 4F state of La, the lowest energy molecular state of AcCO that 4F of Ac forms (4Σ−) lies 14 kcal mol−1 above the 12Π ground state of AcCO, which is clearly due to the higher energy gap between 2D [nd1(n + 1)s2] and 4F [nd2(n + 1)s1] of Ac compared to that of La. The AcCO(12Π) is derived from the ground state fragments [i.e., Ac(2D) + CO(X1Σ+)]. The spin–orbit effect accounted zero-point energy corrected D0 of the 12Π1/2 ground state of AcCO is ∼5 kcal mol−1. The 12Π and 4Σ− of AcCO have 1σ21π1 and 1σ11π2 electron configurations respectively, similar to the configurations of 12Π and 14Σ− of LaCO. The ground state of the side-bonded Ac(η2-CO) (2A′) lies 6 kcal mol−1 above the 12Π of AcCO. A 14 kcal mol−1 transition state barrier lies in between AcCO → Ac(η2-CO) conversion which is lower by ∼5 kcal mol−1 compared to the energy barrier of the LaCO → La(η2-CO) transition. The side-bonded Ac(η2-CO) bears higher ionic character compared to the linear AcCO which is consistent with the observations of the La case.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The support of the Los Alamos National Laboratory (LANL) Laboratory Directed Research and Development program Project No. 20240737PRD1 is acknowledged. This research used resources provided by the LANL Institutional Computing Program, which is supported by the U.S. Department of Energy National Nuclear Security Administration under Contract No. 89233218CNA000001.References
- G. R. Choppin, J. Radioanal. Nucl. Chem., 2007, 273, 695–703 CrossRef.
- K. De Jesus, R. Rodriguez, D. L. Baek, R. V. Fox, S. Pashikanti and K. Sharma, J. Mol. Liq., 2021, 336, 116006 CrossRef.
- R. M. Cox, A. Kafle, P. B. Armentrout and K. A. Peterson, J. Chem. Phys., 2019, 151, 034304 CrossRef.
- P. B. Armentrout and K. A. Peterson, Inorg. Chem., 2020, 59, 3118–3131 CrossRef PubMed.
- Z. Zhu, M. Marshall, K. H. Bowen and K. A. Peterson, J. Chem. Phys., 2022, 156, 054305 CrossRef.
- M. Vasiliu, K. A. Peterson, M. Marshall, Z. Zhu, B. A. Tufekci, K. H. Bowen and D. A. Dixon, J. Phys. Chem. A, 2022, 126, 198–210 CrossRef.
- A. Kafle, P. B. Armentrout, S. R. Battey and K. A. Peterson, Inorg. Chem., 2021, 60, 10426–10438 CrossRef PubMed.
- I. R. Ariyarathna and E. Miliordos, Phys. Chem. Chem. Phys., 2019, 21, 24469–24477 RSC.
- G. F. de Melo, M. Vasiliu, M. Marshall, Z. Zhu, B. A. Tufekci, S. M. Ciborowski, M. Blankenhorn, R. M. Harris, K. H. Bowen and D. A. Dixon, J. Phys. Chem. A, 2022, 126, 4432–4443 CrossRef PubMed.
- G. F. de Melo, M. Vasiliu, G. Liu, S. Ciborowski, Z. Zhu, M. Blankenhorn, R. Harris, C. Martinez-Martinez, M. Dipalo, K. A. Peterson, K. H. Bowen and D. A. Dixon, J. Phys. Chem. A, 2022, 126, 7944–7953 CrossRef.
- J. G. F. Romeu, A. R. E. Hunt, G. F. de Melo, K. A. Peterson and D. A. Dixon, J. Phys. Chem. A, 2024, 128, 5586–5604 CrossRef.
- G. F. de Melo and D. A. Dixon, J. Phys. Chem. A, 2023, 127, 1588–1597 CrossRef.
- G. F. de Melo, M. Vasiliu, G. Liu, S. Ciborowski, Z. Zhu, M. Blankenhorn, R. Harris, C. Martinez-Martinez, M. Dipalo, K. A. Peterson, K. H. Bowen and D. A. Dixon, J. Phys. Chem. A, 2022, 126, 9392–9407 CrossRef PubMed.
- G. Hong, X. Lin, L. Li and G. Xu, J. Phys. Chem. A, 1997, 101, 9314–9317 CrossRef.
- Q. Xu, L. Jiang and R. Q. Zou, Chem. – Eur. J., 2006, 12, 3226–3232 CrossRef.
- L. Jiang and Q. Xu, J. Phys. Chem. A, 2007, 111, 3271–3277 CrossRef.
- G. Zhang, Z. H. Li, W. N. Wang and K. N. Fan, J. Phys. Chem. A, 2007, 111, 11894–11903 CrossRef PubMed.
- W. Xu, X. Jin, M. Chen, P. Pyykkö, M. Zhou and J. Li, Chem. Sci., 2012, 3, 1548–1554 RSC.
- D. R. Lide, CRC Handbook of Chemistry and Physics, 93rd edn, CRC Press, New York, 2012 Search PubMed.
- R. C. Ropp, Encyclopedia of the Alkaline Earth Compounds, Elsevier, Netherlands, 2013, pp. 25–104 DOI:10.1016/B978-0-444-59550-8.00002-8.
- A. Kramida, Y. Ralchenko and J. Reader, NIST Atomic Spectra Database (Version 5.3), National Institute of Standards and Technology, Gaithersburg, MD, 2015. https://physics.nist.gov/asd Search PubMed.
- M. Zhou, L. Andrews, J. Li and B. E. Bursten, J. Am. Chem. Soc., 1999, 121, 9712–9721 CrossRef CAS.
- J. Li, B. E. Bursten, M. Zhou and L. Andrews, Inorg. Chem., 2001, 40, 5448–5460 CrossRef CAS PubMed.
- I. R. Ariyarathna, Y. Cho, C. Duan and H. J. Kulik, Phys. Chem. Chem. Phys., 2023, 25, 26632–26639 RSC.
- J. P. Perdew, Phys. Rev. B: Condens. Matter Mater. Phys., 1986, 33, 8822–8824 CrossRef PubMed.
- A. D. Becke, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098–3100 CrossRef CAS.
- B. Miehlich, A. Savin, H. Stoll and H. Preuss, Chem. Phys. Lett., 1989, 157, 200–206 CrossRef CAS.
- F. J. Devlin, J. W. Finley, P. J. Stephens and M. J. Frisch, J. Phys. Chem., 1995, 99, 16883–16902 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 CrossRef PubMed.
- H. S. Yu, X. He and D. G. Truhlar, J. Chem. Theory Comput., 2016, 12, 1280–1293 CrossRef CAS PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS.
- M. Ernzerhof and G. E. Scuseria, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2007, 120, 215–241 Search PubMed.
- H. S. Yu, X. He, S. L. Li and D. G. Truhlar, Chem. Sci., 2016, 7, 5032–5051 RSC.
- M. A. Rohrdanz, K. M. Martins and J. M. Herbert, J. Chem. Phys., 2009, 130, 054112 CrossRef.
- T. Yanai, D. P. Tew and N. C. Handy, Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- J. D. Chai and M. Head-Gordon, J. Chem. Phys., 2008, 128, 084106 CrossRef PubMed.
- H. J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2011, 2, 242–253 Search PubMed.
- H. J. Werner, P. J. Knowles, F. R. Manby, J. A. Black, K. Doll, A. Hesselmann, D. Kats, A. Kohn, T. Korona, D. A. Kreplin, Q. Ma, T. F. Miller III, A. Mitrushchenkov, K. A. Peterson, I. Polyak, G. Rauhut and M. Sibaev, J. Chem. Phys., 2020, 152, 144107 CrossRef CAS.
- H.-J. Werner and P. J. Knowleset al., MOLPRO, version 2, a package of ab initio programs, 2023, https://www.molpro.net Search PubMed.
- H.-J. Werner and P. J. Knowles, J. Chem. Phys., 1988, 89, 5803–5814 CrossRef CAS.
- P. J. Knowles and H.-J. Werner, Chem. Phys. Lett., 1988, 145, 514–522 CrossRef CAS.
- K. R. Shamasundar, G. Knizia and H. J. Werner, J. Chem. Phys., 2011, 135, 054101 CrossRef CAS PubMed.
- K. Raghavachari, G. W. Trucks, J. A. Pople and M. Head-Gordon, Chem. Phys. Lett., 1989, 157, 479–483 CrossRef CAS.
- Q. Lu and K. A. Peterson, J. Chem. Phys., 2016, 145, 054111 CrossRef PubMed.
- T. H. Dunning, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef CAS.
- W. A. de Jong, R. J. Harrison and D. A. Dixon, J. Chem. Phys., 2001, 114, 48–53 CrossRef CAS.
- H.-J. Werner and P. J. Knowles, J. Chem. Phys., 1985, 82, 5053–5063 CrossRef CAS.
- P. J. Knowles and H.-J. Werner, Chem. Phys. Lett., 1985, 115, 259–267 CrossRef CAS.
- D. A. Kreplin, P. J. Knowles and H. J. Werner, J. Chem. Phys., 2019, 150, 194106 CrossRef.
- D. A. Kreplin, P. J. Knowles and H. J. Werner, J. Chem. Phys., 2020, 152, 074102 CrossRef CAS.
- S. R. Langhoff and E. R. Davidson, Int. J. Quantum Chem., 1974, 8, 61–72 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Gaussian Inc., Wallingford CT, 2016 Search PubMed.
- X. Cao and M. Dolg, J. Chem. Phys., 2001, 115, 7348–7355 CrossRef CAS.
- X. Cao and M. Dolg, J. Mol. Struct.: THEOCHEM, 2002, 581, 139–147 CrossRef CAS.
- C. Koukounas, S. Kardahakis and A. Mavridis, J. Chem. Phys., 2005, 123, 074327 CrossRef PubMed.
- M. Zhou and L. Andrews, J. Phys. Chem. A, 1999, 103, 2964–2971 CrossRef CAS.
- I. R. Ariyarathna and E. Miliordos, J. Phys. Chem. A, 2017, 121, 7051–7058 CrossRef CAS.
- G. Knizia, J. Chem. Theory Comput., 2013, 9, 4834–4843 CrossRef CAS.
- I. R. Ariyarathna, Phys. Chem. Chem. Phys., 2024, 26, 21099–21109 RSC.
- I. R. Ariyarathna, Phys. Chem. Chem. Phys., 2024, 26, 22858–22869 RSC.
- I. R. Ariyarathna, C. Duan and H. J. Kulik, J. Chem. Phys., 2022, 156, 184113 CrossRef CAS PubMed.
- I. R. Ariyarathna, J. Comput. Chem., 2024, 45, 2530–2538 CrossRef CAS.
- I. R. Ariyarathna and E. Miliordos, Phys. Chem. Chem. Phys., 2018, 20, 12278–12287 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1 and S2 list DFT De, IE, and % DFT errors of LaCO(14Σ−); Fig. S1 and S2 illustrate the select molecular orbitals of La(η2-CO) and Ac(η2-CO); Table S3 provides the Cartesian coordinates of the transition states of the linear to side-bonded structures; Fig. S3 illustrates the spin–orbit coupling curves of AcCO. See DOI: https://doi.org/10.1039/d4cp03132f |
| This journal is © the Owner Societies 2024 |