DFT and machine learning guided investigation into the design of new dual-atom catalysts based on α-2 graphyne†
Chandra
Chowdhury
 *a,
Esackraj
Karthikraja
ab and
Venkatesan
Subramanian
bc
*a,
Esackraj
Karthikraja
ab and
Venkatesan
Subramanian
bc
aAdvanced Materials Laboratory, CSIR-Central Leather Research Institute (CSIR-CLRI), Sardar Patel Road, Adyar, Chennai 600 020, India. E-mail: pc.chandra12@gmail.com
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad-201002, India
cIndian Institute of Technology Madras, Sardar Patel Road, Adyar, Chennai 600 036, India
First published on 16th September 2024
Abstract
The realm of atomic catalysts has witnessed notable advancements; yet, the predominant focus remains on single atomic catalysts (SACs). The exploration and successful implementation of dual atomic catalysts (DACs) pose intricate challenges, primarily concerning thermodynamic stability and optimal metallic composition. To address these issues, we present a comprehensive theoretical investigation of α-2 graphyne (GPY)-based DACs, synthesized in-house with a keen focus on formation stability. Density functional theory (DFT) simulations were leveraged to ascertain each DAC structure's stability, considering numerous transition metal permutations totalling about 823 DACs. Furthermore, we developed a machine learning (ML) model that predicts stability based solely on the physical characteristics of the constituent elements in the DACs, thus eliminating the need for extensive DFT calculations. Our findings not only offer detailed insights into atomic interactions but also highlight promising candidates for DACs, pushing beyond traditional trial-and-error synthesis approaches. This study fosters a deeper understanding of DACs and paves new pathways for exploring atomic catalysts for practical applications.
1 Introduction
Human society is confronted with increasingly serious challenges of resource depletion and environmental degradation as a result of the continued expansion of industrialisation and the growth of the world's population. The overuse of fossil fuels like oil, coal, and natural gas has exacerbated the already dire energy problem and increased environmental degradation.1 Hence, a crucial area of study in the realm of catalysis and energy research is the investigation and development of clean, renewable energy sources that can replace fossil fuels.2,3 Studies on clean energy storage and conversion technologies such as metal–air batteries,4 electrolytic cells,5 and fuel cells6 are particularly intriguing. These devices rely on charge transfer processes and electrocatalytic reactions that take place on the electrode material for the conversion of electrical and chemical energies, such as oxygen reduction and evolution reactions, hydrogen evolution/oxidation reactions, etc.7–9 The primary electrode materials in these devices are electrocatalysts, and the thermodynamic and kinetic processes of electrocatalytic reactions are intimately tied to the physical characteristics and chemical states of electrocatalysts.Nano-engineering-based solutions have been presented to create electrocatalysts with enhanced performance in response to rising expectations about catalyst efficiency and activity.10–13 Some noble-metal surfaces, such as Pt, Pd, Rh, and Au, have been shown in prior research to be capable of catalysing CO oxidation.14–16 However, their use in large-scale processes is constrained by their high operating temperature, poor durability, and high cost. It is well known that switching from bulk materials to supported nanoparticles improves the catalytic performance in many reactions including the CO oxidation reaction.17 The supported metal nanoparticles’ catalytic activity, though, can be significantly shape- and size-dependent.18,19 Additionally, because only the surface atoms of the metals are active for catalysis, the overall efficiency is quite poor on a per metal atom basis. Therefore, downsizing the metal nanostructures to well-defined, atomically scattered metal active centres, that is, single-atom catalysts (SACs),20,21 which is the ultimate goal of fine dispersion, is the most efficient technique to utilise every metal atom of supporting metal catalysts. Some examples of SACs, including single Au/Pt/Ir/Ni/Os atoms on FeOx, Co3O4, CeO2, Al2O3, and MgO surfaces, have been successfully fabricated using metal oxide supports since the first SAC, single Pt atoms on FeOx, was reported in 2011.22,23 Despite the fact that SACs have more significant uses than metal nanoclusters, there are a number of significant SAC-related difficulties that raise questions about how well they will be used.24 For example, because of the low metal loading, the whole catalyst is sometimes required to have a heavy loading, which makes the mass-transfer resistance worse.25 Along with this, the consistency of the SACs is still another significant factor to consider. For surface catalytic reactions that require ensemble sites, such as C–C dissociation reactions, SACs can't be a perfect choice as they do not have any ensemble sites.26,27 In addition to the previously described issues, one significant constraint is that because SACs have only one catalytic site, reactions involving numerous reactants cannot take place because competing adsorption would significantly reduce the catalytic activity on a single-atom structure, as for example in the CO oxidation reaction.
Due to their greater versatility in catalytic tuning than SACs, dual-atom catalysts (DACs), an outgrowth of SACs, have recently received increased attention. DACs with a low number of bridge active sites can both improve the metal loading and the percentage of the active centre while maintaining the same level of metal consumption.28,29 Moreover, DACs have a distinct competitive edge due to their metal cooperativity.30 The interaction and stabilisation of two neighbouring metal atoms can increase the stability and synthesisability of DACs.31 The interest in DACs intensified following the groundbreaking work of He et al., who successfully fabricated and characterised graphene-supported Fe dopant pairs.32 An additional potential method to achieve DACs involves using MOFs with various metal precursors.33,34 In both theoretical and experimental research, a growing number of homonuclear and heteronuclear DACs are being used. Nevertheless the accurate characterization, suppression of metal agglomeration, and management of metal atom anchoring provide substantial hurdles to the DACs' ongoing advances.35 Furthermore, the numerous combinations of all the transition metals (TMs) present a challenge to such promising DAC techniques, necessitating an astronomically high cost to individually test the effectiveness of each catalyst. To appropriately examine and screen the most viable and probable choices, the ideal solution is to integrate theoretical calculations with machine learning algorithms, which can provide the essential direction for trials.
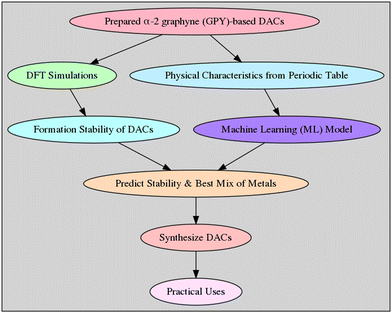
The crucial element to ensuring the stable isolation of surface metal atoms as active sites and achieving high electrocatalytic performances is the strong contacts between the anchoring metal atom and the support material.36 This is the most important foundation for a good atomic catalyst. A novel kind of substrate with distinctive low-dimension structures and coordination environments has been made available by a carbon-based support. Methane has been successfully activated on a dual-atom catalyst supported by graphene, as recently demonstrated.37 Many research studies have used carbon-based materials as the basis for atomic catalysts to activate various kinds of significant catalytic and electrocatalytic events.38,39 Graphdiyne (GDY),40 one of the several carbon-based support materials, has emerged as one of the most promising contenders. Most significantly, GDY contains both sp2 and sp hybridised carbon atoms with high levels of conjugations; the special sp hybridisation enhances intermediate adsorption and coordination of metal atoms during electrocatalysis. Subramanian and coworkers41 created a special type of α-graphyne structure, namely α-2 graphyne, by inclusively introducing acetylene linkers into the graphyne precursor and it has been shown to be very interesting in terms of electronic properties. Also, due to the inclusion of acetylene linkers, there are increasing numbers of sp hybridisations, which eventually can enhance its application as a support material for catalytic applications. Motivated by the current research status, herein we have conducted parallel theoretical research on α-2 graphyne for DACs by taking into account all possible TM combinations. It is to be noted that there are a few previous studies where the s block alkaline-earth metal catalysts have also been extensively studied42–45 and in the future we have a plan to study the details using the alkaline-earth metals as well. The overall idea of the manuscript is illustrated in Fig. 1.
2 Computational details
2.1 Details about DFT calculations
The projector augmented wave approach within the framework of density functional theory (DFT), as implemented in the Vienna ab initio simulation program (VASP), was used for all electronic structure computations.46 The Perdew–Burke–Ernzerhof functional was used as a parameter to define the exchange–correlation energy within the framework of the generalised gradient approximation.47 A plane wave basis set was employed with an energy threshold of 450 eV. The Grimme (DFT-D3) technique, which employs a semiempirical correction methodology, was able to take into account van der Waals interactions.48 The influence of spin polarisation was accounted for in all calculations. The energy and force convergence criteria were set to 10−4 eV and 10−3 eV Å−1, respectively. The Brillouin region was sampled using a 5 × 5 × 1 Monkhorst–Pack special k-point mesh for structural relaxation and a 7 × 7 × 1 mesh for density of state (DOS) computations. To prevent the introduction of any unnatural interactions caused by periodic images, a vacuum of roughly 15 Å was established between two neighbouring graphyne layers.2.2 Details about the ML model
Our analysis includes 823 different possible setups. Without resorting to any further first-principles calculations, we construct a high-dimensional feature space by utilising the properties of the relevant systems obtained from the periodic table. As a result, we use a set of 40 features for target prediction as our primary dataset. In order to ensure that our machine learning models produce consistent results regardless of the scale or size of the underlying dataset, we convert the features before using the dataset. To pick the most relevant features, we built a Pearson correlation using permutation feature importance analysis. For feature importance analysis we used the SHapley Additive exPlanations (SHAP) technique. The SHAP values assigned to each feature indicate the alteration in the anticipated model prediction when the model is conditioned on that specific characteristic. The SHAP value provides an explanation for each feature by quantifying its contribution to the discrepancy between the average prediction made by the model and the actual prediction for a given instance. We use a wide range of supervised machine learning algorithms, such as multiple linear regression (MLR), random forest models (RF), ridge regression (RR), least absolute shrinkage and selection operator (LASSO), extra trees (ET), and gradient boosting (GB), to make predictions about the characteristics.49–54We used root mean squared error (RMSE) as our loss metric, which is determined using eqn (1), where yi, 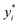 and m denote the true values, predicted values and the number of samples in the dataset, respectively.
and m denote the true values, predicted values and the number of samples in the dataset, respectively.
 | (1) |
For all machine learning training and prediction, we leaned on the Scikit-learn tool.55
3 Results and discussion
3.1 Structure of the α-2 graphyne (GPY) system
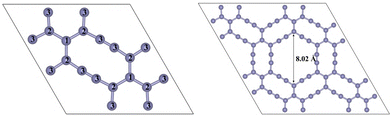
The GPY unit cell contains 20 carbon atoms and the optimised unit cell has a hexagonal lattice with a = b = 9.38 Å, which is consistent with that determined in a previous report.41 There are three different bond lengths and three different bond angles present in the unit cell, as illustrated in Table 1. These bond lengths and angles correspond to the three different hybridizations of C atoms (sp–sp, sp–sp2, and sp2–sp2), which agrees well with those obtained in a previous report.41 In the 2 × 2 supercell the largest distance between the two corner positions is 8.02 Å in the hexagonal vacancy site as shown in Fig. 2. Previously it was shown that this system was stable at room temperature using first-principles molecular dynamics (FPMD) simulations and for investigating its dynamic stability phonon calculations were done, also showing a similar cohesive energy to those of α-, β- and γ-graphyne systems.56 It also has a similar areal density to that of a graphene system.| System | Bond length (Å) | Bond angle (°) |
|---|---|---|
| α-2 graphyne | 1.44 (C1–C2) | 120 (C2–C1–C2) |
| 1.38 (C2–C3) | 114 (C3–C2–C3) | |
| 1.22 (C3–C3) | 177 (C2–C3–C3) |
3.2 Geometric structure and stability of DACs
The DACs have been successfully formed by anchoring metal dimers to a variety of 2D materials, including graphene, graphitic carbon nitride (g-C3N4), nitrogen-doped carbons, and rectangular-shaped expanded phthalocyanine.32,57–59 Recently, using first-principles calculations, researchers have explored γ-graphyne's (γ-GY's) potential as a substrate for metals in the fourth and fifth periods under single-atom and dual-atom concentration modes and eventually a few dual atomic sites have been found to be very active for the CO oxidation reaction.60 The thermodynamic trends of formation, as the most essential aspect, should be one of the most critical criteria to determine the synthesis possibilities in experiments. We have taken into account all the 3d, 4d, and 5d transition metals from group IVB to IB, for a grand total of 823 possible combinations of metal atoms, which include both homonuclear and heteronuclear combinations, in order to create dual atom catalysts.We have accounted for all conceivable vacancy sites in the structure by considering a α-2 graphyne unit cell with a 2 × 2 configuration. We found that only the largest hexagonal vacancy position is suitable for accommodating metal atoms, wherein placing metal atoms in other positions deformed the original structure of GPY. The initial metal is shown to have been inserted into the big hexagonal cavity and to have formed bonds with the adjacent carbon atoms in Fig. 3. The second metal also forms connections with the carbon atoms since it was positioned similarly. When examining DACs in detail, it is clear that there are two distinct ways in which metal atoms can remain in the vacant sites. Smaller-radius metal atoms (3d or early 4d) arrange themselves in a flat plane, while larger-radius metal atoms buckle a bit, with one atom remaining in the same plane as the substrate and the other below it. Sc–Sc, Zr–Zr, and Hf–Hf DACs all exhibit extremely comparable bond length variations, and under most conditions, we observe that metal atoms in the same plane within the pore of the GPY are stabilised. Distinct types of distortion are observed with the heterocoupling DACs. Because of variations in the ionic radius and electronic structure, the atomic distributions are asymmetric, which in turn distorts the GPY bonding. For 4d–5d and 5d–5d hetero-coupling DACs, we find that the metal is separated from the GPY due to the strong repulsive force, resulting in dangling metal sites on the GPY. The structure depicted in Fig. 3 turned out to be the energetically most favourable one after we investigated other sites in the GPY surface for anchoring the metal atoms. The constructed metal dimers in the DACs show a bond length ranging from 2.04 Å to 4.10 Å, resulting in a wide variety of DAC structures. Table S1 (ESI†) provides some values for bond lengths.
 | ||
| Fig. 3 Representation of the formation of dual atom catalysts. The different combinations of metals lead to varied interactions and formation of different DACs occurs. | ||
We have created a variety of metrics for calculating thermodynamic stabilities in order to make direct comparisons between the formation preferences. The binding energy (Eb) was calculated to ascertain the likelihood of the uniform distribution and it was calculated using the following equation:
 | (2) |
 , EGPY and
, EGPY and  represent the energies of the metal intercalated system, bare GPY system and isolated metal atoms, respectively. From Fig. 4, upper left panel, it is seen that most of the considered DACs show negative binding energies, which confirms the strong binding of the metal atoms with the substrate structure, which is important for the stability of the DACs. Anchoring two TM metals from the same row appears to be the most promising method due to the similarities in atomic radii and electronic structures. After careful evaluation of binding energies, it is seen that 5d–5d combinations showed the highest binding energy followed by 4d–5d, 3d–5d and 3d–4d combinations. 3d–3d metal combinations showed lower binding energy compared to the other combinations and this corresponds well with the size of the TM atoms. To learn more about the strong binding preferences of the DACs, we made box plots for different combinations of DACs that show the energy change differences very clearly. From Fig. 5 it is seen that the 5d–5d combination shows the least variations in energy suggesting a higher synthesis probability. Also, it is seen that with the introduction of heteroatoms as for example, 3d–5d combinations, the heteronuclear DACs show a higher stability than the homonuclear counterpart and this effect is more profound for early TM atoms. Electronic structure properties of both homonuclear (MM@GPY) and heteronuclear (MM′@GPY) DACs were validated by Bader charge analysis. Table S2 (ESI†) shows that a large amount of charge is transferred from the metal dimers to the GPY surface, leading to the formation of strong-polarized and hybridised metal–C bonds. Consistent with the trend of electronegativity (Table S3, ESI†), our modelled DACs show a significant decreasing trend of charge transfer from left to right in the same period, suggesting that the increased number of unpaired electrons in metal atoms favours the charge transfer to the surrounding atoms.
represent the energies of the metal intercalated system, bare GPY system and isolated metal atoms, respectively. From Fig. 4, upper left panel, it is seen that most of the considered DACs show negative binding energies, which confirms the strong binding of the metal atoms with the substrate structure, which is important for the stability of the DACs. Anchoring two TM metals from the same row appears to be the most promising method due to the similarities in atomic radii and electronic structures. After careful evaluation of binding energies, it is seen that 5d–5d combinations showed the highest binding energy followed by 4d–5d, 3d–5d and 3d–4d combinations. 3d–3d metal combinations showed lower binding energy compared to the other combinations and this corresponds well with the size of the TM atoms. To learn more about the strong binding preferences of the DACs, we made box plots for different combinations of DACs that show the energy change differences very clearly. From Fig. 5 it is seen that the 5d–5d combination shows the least variations in energy suggesting a higher synthesis probability. Also, it is seen that with the introduction of heteroatoms as for example, 3d–5d combinations, the heteronuclear DACs show a higher stability than the homonuclear counterpart and this effect is more profound for early TM atoms. Electronic structure properties of both homonuclear (MM@GPY) and heteronuclear (MM′@GPY) DACs were validated by Bader charge analysis. Table S2 (ESI†) shows that a large amount of charge is transferred from the metal dimers to the GPY surface, leading to the formation of strong-polarized and hybridised metal–C bonds. Consistent with the trend of electronegativity (Table S3, ESI†), our modelled DACs show a significant decreasing trend of charge transfer from left to right in the same period, suggesting that the increased number of unpaired electrons in metal atoms favours the charge transfer to the surrounding atoms.
 | ||
| Fig. 4 Mapping of binding energy (top left), aggregation energy (top right), stabilization energy (bottom left) and anchoring energy (bottom right) of all the DAC combinations. | ||
 | ||
| Fig. 5 The box plots of each mix of transition metals, which mainly indicate the distribution and variation range of the binding energies. | ||
The aggregation energy is a crucial consideration for DAC stability alongside the adsorption energy and this is calculated using the following equation:
| Eagg = (Eb − Ecoh), | (3) |
| Ecoh = (ETM-bulk − ETM-single), | (4) |
 | (5) |
As can be shown in Fig. 4, most metal atoms would rather form the DACs than two separate SACs. These findings show that the experimental synthesis, which involves a mixture of metal precursors, favours the formation of a DAC at a single, optimal anchoring site over the formation of an isolated SAC at many anchoring sites. Careful examination of the data reveals that although some DACs have a low binding energy, they are less selective for DACs than isolated SACs due to a positive stabilisation energy. These findings provide further evidence that strong thermodynamic stability is not sufficient to ensure the synthesis of DACs. A further critical aspect is the rivalry for selection between the SAC and DAC.
DACs’ stability can also be described in terms of something called “anchoring energy”, which is the amount of energy needed to anchor a second metal atom within the DAC and this is calculated as follows:
 | (6) |
From Fig. 4, it is seen that in contrast to the stabilisation energies where most of the DACs show negative values, for a higher number of DACs the anchoring energy values are positive. The stabilisation and anchoring energies are very different, and therefore it makes sense to anchor two different metal atoms at once as a synthesis strategy. In contrast, the energy cost of adding the second metal atom to the produced SAC is significantly larger.
The interaction between the d-orbitals of the two metal atoms and the support in DACs results in a distinctive electronic environment that affects the adsorption energies of reactants and intermediates. This electronic environment is essential for catalytic activity. Using the traditional d-band centre method, we investigate the electroactivity in depth, in addition to its thermodynamic stability. The d-band centres of all possible DAC permutations are displayed in Fig. 6. Instead of regular downshifting of d band centres with more filled d-orbitals, we found a “hump” type of variation. To investigate it more clearly, we considered Sc-3d DAC combinations and we found that middle TM-based DACs demonstrate a valley-like d-band center trend, supporting a low electroactivity. More importantly, the introduction of hetero-transition metals has evidently lifted the d-band center even for the fully occupied d10 element, which indicates the evident modulations by the d-electrons from transition metals. Meanwhile, we also compare the d-band center of the DAC and SAC. Interestingly, the TM–TM DACs show a distinct d-band center with the SAC, indicating the coupling effect even between similar TM metal atoms. The d-band centre is upshifted by the addition of early TMs and shifted down by the addition of late TMs, respectively, providing an explicit direction for us to predict the electroactivity of DACs based on the change in the d-band centre in TM–TM DACs, which can achieve the optimal electronic environment for electrocatalysis by balancing the d-band centre position.
 | ||
| Fig. 6 Mapping of the d-band centre of all the DACs (left panel) and variation of the d-band centre with introduction of heteroatoms (right panel). | ||
In order to enhance the thermal stability of the DACs, we conducted AIMD simulations for specific DACs (Sc–Sc, Y–Y, and Hf–Hf). In these simulations, the forces were computed based on the electronic configuration in the ground state at each iteration of the molecular dynamics (MD) process. A molecular dynamics (MD) simulation was conducted using the NVT ensemble at a temperature of 300 K. The simulation duration was 6 ps, and a time step of 1 fs was employed. The findings indicate that the overall energy fluctuations of DACs remain relatively constant, and negligible atomic displacements from the equilibrium position are detected, as depicted in Fig. 7. The findings of this study provide evidence supporting the thermal stability of the digital-to-analog converters (DACs) when exposed to room temperature conditions.
 | ||
| Fig. 7 Total energy fluctuations during AIMD simulations for Sc–Sc (top left), Y–Y (top right) and Hf–Hf (bottom) DAC systems. | ||
We have found that TM–TM DACs are the most stable DACs by taking into account the present thermodynamic results. However, the most stable arrangements typically involve giving up some of the DAC's electroactivity. In order to narrow down the pool of potential DACs, we have focused mostly on making direct comparisons between their electrical architectures. We observed that heteronuclear DACs offer the best chance of achieving a high electroactivity for electron transmission. Our research suggests that this work provides useful references for determining the best element combinations to use in future DAC trials.
3.3 Electrochemical stability and activity
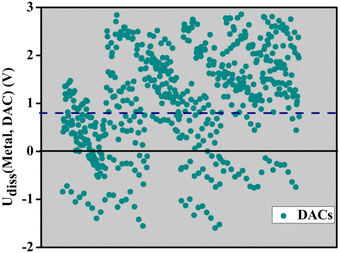
The dissolution potential (Udiss) is a crucial factor that determines the electrochemical stability of materials, especially in applications like batteries, fuel cells, and electrolysis systems. The term refers to the voltage threshold at which a substance starts to dissolve into the electrolyte, potentially causing deterioration of the electrode and compromising the material's structural integrity. Materials having a high dissolution potential are capable of enduring greater operational voltages without dissolving, which leads to a longer lifespan and consistent performance. On the other hand, a low dissolving potential indicates that the material is easily dissolved at relatively low voltages. This can lead to quick deterioration, decreased efficiency, and potential malfunction of the electrochemical system. Hence, it is crucial to comprehend and enhance the ability of electrode materials to dissolve in order to create long-lasting and effective electrochemical devices. The overall dependability and effectiveness of electrochemical systems in many applications can be improved by making sure that materials have a high dissolving potential, which enhances their stability and lifetime. In order to assess the appropriateness of our predicted DACs as electrode materials for fuel cells, we initially computed the dissolving potentials for each individual DAC. The dissolution potential has been calculated using the following equation: | (7) |
3.4 Prediction using the machine learning model
Despite the extensive theoretical calculations conducted on the thermodynamic stability and electrical structures of GPY-based DACs, there is still a need for more analysis and study to understand the underlying causes of the observed differences. The machine learning technique possesses significant capabilities to predict probable qualities, so serving as a logical design guidance for future experimental synthesis. In order to validate the theoretical simulated outcomes, we additionally incorporate the machine learning approach.As discussed above we used a dataset splitting technique to build our machine learning model. We split the data into two groups of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, keeping 80% for training and 20% for testing. This method made sure that the model was trained on the data while keeping a different set for checking. The 165 data points that made up our training set were chosen at random to avoid any intentional bias that might have skewed the model's performance. We did this random splitting and training process 100 times to make our results even more reliable and accurate. This way, we could average the results and lessen the effect of any outliers from a single split. This iterative process made the evaluation of the model's ability to guess more accurate. This gives a full picture of how well the model worked with different training datasets. Table 2 shows a comparison of the average performance metrics of various machine learning models based on these 100 experiments. As demonstrated, Gradient Boosting (GB) showed the best performance with the lowest MSE, RMSE, and MAE values. On the other hand, Lasso had the highest errors on all three metrics among the tested models.
20, keeping 80% for training and 20% for testing. This method made sure that the model was trained on the data while keeping a different set for checking. The 165 data points that made up our training set were chosen at random to avoid any intentional bias that might have skewed the model's performance. We did this random splitting and training process 100 times to make our results even more reliable and accurate. This way, we could average the results and lessen the effect of any outliers from a single split. This iterative process made the evaluation of the model's ability to guess more accurate. This gives a full picture of how well the model worked with different training datasets. Table 2 shows a comparison of the average performance metrics of various machine learning models based on these 100 experiments. As demonstrated, Gradient Boosting (GB) showed the best performance with the lowest MSE, RMSE, and MAE values. On the other hand, Lasso had the highest errors on all three metrics among the tested models.
| Model | Avg MSE | Avg RMSE | Avg MAE |
|---|---|---|---|
| XGB | 0.139 | 0.372 | 0.270 |
| Random forest | 0.226 | 0.474 | 0.363 |
| Extra trees | 0.227 | 0.475 | 0.358 |
| Decision tree | 0.547 | 0.738 | 0.562 |
| Linear regression | 0.545 | 0.737 | 0.604 |
| GB | 0.125 | 0.352 | 0.264 |
| RD | 0.575 | 0.757 | 0.619 |
| ADB | 0.677 | 0.821 | 0.649 |
| Lasso | 0.835 | 0.912 | 0.728 |
Fig. 10 shows the fifteen most important variables of our model. Here, we define significance as the average absolute SHAP value of all the data points, where red denotes high values and blue denotes the low ones; the colours in the graphic represent the value of the input variable. Red on the right (left) side of the plot indicates a positive (negative) connection with the target. From the SHAP analysis, it is seen that features like the group number, number of d electrons, radius of the d orbital, ionic radius, electronegativity, and electron affinity play an important role and have negative correlations with target properties. This can be explained from chemical intution as well. As discussed previously, electronegativity and electron affinity play a major role in transferring electrons to the substrate, which in turn determines the adsorption preferences. An increase in the number of d electrons at the active metal site has a dramatic effect on the charge transfer and adsorption energies of chemical intermediates. There are other ways to figure out how important a feature is besides the SHAP method. The permutation-based feature importance analysis, depicted in the top right panel of Fig. 10, shows that all of the input features have a satisfactory importance value, in good agreement with SHAP feature importance analysis. The feature–feature correlation map of the input features is displayed in the bottom panel of Fig. 10, suggesting that a few atomic attributes are linked. While building the model, we do not include the highly associated features that the Pearson correlation heatmap suggests.
 | ||
| Fig. 10 Feature importance analysis using the SHAP method (top left) and permutation feature importance (top right). Heatmap of the Pearson correlation matrix is shown in the bottom panel. | ||
Our DFT models are in good agreement with those obtained using GB-based machine learning, demonstrating that thermodynamic properties may be easily predicted, but electronic properties show far more intricate contributions from various sources. The GB method, which is based on machine learning, has made it possible to construct complex multi-atomic catalysts with a wide variety of possible configurations. Machine learning-driven studies are inadequate, especially when it comes to elucidating the underlying interactions between the orbitals of the anchoring metals, and therefore trustworthy theoretical calculation-guided investigations are still necessary.
4 Conclusion
Single atom catalysts (SACs) have experienced a number of changes and have taken part in a variety of chemical reactions throughout the history of catalysis. However, notable developments in experimental strategies and methodologies for characterising these catalysts are responsible for the recent spike in study interest. By utilising the combined strength of machine learning techniques and DFT, we suggest that TM–TM DAC systems are a potential class of catalysts. It is expected that these systems will demonstrate a high degree of electroactivity together with remarkable durability over extended periods of use. We rank hetero-coupling TM–TM DACs as the best option in our methodology. This approach involves combining different transition metal elements within DAC structures. By doing so, we aim to achieve not only thermodynamic stability but also superior electroactivity, with particular emphasis on the interactions between d-orbitals, known as d–d couplings. We also include predictions based on gradient boosting (GB), which support the importance of d–d orbital interactions in influencing electroactivity. In addition, we emphasise that intrinsic physicochemical characteristics play a crucial role in determining thermodynamic stability. Taking into account the existing constraints on SACs, our work provides an important direction and a clear way ahead. We suggest creating DACs using graphyne (GPY), which has ideal electroactivity and a high likelihood of successful experimental synthesis. This work not only sets the stage for GPY-based DACs but also creates new opportunities for future research projects examining multi-atom catalyst (AC) systems. In addition to that, the aforementioned methodology can be employed for the purpose of conducting high throughput screening of DACs on alternative supports as well.Data availability
Data will be made available upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
C. C. gratefully acknowledges her sincere gratitude to Prof. Dr. Felix Studt for his generous support. C. C. acknowledges the SERB, India (PDF/2021/003445) for the funding. K. E. and Prof. V. S. acknowledge the Science and Engineering Research Board (SERB), Department of Science and Technology, New Delhi, for funding through the project entitled “First Principle Design and Development of Multifunctional van der Waals and Non-van der Waals solids” (No. CRG/2019/003876). The authors acknowledge Dr Naga Venkateswara Rao Nulakani for providing the structure of α-2 graphyne. CSIR-CLRI communication number is 2025.References
- L. Zhang, M. Xu, H. Chen, Y. Li and S. Chen, Globalization, green economy and environmental challenges: State of the art review for practical implications, Front. Environ. Sci., 2022, 10, 870271 CrossRef.
- O. Ellabban, H. Abu-Rub and F. Blaabjerg, Renewable energy resources: Current status, future prospects and their enabling technology, Renewable Sustainable Energy Rev., 2014, 39, 748–764 CrossRef.
- H. Lund, Renewable energy strategies for sustainable development, Energy, 2007, 32(6), 912–919 CrossRef.
- M. A. Rahman, X. Wang and C. Wen, High energy density metal-air batteries: A review, J. Electrochem. Soc., 2013, 160(10), A1759 CrossRef CAS.
- A. P. Ferreira, R. C. Oliveira, M. M. Mateus and D. M. Santos, A review of the use of electrolytic cells for energy and environmental applications, Energies, 2023, 16(4), 1593 CrossRef CAS.
- S. M. M. Ehteshami and S. Chan, The role of hydrogen and fuel cells to store renewable energy in the future energy network-potentials and challenges, Energy Policy, 2014, 73, 103–109 CrossRef CAS.
- A. Kulkarni, S. Siahrostami, A. Patel and J. K. Nørskov, Understanding catalytic activity trends in the oxygen reduction reaction, Chem. Rev., 2018, 118(5), 2302–2312 CrossRef CAS PubMed.
- N. T. Suen, S. F. Hung, Q. Quan, N. Zhang, Y. J. Xu and H. M. Chen, Electrocatalysis for the oxygen evolution reaction: Recent development and future perspectives, Chem. Soc. Rev., 2017, 46(2), 337–365 RSC.
- D. Strmcnik, P. P. Lopes, B. Genorio, V. R. Stamenkovic and N. M. Markovic, Design principles for hydrogen evolution reaction catalyst materials, Nano Energy, 2016, 29, 29–36 CrossRef CAS.
- J. Bao, W. Liu, Y. Zhou, T. Li, Y. Wang and S. Liang, et al., Interface nanoengineering of PdNi-S/C nanowires by sulfite-induced for enhancing electrocatalytic hydrogen evolution, ACS Appl. Mater. Interfaces, 2019, 12(2), 2243–2251 CrossRef PubMed.
- E. Cuniberto, A. Alharbi, T. Wu, Z. Huang, K. Sardashti and K. D. You, et al., Nano-engineering the material structure of preferentially oriented nano-graphitic carbon for making high-performance electrochemical micro-sensors, Sci. Rep., 2020, 10(1), 9444 CrossRef CAS PubMed.
- D. Feng, L. Zhou, T. J. White, A. K. Cheetham, T. Ma and F. Wei, Nanoengineering metal-organic frameworks and derivatives for electrosynthesis of ammonia, Nano-Micro Lett., 2023, 15(1), 203 CrossRef CAS PubMed.
- J. L. Fiorio, M. L. Gothe, E. C. Kohlrausch, M. L. Zardo, A. A. Tanaka and R. B. de Lima, et al., Nanoengineering of catalysts for enhanced hydrogen production, Hydrogen, 2022, 3(2), 218–254 CrossRef CAS.
- C. Feng, X. Liu, T. Zhu and M. Tian, Catalytic oxidation of CO on noble metal-based catalysts, Environ. Sci. Pollut. Res. Int., 2021, 28, 24847–24871 CrossRef CAS PubMed.
- A. Eichler, CO oxidation on transition metal surfaces: Reaction rates from first principles, Surf. Sci., 2002, 498(3), 314–320 CrossRef CAS.
- M. Ackermann, T. Pedersen, B. Hendriksen, O. Robach, S. Bobaru and I. Popa, et al., Structure and reactivity of surface oxides on Pt (110) during catalytic CO oxidation, Phys. Rev. Lett., 2005, 95(25), 255505 CrossRef CAS PubMed.
- K. Ding, A. Gulec, A. M. Johnson, N. M. Schweitzer, G. D. Stucky and L. D. Marks, et al., Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts, Science, 2015, 350(6257), 189–192 CrossRef CAS PubMed.
- T. Schalow, B. Brandt, D. E. Starr, M. Laurin, S. K. Shaikhutdinov and S. Schauermann, et al., Size-dependent oxidation mechanism of supported Pd nanoparticles, Angew. Chem., Int. Ed., 2006, 45(22), 3693–3697 CrossRef CAS PubMed.
- K. Judai, S. Abbet, A. S. Wörz, U. Heiz and C. R. Henry, Low-temperature cluster catalysis, J. Am. Chem. Soc., 2004, 126(9), 2732–2737 CrossRef CAS PubMed.
- X. F. Yang, A. Wang, B. Qiao, J. Li, J. Liu and T. Zhang, Single-atom catalysts: A new frontier in heterogeneous catalysis, Acc. Chem. Res., 2013, 46(8), 1740–1748 CrossRef CAS PubMed.
- S. Liang, C. Hao and Y. Shi, The power of single-atom catalysis, ChemCatChem, 2015, 7(17), 2559–2567 CrossRef CAS.
- H. Wei, X. Liu, A. Wang, L. Zhang, B. Qiao and X. Yang, et al., FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes, Nat. Commun., 2014, 5(1), 5634 CrossRef CAS PubMed.
- J. Lin, A. Wang, B. Qiao, X. Liu, X. Yang and X. Wang, et al., Remarkable performance of Ir1/FeOx single-atom catalyst in water gas shift reaction, J. Am. Chem. Soc., 2013, 135(41), 15314–15317 CrossRef CAS PubMed.
- J. Li, C. Chen, L. Xu, Y. Zhang, W. Wei and E. Zhao, et al., Challenges and perspectives of single-atom-based catalysts for electrochemical reactions, JACS Au, 2023, 3(3), 736–755 CrossRef CAS PubMed.
- K. Rigby and J. H. Kim, Deciphering the issue of single-atom catalyst stability, Curr. Opin. Chem. Eng., 2023, 40, 100921 CrossRef.
- A. M. Gabelnick, A. T. Capitano, S. M. Kane, J. L. Gland and D. A. Fischer, Propylene oxidation mechanisms and intermediates using in situ soft X-ray fluorescence methods on the Pt (111) surface, J. Am. Chem. Soc., 2000, 122(1), 143–149 CrossRef CAS.
- J. Koop and O. Deutschmann, Detailed surface reaction mechanism for Pt-catalyzed abatement of automotive exhaust gases, Appl. Catal., B, 2009, 91(1–2), 47–58 CrossRef CAS.
- T. Pu, J. Ding, F. Zhang, K. Wang, N. Cao and E. J. M. Hensen, et al., Dual atom catalysts for energy and environmental applications, Angew. Chem., Int. Ed., 2023, e202305964 CAS.
- R. Li and D. Wang, Superiority of dual-atom catalysts in electrocatalysis: One step further than single-atom catalysts, Adv. Energy Mater., 2022, 12(9), 2103564 CrossRef CAS.
- W. Xu, Y. Wang, C. Zhang, X. Ma, J. Wu and Y. Liu, et al., Insights into the electronic structure coupling effect of dual-metal atomic electrocatalytic platform for efficient clean energy conversion, Chem. Eng. J., 2023, 461, 141911 CrossRef CAS.
- Y. Ying, X. Luo, J. Qiao and H. Huang, More is different: Synergistic effect and structural engineering in double-atom catalysts, Adv. Funct. Mater., 2021, 31(3), 2007423 CrossRef CAS.
- Z. He, K. He, A. W. Robertson, A. I. Kirkland, D. Kim and J. Ihm, et al., Atomic structure and dynamics of metal dopant pairs in graphene, Nano Lett., 2014, 14(7), 3766–3772 CrossRef CAS PubMed.
- J. Sun, L. Tao, C. Ye, Y. Wang, G. Meng and H. Lei, et al., MOF-derived Ru1Zr1/Co dual-atomic-site catalyst with promoted performance for Fischer-Tropsch synthesis, J. Am. Chem. Soc., 2023, 145(13), 7113–7122 CrossRef CAS PubMed.
- C. C. Hou, H. F. Wang, C. Li and Q. Xu, From metal-organic frameworks to single/dual-atom and cluster metal catalysts for energy applications, Energy Environ. Sci., 2020, 13(6), 1658–1693 RSC.
- C. Chen, M. Sun, K. Wang and Y. Li, Dual-metal single-atomic catalyst: The challenge in synthesis, characterization, and mechanistic investigation for electrocatalysis, SmartMat, 2022, 3(4), 533–564 CrossRef CAS.
- W. Wan, Y. Zhao, S. Wei, C. A. Triana, J. Li and A. Arcifa, et al., Mechanistic insight into the active centers of single/dual-atom Ni/Fe-based oxygen electrocatalysts, Nat. Commun., 2021, 12(1), 5589 CrossRef CAS PubMed.
- C. Wu, W. Yang, J. J. Wang, H. Li and I. D. Gates, Methane activation on dual-atom catalysts supported on graphene, Chem. Commun., 2021, 57(91), 12127–12130 RSC.
- Y. Zheng, Y. Jiao, L. H. Li, T. Xing, Y. Chen and M. Jaroniec, et al., Toward design of synergistically active carbon-based catalysts for electrocatalytic hydrogen evolution, ACS Nano, 2014, 8(5), 5290–5296 CrossRef CAS PubMed.
- J. Hong, M. Chen, L. Zhang, L. Qin, J. Hu and X. Huang, et al., Asymmetrically coupled Co single-atom and Co nanoparticle in double-shelled carbon-based nanoreactor for enhanced reversible oxygen catalysis, Chem. Eng. J., 2023, 455, 140401 CrossRef CAS.
- Y. Fang, Y. Liu, L. Qi, Y. Xue and Y. Li, 2D graphdiyne: An emerging carbon material, Chem. Soc. Rev., 2022, 51(7), 2681–2709 RSC.
- N. V. R. Nulakani and V. Subramanian, A theoretical study on the design, structure, and electronic properties of novel forms of graphynes, J. Phys. Chem. C, 2016, 120(28), 15153–15161 CrossRef CAS.
- M. Sun, H. H. Wong, T. Wu, A. W. Dougherty and B. Huang, Stepping out of transition metals: Activating the dual atomic catalyst through main group elements, Adv. Energy Mater., 2021, 11(30), 2101404 CrossRef CAS.
- D. Wu, P. Lv, J. Wu, B. He, X. Li and K. Chu, et al., Catalytic active centers beyond transition metals: Atomically dispersed alkaline-earth metals for the electroreduction of nitrate to ammonia, J. Mater. Chem. A, 2023, 11(4), 1817–1828 RSC.
- D. Wu, J. Wu, H. Li, W. Lv, Y. Song and D. Ma, et al., Unlocking the potential of alkaline-earth metal active centers for nitrogen activation and ammonia synthesis: The role of s-d orbital synergy, J. Mater. Chem. A, 2024, 12(7), 4278–4289 RSC.
- Y. Zang, Q. Wu, S. Wang, B. Huang, Y. Dai and Y. Ma, Coupling a main-group metal with a transition metal to create biatom catalysts for nitric oxide reduction, Phys. Rev. Appl., 2023, 19(2), 024003 CrossRef CAS.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54(16), 11169 CrossRef CAS PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized gradient approximation made simple, Phys. Rev. Lett., 1996, 77(18), 3865 CrossRef CAS PubMed.
- S. Grimme, Semiempirical GGA-type density functional constructed with a long-range dispersion correction, J. Comput. Chem., 2006, 27(15), 1787–1799 CrossRef CAS PubMed.
- G. K. Uyank and N. Güler, A study on multiple linear regression analysis, Procedia Soc. Behav. Sci., 2013, 106, 234–240 CrossRef.
- E. Schulz, M. Speekenbrink and A. Krause, A tutorial on Gaussian process regression: Modelling, exploring, and exploiting functions, J. Math. Psychol., 2018, 85, 1–16 CrossRef.
- V. Svetnik, A. Liaw, C. Tong, J. C. Culberson, R. P. Sheridan and B. P. Feuston, Random forest: A classification and regression tool for compound classification and QSAR modeling, J. Chem. Inf. Comput. Sci., 2003, 43(6), 1947–1958 CrossRef CAS PubMed.
- K. Vu, J. C. Snyder, L. Li, M. Rupp, B. F. Chen and T. Khelif, et al., Understanding kernel ridge regression: Common behaviors from simple functions to density functionals, Int. J. Quantum Chem., 2015, 115(16), 1115–1128 CrossRef CAS.
- D. W. Marquardt and R. D. Snee, Ridge regression in practice, Am. Stat., 1975, 29(1), 3–20 CrossRef.
- F. Emmert-Streib and M. Dehmer, High-dimensional LASSO-based computational regression models: Regularization, shrinkage, and selection, Mach. Learn Knowl. Extr., 2019, 1(1), 359–383 CrossRef.
- F. Pedregosa, G. Varoquaux, A. Gramfort, V. Michel, B. Thirion and O. Grisel, et al., Scikit-learn: Machine learning in Python, J. Mach. Learn. Res., 2011, 12, 2825–2830 Search PubMed.
- A. R. Puigdollers, G. Alonso and P. Gamallo, First-principles study of structural, elastic and electronic properties of α -, β -and γ -graphyne, Carbon, 2016, 96, 879–887 CrossRef CAS.
- S. Tian, Q. Fu, W. Chen, Q. Feng, Z. Chen and J. Zhang, et al., Carbon nitride supported Fe2 cluster catalysts with superior performance for alkene epoxidation, Nat. Commun., 2018, 9(1), 2353 CrossRef PubMed.
- L. Zhang, J. M. T. A. Fischer, Y. Jia, X. Yan, W. Xu and X. Wang, et al., Coordination of atomic Co-Pt coupling species at carbon defects as active sites for oxygen reduction reaction, J. Am. Chem. Soc., 2018, 140(34), 10757–10763 CrossRef CAS PubMed.
- O. Matsushita, V. M. Derkacheva, A. Muranaka, S. Shimizu, M. Uchiyama and E. A. Lukyanets, et al., Rectangular-shaped expanded phthalocyanines with two central metal atoms, J. Am. Chem. Soc., 2012, 134(7), 3411–3418 CrossRef CAS PubMed.
- Z. Zhang, L. Zhang, X. Wang, Y. Feng, X. Liu and W. Sun, Rational design of graphyne-based dual-atom site catalysts for CO oxidation, Nano Res., 2023, 16(1), 343–351 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Table showing bond lengths of a few DACs, charge transfer analysis, trend in electronegativity, and cohesive energy. See DOI: https://doi.org/10.1039/d4cp03171g |
| This journal is © the Owner Societies 2024 |