Developing a biosensing prototype utilising a 7CB liquid crystal for human insulin detection†
Athul
Satya
and
Ayon
Bhattacharjee
 *
*
Department of Physics, National Institute of Technology, Bijni Complex, Laitumkhrah, Shillong, Meghalaya 793003, India. E-mail: ayonbh@nitm.ac.in; Tel: +91 9485177033
First published on 18th November 2024
Abstract
This paper presents a novel prototype for human insulin detection using a 4-heptyl-4-biphenylcarbonitrile liquid crystal (7CB-LC). Human insulin is essential for regulating blood glucose levels and facilitating the metabolism of carbohydrates, lipids, and proteins. Insufficient insulin can lead to hyperglycemia, where cells cannot utilise glucose effectively for energy production. Prolonged hyperglycemia can affect the nervous and cardiovascular systems. Our work investigates the scope of using 7CB-LC as a prototype for the label-free detection of human insulin. Both temperature and time-dependent studies conducted using a polarising optical microscope (POM) on human insulin in the concentration range from 25 μM to 500 μM showed that human insulin interacting with 7CB-LC produces radial, twisted-radial, pre-radial and bipolar textures. A detection limit of 25 μM was observed since no distinguishable textures were observed below this concentration. An RGB (red, green, and blue) and grey index study showed a positive correlation graph with an R2 value of 0.97279, proving the selectivity of the proposed biosensor. Molecular docking and Raman spectroscopy studies were conducted to learn more about the interaction between insulin and 7CB-LC at the molecular level. Docking studies revealed how the position of the 7CB core and tail ends interacted with amino acid residues of insulin. Raman spectroscopy studies investigated the segmental mobility of different parts of LC and changes occurring in the core and terminal regions due to insulin interactions. Vibrational studies conducted using Raman spectroscopy analysed the change in 7CB-LC parameters such as the peak position (PP), line width (LW) and integrated intensity (II) on interacting with human insulin. This unique prototype technique shows how 7CB-LC can potentially be employed in biosensing to detect human insulin since it provides better visualisation in a label-free detection method.
1. Introduction
Liquid crystals (LCs) are a specific state of matter that are anisotropic and exhibit the properties of both solids and liquids.1 Based on the transition mode to mesophases, LCs can be classified into thermotropic LCs and lyotropic LCs. LCs, as biosensors, were developed for the first time by Abbot et al. in 1998 to detect anti-Bi-IgG (anti-bispecific immunoglobin G) and avidin.2 Our work investigates the scope of using a 4-heptyl-4-biphenylcarbonitrile (7CB) LC for the label-free detection of human insulin. Human insulin is a peptide hormone synthesised by pancreatic beta cells within the islets of Langerhans. It is integral in maintaining blood glucose levels and overseeing the metabolism of carbohydrates, lipids, and proteins.3 It facilitates cell division and growth by exerting mitogenic influences. Human insulin shortage can cause various health issues, mostly due to the cell's inability to use glucose as an energy source. This can cause hyperglycemia, which can develop into diabetes mellitus and affect the nervous and cardiovascular systems.3,4Traditional insulin detection methods include enzyme-linked immunosorbent assay (ELISA), fully automated immunoassay platforms and chromatography techniques. In addition to these, tests such as the arginine simulation test and hyperglycemic clamp test help in evaluating insulin secretion in the human body. These methods require complicated instrumentation techniques, are time-consuming, and are costly for the day-to-day insulin monitoring.5 An aptamer-based biosensor was developed for insulin detection by Yoshida et al. in 2009, employing a process known as SELEX (systematic evolution of a ligand by exponential enrichment). In this process, DNA aptamers were isolated against insulin to select IGA3 (insulin growth aptamer) because of its high affinity towards insulin. IGA3 was later used in an enzyme system with a thrombin-inhibiting aptamer, which releases thrombin when insulin binds.6 In 2020, Piletsky et al. developed a molecular imprinting (MIP) based insulin biosensor using insulin MIP nanoparticles, immobilising them on a screen-printed electrode made of platinum for insulin detection.7
In efforts to reduce the costs associated with producing insulin detection methods, label-free detection techniques were developed. Notably, LC label-free detectors emerged as significant advancements, as they streamline electronics by employing LCs as electrochemical or optical transducers. In 2023, Servarayan et al. introduced a label-free fluorescence-based biosensor for insulin detection in human serum, employing naturally occurring chromene mimic receptors.8 Label-free detection of insulin using a LC was introduced by Chen and colleagues, developing a label-free aptamer-based optical LC biosensor for insulin detection using a 4-cyano-4-pentyl biphenyl (5CB) LC. This biosensor utilised an aptamer (IGA3) adsorbed to cetyltrimethylammonium bromide (CTAB) and detected insulin-induced conformational changes at the aqueous–liquid crystal interface using polarised optical microscopy (POM).9 All these detection techniques, besides being label-free, used aptamers and other chemical reagents to attain selectivity and high sensitivity. Besides the positive correlation observed between grey scale analysis and concentration, the ability of Chen et al.'s biosensor to accurately quantify insulin remains limited. Conversely, our biosensor model demonstrates a quantitative detection method using less chemical reagents and responds to both time and temperature parameters, showing improved potential for precise sample analysis.
Our research explores the potential of detecting human insulin utilising 7CB-LC without aptamers. Currently, there are no biosensors utilising 7CB-LC for label-free detection of insulin. Optical studies conducted using POM showed that 7CB-LC on interacting with human insulin produces schlieren textures having radial, twisted-radial, pre-radial and bipolar textures. Both time and temperature studies were conducted to know the appropriate temperature for the developed biosensor. Concentrations of insulin ranging from 25 μM to 500 μM were studied. The temperature studies show that temperatures ranging from 22 °C to 40 °C are the most appropriate for the proposed biosensor. The quantitative analysis measured the greyscale intensity (GI) values of the POM images, and a correlation graph was plotted between the insulin concentrations and GI values. The correlation graph showed a positive correlation, having an R2 value of 0.97279. The prototype biosensor demonstrated a detection limit of 25 μM, as distinguishable textures were not observed below this concentration.
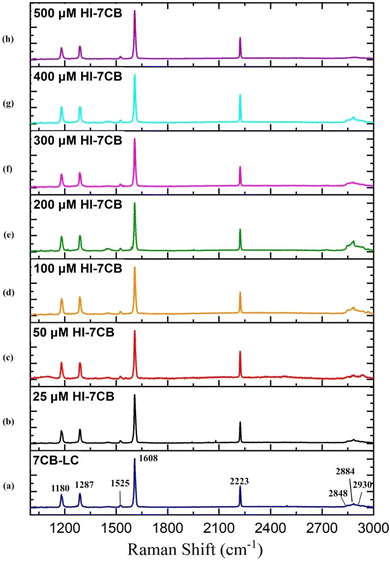
To learn more about the interaction between insulin and 7CB-LC at the molecular level, molecular docking studies using auto dock tools and vibrational studies using Raman spectroscopy were conducted. The docking studies showed that amino acid residues such as leucine (Leu) 15, arginine (Arg) 22, phenylalanine (Phe) 24, 25 and tyrosine (Tyr) 26 present in human insulin were found to form hydrophobic interactions with 7CB-LC. Samples were analysed within a range of 1000–3200 cm−1 using Raman spectroscopy, and six significant peaks were observed at wavelengths 1180 cm−1, 1287 cm−1, 1525 cm−1, 1608 cm−1, 2223 cm−1 and 2848 cm−1. Vibrational analyses revealed that interaction between 7CB and human insulin induces a notable symmetric–asymmetric stretch in the (C–H) aliphatic chains and (C–C) aromatic rings of 7CB-LC, particularly in the presence of the phenylalanine (Phe) amino acid residue found in insulin. The overall studies show the possibility of developing a label-free biosensor using 7CB-LC to detect human insulin. Despite the average human insulin concentration in the body being 90 pmol L−1, our biosensor serves as a pioneering prototype model for utilising 7CB-LC in detecting human insulin.
2. Methodology
2.1 Materials
Sigma Aldrich provided the key raw materials, which included human insulin, 4-heptyl-4-biphenylcarbonitrile (7CB), and dimethyl octadecyl [3-(trimethoxysilyl)propyl] ammonium chloride (DMOAP). The materials were used without any purification. Insulin solutions at various concentrations were prepared using phosphate-buffered saline (PBS) with a buffer strength of 20 mM and a pH of 7.4. The insulin molar extinction coefficient (εmolar) was 5734 M−1 cm−1 at 280 nm, measured with a UV-Vis spectrophotometer (Shimadzu UVPC 2450 Corporation, Tokyo, Japan). Fig. 1(a) depicts the molecular structure of human insulin: chains A and B. Chain A comprises 21 amino acids, whereas chain B has 30 amino acids. The chemical structure of 7CB is shown in Fig. 1(b).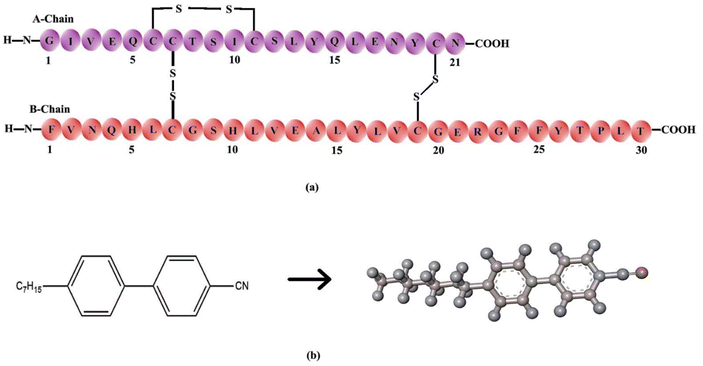 | ||
| Fig. 1 (a) Primary structure of insulin showing the amino acid sequence of chain A and chain B, (b) chemical structure of the 4-heptyl-4-biphenylcarbonitrile (7CB) liquid crystal. | ||
2.2 Method of preparation of a DMOAP-coated glass slide
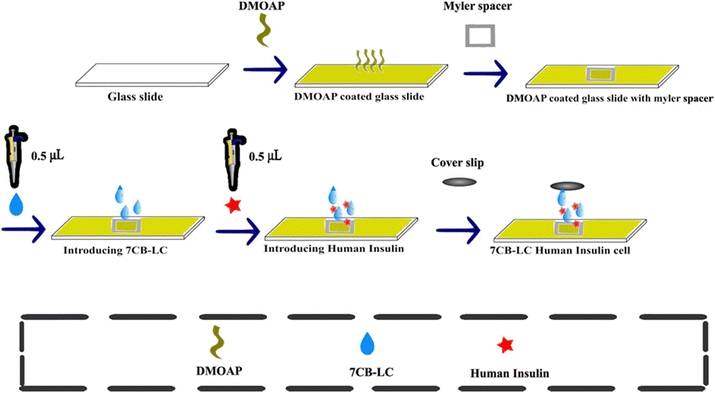
1% (v/v) DMOAP solution was prepared by mixing 1 mL of DMOAP with 100 mL of deionised water. Glass slides were cleaned using a solution of ethanol and deionised water, followed by ultrasonication and baking at 80 °C for 15 minutes. After cleaning, the glass slides were immersed in the DMOAP solution for 15 minutes and then baked again for another 15 minutes. This process resulted in DMOAP-coated glass slides that facilitate the homeotropic alignment of the liquid crystal.2.3 Method of preparation of insulin-7CB cell
To construct 7CB-insulin cells, glass slides were coated with DMOAP solution. 0.5 μL of 7CB-LC was added above the DMOAP-coated glass slide. A Myler spacer with a square inlet was kept above the DMOAP-coated glass slide, which acted as a spacer. Later, the same volume of insulin was introduced above the LC droplet using a micropipette. The resulting insulin-7CB droplet was let to settle for a few seconds. Later, the sample was sealed with a coverslip to create LC-insulin cells, as illustrated in Fig. 2. The cells were made with concentrations ranging from 25 μM to 500 μM. A cover slip was employed to maintain the stability of textures for a significant duration. Normal cover slips lacking the DMOAP coating were chosen to prevent double twisting in the arrangement of LC molecules, which could disrupt homeotropic alignment. Coverslips were specifically used to preserve texture stability on pre-coated glass slides.2.4 Characterisation techniques
3. Results and discussion
3.1 POM study of the 7CB-insulin interacting texture
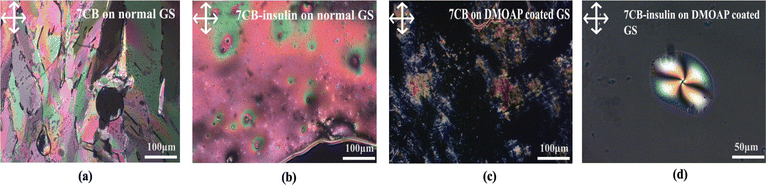
(a) 7CB-LC in a normal glass slide. The optical texture of 7CB-LC in a standard glass slide was studied using POM. A hexagonal texture was observed, as shown in Fig. 3(a).
(b) 7CB-LC interacting with human insulin on a normal glass slide. 7CB-LC, while interacting with human insulin on a normal glass slide, does not show any specific textures as shown in Fig. 3(b). Since the glass slides were not coated with DMOAP, LCs were poorly aligned, resulting in fewer interactions with human insulin.
(c) 7CB-LC on a DMOAP-coated glass slide. 7CB-LC on a normal glass slide shows a lamellar texture under the POM, as shown in Fig. 3(c).
(d) 7CB-LC interacting with human insulin on a DMOAP-coated glass slide. The homeotropic arrangement of 7CB-LCs generated by the DMOAP coating gives a better alignment for the LCs. 7CB-LC interacting with human insulin in a DMOAP-coated glass slide generated a twisted radial texture with four schlieren brushes and a point defect at the centre, as shown in Fig. 3(d).
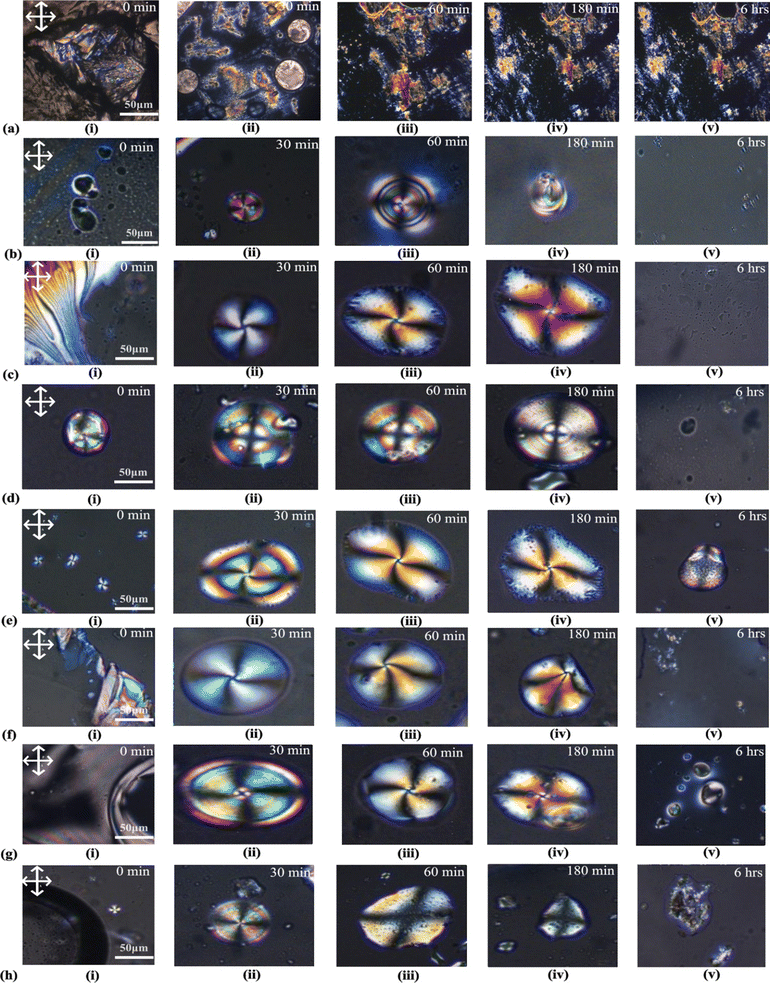
The POM analysis of blank cells shows that 7CB on a DMOAP-coated glass slide shows a lamellar texture, as shown in Fig. 5(a).
At a concentration of 25 μM, no discernible textures were observed initially (0 minutes). However, after 30 minutes, a radial texture with four Schlieren brushes and a point defect at the centre was detected, as depicted in Fig. 5(b-ii). This radial texture persisted until 60 minutes, after which it disappeared. On increasing the concentration to 50 μM, thread-like textures were observed within 0 minutes. After 30 minutes, a twisted radial texture featuring four schlieren textures with a central point defect was detected, as illustrated in Fig. 5(c-ii). This twisted radial texture persisted until 180 minutes, as depicted in Fig. 5(c-iii–iv), before disappearing after 6 hours. Similar to the observation at a concentration of 25 μM, a radial texture was observed at a concentration of 100 μM, as depicted in Fig. 5(d).
At both 200 μM and 400 μM concentrations of insulin interacting with 7CB-LC, as shown in Fig. 5(e) and (g), a twisted radial texture was observed till 180 minutes. At a concentration of 300 μM insulin, a twisted radial texture was observed at 30 minutes, as illustrated in Fig. 5(f-ii). Subsequently, this radial texture transformed into a pre-radial texture characterised by four Schlieren brushes and a point defect at the top corner, as depicted in Fig. 5(f-iii). Similar to 100 μM concentration, a radial texture was observed in the case of 500 μM insulin concentration, as shown in Fig. 5(h).
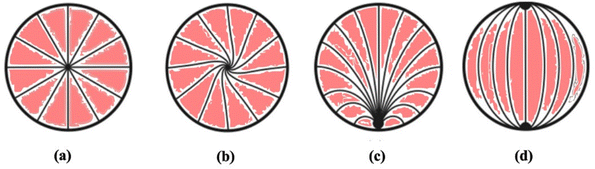
Three main types of director orientations, such as radial, twisted radial and pre-radial textures, were observed for insulin concentrations between 25 μM and 500 μM. It was seen that, for both 200 μM and 500 μM concentrations of insulin, radial schlieren textures started to appear instantly within 0 minutes. At concentrations exceeding 500 μM, distinct liquid crystalline textures were not observed. Increased protein concentration results in protein crowding or aggregation within the protein structure. This aggregation causes steric hindrance, reducing the protein's interaction with LC molecules. Investigating the interactions between liquid crystals and various protein concentrations is essential for enhancing the sensitivity of detection techniques. This understanding plays a pivotal role in characterising the nature and strength of protein–liquid crystal interactions, which is valuable for developing biosensors and gaining insights into biological mechanisms.14,15
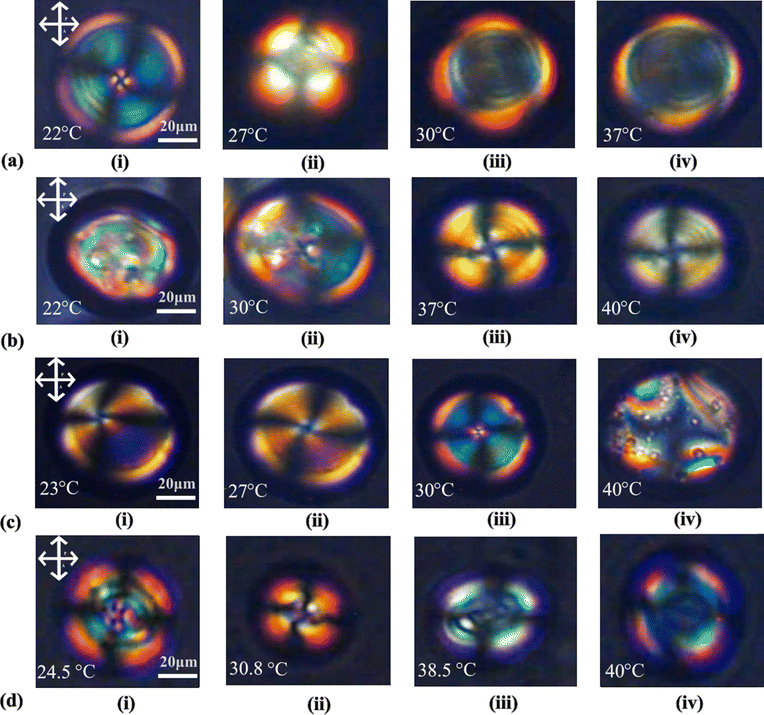
At a concentration of 200 μM insulin, interaction with 7CB-LC at 22 °C results in a twisted radial texture characterised by four schlieren brushes and a central point defect, as depicted in Fig. 6(a-i). Upon raising the temperature to 27 °C, this twisted radial texture transitions into a radial texture, as shown in Fig. 6(a-ii). At temperatures around 30 °C and above, a bipolar texture is observed, as illustrated in Fig. 6(a-iii). On increasing the insulin concentration to 300 μM, concentric textures were observed till 30 °C, as shown in Fig. 6(b-i and ii). By 37 °C and above, these concentric textures were converted into a twisted radial texture, as shown in Fig. 6(b-iii–iv). In the case of 400 μM concentration of insulin, a pre-radial texture having four schlieren brushes and a point defect at the top corner was observed, as shown in Fig. 6(c-i). Increasing the temperature above 27 °C, this pre-radial texture was converted to a twisted radial texture, as shown in Fig. 6(c-ii–iii). For a concentration of 500 μM, a twisted radial texture with four schlieren brushes persisted until reaching 30.8 °C, as illustrated in Fig. 6(d-ii). As the temperature increased to 38.5 °C, the twisted radial textures began transitioning into a bipolar texture, and at 40 °C, a bipolar texture was fully observed, as depicted in Fig. 6(d-iv).
The investigation of temperature dynamics in LC-protein complexes assists in finding the appropriate temperature range for sensor performance and identifying temperature-sensitive regions important for certain biomolecular interactions. This insight increases biosensor sensitivity and selectivity, allowing for developing temperature-responsive biosensors capable of precisely and efficiently detecting and quantifying target molecules.
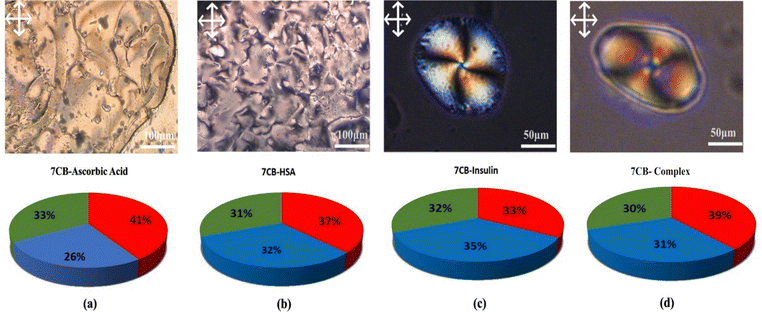
In both cases, a twisted radial texture having four Schlieren brushes and a point defect at the centre was observed, as shown in Fig. 7(c) and (d). To have a more scientific point of view, the RGB (red, green, blue) percentage values were measured as shown in Fig. 7. The RGB percentage values of POM images indicate that the more equal the distribution of RGB values is, the more successful the proteins disrupt the LC molecules. Fig. 7 shows that the RGB values are more equal in the case of insulin interacting with 7CB-LC.16 These results show that human insulin shows enough selectivity towards 7CB-LC. Due to insufficient facilities for disposing of biohazards, our experiments were restricted to commercial samples.
POM observations indicate that no discernible textures were evident when human insulin interacts with 7CB-LC at concentrations below 25 μM. This implies that concentrations below 25 μM do not disturb the organised molecular arrangement of the LC. Therefore, the proposed prototype biosensor is observed to have a detection limit of 25 μM.
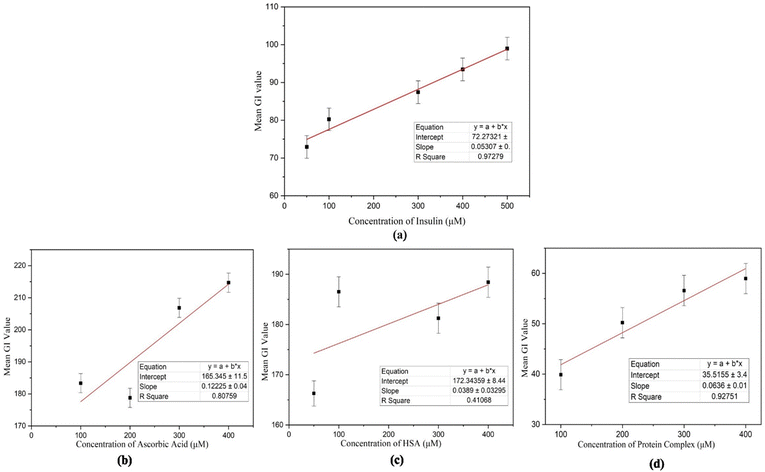
The concentrations were tested three times under the same experimental conditions, yielding comparable optical images and thus demonstrating partial reproducibility in the recorded results. Multiple measurements (3 times) were taken using ImageJ software to calculate the average grey-scale intensity (GI) within POM pictures to quantify the performance of the LC biosensor. Fig. 8 illustrates the calibration curve, which displays the average GI versus insulin concentration values ranging from 50 μM to 500 μM. This association was linear over the wide concentration range, with a significant correlation value (R2) of 0.97279.
To confirm the specificity of human insulin detection, an interference study was conducted by analyzing the interactions of other proteins with 7CB-LC. The POM images of ascorbic acid, HSA, and a protein mixture (concentrations ranging from 50 μM to 400 μM) were captured (Fig. S1, ESI†), and GI calibration graphs were plotted, as shown in Fig. 8(b)–(d). Results indicated that the R2 values for 7CB-ascorbic acid (0.80759) and 7CB-HSA (0.41068) interactions were relatively lower, demonstrating limited specificity. In contrast, the 7CB-protein mixture containing human insulin yielded a significantly higher R2 value of 0.92751, confirming its specificity for quantitative analysis. Table 1 illustrates how our biosensor differs from previously reported label-free biosensors.
| Type of biosensor | Sample | Linear range | Detection limit | Ref. |
|---|---|---|---|---|
| Surface plasmon resonance (SPR) immuno-sensor (graphene field effect transistor) | Buffer solution and human serum | 2.247–674.1 pmol L−1 | 2.247 pmol L−1 | 17 |
| Electrochemical impedance spectroscopy | Buffer solution and human serum | 5 pmol L−1–50 nmol L−1 | 1.2 pmol L−1 | 18 |
| LC-biosensor, (5CB-LC) | Buffer solution, human serum, and IGA3 | 0.1–1.0 nmol L−1 | 0.1 nmol L−1 | 9 |
| Fluorescence-based biosensor | Chromene mimic receptors, buffer solution, and human serum | 10 fmol L−1–600 pmol L−1 | 7.07 fmol L−1 | 8 |
| Interferometric reflectance spectroscopy (IRS) | Buffer solution, human islets, antibodies, and aptamers | 11.235–112.35 nmol L−1 | 2.247 pmol L−1 | 19 |
| LC-biosensor (7CB-LC) | Buffer solution and human serum | 25 μM–500 μM | 25 μmol L−1 | Reported here |
3.2 Molecular docking study of 7CB-LC interacting with human insulin
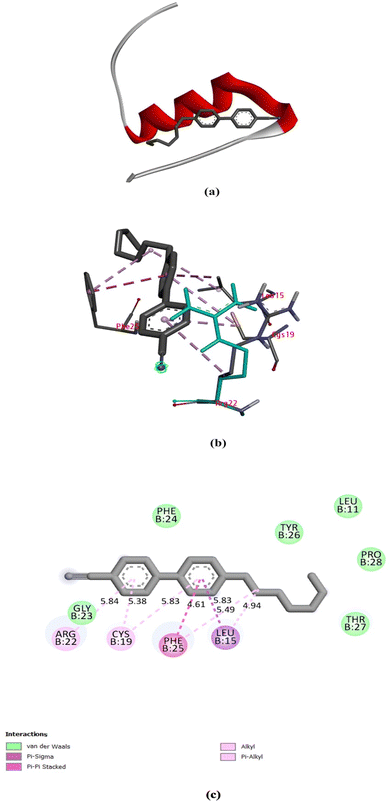
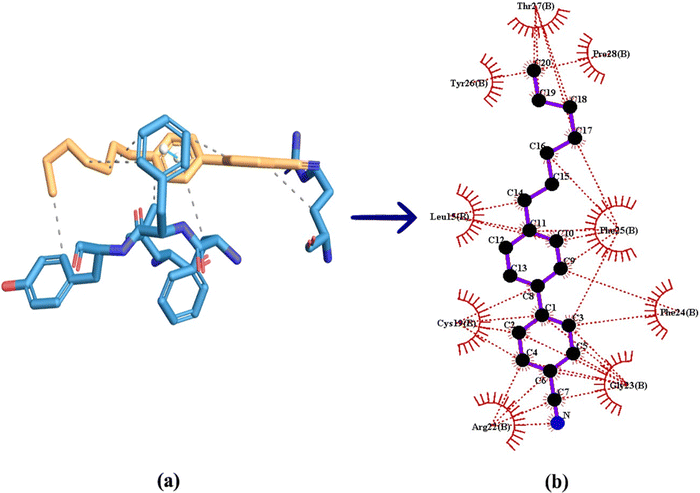
Molecular docking can be considered as an optimisation problem, with the objective of determining the best-fit orientation of a ligand that binds to a specific protein. This technique entails altering the arrangement of both the protein and ligand to reach an ideal fit, similar to a “hand-in-glove” analogy rather than a “lock-and-key” paradigm.20 In the case of 7CB-LC human insulin docking, 7CB is the ligand, which is flexible and human insulin is the rigid protein. Docking studies reveal the minimum binding energy site between the ligand and the protein, providing insights into their interaction and aiding in understanding the formation of specific textures observed under POM. Different types of molecular docking methods exist, such as geometric matching, stimulation-based, fragmentation, and conformational ensemble methods.21 Among these methods, we follow the simulation-based method in which the protein and ligand are prepared using docking software. This method allows the ligand to find its minimum binding energy position at the protein active site through conformational changes. This kind of blind docking approach was used in our study for a flexible docking between 7CB and insulin. An in-depth detailed exploration of the docking mechanism is given in the ESI,† Section S1.The human insulin structure obtained from the protein data bank consists of four chains: A, B, C, and D. Among the four chains, only chain B was considered for docking. By utilising a single chain, the docking process is streamlined, allowing for a more targeted examination of a particular protein structure without the added complexity of multiple chains. Blind docking was conducted using Auto Dock tool 1.5.7, which generated a data log (DLG) file containing all the details regarding the 7CB-insulin docking. Among the binding sites, the minimum binding energy site having an energy of −6.62 kcal mol−1 was considered for docking.
7CB-LC docked at the minimum binding energy site of insulin chain B is shown in Fig. 9(a). Numerous amino acid residues of human insulin were found around the 7CB ligand docking site (Fig. S2, ESI†), among which amino acid residues such as arginine (Arg) 22, cysteine (Cys) 19, phenylalanine (Phe) 25 and leucine (Leu) 15 of insulin were found to form interactions with 7CB at the minimum binding energy site as shown in Fig. 9(b). The amino acid residue arginine (Arg) 22 of human insulin formed a π-alkyl interaction with the first benzene ring of 7CB-LC, having a bond distance of 5.84 Å, as shown in Fig. 9(c). In addition to this, phenylalanine (Phe) 25 formed a π–π stacking having a bond distance of 4.61 Å with the second benzene ring of the 7CB ligand. The amino acid residue leucine (Leu) 15 formed an alkyl interaction having a bond distance of 4.94 Å with the C15 carbon atom of the 7CB ligand. Cysteine (Cys) 19 formed a π-alkyl interaction with both benzene rings of 7CB-LC, with a bond distance of 5.38 Å to the first benzene ring and 5.83 Å to the second benzene ring, as illustrated in Fig. 9(c).
All possible hydrophobic interactions made by the amino acid residues of human insulin surrounding the 7CB ligand are shown in Table 2 and Fig. 10(b). The amino acid reside phenylalanine (Phe) 25 forming a π-stacking interaction between human insulin and the 7CB ligand is shown in Table 3. Amino acid residues such as leucine (Leu) 15, arginine (Arg) 22, phenylalanine (Phe) 24,25 and tyrosine (Tyr) 26 were found to form hydrophobic interactions. In addition to this, van der Waals interactions were observed to be formed by amino acid residues such as phenylalanine (Phe) 24, leucine (Leu) 11, threonine (Thr) 27, tyrosine (Tyr) 26, proline (Pro) 28 and glycine (Gly) 23 as shown in Fig. 9(c).
| Residue no | Residue type | Distance (Å) | Ligand atom | Protein atom |
|---|---|---|---|---|
| 15B | Leucine (Leu) | 3.34 | 329 | 141 |
| 22B | Arginine (Arg) | 3.77 | 322 | 212 |
| 24B | Phenylalanine (Phe) | 3.20 | 327 | 245 |
| 25B | Phenylalanine (Phe) | 3.86 | 335 | 260 |
| 25B | Phenylalanine (Phe) | 3.83 | 334 | 262 |
| 25B | Phenylalanine (Phe) | 3.56 | 321 | 261 |
| 26B | Tyrosine (Tyr) | 3.25 | 338 | 272 |
| Residue no. | Residue type | Distance (Å) | Angle | Stacking type | Ligand atoms |
|---|---|---|---|---|---|
| 25B | Phenylalanine (Phe) | 4.48 | 21.94 | P | 326, 327, 328, 329, 330, 331 |
In addition to hydrophobic interactions, properties such as aromaticity, H-bonds, solvent accessible area (SAS) and hydrophobicity were measured, as shown in Fig. 11. Aromatic interactions play a crucial role in influencing the binding affinity of ligands to proteins. They stabilise complexes, improve specificity, serve as binding hot spots, contribute energetically, and provide structural stability.22 Aromatic amino acid residues such as phenylalanine (Phe), tyrosine (Tyr), tryptophan (Trp), and histidine (His) in the protein show aromatic properties towards the ligand in molecular docking. As shown in Fig. 11(a), amino acid residues phenylalanine (Phe) 15, leucine (Leu) 15 and arginine (Arg) 22 showed aromatic properties around the 7CB ligand. Among these, phenylalanine (Phe) 15 was found to form an edge-to-face interaction with the 7CB ligand. In this type of interaction, the edge of one aromatic ring of Phe (15) aligns with the face of another aromatic ring of the 7CB-ligand.23 Other types of aromatic interactions in protein–ligand docking include π–π stacking, CH–π stacking and face-to-face π-stacking.24
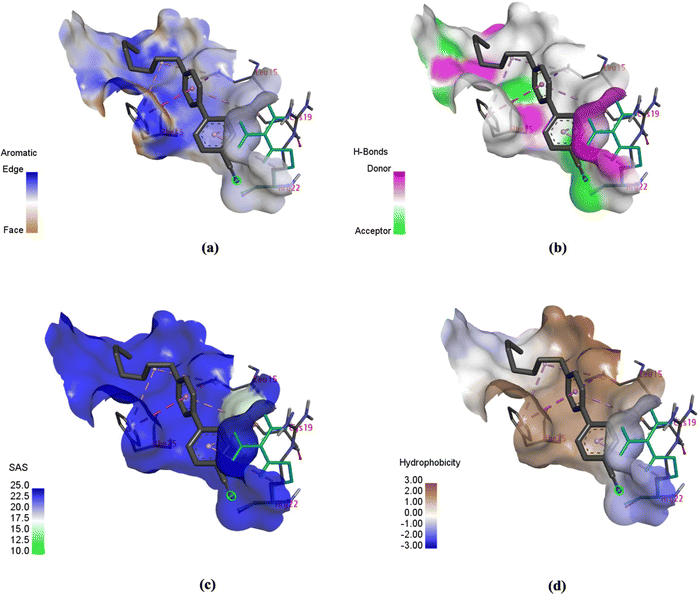 | ||
| Fig. 11 Schematic of (a) aromaticity, (b) H-bonds, (c) solvent-accessible area (SAS), and (d) hydrophobicity exhibited by the human insulin (chain B) docked with 7CB-LC (visualised using DSV). | ||
Fig. 11(b) shows the H-bonds observed around the 7CB-insulin binding site. It can be seen that only the amino acid residue phenylalanine (Phe) 15 was found to be an H-bond donor in nature. Hydrogen bonds identified within ligand–protein binding sites are crucial in dictating the binding affinity and specificity of the interaction. They play a major role in the disruption of hydrogen bonds within the water molecules and the establishment of fresh hydrogen bonds between the protein and the ligand, thereby influencing the strength and selectivity of binding interactions.25,26
Solvent-accessible surface (SAS) is vital in protein–ligand interactions, measuring amino acid exposure to the surrounding environment, encompassing both the solvent and protein core. It aids in understanding how molecular interactions are influenced by the accessibility and arrangement of amino acids within the protein structure. The reduction in ligand SAS upon binding is a crucial indicator of ligand burial within the binding pocket. SAS calculations are important for elucidating protein–ligand interactions, enabling the identification of binding sites, estimation of binding affinities, and advancement of novel sampling algorithms for molecular design.27,28 In the case of 7CB-insulin docking, amino acid residues such as phenylalanine (Phe) 15, leucine (Leu) 15 and arginine (Arg) 22 showed SAS properties, as shown in Fig. 11(c).
Hydrophobic interactions between the hydrophobic regions of proteins and ligands enhance the stability and specificity of the binding complex in molecular docking. The hydrophobic effect, stemming from the behaviour of hydrophobic molecules to cluster in water-based surroundings, is crucial in governing protein–ligand interactions.29,30 In the case of 7CB-insulin docking, amino acid residues such as phenylalanine (Phe) 15 and leucine (Leu) 15 were found to be hydrophobic and arginine (Arg) 22 hydrophilic, as shown in Fig. 11(d).
Molecular docking complements the POM studies since it provides detailed insights into the molecular-level interaction between proteins and ligands. Docking offers insights into ligand binding sites, identifies the interactions occurring at these sites, and facilitates the visualisation of ligand–protein binding on a molecular scale. It helps identify the change in the position of 7CB tail ends at which the amino acid residues of the insulin interact. Raman studies are conducted to learn more about the segmental mobility of different parts of LC and changes happening in the core and terminal changes due to insulin interactions. Raman studies provide structural information regarding conformational changes and changes in vibrational modes that 7CB-LC undergoes on docking with different concentrations of human insulin.
3.3 Investigation of the 7CB–human insulin complex using Raman spectroscopy
Conformational and structural alterations in the vibrational modes of 7CB-LC induced by various concentrations of human insulin are investigated using Raman spectroscopy. The analysis includes examining shifts in the peak position, changes in the line width, and variations in intensity. The peak position in the Raman spectrum corresponds to the specific vibrational mode of the molecule. It gives information regarding the energy linked with each vibrational mode. The frequency of the Raman peak is directly related to the energy of the vibrational mode. This relationship between the frequency of the Raman peak and the energy of vibrational mode in the Raman spectra helps identify vibrations in specific functional groups. The peak position acts as a fingerprint for each sample.31Line width in the Raman spectra provides information about a change in the lifetime, energy distribution and broadening of vibrational modes with respect to a change in the concentration of the sample. Integrated intensity in the Raman spectra helps measure the amount of light scattered by the sample at a specific frequency of vibrations. It measures the overall magnitude of a specific vibrational mode in the Raman spectra.32 Measuring integrated intensity provides insights into the change in structural characteristics that the sample undergoes with respect to the change in the concentration of the sample.33
The Raman spectra of pure 7CB-LC show that major peaks were observed at wavelengths 1180 cm−1, 1287 cm−1, 1525 cm−1, 1608 cm−1, 2223 cm−1 and 2848 cm−1, as shown in Fig. 12(a). Changes in the Raman spectra of 7CB-LC on adding different concentrations of insulin, such as 25 μM, 50 μM, 100 μM, 200 μM, 300 μM, 400 μM, and 500 μM, are shown in Fig. 12(b). For a detailed analysis, the spectral regions were segmented into three intervals: a vibrational region of 1160 cm−1 to 1320 cm−1, a vibrational region of 1580 cm−1 to 2235 cm−1, and a vibrational region of 2853 cm−1 to 3000 cm−1. A detailed mechanism exploration of Fig. 12 is given in Section S2 in the ESI.†
| ν – stretching, ν-s – symmetric stretching, and ν-as – asymmetric stretching. | |||
|---|---|---|---|
| Pure 7CB-LC (Δν) cm−1 | Band assignment for pure 7CB-LC | 7CB-LC bonded with insulin concentrations (25 μM, 50 μM, 100 μM, 200 μM, 300 μM, 400 μM, and 500 μM) (Δν) cm−1 | Band assignment for the 7CB–human insulin complex |
| 1180 | Aromatic C–H in-plane deformation | 1180.19, 1180.13, 1180.24, 1179.82, 1179.95, 1179.94, and 1179.97 | Aromatic C–H in-plane deformation in the presence of phenylalanine (Phe) |
| 1287 | ν(C–C) of biphenyl | 1287.20, 1286.59, 1286.95, 1286.96, 1286.74, 1286.85, and 1286.94 | Amide III bands (involves significant C–N stretching, N–H bending and C–C stretching) |
| 1525 | ν(C–C) of aromatic rings | 1525.51, 1525.58, 1525.54, 1525.40, 1525.31, 1525.39, and 1525.39 | Amide II bands (involves significant C–N stretching, N–H bending, and C–C stretching) |
| 1608 | ν(C–C) of aromatic rings | 1608.46, 1608.43, 1608.43, 1608.29, 1608.18, 1608.34, and 1608.26 | ν(C–C) of aromatic rings in the presence of phenylalanine (Phe) |
| 2223 |
ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) N) |
2223.25, 2223.44, 2223.42, 2223.23, 2223.15, 2223.18, and 2223.20 |
ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) N) |
| 2848 | ν-s(C–H) in aliphatic chains | 2848.71, 2848.62, 2864.51, 2848.83, 2848.84, 2867.62, and 2848.20 | ν-s(C–H) in aliphatic chains (CH2) along with phenylalanine (Phe) in insulin |
| 2884 | ν-s(C–H) in aliphatic chains | 2882.57, 2885.49, 2883.69, 2877.86, 2876.92, 2882.89, and 2886.65 | ν-s(C–H) in aliphatic chains (CH2) along with phenylalanine (Phe) in insulin |
| 2930 | ν-as(C–H) in aliphatic chains | 2936.35, 2940.58, 2936.61, 2930.57, 2922.84, 2951.58, and 2946.01 | ν-as(C–H) in aliphatic chains (CH3) along with phenylalanine (Phe) in insulin |
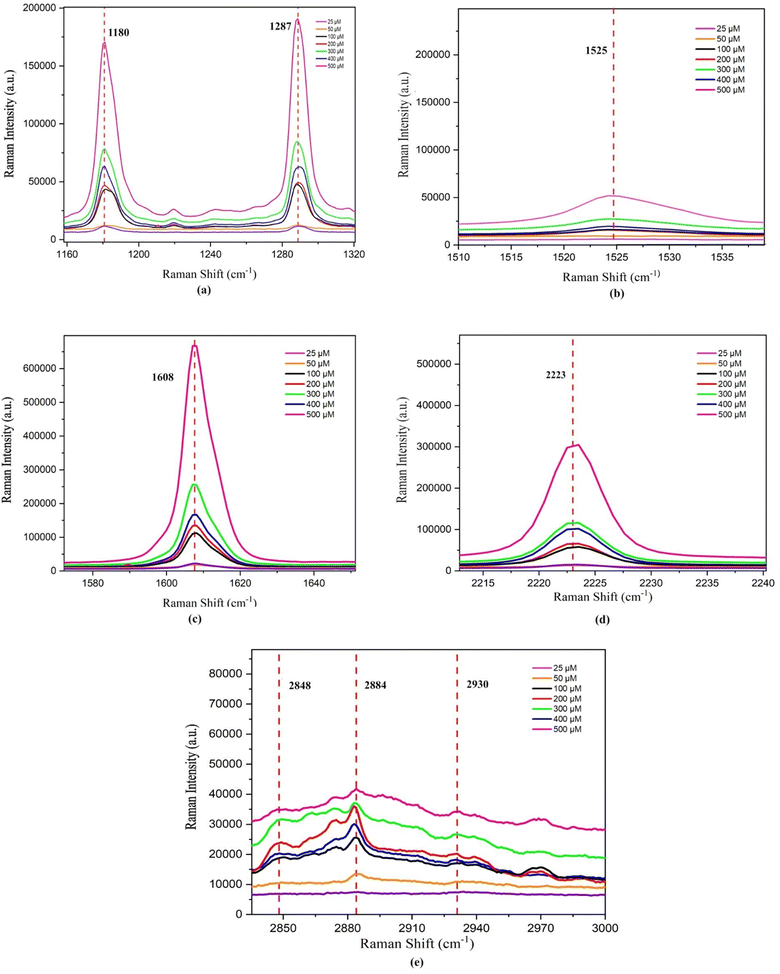
For lower concentrations ranging from 25 μM to 100 μM, the shift in PP was towards a higher frequency. However, for concentrations between 200 μM and 500 μM, the PP shift moved towards a lower frequency, as illustrated in Fig. 13(a). This shift in PP happens because the molecule's vibrational behaviour is affected when the sample's concentration changes. This difference in vibrational behaviour changes the interaction between the LC and insulin, leading to a shift in PP. A variation in the chemical environment and molecular interactions can cause a change in vibrational energy, which leads to a shift in PP.
The linewidth (LW) analysis reveals a gradual decrease in the LW for the 1180 cm−1 peak till 400 μM concentration of HI, and beyond which it increases. Similarly, for the 1287 cm−1 peak, a decrease in the LW was observed until a concentration of 100 μM, beyond which it remained constant, as shown in Fig. 14(b). A decrease in the LW indicated that the sample has a well-defined isolated vibrational mode. The increase in the LW indicated the broadening of the LW due to the change in molecular interactions and vibrational energy resulting from different sample concentrations.
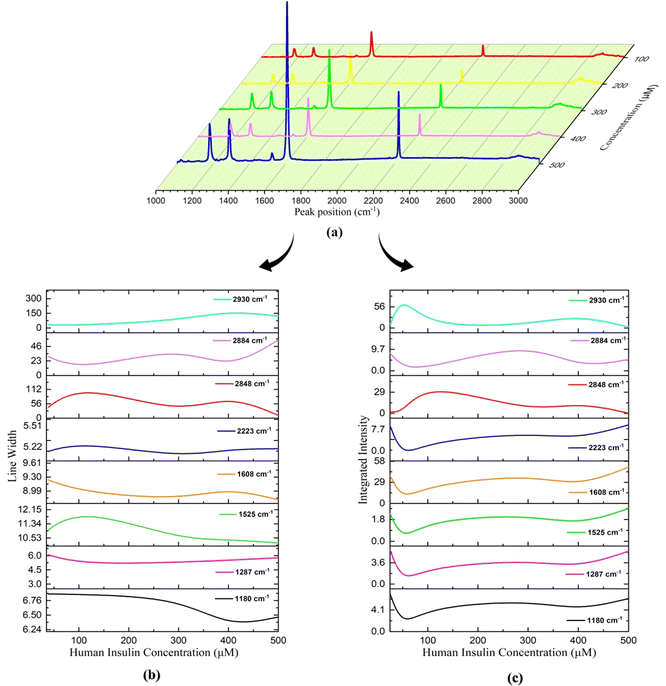 | ||
| Fig. 14 Variation in Raman parameters for different concentrations of the 7CB–insulin complex: (a) peak position, (b) line width, and (c) integrated intensity. | ||
Integrated intensity (II) analyses of the peaks in this range reveal a consistent trend. Since there were changes in the background as we vary concentrations, the intensities were normalised to understand the relative intensities of peaks at different concentrations. The normalised intensities were treated as percentage contributions and were termed integrated intensities. The II initially decreases up to 50 μM, then increases to 200 μM concentration of HI. Subsequently, there is another decrease until 400 μM, followed by an increase till 500 μM. These trends are represented in Fig. 14(c).
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) stretching observed in 7CB-LC. 7CB-LC, when interacting with insulin, showed a shift in these peaks, as shown in Table 4.
N) stretching observed in 7CB-LC. 7CB-LC, when interacting with insulin, showed a shift in these peaks, as shown in Table 4.
The alteration in the PP at 1525 cm−1 is attributed to the presence of amide II bands, specifically involving C–C stretching within insulin. The change in the PP at 1608 cm−1 corresponds to the stretching of aromatic ring (C–C) bonds, indicative of the phenylalanine (Phe) amino acid in insulin. Additionally, the shift in the PP at 2223 cm−1 is due to the stretching of the cyano (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) group in the presence of insulin. A shift in the PP towards a higher frequency range was observed up to 100 μM concentrations of HI, beyond which the PP shifts towards a lower frequency, as illustrated in Fig. 13(b)–(d) and Table 4. LW analysis of 1525 cm−1 shows an increase in the LW till 100 μM, beyond which it decreases. In the LW analysis of 1608 cm−1, a decline in the LW is noted up to a concentration of 300 μM. Beyond this concentration, up to 400 μM, the LW increased, as depicted in Fig. 14(b). Subsequently, from 400 μM to 500 μM concentration, the LW decreased once more. LW analysis of 2223 cm−1 shows an increase in the LW till 100 μM, beyond which it decreases till 300 μM and increases afterwards. The II analysis of the three peaks within this range demonstrates a consistent pattern similar to what was observed in the vibrational region spanning 1160 to 1320 cm−1 and is illustrated in Fig. 14(c). The consistent pattern observed in the range from 1160 to 2223 cm−1 can be attributed to the composition of the 7CB-LC core, as indicated in Table 4. This core portion of 7CB-LC exhibits higher organisation levels than the terminal tail. When interacting with varying insulin concentrations, the core undergoes reorientation to achieve a more favourable configuration.
N) group in the presence of insulin. A shift in the PP towards a higher frequency range was observed up to 100 μM concentrations of HI, beyond which the PP shifts towards a lower frequency, as illustrated in Fig. 13(b)–(d) and Table 4. LW analysis of 1525 cm−1 shows an increase in the LW till 100 μM, beyond which it decreases. In the LW analysis of 1608 cm−1, a decline in the LW is noted up to a concentration of 300 μM. Beyond this concentration, up to 400 μM, the LW increased, as depicted in Fig. 14(b). Subsequently, from 400 μM to 500 μM concentration, the LW decreased once more. LW analysis of 2223 cm−1 shows an increase in the LW till 100 μM, beyond which it decreases till 300 μM and increases afterwards. The II analysis of the three peaks within this range demonstrates a consistent pattern similar to what was observed in the vibrational region spanning 1160 to 1320 cm−1 and is illustrated in Fig. 14(c). The consistent pattern observed in the range from 1160 to 2223 cm−1 can be attributed to the composition of the 7CB-LC core, as indicated in Table 4. This core portion of 7CB-LC exhibits higher organisation levels than the terminal tail. When interacting with varying insulin concentrations, the core undergoes reorientation to achieve a more favourable configuration.
For the case of 2848 cm−1, a rise in the peak position (PP) and linewidth (LW) was observed until reaching 100 μM, after which they decreased. Subsequently, from 300 μM to 400 μM, there was a noticeable increase in both the PP and LW, followed by a decrease thereafter, as depicted in Fig. 13(e) and 14(a). In the case of 2884 cm−1, an increase in the PP is observed until it reaches 50 μM. Beyond this concentration, the PP shifts towards lower frequencies. However, beyond 400 μM, there is a shift towards higher frequencies in the PP, as illustrated in Fig. 13(e). Regarding the peak at 2884 cm−1, the LW and II both show a similar trend, as shown in Fig. 14(b) and (c). A decrease in the LW until 100 μM was observed, after which it increased. Subsequently, it starts decreasing again from 300 μM and increases above 400 μM. A trend similar to the LW was observed in the case of II, as shown in Fig. 14(c).
The PP analysis of the peak 2884 cm−1 shows an increase in the shift to a higher frequency range till 50 μM, beyond which it decreases and then increases on reaching 400 μM concentration of HI. A trend opposite to 2848 cm−1 was observed in the case of the LW and II analysis of 2884 cm−1, as shown in Fig. 14(b) and (c). A similar trend in frequency shift was observed in the PP analysis of the peak at 2930 cm−1, as shown in Table 4. The peak at 2930 cm−1 occurs due to the asymmetric stretching of (C–H) in aliphatic chains (CH3) along with phenylalanine (Phe) in insulin. The observed trends in the II across this region can be explained by the composition of the aliphatic chains within the 7CB-LC, as detailed in Table 4. These terminal tails undergo reconfiguration in response to interactions with varying concentrations of human insulin, thus demonstrating the observed patterns. The LW increases gradually till 400 μM and decreases beyond it, as shown in Fig. 14(b). The II analysis shows that II increases till 50 μM concentration of HI and beyond which it decreases till 200 μM. An increase in II was observed till 400 μM, which decreases upon reaching a higher concentration, as shown in Fig. 14(c). Raman studies reveal that the aliphatic chains of 7CB-LC are highly sensitive to human insulin concentrations. This is demonstrated by irregular changes in the integrated intensity data beyond the 2853 cm−1 range.
4. Conclusion
In this study, a novel prototype for label-free detection of human insulin detection using a 7CB-liquid crystal is developed. 7CB, when interacting with human insulin on a DMOAP-coated glass substrate, showed a distinct director orientation for different concentrations of human insulin. Concentrations ranging from 25 μM to 500 μM were studied under a polarising optical microscope, and textures such as radial, twisted-radial, pre-radial and bipolar were observed. A selectivity study conducted using Image J analysis showed a positive correlation graph between the average grey scale index and the insulin concentration, having an R2 value of 0.97279. Molecular docking and Raman spectroscopy studies conducted for different concentrations of human insulin interacting with 7CB-LC showed that the aliphatic chains and the biphenyl rings of 7CB-LC undergo symmetric and asymmetric stretching in the presence of amino acid residues such as phenylalanine (Phe) present in human insulin. A change in the peak position, line width and integrated intensity in the 7CB-LC spectra was observed due to the interaction of human insulin. Raman studies indicate that the aliphatic chains of 7CB-LC exhibit greater sensitivity to human insulin concentrations, as evidenced by the irregular changes in the integrated intensity data beyond the 2853 cm−1 range. A detection limit of 25 μM was observed for the proposed prototype, which is a limitation since it does not meet the required detection limit. This study provides a unique way of utilising 7CB-LC for human insulin detection using both time and temperature sensing methods. It was observed that among all the LC sensors existing for human insulin detection, 7CB-LC shows better stability and visualisation in terms of the label-free detection method since it shows significant LC–human insulin interaction textures with respect to both time and temperature. However, efforts are ongoing to enhance the biosensor to meet essential detection criteria, aiming for achieving improved performance in upcoming experiments.Data availability
The data supporting the findings of this study are available within the article and its ESI.† Additional datasets generated and analysed during the current study are available from the corresponding author upon reasonable request. Any other data related to this study can be obtained from the authors upon request, ensuring compliance with data protection and confidentiality agreements.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the DST-FIST Project. [File no SR/FST/PS-I/2019/74] for the financial assistance and the Department of Physics, National Institute of Technology Meghalaya, for the instrumentation facilities for polarising optical microscopy and Raman Spectroscopy.References
- S. Chandrasekhar, Liquid Crystals, 1992 Search PubMed.
- V. K. G. Abbot, Optical Amplification of Ligand-Receptor, Science, 1998, 279, 2077–2080 CrossRef PubMed.
- M. S. Rahman, K. S. Hossain, S. Das, S. Kundu, E. O. Adegoke, M. A. Rahman, M. A. Hannan, M. J. Uddin and M. G. Pang, Int. J. Mol. Sci., 2021, 22 Search PubMed.
- G. Wilcox, Insulin and Insulin Resistance, 2005, vol. 26 Search PubMed.
- S. Y. Park, J. F. Gautier and S. Chon, Diabetes Metab. J., 2021, 45, 641–654 CrossRef PubMed.
- W. Yoshida, E. Mochizuki, M. Takase, H. Hasegawa, Y. Morita, H. Yamazaki, K. Sode and K. Ikebukuro, Selection of DNA aptamers against insulin and construction of an aptameric enzyme subunit for insulin sensing, Biosens. Bioelectron., 2009, 24, 1116–1120 CrossRef CAS PubMed.
- A. Garcia Cruz, I. Haq, T. Cowen, S. Di Masi, S. Trivedi, K. Alanazi, E. Piletska, A. Mujahid and S. A. Piletsky, Design and fabrication of a smart sensor using in silico epitope mapping and electro-responsive imprinted polymer nanoparticles for determination of insulin levels in human plasma, Biosens. Bioelectron., 2020, 169, 112536 CrossRef CAS PubMed.
- K. L. Servarayan, E. Sundaram, A. Manna and V. Sivasamy, Label free optical biosensor for insulin using naturally existing chromene mimic synthesized receptors: A green approach, Anal. Chim. Acta, 2023, 1239 Search PubMed.
- J. Chen, Z. Liu, R. Yang, M. Liu, H. Feng, N. Li, M. Jin, M. Zhang and L. Shui, A liquid crystal-based biosensor for detection of insulin driven by conformational change of an aptamer at aqueous-liquid crystal interface, J. Colloid Interface Sci., 2022, 628, 215–222 CrossRef CAS PubMed.
- G. M. Morris and M. Lim-Wilby, Molecular Docking, Methods Mol. Biol., 2008, 443, 365–382 CrossRef CAS PubMed.
- L. F. Gonçalves Dias, S. Stamboroski, M. Noeske, D. Salz, K. Rischka, R. Pereira, M. Do Carmo Mainardi, M. H. Cardoso, M. Wiesing, E. S. Bronze-Uhle, R. B. Esteves Lins and P. N. Lisboa-Filho, New details of assembling bioactive films from dispersions of amphiphilic molecules on titania surfaces, RSC Adv., 2020, 10, 39854–39869 RSC.
- M. Škarabot, E. Osmanagič and I. Muševič, Surface anchoring of nematic liquid crystal 8OCB on a DMOAP-silanated glass surface, Liq. Cryst., 2006, 33, 581–585 CrossRef.
- O. O. Prishchepa, V. Y. Zyryanov, A. P. Gardymova and V. F. Shabanov, Optical textures and orientational structures of nematic and cholesteric droplets with heterogeneous boundary conditions, Mol. Cryst. Liq. Cryst., 2008, 489, 84/[410]–93/[419] CrossRef.
- M. Cieplak, R. Węgłowski, Z. Iskierko, D. Węgłowska, P. S. Sharma, K. R. Noworyta, F. D'souza and W. Kutner, Sensors, 2020, 20, 1–12 CrossRef PubMed.
- Y. Y. Luk, M. L. Tingey, D. J. Hall, B. A. Israel, C. J. Murphy, P. J. Bertics and N. L. Abbott, Using liquid crystals to amplify protein-receptor interactions: Design of surfaces with nanometer-scale topography that present histidine-tagged protein receptors, Langmuir, 2003, 19, 1671–1680 CrossRef CAS.
- M. H. Pourasl, A. Vahedi, H. Tajalli, B. Khalilzadeh and F. Bayat, Liquid crystal-assisted optical biosensor for early-stage diagnosis of mammary glands using HER-2, Sci. Rep., 2023, 13, 6847 CrossRef CAS PubMed.
- Z. Hao, Y. Zhu, X. Wang, P. G. Rotti, C. Dimarco, S. R. Tyler, X. Zhao, J. F. Engelhardt, J. Hone and Q. Lin, Real-Time Monitoring of Insulin Using a Graphene Field-Effect Transistor Aptameric Nanosensor, ACS Appl. Mater. Interfaces, 2017, 9, 27504–27511 CrossRef CAS PubMed.
- M. Xu, X. Luo and J. J. Davis, The label free picomolar detection of insulin in blood serum, Biosens. Bioelectron., 2013, 39, 21–25 CrossRef CAS PubMed.
- R. Chhasatia, M. J. Sweetman, F. J. Harding, M. Waibel, T. Kay, H. Thomas, T. Loudovaris and N. H. Voelcker, Non-invasive, in vitro analysis of islet insulin production enabled by an optical porous silicon biosensor, Biosens. Bioelectron., 2017, 91, 515–522 CrossRef CAS PubMed.
- W. Jorgensen, Rusting of the lock and key model for protein-ligand binding, Science, 1991, 254, 954–955 CrossRef CAS PubMed.
- X.-Y. Meng, H.-X. Zhang, M. Mezei and M. Cui, Molecular Docking: A powerful approach for structure-based drug discovery, Curr. Comput.-Aided Drug Des., 2011, 7(2), 146–157 CrossRef CAS PubMed.
- M. Brylinski, Aromatic interactions at the ligand–protein interface: Implications for the development of docking scoring functions, Chem. Biol. Drug Des., 2018, 91, 380–390 CrossRef CAS PubMed.
- G. Ercolani and P. Mencarelli, Role of face-to-face and edge-to-face aromatic interactions in the inclusion complexation of cyclobis(paraquat-p-phenylene): A theoretical study, J. Org. Chem., 2003, 68, 6470–6473 CrossRef CAS PubMed.
- E. Lanzarotti, L. A. Defelipe, M. A. Marti and A. G. Turjanski, Aromatic clusters in protein–protein and protein–drug complexes, J. Cheminf., 2022, 7, 100079 Search PubMed.
- E. Nittinger, T. Inhester, S. Bietz, A. Meyder, K. T. Schomburg, G. Lange, R. Klein and M. Rarey, Large-Scale Analysis of Hydrogen Bond Interaction Patterns in Protein-Ligand Interfaces, J. Med. Chem., 2017, 60, 4245–4257 CrossRef CAS PubMed.
- H. Zhao and D. Huang, Hydrogen bonding penalty upon ligand binding, PLoS One, 2011, 6(6), 19923 CrossRef PubMed.
- K. Konstantinidis, I. Karakasiliotis, K. Anagnostopoulos and G. C. Boulougouris, On the estimation of the molecular inaccessible volume and the molecular accessible surface of a ligand in protein-ligand systems, Mol. Syst. Des. Eng., 2021, 6, 946–963 RSC.
- E. Durham, B. Dorr, N. Woetzel, R. Staritzbichler and J. Meiler, Solvent accessible surface area approximations for rapid and accurate protein structure prediction, J. Mol. Model., 2009, 15, 1093–1108 CrossRef CAS PubMed.
- J. Li, C. Hou, M. Wang, C. Liao, S. Guo, L. Shi, X. Ma, H. Zhang, S. Jiang, B. Zheng, L. Ye, L. Yang and X. He, A Hydrophobic-Interaction-Based Mechanism Triggers Docking between the SARS-CoV-2 Spike and Angiotensin-Converting Enzyme 2, Glob. Chall., 2020, 4(12), 2000067 CrossRef PubMed.
- Y. Cao and L. Li, Improved protein-ligand binding affinity prediction by using a curvature-dependent surface-area model, Bioinformatics, 2014, 30, 1674–1680 CrossRef CAS PubMed.
- Y. C. Cho and S. Il Ahn, Fabricating a Raman spectrometer using an optical pickup unit and pulsed power, Sci. Rep., 2020, 13, 6847 Search PubMed.
- S. Maeda and C. H. Haruhiko Kataoka, Effects of laser linewidth on the coherent Anti–Stokes Raman spectroscopy spectral profile, 1982, vol. 36 Search PubMed.
- J. C. Evans and H. J. Bernstein, INTENSITY IN THE RAMAN EFFECT: V. THE EFFECT OF INTERMOLECULAR INTERACTION ON THE RAMAN SPECTRUM OF CARBON DISULPHIDE, Can. J. Chem., 1956, 34(8), 1127–1133 CrossRef CAS.
- D. Tuschel, Exploring Resonance Raman Spectroscopy, Spectroscopy, 2018, 33, 12–19 CAS.
- D. Kurouski, M. Sorci, T. Postiglione, G. Belfort and I. K. Lednev, Detection and structural characterization of insulin prefibrilar oligomers using surface enhanced Raman spectroscopy, Biotechnol. Prog., 2014, 30, 488–495 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cp03205e |
| This journal is © the Owner Societies 2024 |