Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advancing nanotechnology for neoantigen-based cancer theranostics
Jianhua
Zou†
 *ab,
Yu
Zhang†
ab,
Yuanbo
Pan
ab,
Zhengwei
Mao
*ab,
Yu
Zhang†
ab,
Yuanbo
Pan
ab,
Zhengwei
Mao
 *efg and
Xiaoyuan
Chen
*efg and
Xiaoyuan
Chen
 *abcd
*abcd
aDepartments of Diagnostic Radiology, Surgery, Chemical and Biomolecular Engineering, and Biomedical Engineering, Yong Loo Lin School of Medicine and College of Design and Engineering, National University of Singapore, Singapore, 119074, Singapore. E-mail: zoujh-93@nus.edu.sg; chen.shawn@nus.edu.sg
bNanomedicine Translational Research Program, NUS Center for Nanomedicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117597, Singapore
cClinical Imaging Research Centre, Centre for Translational Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 117599, Singapore
dInstitute of Molecular and Cell Biology, Agency for Science, Technology, and Research (A*STAR), 61 Biopolis Drive, Proteos, Singapore 138673, Singapore
eMOE Key Laboratory of Macromolecular Synthesis and Functionalization, Department of Polymer Science and Engineering, Zhejiang University, Hangzhou, Zhejiang 310027, P. R. China. E-mail: zwmao@zju.edu.cn
fDepartment of Hepatobiliary and Pancreatic Surgery, The Second Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, Zhejiang 310009, P. R. China
gKey Laboratory of Precision Diagnosis and Treatment for Hepatobiliary and Pancreatic Tumour of Zhejiang Province, Hangzhou, Zhejiang 310009, P. R. China
First published on 21st February 2024
Abstract
Neoantigens play a pivotal role in the field of tumour therapy, encompassing the stimulation of anti-tumour immune response and the enhancement of tumour targeting capability. Nonetheless, numerous factors directly influence the effectiveness of neoantigens in bolstering anti-tumour immune responses, including neoantigen quantity and specificity, uptake rates by antigen-presenting cells (APCs), residence duration within the tumour microenvironment (TME), and their ability to facilitate the maturation of APCs for immune response activation. Nanotechnology assumes a significant role in several aspects, including facilitating neoantigen release, promoting neoantigen delivery to antigen-presenting cells, augmenting neoantigen uptake by dendritic cells, shielding neoantigens from protease degradation, and optimizing interactions between neoantigens and the immune system. Consequently, the development of nanotechnology synergistically enhances the efficacy of neoantigens in cancer theranostics. In this review, we provide an overview of neoantigen sources, the mechanisms of neoantigen-induced immune responses, and the evolution of precision neoantigen-based nanomedicine. This encompasses various therapeutic modalities, such as neoantigen-based immunotherapy, phototherapy, radiotherapy, chemotherapy, chemodynamic therapy, and other strategies tailored to augment precision in cancer therapeutics. We also discuss the current challenges and prospects in the application of neoantigen-based precision nanomedicine, aiming to expedite its clinical translation.
Key learning points1. Neoantigens can be generated from single-nucleotide variants, gene fusions, insertions or deletions, splice mutations, mitochondrial DNA mutations and viruses.2. The combination of nanotechnology and neoantigens can facilitate neoantigen release, promote neoantigen delivery to APCs, augment neoantigen uptake, shield neoantigens from protease degradation, and optimize interactions between neoantigens and the immune system. 3. Different therapeutic modalities (e.g., PDT, PTT, RT, CT, and CDT) can induce ICD to release tumor-specific antigens (TSAs) that can be internalized and cross-presented by APCs, thus promoting the maturation of DCs to activate the immune responses and inhibit tumor recurrence and metastasis. |
1. Introduction
Cancer represents a significant global public health challenge1,2 and approximately 19 to 20 million individuals receive a cancer diagnosis worldwide, leading to around 10 million cancer-related deaths annually.2 Existing cancer treatments include chemotherapy (CT), surgery, radiotherapy (RT), and immunotherapy (IT).3 CT can activate various signaling pathways and promote the release of inflammatory agents.4 Inflammation, however, serves as an innate immune response, inhibiting tumour growth but also creating conditions favorable for tumour promotion and recurrence.4 Surgical resection is the preferred approach for treating locally malignant solid tumours.5 However, it can potentially accelerate tumour recurrence or metastasis by stimulating residual tumour cell growth.5 RT inflicts macromolecular damage that hampers tumour cell proliferation while affecting non-malignant cells.6 Nevertheless, RT can activate multiple cytoprotective pathways, reducing its overall therapeutic efficacy.6 Immune checkpoint molecules, such as programmed cell death ligand 1 (PD-L1), are not exclusive to tumour cells; they are also expressed in normal tissues like the liver, skin, lung, intestine, and endocrine glands, leading to immune-related adverse events during immune checkpoint-based immunotherapy. Additionally, only a small percentage of cancer patients exhibit immune checkpoint expression.7 This differential expression across tumours diminishes the effectiveness of immune checkpoint inhibitors (ICIs) when used as monotherapy. Furthermore, a notable challenge faced by current cancer therapies is their limited ability to specifically target tumours. Therapeutic agents often struggle to accumulate effectively within tumour tissues, resulting in suboptimal therapeutic outcomes. Therefore, ongoing efforts are necessary to explore innovative therapeutic approaches.Neoantigens usually originate from somatic mutations in cancer cells and virus-driven antigens, and the quantity and forms of mutations vary with cancer types.7–9 The somatic mutations mainly include single nucleotide variants (SNVs), insertions or deletions (INDEL), gene fusions (GFs), splice mutations and mitochondrial DNA (mtDNA) mutations.8–10 The operational principle of neoantigens can be generally outlined as follows: the tumour microenvironment (TME) contains an infiltrate of immune cells, primarily antigen-presenting cells (APCs), such as B cells, macrophages, and dendritic cells (DCs).8 Neoantigens are initially taken up by APCs through various ways, including the macropinocytosis of soluble antigens, the phagocytosis of tumour cells by tumour-associated macrophages, the receptor-mediated uptake of apoptotic vesicles by DCs, or the ingestion of immunocomplexes through fragment crystallizable receptors.8 As a result, these mature DCs activate CD4+ and cytotoxic CD8+ T cells specific to the neoantigens, thereby triggering an immune response.11–16 Neoantigens trigger T-cell responses that are specific to the tumour, bypassing the tolerance mechanisms that typically apply to self-epitopes. This mechanism ultimately amplifies the effectiveness of tumour therapy.9 Furthermore, the sustained presence of neoantigen-specific T-cell responses and their ability to establish immunological memory post-therapy offer the potential for long-term prevention of tumour recurrence.9 Additionally, neoantigens based on the tumour cell membrane exhibit significant homologous targeting ability in the process of tumour therapy.17 Cancer with genetic diversity not only presents differences between individuals but also intensifies as the disease advances.18 Consequently, the effectiveness of standard treatment approaches varies significantly from one patient to another, underscoring the pressing need for precision therapy. The specificity of neoantigens holds promise for personalized tumour treatment, offering tremendous potential for precision therapy.19 Therefore, there is an urgent demand for precision neoantigen-based therapies for curbing tumour growth, preventing recurrence, and halting metastasis.
Recently, the development of nanotechnology has played a significant role in improving the efficacy of precision neoantigen-based cancer theranostics.20 The benefits of utilizing nanotechnology in neoantigen-based therapies are primarily evident in the following aspects: first, nanomaterials enhance the spontaneous release of endogenous neoantigens during tumour therapy.21,22 They facilitate the efficient delivery of neoantigens to APCs and enhance their uptake by DCs.23,24 In addition, nanomaterials provide protection to neoantigens, preventing their degradation and thereby bolstering anti-tumour immune responses.21,24 Last but not least, they can extend the interaction between neoantigens and the immune system.17,25,26 The latest advances in the discovery and research of neoantigens has expedited the development of tumour therapeutics, including cancer vaccines, antibody-based therapeutics, and adoptive cell therapies, especially for malignant solid tumours, which have significantly enhanced the anti-tumour efficacy.27–29 Typically, Zhao et al. constructed MPO nanovaccines to promote the delivery of exogenous neoantigens to lymph nodes (LNs), improve cross-presentation of exogenous neoantigens and lysosomal escape, boost immunogenic cell death (ICD) to release endogenous tumour neoantigens as well as repolarize macrophages to an M1 phenotype.30 To enhance the efficacy of precision neoantigen-based therapy, Xu et al. constructed F13-PEI/Mem based on fluoroalkane-grafted cationic polymer F-PEI (fluoroalkane-grafted polyethyleneimine), which effectively prevented post-surgery tumour relapse and metastasis in combination with ICB.31 Overall, rapidly advancing nanotechnology is of tremendous significance.
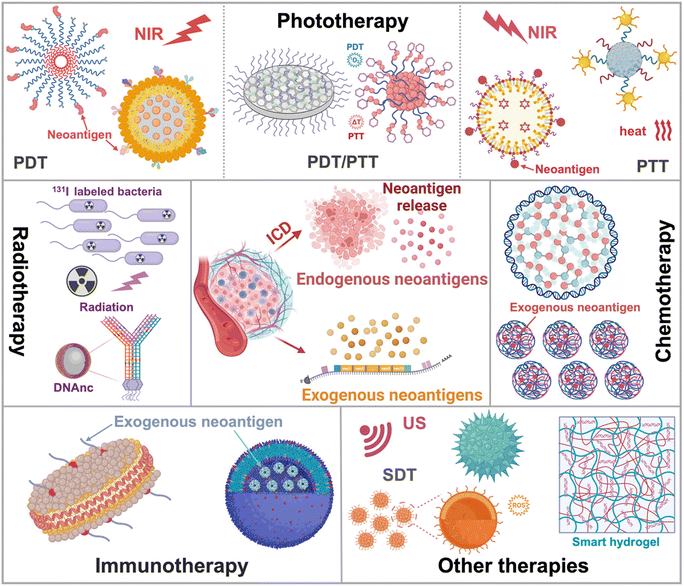
This tutorial review provides an overview of neoantigen sources and their mechanisms as tumour therapeutics. It also highlights advanced nanotechnology for different therapeutic modalities, including phototherapy, radiotherapy, chemotherapy, immunotherapy and other therapies (Fig. 1). The working mechanisms have also been summarized, encompassing the promotion of neoantigen release, safeguarding neoantigen integrity, enhancing targeted neoantigen delivery, and extending neoantigen efficacy within the tumour microenvironment for precision neoantigen-based therapy. The design and preparation of nanomaterials have been summarized. Furthermore, it addresses the current challenges in neoantigen-based therapy and outlines potential future prospects in the clinic.
2. Sources of cancer neoantigens and the mechanisms of neoantigen-induced immune responses against cancer
Neoantigens originate from somatic mutations that are specific to tumour cells, expressing genes that are absent in normal tissue genomes.32,33 These mutations give rise to tumour-specific neoepitopes presented on major histocompatibility complex class I (MHC I) molecules.8 Tumour-specific neoepitopes, displayed by MHC I, can be recognized by tumour-infiltrating lymphocytes (TILs) in individuals with cancer and represent appealing targets for adoptive T-cell therapy.34 In this section, we will delineate the diverse sources of cancer neoantigens, including single-nucleotide variants, gene fusion mutations, insertion or deletion mutations, splice mutations, and mitochondrial DNA mutations. Additionally, we will explore the mechanisms behind neoantigen-induced immune responses, aiming to achieve precision in cancer therapeutics.2.1 Sources of cancer neoantigens
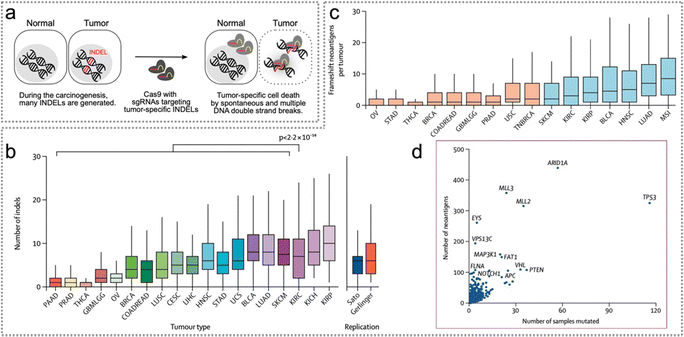
Neoantigens emerge because of non-synonymous somatic mutations, which encompass various genetic alterations such as single-nucleotide variants, gene fusions, insertions or deletions, splice mutations, and mitochondrial DNA mutations.29,35–37 These mutations become expressed within cancerous cells due to the genomic instability characteristic of cancer. This instability leads to the accumulation of non-synonymous somatic genetic changes, ultimately giving rise to tumour-specific mutated peptides, commonly referred to as tumour-specific neoantigens.38 Genes with a higher burden of mutation-derived neoantigens exhibit a greater diversity and frequency of non-synonymous somatic mutations.39 Furthermore, tumour neoantigens can also arise from mutations resulting from viral integration events within tumour cells.29 In this part, the different sources of neoantigens will be discussed.In the Pan-Cancer Analysis of Whole Genomes (PCAWG) database, the median variance in SNV rates, observed in 1 million base pair (Mb) windows across thirty-seven different cancers, was 94.6% (Fig. 2a).42 Positive selection detected mutations in the Neuroblastoma RAS viral (v-ras) cancer gene homolog (NRAS) coding region, noncoding mutations in the telomerase reverse transcriptase (TERT) promoter, and splice site SNVs in Von Hippel-Lindau (VHL) (Fig. 2b). Overall, intronic recessive splicing SNVs accounted for 4.5% of excess (potentially driver) SNVs in tumour suppressor genes (TSGs), a magnitude similar to the 7.4% observed for canonical spliced SNVs (Fig. 2c). Additionally, there was a significant co-occurrence of SNVs in TSGs and oncogenic or protein-truncated SNVs (Fig. 2d).42
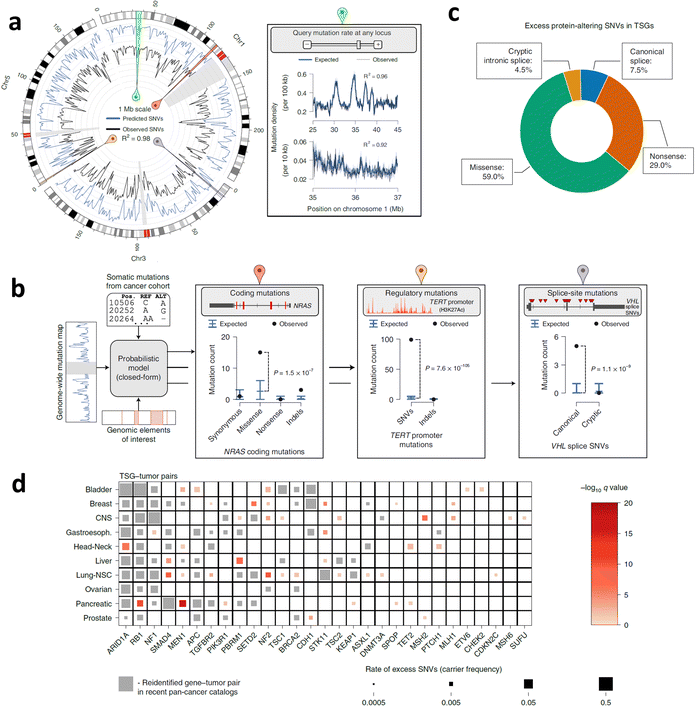 | ||
| Fig. 2 SNVs across cancer types and neoantigen predictions. (a) The SNV map of whole genome neutrophils and the SNV density observed in the 1 Mb window of the PCAWG queue (n = 2279 cases). (b) The illustrations of burden testing for coding mutations in NRAS, noncoding mutations in TERT promoters, and SNV splicing sites in VHL employing the PCAWG dataset. (c) The ratio of excessive SNVs in TSGs was contributed by each protein changing the SNV classification. (d) TSG–tumour matched pairs have a remarkable encumbrance of carcinogenic or protein-truncation SNVs. Reproduced with permission from ref. 42. Copyright 2022, Springer Nature Ltd. | ||
Through the application of whole-genome sequencing (WGS) and RNA sequencing (RNA-seq), researchers identified an original GF in head and neck squamous cell carcinoma (HNSCC). This GF was proven to generate neoantigens that effectively trigger cytotoxic T cell responses.44 In the WGS analysis of 28 frozen tissues from primary HNSCC tumours, a low rate of non-synonymous mutations was observed, including an average of 0.47 mutations per 1 megabase, along with 14 single nucleotide variations (SNVs) distributed among 12 genes. Notably, they identified one original in-frame GF known as DEK–AFF2 (Fig. 3a and b).
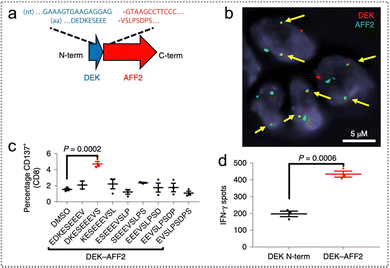 | ||
| Fig. 3 Gene fusion and neoantigen predictions. (a) The DEK–AFF2 GF map displays amino acid (AA) and nucleotide sequences around connect points indicated. (b) FISH test of DEK–AFF2 GF; the arrow points denote colocalization of AFF2 and DEK. (c) The CD137 expression after co-culture of MSK-HN1 T cells with autogenous peripheral blood mononuclear cells (PBMCs) pulsed with 10 μM AFF2–DEK-derived or DEK–AFF2-derived peptides, which was analyzed via flow cytometry. (d) The abundance of IFN-γ spots in MSK-HN1T cells following co-culture with SCC-9 cells expressing DEK–AFF2 or DEK-N-term (n = 3) (Dunnett correction performed after one-way ANOVA for multiple comparisons). Reproduced with permission from ref. 44. Copyright 2019, Springer Nature Ltd. | ||
Furthermore, when autologous peripheral blood mononuclear cells (PBMCs) were exposed to DKESEEEVS (DEK–AFF2 fusion-derived peptides), T cells were stimulated and activated, leading to an increased secretion of interferon-gamma (IFN-γ) and an elevated proportion of CD137-positive CD8+ T cells (Fig. 3c). Moreover, the secretion of IFN-γ was significantly enhanced when the constructed DEK–AFF2 fusion was introduced into SCC-9 cells, providing further confirmation that DEK–AFF2 GFs induce the activation of cytotoxic T cell immune responses (Fig. 3d). These findings underscore the significance of gene fusions as potential targets for immunotherapy in the context of cancer treatment.
 | ||
| Fig. 4 INDEL mutation across cancer types and neoantigen predictions. (a) Schematic illustration of the cancer-specific insertions–deletions attacker (CINDELA). Reproduced with permission from ref. 49. Copyright 2022, PNAS. (b) Absolute count of insertion or deletion mutations in 19 solid tumour types derived from TCGA. (c) Neoantigens derived from indel-induced frameshift. (d) Relapse genes with frameshift indel-derived neoantigens. Reproduced with permission from ref. 48. Copyright 2017, Elsevier. | ||
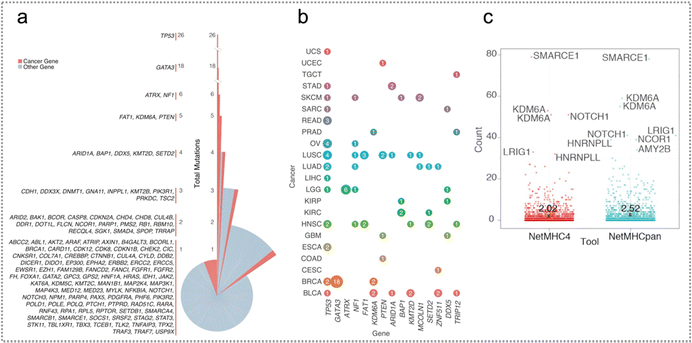 | ||
| Fig. 5 Splice mutations across cancer types and genes and neoantigen predictions. (a) The mutation distribution generated by splicing sites in every gene is divided by total mutations in every gene. The splice site of TP53 generated the highest number of mutations, followed by GATA3 and ATRX. (b) The genes with the largest quantity of mutations at the pan-cancer splice-sites. The size of the circle is related to total mutations in every gene and is colored according to tumour class. The mutations generated by splicing sites in TP53 exist in numerous tumour classes, while mutations in GATA3 and ATRX are particular to BRCA and LGG, respectively. (c) Neoantigen distribution calculated by NetMHCpan and NetMHC4. Reproduced with permission from ref. 53. Copyright 2018, Cell Press. | ||
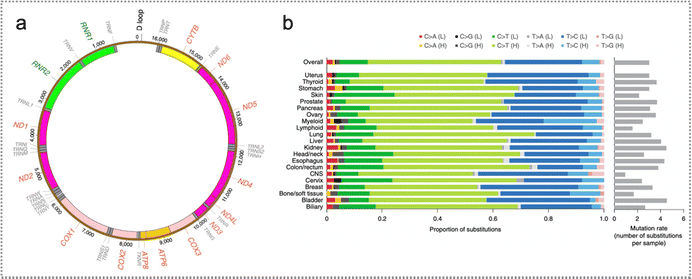 | ||
| Fig. 6 Mutation in mitochondrial DNA. (a) The outlook of mtDNA somatic replacements. Mitochondrial genome coordinates are indicated by numbers. (b) Highly consistent mtDNA mutational profiles spanning 21 cancer tissues. The average quantity of somatic replacements in each sample is also displayed. Reproduced with permission from ref. 58. Copyright 2020, Springer Nature Ltd. | ||
2.2 Endogenous and exogenous neoantigens
According to the different sources of production, neoantigens can be divided into endogenous neoantigens and exogenous neoantigens. Endogenous neoantigens are mainly released by the ICD of tumour cells induced by different therapeutic modalities, such as phototherapy, radiotherapy, and chemotherapy.71,72 Tumour cells undergo the ICD process, during which they release endogenous neoantigens and damage-associated molecular patterns (DAMPs), including molecules like calreticulin (CRT), heat shock proteins (HSP), high-mobility group box1 (HMGB1), and adenosine triphosphate (ATP).72 ICD in cancer cells can activate the immune response, and thus effectively eliminate tumours. Exogenous neoantigens primarily consist of neoantigen peptides synthesized based on amino acid sequences of neoantigens (e.g., ovalbumin, a model antigen) and tumour cell membranes.31,73Possible unique neoantigens were identified by performing computational analysis on the whole exome sequencing (WES) data and RNA-seq data from tumour cells, and their validity was confirmed using enzyme-linked immunospot (ELISPOT) assays (Fig. 7).74,75 Subsequently, exogenous neoantigen peptides can be chemically synthesized based on the amino acid sequence of these neoantigens.74–76 Additionally, tumour cell membranes can also serve as a source of exogenous neoantigens because they contain tumour holoantigen proteins with similar targeting capabilities.73,77
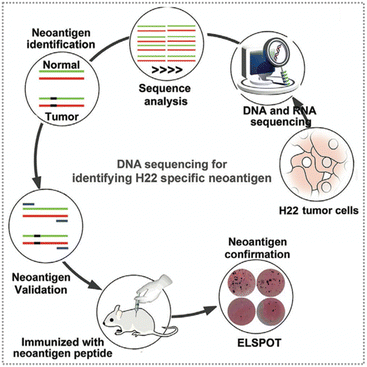 | ||
| Fig. 7 Schematic diagram of the neoantigen screening and identification process. The specific neoantigens of H22 tumour cells were screened by employing WES and RNA-seq, and the potential neoantigens were predicted through computer analysis and their immunogenicity was confirmed by ELISPOT assay. Reproduced with permission from ref. 74. Copyright 2021, Wiley. | ||
2.3 Mechanisms of neoantigen-induced immune responses
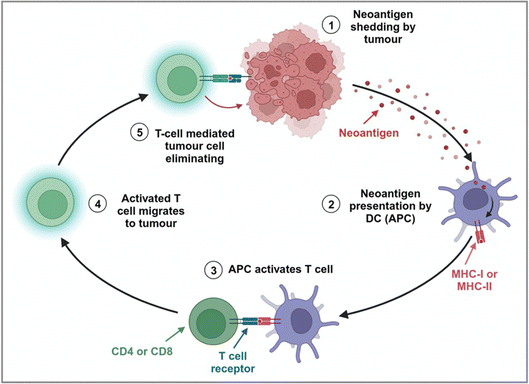
Effective immunity against malignancy in humans is strongly related to the existence of endogenous T cells targeting tumour-specific neoantigens.7 However, tumour-induced immunosuppression and immune resistance in the TME pose a major challenge to realize complete elimination of human malignancies.78 After treatment, tumour cells undergo spontaneous ICD, leading to the release of tumour-specific antigens (TSAs). Within the TME, these tumour antigens can be internalized and cross-presented by APCs, such as DCs.78 This process not only promotes the maturation of DCs but also facilitates the uptake, processing, and presentation of antigens on major histocompatibility complex (MHC) or human leukocyte antigen (HLA) molecules.12,78 Then, DCs loaded with antigens move to the secondary lymphoid organs.78 The interplay between MHC-peptide compound-T cell receptor (TCR) and homologous receptor–ligand pairs induces the secretion of cytokines by DCs to stimulate T cells. The responses of CD8+ T cells are magnified by IL-2 secreted from CD4+ T cells. Then, the activated T cells enter the TME and kill the tumour cells (Fig. 8).78The immune reactions of MHC-II-restricted CD4+ T cells to tumour neoantigens have been reported in the context of tumour immunotherapy.75,79 Oliveira et al. revealed that the helper and regulatory CD4+ T cells specifically respond to human melanoma HLA-II-restricted neoantigens.80 Notably, single-cell TCR sequencing (scTCR-seq) analysis of 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 869 CD4+ TILs from four melanoma patients (Pt-A, -B, -C, and -D) revealed that the cellular phenotypes within the expanded CD4+ TIL TCR clonotype primarily included the exhaustion phenotype (TEX), non-depleted memory phenotype (TNExM) and T regulatory phenotype (TReg). The amplification of CD4+ TReg TILs was higher in the HLA-II-positive (HLA-IIpos) tumour TME. Besides, CD8+ cytotoxic T lymphocytes’ (CTL) responses to MHC-I-restricted neoantigens have also been demonstrated in both human and mouse tumour immunotherapy.29,81,82 In addition, Sumit et al. have provided evidence for increased intratumoral CD8+ T cell density, an IFN-γ responsive gene signature, and robust antigen-specific T cell responses in clinical cases of prostate cancer.81 Recently, Puig-Saus et al. also demonstrated that polyclonal CD8+ T cells in tumours and peripheral blood are positively correlated with immunotherapy efficacy for human melanoma and can repeatedly recognize tumour-specific neoantigens during therapy.83 Meanwhile, some reports have proved that both CD4+ and CD8+ T cells specifically respond to tumour neoantigens in human and mouse tumour immunotherapy.84–87 For example, Spencer et al. revealed that neoantigen-specific CD4+ T cells provided help to CD8+ T cells, such as driving the amplification of CD8+ T cells to realize squamous cell carcinoma (SCC) immunotherapy.86 Additionally, Ott et al. demonstrated that patients with advanced malignant solid tumours (including melanoma, bladder cancer and non-small cell lung cancer) exhibited neoantigen-specific CD4+ and CD8+ T cell responses after immunotherapy with personalized neoantigen-based vaccine combined with PD-1 blocking.88
869 CD4+ TILs from four melanoma patients (Pt-A, -B, -C, and -D) revealed that the cellular phenotypes within the expanded CD4+ TIL TCR clonotype primarily included the exhaustion phenotype (TEX), non-depleted memory phenotype (TNExM) and T regulatory phenotype (TReg). The amplification of CD4+ TReg TILs was higher in the HLA-II-positive (HLA-IIpos) tumour TME. Besides, CD8+ cytotoxic T lymphocytes’ (CTL) responses to MHC-I-restricted neoantigens have also been demonstrated in both human and mouse tumour immunotherapy.29,81,82 In addition, Sumit et al. have provided evidence for increased intratumoral CD8+ T cell density, an IFN-γ responsive gene signature, and robust antigen-specific T cell responses in clinical cases of prostate cancer.81 Recently, Puig-Saus et al. also demonstrated that polyclonal CD8+ T cells in tumours and peripheral blood are positively correlated with immunotherapy efficacy for human melanoma and can repeatedly recognize tumour-specific neoantigens during therapy.83 Meanwhile, some reports have proved that both CD4+ and CD8+ T cells specifically respond to tumour neoantigens in human and mouse tumour immunotherapy.84–87 For example, Spencer et al. revealed that neoantigen-specific CD4+ T cells provided help to CD8+ T cells, such as driving the amplification of CD8+ T cells to realize squamous cell carcinoma (SCC) immunotherapy.86 Additionally, Ott et al. demonstrated that patients with advanced malignant solid tumours (including melanoma, bladder cancer and non-small cell lung cancer) exhibited neoantigen-specific CD4+ and CD8+ T cell responses after immunotherapy with personalized neoantigen-based vaccine combined with PD-1 blocking.88
Although immunogenic cell death (ICD) shows promise as a therapeutic approach in cancer treatment, it is not without its constraints. Some notable limitations can be summarized as follows. First, the heterogeneity of cancer cells within a tumor poses a challenge, as not all cells will undergo ICD in response to treatment. Some cells might develop resistance mechanisms, allowing non-immunogenic cells to survive and potentially leading to tumor relapse. Second, the TME can be immunosuppressive due to factors like regulatory T cells, myeloid-derived suppressor cells, and cytokines, creating an inhibitory setting. This environment may restrict the effectiveness of the immune response triggered by ICD. Effective immune responses require the recognition of specific antigens on cancer cells. Some tumors may have limited antigenicity, lacking the necessary markers for effective targeting by the immune system, even when ICD occurs. Third, immunosuppressive checkpoint pathways: cancer cells can exploit immune checkpoint pathways (e.g., PD-1/PD-L1 and CTLA-4) to evade the immune response. Despite induction of ICD, these checkpoints can impede the immune system's ability to mount a sustained and effective attack on cancer cells. In addition, cancer cells can evolve and develop mechanisms to escape immune surveillance, including downregulating antigen expression, mutating to avoid immune recognition, or altering the TME to resist immune attack. Last but not least, ICD may be less effective in advanced cancer stages when tumor burden is high and the immune system is compromised. The presence of numerous immunosuppressive cells in the tumor microenvironment may further hinder the success of immunotherapy. Implementing ICD-based therapies can face logistical challenges, including issues related to the efficient delivery of treatment and the potential for adverse effects.
Despite these challenges, researchers are actively exploring strategies to overcome these limitations and improve the efficacy of ICD-based approaches. Combining ICD-inducing therapies with other immunotherapeutic or targeted strategies may provide a more comprehensive and effective treatment approach for cancer patients.
2.4 Neoantigen-based immunotherapy
Immunotherapy harnesses the immune system to eliminate cancer cells via boosting or suppressing the immune responses, which has shown significant therapeutic efficacy in many malignancies.89 This section will discuss various immunotherapy strategies combined with tumour neoantigens for achieving precision neoantigen-based cancer immunotherapy.Cancer vaccines can induce tumour specific immunoreactions and have been applied in immunotherapy for various cancers.27 Currently, cancer nanovaccines mainly deliver exogenous neoantigens and immune adjuvants to APCs to stimulate and proliferate tumour-specific T cells. Nanovaccines have shown remarkable efficacy in tumour immunotherapy. Among them, the presentation efficiency of neoantigens is closely related to the therapeutic effect of cancer nanovaccines.27 The effects of APC activation and cytotoxic T cells, however, still need to be improved. Different nanoformulations have been developed to solve this problem. Typically, Xu et al. constructed F13-PEI/Mem nano-vaccine based on fluoroalkane-grafted cationic polymer F-PEI (fluoroalkane-grafted polyethyleneimine), which effectively prevented post-surgery tumour relapse and metastasis in combination with ICB (Fig. 9a).31 F13-PEI/Mem obviously suppressed the proliferation of distant tumours compared to those immunized with Mem alone and unimmunized (Fig. 9b). The synergistic treatment of F13-PEI/Mem and anti-PD1 further promoted the antitumour efficacy and significantly prolonged the disease-free survival of mice (Fig. 9c). However, cationic F13-PEI may also bring about potential systemic cytotoxicity in the long term. Moon et al. developed high-density lipoprotein-based nanodiscs (NDs) which can enhance the delivery efficiency of tumour neoantigens/adjuvants to LNs and induce T cell reactions.90 Nanodiscs eliminated established MC-38 and B16F10 tumours when combined with anti-PD-1 and anti-CTLA-4 therapy.
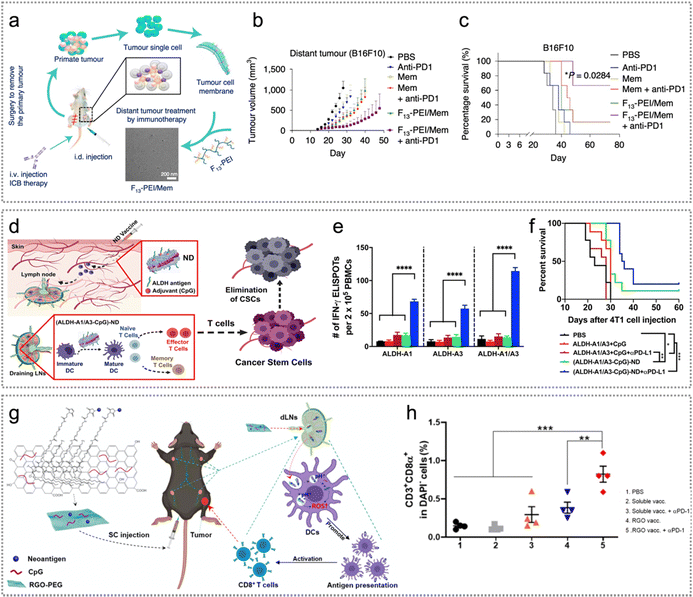 | ||
| Fig. 9 Neoantigen-based immunotherapy. (a) Illustration of F13-PEI/Mem NPs from tumour cytomembranes derived from surgically excised tumours as individual tumour nanovaccines, which were adopted in cooperation with ICB therapy to suppress tumour proliferation. (b) and (c) Distant tumour proliferation curves (b) of B16F10 tumours and disease-free survival of mice (c) in various treatment groups of mice. Reproduced with permission from ref. 31. Copyright 2020, Springer Nature Ltd. (d) ND vaccination illustration for ALDHhigh CSCs. (e) The outcomes from an IFN-γ ELISPOT assay conducted using PBMCs collected on day 15, followed by in vitro restimulation with the specified peptides. (f) The overall survival of 4T1 tumour-bearing mice treated with (ALDH-A1/A3-CpG)-ND or a soluble mixture of ALDH-A1/A3 (15.5 nmol per dose per antigen peptide) and CpG (15 μg per dose) on day 1 and day 8. Reproduced with permission from ref. 91. Copyright 2020, American Chemical Society. (g) Schematic representation of RGO(CpG)-PEG-neoantigen for LN-targeted delivery of antigens and adjuvants. (h) At day 20, tumour infiltrating CD8α+ T cells in different therapeutic groups, including control, soluble vaccine with/without anti-PD-1 and RGO vaccine with/without anti-PD-1. Reproduced with permission from ref. 92. Copyright 2020, American Chemical Society. | ||
The proliferation of cancer stem cells (CSCs) is a significant contributor to tumour recurrence and metastasis. While CSCs have been identified in various types of cancer, further research is required to develop effective strategies for targeting and eliminating CSCs in vivo.93 Among the various surface markers of CSCs, aldehyde dehydrogenase (ALDH) is a well-known one. For efficient delivery of ALDH epitope peptides to APCs, Moon et al. constructed (ALDH-A1/A3-CpG)-ND for eliciting T cell reactions directly against ALDHhigh CSCs.91 (ALDH-A1-CpG)-ND induced strong ALDH-specific T cell reactions, reduced ALDH-specific CSCs and exhibited significant anti-tumour effects in 4T1 mastocarcinoma and D5 melanoma mouse models (Fig. 9d). Remarkably, the combination of ND vaccine and anti-PD-L1 in immunotherapy showed synergistic effects by significantly inhibiting tumour growth and extending the survival of mice, surpassing the outcomes of other treatment groups (Fig. 9e and f).91 Reduced graphene oxide nanosheets (RGO) have been widely studied for drug delivery, including photothermal immunotherapy and cancer vaccines; however, they are nondegradable, and the potential concerns of long-term exposure remain. Moon et al. also developed a PEGylated RGO nanoplatform (RGO-PEG) (20–30 nm in diameter) as a convenient, biodegradable nanoplatform for loading of neoantigens and the CpG oligodeoxynucleotide (CpG ODN, a Toll-like receptor-9 agonist). The ROS-inducing RGO-PEG nanovaccine system can support highly modular and facile production of personalized neoantigen vaccines. After just a single round of vaccination, it remarkably improved the rate of CD3+/CD8α+ T cells in tumours as well as significantly activated DCs (Fig. 9g and h).92
3. Advantages of nanotechnology
The advantages of nanotechnology based neoantigens are generally summarized as follows: first, nanomaterials enhance endogenous neoantigen release during tumour therapy. They can significantly improve ICD of tumour cells by triggering DNA structure destruction to release numerous neoantigens.23,25 The process can be induced by phototherapy/radiotherapy through ROS or hyperthermal production,21,22,25,26,94–101 chemotherapy/chemodynamic therapy, and sonodynamic therapy.30,102–105 Xie et al. developed programmable cytosine-phosphate-guanine (CPG) DNA nanoclusters (DNAnc) for promoting ICD of tumour cells, thus releasing abundant tumour endogenous neoantigens.94 In addition, nanomaterials can efficiently capture tumour neoantigens through hydrophobic interactions or due to the presence of different functional groups, such as maleimide, amino, and catechol. They facilitate the delivery of these neoantigens to APCs,25,26,32 improve the cross-presentation of neoantigens, and enhance their uptake by DCs.92,98,105,106 Consequently, this promotes the maturation of DCs and augments the effectiveness of anti-tumour immune responses.23,30,101,106,107 To mention a typical example, Li et al. constructed 1-MT@OMV-Mal to activate systemic immune responses against the tumour by coordinating endogenous neoantigen capture and immune modulation, thereby significantly suppressing both primary and distant tumours.108 Nanomaterials also protect neoantigens from degradation, and thereby boost anti-tumour immune therapy.21,24,30 To achieve this goal, Li et al. constructed hydrophobic microdomains of neoantigens loaded with 2-formylphenylboronic acid-nanochaperone (PBA-nChap) to protect neoantigens from clearance by protease.24 Last but not least, nanomaterials prolong the interaction between neoantigens and the immune system. Concretely, the enhanced permeability and retention effect (EPR) of nanomaterials significantly prolongs the residence time of neoantigens in the TME and the interaction time between neoantigens and immune cells, thus enhancing anti-tumour immune responses.17,25,26 Recently, Dai et al. developed PCN-224-chloroquine@cancer cell membranes (PCN-CQ@CCM) to significantly enhance homologous tumour-targeting capability, avoid phagocytosis of macrophages, as well as prolong the residence time of exogenous neoantigens in the TME, thus realizing precision targeted therapy.174. Neoantigen based different therapeutic modalities
4.1 Neoantigen-based phototherapy
Over the past several decades, phototherapy has emerged as an attractive treatment for cancer.109 Light-activated, photosensitizer-based phototherapies have made a profound impact on tumour elimination in numerous cancer cases.109,110 Cancer nanomedicine has embraced two leading-edge nanotechnologies: photodynamic therapy (PDT) targets specific lesions for chemical destruction, and photothermal therapy (PTT) induces localized thermal damage.109–111 Improving the tumour specificity of phototherapy could produce a better therapeutic effect and less side effects. During the phototherapy process, ROS or hyperthermia produced by photosensitizers under irradiation induces ICD of tumour cells to release abundant endogenous neoantigens, which trigger robust immune responses.26,71,76 Additionally, exogenous neoantigens loaded with phototherapeutic agents also play a synergistic role in tumour therapy.76,97,112 In this part, we will discuss the combination of neoantigen and phototherapy in tumour therapy.Soild tumors may suffer from hypoxia but PDT is usually dependent on the oxygen level in tumors. Designing hypoxia responsive nanomaterials may have the potential for enhanced theranostics. Inspired by this, Liang and co-workers constructed a light-activatable immunological adjuvant (LIA) consisting of a hypoxia-responsive amphiphilic dendrimer (HAD) NP loaded with Ce6 (Fig. 10a and b).26 Strikingly, the LIA promoted endogenous tumour neoantigen release to form an in situ cancer vaccine and activated DCs, thereby inducing tumour cell death and endogenous tumour neoantigen release. At the same time, the hypoxic environment led to the rapid reduction of the 2-nitroimidazole group of the dendrimer to 2-aminoimidazole (rHAD). LIA plus laser therapy effectively enhanced the proportion of CD8+ and CD4+ T cells in infiltrating distal tumours and spleen, suppressing both primary and distant tumours (Fig. 10c and d). Meanwhile, LIA + laser therapy significantly promoted T cells (CD8+ and CD4+ T cells) secreting IFN-γ in distant tumours as well as obviously decreased the ratio of CD4+/CD25+/foxp3+ T cells (Fig. 10e).
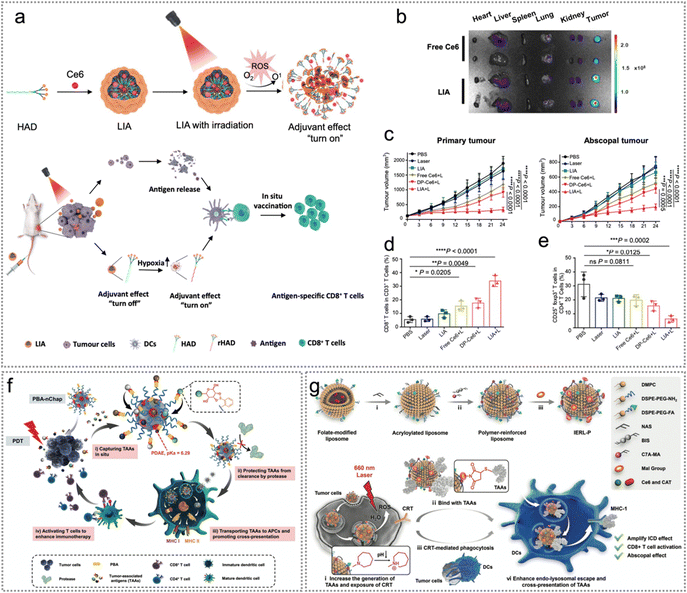 | ||
| Fig. 10 Endogenous neoantigen-based photodynamic therapy. (a) The scheme of a light-activatable immunological adjuvant (LIA) for enhanced orthotopic cancer vaccination. (b) The fluorescence images of heart, liver, spleen, lung, kidney and tumour tissues at 24 h after injection. (c) The growth curves of 4T1 primary and distant tumours. (d) and (e) Statistical analysis of CD3+/CD8+ T cell percentage in the spleen (d), as well as the proportion of CD4+/CD25+/foxp3+ CD4+ T cells (e). Reproduced with permission from ref. 26. Copyright 2021, Springer Nature Ltd. (f) The schematic diagram of PBA-nChap capturing TAAs in situ and boosting tumour immune therapy. Reproduced with permission from ref. 24. Copyright 2022, Wiley. (g) IERL-Ps promote ICD induced by PDT by promoting cross-priming. Reproduced with permission from ref. 21. Copyright 2022, Wiley. | ||
The efficacy of neoantigen-based therapy is limited by the specificity and effectiveness of neoantigens and the side effects of adjuvants.15,118 To improve the anti-tumour immune reactions of neoantigen-based PDT while reducing the complexity of nano-vaccines, Luo et al. synthesized self-assembling, endogenous neoantigen-harvesting and molecular activator nano-vaccine Mal-PEG5000-b-PC7A45 NPs (Mal-NPs) for enhancing cancer immunotherapy.119 Maleimide is capable of capturing antigens through Michael reactions between thiol and maleimide groups.119 Then the endogenous tumour neoantigens were harvested by Mal-NPs. The synthesized nano-vaccine could promote DC maturation and rapid accumulation in lymph nodes (LNs), thereby improving the amplification of cytotoxic CD8+ T cells.119 To capture neoantigens in situ, protect neoantigens from protease degradation and promote cross-presentation of neoantigens, Li et al. constructed 2-formylphenylboronic acid-nanochaperone (PBA-nChap) and used it in combination with PDT for personalized cancer immunotherapy.24 Notably, PBA-nChap could effectively capture endogenous tumour neoantigens in situ through hydrophobic interactions, thereby significantly protecting neoantigens from degradation and delivering the neoantigens to APCs. It was worth noting that PBA could recognize the main categories of neoantigens (glycoproteins) and recruit them to the vicinity of the hydrophobic microdomain, thus improving the capture efficiency of neoantigens. Furthermore, PBA-nChap transported neoantigens to APCs and escaped from lysosomes to facilitate antigen cross-presentation, therefore stimulating T cells to boost immune responses (Fig. 10f).
Additionally, to improve the cross-presentation of tumour endogenous neoantigens and ICD-related anti-tumour immune response after PDT, Zhao et al. constructed an immune-enhancing polymer-reinforced liposome (IERL).21 The IERL avoided the interception of neoantigens by internal lysosomes and significantly enhanced the cross-presentation of neoantigens, thereby systematically activating anti-tumour immune responses. IERL-Ps generated numerous ROS in tumour cells upon laser irradiation, and then induced ICD of tumour cells to release abundant tumour endogenous neoantigens. The released neoantigens were covalently bound to the Mal group on the surface of IERL-P to achieve efficient capture of neoantigens. After being ingested by DCs, the IERL avoided the interception of neoantigens in inner lysosomes, which was ascribed to the protonation of the 2-(hexamethyleneimino) (C7A) group (proton sponge effect, PSE) (Fig. 10g).21
Exogenous neoantigens co-loaded with photosensitizers can be identified by DCs and presented to cytotoxic T cells, thereby boosting homologous tumour targeting ability, immunoreactions, and the elimination of neoantigen-expressing cancer cells.17,76,77 PDT, in return, induces ICD of tumour cells to release endogenous tumour neoantigens, thus boosting immune responses.76,112 Therefore, the combination of PDT and exogenous neoantigens may work synergistically in tumour therapy. Wang and co-workers constructed a neoantigen nanovaccine mD@cSMN based on an acid/photo-sensitive DC membrane to potentiate anti-tumour immune response for hepatocellular carcinoma immunotherapy (Fig. 11a).76 MnO2 can be degraded in the TME and release oxygen for the relief of hypoxia. Therefore, Zhang et al. constructed a nanostructure made of a MnO2 nano-sheet-coated metal–organic framework core and a cancer cell membrane shell (CM-MMNPs).77 CM-MMNPs improved the homologous tumour targeting ability in the hypoxic TME. Tumour cell membranes were coated on the outside of MMNPs to generate CM-MMNPs (Fig. 11b). CM-MMNPs enhanced the tumour-targeting capability and increased the oxygen content in tumour cells, thus significantly improved the efficacy of PDT. Since the phototherapeutic efficacy can be enhanced by avoiding the phagocytosis of macrophages, PCN-CQ@CCM17 was developed by Dai and collaborators to homologously adhere to tumour cells and evade the phagocytosis of macrophages, thus improving the retention of nanomaterials in the TME. This phenomenon was attributed to homologous targeting capability and immunological escape of oral squamous cell carcinoma (OSCC). Furthermore, PCN-CQ was activated in cancer cells under 660 nm laser irradiation, leading to the generation of ROS that induced apoptosis in cancer cells. And the released CQ could simultaneously inhibit autophagy effectively (Fig. 11c).
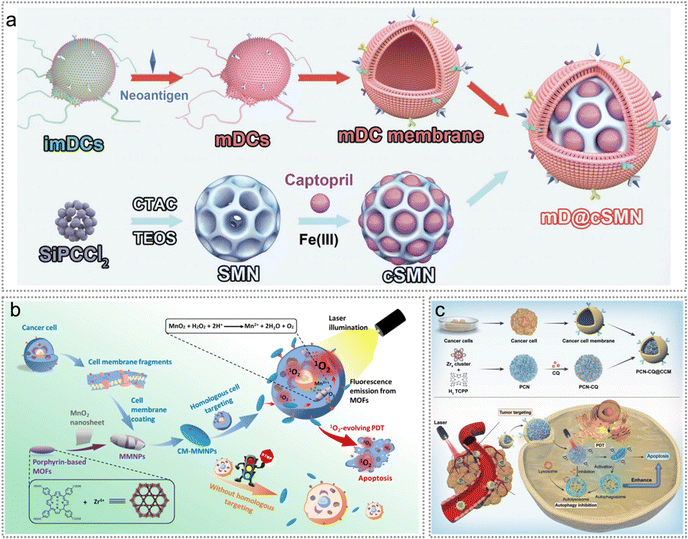 | ||
| Fig. 11 Exogenous neoantigen-based photodynamic therapy. (a) Schematic diagram of the preparation of the mD@cSMN nanovaccine. Reproduced with permission from ref. 76. Copyright 2022, Wiley. (b) CM-MMNPs for homologous targeting and MRI/fluorescence dual-mode imaging guided PDT against cancer cells. Reproduced with permission from ref. 77. Copyright 2019, American Chemical Society. (c) Schematic illustration of PCN-CQ@CCM for PDT induced autophagy inhibition to boost immunotherapy. Reproduced with permission from ref. 17. Copyright 2022, The Royal Society of Chemistry. | ||
The immunosuppressive TME substantially diminishes both the immune responses and the effectiveness of PTT based on endogenous neoantigens.71 Since TME modulation has some potential to transform the “cold” tumours to “hot” ones, Liu et al. constructed a multi-responsive immune adjuvant nanoparticle R837@MSN-mannose-AuNPs-Glu/Lys (RMmAGL) for tumour-specific PTT.22 Such NPs polarized macrophages from an immunosuppressive to an inflammatory phenotype, and thereby effectively boosted the efficacy of anti-tumour therapy. Besides, RMmAGL responded simultaneously to pH, enzymes and NIR laser for the treatment of metastatic malignancies (Fig. 12a).22 The innate immune clearance mechanisms in vivo swiftly removed endogenous tumour neoantigens released from apoptotic tumour cells, resulting in the ineffective activation of anti-tumour immune responses and the inability to induce abscopal effects. Recently, a variety of nanocarriers with antigen-capturing functions have been constructed based on chemically grafted protein-capturing moieties, including amine, catechol and maleimide.119,122,123 These nanocarriers not only extended the retention period of endogenous tumour neoantigens at the tumour site but also delivered tumour antigens to DCs, eliciting robust immune responses. Typically, Li et al. constructed a Mn-ONc-A-malF127 coordination nanovaccine for complete eradication of the primary and distant tumours.95 Mn-ONc-A-malF127 nanomicelles significantly increased the secretion of IFN-β and TNF-α in BMDCs. Notably, Mn-ONc-A-malF127 nanomicelles can co-deliver Mn2+ and ABZI, thereby effectively activating the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (cGAS-STING) pathway (Fig. 12b). In another example, Li et al. constructed a multifunctional orthotopic nanovaccine (1-MT@OMV-Mal) with neoantigen-capturing and immunomodulatory capabilities to boost immune-mediated tumour elimination after PTT (Fig. 12c).108 1-Methyltryptophan (1-MT) could effectively suppress IDO-mediated transformation of tryptophan (Trp) to kynurenine (Kyn), and significantly reduce the proportion of tumour-infiltrating CD3+/CD4+/Foxp3+ Tregs in CT26-luc tumour-bearing mice, thereby inhibiting the Tregs-mediated immunosuppressive microenvironment (Fig. 12c).
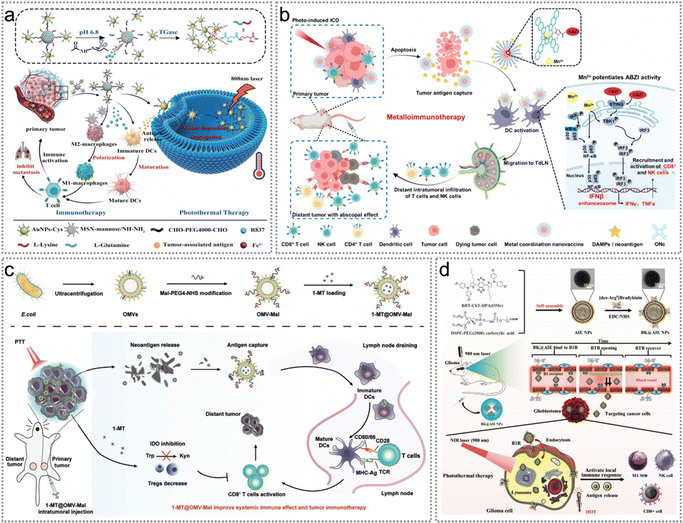 | ||
| Fig. 12 Endogenous neoantigen-based photothermal therapy. (a) Schematic illustration of the treatment procedure of pH–enzyme–NIR multi-stimuli responsive immune-adjuvant NPs (R837@MSN-mannose-AuNPs-Glu/Lys, RMmAGL), which combines tumour-specific PTT and photothermal-assisted immunotherapy. Reproduced with permission from ref. 22. Copyright 2023, Wiley. (b) Schematic of manganese-coordinated micelles for PTT-mediated ICD, tumour antigen capture, and improved cGAS-STING pathway stimulation for the eradication of primary and distant tumours. Reproduced with permission from ref. 95. Copyright 2022, American Chemical Society. (c) Illustration of construction of 1-MT@OMV-Mal as an orthotopic vaccine for PTT induced neoantigen release. Reproduced with permission from ref. 108. Copyright 2022, Wiley. (d) Schematic diagram of constructing BK@AIE NPs for PTT and topical immune-response stimulation. Reproduced with permission from ref. 96. Copyright 2021, Wiley. | ||
Second near-infrared (NIR-II) PTT, featuring reduced photon scattering and deeper penetration depth, can induce ICD more effectively against malignant solid tumours.124,125 NIR-II photothermal agents based on various organic (e.g., small molecules, lanthanide compounds, conjugated polymers) and inorganic materials (e.g., transition metals, quantum dots) have been extensively developed.121,126–132 NIR-II AIE luminogens (AIEgens) stand out as exemplary organic photothermal agents as they display amplified fluorescence, remarkable fluorescence stability, improved tissue penetration, and efficient hyperthermia generation when exposed to NIR-II light irradiation.133 Benefiting from these, Zhang et al. synthesized photostable and strongly absorbing bradykinin (BK) AIE nanoparticles (BK@AIE NPs) in the NIR-II window for accurate photothermal elimination of deep-seated tumour and activation of topical immune response (Fig. 12d).96 Under NIR-II laser irradiation, BK@AIE NPs boosted endogenous tumour neoantigen release and reversed the immunosuppressive TME by improving the proportion of T cells (CD3+, CD4+ and CD8+ T), M1 MΦ and NK cells, thereby enhancing the therapeutic effect (Fig. 12d).
Similarly, exogenous neoantigens can be co-delivered with the photothermal agents as well. Indocyanine green (ICG), a near-infrared (NIR) dye, holds great promise for application in both PDT and PTT due to its favorable optical properties.134 Since ICG is the sole NIR agent approved by the United States Food and Drug Administration (FDA) for clinical use,135 Pan et al. constructed an ovalbumin-indocyanine green (OVA-ICG) multifunctional nanovaccine for tumour photothermal-immunotherapy (Fig. 13a).136 OVA-ICG effectively elevated tumour temperature to promote tumour elimination due to the synergistic effect of exogenous neoantigen immunotherapy and PTT. OVA-ICG treated mice exhibited significant increases in both the amount of CD8+ cytotoxic T cells in the tumour and the concentration of specific anti-OVA-immunoglobulin G (IgG) in serum, revealing excellent anti-tumour immunotherapeutic efficacy.136 To achieve better photothermal therapeutic efficacy, Li et al. constructed a mesoporous polydopamine-R848@ cancer cell membrane (MPDA-R848@CM) nanovaccine for tumour therapy and preventing postoperative tumour relapse (Fig. 13b).97 The combination of MPDA-R848@CM and photothermal therapy (PTT) significantly amplified the maturation of BMDCs, compared to MPDA-R848@CM alone. This enhancement can be attributed to the release of endogenous tumour neoantigens from apoptotic tumour cells and the abundant supply of R848 from MPDA-R848@CM nanoparticles.97
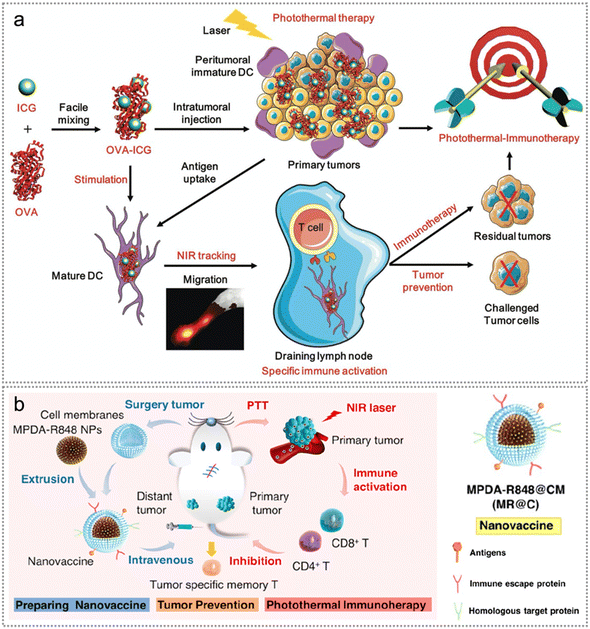 | ||
| Fig. 13 Neoantigen-based photothermal therapy. (a) Illustration of the manufacturing and elucidation of the OVA–ICG nanovaccine for anti-tumour photothermal-immunotherapy, DC maturation/tracking, and tumour prevention. Reproduced with permission from ref. 136. Copyright 2018, Wiley. (b) Illustration of MR@C NPs nanovaccine for tumour therapy and prevention of postoperative tumour relapse. Reproduced with permission from ref. 97. Copyright 2022, American Chemical Society. | ||
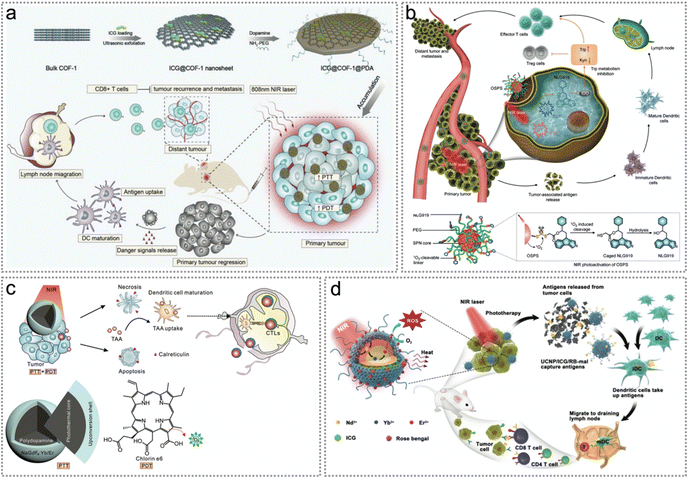 | ||
| Fig. 14 Neoantigen-based synergistic photodynamic/photothermal therapy. (a) Schematic illustration of the fabrication procedure and photoimmunotherapy activity of ICG@COF-1@PDA nanosheets. Reproduced with permission from ref. 98. Copyright 2019, Wiley. (b) The structure of OSPS and its NIR photoactivation mechanism. Photoactivation of OSPS produces synergistic therapeutic effects, including phototherapy and checkpoint blockade immunotherapy. Reproduced with permission from ref. 99. Copyright 2019, Wiley. (c) Illustration of the design of core–shell particles for synergistic phototherapy to release tumour neoantigens, trigger the maturation of DCs and activate CTLs and memory T cells. Reproduced with permission from ref. 100. Copyright 2019, Wiley. (d) Synthesis of the NIR-triggered antigen-harvesting nanoplatform to in situ capture and reserve endogenous neoantigens by reactions with maleimide for synergistic photo-immunotherapy. Reproduced with permission from ref. 25. Copyright 2019, Wiley. | ||
Apart from organic semiconducting compounds, upconversion nanoparticles (UCNPs) benefit from deeper tissue penetration, better optical stability and lower autofluorescence background.141,142 Yan et al. constructed polydopamine@NaGdF4:Yb/Er-polyethylene glycol/chlorin e6 (PDA@UCNP-PEG/Ce6) NPs for boosting primary tumour ablation and antitumour immunity (Fig. 14c and d).100 Under 980 nm laser irradiation, PDT/PTT promoted the ablation of primary tumours and increased the exposure of CRT on the cell surface. Tumour neoantigens were released during synergistic phototherapy, triggering the maturation of DCs and activating CTLs and memory T cells, therefore suppressing tumour recurrence and metastasis (Fig. 14c). To achieve better PDT/PTT efficacy, Wang et al. developed a near-infrared light triggered antigen-capture nanoplatform NaYF4:Yb/Er@NaYF4:Nd/ICG/rose bengal-maleimide (UCNP/ICG/RB-mal) for synergistic photoimmunotherapy (Fig. 14d).25 As a NIR fluorescent dye, ICG improved the upconversion luminescence for PTT while RB was used to induce PDT. Importantly, due to the antigen-trapping ability of maleimide, endogenous neoantigens could be trapped and reserved in situ. Overall, the synergistic PTT/PDT of light-activated UCNP/ICG/RB-mal can induce immune responses for tumour-specific immunotherapy.25
4.2 Neoantigen-based radiotherapy
Radiotherapy (RT), an important strategy in clinical anticancer treatment, is used in more than 50% of all patients with cancer.6,143–145 Radiosensitizers are chemical substances or agents that can improve the killing effect on cancer cells by expediting DNA structure destruction and indirectly generating free radicals. Several studies have reported the synthesis of efficient radiosensitizers with low toxicity.144 RT-triggered DNA structure destruction and ROS production promote tumour cell necrosis and apoptosis, thereby releasing tumour endogenous neoantigens.146–148 Similar to that in phototherapy, the captured endogenous neoantigens can be delivered to APCs, thereby promoting DC maturation and triggering a powerful immune reaction to enhance the efficacy of RT.106 This section will discuss various applications of radiosensitizers to enable neoantigen-based precision tumour RT.Similar to PDT, RT also can promote tumour cell apoptosis and release tumour endogenous neoantigens, which can serve as an in situ cancer vaccine.147 Serving as natural carriers, bacteria possess notable advantages in tumor-targeted delivery and in eliciting immune responses.149 Antigen-capturing (AC) bacteria were prepared by coating Salmonella (VNP20009) with polyamidoamine dendrimer NPs by Wang et al. to capture neoantigens and deliver them to the DCs in tumour. Notably, neoantigens were captured by positively charged AC bacteria through ionic interactions and spontaneously moved out of the tumour core to activate normal DCs in the surrounding tumour margin tissues, reversing the immunosuppressive microenvironment and promoting the efficacy of radiotherapy (Fig. 15a).106 Intratumoral injection of AC bacteria (B+) after RT significantly suppressed the proliferation of primary tumours and secondary tumours, and effectively enhanced the local therapeutic effect of radiotherapy. Similarly, Pei et al. constructed an inactivated and attenuated Salmonella (VNP20009) vector with 131I labeling (131I-VNP).107 Remarkably, DNA fragments generated by internal radioisotope therapy (IRT) and bacteria further activated the cGAS-STING pathway, initiating innate immune assisted anti-tumour immune response. Besides, ICD co-induced by IRT and bacteria significantly facilitated the release and cross-presentation of tumour endogenous neoantigens.
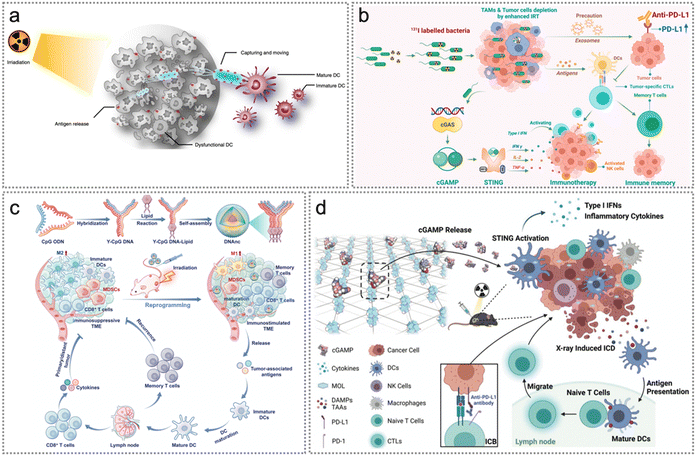 | ||
| Fig. 15 Neoantigen-based radiotherapy. (a) Illustration of radiotherapy induced neoantigen capture and delivery to APCs in tumour. Reproduced with permission from ref. 106. Copyright 2022, Springer Nature Ltd. (b) The mechanism of inactivated bacteria boosting multiple anti-tumour immune responses in radioimmunotherapy. Reproduced with permission from ref. 107. Copyright 2022, American Chemical Society. (c) Illustration of DNAnc self-assembly and the antitumour mechanism of DNAnc. Radiotherapy repolarizes M2-like macrophages into M1-like ones and inhibits the proliferation and migration of the residual tumour cells. Reproduced with permission from ref. 94. Copyright 2023, Wiley. (d) Summary of the mechanism of synergistic radiosensitization and immune activation of cGAMP/MOL. Reproduced with permission from ref. 101. Copyright 2022, Wiley. | ||
Excessive accumulation of tumour-associated macrophages (TAMs) in irradiated neoplasms will occur during radiotherapy. Nonetheless, excessive TAMs promote the restoration of blood flow in irradiated tumours, ultimately leading to tumor recurrence.150,151 CpG oligonucleotides (CpG ODNs), effective immunostimulatory elements, are naturally able to polarize TAMs and enhance immunoreactions to effectively treat cancer.152,153 Encouraged by this, Xie et al. constructed a programmable CpG DNA nanocluster (DNAnc) which was self-assembled from Y-shaped double-stranded DNA vectors loaded with CpG-ODNs under special complementary base pairing rules (Fig. 15c).94 DNAnc reversed the TME and induced tumour cell ICD to release abundant endogenous tumour neoantigens, thus improving long-term anti-tumour immunity. Notably, RT + DNAnc completely suppressed the recurrence of newly inoculated CT26 tumours.94 Additionally, Luo et al. constructed a two-dimensional nanoplatform cGAMP/MOL based on nanoscale metal organic layers (MOLs) to maximize the surface area in contact with tumours during the distinct radiotherapy–radiodynamic therapy (RT–RDT) process.101 cGAMP/MOL achieved effective radiosensitization during radiotherapy, while cGAMP (STING agonist) promoted STING pathway activation.101 cGAMP/MOL plus 2 Gy X-ray therapy triggered apoptosis of murine colon cancer MC38 cells while inducing rapid secretion of type-I interferons and inflammatory cytokines containing IFN-β, interleukin 6 (IL-6) and TNF-α. Overall, cGAMP/MOL served as a nanovehicle of STING agonists and a powerful radiosensitizer in tumour stroma (Fig. 15d).101
4.3 Neoantigen-based chemotherapy/chemodynamic therapy (CDT)
Chemotherapy (CT) has been employed as the standard approach in cancer therapy for a long time154 while chemodynamic therapy (CDT) is an emerging therapeutic approach based on Fenton or Fenton-like reactions.155 Similar to photo/radiotherapeutic agents, both chemo- and chemodynamic therapeutic drugs can trigger ICD of tumour cells, thereby releasing tumour endogenous neoantigens and DAMPs.155–159 Besides, the combination of exogenous neoantigens (including synthetic neoantigen peptides, proteins and the tumour cell membrane) with CT or CDT facilitates immune escape, promotes intratumoral accumulation and enhances anti-tumour immune responses.30,71,84,160–163 In this part, we will discuss various strategies for developing therapeutic agents to enable exo/endogenous neoantigen-based cancer CT/CDT.Malignant solid tumours with low immunogenicity continue to exhibit resistance to existing immunotherapies. Although conventional chemotherapy can induce ICD, the anti-tumour effect is not satisfactory in malignant solid tumours.78,164 To improve the therapeutic efficacy, Wang et al. developed a GM-CSF/Dox-iRGD/CpG gel (a macroporous alginate gel) loaded with the doxorubicin-iRGD (CRGDKGPDC, a nine-AA cyclic peptide) conjugate, granulocyte macrophage colony-stimulating factor (GM-CSF) and CpG oligonucleotides for in situ chemoimmunotherapy against poorly immunogenic tumours.102 Excitingly, the GM-CSF/Dox-iRGD/CpG gel promoted ICD to facilitate endogenous tumour neoantigen release, enhanced DC recruitment and activation and repolarized TAMs into the pro-inflammatory M1 phenotype, thus boosting anti-tumour immune responses (Fig. 16a).
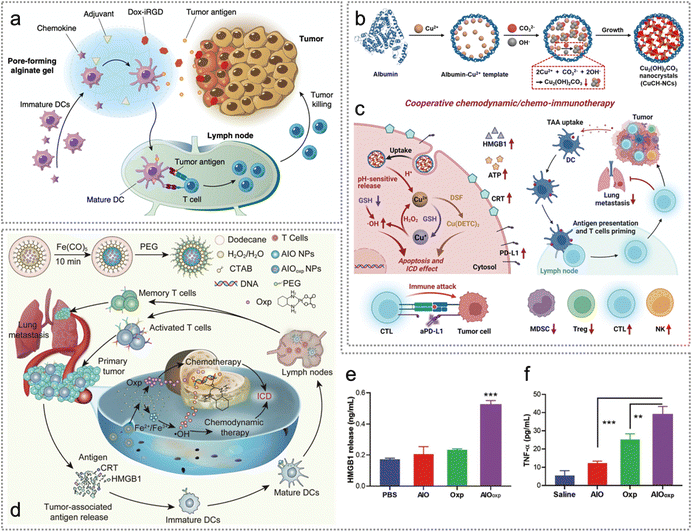 | ||
| Fig. 16 Neoantigen-based chemo-/chemodynamic therapy. (a) Schematic illustration of cancer vaccine consisting of Dox-iRGD, adjuvants and chemokines for chemo-immunotherapy. Reproduced with permission from ref. 102. Copyright 2020, Springer Nature Ltd. (b) Synthetic procedure of CuCH-NCs. (c) In vivo synergistic chemodynamic/chemoimmunotherapy of CuCH-NCs combined with DSF and anti-PD-L1. Reproduced with permission from ref. 103. Copyright 2022, Wiley. (d) Schematic representation of AIOoxp NPs as orthotopic cancer vaccines for chemo/chemodynamic therapy-mediated ICD. (e) The concentrations of released HMGB1 from 4T1 cells treated with PBS, AIO, Oxp and AIOoxp. (f) The secretion levels of TNF-α by splenocytes from tumour-bearing mice treated with saline, AIO, Oxp and AIOoxp. Reproduced with permission from ref. 104. Copyright 2021, The Royal Society of Chemistry. | ||
Nanozymes are nanomaterials with inherent enzyme-like catalytic activity.165 Based on the catalytic mechanism of nanozymes, they can be divided into hydrolase nanozymes, peroxidase nanozymes, oxidase nanozymes, and superoxide dismutase nanozymes.165 Nanozymes have the advantages of high catalytic stability, low manufacturing cost and easy modification.165,166 Since the catalytic capability of nanozymes can be modulated by different microenvironments,165,167,168 Li et al. constructed a pH-activatable zymogen, namely, biomineralized copper(II) carbonate hydroxide nanocrystals (CuCH-NCs), for tumour-selective chemodynamic/chemoimmunotherapy against triple-negative breast cancer (TNBC) (Fig. 16b and c).103 As a pH-activatable zymogen, CuCH-NCs triggered the release of Cu2+ in the acidic TME, and Cu2+ was spontaneously reverted to Cu+ by glutathione (GSH) and H2O2 was catalyzed to generate OH˙. In addition, OH˙ induced damage to tumour cells, and stimulated nontoxic disulfiram (DSF) to inhibit cancer cell proliferation and promote apoptosis.103 Apart from Cu(II) based nanomaterials, Fe(II) or Fe(III) based ones can also induce CDT. For example, Ding et al. developed amorphous iron oxide (AIO, Fe2O3)-packaged oxaliplatin (AIOoxp) NPs, which induced dual ICD effectively through the synergistic effect of chemotherapy and chemodynamic therapy.104 Enhanced ICD-induced release of numerous neoantigens and DAMPs (e.g., CRT and HMGB) effectively promotes DC maturation and T cell (CD4+ and CD8+ T) activation (Fig. 16d and e). Furthermore, AIOoxp also significantly boosted the secretion of TNF-α and IL-6 (Fig. 16f).
Tumour heterogeneity and enzymatic degradation significantly reduce the efficacy of neoantigens in clinical tumour therapy.169 To overcome this barrier, Liu et al. constructed a personalized neoantigen nanovaccine (PNVAC) to enhance tumour immunotherapy (Fig. 17a).23 The fluorescence intensity of PNVAC in bone marrow-derived dendritic cells (BMDCs) dropped significantly after incubation with caveolin inhibitor WL-47 and dynamin inhibitor dynasore, indicating that PNVAC uptake mainly depends on caveolin and dynamin (Fig. 17a). Once ingested, the free vaccine was mainly distributed in lysosomes and easily degraded. Part of PNVAC was shown to be located on the surface of Golgi apparatus, and this is responsible for post-translational modification, indicating that nanotechnology is conducive to lysosomal escape and antigen presentation (Fig. 17b). A phase I clinical trial (ChiCTR1800017319) was initiated to evaluate the immunogenicity, safety, and prophylactic effect of PNVAC in preventing tumor recurrence in patients diagnosed with gastric cancer after surgery, including IIIB/IIIC/IVA. It was worth mentioning that PNVAC induced T cell reactions in all 275 patients, with 256 patients experiencing new immune reactions. The immunized antigens elicited T cell responses in all patients and 93.1% of patients generated novel immune responses. Furthermore, 77.8% (214/275) of immunized peptides induced positive T cells because the IFN-γ secretion was double than that of the control group (Fig. 4c). An ex vivo IFN-γ cytometric bead array (CBA) of the immunized neoantigens induced primary responses and 43.5% (81/186) induced novel antigen reactive responses after treatment (Fig. 4d). In some cases, the reactivity of T cells was assessed using in vitro IFN-γ CBA assay. The primary and novel responses to the given neoantigens were found to be 42.7% (38/39) and 42.7% (38/39), respectively (Fig. 4d). This work provides a feasible strategy with excellent biosafety for developing neoantigen vaccines to delay gastric cancer recurrence after surgery.
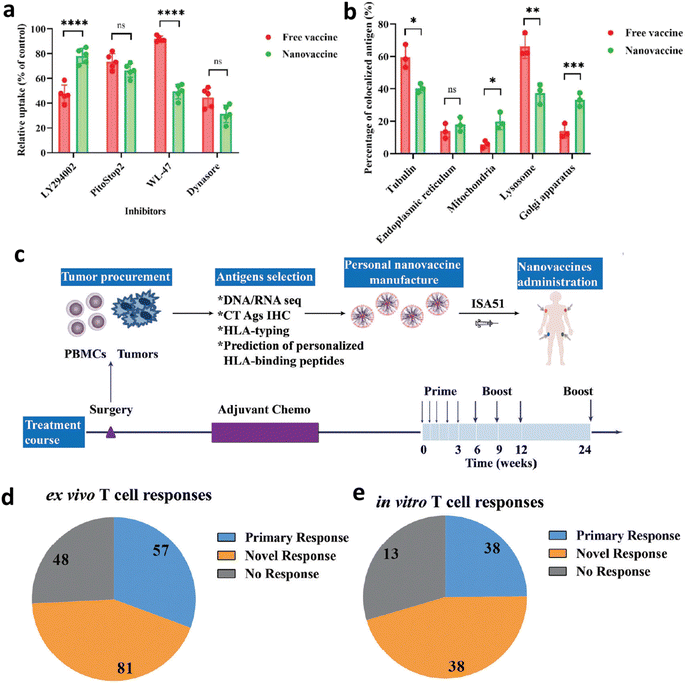 | ||
| Fig. 17 Exogenous neoantigen-based chemotherapy. (a) Quantification of the fluorescence intensity of the cells incubated with four inhibitors (micropinocytosis inhibitor: LY294002, clathrin inhibitor: PitoStop2, caveolin inhibitor: WL-47, dynamin inhibitor: dynasore) before incubation with PNVAC. (b) Quantification of fluorescence intensity of endoplasmic reticulum, mitochondria, lysosomes, or Golgi apparatus after mouse BMDC incubation with the free vaccine or PNVAC by total internal reflection fluorescence microscopy. (c) Procedure of the Phase I clinical trial. First, tumor somatic mutations of gastric cancer were identified using WES and gene expression was confirmed using RNA-seq. Then the cancer-testis antigen (CTA) expression was investigated by immunochemical staining and immunized neoantigens were identified based on HLA binding affinity predictions and manufactured into PNVAC. PNVAC and adjuvant Montanide ISA 51 were subcutaneously injected at four designated sites. (d) Ex vivo and (e) in vitro stimulation. The patients’ immune responses to PNVAC were evaluated by detecting and monitoring IFN-γ released from peripheral blood mononuclear cells (PBMCs) using CBA assay. The IFN-γ expression of mutant peptide-stimulated PBMCs greater than twice the negative control was considered to have positive immune responses. Reproduced with permission from ref. 23. Copyright 2022, Wiley. | ||
To maximize effectiveness after vaccination, Zhao et al. constructed MPO nanovaccines to enhance antitumour immunity by promoting the vaccine cascade and inducing ICD.30 Specifically, MPO nanovaccines promoted exogenous neoantigen delivery to LNs, improved cross-presentation of exogenous neoantigens and lysosomal escape, boosted tumour cell ICD to release endogenous neoantigens as well as repolarized macrophages to an M1 phenotype (Fig. 18a and b). Additionally, MPO effectively repolarized macrophages to an M1 phenotype (CD11b+F4/80+CD86+) with anti-tumour capabilities while reducing the proportion of the M2 phenotype (CD11b+F4/80+CD206+) (Fig. 18c). Overall, MPO effectively inhibited tumour proliferation and enhanced the survival rate, which was attributed to its immunomodulatory effect and enhanced antitumour immunity.30
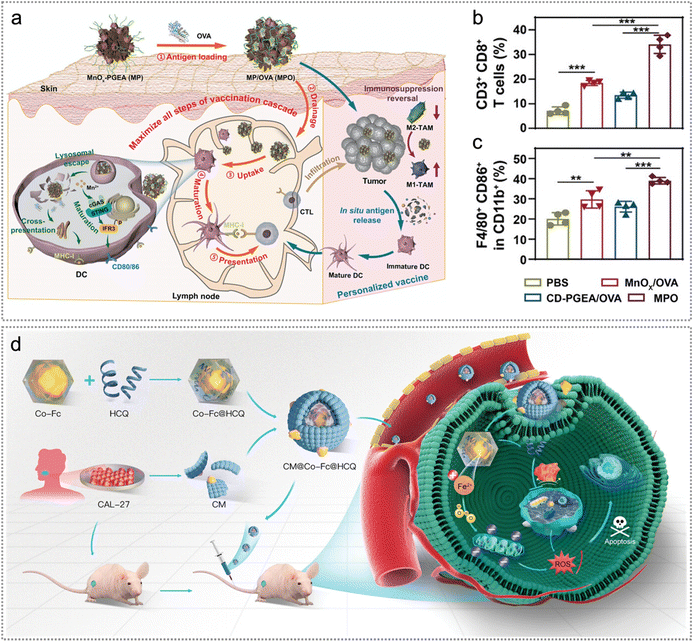 | ||
| Fig. 18 Exo/endogenous neoantigen-based chemotherapy/chemodynamic therapy. (a) Illustration of MPO for promoting exogenous neoantigen delivery to LNs, improving cross-presentation of exogenous neoantigens and lysosomal escape, boosting endogenous neoantigen release as well as repolarizing macrophages to an M1 phenotype. (b) and (c) The quantification of CD8+ T cells (gated on CD3+ T cells) in tumours (b) and M1 macrophages (CD11b+F4/80+CD86+) in tumours (c) on day 25 (n = 4). Reproduced with permission from ref. 30. Copyright 2023, Wiley. (d) Schematic illustration of the preparation procedure and intracellular mechanism of CM@Co-Fc@HCQ NPs. Reproduced with permission from ref. 160. Copyright 2023, Wiley. | ||
Side effects such as systemic chemotherapy and multidrug resistance are the most frequently encountered issues in cancer chemotherapy.170 It remains a huge challenge to improve therapeutic efficacy as well as minimize the adverse effect. Inspired by this, Chen et al. developed cancer cell membranes (CM)@cobalt-ferrocene metal–organic framework (Co-Fc)@hydroxychloroquine (HCQ) (CM@Co-Fc@HCQ) for effective ablation of OSCC (Fig. 18d).160 Notably, CM@Co-Fc@HCQ significantly enhanced tumour-targeting ability, accumulated at tumour locations and facilitated immune escape, thereby boosting therapeutic efficacy and effectively minimizing the adverse effects from systemic chemotherapy. Acting as external neoantigens, CMs derived from CAL-27 cell lines markedly promoted immune evasion, consequently amplifying the accumulation of CM@Co-Fc@HCQ within the tumor (Fig. 18d). Moreover, the enhanced tumour-targeting ability of CM@Co-Fc@HCQ significantly reduced the side effects of systemic chemotherapy.
5. Neoantigen-based other therapies
Besides PDT, PTT, RT, CT and CDT, sonodynamic therapy (SDT) can also promote highly cytotoxic ROS generation to induce ICD of tumour cells, triggered by ultrasound (US).171–174 Due to the fact that SDT can specially stimulate tumour immune reactions, Lu et al. developed MnO2-poly(I:C)@COF nanoparticles for reshaping the TME and activating immune responses to enhance SDT.105 Enhanced SDT significantly amplified the ICD effect and promoted cancer cell death, releasing an abundance of tumour endogenous antigens and other damage-related model molecules. Besides, GSH depletion triggered the production of Mn2+ and the released poly(I:C) further promoted the production of tumour endogenous antigens. Furthermore, tumour endogenous antigens promoted DC maturation and cytokine secretion (Fig. 19a). Such NPs effectively suppressed the proliferation of both primary and distant tumours as well as significantly increased various cytokines’ expression (Fig. 19b–d).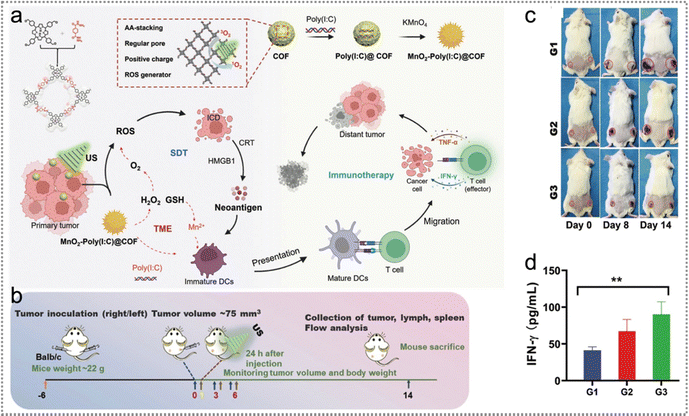 | ||
| Fig. 19 Neoantigen-based sonodynamic therapy. (a) The schematic diagram of the reasonable construction of MnO2-poly(I:C)@COF NPs to boost SDT by reshaping the TME and stimulating immune responses. (b) Schematic diagram of the experimental project of the bilateral model strategy for 4T1 subcutaneous tumours. (c) Representative visual pictures of various therapy groups on days 0, 8, and 14. (d) Cytokine levels of IFN-γ in the blood serum of mice were measured on the final day after multiple therapeutics. Reproduced with permission from ref. 105. Copyright 2022, Wiley. | ||
Tumour proliferation and survival depend on sustenance provided by the host.175 Besides, general tumour cells consume sustenance significantly faster than normal cells.176 Starvation therapy is a non-invasive cancer treatment strategy by effectively interrupting the nutrient supply to tumour cells and starving them to death.176,177 Since the combination of starvation therapy and other therapies (e.g., PDT, PTT, CDT) will further improve the efficacy of tumour therapy,176,177 Chang et al. constructed a multifunctional cascade biological reactor based on hollow mesoporous Cu2MoS4 (CMS) with glucose oxidase (GOx) for starvation photo-/chemodynamic/immuno-therapy of cancer (Fig. 20a).178 PEGylated CMS@GOx elicited a vaccine-like immune reaction by tumour endogenous antigens released from the elimination of the primary tumour. Moreover, PEGylated CMS@Gox (anti-CTLA4) triggered a strong immune response to effectively inhibit tumour metastasis (Fig. 20b).
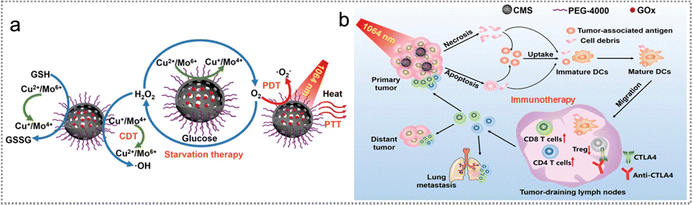 | ||
| Fig. 20 Neoantigen-based starvation therapy. (a) Illustration of PEGylated CMS@GOx for PTT/PDT/CDT/starvation therapy by photogeneration of superoxide radicals, depletion of GSH and Fenton-like reaction. (b) The mechanism of CMS@Gox activated antitumour immune response. The NPs elicited a vaccine-like immune reaction triggered by endogenous antigens released from the primary tumour. Reproduced with permission from ref. 178. Copyright 2019, Wiley. | ||
6. Prospects and challenges
In this tutorial review, we have provided an overview of the origins of neoantigens and their mechanisms as potential cancer therapeutics. Additionally, we have outlined strategies to improve the effectiveness of neoantigen-based treatments, which encompass facilitating neoantigen release, enhancing neoantigen capture, promoting delivery to APCs, protecting them from degradation, prolonging retention time, and increasing the immunogenicity of neoantigens (Table 1). Meanwhile, the complete names and abbreviations are listed in Table 2.| Strategy | Nanoparticles | Therapeutic types | Cell lines | Ref. |
|---|---|---|---|---|
| Facilitating neoantigen release | LIA | PDT | 4T1 | 26 |
| RMmAGL | PTT | LoVo | 22 | |
| BK@AIE NPs | PTT | U87-MG | 96 | |
| ICG@COF-1@PDA | PDT/PTT | 4T1 | 98 | |
| OSPS | PDT/PTT | 4T1 | 99 | |
| PDA@UCNP-PEG/Ce6 | PDT/PTT | 4T1 | 100 | |
| 131I-VNP | RT | CT26 | 107 | |
| cGAM/MOL | RT-RDT | MC38, CT26 | 101 | |
| DNAnc | RT | 4T1, CT26 | 94 | |
| GM-CSF + Dox-iRGD + CpG gel | CT | 4T1 | 102 | |
| CuCH-NCs | CDT/CIT | 4T1, MDA-MB-231, B16F10 | 103 | |
| MnO2-poly(I:C)@COF | SDT | 4T1 | 105 | |
| MPO | CDT | B16-OVA | 30 | |
| AIOoxp | CT/CDT | 4T1 | 104 | |
| MR@C | PTT | 4T1 | 97 | |
| PBA-nChap | PDT | B16F10 | 24 | |
| IERL-Ps | PDT | 4T1 | 21 | |
| Enhancing neoantigen capture | Mal-PEG5000-b-PC7A45 | PDT | CT26, B16F10 | 119 |
| Mn-ONc-A-malF127 | PTT | CT26 | 95 | |
| 1-MT@OMV-Mal | PTT | CT26 | 108 | |
| AC bacteria | RT | CT26, 4T1, B16F10 | 106 | |
| UCNP/ICG/RB-mal | PDT/PTT | 4T1 | 25 | |
| PBA-nChap | PDT | B16F10 | 24 | |
| IERL-Ps | PDT | 4T1 | 21 | |
| Promoting neoantigen delivery to APCs | mD@cSMN | PDT | H22 | 76 |
| AC bacteria | RT | CT26, 4T1, B16F10 | 106 | |
| Mn-ONc-A-malF127 | PTT | CT26 | 95 | |
| F13-PEI/Mem | IT | B16F10, 4T1 | 31 | |
| (ALDH-A1/A3-CpG)-ND | IT | 4T1, D5 | 91 | |
| RGO(CpG)-PEG | IT | MC38, B16F10 | 92 | |
| MPO | CDT | B16-OVA | 30 | |
| MR@C | PTT | 4T1 | 97 | |
| PBA-nChap | PDT | B16F10 | 24 | |
| Protecting neoantigens from degradation | PBA-nChap | PDT | B16F10 | 24 |
| IERL-Ps | PDT | 4T1 | 21 | |
| Prolonging the retention time of neoantigens | LIA | PDT | 4T1 | 26 |
| UCNP/ICG/RB-mal | PDT/PTT | 4T1 | 25 | |
| MPO | CDT | B16-OVA | 30 | |
| Increasing the immunogenicity of neoantigens | AC bacteria | RT | CT26, 4T1, B16F10 | 106 |
| PBA-nChap | PDT | B16F10 | 24 | |
| IERL-Ps | PDT | 4T1 | 21 |
| Full name | Abbreviation | Full name | Abbreviation |
|---|---|---|---|
| Single nucleotide variant | SNV | Interferon gamma | IFN-γ |
| Insertions or deletions | INDEL | Enzyme-linked immunospot assay | ELISPOT |
| Mitochondrial DNA | mtDNA | Single-cell TCR sequencing | scTCR-seq |
| Antigen-presenting cell | APC | Hydroxyl radical | ˙OH |
| Dendritic cell | DC | Superoxide radical | ˙O2 |
| Chlorin e6 | Ce6 | Hydrogen peroxide | H2O2 |
| Light-activatable immunological adjuvant | LIA | Tumour associated antigen | TAA |
| Reactive oxygen species | ROS | Polyethylene glycol | PEG |
| Near-infrared | NIR | 1-Methyltryptophan | 1-MT |
| Hypoxia-responsive amphiphilic dendrimer | HAD | Tryptophan | Trp |
| Upconversion nanoparticle | UCNP | Kynurenine | Kyn |
| Photodynamic therapy | PDT | Bradykinin | BK |
| Photothermal therapy | PTT | Indocyanine green | ICG |
| Covalent organic framework | COF | Polydopamine | PDA |
| Immunogenic cell death | ICD | Organic semiconductor pro-nanostimulator | OSPS |
| Nanodisc | ND | Regulatory T | Treg |
| Major histocompatibility complex | MHC | Rose Bengal | RB |
| Tumour-infiltrating lymphocyte | TIL | Upconversion luminescence | UCL |
| Single-nucleotide alteration | SNA | Radiotherapy | RT |
| Pan-cancer analysis of whole genomes | PCAWG | Antigen-capturing | AC |
| Neuroblastoma RAS viral oncogene homolog | NRAS | Cancer stem cell | CSC |
| Telomerase reverse transcriptase | TERT | Aldehyde dehydrogenase | ALDH |
| Von Hippel-Lindau | VHL | Sonodynamic therapy | SDT |
| Tumour suppressor gene | TSG | Ultrasound | US |
| Gene fusion | GF | Granulocyte macrophage colony-stimulating factor | GM-CSF |
| Head and neck squamous cell carcinoma | HNSCC | Chemodynamic therapy | CDT |
| Amino acid | AA | Personalized neoantigen nanovaccine | PNVAC |
| Whole-exome sequencing | WES | N-Ethyl-hexamethyleneimine | C7A |
| Immune checkpoint blockade | ICB | Oral squamous cell carcinoma | OSCC |
| Tumour microenvironment | TME | Immunoglobulin G | IgG |
| Tumour-specific antigen | TSA | Triple-negative breast cancer | TNBC |
| Human leukocyte antigen | HLA | Amorphous iron oxide | AIO |
| T cell receptor | TCR | Adenosine triphosphate | ATP |
| RNA sequencing | RNA-seq | Hepatitis B virus | HBV |
| Radiodynamic therapy | RDT | Human papillomavirus | HPV |
| Cytotoxic T lymphocyte | CTL | Merkel cell polyomavirus | MCPyV |
| Squamous cell carcinoma | SCC | Merkel cell cancer | MCC |
| Peripheral blood mononuclear cell | PBMC | Oxaliplatin | Oxp |
| Metal organic layer | MOL | High-mobility group box 1 | HMGB1 |
| Photosensitizer | PS | Proton sponge effect | PSE |
| Calreticulin | CRT | Epstein–Barr virus | EBV |
| Interleukin 6 | IL-6 | Hepatitis C virus | HCV |
| Tumour-associated macrophage | TAM | Kaposi's sarcoma-associated herpesvirus | KSHV |
| DNA nanocluster | DNAnc | Human T-cell lymphotropic virus type 1 | HTLV1 |
| Cytosine-phosphate-guanine | CpG | Cyclic GMP-AMP synthase-stimulator | cGAS |
| Oligonucleotide | ODN | Reduced graphene oxide | RGO |
| Doxorubicin | Dox | Chloroquine | CQ |
| Glutathione | GSH | Ovalbumin | OVA |
| Disulfiram | DSF | Food and Drug Administration | FDA |
| Mesoporous polydopamine | MPDA | ||
| 2-Formylphenylboronic acid | PBA | Internal radioisotope therapy | IRT |
| Immune-enhancing polymer-reinforced liposome | IERL | Stimulator of interferon gene | STING |
Neoantigens are able to evade central tolerance, effectively inhibiting tumour progression, metastasis, and relapse in the treatment of diverse cancers. They accomplish this by facilitating the maturation of APCs and eliciting immune responses. ICD can be induced by ROS generated by PDT, RT, CDT, and SDT, the hyperthermia induced by PTT, as well as double-strand DNA breaks caused by RT and CT,179 thereby releasing sufficient tumour neoantigens. Besides, exo/endogenous neoantigens could increase the delivery efficiency of neoantigens to APCs, improve the adsorption of neoantigens by APCs and promote antigen presentation, further inducing the maturation of DCs and effectively activating effective T cells. In addition, the EPR effect of NPs promotes the binding of neoantigens to tumor cells or APCs, prolonging the retention time in the TME and resulting in a long-term immune response.25,26,30,164 The combination of nanomedicine and neoantigens synergistically augmented the therapeutic efficacy because multiple therapeutic modalities could significantly reshape the TME, including suppressing Tregs cell proliferation, promoting CD8+ T cell and NK cell proliferation, repolarizing macrophages from the M2 phenotype to M1 phenotype as well as enhancing the release of cytokines, including IFN-β, IL-2, TNF-α, etc. The reshaping of the TME can accurately and effectively inhibit tumour growth, recurrence and metastasis.180
Although neoantigen-based therapeutics have achieved certain achievements in precision therapy, there are still many challenges in the clinic. First, tumour heterogeneity is a notable characteristic, evident both among different individuals and within the development of the same tumour. Consequently, the accurate identification and selection of neoantigens are pivotal for achieving clinical precision and personalized treatments.27 At present, identification of neoantigens mainly relies on deep sequencing,118 bioinformatics prediction and protein mass spectrometry identification technology,19,181–184 whose duration is relatively longer. In addition, the efficacy of neoantigens varies from each other, necessitating the screening of neoantigens with the highest affinity for HLA. Hence, advanced neoantigen identification technology should be developed to accelerate the clinical translation. Second, although mouse tumour models have been universally employed, the cancer cell lines lack the characteristic of tumour heterogeneity. A majority of the tumours adopted in the research are subcutaneous tumours, which are easy to operate and monitor but cannot mimic the human tumour and TME nicely. Humanized mice are costly, and presently, researchers create tumour models by grafting human carcinoma cell lines or patient-derived tumour tissues into immunodeficient mice. However, notable disparities exist in the immune systems of mice and humans, making it challenging to accurately reflect the immune responses and therapeutic outcomes in humans. Therefore, more rationalized big animal tumour models (e.g., pig, dog, monkey) need to be developed for neoantigen-based therapy. The rapid development of nanotechnology has shown an excellent synergistic effect, including reshaping the TME, promoting the release of neoantigens, enhancing the adsorption of neoantigens by APCs, antigen presentation, etc. However, there are still numerous challenges to overcome before clinical translation. The in vivo biosafety evaluation of most nanomaterials has been evaluated based on animal models and the evaluation cycle is relatively short. In addition, the assessment of biosafety mainly focuses on main organs, without considering the overall body (e.g., brain, skin, eye, skeleton, and muscle). Currently, most of the current research is focused on the innovation of nanomaterials. Although innovative nanomaterials have revealed excellent synergistic effects in neoantigen-based therapeutics, there is still a long way to go before FDA approval and certification. Therefore, it is necessary to simultaneously consider the innovation of nanomaterials and the feasibility of clinical applications.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the National University of Singapore (NUHSRO/2020/133/Startup/08, NUHSRO/2023/008/NUSMed/TCE/LOA, NUHSRO/2021/034/TRP/09/Nanomedicine), National Medical Research Council (CG21APR1005, MOH-001388-00), Singapore Ministry of Education (MOE-000387-00), National Research Foundation (NRF-000352-00), the Open Fund Young Individual Research Grant of Singapore (MOH-001127-01), National Natural Science Foundation of China (Grant No. 52273152), and the Starry Night Science Fund of the Zhejiang University Shanghai Institute for Advanced Study (Grant No. SN-ZJU-SIAS-006).References
- R. L. Siegel, K. D. Miller, N. S. Wagle and A. Jemal, CA-Cancer J. Clin., 2023, 73, 17–48 CrossRef PubMed.
- B. S. Chhikara and K. Parang, Chem. Biol. Lett., 2023, 10, 451 Search PubMed.
- H. Petrowsky, R. Fritsch, M. Guckenberger, M. L. De Oliveira, P. Dutkowski and P. A. Clavien, Nat. Rev. Gastroenterol. Hepatol., 2020, 17, 755–772 CrossRef PubMed.
- N. Behranvand, F. Nasri, R. Zolfaghari Emameh, P. Khani, A. Hosseini, J. Garssen and R. Falak, Cancer Immunol. Immunother., 2022, 71, 507–526 CrossRef PubMed.
- T. Kadosawa and A. Watabe, Vet. J., 2015, 205, 175–179 CrossRef CAS PubMed.
- G. Petroni, L. C. Cantley, L. Santambrogio, S. C. Formenti and L. Galluzzi, Nat. Rev. Clin. Oncol., 2022, 19, 114–131 CrossRef CAS PubMed.
- T. N. Schumacher and R. D. Schreiber, Science, 2015, 348, 69–74 CrossRef CAS PubMed.
- F. Lang, B. Schrors, M. Lower, O. Tureci and U. Sahin, Nat. Rev. Drug Discovery, 2022, 21, 261–282 CrossRef CAS PubMed.
- E. Blass and P. A. Ott, Nat. Rev. Clin. Oncol., 2021, 18, 215–229 CrossRef PubMed.
- E. A. Schon, S. DiMauro and M. Hirano, Nat. Rev. Genet., 2012, 13, 878–890 CrossRef CAS PubMed.
- N. McGranahan, A. J. Furness, R. Rosenthal, S. Ramskov, R. Lyngaa, S. K. Saini, M. Jamal-Hanjani, G. A. Wilson, N. J. Birkbak and C. T. Hiley, Science, 2016, 351, 1463–1469 CrossRef CAS PubMed.
- M. Yarchoan, B. A. Johnson, 3rd, E. R. Lutz, D. A. Laheru and E. M. Jaffee, Nat. Rev. Cancer, 2017, 17, 209–222 CrossRef CAS PubMed.
- M. Peng, Y. Mo, Y. Wang, P. Wu, Y. Zhang, F. Xiong, C. Guo, X. Wu, Y. Li, X. Li, G. Li, W. Xiong and Z. Zeng, Mol. Cancer, 2019, 18, 128 CrossRef PubMed.
- M. C. Sellars, C. J. Wu and E. F. Fritsch, Cell, 2022, 185, 2770–2788 CrossRef CAS PubMed.
- C. S. Shemesh, J. C. Hsu, I. Hosseini, B. Q. Shen, A. Rotte, P. Twomey, S. Girish and B. Wu, Mol. Ther., 2021, 29, 555–570 CrossRef CAS PubMed.
- P. G. Coulie, B. J. Van den Eynde, P. van der Bruggen and T. Boon, Nat. Rev. Cancer, 2014, 14, 135–146 CrossRef CAS PubMed.
- H. Dai, H. Yan, F. Dong, L. Zhang, N. Du, L. Sun, N. Li, G. Yu, Z. Yang, Y. Wang and M. Huang, Biomater. Sci., 2022, 10, 1456–1469 RSC.
- I. Dagogo-Jack and A. T. Shaw, Nat. Rev. Clin. Oncol., 2018, 15, 81–94 CrossRef CAS PubMed.
- V. Leko and S. A. Rosenberg, Cancer Cell, 2020, 38, 454–472 CrossRef CAS PubMed.
- L. Scheetz, K. S. Park, Q. Li, P. R. Lowenstein, M. G. Castro, A. Schwendeman and J. J. Moon, Nat. Biomed. Eng., 2019, 3, 768–782 CrossRef CAS PubMed.
- Y. Zhao, Z. Chen, Q. Li, X. Cao, Q. Huang, L. Shi and Y. Liu, Adv. Funct. Mater., 2022, 32, 2209711 CrossRef CAS.
- T. Liu, M. Zhu, X. Chang, X. Tang, P. Yuan, R. Tian, Z. Zhu, Y. Zhang and X. Chen, Adv. Mater., 2023, e2300086 CrossRef PubMed.
- Q. Liu, Y. Chu, J. Shao, H. Qian, J. Yang, H. Sha, L. Cen, M. Tian, Q. Xu, F. Chen, Y. Yang, W. Wang, K. Wang, L. Yu, J. Wei and B. Liu, Adv. Sci., 2022, 10, e2203298 CrossRef PubMed.
- X. Li, Y. Zhang, X. Wu, J. Chen, M. Yang, F. Ma and L. Shi, Small, 2022, 18, e2203100 CrossRef PubMed.
- M. Wang, J. Song, F. Zhou, A. R. Hoover, C. Murray, B. Zhou, L. Wang, J. Qu and W. R. Chen, Adv. Sci., 2019, 6, 1802157 CrossRef PubMed.
- Y. Wang, N. Gong, C. Ma, Y. Zhang, H. Tan, G. Qing, J. Zhang, Y. Wang, J. Wang, S. Chen, X. Li, Q. Ni, Y. Yuan, Y. Gan, J. Chen, F. Li, J. Zhang, C. Ou, Y. Zhao, X. Liu and X. J. Liang, Nat. Commun., 2021, 12, 4964 CrossRef CAS PubMed.
- M. J. Lin, J. Svensson-Arvelund, G. S. Lubitz, A. Marabelle, I. Melero, B. D. Brown and J. D. Brody, Nat. Cancer, 2022, 3, 911–926 CrossRef CAS PubMed.
- S. Shang, Y. Zhao, K. Qian, Y. Qin, X. Zhang, T. Li, L. Shan, M. Wei, J. Xi and B. Tang, Biomed. Pharmacother., 2022, 151, 113118 CrossRef CAS PubMed.
- N. Xie, G. Shen, W. Gao, Z. Huang, C. Huang and L. Fu, Signal Transduction Targeted Ther., 2023, 8, 9 CrossRef CAS PubMed.
- X. Zhao, J. Zhang, B. Chen, X. Ding, N. Zhao and F. J. Xu, Small Methods, 2023, 7, e2201595 CrossRef PubMed.
- J. Xu, J. Lv, Q. Zhuang, Z. Yang, Z. Cao, L. Xu, P. Pei, C. Wang, H. Wu, Z. Dong, Y. Chao, C. Wang, K. Yang, R. Peng, Y. Cheng and Z. Liu, Nat. Nanotechnol., 2020, 15, 1043–1052 CrossRef CAS PubMed.
- A. C. Huang and R. Zappasodi, Nat. Immunol., 2022, 23, 660–670 CrossRef CAS PubMed.
- W. Ma, B. Pham and T. Li, Clin. Exp. Metastasis, 2022, 39, 51–60 CrossRef PubMed.
- J. J. Gartner, M. R. Parkhurst, A. Gros, E. Tran, M. S. Jafferji, A. Copeland, K. I. Hanada, N. Zacharakis, A. Lalani, S. Krishna, A. Sachs, T. D. Prickett, Y. F. Li, M. Florentin, S. Kivitz, S. C. Chatmon, S. A. Rosenberg and P. F. Robbins, Nat. Cancer, 2021, 2, 563–574 CrossRef CAS PubMed.
- C. C. Smith, S. R. Selitsky, S. Chai, P. M. Armistead, B. G. Vincent and J. S. Serody, Nat. Rev. Cancer, 2019, 19, 465–478 CrossRef CAS PubMed.
- A. H. Capietto, R. Hoshyar and L. Delamarre, Int. J. Mol. Sci., 2022, 23, 10131 CrossRef CAS PubMed.
- J. B. Stewart and P. F. Chinnery, Nat. Rev. Genet., 2021, 22, 106–118 CrossRef CAS PubMed.
- A. Mauriello, R. Zeuli, B. Cavalluzzo, A. Petrizzo, M. L. Tornesello, F. M. Buonaguro, M. Ceccarelli, M. Tagliamonte and L. Buonaguro, Cancers, 2019, 11, 1824 CrossRef CAS PubMed.
- H. Liang, Y. Xu, M. Chen, J. Zhao, W. Zhong, X. Liu, X. Gao, S. Li, J. Li, C. Guo, H. Jia and M. Wang, Front. Immunol., 2021, 12, 749461 CrossRef CAS PubMed.
- Y. K. Bozhilov, D. J. Downes, J. Telenius, A. Marieke Oudelaar, E. N. Olivier, J. C. Mountford, J. R. Hughes, R. J. Gibbons and D. R. Higgs, Nat. Commun., 2021, 12, 3806 CrossRef CAS PubMed.
- K. Zhang, R. Deng, H. Gao, X. Teng and J. Li, Chem. Soc. Rev., 2020, 49, 1932–1954 RSC.
- M. A. Sherman, A. U. Yaari, O. Priebe, F. Dietlein, P. R. Loh and B. Berger, Nat. Biotechnol., 2022, 40, 1634–1643 CrossRef CAS PubMed.
- H. Rahi, M. C. Olave, K. J. Fritchie, P. T. Greipp, K. C. Halling, B. R. Kipp and R. P. Graham, Genes Chromosomes Cancer, 2022, 61, 285–297 CrossRef CAS PubMed.
- W. Yang, K. W. Lee, R. M. Srivastava, F. Kuo, C. Krishna, D. Chowell, V. Makarov, D. Hoen, M. G. Dalin, L. Wexler, R. Ghossein, N. Katabi, Z. Nadeem, M. A. Cohen, S. K. Tian, N. Robine, K. Arora, H. Geiger, P. Agius, N. Bouvier, K. Huberman, K. Vanness, J. J. Havel, J. S. Sims, R. M. Samstein, R. Mandal, J. Tepe, I. Ganly, A. L. Ho, N. Riaz, R. J. Wong, N. Shukla, T. A. Chan and L. G. T. Morris, Nat. Med., 2019, 25, 767–775 CrossRef CAS PubMed.
- S. M. Foltz, Q. Gao, C. J. Yoon, H. Sun, L. Yao, Y. Li, R. G. Jayasinghe, S. Cao, J. King, D. R. Kohnen, M. A. Fiala, L. Ding and R. Vij, Nat. Commun., 2020, 11, 2666 CrossRef CAS PubMed.
- D. Weber, J. Ibn-Salem, P. Sorn, M. Suchan, C. Holtstrater, U. Lahrmann, I. Vogler, K. Schmoldt, F. Lang, B. Schrors, M. Lower and U. Sahin, Nat. Biotechnol., 2022, 40, 1276–1284 CrossRef CAS PubMed.
- S. K. Loo, M. E. Yates, S. Yang, S. Oesterreich, A. V. Lee and X. S. Wang, Genes Chromosomes Cancer, 2022, 61, 261–273 CrossRef CAS PubMed.
- S. Turajlic, K. Litchfield, H. Xu, R. Rosenthal, N. McGranahan, J. L. Reading, Y. N. S. Wong, A. Rowan, N. Kanu, M. Al Bakir, T. Chambers, R. Salgado, P. Savas, S. Loi, N. J. Birkbak, L. Sansregret, M. Gore, J. Larkin, S. A. Quezada and C. Swanton, Lancet Oncol., 2017, 18, 1009–1021 CrossRef CAS PubMed.
- T. Kwon, J. S. Ra, S. Lee, I. J. Baek, K. W. Khim, E. A. Lee, E. K. Song, D. Otarbayev, W. Jung, Y. H. Park, M. Wie, J. Bae, H. Cheng, J. H. Park, N. Kim, Y. Seo, S. Yun, H. E. Kim, H. E. Moon, S. H. Paek, T. J. Park, Y. U. Park, H. Rhee, J. H. Choi, S. W. Cho and K. Myung, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2103532119 CrossRef CAS PubMed.
- E. Wang and I. Aifantis, Trends Cancer, 2020, 6, 631–644 CrossRef CAS PubMed.
- R. F. Stanley and O. Abdel-Wahab, Nat. Cancer, 2022, 3, 536–546 CrossRef CAS PubMed.
- S. C. Bonnal, I. Lopez-Oreja and J. Valcarcel, Nat. Rev. Clin. Oncol., 2020, 17, 457–474 CrossRef PubMed.
- R. G. Jayasinghe, S. Cao, Q. Gao, M. C. Wendl, N. S. Vo, S. M. Reynolds, Y. Zhao, H. Climente-Gonzalez, S. Chai, F. Wang, R. Varghese, M. Huang, W. W. Liang, M. A. Wyczalkowski, S. Sengupta, Z. Li, S. H. Payne, D. Fenyo, J. H. Miner, M. J. Walter, N. Cancer Genome Atlas Research, B. Vincent, E. Eyras, K. Chen, I. Shmulevich, F. Chen and L. Ding, Cell Rep., 2018, 23, 270–281 e273 CrossRef CAS PubMed.
- S. Matsushima, M. Ajiro, K. Iida, K. Chamoto, T. Honjo and M. Hagiwara, Sci. Trans. Med., 2022, 14, eabn6056 CrossRef CAS PubMed.
- S. X. Lu, E. De Neef, J. D. Thomas, E. Sabio, B. Rousseau, M. Gigoux, D. A. Knorr, B. Greenbaum, Y. Elhanati, S. J. Hogg, A. Chow, A. Ghosh, A. Xie, D. Zamarin, D. Cui, C. Erickson, M. Singer, H. Cho, E. Wang, B. Lu, B. H. Durham, H. Shah, D. Chowell, A. M. Gabel, Y. Shen, J. Liu, J. Jin, M. C. Rhodes, R. E. Taylor, H. Molina, J. D. Wolchok, T. Merghoub, L. A. Diaz, Jr., O. Abdel-Wahab and R. K. Bradley, Cell, 2021, 184, 4032–4047 e4031 CrossRef CAS PubMed.
- J. R. Friedman and J. Nunnari, Nature, 2014, 505, 335–343 CrossRef CAS PubMed.
- S. Anderson, A. T. Bankier, B. G. Barrell, M. H. de Bruijn, A. R. Coulson, J. Drouin, I. C. Eperon, D. P. Nierlich, B. A. Roe and F. Sanger, Nature, 1981, 290, 457–465 CrossRef CAS PubMed.
- Y. Yuan, Y. S. Ju, Y. Kim, J. Li, Y. Wang, C. J. Yoon, Y. Yang, I. Martincorena, C. J. Creighton, J. N. Weinstein, Y. Xu, L. Han, H. L. Kim, H. Nakagawa, K. Park, P. J. Campbell, H. Liang and P. Consortium, Nat. Genet., 2020, 52, 342–352 CrossRef CAS PubMed.
- M. P. Thompson and R. Kurzrock, Clin. Cancer Res., 2004, 10, 803–821 CrossRef CAS PubMed.
- C. Song, J. Lv, Y. Liu, J. G. Chen, Z. Ge, J. Zhu, J. Dai, L. B. Du, C. Yu, Y. Guo, Z. Bian, L. Yang, Y. Chen, Z. Chen, J. Liu, J. Jiang, L. Zhu, X. Zhai, Y. Jiang, H. Ma, G. Jin, H. Shen, L. Li, Z. Hu and G. China Kadoorie Biobank Collaborative, JAMA Netw. Open, 2019, 2, e195718 CrossRef PubMed.
- G. Castello, S. Scala, G. Palmieri, S. A. Curley and F. Izzo, Clin. Immunol., 2010, 134, 237–250 CrossRef CAS PubMed.
- A. Wieland, M. R. Patel, M. A. Cardenas, C. S. Eberhardt, W. H. Hudson, R. C. Obeng, C. C. Griffith, X. Wang, Z. G. Chen, H. T. Kissick, N. F. Saba and R. Ahmed, Nature, 2021, 597, 274–278 CrossRef CAS PubMed.
- M. Eckhardt, W. Zhang, A. M. Gross, J. Von Dollen, J. R. Johnson, K. E. Franks-Skiba, D. L. Swaney, T. L. Johnson, G. M. Jang, P. S. Shah, T. M. Brand, J. Archambault, J. F. Kreisberg, J. R. Grandis, T. Ideker and N. J. Krogan, Cancer Discovery, 2018, 8, 1474–1489 CrossRef CAS PubMed.
- E. A. Mesri, E. Cesarman and C. Boshoff, Nat. Rev. Cancer, 2010, 10, 707–719 CrossRef CAS PubMed.
- N. A. Krump and J. You, Front. Microbiol., 2021, 12, 739695 CrossRef PubMed.
- M. Matsuoka and K. T. Jeang, Nat. Rev. Cancer, 2007, 7, 270–280 CrossRef CAS PubMed.
- A. Morales-Sanchez and E. M. Fuentes-Panana, Viruses, 2014, 6, 4047–4079 CrossRef CAS PubMed.
- C. M. Bollard, S. Gottschalk, V. Torrano, O. Diouf, S. Ku, Y. Hazrat, G. Carrum, C. Ramos, L. Fayad, E. J. Shpall, B. Pro, H. Liu, M. F. Wu, D. Lee, A. M. Sheehan, Y. Zu, A. P. Gee, M. K. Brenner, H. E. Heslop and C. M. Rooney, J. Clin. Oncol., 2014, 32, 798–808 CrossRef CAS PubMed.
- L. M. Draper, M. L. M. Kwong, A. Gros, S. Stevanović, E. Tran, S. Kerkar, M. Raffeld, S. A. Rosenberg and C. S. Hinrichs, Clin. Cancer Res., 2015, 21, 4431–4439 CrossRef CAS PubMed.
- B. Gomez, L. He, Y. C. Tsai, T. Wu, R. P. Viscidi and C.-F. Hung, Cell Biosci., 2013, 3, 1–8 CrossRef PubMed.
- J. Huang, B. Yang, Y. Peng, J. Huang, S. H. D. Wong, L. Bian, K. Zhu, X. Shuai and S. Han, Adv. Funct. Mater., 2021, 31, 2011171 CrossRef CAS.
- Q. Chen, C. Li and Q. Wang, Small Methods, 2023, 7, e2201457 CrossRef PubMed.
- H. Ren, W. Jia, Y. Xie, M. Yu and Y. Chen, Chem. Soc. Rev., 2023, 52, 5172–5254 RSC.
- D. Zhang, Z. Lin, M. Wu, Z. Cai, Y. Zheng, L. He, Z. Li, J. Zhou, L. Sun, G. Chen, Y. Zeng, J. Li, J. Liu, H. Yang and X. Liu, Adv. Sci., 2021, 8, 2003504 CrossRef CAS PubMed.
- P. A. Ott, Z. Hu, D. B. Keskin, S. A. Shukla, J. Sun, D. J. Bozym, W. Zhang, A. Luoma, A. Giobbie-Hurder, L. Peter, C. Chen, O. Olive, T. A. Carter, S. Li, D. J. Lieb, T. Eisenhaure, E. Gjini, J. Stevens, W. J. Lane, I. Javeri, K. Nellaiappan, A. M. Salazar, H. Daley, M. Seaman, E. I. Buchbinder, C. H. Yoon, M. Harden, N. Lennon, S. Gabriel, S. J. Rodig, D. H. Barouch, J. C. Aster, G. Getz, K. Wucherpfennig, D. Neuberg, J. Ritz, E. S. Lander, E. F. Fritsch, N. Hacohen and C. J. Wu, Nature, 2017, 547, 217–221 CrossRef CAS PubMed.
- Y. Wang, Q. Zhao, B. Zhao, Y. Zheng, Q. Zhuang, N. Liao, P. Wang, Z. Cai, D. Zhang, Y. Zeng and X. Liu, Adv. Sci., 2022, 9, e2105631 CrossRef PubMed.
- D. Zhang, Z. Ye, L. Wei, H. Luo and L. Xiao, ACS Appl. Mater. Interfaces, 2019, 11, 39594–39602 CrossRef CAS PubMed.
- M. Saxena, S. H. van der Burg, C. J. M. Melief and N. Bhardwaj, Nat. Rev. Cancer, 2021, 21, 360–378 CrossRef CAS PubMed.
- D. E. Speiser, O. Chijioke, K. Schaeuble and C. Munz, Nat. Cancer, 2023, 4, 317–329 CrossRef CAS PubMed.
- G. Oliveira, K. Stromhaug, N. Cieri, J. B. Iorgulescu, S. Klaeger, J. O. Wolff, S. Rachimi, V. Chea, K. Krause, S. S. Freeman, W. Zhang, S. Li, D. A. Braun, D. Neuberg, S. A. Carr, K. J. Livak, D. T. Frederick, E. F. Fritsch, M. Wind-Rotolo, N. Hacohen, M. Sade-Feldman, C. H. Yoon, D. B. Keskin, P. A. Ott, S. J. Rodig, G. M. Boland and C. J. Wu, Nature, 2022, 605, 532–538 CrossRef CAS PubMed.
- S. K. Subudhi, L. Vence, H. Zhao, J. Blando, S. S. Yadav, Q. Xiong, A. Reuben, A. Aparicio, P. G. Corn and B. F. Chapin, Sci. Trans. Med., 2020, 12, eaaz3577 CrossRef CAS PubMed.
- G. Oliveira, K. Stromhaug, S. Klaeger, T. Kula, D. T. Frederick, P. M. Le, J. Forman, T. Huang, S. Li, W. Zhang, Q. Xu, N. Cieri, K. R. Clauser, S. A. Shukla, D. Neuberg, S. Justesen, G. MacBeath, S. A. Carr, E. F. Fritsch, N. Hacohen, M. Sade-Feldman, K. J. Livak, G. M. Boland, P. A. Ott, D. B. Keskin and C. J. Wu, Nature, 2021, 596, 119–125 CrossRef CAS PubMed.
- C. Puig-Saus, B. Sennino, S. Peng, C. L. Wang, Z. Pan, B. Yuen, B. Purandare, D. An, B. B. Quach, D. Nguyen, H. Xia, S. Jilani, K. Shao, C. McHugh, J. Greer, P. Peabody, S. Nayak, J. Hoover, S. Said, K. Jacoby, O. Dalmas, S. P. Foy, A. Conroy, M. C. Yi, C. Shieh, W. Lu, K. Heeringa, Y. Ma, S. Chizari, M. J. Pilling, M. Ting, R. Tunuguntla, S. Sandoval, R. Moot, T. Hunter, S. Zhao, J. D. Saco, I. Perez-Garcilazo, E. Medina, A. Vega-Crespo, I. Baselga-Carretero, G. Abril-Rodriguez, G. Cherry, D. J. Wong, J. Hundal, B. Chmielowski, D. E. Speiser, M. T. Bethune, X. R. Bao, A. Gros, O. L. Griffith, M. Griffith, J. R. Heath, A. Franzusoff, S. J. Mandl and A. Ribas, Nature, 2023, 615, 697–704 CrossRef CAS PubMed.
- M. M. Awad, R. Govindan, K. N. Balogh, D. R. Spigel, E. B. Garon, M. E. Bushway, A. Poran, J. H. Sheen, V. Kohler, E. Esaulova, J. Srouji, S. Ramesh, R. Vyasamneni, B. Karki, T. E. Sciuto, H. Sethi, J. Z. Dong, M. A. Moles, K. Manson, M. S. Rooney, Z. S. Khondker, M. DeMario, R. B. Gaynor and L. Srinivasan, Cancer Cell, 2022, 40, 1010–1026 e1011 CrossRef CAS PubMed.
- E. Alspach, D. M. Lussier, A. P. Miceli, I. Kizhvatov, M. DuPage, A. M. Luoma, W. Meng, C. F. Lichti, E. Esaulova, A. N. Vomund, D. Runci, J. P. Ward, M. M. Gubin, R. F. V. Medrano, C. D. Arthur, J. M. White, K. C. F. Sheehan, A. Chen, K. W. Wucherpfennig, T. Jacks, E. R. Unanue, M. N. Artyomov and R. D. Schreiber, Nature, 2019, 574, 696–701 CrossRef CAS PubMed.
- S. E. Brightman, A. Becker, R. R. Thota, M. S. Naradikian, L. Chihab, K. S. Zavala, A. L. Ramamoorthy Premlal, R. Q. Griswold, J. S. Dolina, E. E. W. Cohen, A. M. Miller, B. Peters and S. P. Schoenberger, Nat. Immunol., 2023, 24, 1345–1357 CrossRef CAS PubMed.
- J. S. Dolina, J. Lee, S. E. Brightman, S. McArdle, S. M. Hall, R. R. Thota, K. S. Zavala, M. Lanka, A. L. Ramamoorthy Premlal, J. A. Greenbaum, E. E. W. Cohen, B. Peters and S. P. Schoenberger, J. Clin. Invest., 2023, 133, e164258 CrossRef PubMed.
- P. A. Ott, S. Hu-Lieskovan, B. Chmielowski, R. Govindan, A. Naing, N. Bhardwaj, K. Margolin, M. M. Awad, M. D. Hellmann, J. J. Lin, T. Friedlander, M. E. Bushway, K. N. Balogh, T. E. Sciuto, V. Kohler, S. J. Turnbull, R. Besada, R. R. Curran, B. Trapp, J. Scherer, A. Poran, D. Harjanto, D. Barthelme, Y. S. Ting, J. Z. Dong, Y. Ware, Y. Huang, Z. Huang, A. Wanamaker, L. D. Cleary, M. A. Moles, K. Manson, J. Greshock, Z. S. Khondker, E. Fritsch, M. S. Rooney, M. DeMario, R. B. Gaynor and L. Srinivasan, Cell, 2020, 183, 347–362 e324 CrossRef CAS PubMed.
- S. L. Gupta, S. Basu, V. Soni and R. K. Jaiswal, Mol. Biol. Rep., 2022, 49, 9903–9913 CrossRef CAS PubMed.
- R. Kuai, L. J. Ochyl, K. S. Bahjat, A. Schwendeman and J. J. Moon, Nat. Mater., 2017, 16, 489–496 CrossRef CAS PubMed.
- A. Hassani Najafabadi, J. Zhang, M. E. Aikins, Z. I. Najaf Abadi, F. Liao, Y. Qin, E. B. Okeke, L. M. Scheetz, J. Nam, Y. Xu, D. Adams, P. Lester, T. Hetrick, A. Schwendeman, M. S. Wicha, A. E. Chang, Q. Li and J. J. Moon, Nano Lett., 2020, 20, 7783–7792 CrossRef CAS PubMed.
- C. Xu, H. Hong, Y. Lee, K. S. Park, M. Sun, T. Wang, M. E. Aikins, Y. Xu and J. J. Moon, ACS Nano, 2020, 14, 13268–13278 CrossRef CAS PubMed.
- E. Batlle and H. Clevers, Nat. Med., 2017, 23, 1124–1134 CrossRef CAS PubMed.
- Y. Xie, H. Li, L. Xu, H. Zou, X. Wang, X. He, Q. Tang, Y. Zhou, X. Zhao, X. Chen, H. Liu, J. Pu, D. Luo and P. Liu, Adv. Mater., 2023, e2208546 CrossRef PubMed.
- J. Li, H. Ren, Q. Qiu, X. Yang, J. Zhang, C. Zhang, B. Sun, J. F. Lovell and Y. Zhang, ACS Nano, 2022, 16, 16909–16923 CrossRef CAS PubMed.
- M. Zhang, W. Wang, M. Mohammadniaei, T. Zheng, Q. Zhang, J. Ashley, S. Liu, Y. Sun and B. Z. Tang, Adv. Mater., 2021, 33, e2008802 CrossRef PubMed.
- T. Li, G. Chen, Z. Xiao, B. Li, H. Zhong, M. Lin, Y. Cai, J. Huang, X. Xie and X. Shuai, Nano Lett., 2022, 22, 3095–3103 CrossRef CAS PubMed.
- S. Gan, X. Tong, Y. Zhang, J. Wu, Y. Hu and A. Yuan, Adv. Funct. Mater., 2019, 29, 1902757 CrossRef CAS.
- J. Li, D. Cui, J. Huang, S. He, Z. Yang, Y. Zhang, Y. Luo and K. Pu, Angew. Chem., Int. Ed., 2019, 58, 12680–12687 CrossRef CAS PubMed.
- S. Yan, X. Zeng, Y. Tang, B. F. Liu, Y. Wang and X. Liu, Adv. Mater., 2019, 31, e1905825 CrossRef PubMed.
- T. Luo, G. T. Nash, X. Jiang, X. Feng, J. Mao, J. Liu, A. Juloori, A. T. Pearson and W. Lin, Adv. Mater., 2022, 34, e2110588 CrossRef PubMed.
- H. Wang, A. J. Najibi, M. C. Sobral, B. R. Seo, J. Y. Lee, D. Wu, A. W. Li, C. S. Verbeke and D. J. Mooney, Nat. Commun., 2020, 11, 5696 CrossRef CAS PubMed.
- T. Li, Y. Zhang, J. Zhu, F. Zhang, A. Xu, T. Zhou, Y. Li, M. Liu, H. Ke, T. Yang, Y. Tang, J. Tao, L. Miao, Y. Deng and H. Chen, Adv. Mater., 2023, 35, e2210201 CrossRef PubMed.
- B. Ding, P. Zheng, D. Li, M. Wang, F. Jiang, Z. Wang, P. Ma and J. Lin, Nanoscale, 2021, 13, 10906–10915 RSC.
- Z. Lu, S. Bai, Y. Jiang, S. Wu, D. Xu, Y. Chen, Y. Lan, Y. An, J. Mao, X. Liu and G. Liu, Adv. Funct. Mater., 2022, 32 Search PubMed.
- W. Wang, H. Xu, Q. Ye, F. Tao, I. Wheeldon, A. Yuan, Y. Hu and J. Wu, Nat. Biomed. Eng., 2022, 6, 44–53 CrossRef CAS PubMed.
- P. Pei, Y. Zhang, Y. Jiang, W. Shen, H. Chen, S. Yang, Y. Zhang, X. Yi and K. Yang, ACS Nano, 2022, 16, 11325–11337 CrossRef CAS PubMed.
- Y. Li, K. Zhang, Y. Wu, Y. Yue, K. Cheng, Q. Feng, X. Ma, J. Liang, N. Ma, G. Liu, G. Nie, L. Ren and X. Zhao, Small, 2022, 18, e2107461 CrossRef PubMed.
- Z. Xie, T. Fan, J. An, W. Choi, Y. Duo, Y. Ge, B. Zhang, G. Nie, N. Xie, T. Zheng, Y. Chen, H. Zhang and J. S. Kim, Chem. Soc. Rev., 2020, 49, 8065–8087 RSC.
- Q. Chen, M. Chen and Z. Liu, Chem. Soc. Rev., 2019, 48, 5506–5526 RSC.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef PubMed.
- Z. Wang, L. Chen, Y. Ma, X. Li, A. Hu, H. Wang, W. Wang, X. Li, B. Tian and J. Dong, J. Nanobiotechnol., 2021, 19, 243 CrossRef CAS PubMed.
- L. Fang, Z. Zhao, J. Wang, P. Zhang, Y. Ding, Y. Jiang, D. Wang and Y. Li, Sci. Adv., 2020, 6, eaba4024 CrossRef CAS PubMed.
- Y. Sun, Y. Zhang, Y. Gao, P. Wang, G. He, N. T. Blum, J. Lin, Q. Liu, X. Wang and P. Huang, Adv. Mater., 2020, 32, e2004481 CrossRef PubMed.
- V. N. Nguyen, Z. Zhao, B. Z. Tang and J. Yoon, Chem. Soc. Rev., 2022, 51, 3324–3340 RSC.
- B. Ji, M. Wei and B. Yang, Theranostics, 2022, 12, 434–458 CrossRef CAS PubMed.
- D. Kessel and N. L. Oleinick, Photochem. Photobiol., 2018, 94, 213–218 CrossRef CAS PubMed.
- L. Lybaert, S. Lefever, B. Fant, E. Smits, B. De Geest, K. Breckpot, L. Dirix, S. A. Feldman, W. van Criekinge, K. Thielemans, S. H. van der Burg, P. A. Ott and C. Bogaert, Cancer Cell, 2023, 41, 15–40 CrossRef CAS PubMed.
- Z. Luo, T. He, P. Liu, Z. Yi, S. Zhu, X. Liang, E. Kang, C. Gong and X. Liu, Adv. Healthcare Mater., 2021, 10, e2002080 CrossRef PubMed.
- Q. Zheng, X. Liu, Y. Zheng, K. W. K. Yeung, Z. Cui, Y. Liang, Z. Li, S. Zhu, X. Wang and S. Wu, Chem. Soc. Rev., 2021, 50, 5086–5125 RSC.
- C. Xu and K. Pu, Chem. Soc. Rev., 2021, 50, 1111–1137 RSC.
- Y. Min, K. C. Roche, S. Tian, M. J. Eblan, K. P. McKinnon, J. M. Caster, S. Chai, L. E. Herring, L. Zhang, T. Zhang, J. M. DeSimone, J. E. Tepper, B. G. Vincent, J. S. Serody and A. Z. Wang, Nat. Nanotechnol., 2017, 12, 877–882 CrossRef CAS PubMed.
- B. N. Yalamandala, T. M. H. Huynh, M. R. Chiang, W. H. Weng, C. W. Chang, W. H. Chiang and S. H. Hu, Adv. Funct. Mater., 2022, 33, 2210644 CrossRef.
- Y. Ma, Y. Zhang, X. Li, Y. Zhao, M. Li, W. Jiang, X. Tang, J. Dou, L. Lu, F. Wang and Y. Wang, ACS Nano, 2019, 13, 11967–11980 CrossRef CAS PubMed.
- X. Wang, Y. Ma, X. Sheng, Y. Wang and H. Xu, Nano Lett., 2018, 18, 2217–2225 CrossRef CAS PubMed.
- S. Jiang, J. Lin and P. Huang, Adv. Healthcare Mater., 2023, 12, e2202208 CrossRef PubMed.
- Y. Yang, Y. Chen, P. Pei, Y. Fan, S. Wang, H. Zhang, D. Zhao, B. Z. Qian and F. Zhang, Nat. Nanotechnol., 2023, 18, 1195–1204 CrossRef CAS PubMed.
- Y. Chen, S. Wang and F. Zhang, Nat. Rev. Bioeng., 2023, 1, 60–78 CrossRef.
- J. Zou, L. Li, J. Zhu, X. Li, Z. Yang, W. Huang and X. Chen, Adv. Mater., 2021, 33, e2103627 CrossRef PubMed.
- H. Li, Y. Zhong, S. Wang, M. Zha, W. Gu, G. Liu, B. Wang, Z. Yu, Y. Wang, K. Li, Y. Yin, J. Mu and X. Chen, Nano Res., 2022, 16, 2895–2904 CrossRef.
- J. He, S. Hua, D. Zhang, K. Wang, X. Chen and M. Zhou, Adv. Funct. Mater., 2022, 32, 2208028 CrossRef CAS.
- C. Cui, C. Wang, Q. Fu, J. Song, J. Zou, L. Li, J. Zhu, W. Huang, L. Li, Z. Yang and X. Chen, Acta Biomater., 2022, 140, 601–609 CrossRef CAS PubMed.
- W. Xu, D. Wang and B. Z. Tang, Angew. Chem., Int. Ed., 2021, 60, 7476–7487 CrossRef CAS PubMed.
- M. Overchuk, R. A. Weersink, B. C. Wilson and G. Zheng, ACS Nano, 2023, 17, 7979–8003 CrossRef CAS PubMed.
- Z. Hu, C. Fang, B. Li, Z. Zhang, C. Cao, M. Cai, S. Su, X. Sun, X. Shi, C. Li, T. Zhou, Y. Zhang, C. Chi, P. He, X. Xia, Y. Chen, S. S. Gambhir, Z. Cheng and J. Tian, Nat. Biomed. Eng., 2020, 4, 259–271 CrossRef PubMed.
- J. Pan, Y. Wang, C. Zhang, X. Wang, H. Wang, J. Wang, Y. Yuan, X. Wang, X. Zhang, C. Yu, S. K. Sun and X. P. Yan, Adv. Mater., 2018, 30, 1704408 CrossRef PubMed.
- A. Paul, P. N. M. P. Božidar Šarler, A. Narasimhan and S. K. Das, Int. J. Numer. Methods Heat Fluid Flow, 2016, 26, 461–476 CrossRef.
- D. C. M. Vyas, S. Kumar and A. Srivastava, Int. J. Heat Mass Transfer, 2016, 99, 122–140 CrossRef.
- R. Liang, L. Liu, H. He, Z. Chen, Z. Han, Z. Luo, Z. Wu, M. Zheng, Y. Ma and L. Cai, Biomaterials, 2018, 177, 149–160 CrossRef CAS PubMed.
- J. Li and K. Pu, Chem. Soc. Rev., 2019, 48, 38–71 RSC.
- Y. Zhang, X. Zhu and Y. Zhang, ACS Nano, 2021, 15, 3709–3735 CrossRef CAS PubMed.
- L. Cheng, C. Wang and Z. Liu, Nanoscale, 2013, 5, 23–37 RSC.
- A. R. Parikh, A. Szabolcs, J. N. Allen, J. W. Clark, J. Y. Wo, M. Raabe, H. Thel, D. Hoyos, A. Mehta, S. Arshad, D. J. Lieb, L. C. Drapek, L. S. Blaszkowsky, B. J. Giantonio, C. D. Weekes, A. X. Zhu, L. Goyal, R. D. Nipp, J. S. Dubois, E. E. Van Seventer, B. E. Foreman, L. E. Matlack, L. Ly, J. A. Meurer, N. Hacohen, D. P. Ryan, B. Y. Yeap, R. B. Corcoran, B. D. Greenbaum, D. T. Ting and T. S. Hong, Nat. Cancer, 2021, 2, 1124–1135 CrossRef CAS PubMed.
- D. Komorowska, T. Radzik, S. Kalenik and A. Rodacka, Int. J. Mol. Sci., 2022, 23, 10627 CrossRef CAS PubMed.
- Y. Pan, W. Tang, W. Fan, J. Zhang and X. Chen, Chem. Soc. Rev., 2022, 51, 9759–9830 RSC.
- L. Gong, Y. Zhang, C. Liu, M. Zhang and S. Han, Int. J. Nanomed., 2021, 16, 1083–1102 CrossRef PubMed.
- J. Wang, Z. Li, Z. Wang, Y. Yu, D. Li, B. Li and J. Ding, Adv. Funct. Mater., 2020, 30, 1124–1135 Search PubMed.
- J. M. Price, A. Prabhakaran and C. M. L. West, Nat. Rev. Clin. Oncol., 2023, 20, 83–98 CrossRef CAS PubMed.
- S. Zhou, C. Gravekamp, D. Bermudes and K. Liu, Nat. Rev. Cancer, 2018, 18, 727–743 CrossRef CAS PubMed.
- A. Mantovani, F. Marchesi, A. Malesci, L. Laghi and P. Allavena, Nat. Rev. Clin. Oncol., 2017, 14, 399–416 CrossRef CAS PubMed.
- M. J. Pittet, O. Michielin and D. Migliorini, Nat. Rev. Clin. Oncol., 2022, 19, 402–421 CrossRef PubMed.
- X. Tang, C. Mo, Y. Wang, D. Wei and H. Xiao, Immunology, 2013, 138, 93–104 CrossRef CAS PubMed.
- R. Yuan, S. Li, H. Geng, X. Wang, Q. Guan, X. Li, C. Ren and X. Yuan, Int. Immunopharmacol., 2017, 49, 30–37 CrossRef CAS PubMed.
- Brianna and S. H. Lee, Med. Oncol., 2023, 40, 88 CrossRef CAS PubMed.
- Q. Meng, B. Ding, P. Ma and J. Lin, Small Methods, 2023, 7, e2201406 CrossRef PubMed.
- L. Galluzzi, A. Buque, O. Kepp, L. Zitvogel and G. Kroemer, Nat. Rev. Immunol., 2017, 17, 97–111 CrossRef CAS PubMed.
- J. Guo, Y. Zou and L. Huang, Small Methods, 2023, 7, e2201307 CrossRef PubMed.
- P. Zhao, H. Li and W. Bu, Angew. Chem., Int. Ed., 2023, 62, e202210415 CrossRef CAS PubMed.
- L. Galluzzi, J. Humeau, A. Buque, L. Zitvogel and G. Kroemer, Nat. Rev. Clin. Oncol., 2020, 17, 725–741 CrossRef PubMed.
- J. Chen, Z. Zhu, Q. Pan, Y. Bai, M. Yu and Y. Zhou, Adv. Funct. Mater., 2023, 33, 2300235 CrossRef CAS.
- Y. Hong, C. Wang, H. Wang, Y. Gao, J. Wu and J. Xia, J. Chem. Technol. Biotechnol., 2023, 98, 1781–1790 CrossRef CAS.
- Y. Liu, R. Chang, R. Xing and X. Yan, Small Methods, 2023, 7, e2201708 CrossRef PubMed.
- Y. Guo, Y. Fan, Z. Wang, G. Li, M. Zhan, J. Gong, J. P. Majoral, X. Shi and M. Shen, Adv. Mater., 2022, 34, e2206861 CrossRef PubMed.
- J. Nam, S. Son, K. S. Park, W. Zou, L. D. Shea and J. J. Moon, Nat. Rev. Mater., 2019, 4, 398–414 CrossRef.
- D. Jiang, D. Ni, Z. T. Rosenkrans, P. Huang, X. Yan and W. Cai, Chem. Soc. Rev., 2019, 48, 3683–3704 RSC.
- H. Wang, K. Wan and X. Shi, Adv. Mater., 2019, 31, e1805368 CrossRef PubMed.
- W. Zhang, J. Liu, X. Li, Y. Zheng, L. Chen, D. Wang, M. F. Foda, Z. Ma, Y. Zhao and H. Han, ACS Nano, 2021, 15, 19321–19333 CrossRef CAS PubMed.
- N. M. Phan, T. L. Nguyen and J. Kim, Tissue Eng. Regener. Med., 2022, 19, 237–252 CrossRef CAS PubMed.
- C. Peres, A. I. Matos, L. I. F. Moura, R. C. Acurcio, B. Carreira, S. Pozzi, D. Vaskovich-Koubi, R. Kleiner, R. Satchi-Fainaro and H. F. Florindo, Adv. Drug Delivery Rev., 2021, 172, 148–182 CrossRef CAS PubMed.
- A. Parmar, M. Macluskey, N. Mc Goldrick, D. I. Conway, A. M. Glenny, J. E. Clarkson, H. V. Worthington and K. K. Chan, Cochrane Database Syst. Rev., 2021, 12, CD006386 Search PubMed.
- J. Li, Y. Luo and K. Pu, Angew. Chem., Int. Ed., 2021, 60, 12682–12705 CrossRef CAS PubMed.
- S. Son, J. Kim, J. Kim, B. Kim, J. Lee, Y. Kim, M. Li, H. Kang and J. S. Kim, Chem. Soc. Rev., 2022, 51, 8201–8215 RSC.
- D. Wang, L. Lin, T. Li, M. Meng, K. Hao, Z. Guo, J. Chen, H. Tian and X. Chen, Adv. Mater., 2022, 34, e2205924 CrossRef PubMed.
- Y. Wang, F. Gong, Z. Han, H. Lei, Y. Zhou, S. Cheng, X. Yang, T. Wang, L. Wang, N. Yang, Z. Liu and L. Cheng, Angew. Chem., Int. Ed., 2023, 62, e202215467 CrossRef CAS PubMed.
- N. Kanarek, B. Petrova and D. M. Sabatini, Nature, 2020, 579, 507–517 CrossRef CAS PubMed.
- L. H. Fu, C. Qi, Y. R. Hu, J. Lin and P. Huang, Adv. Mater., 2019, 31, e1808325 CrossRef PubMed.
- S. Yu, Z. Chen, X. Zeng, X. Chen and Z. Gu, Theranostics, 2019, 9, 8026–8047 CrossRef CAS PubMed.
- M. Chang, M. Wang, M. Wang, M. Shu, B. Ding, C. Li, M. Pang, S. Cui, Z. Hou and J. Lin, Adv. Mater., 2019, 31, e1905271 CrossRef PubMed.
- M. C. Rodrigues, J. A. V. Morais, R. Ganassin, G. R. T. Oliveira, F. C. Costa, A. A. C. Morais, A. P. Silveira, V. C. M. Silva, J. P. F. Longo and L. A. Muehlmann, Pharmaceutics, 2022, 14, 1564 CrossRef CAS PubMed.
- R. Cheng and H. A. Santos, Adv. Healthcare Mater., 2023, 12, e2202063 CrossRef PubMed.
- R. G. Gupta, F. Li, J. Roszik and G. Lizee, Cancer Discovery, 2021, 11, 1024–1039 CrossRef CAS PubMed.
- Y. Xu, G. H. Su, D. Ma, Y. Xiao, Z. M. Shao and Y. Z. Jiang, Signal Transduction Targeted Ther., 2021, 6, 312 CrossRef PubMed.
- Y. Zhao, A. V. Baldin, O. Isayev, J. Werner, A. A. Zamyatnin, Jr. and A. V. Bazhin, Vaccines, 2021, 9, 85 CrossRef CAS PubMed.
- C. Chong, G. Coukos and M. Bassani-Sternberg, Nat. Biotechnol., 2022, 40, 175–188 CrossRef CAS PubMed.
Footnote |
| † These authors contribute equally. |
| This journal is © The Royal Society of Chemistry 2024 |