Biotemplated heterostructure materials: opportunities for the elaboration of new photocatalysts and selective-oxidation catalysts
Xiaoqian
Ma
a,
Xiaoli
Bai
a,
Xiaohong
Chen
c,
Chunyan
Zhang
a,
Junyang
Leng
a,
Anlong
Zhang
b,
Daomei
Chen
b and
Jiaqiang
Wang
 *ab
*ab
aSchool of Chemical Sciences & Technology, Yunnan University, Kunming, 650091, P. R. China. E-mail: jqwang@ynu.edu.cn; Fax: + 86 871 65031567
bSchool of Materials and Energy, Yunnan University, Kunming 650091, P. R. China
cInstitute of International Rivers and Eco-security, Yunnan University, Kunming, 650091, PR China
First published on 1st November 2023
Abstract
Natural biological materials display a large number of sophisticated nanostructures that are difficult to acquire even using the most technologically advanced synthetic methodologies. Since the hierarchical porosity and structure of a catalyst including photocatalysts are becoming increasingly important in the design of catalysts, the potential of biological components of nanoscale dimension for the synthesis of catalysts with novel types of heterostructure and improved activities is being actively explored. This review summarizes the recent advances in the synthesis of nano/microstructures using biotemplates obtained from various types of plants such as Hydrilla, cyanobacteria, reeds (Phragmites communis), leaves, tobacco, and rubber latex. In addition, the applications of synthesized materials in photocatalytic hydrogen production, photocatalytic reduction of Cr(VI), photodegradation of organic pollutants, and catalytic selective oxidation of tetralin, limonene, and cyclohexane have been highlighted. The key issues to improve the catalytic efficiency during the biotemplating procedure are discussed. More importantly, outlooks towards these emerging synthetic approaches have been presented from the perspective of practical application and future challenges are proposed.
1. Introduction
Generally, the morphology of a catalyst, which determines both surface atomic arrangement and the structural properties of materials, is an important factor affecting the catalytic as well as the photocatalytic performance. Enormous efforts have been devoted to the study of shape-controlled synthesis methods to enhance catalytic performance.1–5 In particular, catalytic selective oxidation is a key basis for the exploration and development of new green and sustainable chemical processes6 since currently about 30% of important chemicals such as alcohols, aldehydes, organic acids, and epoxides are obtained through selective oxidation processes.7,8 For example, nanorod, nanocube, and nano octahedra CeO2 supports were found to exhibit different activities of CeO2-supported catalysts.9 A series of MIL-53(Fe) nanocrystals (MIL-53(Fe)-xH) with different sizes and morphology were also found to be closely related to the performance of the resulting MIL-53(Fe)-xH.10 Three kinds of MCM-41-type silica materials with particles, helices, and spheres exhibit varied catalytic activities.11The helical mesostructures are superior to the other two morphologies in terms of catalytic activity and reusability. Again, the synthesis of a catalyst with optimum morphology is a key step in the development of a more efficient and reusable catalyst.
Photocatalysis has attracted tremendous attention due to its potential in solving environmental and energetic problems by sustainably using inexhaustible solar energy. Similar to conventional thermal catalysts, various morphologies have been observed in nanorods, nanotubes, nanocubes, nanopolyhedra, and nanosheets. These unique morphologies exhibit distinct surface electronic structures, such as band gaps, band edge potentials, and electrical conductivity. Additionally, they possess surface built-in electric fields, reaction centers, and reactant adsorption sites.12 In most cases, improving the light-harvesting capacity and reducing recombination centers through microscopic morphology modification is beneficial. This can be achieved by mediating surface properties such as chemical states, electronic characteristics, and active sites. For instance, researchers have successfully synthesized unique urchin-like hollow titania spheres, which possess a tunable interior structure. This distinctive morphology leads to a significant enhancement in their photocatalytic activity. This improvement can be attributed to the multiple reflections of UV light within the voids of the sphere.2,3,13 Hence, the significance of morphology in determining the surface characteristics of photocatalysts is apparent. Fabricating heterostructure photocatalysts with diverse morphologies is an effective approach to enhance the separation of photogenerated electron–hole pairs, ultimately improving the overall photocatalytic efficiency. Extensive research has been conducted on the preparation of morphology-oriented TiO2-based materials and their subsequent application in photocatalysis.14,15
Although the synthesis of various catalysts with different morphologies has become a hot topic, it has remained challenging to obtain catalysts simultaneously with the desired size and specific morphologies so far. It is of great interest in many fields to tune the morphology of inorganic materials via simple methods, while it is hard to prepare nanostructured materials such as TiO2 with hierarchical morphologies.16 In summary, tailoring the properties of catalysts through engineering their morphologies has proven to be an effective approach. While significant efforts have been made in synthesizing catalysts with various morphologies, such as photocatalysts, the current methods still face inherent drawbacks. These include the need for multi-step procedures or the use of unfriendly reagents, which greatly hinder their potential application. To address this issue, it is crucial to explore simpler and more environmentally friendly approaches in future research for synthesizing catalysts with different morphologies. In contrast, nature serves as an exceptional teacher, inspiring scientists and engineers to seek innovative solutions. It offers a wide range of intricate nanostructures that are challenging to replicate even with the most advanced synthetic techniques. For example, seashells and lotus leaves, composed of ordinary materials, possess remarkable properties due to their unique structures. These intricate designs found in nature have stimulated extensive investigations into the synthesis of materials with precise structures and morphologies, aiming to achieve novel or enhanced catalytic properties.17 At the same time, this kind of material has the advantages of sustainable regeneration and being green. Therefore, nature also provides model architects to design and fabricate nanomaterials by using biological components of nanoscale dimensions.15 Biotemplating is an emerging, unique approach to the preparation and organization of nanostructures into well-defined architectures.18 Unfortunately, limited reviews systematically summarize and critically discuss biotemplated catalytic materials including photocatalysts.
Furthermore, while most reviews and feature articles mainly focus on the synthesis and influence of catalysts, including photocatalysts with controlled morphology, this feature article aims to provide a comprehensive discussion on the fundamental concepts and the latest advancements in morphology control of various photocatalysts as well as selective catalysts prepared through templating methods. Additionally, we will delve into the comparative analysis of biotemplated catalysts, exploring their synthesis methods in detail. Special emphasis will be placed on the current understanding of replicating hierarchical biological materials into catalysts, with a particular focus on algae and plants as templates. The methods to enhance the catalytic including photocatalytic activity, such as self-doping elements from biotemplates, transition metal doping, and microstructural building unit design, are also described. Furthermore, the applications of synthesized materials in photocatalytic hydrogen production, photocatalytic reduction of Cr(VI), photodegradation of organic pollutants, and catalytic selective oxidation of tetralin, limonene, and cyclohexane are briefly presented. In general, biological templates have been used to synthesize only photocatalysts and a few selective oxidation catalysts. Finally, the prospects and challenges of biotemplated catalysts are proposed.
2. Biotemplating
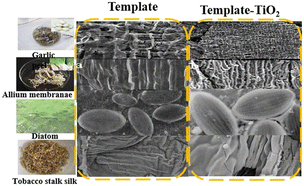
In general, a template is a type of central structure in networking that provides porous sites for material synthesis. Upon removal of the template, which is used as a sacrificial material, targeted materials with well-defined morphologies and hierarchical porous structures can be created, exhibiting a shape and size similar to that of the removed templates. As a result, template methods, including soft templates and hard templates, are often adopted approaches to synthesize three-dimensional nanomaterials with complex structures due to their unique versatility and functionality.19,20 Since the structure of the resulting targeted materials will directly depend on the properties of the template utilized, the template method provides good possibilities for designing the components and structure of templates.21–23 Currently, the components and structure of templates have gained considerable attention and are now recognized as excellent protocols for the synthesis of nanomaterials. These processes have been demonstrated to be superior to template-free methods because of their capability to achieve a high repetition rate and precise control over the morphology, structure, and size of the resulting materials. Templates can be classified into two categories based on their structure: hard templates and soft templates.24–26 Natural biomaterials have a great diversity of unusual structures with well-distributed pores, complex morphologies, and various functionalities, which are potentially good templates for preparing advanced functional materials (Fig. 1).27–29 Recently, the field of biotemplating has emerged as a promising strategy for synthesizing inorganic materials with a hierarchical structure. Unlike conventional template synthesis, biotemplating utilizes biological-based templates, which are not only energy-conserving and environmentally friendly but also readily available at low costs. Moreover, biotemplating offers greener protocols compared to traditional synthetic techniques, since they can be easily obtained via a sustainable biosynthesis process on a large scale, and the preparation can be operated under mild conditions and requires minimal instrumentation.17–20,30Furthermore, biotemplate methods can make maximal use of natural materials including biomass wastes. The special morphology with the desired physical and chemical properties and specific application of inorganic materials would be obtained from biotemplates due to their structural and characteristic features. Among these biotemplates, eggshells,31,32 viruses,33 scallion roots,34 leaves,35 butterfly wings36 and sawdust37 have been explored to fabricate Co3O4 materials with different morphologies.
Overall, a biological template is a new method and way to make special structural materials by using natural biological structures. At present, in addition to TiO2, the biological template method has also been employed to prepare CdS,38 ZnO,39 g-C3N4@C,40 Ag–ZnO (ref. 41) and semiconductor composite materials.42 The synthesis methods of biological templates include hydrothermal method, sol–gel method, solution method, etc.42 Thus, the key of this method is to further improve the charge separation efficiency and enhance the photocatalytic performance by controlling the structure and morphology of the catalyst. As shown in Fig. 2, it is revealed that duplicating and constructing the macrostructural morphology of biotemplates with designed microstructural building units was beneficial to providing increased number of active sites, promoting the separation efficiency of photo-generated electrons and reducing the charge-transfer resistance.43
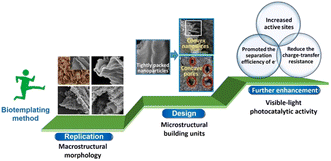 | ||
| Fig. 2 Schematic illustration of the enhanced visible light photocatalytic activity obtained via further processing of the biotemplating method. Copyright 2022, Elsevier. | ||
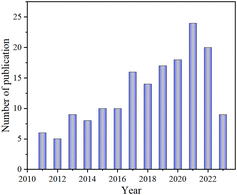
Furthermore, the incorporation of specific elements into the final desired products through the self-doping process of biotemplates imparts unique activity characteristics, attributed to their electron-donating or electron-withdrawing abilities. In catalytic applications, it becomes essential to effectively distribute a fixed amount of materials at specific reaction sites. Therefore, investigating the diffusion properties and surface functional groups of biotemplated materials is crucial.22 The selection of an appropriate template is of utmost importance as it offers valuable insights into the reactivity and transport behavior within the catalysts' pores. We searched the literature within this decade with the keywords “biological template” and “photocatalysis” in the Web of Science (date of search: August 06, 2023); there has been an almost linear increase in the number of publications despite the slight decrease in 2022 (Fig. 3), indicating their potential for future advancements in this field and also that they have received more and more attention.
Biotemplated materials have been used in catalytic selective oxidation since they often exhibited excellent selectivity. However, the available literature on this topic is currently very limited. Up to August 24, 2023, there have been only 24 papers on selective oxidation using biological templates since 2006. This implies that this area is not being paid enough attention. Herein, we will give some examples of biotemplate methods used for the synthesis of catalysts as well as photocatalysts. Then, we will introduce aquatic organisms (algae), reed leaves, tobacco, plant extracts and latex as biological templates for preparation and application.
3. Synthesis and applications of biotemplated photocatalysts
3.1 Aquatic organisms (algae)
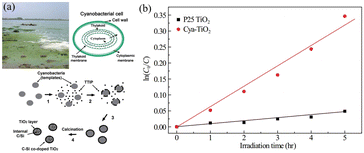
Cyanobacteria, also known as blue-green algae, are considered to be some of the oldest organisms in evolution. They are commonly found in eutrophic water, where they efficiently carry out photosynthesis by harnessing solar light. Taking inspiration from the features of cyanobacteria, particularly Microcystis species, our research group successfully utilized cyanobacteria as a biotemplate to synthesize TiO2 with similar morphology. Despite the fragility of unicellular structures and the high water content in cyanobacterial cells, the resulting TiO2 exhibited a comparable morphology to the original template.44Through this approach, we discovered that self-doping biogenic cyanobacteria, along with the inherited morphology from cyanobacteria, significantly enhanced the visible light absorbance of TiO2. In fact, the cyanobacteria–TiO2 photocatalyst exhibited even higher visible-light photocatalytic activity compared to the commercial titanium dioxide photocatalyst, Degussa P25, in the degradation of rhodamine B (RhB). This finding demonstrates a strong correlation with the pseudo-first-order reaction kinetics, as illustrated in Fig. 4.
The determined reaction rate constant (k) for RhB degradation was found to be 0.06 h−1 for cyanobacteria–TiO2 and 0.01 h−1 for P25. These results exhibit the superior photocatalytic activity of cyanobacteria–TiO2 under visible-light irradiation in comparison to P25. This finding highlights the enhanced performance of cyanobacteria–TiO2 as a photocatalyst. This innovative strategy not only opens up new possibilities for the utilization of cyanobacteria but also offers an environmentally friendly and highly appealing method to reuse this valuable resource.
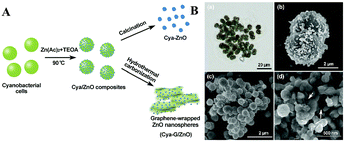
At the beginning of our study, we attempted to utilize cyanobacterial cells as templates for the synthesis of ZnO nanoparticles. Surprisingly, these cells not only served as templates for ZnO growth but also acted as a carbon source for graphene sheets. As a result, we successfully obtained graphene-wrapped ZnO nanospheres containing chlorophyll.45 Scanning electron microscopy (SEM) images of our samples are presented in Fig. 5. In Fig. 5A, spherical cyanobacterial cells with diameters of approximately 7 mm to 8 mm can be observed. Fig. 5B displays the cyanobacteria/ZnO composite, where numerous nanoscale ZnO particles are bound to the adhered cells. After treatment in an alkaline liquid at 90 °C, the cell size decreased to approximately 4 mm. Subsequent calcination eliminated the organic matter, leading to the aggregation of ZnO particles attached to the cells. These aggregates formed spherical structures reminiscent of Ferrero Rocher chocolates, with diameters ranging from 500 nm to 700 nm. Alternatively, by subjecting the cyanobacteria/ZnO composite to hydrothermal carbonization without calcination, we obtained graphene-wrapped ZnO nanoparticles, referred to as Cya-G/ZnO. Fig. 5B(d) demonstrates the close contact between the graphene sheets and the wrapped ZnO nanospheres, which are similar in size to those found in the cyanobacteria/ZnO composite (Fig. 5B(b)), and the arrows emphasize the existence of folded graphene sheets.
Compared to traditional chemical synthesis methods for graphene–semiconductor composites, this strategy offers a more economical and environmentally friendly approach.
The process is mild and simple, eliminating the need for pre-synthesized graphite. Furthermore, the strategy could arouse some interest and the technique could be extended to other graphene-based materials.
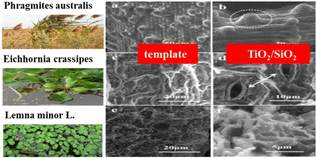
3.2 Reed leaves
Due to biodiversity, nature has inspired many unique structures. Leaves of aquatic plants are typically thin and flexible, possessing excellent mass transport and light-harvesting capabilities, making them ideal templates for constructing bio-inspired hierarchical photocatalysts. Three typical aquatic plant leaves, namely reed (Phragmites australis), water hyacinth (Eichhornia crassipes), and duckweed (Lemna minor L.), were chosen as biotemplates and sources of silica for the synthesis of biomorphic TiO2/SiO2 photocatalysts. These photocatalysts exhibit enhanced light-harvesting abilities and possess a larger specific surface area compared to P25, thanks to the incorporation of Si (as shown in Fig. 6). The TiO2/SiO2 composites exhibit primarily an anatase phase and possess a mesoporous structure, leading to a significantly large specific surface area. Furthermore, these composites demonstrate exceptional light-harvesting efficiency, especially in the visible light range, surpassing the performance of the commercial Degussa P25 (photocatalytic activities were studied by degrading gentian violet (GV) dye in the presence of TiO2/SiO2 samples, P25, and TiO2-p (TiO2 particles prepared without a template) under simulated sunlight irradiation. The degradation rate of TiO2/SiO2 samples, P25, and TiO2-p is 72%, 42.4%, and 24.5%, respectively). Photocatalytic degradation experiments using gentian violet as the target were conducted under simulated solar irradiation. The TiO2/SiO2 samples, templated using reed and water hyacinth leaves, demonstrate excellent photocatalytic activity. In contrast, the samples synthesized from duckweed exhibit inferior degradation performance compared to P25.46This observation suggests that the enhanced photocatalytic activity is a result of the synergistic effect of SiO2 incorporation and the structural construction achieved through biotemplating. Furthermore, this heterogeneous catalyst can be easily recycled and reused twice without any significant loss of activity or selectivity. These characteristics make it an environmentally friendly and economically viable alternative for practical applications.
Nanostructured heterojunction photocatalysts are considered very promising for various applications.47–52 For instance, a nanocomposite consisting of anatase TiO2 and wurtzite ZnO nanoparticles was successfully developed via a simple sol–gel and hydrolysis process using the biological template celery stalk.48 A novel three-dimensional spherical LaFeO3/Bi2O3 heterojunction was successfully constructed through a two-step synthesis including a biological template method and hydrothermal synthesis.47 The formation of heterojunction delayed the recombination of electron–hole pairs and improved the efficiency of electron–hole separation in the composite catalyst. Hollyhock stem was used as a biological template to prepare a porous CdS–g-C3N4/C composite with n–n heterojunction.49 Photoelectron–hole pairs are easily separated, which results in a longer life and a stronger activity in the photocatalytic reaction. The construction of heterojunction structure by a residual template method provides a new way for the preparation of cheap and stable photocatalysts.50,51 Kapok fibre was used as a biotemplate and for in situ C doping in g-C3N4 and TiO2 during the formation of core–shell heterojunction photocatalysts.52
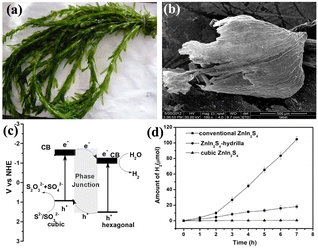
Moreover, the phase structure is a major factor that influences the activity of a photocatalyst. As early as 2012, two ZnIn2S4 polymorphs were successfully prepared; the result shows that different pathways for the degradation of RhB as well as different activity orders for the degradation of RhB and methyl orange (MO) were observed over hexagonal ZnIn2S4 microspheres and cubic ZnIn2S4 nanoparticles.53 Obviously, the formation of a proper phase junction has been shown to effectively promote interfacial charge transfer and separation. In the case of ZnIn2S4 ternary chalcogenide, it is possible to form proper junctions even in the presence of hexagonal and cubic phases. This phenomenon can potentially lead to an improvement in photocatalytic activity. Owing to the metastability of cubic ZnIn2S4, it is a challenge to form a hexagonal–cubic junction of ZnIn2S4. Since it is very difficult to achieve the phase transformation of ZnIn2S4 by conventional methods, it is necessary to find a simple and mild method. Hydrilla verticillata (L.f.) Royle, a submersed aquatic weed belonging to the monocot family Hydrocharitaceae (order Alismatales), is a widely distributed species. It has been observed that replicas derived from the leaves of Hydrilla possess effective light harvesting ability under water. In 2016, by employing a biomimetic approach, ZnIn2S4 with a hexagonal cubic phase structure was prepared using Hydrilla as templates, as depicted in Fig. 7. This material demonstrated remarkable photocatalytic hydrogen evolution activity under visible light, surpassing those with solely hexagonal or cubic phase structures. The measured H2 generation rate for conventional ZnIn2S4 was 2.5 μmol h−1, while the rate for ZnIn2S4Hydrilla was greatly increased to 14.9 μmol h−1. The enhanced photocatalytic performance of ZnIn2S4, a ternary chalcogenide photocatalyst, can be attributed to its efficient charge separation and transfer facilitated by the hexagonal cubic phase structure. Additionally, the unique Hydrilla leaf structure of ZnIn2S4 contributes to its excellent light-harvesting properties. Notably, this study presents the first successful synthesis of ZnIn2S4 with hexagonal–cubic phase junctions using a biotemplating method based on Hydrilla. This demonstrates the elegance of utilizing biotemplates to enhance the properties of materials for advanced applications by the formation of heterojunction photocatalysts.42
Thus, an opportunity to apply ZnIn2S4 with hexagonal cubic phase structures in other related fields such as solar cells could be opened up. In addition, a black TiO2/CdS heterogeneous catalyst material with leaf structure has been successfully prepared to achieve efficient photocatalytic hydrogen evolution by using inorganic leaves as a biological template.54 Hence, biotemplating provides a new and easy way to renew solar energy production.55
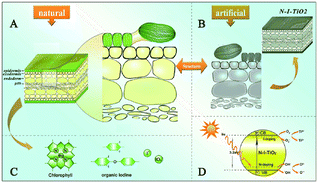
Kelp possesses the ability to optimize sunlight absorption, thus enhancing the efficiency of light harvesting. This is attributed to the majority of its chromophores being located in the epidermis. Furthermore, kelp is rich in natural iodine as well as organic iodine, including I−, and IO3−. These iodine compounds are anticipated to self-dope the synthesized samples, resembling the behavior of nitrogen.56 A novel approach has been proposed to fabricate biotemplated TiO2 with highly efficient light harvesting and photocatalytic properties, inspired by the intricate architecture of kelp at macro, micro, and nanoscale levels. This involves replicating the hierarchical structure of kelp and utilizing the self-doping of iodine and nitrogen present in the original plant (Fig. 8).57
The unique hierarchical architecture of kelp enables enhanced light harvesting through increased absorption and multiple scattering of light, resulting in excellent photocatalytic properties. A clear red shift in the band gap absorption edge is observed when comparing the biogenic TiO2 with common N–TiO2 synthesized under the same conditions but without a template. Notably, the biogenic TiO2 exhibits superior photocatalytic activity, as demonstrated by its ability to degrade methylene blue under solar energy irradiation. This approach introduces a new strategy for mimicking nature's intricate designs in the creation of materials that are efficient for photocatalysis and artificial photosynthesis systems.
3.3 Tobacco
A large amount of biomass materials of tobacco stems is disposed of worldwide including the authors' hometown, one of the biggest tobacco-growing areas, annually and causes a waste of natural plant resources and serious environmental pollution. Tobacco stem strips (TSS) are a by-product of the tobacco industry and are often disposed of. Tobacco stem silks are also a common tobacco waste, often burned off in stacks or used as mulch. Tobacco wastes, which are renewable and abundantly available, can be converted into valuable products such as activated carbon, biochar, bio-oil, and biogas. Tobacco leaves have rough surfaces and layered veins, making them suitable as a biological template. These discarded plant parts can be harvested in large quantities at low cost, making the process energy-efficient and environmentally friendly. Moreover, the use of biotemplates, such as tobacco leaves, can effectively enhance the photocatalytic activity of TiO2.56 Producing nano-sized catalysts with specific functionalities has gained significant attention in academic research because the materials synthesized with biotemplates have shown improved efficiency in degrading pollutants through photocatalysis. Therefore, it is possible to adjust the microscopic morphology of nanomaterials by using natural biomass.57The hard biotemplating method is an effective strategy to synthesize inorganic materials including TiO2 inherited the morphologies from mother templates, but very little attention has been given to the further fabrication of microstructural building units. At present, the conventional hard biotemplating method to synthesize TiO2, principally prepared through sol–gel processing, has often focused on only the replication of microstructural morphology from templates. That means the synthesized TiO2 exhibited closely packed nanoparticle subunits with relatively smooth nontextured surfaces though it inherited the microstructural morphologies of templates. In the other, the biotemplated TiO2 microstructural morphology assembled by the controllable subunits to obtain designed nanotextured surface had not been reported, let alone their photocatalytic degradation of tetracycline antibiotic. Furthermore, surface texturing has emerged as a viable option for surface engineering, resulting in significant improvement in a variety of properties including photocatalytic activities. Interestingly, as shown in Fig. 9, we have constructed for the first time a bio-derived TiO2 hierarchical architecture with nanowires or pore substructures via a new solvothermal method. The addition of glycerol or HF during the preparation process plays a crucial role in regulating the microstructural building units, where glycerol facilitates the growth and assembly of protruding nanowires, and HF etching generates concave-shaped porous structures.43
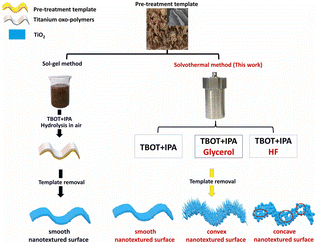 | ||
| Fig. 9 Schematic illustration of the synthetic processes of conventional solvothermal and newly developed solvothermal processes. Copyright 2022, Elsevier. | ||
This strategy achieves a leap from replicating macroscopic structures to designing microstructural building units in traditional hard-template methods. The catalyst exhibits a remarkable enhancement in the efficiency of visible light photocatalytic degradation of tetracycline. This improvement can be attributed to the increased number of active sites, improved electron separation efficiency, and reduced charge transfer resistance. The utilization of biotemplate TiO2, which consists of concave pore microstructural building units, leads to a remarkable 22.0-fold enhancement compared to pure TiO2 without the template. Furthermore, it achieves a 5.5-fold enhancement compared to the conventional sol–gel biotemplating method for TiO2 synthesis, and a 4.4-fold enhancement compared to the solvothermal biotemplate method that synthesizes the TiO2 without the addition of glycerol or HF. The application of biotemplate titanium dioxide with designed microstructural building units in wastewater treatment and other areas becomes available.53
We summarized the TiO2-based nanomaterials for removal of various pollutants as shown in Table 1. Doping transition metal ions into TiO2 can modify its crystal structure, thereby altering the band gap and lattice parameters, which leads to an improvement in photocatalytic activity. Fig. 10 shows the successful synthesis of an efficient Co-doped TiO2 photocatalyst using a one-pot impregnation method. Titanium butoxide (TBOT) and Co(NO3)2·6H2O were used as the sources of Ti and Co, respectively. The waste template synthesis strategy (TSS) was employed for morphology control. The synthesized material, including mesoporous TiO2 synthesized using waste TSS as a template, was employed for the degradation of tetracycline hydrochloride (TCH) under visible light (420–800 nm). However, the photocatalytic activity of these materials, including Co-doped mesoporous titanium dioxide or TiO2, was not found to be significant.
| Pollutants | Photocatalysts | Condition | Performance (%) | Ref. |
|---|---|---|---|---|
| Rhodamine B | MMT/TiO2-NTs | UV light | 90 | 58 |
| Rhodamine B | γ-Fe2O3/TiO2 | Visible light | 85 | 59 |
| Rhodamine B | TiO2/C-doped-cyanobacteria | Visible light | 90 | 44 |
| MB | TiO2@CdS | Visible light | 99 | 60 |
| MO | TiO2–Au | UV light | 73.2 | 61 |
| Tetracycline | TiO2@AC | UV light | 87.9 | 62 |
| Tetracycline | Bi2O3–TiO2/PAC | UV light | 72 | 63 |
| Cr(VI) | SiO2/TiO2 | Visible light | 100 | 64 |
| Cr(VI) | 2D coordination polymer | UV light | 96 | 65 |
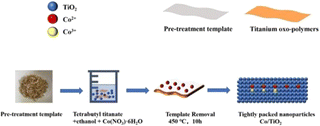 | ||
| Fig. 10 Schematic diagram of the synthesis through the one-pot impregnation method. Copyright 2023, MDPI. | ||
However, the photocatalytic degradation of TCH was significantly enhanced when co-doped mesoporous TiO2 was synthesized using waste TSS as a template, achieving an impressive removal rate of 86%. This study improved the photocatalytic efficiency by adjusting the Co2+/Co3+ ratio, a method that is rarely reported. Due to the unique chemical structure of Ti–O–Co, the energy gap of TiO2 is reduced. This reduction in the energy gap leads to a redshift in the absorption edge, effectively lowering the excitation energy required for the generation of electron–hole pairs. Consequently, the efficiency of electron–hole separation is enhanced, making the microstructure biotemplate TiO2 a more effective catalyst for the visible-light-driven degradation of TCH. This research illustrates the elegance of utilizing waste templates to synthesize TiO2 photocatalysts with enhanced visible light activity.66
3.4 Latex
Although biotemplates have been used to prepare mesoporous photocatalysts, the potential of utilizing natural latex secreted by plants as a template for synthesizing porous materials, particularly for achieving high photocatalytic activity under solar light, remains unexplored. Building upon previous research findings, a novel approach was developed by using natural latex as a structure-directing agent to synthesize thermally stable mesoporous TiO2, as depicted in Fig. 11. The resulting TiO2 demonstrated exceptional photocatalytic efficiency in the degradation of phenol and RhB under solar light.67 Furthermore, the addition of acetylacetone (ACA) during the synthesis process significantly enhanced the photocatalytic activity, resulting in a threefold increase compared to the original material. This reveals a new role of natural latex as a template for the preparation of other mesoporous materials. Consequently, it holds great potential for applications in wastewater treatment, dye-sensitized solar cells, and various other fields.68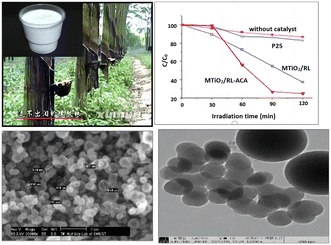 | ||
| Fig. 11 Schematic diagram of natural rubber latex templated cobalt doped mesoporous alumina. Copyright 2011, Elsevier. | ||
In addition, the use of organic pigments to sensitize TiO2 nanocomposites is a convenient method for developing highly efficient visible light photocatalysts. This is because the organic pigment molecules adsorbed on the surface of TiO2 are responsible for absorbing visible light.69,70 Natural pigments, in comparison to synthetic pigments, are easier to obtain, cost less, and are less toxic, making them more environmentally friendly. Due to their non-toxicity and availability, natural plant dyes have gained widespread attention in the scientific community. Therefore, this study proposes a new strategy for preparing TiO2 aerogel nanocomposites. Instead of the traditional method of adsorbing organic dyes, this study focuses on incorporating natural plant dyes, specifically anthocyanin from red cabbage (RCP), as a structure-directing agent. The research findings indicate that pure TiO2 aerogel nanocomposites show no significant activity in the photocatalytic reduction of Cr(VI). However, when RCP is inserted into the TiO2 aerogel nanocomposites, they exhibit greatly enhanced photocatalytic reduction activity under visible light irradiation.
The photocatalytic activity of TiO2 aerogel nanocomposites with RCP insertion is approximately 2 times higher than that of conventional photoactive TiO2 aerogel-composite materials containing RCP, and 12.3 times higher than that of pure TiO2 aerogels. Furthermore, the RCP-inserted nanocomposites demonstrate good stability and can be reused for at least three cycles without significant loss of activity. These findings provide new insights for the preparation of anatase TiO2 aerogel nanocomposites, which serve as efficient visible light photocatalysts for environmental applications, particularly in wastewater treatment.71
Chlorophyll-sensitized TiO2 was considered a promising photocatalyst, but it is largely limited by the complex chlorophyll purification process and the addition of extra-hole scavengers in the system. In the beginning, we attempted to utilize chlorophyll as the template for synthesizing mesoporous TiO2 and made certain modifications to prepare TiO2 aerogel instead of conventional mesoporous TiO2. By chance, we achieved two significant outcomes. Firstly, we obtained mesoporous TiO2 entrapped with chlorophyll under autogenous pressure, rather than using supercritical conditions. Secondly, we observed an enhanced photocatalytic reduction activity of Cr(VI) due to the synergistic effect between TiO2 and chlorophyll. This indicates that chlorophyll was incorporated into mesoporous TiO2in situ during the synthesis of TiO2, without the need for any additional co-agents. Furthermore, the efficiency of mesoporous TiO2 materials incorporated with chlorophyll was found to be higher compared to those prepared by conventional photosensitization.
Fig. 12 illustrates the absorption spectra of chlorophyll-incorporated TiO2 (TiO2/Chl), which exhibit two distinct absorption bands near 407 nm and 668 nm, closely resembling the characteristic absorption peaks of chlorophyll.
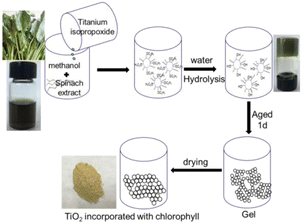 | ||
| Fig. 12 The general route of synthesis of titania is by using spinach extract. Copyright 2021, Wiley. | ||
In the absence of a sacrificial agent, TiO2 incorporated with chlorophyll, synthesized at 185 °C for 1.5 hours (TiO2/Chl-185), demonstrated a significantly higher photocatalytic rate for the reduction of Cr(VI). Specifically, this rate was approximately 29 times and 20 times higher than that of TiO2 and TiO2 adsorbing chlorophyll (TiO2/ads Chl), respectively. These findings highlight the potential of mesoporous TiO2 templated and confined with chlorophyll as a novel approach for the photocatalytic removal of Cr(VI).72
4. Synthesis and catalytic selective oxidation applications of biotemplated catalysts
4.1 Latex
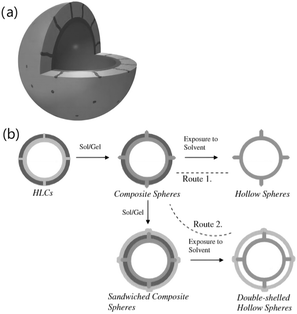
Selective oxidation of olefins is an attractive process for the fine chemical industry, but it continues to be a challenge to develop “green and sustainable” and efficient catalysts due to the disadvantages associated with the present methods such as hazardous residue, low efficiency and recycling.The origin of natural rubber latex is mainly in tropical areas, such as Hainan in China, as well as in Southeast Asia, Africa, and the tropical areas of the Americas. Natural rubber latex is usually grown in sufficient sunlight. Like other green plants, rubber trees can be seen as a plant factory of solar energy; therefore, natural latex has good application prospects in the synthesis of efficient solar photocatalysts,73–75 although there have been some reports using a series of latex templates as template preparation catalyst materials. For example, a composite hollow sphere with a complex structure was successfully synthesized by using hollow latex cages (HLCs) as a template for the sol–gel method,76 as shown in Fig. 13. A submicron titanium dioxide hollow sphere with adjustable shell thickness and smooth surface has been successfully synthesized using the sol–gel method to sulfonate polystyrene (PS) latex particles as a template.77
In a single-step synthesis, an inorganic microcapsule containing organic liquid or polymer is created by complexing disc-shaped silica particles with a cationic surfactant stabilized microemulsion emulsion. The resulting structure remains intact even after depolymerization and evaporation of a polymeric organic template. To fabricate CdS hollow spherical particles of varying sizes, the core/shell fabrication method is employed using poly(styrene-acrylic acid) (PSA) latex particles as a template. In the template synthesis, the emulsifier used initially is the sodium salt of extractant P204 (di-(2-ethylhexyl)phosphoric acid).78
However, prior to this study, the utilization of natural rubber latex as a template for synthesizing mesoporous materials had not been explored. Additionally, the synthesis methods for alumina using biopolymer templates are currently limited. In particular, there has been scarce exploration of the catalytic properties of such templates, let alone a comparison with traditional chemical templates. Natural rubber is a type of natural polymer compound primarily composed of cis-1.4 polyisoprene. Typically, inorganic materials can serve as precursor synthetic materials for natural rubber. Nevertheless, in order to achieve the desired inorganic replica at the nanoscale, the biological templates need to be made accessible, often requiring a final thermal treatment that poses the risk of nanostructure collapse.78
Using natural rubber latex as a biological template, we synthesized cobalt-doped mesoporous alumina (Co/MAl2O3) and employed it as a catalyst for the oxidation of tetralin.73 Under mild conditions, this catalyst has demonstrated exceptional efficiency in the conversion of tetral into tetralone, marking a significant achievement. With an impressive substrate conversion rate of 80.3% and a remarkable selectivity of 74.5% towards tetralone, it has outperformed other catalysts, including cobalt-doped mesoporous silica synthesized using natural latex as a template (Co/MSiO2/RL) and cobalt-doped alumina synthesized using the traditional chemical template P123 (Co/MAl2O3/P123).79 We also investigated the effect of catalyst concentration on the reaction. Notably, when using a 40 mg sample as the catalyst, we achieved the highest conversion of tetralin and selectivity towards tetralone. Furthermore, our catalyst exhibited heterogeneous behavior, as confirmed by a fast hot catalyst filtration experiment. Additionally, we found that the catalyst could be recycled multiple times without significant loss of activity. The synthetic strategy presented in this study can be extended to the synthesis of other mesoporous materials, such as SiO2 and TiO2, with similar success.80 Obviously, the synthetic strategy demonstrated here could also be extended to syntheses of other mesoporous materials, such as TiO2, which are also successful. Moreover, the method developed in this work may represent a new method for the synthesis of mesoporous alumina with an efficient catalyst for the oxidation of tetralin to 1-tetralone under relatively mild reaction conditions.
4.2 Reed leaves
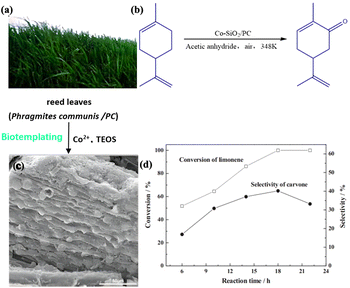
Carvone, a compound obtained through the oxidation of limonene, holds significant value due to its versatile applications in various industries such as food additives, fragrances, and pharmaceuticals. However, the oxidation of limonene produces various by-products, leading to the considerably low selectivity of carvone in heterogeneous catalytic processes. Therefore, considering the economic and environmental factors, there is an urgent need to develop highly selective catalysts for the mild production of carvone.To address this issue, researchers utilized reed leaves (Phragmites communis) as templates for synthesizing cobalt-doped mesoporous silica (Co/SiO2/PC).81 The catalyst employed air as an oxidant and acetic anhydride as a solvent for the conversion of limonene to carvone with allyl (Fig. 14).
The microstructure of Co/SiO2/PC exhibited a well-distributed and compact pore system, accurately replicating the original hierarchical structure found in reed leaves, including the sophisticated vascular bundle and stomata. Experimental results demonstrated that the substrate conversion reached 100%, while the product selectivity was measured at 40.2%. Additionally, a fast hot catalyst filtration experiment confirmed the heterogeneous nature of the catalyst, which could be easily recycled and reused twice without significant loss of activity and selectivity. The reaction was carried out under mild conditions with cheap and environmentally benign air as an oxidant. The synthetic strategy could be extended to other mesoporous materials and could open up new perspectives on the utilization of a variety of abundant plants of which reed is just a prototype in the synthesis of heterogeneous catalysts for selective oxidation. Therefore, the controllable preparation of the structure and morphology of the catalyst is significant to improve the charge separation efficiency and photocatalytic performance.
4.3 Tobacco
Catalytic oxidation of CO at low temperatures poses a significant challenge, as it plays a crucial role in pollution control for various applications such as automobile exhausts, cigarette combustion, and many industrial processes.82 One promising solution to this challenge is the utilization of a cerium–zirconium solid solution, which exhibits excellent oxygen storage properties and thermal stability. This material finds applications in fuel cells, catalytic combustion of methane, catalytic purification of chlorine-containing VOCs, as well as motor vehicle exhaust catalytic purification, among other fields. Oxygen storage properties and redox properties of cerium–zirconium solid solution are closely related to factors such as its oxygen vacancy concentration, the mobility of oxygen vacancies, surface area, and the forming of anion defects. In order to further improve the oxidation ability of cerium–zirconium solid solution catalytic, the preparation of cerium zirconium solid solution is the most crucial in many research methods. Furthermore, it remains a challenge to develop cerium–zirconium solid solution catalysts with a lower T50, which refers to the light-off temperature, i.e., the temperature at which 50% CO conversion is achieved. Additionally, the oxygen storage capacity (OSC), reducibility, and catalytic activity of cerium–zirconium solid solution are closely dependent on their microstructures, including crystal size, orientation, and morphology.83–85 Biotemplating has emerged as an attractive alternative for controlling and influencing interfacial properties. However, the application of biotemplated materials in selective oxidation is still relatively restricted.We synthesized a range of cerium–zirconium solid solutions using templates derived from tobacco leaves, stems, and stem-silk.77 These biotemplated materials exhibited excellent catalytic properties for CO oxidation at lower temperatures. Notably, the cerium–zirconium solid solution prepared using tobacco leaves as a template showed a high oxygen storage capacity (OSC) of up to 1976 mmol g−1. The stem-silk/CZ50 sample demonstrated the highest catalytic activity for CO oxidation, which can be attributed to its unique morphology. This work presents a novel strategy for utilizing biotemplates in the synthesis of cerium–zirconium solid solution catalysts.86
On the other hand, it will be interesting to investigate the possible effect of the partial removal of the plant biotemplate. Very recently, we demonstrated a facile one-pot pyrolysis technique for the in situ growth of Cu2O nanoparticles on Bougainvillea glabra (BGC)-derived carbon nanostructures.87 This method significantly enhanced the stability of Cu2O. Our synthesis strategy offers several advantages. Firstly, we employed a metal hydrolysis reaction, which differs from the previously reported use of other reductants or stabilizing agents. Secondly, the current strategy involves facile generation of a porous carbon support and well-dispersed Cu2O nanoparticles simultaneously, achieved through a one-pot pyrolysis process. Thirdly, this synthesis process has the potential to be advantageous for the large-scale production of Cu2O derived from B. glabra carbon-based materials. Transforming a low-value and seemingly worthless bio-material, effectively enhances the efficiency of Cu2O catalysts and expands their practical applications (Fig. 15). Furthermore, the optimal B. glabra-derived carbon-supported Cu2O catalyst demonstrates both high activity and stability in the selective oxidation of cyclohexane, leading to the production of valuable cyclohexanone and cyclohexanol.
 | ||
| Fig. 15 (A) Schematic illustration of the formation of Cu2O/BGC composites. (B) SEM images of (a–c) Cu2O/BGC-0.2; (d) Cu2O/BGC-DR; (e) Cu2O/AC-DR; and (f) Cu2O/G-DR. Copyright 2022, Elsevier. | ||
Compared to the deposition-reduction method used to prepare pure Cu2O and Cu2O/BGC, significant improvements were observed, the turnover number (TON) of the Cu2O catalyst supported on B. glabra-derived carbon was increased by 12.2-fold and 1.3-fold, respectively. This indicates the superior performance of the Cu2O catalyst supported on B. glabra-derived carbon. To confirm the heterogeneous nature of the catalyst, a hot filtration experiment was conducted, which demonstrated that the sample could be reused five times without any significant loss of activity. The selective oxidation of cyclohexane to produce valuable cyclohexanone and cyclohexanol is considered one of the most desirable organic transformations. However, it remains a challenging task due to the high dissociation energy of C (sp3)–H bonds.
As we are aware, the use of biomass containing nitrogen and metal elements to prepare a carbon catalyst can help avoid the need for a separate doping process.88 Kelp biochar catalysts have demonstrated outstanding catalytic performance when used with peroxymonosulfate (PMS). Kelp, a representative type of algae, is abundant in nitrogen and offers advantages such as low cost and availability, making it an ideal choice for the production of N-doped biochar. To prepare a high-efficiency catalyst for the activation of PMS in the degradation of ofloxacin (OFL), kelp biomass was utilized as a precursor (Fig. 16). This greatly addresses the issue of limited reusability commonly found in traditional carbon materials, thanks to its graphite-like structure, high graphite N2 content, and exceptionally large specific surface area. It was observed that the inclusion of graphite N in kelp biochar resulted in remarkable catalytic degradation ability, independent of metal elements. Furthermore, this catalyst demonstrated remarkable versatility, delivering exceptional degradation performance of OFL under varying pH conditions and even in the presence of high concentrations of background anions. After three degradation cycles, the catalyst maintained a degradation rate above 98%, demonstrating excellent reusability.
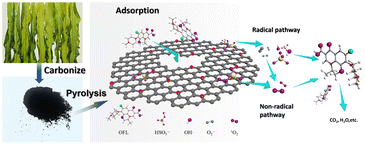 | ||
| Fig. 16 Schematic illustration of the kelp-derived N-doped biochar activated peroxymonosulfate for ofloxacin degradation. Copyright 2019, Elsevier. | ||
5. Synthesis and photocatalytic selective oxidation applications of biotemplated photocatalysts
Photocatalytic selective oxidation of cyclohexane presents an energy-saving and sustainable alternative to traditional thermal catalysis. Currently, the photocatalytic selective oxidation properties of biotemplated materials are seldom explored. For example, a solvent-thermal-assisted biotemplating method to prepare a vanadium-doped TiO2 catalyst was reported, while glycerol was used to regulate the surface ultrastructure of the catalyst.89Fig. 17 shows a schematic diagram of the bio-inspired V–TiO2 for visible-light photocatalytic selective oxidation of cyclohexane. Our findings indicate that the use of a biotemplate, surface ultrastructure regulation through glycerol, and vanadium doping all contribute to improving the photocatalyst's performance.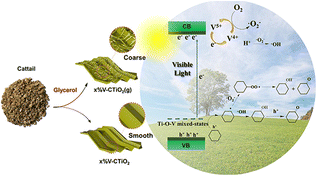 | ||
| Fig. 17 Schematic diagram of bio-inspired V–TiO2 for visible-light photocatalytic selective oxidation of cyclohexane. Copyright 2023, Elsevier. | ||
Among the samples tested, the biotemplated TiO2 doped with 2% vanadium (2% V–CTiO2 (g)) demonstrated the highest cyclohexane conversion rate (0.98%). This rate was significantly higher compared to those of the TiO2 samples without vanadium doping, surface ultrastructure adjustment, and biotemplating. Specifically, the cyclohexane conversion rate of 2% V–CTiO2 (g) was 7.0, 8.9, and 49 times higher than that of the TiO2 samples without vanadium doping, surface ultrastructure adjustment, and biotemplating, respectively. Characterization and density functional theory (DFT) calculations confirmed that the unique morphology, oxygen vacancies, and high surface area of the catalyst were achieved through biotemplating and the use of glycerol. The introduction of vanadium doping not only modified the band structure of TiO2 but also facilitated the interconversion of V4+ and V5+ during the photocatalytic process. This study presents a novel and environmentally friendly approach to the conversion of cyclohexane (Fig. 17).
A variety of TiO2–CuO solids were successfully synthesized using olive leaves as biotemplates. These materials were then tested for hydrogen production through glycerol photo-reforming. The results indicated that the incorporation of copper led to a decrease in the band gap value.90
The structural morphology of the catalyst has a significant effect on its catalytic performance. At present, the biological template method is the most direct and effective research method for material morphology and structure control in the field of inorganic material preparation. Recently, new types of catalytic materials were prepared using maize straw as a template,44,91 and the composition, structure, morphology, and electrochemical properties of the materials were analyzed by a series of characterization methods and tetracycline as the target pollutant was used to investigate the catalytic activity and stability of each material. The research results will provide a theoretical basis and technical support for the development of an economical and efficient refractory organic wastewater treatment technology. A novel TiO2 was prepared by a biotemplate method using natural biomass material-maize straw as a template (MST-TiO2).92 The catalytic activity of the prepared MST-TiO2 photocatalytic material for the degradation of tetracycline in a homogeneous UV-Fenton system was first investigated. It was found that the template controlled the structure and morphology of MST-TiO2, which formed the hierarchical porous structure of the straw and restricted the growth of the sample. At the same time, a small amount of carbon was doped into the MST-TiO2 lattice (Fig. 18). It revealed that at pH 4.5, the initial concentration of H2O2 is 10 mmol L−1, molar ratio of Fe2+/H2O2 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, dosage of catalyst is 1.0 mg L−1, initial concentration of tetracycline is 50 mg L−1, and degradation rate of tetracycline by UV-Fenton after 40 min is 99%. The main active radical group in the UV/Fenton/MST-TiO2 system is ·OH. The Fe formed in the system can accept photo-generated electrons as an electron acceptor, which will not only inhibit photo-generated holes and electron recombination but also accelerate the conversion of Fe to Fe2+ in the system.
10, dosage of catalyst is 1.0 mg L−1, initial concentration of tetracycline is 50 mg L−1, and degradation rate of tetracycline by UV-Fenton after 40 min is 99%. The main active radical group in the UV/Fenton/MST-TiO2 system is ·OH. The Fe formed in the system can accept photo-generated electrons as an electron acceptor, which will not only inhibit photo-generated holes and electron recombination but also accelerate the conversion of Fe to Fe2+ in the system.
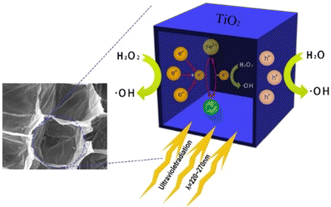 | ||
| Fig. 18 Schematic of the proposed mechanism for the UV/Fenton/T-TiO2 hybrid process for TC degradation. Copyright 2019, Elsevier. | ||
In addition, TiO2 modified with CeOx using spent coffee as a biomass sacrificial template was successfully prepared, which was applied to the selective oxidation of benzaldehyde. The selectivity towards benzaldehyde was ca. 75%, 3 times higher than the selectivity value registered for the benchmarked P25 or the bare prepared TiO2.93 It is known that the biotemplated synthesis method consists of physical and chemical reactions between the template and precursors that influence the growth, nucleation and assembly of inorganic grains. The advantages of photocatalysts synthesized by biological templates over traditional catalysts may be more due to the adjustment of morphology and microstructure, further increasing the charge separation efficiency and improving the photocatalytic performance.38–42 For example, when rice husk was used as a biological template, the confinement effect allows reactants to grow within a porous and intimate integration. The self-doping of the elements caused by the introduction of rice husk significantly promotes the light absorption range of TiO2.94 The confinement effect caused by the well-developed pore structure of rice husk makes the semiconductors tightly bond and effectively promotes the formation of heterojunction structures; the electron–hole pairs can be separated in space more efficiently because of heterojunction structure.95 Therefore, the flexible design and architecture of active heterojunction catalysts is still a significant topic.
Conclusions
In recent decades, bio-inspired methods have played a significant role in advancing the synthesis and application of innovative materials at both macro and nano scales, particularly in the fields of science and engineering, including materials science. This review presents an overview of the synthesis, properties and applications of nanomaterials prepared by biological templates. The greatest feature of biotemplating technology is providing high flexibility, mild operating conditions, precise control of the morphology and structure, prevention of nanomaterial agglomeration, regular morphology, good dispersibility, large specific surface area, a wide range of sources, low cost, and green and sustainable regeneration. This review aims to provide readers with a comprehensive understanding of biotemplating technology, specifically focusing on biotemplated photocatalysts and very limited catalytic selective oxidation. Despite the growing body of literature and patents on this subject, it is important to acknowledge that biotemplated catalytic systems are still in their early stages. In order to bridge the gap with conventional template technologies, these systems need to overcome several practical challenges:(1) The first challenge is the choice of a suitable biotemplate. Each template has its advantages while the availability, quantity and reproducibility should be considered. We note that each class of templates has its mechanisms and characteristics that influence the structure of nanomaterials. The purpose of introducing templates is to control size (1D, 2D, 3D), surface area, porosity, and phase or to prevent agglomeration by designing surfaces suitable for nanomaterial growth, thereby increasing the performance of catalytic materials.96–98
(2) The second challenge is a poor understanding of the nature of biotemplated catalysts. Although this technology has been widely studied and applied, it needs further research in both theoretical research and practical application of mechanisms.99 Up to now, there is little literature on the use of photocatalysts prepared by biological templates for the synergistic effect of catalytic oxidation. Careful control of synthetic and reaction parameters such as energy density, electron energy distribution function, electron temperature, the effect of different biotemplates, and the comparison with traditional templates should warrant a separate study and be conducted in future research. More efforts should be devoted to the theoretical simulation. Hence, more in situ characterization methods are highly beneficial to directly monitor the preparation and catalytic processes. These will supply helpful knowledge to develop new catalysts. Furthermore, these in situ characterization methods should be integrated with theoretical calculations to unveil these complex processes. Meanwhile, further exploration is needed to understand the influence of material morphology on photocatalytic performance, particularly concerning the role of active sites.100,101 Additionally, a deeper understanding of the mechanisms governing morphology and other properties is crucial for targeted applications in optoelectronics, energy generation, environmental remediation, and sensors. In addition to the traditional one-route procedure, recent advancements have been made by employing strategies that combine multiple preparation methods.
(3) The study of artificial photosynthesis via biotemplated photocatalysts could cause close attention in the future. We report for the first time that minerals in plant leaf ashes after the removal of three key aspects (antenna, reaction center, and quinine pool) of a primary process in natural photosynthesis have abilities to carry out two important photocatalytic reactions in vitro: photodegradation of organic dyes and photoreduction of 2,3,5-triphenyl tetrazolium chloride under solar light irradiation.102 That is, the photocatalysts were obtained by planting instead of chemical synthesis. Moreover, our findings demonstrate the potential of utilizing both soluble and insoluble minerals present in plant leaf ashes to create advanced photocatalysts. We also successfully obtained enzymatic mimics directly from calcined plant leaves, eliminating the need for chemical synthesis. The presence of dehydrogenase-like activities in the plant leaf ashes further suggests that minerals may play a crucial role in driving dehydrogenation reactions during biological and chemical evolution. We believe this strategy opens a new way for the environmentally friendly syntheses of solar-light-driven photocatalysts at low cost and large scale.
(4) One of the key challenges in the field of biotemplating technology and its catalytic applications is to shift the academic perspective. To transit from laboratory-scale research to large-scale manufacturing of biotemplated systems, the overall cost, enhanced energy efficiency, and optimized utilization of chemicals should be considered.103 Research efforts will primarily focus on developing scalable manufacturing processes, optimizing catalyst production, and exploring cost-effective approaches for the mass production of highly efficient catalysts.104–106 Future research in this field aims to develop environmentally friendly synthetic protocols, cost-effective templates, and innovative strategies that offer improved control over morphological characteristics. The following questions should be paid attention to: is it possible to reduce the amount of water and other chemicals used in the preparation? Can the used solvent and chemicals be reused or recycled? Is there any scale procedure to produce specified catalysts with corresponding biotemplates? For instance, in the future, a production line specified for soft and hard biotemplates, respectively, it is expected to reduce costs such as electricity.
(5) In the future, photocatalytic selective oxidation will be a field in which biotemplating materials may fully display their capabilities. Since the catalytic properties of biotemplating materials were seldom explored compared with intensive photocatalytic studies, the combination of photocatalysis and conventional thermal catalytic selective oxidation will produce unexpected results by using biotemplated heterostructure materials.107,108
In conclusion, considering the growing research interest and promising achievements, we are optimistic that the biotemplate strategy can offer a potential solution to the current bottlenecks in catalysis, particularly in the field of photocatalysis. Heterostructure materials could be applicable to a wide range of catalytic applications, enabling the synthesis of a new generation of catalysts. Future research, including some fundamental aspects, will require improved synthesis techniques to allow the synthesis of complex nanostructures at smaller sizes. However, despite the numerous challenges, research in this field presents a wealth of opportunities to delve into novel scientific discoveries. It is highly desirable to develop new strategies to design more efficient biotemplated catalysts as well as photocatalysts with high catalytic activity. The practical application of biotemplates in the synthesis of different catalysts is another important engineering objective. With the objectives being actualized, it is expected to acquire wide practical applications in the future.
Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22062026), the Yunling Scholar (YNWR-YLXZ-2019-002), and the Key Laboratory of Advanced Materials for Wastewater Treatment of Kunming.Notes and references
- R. A. C. Amoresi, R. C. Oliveira, N. L. Marana, P. B. De Almeida, P. S. Prata, M. A. Zaghete, E. Longo, J. R. Sambrano and A. Z. Simoes, ACS Appl. Nano Mater., 2019, 2, 6513–6526 CrossRef CAS.
- Z. L. Wu, M. J. Li, J. Howe, H. M. Meyer and S. H. Overbury, Langmuir, 2010, 26, 16595–16606 CrossRef CAS.
- Y. Ding, Y. Gao, Z. L. Wang, N. Tian, Z. Y. Zhou and S. G. Sun, Appl. Phys. Lett., 2007, 91, 121901–121903 CrossRef.
- S. J. Wang, J. Q. Zhang, B. Li, H. Q. Sun, S. B. Wang and X. G. Duan, J. Environ. Chem. Eng., 2022, 10, 107438–107449 CrossRef CAS.
- J. L. Wang, C. Wan, D. Cheng, D. G. Cheng, F. Q. Chen and X. L. Zhan, Appl. Surf. Sci., 2021, 565, 150609–150621 CrossRef CAS.
- A. C. Mecha and M. N. Chollom, Environ. Chem. Lett., 2020, 18, 1491–1507 CrossRef CAS.
- H. Van Lente, C. Spitters and A. Peine, Technol. Forecast. Soc. Change, 2013, 80, 1615–1628 CrossRef.
- D. W. Wang, M. A. Mueses, J. A. Marquez, F. Machuca-Martinez, I. Grcic, R. P. M. Moreira and G. L. Puma, Water Res., 2021, 202, 117421–117443 CrossRef CAS PubMed.
- W. W. Jie, Y. M. Liu, W. Y. Deng, Q. Liu, M. Qiu, S. W. Liu, J. Q. Hu and L. Gong, J. Solid State Chem., 2022, 311, 123109–123117 CrossRef CAS.
- X. X. Zheng, S. H. Qi, Y. N. Cao, L. J. Shen, C. Au and L. L. Jiang, Chin. J. Catal., 2021, 42, 279–287 CrossRef CAS.
- A. I. Carrillo, E. Serrano, R. Luque and J. Garcia-Martinez, Appl. Catal., A, 2013, 453, 389–390 CrossRef.
- D. Rodriguez-Padron, R. Luque and M. J. Munoz-Batista, Top. Curr. Chem., 2020, 378, 1–28 CrossRef PubMed.
- H. X. Li, Z. F. Bian, J. Zhu, D. Q. Zhang, G. S. Li, Y. N. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406–8407 CrossRef CAS PubMed.
- S. Sultana, S. Mansingh and K. M. Parida, Mater. Adv., 2021, 2, 6942–6983 RSC.
- X. Y. Qi, Y. L. He, Y. Yao, Y. R. Li, L. Zhang, M. Geng, H. Wei and H. B. Chu, Catal. Sci. Technol., 2022, 12, 1313–1323 RSC.
- Q. Hao, G. H. Jia, W. Wei, A. Vinu, Y. Wang, H. Arandiyan and B. J. Ni, Nano Res., 2020, 13, 18–37 CrossRef CAS.
- S. J. Wang, J. Q. Zhang, B. Li, H. Q. Sun, S. B. Wang and X. G. Duan, J. Environ. Chem. Eng., 2022, 10, 107438–107449 CrossRef CAS.
- B. A. Krajina, A. C. Proctor, A. P. Schoen, A. J. Spakowitz and S. C. Heilshorn, Prog. Mater. Sci., 2018, 91, 1–23 CrossRef CAS.
- Y. C. Miao, Z. B. Zhai, J. He, B. Li, J. J. Li and J. Q. Wang, Mater. Sci. Eng., C, 2010, 30, 839–846 CrossRef CAS.
- S. Zhu, N. Q. Zhao, J. J. Li, X. Y. Deng, J. W. Sha and C. N. He, Nano Today, 2019, 29, 100796–100817 CrossRef CAS.
- A. H. Lu and F. Schuth, Adv. Mater., 2006, 18, 1793–1805 CrossRef CAS.
- C. D. Liang, Z. J. Li and S. Dai, Angew. Chem., Int. Ed., 2008, 47, 3696–3717 CrossRef CAS.
- V. Malgras, J. Tang, J. Wang, J. Kim, N.-L. Torad, S. Dutta, K. Ariga, M. S. A. Hossain, Y. Yamauchi and K. C. W. Wu, J. Nanosci. Nanotechnol., 2019, 19, 3673–3685 CrossRef CAS PubMed.
- K. Takahashi, S. J. Limmer, Y. Wang and G. Z. Cao, J. Phys. Chem. B, 2004, 108, 9795–9800 CrossRef CAS.
- Q. Xue and Q. Zhang, Nanomaterials, 2019, 9, 1–14 CAS.
- A. Kaur, B. Bajaj, A. Kaushik, A. Saini and D. Sud, Mater. Sci. Eng., B, 2022, 286, 116005–116026 CrossRef CAS.
- X. Zhou, F. Cao, J. Li, W. Shen and J. Liu, J. Alloys Compd., 2016, 688, 354–361 CrossRef CAS.
- S. S. Fan, M. G. Zhao, L. J. Ding, Y. Ma, J. J. Liang, X. T. Wang, Y. W. Song and S. G. Chen, Sens. Actuators, B, 2016, 237, 373–379 CrossRef CAS.
- Y. Liu, B. J. Lv, P. P. Li, Y. L. Chen, B. F. Gao and B. Z. Lin, Mater. Lett., 2018, 210, 231–234 CrossRef CAS.
- T. X. Fan, S. K. Chow and D. Zhang, Prog. Mater. Sci., 2009, 54, 542–659 CrossRef CAS.
- R. Mallampati and S. Valiyaveettil, Nanoscale, 2013, 5, 3395–3399 RSC.
- S. S. Fan, M. G. Zhao, L. J. Ding, J. J. Liang, J. Chen, Y. C. Li and S. G. Chen, J. Electroanal. Chem., 2016, 775, 52–57 CrossRef CAS.
- K. T. Nam, R. Wartena, P. J. Yoo, F. W. Liau, Y. J. Lee, Y. M. Chiang, P. T. Hammond and A. M. Belcher, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 17227–17231 CrossRef CAS PubMed.
- M. Zhang, X. F. Zhang, Z. P. Deng, L. H. Huo and S. Gao, Mater. Lett., 2018, 223, 61–64 CrossRef CAS.
- L. Han, D. P. Yang and A. H. Liu, Biosens. Bioelectron., 2015, 63, 145–152 CrossRef CAS.
- J. J. Zhang, Y. Q. Liang, J. Mao, X. J. Yang, Z. D. Cui, S. L. Zhu and Z. Y. Li, Sens. Actuators, B, 2016, 235, 420–431 CrossRef CAS.
- J. Yang, M. Y. Wang, S. S. Zhao, Y. Y. Liu, W. Zhang, B. W. Wu and Q. Liu, Int. J. Hydrogen Energy, 2019, 44, 870–879 CrossRef CAS.
- P. Devaraji, R. Gao, L. Xiong, L. Y. Xiong, X. J. Jia, L. Huang, W. Chen, S. H. Liu and L. Q. Mao, Int. J. Hydrogen Energy, 2021, 46, 14369–14383 CrossRef CAS.
- M. A. Mohamed, M. F. M. Zain, L. J. Minggu, M. B. Kassim, J. Jaafar, N. A. S. Amin and Y. H. Ng, Appl. Surf. Sci., 2019, 476, 205–220 CrossRef CAS.
- H. F. Aritonang, O. E. Kamea, H. Koleangan and A. D. Wuntu, Polym.-Plast. Technol. Mater., 2020, 59, 1292–1299 CAS.
- M. Mohandesi, M. Tavakolian and M. R. Rahimpour, Ceram. Int., 2022, 47, 22820–22826 CrossRef.
- Y. J. Chen, J. He, J. J. Li, M. Q. Mao, Z. Y. Yan, W. Wang and J. Q. Wang, Catal. Commun., 2016, 87, 1–5 CrossRef CAS.
- L. Jiang, J. He, Y. P. Yang, D. C. Mao, D. M. Chen, W. Wang, Y. J. Chen, V. K. Sharma and J. Q. Wang, J. Alloys Compd., 2021, 898, 162886–162898 CrossRef.
- J. He, G. L. Zi, Z. Y. Yan, Y. L. Li, J. Xie, D. L. Duan, Y. J. Chen and J. Q. Wang, J. Environ. Sci., 2014, 26, 1195–1202 CrossRef CAS.
- J. He, L. Jiang, Y. Chen, Y. J. Chen, Z. F. Luo, Z. Y. Yan and J. Q. Wang, Chem. Commun., 2019, 55, 11410–11413 RSC.
- M. Z. Ge, C. Y. Cao, J. Y. Huang, S. H. Li, Z. Chen, K.-Q. Zhang, S. S. Al-Deyab and Y. K. Lai, J. Mater. Chem. A, 2016, 4, 6772–6801 RSC.
- Z. A. Jia, Q. R. Zeng, Y. B. Sun and W. Feng, J. Nanostruct. Chem., 2023, 13, 481–495 CrossRef CAS.
- K. Wu, X. Dong, J. Zhu, P. Wu, C. Liu, Y. X. Wang, J. N. Wu, J. Hou, Z. Y. Liu and X. H. Guo, J. Mater. Sci., 2018, 53, 11595–11606 CrossRef CAS.
- F. Tang, C. B. Liu, F. Chen, J. C. Qian, Y. B. Qiu, X. R. Meng and Z. G. Chen, Ceram. Int., 2022, 48, 28614–28621 CrossRef CAS.
- Y. Y. Du, L. Zhao, H. Chen, Z. H. Huang, X. He, W. Fang, W. X. Li, F. Q. Zhang and G. H. Wang, J. Mater. Sci.: Mater. Electron., 2018, 29, 20356–20366 CrossRef CAS.
- I. Y. Kaplin, E. S. Lokteva, E. V. Golubina, K. I. Maslakov, N. E. Strokova, S. A. Chernyak and V. V. Lunin, RSC Adv., 2017, 7, 51359–51372 RSC.
- M. A. Mohamed, M. F. M. Zain, L. J. Minggu, M. B. Kassim, J. Jaafar, N. A. S. Amin and Y. H. Ng, Appl. Surf. Sci., 2019, 476, 205–220 CrossRef CAS.
- Y. J. Chen, R. Huang, D. Q. Chen, Y. S. Wang, W. J. Liu, X. N. Li and Z. H. Li, ACS Appl. Mater. Interfaces, 2012, 4, 2273–2279 CrossRef CAS PubMed.
- Z. Y. Yan, J. He, L. Guo, Y. T. Li, D. L. Duan, Y. J. Chen, J. J. Li, F. G. Yuan and J. Q. Wang, Catalysts, 2017, 7, 82–95 CrossRef.
- P. Devaraji, R. C. Gao, L. Y. Xiong, X. J. Jia, L. Huang, W. Chen, S. H. Liu and L. Q. Mao, Int. J. Hydrogen Energy, 2021, 46, 14369–14383 CrossRef CAS.
- G. Kwon, A. Bhatnagar, H. L. Wang, E. E. Kwon and H. Song, J. Hazard. Mater., 2020, 400, 123242–123264 CrossRef CAS PubMed.
- N. Shi, X. H. Li, T. X. Fan, H. Zhou, J. A. Ding, D. Zhang and H. X. Zhu, Energy Environ. Sci., 2011, 4, 172–180 RSC.
- A. S. Martins-da-Silva, J. Torales, R. F. V. Becker, H. F. Moura, M. W. Campos, T. M. Fadalgo, A. Ventriglio and J. M. Castaldelli-Maia, Int. Rev. Psychiatry, 2022, 34, 51–58 CrossRef PubMed.
- T. B. T. Dao, T. T. L. Ha, T. D. Nguyen, H. N. Le, C. N. Ha-Thuc, T. M. L. Nguyen, P. Perre and D. M. Nguyen, Chemosphere, 2021, 280, 130802–130813 CrossRef CAS PubMed.
- F. Z. Mou, L. L. Xu, H. R. Ma, D.-R. Chen and S. H. Wang, Nanoscale, 2012, 4, 4650–4657 RSC.
- H. L. Meng, C. Cui, H. L. Shen, D. Y. Liang, Y. Z. Xue, P. G. Li and W. H. Tang, J. Alloys Compd., 2012, 527, 30–35 CrossRef CAS.
- S. Pradhan, D. Ghosh and S. W. Chen, ACS Appl. Mater. Interfaces, 2009, 1, 2060–2065 CrossRef CAS PubMed.
- A. C. Martins, A. L. Cazetta, O. Pezoti, J. R. B. Souza, T. Zhang, E. J. Pilau, T. Asefa and V. C. Almeida, Ceram. Int., 2017, 43, 4411–4418 CrossRef CAS.
- R. Wang, M. Xu, J. W. Xie, S. Y. Ye and X. L. Song, Colloids Surf., A, 2020, 602, 125048–125058 CrossRef CAS.
- Z. Yan, Z. J. He, M. Li, L. Zhang, Y. Luo, J. He, Y. J. Chen and J. Q. Wang, Catalysts, 2020, 10, 942–956 CrossRef CAS.
- F. X. Wang, X. H. Yi, C. C. Wang and J. G. Deng, Chin. J. Catal., 2017, 38, 2141–2149 CrossRef CAS.
- Q. Li, L. Jiang, Y. Li, X. R. Wang, L. X. Zhao, P. Z. Huang, D. M. Chen and J. Q. Wang, Molecules, 2023, 28, 386–400 CrossRef CAS PubMed.
- J. J. Li, Y. J. Chen, Y. N. Wang, Z. Y. Yan, D. L. Duan and J. Q. Wang, RSC Adv., 2015, 5, 21480–21486 RSC.
- L. J. Foruzin, Z. Rezvani and K. Nejati, Sol. Energy, 2019, 186, 106–112 CrossRef CAS.
- T. T. Yang, Y. Zheng, X. He, Y. D. Hou, L. Wu and X. Z. Fu, Appl. Catal., B, 2018, 221, 223–234 CrossRef CAS.
- F. X. Wang, X. H. Yi, C. C. Wang and J. G. Deng, Chin. J. Catal., 2017, 38, 2141–2149 CrossRef CAS.
- M. C. Wang, A. A. Orr, J. M. Jakubowski, K. E. Bird, C. M. Casey, S. E. Hearon, P. Tamamis and T. D. Phillips, Water Res., 2021, 188, 116534–116544 CrossRef CAS PubMed.
- H. Y. Yang, Y. P. Yang, L. Jiang, Y. J. Chen, J. He, W. Wang and J. Q. Wang, Nano Sel., 2022, 3, 140–146 CrossRef CAS.
- Y. P. Ma, M. L. Zeng, J. He, L. N. Duan, J. F. Wang, J. J. Li and J. Q. Wang, Appl. Catal., A, 2011, 396, 123–128 CrossRef CAS.
- J. J. Li, Z. Q. Li, G. L. Zi, Z. Yao, Z. R. Luo, Y. M. Wang, D. Xue, B. Q. Wang and J. Q. Wang, Catal. Commun., 2015, 59, 233–237 CrossRef CAS.
- M. Yang, J. Ma, Z. W. Niu, X. Dong, H. F. Xu, Z. K. Meng, Z. G. Jin, Y. F. Lu, Z. B. Hu and Z. Z. Yang, Adv. Funct. Mater., 2005, 15, 1523–1528 CrossRef CAS.
- A. Syouflan, Y. Inoue, M. Yada and K. Nakashima, Mater. Lett., 2007, 61, 1572–1575 CrossRef.
- B. Z. Putlitz, K. Landfester, H. Fischer and M. Antonietti, Adv. Mater., 2001, 13, 500–503 CrossRef.
- M. Yang, J. Ma, Z. W. Niu, X. Dong, H. F. Xu, Z. K. Meng, Z. G. Jin, Y. F. Lu, Z. B. Hu and Z. Z. Yang, Adv. Funct. Mater., 2005, 15, 1523–1528 CrossRef CAS.
- P. Benavente, F. Cardenas-Lizana and M. A. Keane, Catal. Commun., 2017, 96, 37–40 CrossRef CAS.
- J. J. Li, Z. Q. Li, G. L. Zi, Z. Yao, Z. R. Luo, Y. M. Wang, D. Xue, B. Q. Wang and J. Q. Wang, Catal. Commun., 2015, 59, 233–237 CrossRef CAS.
- M. Thammachart, V. Meeyoo, T. Risksomboon and S. Osuwan, Catal. Today, 2001, 68, 53–61 CrossRef CAS.
- Q. Wu, X. C. Liu, S. Hou, Q. Li, K. Zhang and Z. Yang, Colloids Surf., A, 2021, 629, 127459–127469 CrossRef CAS.
- G. Z. Chen, F. Rosei and D. L. Ma, Nanoscale, 2015, 7, 5578–5591 RSC.
- Z. Y. Yan, J. He, L. Guo, Y. T. Li, D. L. Duan, Y. J. Chen, J. J. Li, F. G. Yuan and J. Q. Wang, Catalysts, 2017, 7, 82–95 CrossRef.
- G. Q. Zhang, Z. Li, H. Y. Zheng, T. J. Fu, Y. B. Ju and Y. C. Wang, Appl. Catal., B, 2015, 179, 95–105 CrossRef CAS.
- C. J. Xie, Q. Xiong, L. Jiang, Y. F. Wang, Q. Y. Tang, J. He and J. Q. Wang, Appl. Surf. Sci., 2022, 576, 151833–151846 CrossRef CAS.
- Y. M. Huang, G. Li, M. Z. Li, J. J. Yin, N. Meng, D. Zhang, X. Q. Cao, F. P. Zhu, M. Chen, L. Li and X. J. Lyu, Sci. Total Environ., 2021, 754, 141999–142010 CrossRef CAS.
- H. L. Zhou, Y. Shao, Z. Z. Zhou, Y. Yang, J. He, L. Jiang, D. M. Chen, Y. J. Chen, Z. Y. Yan and J. Q. Wang, Appl. Surf. Sci., 2023, 622, 156957–156969 CrossRef CAS.
- J. Martin-Gomez, J. Hidalgo-Carrillo, R. C. Estevez, F. J. Urbano and A. Marinas, Appl. Catal., A, 2021, 620, 118178–118188 CrossRef CAS.
- S. Sotiropoulou, Y. Sierra-Sastre, S. S. Mark and C. A. Batt, Chem. Mater., 2008, 20, 821–834 CrossRef CAS.
- X. D. Yu, X. C. Lin, W. Feng and W. G. Li, Appl. Surf. Sci., 2019, 465, 223–231 CrossRef CAS.
- R. R. Solís, D. Rodríguez-Padrón, M. Á. Martín-Lara, M. Calero, R. Luque and M. J. Muñoz-Batista, Chemosphere, 2023, 311, 136672–136681 CrossRef PubMed.
- J. H. Pan, G. Han, R. Zhou and X. S. Zhao, Chem. Commun., 2011, 47, 6942–6944 RSC.
- L. Qin, M. Liu, Y. Wu, Y. J. Wu, Z. H. Xu, X. W. Guo and G. L. Zhang, Appl. Catal., B, 2016, 194, 50–60 CrossRef CAS.
- I. M. Denekamp, C. Deacon-Price, Z. Zhang and G. Rothenberg, Catal. Sci. Technol., 2020, 10, 6694–6700 RSC.
- K. Li, R. Chen, S. L. Li, S. L. Xie, X. L. Cao, L. Z. Dong, J. C. Bao and Y. Q. Lan, ACS Appl. Mater. Interfaces, 2016, 8, 4516–4522 CrossRef CAS PubMed.
- P. Hesemann, Curr. Opin. Green Sustainable Chem., 2018, 10, 21–26 CrossRef.
- M. Wang, J. Liu, C. Guo, X. Gao, C. Gong, Y. Wang, B. Liu, X. Li, G. Gurzadyan and L. Sun, J. Mater. Chem. A, 2018, 6, 4768–4775 RSC.
- O. Adeyiga, D. Panthi and S. O. Odoh, Catal. Sci. Technol., 2021, 11, 5671–5683 RSC.
- X. Fu, R. Yang, G. Zhou, X. Chen, Y. Liu, J. Chi, X. Li, H. Fang, H. Li and W. Li, Curr. Opin. Green Sustainable Chem., 2022, 35, 100629–100638 CrossRef CAS.
- X. Q. Ma, J. He, Y. Liu, X. L. Bai, J. Y. Leng, Y. Zhao, D. M. Chen and J. Q. Wang, Nanomaterials, 2023, 13, 2260–2277 CrossRef CAS PubMed.
- Z. A. Alothman, D. Rodriguez-Padron, A. Puente-Santiago, S. M. Osman and R. Luque, Curr. Opin. Green Sustainable Chem., 2020, 26, 100373–100380 CrossRef.
- J. Li, G. Z. Xie, J. Jiang, Y. Y. Liu, C. X. Chen, W. X. Li, J. L. Huang, X. L. Luo, M. Xu, Q. P. Zhang, M. Yang and Y. J. Su, Nano Energy, 2023, 108, 108234–108243 CrossRef CAS.
- C. X. Chen, G. Z. Xie, J. Dai, W. X. Li, Y. L. Cai, J. Li, Q. P. Zhang, H. L. Tai, Y. D. Jiang and Y. J. Su, Nano Energy, 2023, 116, 108788–108797 CrossRef CAS.
- Y. J. Su, W. X. Li, X. X. Cheng, Y. H. Zhou, S. Yang, X. Zhang, C. X. Chen, T. N. Yang, H. Pan, G. Z. Xie, G. R. Chen, X. Zhao, X. Xiao, B. Li, H. L. Tai, Y. D. Jiang, L. Q. Chen and F. J. Li, Nat. Commun., 2022, 13, 4867–4879 CrossRef CAS PubMed.
- C. X. Chen, M. J. Jiang, X. L. Luo, H. L. Tai, Y. D. Jiang, M. Yang, G. Z. Xie and Y. J. Su, Sens. Actuators, B, 2022, 370, 132441–132449 CrossRef CAS.
- H. Pan, G. R. Chen, Y. M. Chen, A. Di Carlo, M. A. Mayer, S. P. Shen, C. X. Chen, W. X. Li, S. Subramaniam, H. C. Huang, H. L. Tai, Y. D. Jiang, G. Z. Xie, Y. J. Su and J. Chen, Biosens. Bioelectron., 2023, 222, 114999–115007 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |