Open Access Article
Open Access ArticleMild and selective transformations of amines and alcohols through bioinspired oxidation with nitrous oxide or oxygen†
Bruce A.
Lobo Sacchelli
 ab,
Ruben S. M.
Almeida
ab,
Ruben S. M.
Almeida
 a,
Abdallah G.
Mahmoud
a,
Abdallah G.
Mahmoud
 a,
Dmytro S.
Nesterov
a,
Dmytro S.
Nesterov
 a,
Leandro H.
Andrade
a,
Leandro H.
Andrade
 b,
Ana M. M.
Faisca Phillips
b,
Ana M. M.
Faisca Phillips
 *a,
Elisabete C. B. A.
Alegria
*a,
Elisabete C. B. A.
Alegria
 *ac and
Martin H. G.
Prechtl
*ac and
Martin H. G.
Prechtl
 *ad
*ad
aCentro de Química Estrutural, Instituto Superior Técnico, Universidade de Lisboa, Av. Rovisco Pais 1, 1049-001, Lisboa, Portugal. E-mail: ana.faiscaphillips@tecnico.ulisboa.pt; martin.prechtl@tecnico.ulisboa.pt; Web: https://cqe.tecnico.ulisboa.pt Web: http://www.h2.bio
bDepartamento de Química Fundamental, Instituto de Química, Universidade de São Paulo, Av. Prof. Lineu Prestes, 748 – Butantã, São Paulo – SP 05508-900, Brazil
cDepartamento de Engenharia Química, Instituto Superior de Engenharia de Lisboa, Instituto Politécnico de Lisboa, 1959-007 Lisboa, Portugal. E-mail: elisabete.alegria@isel.pt
dDepartment of Synthesis and Analysis, Albert-Hofmann-Institute for Physiochemical Sustainability, Albert-Schweitzer-Str. 22, 32602 Vlotho, Germany Web: https://www.a-h.institute
First published on 23rd January 2024
Abstract
Herein we report on the catalytic oxidation of amines to nitriles with either pure oxygen or nitrous oxide, using the air- and water-stable organometallic complex {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 which has been previously reported to be active for a series of biomimetic transformations, including formaldehyde dehydrogenase and dismutase, and transfer-hydrogenation reactions like deamination of nitriles to alcohols. Inline with these previous studies we now report on other biomimetic properties of this binuclear ruthenium complex which is able to act as well as nitrous oxide reductase (N2OR) and decompose nitrous oxide in the presence of hydrogen donating molecules like amines and alcohols. This complex can be synthesised from the inexpensive and commercially available precursor [Ru(p-cymene)Cl2]2 or from ruthenium chloride and renewable α-phellandrene which naturally occurs in eucalyptus oil for example. The selectivities and yields can be controlled by solvents, oxidants and temperature. Albeit oxygen is known as a potent oxidant, the observation that the catalyst can both oxidise alcohols or amines and simultaneously decompose the greenhouse gas nitrous oxide is very interesting. In addition, under similar conditions this catalyst is able to convert aromatic alcohols to benzaldehydes. These reactions with an air stable and robust catalyst were easy to carry out and affordable, making them highly practical. Note, in here we report on the oxidation of benzylamines and benzylic alcohols as model substrates for the initial evaluation of these catalytic set-ups.
Introduction
In continuation of our previous reports on ruthenium-catalysed biomimetic dehydrogenation reactions and transfer-hydrogenation reactions,1–9 including formaldehyde dehydrogenase (FADH),1,2,8,9 dismutase5 and deamination of nitriles,7 we have been motivated to further explore the potential of these C1-activating mimicries inspired by other related biological processes. Despite the apparent advantages of acceptorless and oxidant-free conditions, a significant challenge for other chemical transformations and their practical application often lies in the need for inert conditions. However, we can learn from nature that metal containing enzymes are also able to catalyse the oxidation of amines (i.e. methylamine, phenethylamines, aliphatic amines) to using oxygen as oxidant and hydrogen acceptor. For instance copper amine oxidases,8,10,11 enable N-hydroxylation,12 while aldoxime dehydratases convert aldoximes to nitriles.13–15 More specifically nitrogen-activating enzymes involved in biological denitrification processes are a source for inspiration. For example the methane monooxygenase (MMO) and nitrous oxide reductase (N2OR) are interesting systems, where metalloenzymes are able to decompose nitrous oxide in presence of methane as hydrogen donating molecule.16,17 The latter converts also alcohol to aldehyde in denitrifying processes, but not further to carboxylic acid.18 Examples of MMO and N2OR contain dinuclear copper sites, or diiron units in case of MMO.19,20 Interestingly, it has been observed in cytotoxicity studies that N2O is not only converted by N2OR, but N2O exhibited cytoxicity to vitamin B12, and binds to cobalamin resulting in deactivation of B12 in B12-dependent metabolism cycles, while N2O undergoes degradation.21,22 Such (bio)catalytic conversions are also of interest for synthesis and artificial energy conversion since N2O is a greenhouse gas which must be eliminated from the atmosphere.16,23–25 Taking into account that nitrous oxide tends to decompose while acting as hydrogen acceptor during biological alcohol oxidation to aldehyde,18 we are keen to explore nitrous oxide activation with our organometallic biomimetics. And, since N2OR are able to decompose nitrous oxide using different types of hydrogen donating molecules as co-factors, we extended this approach to amines as sacrificial hydrogen source, likewise to use nitrous oxide for the activation of NH- and OH-bonds under oxidative conditions for the formation of C–O and C–N multiple bonds. These oxidative conversions complement well our previous findings on the reductive deaminative conversion of nitriles to alcohols using paraformaldehyde in aqueous solution (Fig. 1).7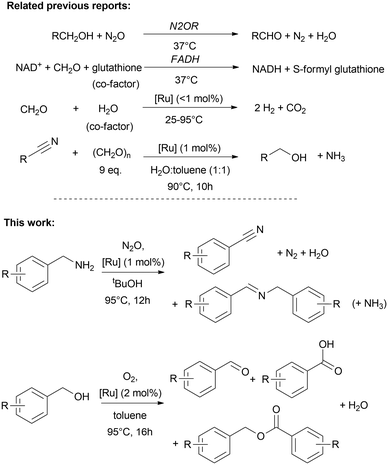 | ||
| Fig. 1 Biological and biomimetic oxidation of C–O and C–N bonds.1,2,7,8,18,29 | ||
Both benzonitriles and benzaldehydes have a wide range of applications, for a broad variety of industries. For example benzonitriles are used in pesticides since the 1970's,26 in the synthesis of benzoguanamine resins,27 or for the synthesis of fluvoxamine, an important antidepressant, that uses 4-(trifluoromethyl)benzonitrile as a key intermediate,28 one of the compounds that we also prepared in this work.
Industrially, nitriles are typically synthesized by ammoxidation, a high-temperature vapor-phase oxidation process involving toluene and ammonia. This occurs in fixed-bed reactors operating at temperatures between 300 and 550 °C, with catalysts ranging from vanadium and molybdenum, to a tungsten–manganese complex.27,30,31 Classically, on a laboratory scale, benzonitriles are conventionally prepared through two main reactions: the Rosenmund–von-Braun reaction from aryl halides at 150–250 °C using Cu(I) cyanide as a cyanating agent,32,33 or the Sandmeyer reaction, starting from benzylamines through diazotization, also using copper cyanide.34 This latter one, is also used in industrial scale to produce antipsychotic drug Fluanxol and the anti-cancer drug neoamphimedine.35,36 Benzaldehydes also play an important role in the industry. For instance, benzaldehyde is the simplest and most important aromatic aldehyde in industry.37,38 It is known for its bitter almond odour and taste, and it is only behind vanillin as the most used flavouring agent, it is also commonly used as a denaturant and as a fragrance, in the cosmetic industry.39 Its industrial process is based on the hydrolysis of benzal chloride, usually using metal salts as catalysts, preferably those of iron or zinc, and could be carried out either continuously or in batch. More recently, the process has been adapted to work continuously with activated carbon as catalyst, resulting In yields over 97%, and can be used for the synthesis of substituted benzaldehydes.37 The aerial oxidation of toluene is another option, but requires very high temperatures and low yields due to the formation of secondary products.38 On the lab scale, benzaldehydes can be produced from a wide range of compounds, most commonly following a similar path to the industrial one, from benzyl chloride or toluene, or being extracted from natural sources, like cinnamon oil.40 Protocols by Yamada,41 Severin,42 Grützmacher43 and their co-workers for lab scale experimentation report on the oxidation of benzylic alcohols,41,42 and also light weight aliphatic alcohols43 using ruthenium complex catalysts and nitrous oxide as terminal oxidant, at elevated temperatures (100–150 °C) with moderate to good yields,41,42 and also very promising high activity at lower temperature (65–80 °C) for the oxidation of light weight aliphatic alcohols with the target to simultaneously reduce nitrous oxide.43
In more recent years, in the field of homogeneous catalysis, Szymczack et al. reported on aceptorless and oxidant-free conditions at 110 °C for the amine to nitrile conversion with moderate yields (17–75%), using a ruthenium hydride NNN-pincer complex further stabilised with two triphenylphosphine. Albeit the acceptorless and oxidant free conditions, the requirement of inert conditions limits the practical application and scalability.44 Another example with a ruthenium NNN-pincer complex has been demonstrated by Bera et al. reporting activity at 70 °C with catalyst loadings as low as 2 mol%.45 Moreover, Achard et al. demonstrated that commercially available [Ru(p-cymene)Cl2]2 catalyses this reaction as well under acceptorless/oxidant-free and inert conditions in dichlorobenzene at 110 °C with moderate yields (23–65%).46 Following this approach, it has been demonstrated by Kannan and Muthaiah that the catalyst performance under inert conditions could be further improved by the addition of hexamethylenetetramine as hydride source to activate [Ru(benzene)Cl2]2 and [Ru(p-cymene)Cl2]2 for the acceptorless dehydrogenation of amines to nitriles with good yields (71–91%).47–49 Other protocols on amine oxidation to nitriles were published by Parvulescu,50 Albrecht51 and their co-workers. The latter group reported their evaluation on the ruthenium catalysed conversion of 4-methylbenzylamine (0.2 mmol) to the corresponding nitrile under oxygen in presence of gaseous ammonia showing good yields for nitrile (85%), but rather high catalyst loadings and temperature (catalyst: 5 mol%, 150 °C). Impressively, Parvulescu50 reported a highly selective (>99 °C) oxidation of amines (0.14 mol) to nitriles at low-temperature (60 °C), but with the requirement of elevated pressure of oxygen (5 bar) or air (25 bar) and relatively high molecular catalyst loadings (ratio: 0.14 mmol amine vs. 0.01 mmol complex; 7 mol%). In a different approach, with a zirconia supported ruthenium catalyst and a strong base the selectivity for imines or nitriles could be controlled.52
Taking into account the above described limitations and our previous demonstrations with bench stable biomimetic catalysts, an appropriate air-stable complex should be capable to catalyse the reaction cascade from amine to nitrile under oxidative conditions without the requirement of inert conditions which will be discussed in more detail below.
Note, in here we report on the oxidation of benzylamines and benzylic alcohols as model substrates for the initial evaluation of these catalytic set-ups with nitrous oxide and oxygen. For the catalytic activation of aliphatic substrates improved modification of the reaction conditions are still required. Additionally we tested also air instead of pure oxygen, but observed lower conversions owing to the lower oxygen content in air which underlined also the requirement of higher concentrations of the respective oxidant in comparison to oxidant-free (inert) conditions. Moreover, for the development of a simplified setup we disregarded the addition of gaseous ammonia to improve the selectivity which is a typical workaround to shift the equilibrium in nitrile reduction and amine oxidation processes.51,53 Instead, since we demonstrated previously that this is also possible by the application of polar protic solvents in (de)hydrogenation processes, we continue to further develop our previous protocols in this field to influence the selectivity in such processes by means of polar-protic or non polar, non protic solvents.4,7,53–56
Results and discussion
Oxidation of the benzylamines
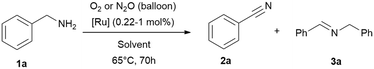
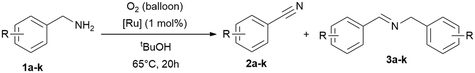
In this work a fast, economic and practical way to synthesise benzonitriles is presented, using simple lab equipment and the easy accessible catalyst {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 (RuBF4), that can be made from the commercially available [Ru(p-cymene)Cl2]2 (Ru2).1The oxidation of benzylamines is known to result in the formation of several by-products,44–49,52 mainly the respective secondary imine. Therefore further optimisation is required for improved reaction conditions avoiding inert conditions and water/air-sensitive catalysts. For this purpose, the benchmark substrate benzylamine was catalytically oxidised with O2 or N2O as terminal oxidant, in order to properly evaluate and investigate the effect of both these gases.
Optimisation of reaction parameters
Drawing insights from our previous studies we learned that a protic polar solvent plays a crucial role in stabilising the cationic species of [RuBF4]: {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4. However, in the current study we need to consider in addition to the nature of the catalyst and substrates, also the oxidising conditions and the gas solubility.57–60 Given these considerations, alcohols emerge as promising additives to water, given their higher solubilities in alcohol. tert-Butanol, being both protic and stable against oxidation under these conditions, appears to be the most suitable alcohol for our purpose in contrast to primary and secondary alcohols which are oxidised under these conditions. To verify our proposal, we performed the reactions in neat amine, water and tert-butanol with [(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 (RuBF4) as catalyst.Thus, we verified the following parameters: (I) solvents, (II) oxidant (N2O vs. O2), (III) catalysts of interest (RuBF4vs. Ru2), (IV) reaction temperature, (V) reaction time, (VI) and (VI) reaction vessel (flask with gas balloon vs. sealed system (high vacuum tube with Teflon valve)). Notably, in systems equipped with balloons as gas reservoirs we avoided higher reaction temperatures owing to the observed potential boil off of the low boiling solvent and the related experimental errors towards reproducibility. The results (Table 1 and ESI:† Table S1) highlight tert-butanol as the most suitable solvent. In this solvent, we achieved the conversion of (>99%) benzylamine (1a) to benzonitrile (2a) with the best selectivity (90%) towards the desired nitrile product. Neat benzylamine turned out to be less suitable, since the high concentration of substrate shifts the reaction towards secondary imine formation, resulting in a mixture of nitrile (2a) and imine (3a) in ratios of approx. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 with little effect of the used oxidant (Table 1: entry 1–2 and ESI:† Table S1). In water (Table 1: entry 3–4 and ESI:† Table S1) the selectivity was similar (4
6 with little effect of the used oxidant (Table 1: entry 1–2 and ESI:† Table S1). In water (Table 1: entry 3–4 and ESI:† Table S1) the selectivity was similar (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 and 5
6 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) and probably related to the miscibility and local concentration of benzylamine in water. In tert-butanol (Table 1: entry 5–6 and ESI:† Table S1) selectivities for nitrile are ca. 90% independent of the used oxidant (balloon). The increased gas solubility in alcohols may contribute to accelerating the catalytic oxidation reaction for nitrile formation compared to reactions in water, which has lower gas solubility. Consequently, we continued the studies with tert-butanol as solvent for the catalytic oxidation of amines to nitriles. Therefore, similar to our previous studies on nitrile hydrogenation7,53,54 the use of protic solvents are beneficial to shift the equilibrium and obtain the primary amine, or in the present case the nitrile rather than the secondary imine which is formed through condensation of the primary imine in presence of the primary amine. In addition one need to consider the miscibility of the organic substrates with the solvents which has influence of the local concentration, thus tBuOH appears more appropriate than water, and of course under neat conditions the concentration of benzylamine is also higher which shifts the equilibrium towards the imine.
5) and probably related to the miscibility and local concentration of benzylamine in water. In tert-butanol (Table 1: entry 5–6 and ESI:† Table S1) selectivities for nitrile are ca. 90% independent of the used oxidant (balloon). The increased gas solubility in alcohols may contribute to accelerating the catalytic oxidation reaction for nitrile formation compared to reactions in water, which has lower gas solubility. Consequently, we continued the studies with tert-butanol as solvent for the catalytic oxidation of amines to nitriles. Therefore, similar to our previous studies on nitrile hydrogenation7,53,54 the use of protic solvents are beneficial to shift the equilibrium and obtain the primary amine, or in the present case the nitrile rather than the secondary imine which is formed through condensation of the primary imine in presence of the primary amine. In addition one need to consider the miscibility of the organic substrates with the solvents which has influence of the local concentration, thus tBuOH appears more appropriate than water, and of course under neat conditions the concentration of benzylamine is also higher which shifts the equilibrium towards the imine.
| Entry | Solvent | t [h] | Oxidant | Conv. [%] | 2 (3)a [%] |
|---|---|---|---|---|---|
| Reaction conditions: benzylamine (1 mmol, except entry 1–2; 4.5 mmol), solvent (1 mL, except entry 1–2: no solvent added), 65 °C, time varying, [Ru] = {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 (RuBF4, 0.01 mmol; 0.22 mol% for entry 1–2 and 1 mol% for entry 3–6), oxidant gas varying (balloon). Conversions and yields were determined by GC and GC-MS analysis with hexadecane as internal standard. Imine quantities and benzylamine conversions were determined by 1H NMR analysis with cyclohexane as internal standard.a Nitrile 2 yield, the major side-product is the secondary imine 3 (yield in brackets). | |||||
| 1 | Neat | 70 | O2 | >99 | 40 (60) |
| 2 | N2O | >99 | 43 (57) | ||
| 3 | H2O | 70 | O2 | >99 | 41 (59) |
| 4 | N2O | >99 | 50 (50) | ||
| 5 | tBuOH | 70 | O 2 | >99 | 91 (9) |
| 6 | N 2 O | >99 | 89 (11) | ||
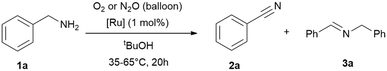
Subsequently, we investigated whether the commercially available [Ru(p-cymene)Cl2]2 (Ru2) could serve as a competitive alternative to RuBF4, under identical conditions (T = 35 °C or 65 °C, 20 h) using tert-butanol as solvent and balloons as reservoirs for the oxidants (Table 2 and ESI:† Table S1). It was observed that, under identical conditions, RuBF4 yielded better results. As a result we continued the studies with this complex. Interestingly, the comparison of reaction times (Table 1 entries 5–6 and Table 2 entries 7–10) suggests that selectivity is time-dependent. The secondary imine is not merely a by-product but a reaction intermediate formed in equilibrium. As soon as free primary imine is formed in equilibrium, the reaction shifts to nitrile formation under oxidative conditions.
| Entry | Cat. | T [°C] | Oxidant | Conv. [%] | 2 (3)b [%] |
|---|---|---|---|---|---|
| Reaction conditions: benzylamine (1 mmol), tert-butanol (1 mL), 20 h, [Ru] = {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 (1 mol%; 0.01 mmol) or [Ru(p-cymene)Cl2]2 (1 mol%; 0.01 mmol), oxidant gas varying (balloon). Conversions and yields were determined by GC and GC-MS analysis with hexadecane as internal standard. Imine quantities and benzylamine conversions were determined by 1H NMR analysis with cyclohexane as internal standard.a Reaction time: 24 hours.b Nitrile 2 yield, the major side-product is the secondary imine 3 (yield in brackets). | |||||
| 1 | Ru2 | 35 | O2 | 10 | 6 (94) |
| 2 | N2O | 6 | 0 (99) | ||
| 3 | Ru2 | 65 | O2 | 20 | 55 (45) |
| 4 | N2O | 10 | 30 (70) | ||
| 5 | RuBF4 | 35 | O2 | 17 | 55 (45) |
| 6 | N2O | 10 | 42 (58) | ||
| 7 | RuBF4 | 65 | O2 | >99 | 71 (29) |
| 8 | N2O | >99 | 67 (33) | ||
| 9 | RuBF4 | 65 | O2 | >99 | 85 (15)a |
| 10 | N2O | >99 | 77 (23)a | ||
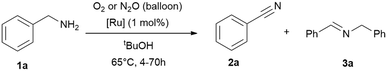
Following the previous observations, we extended our exploration to optimise conversion and selectivities by adjusting the reaction time (4–70 h; Table 3) using RuBF4 as catalyst for the amine oxidation at 65 °C in tert-butanol. The time screening shows that >99% conversion can be reached after 16 h while the selectivity for nitrile is still about 70% (Table 3, entries 6–7). Longer reaction times further improved the selectivity (Table 3, 77–91%), while the results with oxygen are slightly better, but are not significantly more selective. Remarkably, this process offered the simultaneous decomposition and elimination of the greenhouse gas nitrous oxide.
| Entry | t [h] | Oxidant | Conv. [%] | 2 (3)a [%] |
|---|---|---|---|---|
| Reaction conditions: benzylamine (1 mmol), tert-butanol (1 mL), [Ru] = {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 (1 mol%; 0.01 mmol), oxidant gas varying (balloon). Conversions and yields were determined by GC and GC-MS analysis with hexadecane as internal standard. Imine quantities and benzylamine conversions were determined by 1H NMR analysis with cyclohexane as internal standard.a Nitrile 2 yield, the major side-product is the secondary imine 3 (yield in brackets). | ||||
| 1 | 4 | O2 | 26 | 67 (33) |
| 2 | N2O | 19 | 67 (33) | |
| 4 | 8 | O2 | 57 | 74 (26) |
| 5 | N2O | 21 | 67 (33) | |
| 6 | 16 | O2 | >99 | 70 (30) |
| 7 | N2O | >99 | 69 (31) | |
| 8 | 24 | O2 | >99 | 85 (15) |
| 9 | N2O | >99 | 77 (23) | |
| 10 | 70 | O2 | >99 | 91 (9) |
| 11 | N2O | >99 | 89 (11) | |
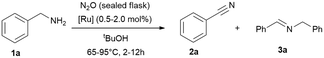
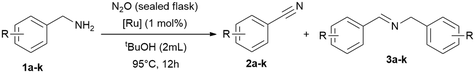
To improve the reaction setup, we considered to substitute the balloon (used as an oxidant reservoir) and perform the reaction in a sealed reaction vessel (a gas-tight tube with a high vacuum Teflon valve) saturated with oxidant (Table 4). The idea is to have a better miscibility of the gaseous oxidants with the solute substrates and the catalyst, and the advantage that the temperature can be increased further. For this reason, we condensed 4.5 mmol nitrous oxide (at −196 °C) onto the reaction mixture and further optimised parameters such as time, temperature and solvent quantity (Table 4). Consequently, we were able to reduce the reaction time in this manner to achieve full conversion. However, the yields remained unchanged, which is most likely due to the considerable high concentration of the substrate and intermediate in solution, leading to the formation of the imine. Consequently, we tested then a more diluted reaction and doubled the amount of solvent (2 mL) and simultaneously varied the catalyst loading (0.5–2.0 mol%), shortened the reaction time further and increase of the reaction temperature to 95 °C, giving high conversions already in 12 h (Table 4, entry 11).
| Entry | t [h] | [Ru] (mol%) | tBuOH [mL] | T [°C] | Conv. [%] | 2 (3)a [%] |
|---|---|---|---|---|---|---|
| Reaction conditions: benzylamine (1 mmol), tert-butanol (1 mL), [Ru] = {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 (1 mol%, 0.01 mmol). Oxidant: nitrous oxide (4.5 mmol) condensed at −196 °C into the gas tight high vacuum tube with Teflon valve. Conversions and yields were determined by GC and GC-MS analysis with hexadecane as internal standard. Imine quantities and benzylamine conversions were determined by 1H NMR analysis with cyclohexane as internal standard.a Nitrile 2 yield, the major side-product is the secondary imine 3 (yield in brackets). | ||||||
| 1 | 2 | 1 | 1 | 65 | 25 | 43 (57) |
| 2 | 4 | 1 | 1 | 65 | 51 | 68 (32) |
| 3 | 4 | 0.5 | 1 | 65 | 37 | 59 (41) |
| 4 | 4 | 0.5 | 2 | 65 | 10 | 19 (81) |
| 5 | 4 | 2 | 1 | 95 | 59 | 38 (62) |
| 6 | 2 | 1 | 2 | 95 | 53 | 65 (35) |
| 7 | 4 | 1 | 2 | 95 | 69 | 67 (33) |
| 8 | 6 | 1 | 2 | 95 | 79 | 64 (36) |
| 9 | 8 | 1 | 2 | 95 | 87 | 70 (30) |
| 10 | 8 | 1 | 1 | 95 | 66 | 69 (31) |
| 11 | 12 | 1 | 2 | 95 | 97 | 70 (30) |
Now equipped with two distinct optimised reaction setups we conducted substrate scope screening with oxygen (balloon) with 1 mol% of RuBF4 as catalyst at 65 °C in tert-butanol (20 h; Table 5), and a second set with nitrous oxide (sealed flask) with 1.0 mol% of RuBF4 as catalyst at 95 °C for 12 hours (Table 4 and Table 6).
| Entry | Benzylamine 1a–k/conv. [%] | Benzonitrile 2a–k/yield [%] | Imine 3a–k/yield [%] | Other byproducts of oxidation |
|---|---|---|---|---|
| Reaction conditions: 1 mol% (0.01 mmol) catalyst RuBF4, 1 mmol for all substrates, 1 mL tBuOH, 65 °C for 20 h. Oxidant gas O2 (balloon). Conversions, selectivity and yields were determined by GC and GC-MS analysis with hexadecane as internal standard. Imine quantities and benzylamine conversions were determined by 1H NMR analysis with cyclohexane as internal standard. n. q.: not quantified (traces).a Reaction time: 24 hours.b In [brackets]: isolated gravimetric yield after column chromatography (refer ESI† for details). | ||||
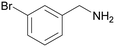
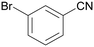
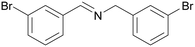
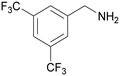
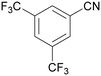
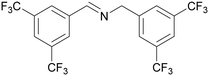
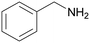
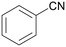
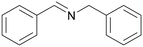
| 1 |
|
|
|
Traces of benzamide and N-benzylidene benzamide |
| >99 | 71 (85)a [71]b | 29 (15)a | ||
| 1a | 2a | 3a | ||
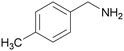
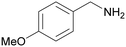
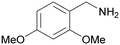
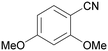
| 2 |
|
|
— | Trace of benzamide |
| >99 | >99 [89]b | |||
| 1b | 2b | |||
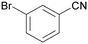
| 3 |
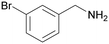
|
|
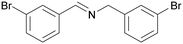
|
Trace of benzamide |
| >99 | 94 [72]b | 6 | ||
| 1c | 2c | 3c | ||
| 4 |
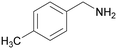
|
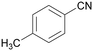
|
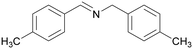
|
Trace of 4-methyl benzamide |
| >99 | 69 | 31 | ||
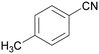
| 1d | 2d | 3d | ||
| 5 |
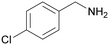
|
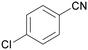
|
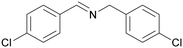
|
Trace of 4-chloro benzamide |
| >99 | 70 | 30 | ||
| 1e | 2e | 3e | ||
| 6 |
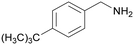
|
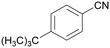
|
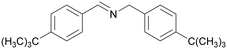
|
Trace of 4-tertbutyl benzamide |
| 84 | 63 | 38 | ||
| 1f | 2f | 3f | ||
| 7 |
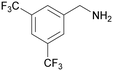
|
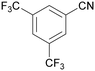
|
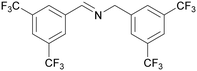
|
Traces of benzamide and N-benzylidene benzamide |
| 72 | >98 | n. q. | ||
| 1g | 2g | 3g | ||
| 8 |
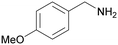
|
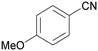
|
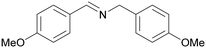
|
Trace of 2-methoxy benzamide |
| 70 | 81 | 19 | ||
| 1h | 2h | 3h | ||
| 9 |
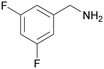
|
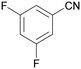
|
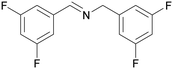
|
Traces of benzamide and N-benzylidene benzamide |
| 53 | 41 | 59 | ||
| 1i | 2i | 3i | ||
| 10 |
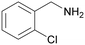
|
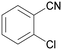
|
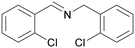
|
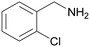
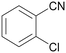
Trace of 2-chloro benzamide |
| 50 | 70 | 30 | ||
| 1j | 2j | 3j | ||
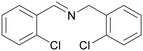
| 11 |
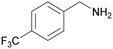
|
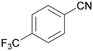
|
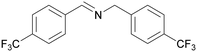
|
Traces of benzamide and N-benzylidene benzamide |
| 39 | 94 | 6 | ||
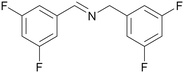
| 1k | 2k | 3k | ||
Scope of benzylamines
Following the optimisation of the reaction parameters (vide supra) for both reaction setups, we extended the substrate scope to benzylamines with different functional groups for the dehydrogenative oxidation under oxygen atmosphere (balloon; Table 5). The reaction tolerates different substituents, including methoxy, alkyl and halide groups. Additionally, both electron-withdrawing and electron-donating groups are well-tolerated, as are varying degrees of substitution at the arene site. The conversions vary from 39 to >99% with selectivities for nitriles of 41 to >99%.With nitrous oxide as oxidant at 95 °C the conversions vary from 65 to >99% with selectivities for nitriles of 33 to 91%. The comparison of the results summarised in the Tables above one can conclude that both oxidants O2 and N2O are suitable for the benzylamine oxidation to benzonitriles with good conversions. However, lower temperature (65 °C) and longer reaction times (20 h) give better selectivities for nitriles than at higher temperature (95 °C) and shorter time (12 h).
Benzyl alcohols oxidation
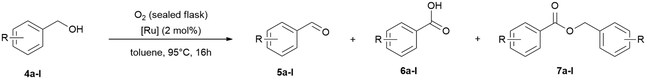
Following these results, we tested ruthenium catalysed selective benzyl alcohol oxidation with oxygen and nitrous oxide. Owing to the higher stability of alcohols in comparison to amines we focused on higher reaction temperatures and increased the catalyst loadings.61 In addition, one need to consider the lower gas solubility at higher temperatures,58–60 and the lower miscibility of benzyl alcohol (4 g/100 mL) with water. Initial tests at 65 °C and 95 °C in water and tBuOH showed that both solvents give poor results with nitrous oxide (balloon) and moderate conversions with oxygen (balloon) in water within 20 h reaction time (ESI† Table S2). Increasing the reaction time to 48 h (ESI† Table S2) for the reaction in water under oxygen, full conversion was observed giving a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of benzaldehyde and benzoic acid. Increasing the catalyst loading to 4 mol% had no significant effect to improve the low conversions, but the aldehyde selectivity improved (ESI† Table S2). In addition, we tested then toluene as solvent with oxygen (balloon and sealed flask), which we used in previous studies for transfer hydrogenation reactions,7 and best results were obtained after 16 h at 95 °C with full conversion and 96% aldehyde selectivity (ESI† Table S2). A test reaction without addition of oxygen under air showed low conversion which underlines the requirement of the oxidant (ESI† Fig. S110). The optimised conditions were then tested with a broader scope of benzyl alcohols (Table 7). The conversions (59% to >99%) and selectivities for benzaldehyde (41% to >99%) are in general very good and tolerates different functional groups, including methoxy, alkyl and halide groups.
1 mixture of benzaldehyde and benzoic acid. Increasing the catalyst loading to 4 mol% had no significant effect to improve the low conversions, but the aldehyde selectivity improved (ESI† Table S2). In addition, we tested then toluene as solvent with oxygen (balloon and sealed flask), which we used in previous studies for transfer hydrogenation reactions,7 and best results were obtained after 16 h at 95 °C with full conversion and 96% aldehyde selectivity (ESI† Table S2). A test reaction without addition of oxygen under air showed low conversion which underlines the requirement of the oxidant (ESI† Fig. S110). The optimised conditions were then tested with a broader scope of benzyl alcohols (Table 7). The conversions (59% to >99%) and selectivities for benzaldehyde (41% to >99%) are in general very good and tolerates different functional groups, including methoxy, alkyl and halide groups.
| Entry | BnOH/conv. [%] | PhCHO/yield [%] | Benzoic acid or benzyl benzoate/yield [%] |
|---|---|---|---|
| Reaction conditions: 2 mol% (0.01 mmol) catalyst RuBF4, 0.5 mmol for all substrates, 1 mL toluene, 95 °C for 16 h. Oxidant: oxygen (4.5 mmol) condensed at −196 °C into the gas tight high vacuum tube with Teflon valve. Conversions and yields were determined by GC and GC-MS analysis with hexadecane as internal standard and/or by 1H NMR analysis with cyclohexane as internal standard.a In (brackets): isolated gravimetric yield after column chromatography (refer ESI† for details). | |||
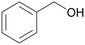
| 1 |
|
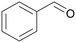
|
— |
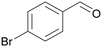
| >99 | >99 (86)a | ||
| 4a | 5a | ||
| 2 |
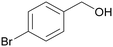
|
|
— |
| >99 | >99 (92)a | ||
| 4b | 5b | ||
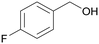
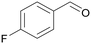
| 3 |
|
|
— |
| >99 | >99 (95)a | ||
| 4c | 5c | ||
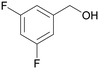
| 4 |
|
|
|
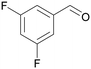
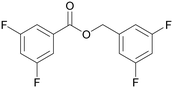
| >99 | 84 | 16 | |
| 4d | 5d | 7d | |
| 5 |
|
|
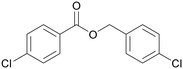
|
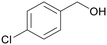
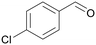
| >99 | 82 | 18 | |
| 4e | 5e | 7e | |
| 6 |
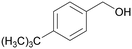
|
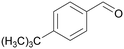
|
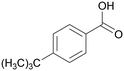
|
| >99 | 64 | 36 | |
| 4f | 5f | 6f | |
| 7 |
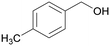
|
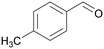
|
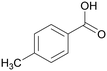
|
| >99 | 48 | 52 | |
| 4g | 5g | 6g | |
| 8 |
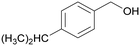
|
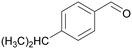
|
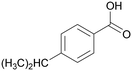
|
| >99 | 41 | 59 | |
| 4h | 5h | 6h | |
| 9 |
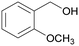
|
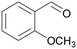
|
— |
| 80 | >99 | ||
| 4i | 5i | ||
| 10 |
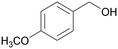
|
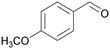
|
— |
| 74 | >99 | ||
| 4j | 5j | ||
| 11 |
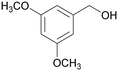
|
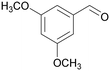
|
— |
| 73 | >99 | ||
| 4k | 5k | ||
| 12 |
|
|
— |
| 59 | >99 | ||
| 4l | 5l | ||
Conclusions
In summary, we assessed the oxidation of various benzylic amines and alcohols using nitrous oxide and oxygen as oxidation agents under mild, with low catalyst loadings and practical reaction conditions, eliminating the need for air or moisture sensitive chemicals or highly sophisticated glassware. The reactions exhibited high selectivities for nitrile or aldehydes production in the presence of {[(p-cymene)Ru](μ-H)(μ-Cl)(μ-HCO2)[Ru(p-cymene)]}BF4 (RuBF4), a catalyst derived from the commercially available [Ru(p-cymene)Cl2]2 (Ru2). Both catalysts are air-stable and readily accessible catalysts.3 Interestingly, nitrous oxide and oxygen showed similar activity for these conversions, offering the simultaneous decomposition of the greenhouse gas nitrous oxide – a common by-product in the chemical industry. Thus the dimeric ruthenium complex acts as N2OR mimic and is able to decompose nitrous oxide in presence of hydrogen donating molecules like alcohols and amines producing nitriles or aldehydes while nitrous oxide acts as hydrogen acceptor and forms nitrogen and water. In addition, one need to underline that the use of protic solvents (tBuOH) for the amine oxidation is beneficial to obtain nitrile since this solvent suits well to dissolve the organic substrates, the gaseous reagents and the catalysts, and lower the local concentrations of organic species in contrast to water and neat conditions. In contrast, for the alcohol oxidation to aldehyde, toluene turned out to be the best solvent which is related to the better solubility of the organic substrates, and of course aldehydes in aqueous solvents are known to form geminal diols in high concentrations which are readily converted into carboxylic acids under dehydrogenative or oxidative conditions.1,62 Moreover, the protocols in the lab scale are flexible, allowing for the use of two oxidation agents with similar activity, advantageous in situations of limited chemical supplies. The authors anticipate that this work will open up new possibilities in the field of homogeneous oxidation reactions with bench stable molecular catalysts and further mechanistic studies about those systems.Author contributions
Synthesis and catalysis: BASL, RSMA, MHGP; characterisation: BASL, RSMA, MHGP, AGM, DSN; concept, funding acquisition, supervision, project administration: MHGP, AMMFP, ECBAA, LHA; writing – original draft: BASL, RSMA, MHGP; writing – review & editing: BASL, RSMA, MHGP; ECBAA.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Fundação para Ciência e Tecnologia (FCT, EXPL/QUI-QOR/1079/2021; 2022.02069.PTDC, UIDB/00100/2020, UIDP/00100/2020, LA/P/0056/2020, IST-ID/086/2018), the Deutsche Forschungsgemeinschaft (DFG 411475421) the Centro de Química Estrutural/Institute of Molecular Sciences, Instituto de Química da Universidade de São Paulo (IQ-USP), Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2019/10762-1), and Conselho Nacional de Desenvolvimento Científico e Tecnológico/Conselho Nacional de Pesquisas (CNPq, 312751/2018-4). The authors are grateful to Instituto Politécnico de Lisboa for the IPL/2023/SMARTCAT_ISEL project.Notes and references
- L. E. Heim, N. E. Schloerer, J. H. Choi and M. H. G. Prechtl, Nat. Commun., 2014, 5, 3621, DOI:10.1038/ncomms4621.
- L. E. Heim, D. Thiel, C. Gedig, J. Deska and M. H. G. Prechtl, Angew. Chem., Int. Ed., 2015, 54, 10308–10312 CrossRef CAS PubMed.
- L. E. Heim, S. Vallazza, D. van der Waals and M. H. G. Prechtl, Green Chem., 2016, 18, 1469–1474 RSC.
- D. van der Waals, L. E. Heim, C. Gedig, F. Herbrik, S. Vallazza and M. H. G. Prechtl, ChemSusChem, 2016, 9, 2343–2347 CrossRef CAS PubMed.
- D. van der Waals, L. E. Heim, S. Vallazza, C. Gedig, J. Deska and M. H. G. Prechtl, Chem. – Eur. J., 2016, 22, 11568–11573 CrossRef CAS PubMed.
- M. N. A. Fetzer, G. Tavakoli, A. Klein and M. H. G. Prechtl, ChemCatChem, 2021, 13, 1317–1325 CrossRef CAS.
- G. Tavakoli and M. H. G. Prechtl, Catal. Sci. Technol., 2019, 9, 6092–6101 RSC.
- G. Tavakoli, J. E. Armstrong, J. M. Naapuri, J. Deska and M. H. G. Prechtl, Chem. – Eur. J., 2019, 25, 6474–6481 CrossRef CAS PubMed.
- M. Trincado, H. Grutzmacher and M. H. G. Prechtl, Phys. Sci. Rev., 2018, 3(5) DOI:10.1515/psr-2017-0013.
- D. M. Dooley, M. A. Mcguirl, D. E. Brown, P. N. Turowski, W. S. Mcintire and P. F. Knowles, Nature, 1991, 349, 262–264 CrossRef CAS PubMed.
- M. C. Pichardo, G. Tavakoli, J. E. Armstrong, T. Wilczek, B. E. Thomas and M. H. G. Prechtl, ChemSusChem, 2020, 13, 882–887 CrossRef CAS PubMed.
- H. Uehleke, Xenobiotica, 1971, 1, 327–338 CrossRef CAS PubMed.
- K.-I. Oinuma, Y. Hashimoto, K. Konishi, M. Goda, T. Noguchi, H. Higashibata and M. Kobayashi, J. Biol. Chem., 2003, 278, 29600–29608 CrossRef CAS PubMed.
- Y. Kato, S. Yoshida, S.-X. Xie and Y. Asano, J. Biosci. Bioeng., 2004, 97, 250–259 CrossRef CAS PubMed.
- S.-X. Xie, Y. Kato, H. Komeda, S. Yoshida and Y. Asano, Biochemistry, 2003, 42, 12056–12066 CrossRef CAS PubMed.
- D. Mahor, Z. Q. Cong, M. J. Weissenborn, F. Hollmann and W. Y. Zhang, ChemSusChem, 2022, 15, e202101116 CrossRef CAS PubMed.
- S. R. Pauleta, M. S. P. Carepo and I. Moura, Coord. Chem. Rev., 2019, 387, 436–449 CrossRef CAS.
- K. Yamaguchi, A. Kawamura, H. Ogawa and S. Suzuki, J. Biochem., 2003, 134, 853–858 CrossRef CAS PubMed.
- M. O. Ross and A. C. Rosenzweig, J. Biol. Inorg. Chem., 2017, 22, 307–319 CrossRef CAS PubMed.
- T. Haltia, K. Brown, M. Tegoni, C. Cambillau, M. Saraste, K. Mattila and K. Djinovic-Carugo, Biochem. J., 2003, 369, 77–88 CrossRef CAS PubMed.
- J. T. Drummond and R. G. Matthews, Biochemistry, 1994, 33, 3732–3741 CrossRef CAS PubMed.
- J. T. Drummond and R. G. Matthews, Biochemistry, 1994, 33, 3742–3750 CrossRef CAS PubMed.
- K. Severin, Chem. Soc. Rev., 2015, 44, 6375–6386 RSC.
- T. L. Gianetti, S. P. Annen, G. Santiso-Quinones, M. Reiher, M. Driess and H. Grützmacher, Angew. Chem., Int. Ed., 2016, 55, 1854–1858 CrossRef CAS PubMed.
- P. Jurt, A. S. Abels, J. J. Gamboa-Carballo, I. Fernández, G. Le Corre, M. Aebli, M. G. Baker, F. Eiler, F. Müller, M. Wörle, R. Verel, S. Gauthier, M. Trincado, T. L. Gianetti and H. Grützmacher, Angew. Chem., Int. Ed., 2021, 60, 25372–25380 CrossRef CAS PubMed.
- P. Lovecka, M. Thimova, P. Grznarova, J. Lipov, Z. Knejzlik, H. Stiborova, T. G. T. Nindhia, K. Demnerova and T. Ruml, BioMed Res. Int., 2015, 2015, 381264 Search PubMed.
- T. Maki and K. Takeda, in Ullmann's Encyclopedia of Industrial Chemistry, 2000 Search PubMed.
- P. Anbarasan, T. Schareina and M. Beller, Chem. Soc. Rev., 2011, 40, 5049–5067 RSC.
- K. Yamaguchi, H. Ogawa, A. Kawamura and S. Suzuki, J. Inorg. Biochem., 2003, 96, 252–252 CrossRef.
- A. Martin, N. V. Kalevaru, B. Lücke and J. Sans, Green Chem., 2002, 4, 481–485 RSC.
- D. D. Dixon and W. F. Burgoyne, Appl. Catal., 1986, 20, 79–90 CrossRef CAS.
- C. F. Koelsch and A. G. Whitney, J. Org. Chem., 1941, 6, 795–803 CrossRef CAS.
- J. X. Wu, B. Beck and R. X. Ren, Tetrahedron Lett., 2002, 43, 387–389 CrossRef CAS.
- S. G. Hammer and M. R. Heinrich, in Comprehensive Organic Synthesis, ed. P. Knochel, Elsevier, Amsterdam, 2nd edn, 2014, pp. 495–516 Search PubMed.
- M. A. Nielsen, M. K. Nielsen and A. Pittelkow, Org. Process Res. Dev., 2004, 8, 1059–1064 CrossRef CAS.
- D. V. LaBarbera, T. S. Bugni and C. M. Ireland, J. Org. Chem., 2007, 72, 8501–8505 CrossRef CAS PubMed.
- F. Brühne and E. Wright, in Ullmann's Encyclopedia of Industrial Chemistry, 2011 Search PubMed.
- J. A. B. Satrio and L. K. Doraiswamy, Chem. Eng. J., 2001, 82, 43–56 CrossRef CAS.
- A. Andersen, Int. J. Toxicol., 2006, 25, 11–27 CrossRef PubMed.
- H. Li, Y. Meng, C. Shu, X. Li, A. A. Kiss and X. Gao, ACS Sustainable Chem. Eng., 2018, 6, 14114–14124 CrossRef CAS.
- K. Hashimoto, Y. Kitaichi, H. Tanaka, T. Ikeno and T. Yamada, Chem. Lett., 2001, 922–923 CrossRef CAS.
- A. G. Tskhovrebov, M. Solari, R. Scopelliti and K. Severin, Organometallics, 2012, 31, 7235–7240 CrossRef CAS.
- J. Bösken, R. E. Rodriguez-Lugo, S. Nappen, M. Trincado and H. Grützmacher, Chem. – Eur. J., 2023, 29, e202203632, DOI:10.1002/chem.202203632.
- K. N. T. Tseng, A. M. Rizzi and N. K. Szymczak, J. Am. Chem. Soc., 2013, 135, 16352–16355 CrossRef CAS PubMed.
- I. Dutta, S. Yadav, A. Sarbajna, S. De, M. Hölscher, W. Leitner and J. K. Bera, J. Am. Chem. Soc., 2018, 140, 8662–8666 CrossRef CAS PubMed.
- T. Achard, J. Egly, M. Sigrist, A. Maisse-François and S. Bellemin-Laponnaz, Chem. – Eur. J., 2019, 25, 13271–13274 CrossRef CAS PubMed.
- M. Kannan, P. Barteja, P. Devi and S. Muthaiah, J. Catal., 2020, 386, 1–11 CrossRef CAS.
- M. Kannan and S. Muthaiah, Organometallics, 2019, 38, 3560–3567 CrossRef CAS.
- M. Kannan and S. Muthaiah, Synlett, 2020, 31, 1073–1076 CrossRef CAS.
- L. Cristian, S. Nica, O. D. Pavel, C. Mihailciuc, V. Almasan, S. M. Coman, C. Hardacre and V. I. Parvulescu, Catal. Sci. Technol., 2013, 3, 2646–2653 RSC.
- M. Olivares, P. Knörr and M. Albrecht, Dalton Trans., 2020, 49, 1981–1991 RSC.
- G. Z. Zhu, S. Shi, X. Feng, L. Zhao, Y. W. Wang, J. Q. Cao, J. Gao and J. Xu, ACS Appl. Mater. Interfaces, 2022, 14(47), 52758–52765 CrossRef CAS PubMed.
- J. H. Choi and M. H. G. Prechtl, ChemCatChem, 2015, 7, 1023–1028 CrossRef CAS.
- H. Konnerth and M. H. G. Prechtl, New J. Chem., 2017, 41, 9594–9597 RSC.
- H. Konnerth and M. H. G. Prechtl, Green Chem., 2017, 19, 2762–2767 RSC.
- M. H. G. Prechtl, J. D. Scholten and J. Dupont, J. Mol. Catal. A: Chem., 2009, 313, 74–78 CrossRef CAS.
- E. Sada, S. Kito and Y. Ito, Ind. Eng. Chem. Fundam., 1975, 14, 232–237 CrossRef CAS.
- C. B. Kretschmer, J. Nowakowska and R. Wiebe, Ind. Eng. Chem., 1946, 38, 506–509 CrossRef CAS.
- J. Tokunaga, J. Chem. Eng. Data, 1975, 20, 41–46 CrossRef CAS.
- D. Tromans, Hydrometallurgy, 1998, 48, 327–342 CrossRef CAS.
- T. Debnath, T. Ash, A. Ghosh, S. Sarkar and A. K. Das, J. Catal., 2018, 363, 164–182 CrossRef CAS.
- J. H. Choi, L. E. Heim, M. Ahrens and M. H. G. Prechtl, Dalton Trans., 2014, 43, 17248–17254 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and instrumental details on synthesis, catalysis and characterisation, including NMR, MS and GC data are available in the ESI. See DOI: https://doi.org/10.1039/d3cy01635h |
| This journal is © The Royal Society of Chemistry 2024 |