Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure and size of complete hydration shells of metal ions and inorganic anions in aqueous solution†
Ingmar
Persson

Department of Molecular Sciences, Swedish University of Agricultural Sciences, P.O. Box 7015, SE-750 07 Uppsala, Sweden. E-mail: ingmar.persson@slu.se; Tel: +46-722164969
First published on 19th August 2024
Abstract
The structures of nine hydrated metal ions in aqueous solution have been redetermined by large angle X-ray scattering to obtain experimental data of better quality than those reported 40–50 years ago. Accurate M–OI and M–(OI–H)⋯OII distances and M–OI(H)⋯OII bond angles are reported for the hydrated magnesium(II), aluminium(III), manganese(II), iron(II), iron(III), cobalt(II), nickel(II), copper(II) and zinc(II) ions; the subscripts I and II denote oxygen atoms in the first and second hydration sphere, respectively. Reported structures of hydrated metal ions in aqueous solution are summarized and evaluated with emphasis on a possible relationship between M–OI–OII bond angles and bonding character. Metal ions with high charge density have M–OI–OII bond angles close to 120°, indicative of a mainly electrostatic interaction with the oxygen atom in the water molecule in the first hydration shell. Metal ions forming bonds with a significant covalent contribution, as e.g. mercury(II) and tin(II), have M–OI–OII bond angles close to 109.5°. This implies that they bind to one of the free electron pairs in the water molecule. Comparison of M–O bond distances of hydrated metal ions in the solid state with one hydration shell, and in aqueous solution with in most cases at least two hydration shells, shows no significant differences. On the other hand, the X–O bond distance in hydrated oxoanions increases by ca. 0.02 Å in aqueous solution in comparison with the corresponding X–O distance in the solid state. A linear correlation is observed between volume, calculated from the van der Waals radius of the hydrated ion, and the ionic diffusion coefficient in aqueous solution. This correlation strongly indicates that monovalent metal ions, except lithium and silver(I), and singly-charged monovalent oxoanions have a single hydration shell. Divalent metal ions, bismuth(III) and the lanthanoid(III) and actinoid(III) ions have two hydration shells. Trivalent transition and tetravalent metal ions have two full hydration shells and portion of a third one. Doubly charged oxoanions have one well-defined hydration shell and an ill-defined second one.
Introduction
Hydrated metal ions and anions bind different number of water molecules in aqueous solution depending on their size and charge. The number of water molecules close to an ion with different measurable physico-chemical parameters to those of bulk water molecules can be regarded as the hydration number. Except for the most weakly hydrated ions with only one hydration shell, these can be divided into hydration shells where on average one water molecule in an inner shell forms hydrogen bonds to two or three water molecules in the next shell, Fig. 1. Strongly polarized water molecules bound to a highly charged metal ion and thus having a depleted charge on hydrogen, bind two water molecules in the second hydration shell, Fig. 1 left panel. When the water molecules are less polarized, each water molecule accepts one and donates two hydrogen bonds to other water molecules outside, as in the second hydration shell of a metal ion and in the hydration shells of anions, Fig. 1.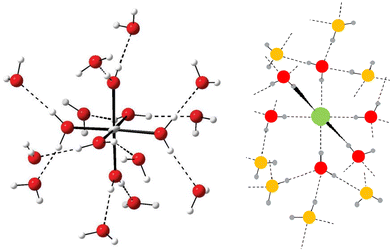 | ||
| Fig. 1 Left panel: Principle structure of a highly charged metal ion coordinating two hydration shells. The figure has been prepared from the crystallographic data in ref. 7. Right panel: Principle structure of a monoatomic anion coordinating a complete first hydration shell (red oxygen atoms) and portion of a second one (orange oxygen atoms). | ||
As part of this study the structures of nine hydrated metal ions have been redetermined in aqueous solution by large angle X-ray scattering (LAXS) to provide improved data to be placed in a general context. LAXS and large angle neutron scattering are more or less the only experimental structure methods that accurately determine long distances and thus can define to determine the broad distance distributions of the second and third hydration shells of hydrated ions in aqueous solution. Furthermore, second hydration shells or portions of them have only been described in very few cases in the solid state, vide infra. Therefore, the structure determination of hydration shells outside the first one is necessarily performed in aqueous solution. Extended X-ray Absorption Fine Structure (EXAFS) cannot be used to determine such distances as both increasing distance and the large Debye–Waller coefficient, σ2, strongly damp the EXAFS signal as discussed in detail elsewhere.1
Double difference infrared (DDIR) spectroscopy in aqueous solution containing 8% HDO, can be used to determine the O–D bond strength of the water molecules not in the aqueous bulk but bound to the ions in an electrolyte.2 The value of the O–D stretching vibration in the HDO molecule, bound to an ion, reflects the strength of the M–O(–D) or X⋯D(–O) bonds, and can be compared to the O–D bond in HDO bulk water, observed at 2509 cm−1.2 If the M–O(–D) or X⋯D(–O) bond is stronger than the (D–)O⋯D(–O) one in the aqueous bulk, the D–O stretching vibration will have a value less than 2509 cm−1. Conversely, when the M–O(–D) or X⋯D(–O) bond is weaker than the intermolecular hydrogen bond in bulk water, the O–D stretching frequency will be higher than 2509 cm−1. The lower the O–D stretching frequency, the stronger M–O(–D) or X⋯D(–O) bond, and vice versa. The X⋯D(–O) bond strength is proportional to the polarization power of anions, while for M–O(–D) bonds the O–D stretching frequencies have specific values, ca. 2535, 2420 and 2200 cm−1.2 These correspond to water in the hydration shell of weakly hydrated metal ions and the outermost hydration shell when several hydration shells are present, in the first hydration shell of metal(II) ions, lanthanoid(III) and bismuth(III) ions or the second hydration shell of tri- and tetra-positive metal ions, and in the first hydration shell of tri- and tetra-positive metal ions, respectively.2 Similar observations have been made for a recently developed vibrational spectroscopic technique, Raman difference with simultaneous curve fitting (RD-SCF).3 RD-SCF uses the O–H stretching vibration in non-bulk water where bands at ca. 3600 and 3300 cm−1 are compared with the corresponding value of bulk water at 3420 cm−1. The bands at ca. 3600 and 3300 cm−1 correspond to weakly bound water, and more strongly bound water molecules in e.g. the first hydration shell of di-and trivalent metal ions, respectively.3 The information from these methods determines whether a metal ion has structure making or breaking properties.4
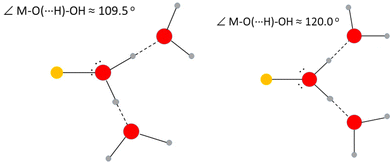
The positions relative to the metal ion of the oxygen atoms in the first and second hydration spheres give the M–OI⋯OII angle. This may be taken to indicate the bonding character of the M–O bond to the water molecules in the first hydration shell. Metal ions involved in an essentially electrostatic interaction are expected to have an M–OI⋯OII angle close to 120°, while metal ions forming significant covalent interactions will bind to one of the free electron-pairs on the water oxygen. This angle is expected to be close to the tetrahedral angle, 109.5°, due to the sp3 hybridization of the water oxygen, Fig. 2.
A series of publications from Voigt et al.5–7 has provided crystal structures of hydrated metal ions containing more water than normally found in solid structures due to crystallization at low temperature. These include MgCl2·12H2O,5 CaBr2·9H2O,6 AlCl3·15H2O, AlBr3·15H2O and AlI3·17H2O.7 AlI3·17H2O has two complete hydration shells with each water in the first hydration shell hydrogen binding two water molecules in the second one with a mean OI–(H)⋯OII distance of 2.663 Å.7 In the compounds MgCl2·12H2O and CaBr2·9H2O, portions of a second hydration shell are observed with mean OI–(H)⋯OII distances of 2.783 and 2.774 Å, respectively.5,6
The present LAXS studies have been performed on 1.5 mol dm−3 aqueous solutions of metal perchlorates in perchloric acid to avoid hydrolysis. This is a compromise between the aim to collect data with as large contribution from the hydrated metal ion to the LAXS signal as possible, and to have sufficient amount of water for complete hydration and thereby limit the interactions between the solute ions. The results show that the hydrated metal ions bind the amount of water they require for complete hydration, while there may be some deficit of water for complete hydration of the perchlorate ions.
One aim of the present study was to collect LAXS data of nine hydrated metal ions in aqueous solution of better quality than data collected 40–50 years ago. While the previously reported M–O bond distances have been largely substantiated, the limited and somewhat scattered estimates of distances to the second hydration sphere, if reported, have been considerably improved. Another aim was to use these data to augment the general knowledge of reported structures of hydrated metal ions and inorganic anions in aqueous solution, with special emphasis on a possible relationship between the M–OI–OII bond angle and bonding character of the metal ion. To support the information from LAXS data, reported vibrational spectroscopy data on O–D or O–H stretching frequencies in water molecules in the first and second hydration spheres have been used as well as reported theoretical simulations of hydrated metal ions in aqueous solution. The correlation between the size of a hydrated ion and its ionic diffusion coefficient is discussed as a means of providing further information about the number of water molecules with different physico-chemical properties to bulk water surrounding an ion.
Experimental
Chemicals
Hexaaquamagnesium(II) perchlorate (Thermo Scientific, 99%), [Mg(H2O)6](ClO4)2, nonaaquaaluminum(III) perchlorate (Thermo Scientific, 99%), [Al(H2O)6](ClO4)3·3H2O, hexaaquamanganese(II) perchlorate (Aldrich, 99%), [Mn(H2O)6](ClO4)2, hexaaquairon(II) perchlorate (Merck, 99%), [Fe(H2O)6](ClO4)2, hexaaquairon(III) perchlorate (Aldrich, 99%), [Fe(H2O)6](ClO4)3, hexaaquacobalt(II) perchlorate (Fluka, 99%), [Co(H2O)6](ClO4)2, hexaaquanickel(II) perchlorate (GFS Chemicals, 99%), [Ni(H2O)6](ClO4)2, hexaaquacopper(II) perchlorate (GFS Chemicals, 99%), [Cu(H2O)6](ClO4)2, hexaaquazinc(II) perchlorate (GFS Chemicals, 99%), [Zn(H2O)6](ClO4)2, and perchloric acid (Merck, 70 weigh%,) HClO4, were used as purchased. All water used was deionized and MilliQ filtered, resistivity >18.2 MΩ cm.Solutions
The solutions were prepared by dissolving weighed amounts of the salts in either 0.1 or 1.0 mol dm−3 perchloric acid for divalent or trivalent metal ion salts, respectively. The concentrations, densities and absorption coefficients of the perchloric acidic aqueous solutions used in the large angle X-ray scattering measurements are summarized in Table 1. Dissolution in perchloric acid was made to avoid hydrolysis reactions.| Solute | [Mn+] | [ClO4−] | [H+] | [H2O] | ρ/g cm−3 | μ/cm−1 |
|---|---|---|---|---|---|---|
| Mg(ClO4)2 | 1.5008 | 3.0767 | 0.0751 | 48.6588 | 1.2086 | 2.709 |
| Al(ClO4)3 | 1.5036 | 5.2107 | 0.6999 | 45.3949 | 1.3754 | 3.732 |
| Mn(ClO4)2 | 1.5024 | 3.0778 | 0.0730 | 44.4087 | 1.1887 | 5.331 |
| Fe(ClO4)2 | 1.5002 | 3.0719 | 0.0715 | 48.8097 | 1.2687 | 5.793 |
| Fe(ClO4)3 | 1.5000 | 5.2000 | 0.7000 | 40.4582 | 1.3306 | 6.650 |
| Co(ClO4)2 | 1.5019 | 3.0748 | 0.0710 | 49.6912 | 1.2896 | 6.342 |
| Ni(ClO4)2 | 1.4999 | 3.0728 | 0.0730 | 49.1668 | 1.2795 | 6.650 |
| Cu(ClO4)2 | 1.4864 | 3.0473 | 0.0745 | 50.2400 | 1.3027 | 7.386 |
| Zn(ClO4)2 | 1.5060 | 3.0850 | 0.0730 | 49.1101 | 1.2901 | 8.027 |
Large angle X-ray scattering
A large-angle θ–θ diffractometer was used to measure the scattering of Mo Kα radiation, λ = 0.7107 Å, from the free surface of the aqueous solutions. The solutions were contained in a Teflon cuvette inside a radiation shield with beryllium windows. After monochromatization of the scattered radiation, by means of a focusing LiF crystal, the intensity was measured at 450 discrete points in the range 1 < θ < 65° (the scattering angle is 2θ). A total of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 counts was accumulated at each angle and the whole angular range was scanned twice, corresponding to a statistical uncertainty of about 0.3%. The divergence of the primary X-ray beam was limited by 1° or 1/4° slits for different θ regions with overlap of some parts of the data for scaling purposes. All data treatment was carried out using the KURVLR program,8 which has been described in detail previously.9 The experimental intensities were normalized to a stoichiometric unit of volume containing one metal atom, using the scattering factors ƒ for neutral atoms, including corrections for anomalous dispersion, Δƒ′ and Δƒ′′,10 and values for Compton scattering.11,12 For a better alignment of the intensity function, a Fourier back-transformation was applied to eliminate spurious (unrelated to any interatomic distances) peaks below 1.2 Å in the radial distribution function.13 Least-squares refinements of the model parameters were performed by means of the STEPLR program14 to minimize the error squares sum U = ∑w(s)·[iexp(s) − ical(s)]2.
000 counts was accumulated at each angle and the whole angular range was scanned twice, corresponding to a statistical uncertainty of about 0.3%. The divergence of the primary X-ray beam was limited by 1° or 1/4° slits for different θ regions with overlap of some parts of the data for scaling purposes. All data treatment was carried out using the KURVLR program,8 which has been described in detail previously.9 The experimental intensities were normalized to a stoichiometric unit of volume containing one metal atom, using the scattering factors ƒ for neutral atoms, including corrections for anomalous dispersion, Δƒ′ and Δƒ′′,10 and values for Compton scattering.11,12 For a better alignment of the intensity function, a Fourier back-transformation was applied to eliminate spurious (unrelated to any interatomic distances) peaks below 1.2 Å in the radial distribution function.13 Least-squares refinements of the model parameters were performed by means of the STEPLR program14 to minimize the error squares sum U = ∑w(s)·[iexp(s) − ical(s)]2.
Results
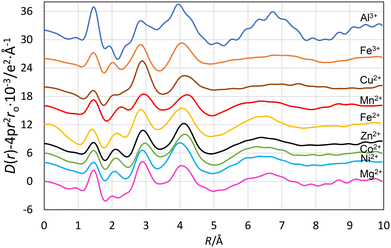
The new radial distribution functions (RDFs) from LAXS measurements for acidic aqueous solutions of magnesium(II), aluminum(III), manganese(II), iron(II), iron(III), cobalt(II), nickel(II), copper(II) and zinc(II) perchlorate show marked similarities with peaks at ca. 1.0, 1.5, 1.9–2.2, 2.9 and ca. 4.1 Å, Fig. 3. The peak at 1.0 Å corresponds to O–H bond distances in the water molecule, and the peak at 2.9 Å to the Oaq–(H)⋯Oaq distance in the aqueous bulk, and distance between the oxygens the first and second hydration shells, OI–(H)⋯OII, in the hydrated metal ion. The peaks at 1.9–2.2 Å and ca. 4.1 Å correspond to the M–OI bond and the M–(OI–H)⋯OII distances in the hydrated metal ions. The peak at 1.45 Å, and the shoulder at 2.4 Å correspond to the Cl–O and O⋯O distances in the perchlorate ion. The fits of the individual systems are shown in Fig. 4, 5 and S1–S7,† and the refined structure parameters are summarized in Table 2. The structures of these and previously reported structures of hydrated metal ions are discussed in detail in the Discussion section, vide infra.| Species/interaction | N | d/Å | b/Å2 | d(M–O) cryst./Å |
|---|---|---|---|---|
| [Mg(H2O)6(H2O)12]2+ | ||||
| Mg–OI | 6 | 2.069(5) | 0.0061(4) | 2.066 |
| Mg(–OI–H)⋯OII | 12 | 4.075(5) | 0.0248(7) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.832(2) | 0.0274(3) | |
| Cl–O | 4 | 1.452(2) | 0.0026(2) | |
| [Al(H2O)6(H2O)12(H2O)n]3+ | ||||
| Al–OI | 6 | 1.891(5) | 0.0039(5) | 1.883 |
| Al(–OI–H)⋯OII | 12 | 4.036(6) | 0.0199(4) | |
| Al(–OI–H⋯OII–H)⋯OIII | 12 | 6.70(3) | 0.030(4) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.820(2) | 0.0302(3) | |
| Cl–O | 4 | 1.453(2) | 0.0024(2) | |
| [Mn(H2O)6(H2O)12]2+ | ||||
| Mn–OI | 6 | 2.174(2) | 0.0059(5) | 2.174 |
| Mn(–OI–H)⋯OII | 12 | 4.258(7) | 0.0352(11) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.825(3) | 0.0255(4) | |
| Cl–O | 4 | 1.451(2) | 0.0025(2) | |
| [Fe(H2O)6(H2O)12]2+ | ||||
| Fe–OI | 6 | 2.124(4) | 0.0057(3) | 2.120 |
| Fe(–OI–H)⋯OII | 12 | 4.092(7) | 0.0317(8) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.832(2) | 0.0282(9) | |
| Cl–O | 4 | 1.452(2) | 0.0025(2) | |
| [Fe(H2O)6(H2O)12]3+ | ||||
| Fe–OI | 6 | 1.999(2) | 0.0040(3) | 1.995 |
| Fe(–OI–H)⋯OII | 12 | 4.012(5) | 0.0307(8) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.794(4) | 0.0283(9) | |
| Cl–O | 4 | 1.454(2) | 0.0026(2) | |
| [Co(H2O)6(H2O)12]2+ | ||||
| Co–OI | 6 | 2.090(2) | 0.0060(5) | 2.087 |
| Co(–OI–H)⋯OII | 12 | 4.072(7) | 0.0325(12) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.825(3) | 0.0256(6) | |
| Cl–O | 4 | 1.452(2) | 0.0026(2) | |
| [Ni(H2O)6(H2O)12]2+ | ||||
| Ni–OI | 6 | 2.057(2) | 0.0053(3) | 2.055 |
| Ni(–OI–H)⋯OII | 12 | 3.981(6) | 0.0304(10) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.834(2) | 0.0266(4) | |
| Cl–O | 4 | 1.453(2) | 0.0025(2) | |
| [Cu(H2O)6(H2O)8]2+ | ||||
| Cu–Oeq | 4 | 1.965(5) | 0.0040(3) | 1.980 |
| Cu–Oax1 | 1 | 2.161(9) | 0.0043(12) | 2.230 |
| Cu–Oax2 | 1 | 2.29(1) | 0.025(1) | 2.36 |
| Cu(–O–H)⋯O | 8 | 4.094(4) | 0.0277(10) | |
| Oeq(–H)⋯OII and Oaq⋯Oaq | 2 | 2.835(2) | 0.0257(3) | |
| Cl–O | 4 | 1.451(2) | 0.0023(2) | |
| [Zn(H2O)6(H2O)12]2+ | ||||
| Zn–OI | 6 | 2.091(5) | 0.0058(3) | 2.088 |
| Zn(–OI–H)⋯OII | 12 | 4.100(5) | 0.0318(8) | |
| OI(–H)⋯OII and Oaq⋯Oaq | 2 | 2.828(3) | 0.0256(5) | |
| Cl–O | 4 | 1.452(2) | 0.0024(2) | |
Discussion
The new LAXS data given above, and previously reported data on other metal ions and anions, can now be placed within the context of what is known broadly of the hydration characteristics of both cations and anions.Hydrated alkali metal ions
A large number of experimental and theoretical simulation studies has been reported on the hydrated lithium ion in aqueous solution.15–19 However, the spread in the reported results is very large, mainly due to the very weak scattering power of lithium in X-ray experiments and there is a large uncertainty in the experimental data. The hydrated lithium ion has been proposed to have tetrahedral or octahedral configuration in aqueous solution or a mixture of both. Tetrahedral coordination is predominant in the solid state with a mean Li–O bond distance of 1.941 Å for 53 structures, Table S1a (ESI†). A single structure each has been reported for the hydrated lithium ion in five- or six-coordination, giving mean Li–O bond distances of 2.044 and 2.144 Å, respectively, Table S1a (ESI†). Several different crystalline polyatomic lithium ion hydrates have described where lithium is in tetrahedral four-coordination, Table S1a (ESI†). The conclusion from these observations in both the solid state and aqueous solution is that the hydrated lithium ion prefers tetrahedral four-coordination with a mean Li–O bond distance of 1.95 Å. Four-coordination of the hydrated lithium ion is also supported by a vibrational spectroscopy study.20 The presence of two O–D stretching bands belonging to the hydrated lithium ion in HDO, one below and one above the O–D stretching frequency in bulk HDO, at 2438 and 2530 cm−1, respectively,2 indicates that the hydrated lithium ion has two hydration shells. This is further supported by a RD-SCF study reporting two O–H stretching frequencies at ca. 3300 and 3600 cm−1, due to water molecules in the first and second hydration sphere, respectively.3 Only one probable Li–(OI–D)⋯OII distance has been reported of 4.2 Å, with the corresponding Li–O and Li–(O)–D distances refined to 1.97 and 2.64 Å, respectively, giving an Li–O–D angle of ca. 125°.20DDIR spectroscopy has shown that the hydrated sodium, potassium, rubidium and cesium ions all have an O–D stretching frequency higher than in bulk water.2 A RD-SCF study only detected O–H frequencies similar to those in bulk water.3 This shows that the M–O bonds are weaker than the O–H/D⋯·O–H/D bonds in bulk water. It is therefore not expected that any second hydration sphere is present around these ions in aqueous solution as the first hydration sphere is not sufficiently polarized to form a second hydration sphere with physical properties different from those in bulk water. LAXS studies have shown that the hydrated sodium, potassium, rubidium and cesium ions have octahedral six-coordinate, irregular seven-coordinate, square antiprismatic eight-coordinate and square antiprismatic eight-coordinate configurations in aqueous solution with mean M–O bond distances of 2.43, 2.79, 2.98 and 3.08 Å, respectively.21,22 The structure determinations of these hydrated ions in aqueous solution do not provide any evidence of a well-defined second hydration shell.21,22 Several experimental structure determinations and theoretical simulations of the hydrated alkali metal ions have been reported, but the spread in M–O bond distances and coordination numbers is large.23 The sodium ion retains its first hydration shell on crystallization with reported di-, tetra-, penta-, hexa- and octahydrates as well as several kinds of polymeric structures where the sodium ion in most cases is octahedrally coordinated, Table S1b (ESI†). The most common sodium hydrate in the solid state is [Na(H2O)6]+ with octahedral coordination and a mean Na–O bond distance of 2.417 Å (28 structures), Table S1b (ESI†). Salts containing potassium, rubidium and cesium ions do crystallize either as anhydrous materials or with a number of water molecules significantly smaller than observed in aqueous solution, Tables S1c–e (ESI†).21
Alkaline earth metal ions
The structure of the hydrated beryllium(II) ion in aqueous solution has been described in a couple of studies.24,25 The reported Be–O bond distances, 1.67 and 1.6 Å, are slightly different from the mean Be–O bond distance in the [Be(H2O)4]2+ ion in the solid state, 1.613 Å (20 structures), Table S1f (ESI†). The studies in aqueous solution report also the presence of a second hydration sphere, most likely with eight water molecules, with a Be–(OI–H)⋯OII distance of 3.7–3.8 Å.24–26 A number of theoretical simulations have been reported,27–30 of which that of D'Incal et al. is in close agreement with the experimental studies with Be–OI and Be–(OI–H)⋯OII distances of 1.61 and 3.70 Å, respectively. A vibrational spectroscopic analysis strongly supports the results of the X-ray scattering and simulation studies.31The Mg–OI bond and Mg–(OI–H)⋯OII distances in the hydrated magnesium(II) ion were refined to 2.069(10) and 4.077(10) Å, respectively, Table 2; the fitted RDF and intensity functions are given in Fig. S1 (ESI†). This Mg–OI bond distance is in close agreement with the mean Mg–OI bond distance in solids containing a hexaaquamagnesium(II) ion, of 2.066 Å, an average of 521 structures, Table S1g (ESI†). The mean value of the OI(–H)⋯OII (55.5%) and Oaq(–H)⋯Oaq (44.5%) distances was refined to 2.832(5) Å, Table 2. The OI(–H)⋯OII distance was estimated as 2.785 Å from such distances in solid compounds,5vide supra, and from the refined mean value of the OI(–H)⋯OII and Oaq(–H)⋯Oaq distances; the Oaq(–H)⋯Oaq distance in dilute aqueous solutions of electrolytes is ca. 2.89 Å.21 The mean Mg–OI⋯OII angle becomes 113.4°, a result in good agreement with previously reported X-ray and neutron scattering and theoretical simualtions of the hydrated magnesium(II) ion in aqueous solution.26,32–35 Smirnov and Trostin reviewed structural studies on the hydrated magnesium(II) ion in aqueous solution up to 2007.26 They concluded that the hydrated magnesium(II) ion binds six water molecules in octahedral fashion with a mean Mg–O bond distance of 2.1 Å, also having a second hydration shell with twelve water molecules at 4.1 Å. Since then, several theoretical simulation studies have been reported supporting the experimental results in this and previous studies.29,32–35 There is a small difference in the results reported in the experimental and simulation studies, the Mg–(OI–H)⋯OII distance being 4.1 and 4.2 Å, respectively, corresponding to Mg–OI⋯OII angles of ca. 115 and 120°, respectively. The simulations seem to result in a Mg–O bond with a higher degree of electrostatic bonding than observed experimentally. The O–D and O–H stretching frequencies of the hydrated magnesium(II) ion in aqueous solution clearly indicates the presence of two hydration shells.2,3
LAXS studies of aqueous solutions of calcium(II) chloride, bromide and iodide, where the salts are fully dissociated, have shown that the hydrated calcium ion is eight-coordinate in square antiprismatic fashion with mean Ca–OI and Ca–(OI–H)⋯OII distances of 2.46 and 4.58 Å, respectively.36 The mean Ca–O bond distance in eight-coordinate hydrated calcium(II) ions in the solid state is 2.476 Å (11 structures), while it is significantly shorter in six- and seven-coordinate species present in crystalline hydrates at 2.324 and 2.401 Å (15 and 18 structures), Table S1h (ESI†), respectively.15 Earlier LAXS studies gave the same Ca–OI and Ca–(OI–H)⋯OII distances but in some cases with lower coordination numbers.23,37 A DFT simulation of the hydrated calcium(II) ion in aqueous solution has given a coordination number close to eight, and indicated the presence of a second hydration shell.30
A combined LAXS and EXAFS study of aqueous strontium(II) perchlorate and chloride solutions has defined eight-coordination in a square antiprismatic fashion with mean Sr–OI and Sr–(OI–H)⋯OII distances of 2.62 and 4.78 Å, respectively.1 Another LAXS study reported a Sr–OI bond distance of 2.64 Å, but a much longer Sr–(OI–H)⋯OII distance of 4.90 Å.38 The mean Sr–O bond distance in the octaaquastrontium(II) ion in the solid state is 2.617 Å (20 structures), while the mean Sr–O bond distances in hexa- and nonaaquastrontium(II) ions are 2.496 Å (3 structures) and 2.658 Å (1 structure), Table S1i (ESI†). The Sr–O bond distance observed in aqueous solution indicates that hydrated strontium ion is eight-coordinate square antiprismatic. A combined MD simulation and EXAFS study supports eight-coordination, but with a shorter Sr–O bond distance, 2.57 Å, than the LAXS studies.39
A LAXS study of an aqueous barium(II) perchlorate solution provided Ba–OI and Ba–(OI–H)⋯OII distances of 2.82 and 4.90 Å, respectively.1 The mean Ba–O bond distances in the octa- and nonaaquabarium(II) ions in the solid state are 2.777 and 2.832 Å (4 structures each), respectively, Table S1j (ESI†). The Ba–O bond distance observed in aqueous solution indicates that the hydrated barium ion is nine-coordinate, even though an equilibrium between eight- and nine-coordination species cannot be excluded. Two combined MD simulation and EXAFS studies provided a similar conclusion.39,40
An RD-SCF study has detected two O–H stretching frequencies of the hydrated magnesium(II), calcium(II), strontium(II) and barium(II) ions both below and above the value of bulk water, showing the presence of two well-defined hydration shells.3 DDIR spectroscopy supports the presence of two hydration shells around all alkaline earth metal ions.2
Group 3 metal ions
The hydrated group 3 metal ions, scandium(III), yttrium(III) and lanthanum(III), differ in their coordination geometry being dicapped trigonal prismatic (8-coordination),41 square antiprismatic (8-coordination)42 and tricapped trigonal prismatic (9-coordination),43 respectively, in both aqueous solution and the solid state. The hydrated scandium(III) ion binds six water molecules in a trigonal prism at 2.17 Å to which twelve water molecules are bound in a second hydration sphere at 4.27 Å.41 The mean value of the OI(–H)⋯OII (47.1%) and Oaq(–H)⋯Oaq (52.9%) distances was refined to 2.825(5) Å. Using this value the OI(–H)⋯OII distance was estimated as 2.752 Å using the Oaq(–H)⋯Oaq distance of 2.89 Å.21 The two water molecules in the capping positions are much more weakly bound at ca. 2.32 and 2.5 Å.41 As the capping water molecules are weakly bound, it is not expected that these are sufficiently polarized to form a second hydration shell with water molecules with different properties than bulk water. A summary of reported solid state structures containing a scandium(III) aqua ion is given in Table S1k (ESI†). It should be noted that the Sc–O bond distance in the capping positions are reported to be identical in some structure determinations. This is due to the fact that the compounds crystallize in space groups with higher symmetry than that of the [Sc(H2O)8]3+ complex.41The hydrated yttrium(III) ion binds eight water molecules at 2.365 Å, and 16 water molecules in a second hydration sphere at 4.40 Å in aqueous solution.42 The mean Y–O bond distance in the octaaquayttrium(III) ion in solid state is 2.353 Å (21 structures), Table S1l (ESI†). No vibrational spectroscopic studies on the hydrated scandium(III) and yttrium(III) ions have been reported.
The hydrated lanthanum(III) ion binds six water molecules in the trigonal prismatic positions at 2.515 Å, and three in the capping positions at 2.66 Å in aqueous solution.43 The water molecules in the prismatic positions hydrogen bind 12 water molecules in a second hydration sphere at 4.40 Å.43 The mean La–O bond distance in the octa- and nonaaqualanthanum(III) ions in the solid state are 2.495 (2 structures) and 2.555 Å (19 structures), Table S1m (ESI†). The RD-SCF spectrum of the hydrated lanthanum(III) ion shows that the band related to weakly coordinated water molecules is more pronounced than, e.g., with the alkaline earth and transition metal ions.3 This means that the water molecules in the second hydration sphere and those in the capping positions have interactions that are weaker than the intermolecular hydrogen bonding in the aqueous bulk and indicates that any second hydration sphere is not present outside the water molecules in the capping positions. The strength of the hydrogen bonds between the first and second hydration spheres of lanthanum(III) is of the same order as that of divalent alkaline earth and transition metal ions,2 and the OI(–H)⋯OII distance is estimated as 2.785 Å, vide supra.
Lanthanoid(III) and actinoid(IV) ions
The hydrated lighter lanthanoid(III) ions, Ce–Er, bind nine water molecules in tricapped trigonal prismatic fashion in aqueous solution and the solid state, while the heavier ones, Ho–Lu, have an increasing water deficit in the capping positions with creasing atomic number in both aqueous solution and the solid trifluoromethanesulfonate salts.44 The Ln–Op (prism) and Ln–Oc (capping) bond distances decrease with increasing atomic number, and the difference between the Ln–Op and Ln–Oc bond distances increases with increasing atomic number.44 The lanthanoid(III) ions with water deficit in the capping positions have different Ln–Oc bond distances, while for the lighter ones with all three capping positions filled, the Ln–Oc bond distances are the same.44 The solid state structures containing a hydrated lanthanoid(III) ion are summarized in Tables S1n–S1z (ESI†). The RD-SCF spectra of the hydrated gadolinium, dysprosium(III) and lutetium(III) ions are similar to that of lanthanum(III).3 DDIR spectra of the hydrated lanthanoid(III) ions are more or less identical to the lanthanum(III) one.2 A limited number of LAXS studies on hydrated lanthanoid(III) ions are reported45,46 as summarized in Table 3. However, it can be assumed that all lanthanoid(III) ions are hydrated in a similar way with a second hydration shell outside the water molecules in the prismatic positions but with most likely no polarized water molecules outside the capping positions.| Ion | CN | d(M–OI) | d(M(–OI))⋯OII | d(OI⋯OII) | ∠M–OI–OII/° | ν(O–D)/cm−1 | Ref. |
|---|---|---|---|---|---|---|---|
| Li+ | 4 | 1.95 | 4.0 | 2.85 | 114.1 | 2438 + 2530 | 2 and 15–20 |
| Na+ | 6 | 2.43 | 2534 | 2 and 21 | |||
| K+ | 7 | 2.79 | 2549 | 21 | |||
| Rb+ | 8 | 2.98 | 2560 | 21 and 22 | |||
| Cs+ | 8 | 3.08 | 2567 | 21 | |||
| Be2+ | 4 | 1.62 | 3.7 | 2.785 | 111.5 | 24–26 | |
| Mg2+ | 6 | 2.069 | 4.077 | 2.785 | 113.4 | 2428 | This work, ref. 2 and 26 |
| Ca2+ | 8 | 2.46 | 4.58 | 2.8 | 121.0 | 2540 | 2 and 36 |
| Sr2+ | 8 | 2.64 | 4.78 | 2.8 | 123.0 | 2529 | 1 and 2 |
| Ba2+ | 9 | 2.82 | 4.90 | 2.8 | 121.4 | 2537 | 1 and 2 |
| Sc3+ | 6 + 1 + 1 | 2.174 + 2.29 + 2.5 | 4.276 | 2.752 | 120.0 | 41 | |
| Y3+ | 8 | 2.365 | 4.41 | 2.725 | 119.9 | 42 | |
| La3+ | 6 + 3 | 2.527 + 2.60 | 4.63 | 2.785 | 121.2 | 2421 | 2 and 43 |
| Ce3+ | 6 + 3 | 2.516 + 2.58 | 4.56 | 2.785 | 118.6 | 45 and 46 | |
| Sm3+ | 6 + 3 | 2.43 + 2.53 | 4.56 | 2.785 | 121.0 | 45 | |
| Tb3+ | 6 + 3 | 2.40 + 2.53 | 4.50 | 2.785 | 120.1 | 45 | |
| Er3+ | 6 + 2.9 | 2.36 + 2.48 | 4.45 | 2.785 | 119.7 | 45 | |
| Th4+ | 6 + 3 | 2.454 | 4.547 | 2.725 | 122.8 | 47–49 | |
| U4+ | 6 + 3 | 2.43 | 4.43 | 2.725 | 118.4 | 52 | |
| Al3+ | 6 | 1.891 | 4.018 | 2.725 | 119.9 | 2200 + 2420 | This work, ref. 2 |
| Ga3+ | 6 | 1.959 | 4.05 | 2.725 | 118.7 | 58 | |
| In3+ | 6 | 2.131 | 4.13 | 2.725 | 116.0 | 58 | |
| Tl3+ | 6 | 2.23 | 4.20 | 2.725 | 115.5 | 59 | |
| Tl+ | 2 + 2 | 2.8 + 3.2 | 60 | ||||
| Sn2+ | 3 | 2.204 | 4.126 | 2.785 | 111.1 | 61 | |
| Pb2+ | 6 | 2.53 | 4.44 | 2.785 | 113.2 | 62 | |
| Bi3+ | 8 | 2.411 | 4.47 | 2.745 | 120.1 | 64 | |
| Cr3+ | 6 | 1.966 | 4.08 | 2.725 | 120.0 | 2200 + 2420 | 2, 58 and 65 |
| Mn2+ | 6 | 2.174 | 4.258 | 2.783 | 116.7 | 2420 | This work, ref. 2 and 72 |
| Fe2+ | 6 | 2.124 | 4.092 | 2.785 | 112.2 | 2419 | This work, ref. 2 and 77 |
| Fe3+ | 6 | 1.999 | 4.012 | 2.746 | 114.5 | This work, ref. 77 | |
| Co2+ | 6 | 2.090 | 4.072 | 2.788 | 112.4 | 2428 | This work |
| Rh3+ | 6 | 2.030 | 4.02 | 2.745 | 113.8 | 2200 + 2420 | 2 and 96–99 |
| Ni2+ | 6 | 2.057 | 3.981 | 2.788 | 109.6 | 2418 + 2530 | This work, ref. 2, 71, 89, 90 and 101 |
| Cu2+ | 6 | 1.965 + 2.16 + 2.32 | 4.094 | 2.787 | 117.9 | 2400 + 2530 | This work, ref. 2 and 109 |
| Ag+ | 2 + 2 + ? | 2.33 + 2.59 + 4 | 4.65 | 2.89 | 125.6 | 118 | |
| Zn2+ | 6 | 2.091 | 4.10 | 2.790 | 113.5 | 2418 | This work, ref. 2 and 120–122 |
| Cd2+ | 6–7 | 2.302 | 4.284 | 2.788 | 114.4 | 2423 | 2 and 126–128 |
| Hg22+ | 6 | 2.242 | 4.515 | 2.89 | 122.7 | 135 | |
| Hg2+ | 6 | 2.342 | 4.198 | 2.785 | 109.6 | 2416 | 2 and 130 |
| VO2+ | 4 + 1 | 1.992 + 2.2 | 4.06 | 2.85 | 115.4 | 137 | |
| VO2+ | 2 + 2 | 1.987 + 2.215 | 4.16 + 4.27 | 2.9 | 117.5 + 114.3 | 137 | |
| UO22+ | 5 | 2.421 | 4.37 | 2.85 | 111.7 | 138 |
The hydrated thorium(IV) ion hydrolyses very easily, and is only present in very acidic aqueous solution. Two LAXS and two EXAFS studies have shown that the mean Th–O bond distance in the hydrated thorium(IV) ion in acidic aqueous solution is ca. 2.46 Å.47–50 The number of bound water molecules differs in the reported studies, mainly due to the difficulties in determining it from LAXS and EXAFS studies. The few reported homoleptic thorium complexes with oxygen donor ligands give mean Th–O bond distances of 2.40, 2.45 and 2.50 Å in eight-, nine- and ten-coordination, respectively,47–51 Table S1aa (ESI†). The reported mean Th–O bond distance strongly indicates that the hydrated thorium(IV) ion binds ca. nine water molecules. The LAXS studies define a second hydration sphere of ca. 18 water molecules at 4.55–4.62 Å, and at least portions of a third hydration shell at 6.75–6.85 Å.47–49 In the only homoleptic aquathorium(IV) complex known in the solid state, [Th(H2O)10]Br4, thorium(IV) binds ten water molecules in bicapped square antiprismatic configuration with a mean Th–O bond distance of 2.50 Å.49
The hydrated uranium(IV) ion binds ca. 8 water molecules at ca. 2.43 Å, and a second hydration shell at ca. 4.43 Å in acidic aqueous solution.52 A summary of reported solid state structures containing an aquauranium or an aquadioxouranium ion is given in Table S1ab (ESI†).
Group 13 metal ions
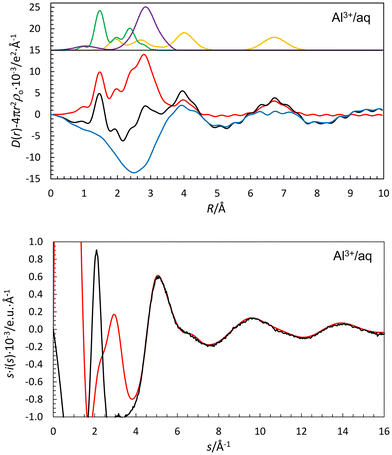
In the present work, the Al–OI and Al–(OI–H)⋯OII distances obtained of 1.891(10) and 4.018(10) Å, respectively, Table 2, are in close agreement with previous LAXS studies.53–55 In addition, portion of a third hydration shell is observed at ca. 6.7 Å, Fig. 3. The fits of the RDF and intensity functions are given in Fig. 4. The Al–OI bond distance is in close agreement with the mean Al–O bond distance in solids containing the hexaaquaaluminum(III) ion, 1.882 Å (99 structures), Table S1af (ESI†). The mean value of both the OI(–H)⋯OII (60.0%) and Oaq(–H)⋯Oaq (40.0%) distances was refined to 2.791(5) Å, Table 2, and the OI(–H)⋯OII distance was estimated as 2.725 Å in the same way as described above, giving an Al–OI⋯OII bond angle of 119.9°. Most Al–OI⋯OII bond angles in solid AlI3·17H2O are in the range 120–125°.7 Theoretical simulation studies support the structure of the hydrated aluminum(III) ion in aqueous solution found in the experimental studies.29,56 A vibrational spectroscopic analysis of the [Al(H2O)6(H2O)12]3+ complex in aqueous solution has shown the high symmetry of the hydrated aluminum(III) ion.57 DDIR spectra show two bands of the hydrated aluminum(III) ion below the O–D stretching of bulk water, 2200 and 2420 cm−1, identifying to HDO molecules in the first and second hydration shells, respectively.2 The presence of significantly polarized water molecules in the second hydration shell strongly indicates the presence of at least parts of a third hydration sphere.The hydrated gallium(III), indium(III) and thallium(III) ions bind six water molecules in octahedral fashion in the first hydration shell, and twelve in well-defined second hydration shells in aqueous solution.58,59 The mean M–OI and M–(O–H)⋯OII distances are 1.959 and 4.05 Å, and 2.131 and 4.13 Å, for gallium(III) and indium(III) ions, respectively.58 The Tl–OI bond distance in the hydrated thallium(III) ion is reported to 2.236 Å, but a Tl–(OI–H)⋯OII distance was not reported even though a significant peak in the RDF was observed at 4.20 Å.59 The mean M–O bond distances in solid compounds containing the hexaaquametal(III) ions are 1.946, 2.124 and 2.230 Å for gallium(III), indium(III) and thallium(III) as a mean of 13, 9 and 1 structures, respectively, Tables S1ag–S1ai (ESI†). No vibrational spectroscopic studies of the hydrated gallium(III), indium(III) and thallium(III) ions in aqueous solution have been reported.
The thallium(I) ion is weakly hydrated without any well-defined coordination number or figure.60 It is not expected that any second hydration shell is present in aqueous solution.
Group 14 and 15 metal ions
The aqueous chemistry of group 14 and 15 metal ions is limited to the oxidation states +II and +III, respectively, which is two below the maximum one. These ions have d10s2 electron configuration. The coordination chemistry of tin(II) and lead(II) is strongly affected by the electron configuration with at least partially filling of the Sn(5s)/Pb(6s)-ligand (np) anti-bonding orbitals causing a large gap in the coordination sphere.61,62 The hydrated tin(II) ion binds three water molecules in a footstool-like configuration with a mean Sn–OI bond distance of 2.21 Å, and a mean OI–Sn–OI bond angle of 78° in both aqueous solution and the solid state,61,63 Table S1aj (ESI†). The mean Sn–OI(–H)⋯OII distance is 4.13 Å in aqueous solution. By assuming an OI–(H)⋯OII distance of 2.785 Å, as in most divalent metal ions, vide supra, the Sn–OI–(H)⋯OII bond angle becomes 111.1°.61The hydrated lead(II) ion binds approximately six water molecules, but no detailed coordination geometry could be given.62 It can be expected that a large part of the coordination sphere is occupied by electrons in the anti-bonding orbital causing a gap in that sphere. The Pb–OI and Pb–(OI–H)⋯OII distances are 2.53 and 4.44 Å, respectively, and assuming an OI–(H)⋯OII distance of 2.785 Å, the mean Pb–(OI–H)⋯OII angle becomes 113.4°.62
The hydrated bismuth(III) ion binds eight water molecules in square antiprismatic fashion in aqueous solution with a mean Bi–OI bond distance of 2.41 Å, and the Bi–(O–H)⋯OII distance is 4.47 Å. Assuming an OI–(H)⋯OII distance of 2.785 Å, the mean Bi–(OI–H)⋯OII angle becomes 120.1°.64 In the solid state, the hydrated bismuth(III) ion is nine-coordinate in a tricapped trigonal prismatic fashion in the trifluoromethanesulfonate salt with Bi–Op and Bi–Oc bond distances of 2.435 and 2.595 Å, respectively.64 No vibrational spectroscopic studies of the hydrated tin(II), lead(II) and bismuth(III) ions in aqueous solution have been reported.
Transition metal ions
The hydrated chromium(III) ion binds six water molecules in octahedral fashion at 1.966 Å, and twelve in a second hydration shell at 4.08 Å.15,58,65 Assuming a mean Cr–(OI–H)⋯OII distance of 2.725 Å, as found for the trivalent aluminium ion, vide supra, the Cr–OI–(H)⋯OII bond angle becomes 120.0°. The mean Cr–O bond distance in hexaaquachromium(III) ions in crystalline compounds is 1.965 Å (24 structures), Table S1ap (ESI†). The results of combined MD simulation and EXAFS studies are in agreement with the LAXS studies.66,67 DDIR spectroscopy shows that the hydrated chromium(III) ion has identical absorption bands to those of aluminum, vide supra, indicating the presence of at least part of a third hydration shell.2 The LAXS studies of the hydrated chromium(III) ion in aqueous solution do however not show any well-defined Cr(–OI–H⋯OII–H)⋯OIII distance.58,65In the present study, the Mn–OI and Mn–(OI–H)⋯OII distances were refined to 2.174(4) and 4.26(2) Å, respectively, Table 2. The fits of the RDF and intensity functions are given in Fig. S2 (ESI†). The mean value of the OI(–H)⋯OII (60.9%) and Oaq(–H)⋯Oaq (39.1%) distances was refined to 2.825(5) Å, Table 2, giving an estimated OI(–H)⋯OII distance of 2.783 Å; the Mn–OI⋯OII angle becomes 116.7(2.0)°. The mean Mn–O bond distance in crystalline compounds containing a hexaaquamanganese(II) ion is 2.174 Å (166 structures), Table S1aq (ESI†). LAXS and EXAFS studies on the hydrated manganese(II) ion in aqueous solution have given Mn–O bond distances in the range 2.17–2.20 Å.68–72 However, there is a large spread in the Mn–(OI–H)⋯OII distances of 4.17–4.43 Å. A theoretical simulation study of the hydrated manganese(II) ion in aqueous solution is good agreement with the experimental result in this study.73 A vibrational spectroscopic analysis of the [Mn(H2O)6(H2O)12]2+ complex in aqueous solution has been reported,74 and a DDIR spectroscopic study has given an O–D stretch of 2420 cm−1 in agreement with the values for other divalent metal ions, showing two hydration shells around the manganese(II) ion in aqueous solution.2
The Fe–OI and Fe–(OI–H)⋯OII distances in the hydrated iron(II) ion in aqueous solution have now been refined to 2.124(8) and 4.092(14) Å, respectively, Table 2. The fits of the RDF and intensity functions are given in Fig. S3 (ESI†). The mean value of the OI(–H)⋯OII (55.3%) and Oaq(–H)⋯Oaq (44.7%) distances was refined to 2.832(5) Å, Table 2, giving an estimated OI(–H)⋯OII distance of 2.785 Å; the Fe–OI⋯OII angle becomes 112.2(1.0)°. The Fe–O bond distance is in close agreement with the mean Fe–O bond distance in crystalline compounds containing the hexaaquairon(II) ion of 2.120 Å (92 structures), Table S1ar (ESI†). LAXS, neutron scattering and EXAFS studies on the hydrated iron(II) ion in aqueous solution have given Fe–O bond distances in the range 2.10–2.13 Å.75–77 The LAXS study gives a much longer Fe–(OI–H)⋯OII distance, 4.30–4.51 Å,75 giving an unrealistically large Fe–OI⋯OII angle. This is also the case for two simulation studies of the hydrated iron(II) ion in aqueous solution.78,79 DDIR spectroscopy gives an O–D stretching frequency at 2420 cm−1 as found with other divalent metal ions, consistent with two hydration shells around the hydrated iron(II) ion.2
The Fe–OI and Fe–(OI–H)⋯OII distances of the hydrated iron(III) ion have been refined to 1.999(4) and 4.012(10) Å, respectively, in this study, Table 2. The fits of the RDF and intensity functions are given in Fig. S4 (ESI†). The mean value of the OI(–H)⋯OII (66.7%) and Oaq(–H)⋯Oaq (33.3%) distances was refined to 2.794(5) Å, Table 2, giving an estimated OI(–H)⋯OII distance of 2.746 Å, and an Fe-OI⋯OII angle of 114.5(0.7)°. The Fe–O bond distance is in close agreement with the mean Fe–O bond distance in crystals containing the hexaaquairon(III) ion of 1.995 Å (21 structures), Table S1ar (ESI†). LAXS and EXAFS studies on the hydrated iron(III) ion in aqueous solution give Fe–O bond distances in the range 1.99–2.05 Å.77,80–86 One simulation study has given slightly longer Fe–OI and Fe–(OI–H)⋯OII distances than in the experimental studies, with the Fe–O bonds deemed stronger than Al–O bonds due to a higher degree of covalency.79 Another simulation study has given Fe–OI and Fe–(OI–H)⋯OII distances in close agreement with the present LAXS data.87
The Co–OI and Co–(OI–H)⋯OII distances of the hydrated cobalt(II) ion in solution have now been refined to 2.090(4) and 4.072(14) Å, respectively, Table 2. The fits of the RDF and intensity functions are given in Fig. S5 (ESI†). The mean value of the OI(–H)⋯OII (54.4%) and Oaq(–H)⋯Oaq (45.6%) distances was refined to 2.835(5) Å, giving an estimated OI(–H)⋯OII distance of 2.788 Å and the Co–OI⋯OII angle becomes of 112.4(1.0)°. The Co–O bond distance is in close agreement with the mean Co–O bond distance in crystals containing a hexaaquacobalt(II) ion of 2.088 Å (446 structures), Table S1at (ESI†). Previous LAXS and EXAFS studies on the hydrated cobalt(II) ion in solution give Co–O bond distances in the range 2.08–2.14 Å but the Co–(OI–H)⋯OII distances are significantly longer, 4.20–4.28 Å,88–91 than in this study resulting in unrealistically wide Co–OI⋯OII angles. Combined MD simulation and EXAFS studies give Co–OI distances close to the LAXS studies of 2.09 Å.92–95 However, several of the simulation studies gave significantly longer Co–(OI–H)⋯OII distances of ca. 4.4 Å, along with unrealistic Co–OI⋯OII angles,91–93 while another gave 4.24 Å and a Co–OI⋯OII angle of 120°.95 The contribution from the second hydration shell to the EXAFS signal was judged not to be significant.92–95 DDIR spectroscopy has given an O–D stretching at 2428 cm−1 in agreement with that of other divalent metal ions, indicative of two hydration shells around the cobalt(II) ion in solution.2
The hydrated rhodium(III) ion binds six water molecules in the first hydration shell at 2.03 Å, and twelve in a second hydration shell at 4.02 Å.96–99 DDIR spectroscopy shows two O–D stretching bands at 2200 and 2420 cm−1,2 strongly indicating the presence of at least part of a third hydration shell. However, this was not confirmed in the reported LAXS studies.96–98 The Rh–O bond distance is in close agreement with the mean Rh–O bond distance in crystals containing a hexaaquarhodium(III) ion of 2.022 Å (4 structures), Table S1au (ESI†).
The Ni–OI and Ni–(OI–H)⋯OII distances in the hydrated nickel(II) ion in solution have been refined to 2.057(4) and 3.981(12) Å, respectively, in the present study, Table 2. The fits of the RDF and intensity functions are given in Fig. S6 (ESI†). The mean value of the OI(–H)⋯OII (54.9%) and Oaq(–H)⋯Oaq (45.1%) distances was refined to 2.834(5) Å, Table 2, giving an estimated OI(–H)⋯OII distance of 2.788 Å and thereby a Ni–OI⋯OII angle of 109.6(0.7)°. The Ni–O bond distance is in close agreement with the mean Ni–O bond distance in crystals containing the hexaaquanickel(II) ion of 2.055 Å (386 structures), Table S1av (ESI†). LAXS and EXAFS studies on the hydrated nickel(II) ion in aqueous solution give Ni–O bond distances in the range 2.05–2.08 Å.71,89,90,100–107 The reported Ni–(OI–H)⋯OII distances are in the range 3.99–4.3 Å,71,89,90,100–107 thus, some are in close agreement with this study,71,89,90,101 while others differ significantly with too long Ni–(OI–H)⋯OII distances for a realistic Ni–OI⋯OII angle. Combined MD simulation and EXAFS studies have given Ni–OI distances close to that of the LAXS studies, 2.05 Å.91,92,107 However, the simulations give significantly longer Ni–(OI–H)⋯OII distances of 4.27–4.4 Å and unrealistically large Ni–OI⋯OII angles.92,93,108 The contribution from the second hydration shell to the EXAFS signal is not significant.92,93,108 The DDIR spectrum showed an O–D stretch at 2418 cm−1 similar to those of other divalent metal ions and indicating two hydration shells for the nickel(II) ion in solution.2
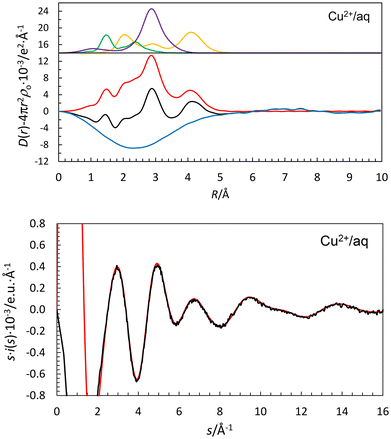
The RDF of the aqueous copper(II) perchlorate solution has a significantly different shape to those of the other hydrated metal ions in this study with significant contributions in the range 2.0–2.4 Å, Fig. 2 and 5. The peaks at 2.0 and 4.15 Å correspond to the Cu–OI and Cu–(OI–H)⋯OII distances in the equatorial positions of the Jahn–Teller distorted hydrated copper(II) ion. The shoulders in the range 2.0–2.4 Å correspond to Cu–O bond distances to water molecules in the axial positions, Cu–Oax, at different distances, as has been shown recently.109,110 The Cu–OI, Cu–Oax1, Cu–Oax2 and Cu–(OI–H)⋯OII distances were refined to 1.965(10), 2.16(2), 2.29(2) and 4.094(8) Å, respectively, which is in good agreement with EXAFS studies applying a model with two different axial bond distances,109,110Table 2. The mean value of the OI(–H)⋯OII (53.3%) and Oaq(–H)⋯Oaq (46.7%) distances was refined to 2.835(5) Å, Table 2, giving an estimated OI(–H)⋯OII distance of 2.787 Å and a Cu–OI⋯OII angle of 117.9(0.9)°. The water molecules in the weak Cu–O bonds in the axial positions are most likely not sufficiently polarized to form a second hydration shell in their vicinity.
The hexaaquacopper(II) complex has several different configurations in the solid state depending on the symmetry of space group in which the compound crystallizes. In space groups without a centre of symmetry or with one not located on copper in the Jahn–Teller distorted [Cu(H2O)6]2+ complex, different Cu–Oax bond distances are observed; the mean Cu–OI, Cu–Oax1 and Cu–Oax2 bond distances in the seven reported structures are 1.983, 2.298 and 2.362 Å, respectively.109 In centrosymmetric space groups the axial Cu–O bond distances are equal and the mean Cu–OI and Cu–Oax bond distances in the 108 reported structures are 1.980 and 2.333 Å, the solid state structures containing a tetra-, penta- or hexaaquacopper(II) ion are summarized in Table S1ax (ESI†), respectively.109 X-ray and neutron scattering, and studies on the hydrated copper(II) ion in aqueous solution have given Cu–OI bond distances in the range 1.94–1.98 Å and Cu–Oax bond distances of ca. 2.3 Å.111–118 The Cu–(OI–H)⋯OII distances in the equatorial positions lie in the range 3.95–4.20 Å.110–117 DDIR spectra show two O–D stretching bands at 2400 and 2530 cm−1,2 indicating a second hydration shell attached to the equatorially bound water molecules, while again the axially bound water molecules are most likely not sufficiently polarized to form a second hydration shell.
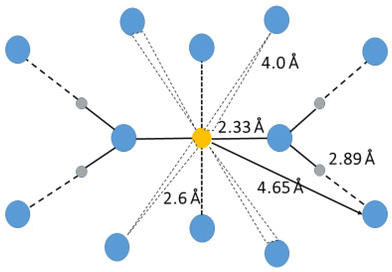
The hydrated silver(I) ion binds two water molecules in a linear fashion in the solid state with a mean Ag–O bond distance of 2.129 Å (6 structures), Table S1ay (ESI†). However, in aqueous solution the mean Ag–O bond distance is significantly longer, 2.33 Å, and a second hydration shell is apparent at 4.65 Å.119 In addition there are ca. two water molecules at ca. 2.6 Å, and another set of water molecules at ca. 4 Å, Fig. 6. The presence of these additional bound water molecules at long distances explains the significantly longer Ag–O bond distance in aqueous solution than in the solid state. The Ag–(OI–H)⋯OII angle of 125.6° is larger than observed for other hydrated metal ions, vide supra, and vide infra. The Ag–OI–HI angle was estimated as 130° in an EXAFS study supporting the result from the LAXS study.119 The loosely bound water molecules most likely repel the water molecules in the second hydration shell giving a larger Ag–(OI–H)⋯OII angle than observed for other hydrated metal ions, Table 3 and Fig. 5, even though the silver(I) ion has soft bonding characteristics. No vibrational spectroscopic studies on the hydrated silver(I) are reported.
The Zn–OI and Zn–(OI–H)⋯OII distances in the hydrated zinc(II) ion in solution have been refined to 2.091(10) and 4.100(10) Å, respectively, in the present study, Table 2. The mean value of the OI(–H)⋯OII (56.2%) and Oaq(–H)⋯Oaq (43.8%) distances was refined to 2.828(7) Å, Table 2, giving an estimated OI(–H)⋯OII distance of 2.780 Å and a Zn–OI⋯OII angle of 113.7(0.8)°. The fits of the RDF and intensity functions are given in Fig. S7 (ESI†). The Zn–O bond distance is in close agreement with the mean Zn–O bond distance in crystals containing the hexaaquazinc(II) ion, 2.088 Å (261 structures), Table S1ba (ESI†). LAXS and EXAFS studies on the hydrated zinc(II) ion in solution give Zn–O bond distances in the range 2.08–2.11 Å.90,91,120–122 Zn–(OI–H)⋯OII distances are in the range 3.95–4.26 Å.90,91,120–122 Simulation studies of the hydrated zinc(II) ion in solution are in good agreement with the experimental studies.30,123–125 The DDIR spectrum shows an O–D stretch at 2418 cm−1 in agreement with other divalent metal ion spectra, indicating two hydration shells around zinc(II) in solution.2
The Cd–OI and Cd–(O–H)⋯OII distances in the hydrated cadmium(II) ion are 2.30 and 4.28 Å.126–128 The mean Cd–O bond distance in solids containing a hexaaquacadmium ion is 2.266 Å (57 structures), Table S1bb (ESI†), while no heptaaquacadmium(II) ion has been found in the solid state so far. There are indications that an equilibrium between hexa– and heptaaquacadmium(II) ions is present in aqueous solution.126,127
The hexaaquamercury(II) ion has a regular octahedral coordination geometry in the solid perchlorate salt, crystallizing a trigonal space group with a Hg–O bond distance of 2.342 Å.129 A combined LAXS and EXAFS study of the hydrated mercury(II) ion in acidic aqueous solution revealed a highly asymmetric bond distance distribution assigned to vibronic coupling of valence states in a so-called pseudo Jahn–Teller effect that induces a distorted configuration around six-coordinate mercury(II) complexes.130 An EXAFS study of solid [Hg(H2O)6](ClO4)2 gave a mean Hg–O bond distance of 2.36 Å, while the peak maximum appears at 2.33 Å.130 The corresponding values for a perchloric acid solution of mercury(II) gave mean and peak maximum Hg–O distances of 2.38 and 2.33 Å, respectively. The crystal structure of [Hg(OS(CH3)2)]6(ClO4)2 shows three pair-wise Hg–O bond distances of 2.317, 2.320 and 2.376 Å,131 supporting a pseudo or second order Jahn–Teller distortion. By applying such a model to the hydrated mercury(II) ion in solution and in the solid perchlorate salt, the experimental observations are fully explained.130 A LAXS study on a perchloric acidic solution of mercury(II) perchlorate gave a mean Hg–O bond distance of 2.34 Å, and a very large displacement factor implying a distribution at least 0.05 Å wider than that expected for a regular octahedral hydrated divalent transition metal ion.130 A second hydration sphere is observed with a mean Hg–(O–H)⋯OII distance of 4.20 Å.130 Other EXAFS studies on the hydrated mercury(II) ion in solution have provided a mean Hg–O bond distance of 2.33 Å, and coordination number of seven.132–134 However, seven-coordination of mercury(II) ion should result in a significantly longer Hg–O bond distance than the reported one.
Each mercury atom in the dimeric mercury(I) ion, [Hg–Hg]2+, binds three water molecules at 2.24 Å, and six water molecules in a second hydration shell at 4.52 Å in aqueous solution.135 The Hg–Hg bond distance was found to be 2.524 Å. In the solid state, each mercury atom binds one water molecule at 2.135 Å, and the Hg–Hg bond distance is 2.501 Å (6 structures), Table S1bc (ESI†). A QMCF MD simulation study of the hydrated mercury(I) ion has provided somewhat longer Hg–Hg and Hg–OI distances than determined experimentally.136
Oxo- and dioxometal ions
The hydrated oxovanadium(IV) or vanadyl(IV) ion, VO2+, binds four water molecules in the equatorial plane almost perpendicular to the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond at 1.99 Å, and a second hydration shell is observed at 4.06 Å.137 One water molecule is weakly bound trans to the V
O double bond at 1.99 Å, and a second hydration shell is observed at 4.06 Å.137 One water molecule is weakly bound trans to the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bond at ca. 2.20 Å. This water molecule is most likely not sufficiently polarized to hydrogen bind to water molecules with different physical properties than bulk water. The V
O double bond at ca. 2.20 Å. This water molecule is most likely not sufficiently polarized to hydrogen bind to water molecules with different physical properties than bulk water. The V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, V–Ocis and V–Otrans bond distances in the solid state are 1.580, 2.026 and 2.187 Å (10 structures), Table S1ao (ESI†).
O, V–Ocis and V–Otrans bond distances in the solid state are 1.580, 2.026 and 2.187 Å (10 structures), Table S1ao (ESI†).
The hydrated dioxovanadium(V) or vanadyl(V) ion VO2+, has two V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in cis-configuration. Two water molecules trans to the V
O bonds in cis-configuration. Two water molecules trans to the V![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds are weakly bound at 2.22 Å, while the two cis-oxido atoms have a mean V–O bond distance of 1.99 Å, and a second hydration sphere at ca. 4.16 Å.137
O bonds are weakly bound at 2.22 Å, while the two cis-oxido atoms have a mean V–O bond distance of 1.99 Å, and a second hydration sphere at ca. 4.16 Å.137
The hydrated dioxouranium(VI) or uranyl(VI) ion, UO22+, double-binds two oxygens, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, in a trans configuration, at 1.77 Å, and five water molecules perpendicular to the O
O, in a trans configuration, at 1.77 Å, and five water molecules perpendicular to the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O unit at 2.42 Å with a second hydration sphere at 4.58 Å.138 An early LAXS study reported similar results.139 The mean U
O unit at 2.42 Å with a second hydration sphere at 4.58 Å.138 An early LAXS study reported similar results.139 The mean U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and U–OI bond distances in crystals containing the pentaaquauranyl(VI) ion are 1.760 and 2.422 Å (7 structures), Table S1ab (ESI†). A combined simulation and EXAFS study reports U–Oaq and U–(Oaq–H)⋯OII distances of 2.46 and 4.61 Å, respectively.140
O and U–OI bond distances in crystals containing the pentaaquauranyl(VI) ion are 1.760 and 2.422 Å (7 structures), Table S1ab (ESI†). A combined simulation and EXAFS study reports U–Oaq and U–(Oaq–H)⋯OII distances of 2.46 and 4.61 Å, respectively.140
Degree of polarization of the second hydration shell of hydrated metal ions
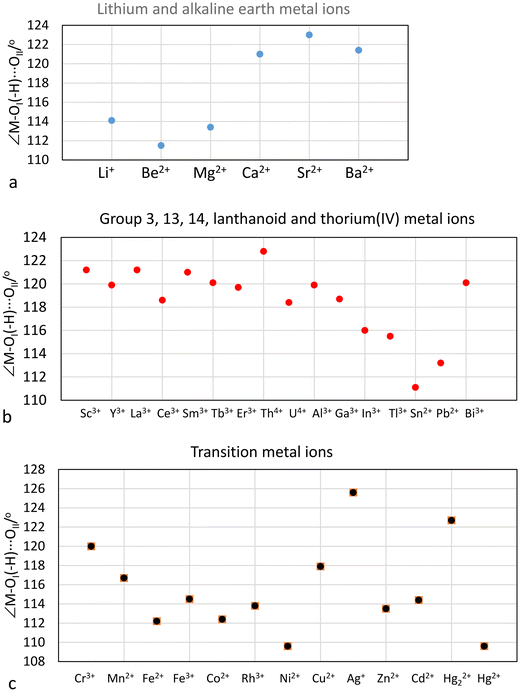
The M–OI, M–(OI–H)⋯OII and OI–(H)⋯OII distances, the M–OI–(H)⋯OII bond angle, and the O–D stretching frequencies from DDIR measurements of hydrated metal ions in aqueous solution are summarized in Table 3. The large hydrated monovalent metal ions, Na+, K+, Rb+, Cs+ and Tl+, have an O–D stretching frequency higher than that of bulk HDO showing that the M–O bond is weaker than the intermolecular hydrogen bonds in bulk water.2 These metal ions have most likely only a single hydration shell, Table 3. Furthermore, LAXS studies have not been able to prove the presence of second hydration sphere around these metal ions.21,60 The lithium ion, all divalent metal ions and the trivalent lanthanoid(III) ions have an O–D stretching frequency of ca. 2420 cm−1, showing that the M–O bond is stronger than the intermolecular hydrogen bonds in bulk water and the presence of two hydration shells,2Table 3. RD-SCF has detected two O–H stretching frequencies for these hydrated metal ions at 3300 and 3600 cm−1 corresponding to the water molecules in the first and second hydration shell, respectively. This shows that the water molecules in the first hydration shell are sufficiently polarized to hydrogen bond to a second hydration shell. A second hydration shell has indeed been detected in LAXS and neutron scattering studies of these metal ions in aqueous solution, Table 3. Tri- and tertravalent metal ions, except lanthanum(III) and the lanthanoid(III) ions, show two O–D stretching frequencies below the one in bulk water, ca. 2200 and 2420 cm−1, Table 3, strongly indicating the presence of at least portions of a third hydration shell. The third hydration shell is expected to have similar properties as the second hydration shell of divalent metal ions as they display similar O–D stretching frequencies, Table 3. At least portions of a third hydration shell has been detected for the aluminum(III) and thorium(IV) ions by LAXS, this work and ref. 47–49, while it is not sufficiently well-defined in the RDF's of the hydrated chromium(III), iron(III) and rhodium(III) ions.The M–OI–(H)–OII angle is assumed to be an indicator of the M–OI bond character, vide supra. The M–OI–(H)–OII angles of hydrated metal ions are summarized in Table 3 and Fig. 7. The small lithium, beryllium and magnesium(II)ions have M–OI–(H)–OII angles in the range 111–114°, indicating a covalent contribution in the M–O bonds, Fig. 7a. On the other hand, the larger alkaline earth metal ions, calcium, strontium and barium, have M–OI–(H)–OII angles close to 120° indicating mainly electrostatic M–O bonds, Fig. 7a. The group 3, 13, lanthanoid(III), thorium(IV) and bismuth(III) ions also have M–OI–(H)–OII angles close to 120°, Fig. 7b. The tin(II) and lead(II) ions have M–OI–(H)–OII angles close to 112° indicating a significant covalent contribution to the M–O bonds, Fig. 6b. The M–OI–(H)–OII angles in the hydrated first row transition metal ions decrease from 120 to 110° for chromium(III) to nickel(II), Fig. 7c. The Cu–OI–(H)–OII angle is surprisingly large, 118°. The d10 metal ions zinc and cadmium have M–OI–(H)–OII angles of 114°, while the mercury(II) ion, known to form bonds with a significant covalent contribution, has a Hg–OI–(H)–OII angle of 110°, Fig. 7c. The reason to the large Ag–OI–(H)–OII angle of 126°, is discussed vide supra.
Hydration of inorganic anions in aqueous solution
The monovalent halide, oxohalo and oxometallate ions, except the fluoride ion, all have an O–D stretching frequency higher than that of bulk water, Table 4, showing that they are weakly hydrated. The di- and trivalent oxoanions have O–D stretching frequencies lower than that of bulk water, and thereby form bonds to water that are stronger than the intermolecular hydrogen bonds in bulk water. The presence of a second hydration should be possible but it has not been possible to detect any second hydration shell by structure methods, except for the vanadate ion.149| Ion | d(X–O)/Å | d(X–(O)⋯Oaq)/Å | d((X–)O⋯(H–)Oaq)/Å | X⋯O⋯Oaq/° | CN | ν(O–D)/cm−1 | Ref. |
|---|---|---|---|---|---|---|---|
| d(X⋯(H)–Oaq)/Å | |||||||
| a Denotes estimated value. | |||||||
| F− | 2.7a | 4 | 2450 | 2 and 141–143 | |||
| Cl− | 3.25 | 6 | 2530 | 2, 23 and 36 | |||
| Br− | 3.36 | 6 | 2550 | 2, 23, 36 and 144 | |||
| I− | 3.61 | 8 | 2570 | 2, 23, 36 and 145 | |||
| NO3− | 1.28 | 3.61 | 3.02 | 107 | 9 | 2595 | 99, 128 and 146 |
| ClO− | 1.662 | 3.85 | 3.045 | 106 | 3 | 147 | |
| ClO2− | 1.591 | 3.881 | 3.045 | 110 | 6 | 148 | |
| ClO3− | 1.501 | 3.770 | 3.021 | 108 | 9 | 148 | |
| ClO4− | 1.453 | 3.757 | 3.046 | 109 | 12 | 2630 | 2, 62 and 148 |
| BrO3− | 1.671 | 4.068 | 2.987 | 119 | 6 | 148 | |
| IO3− | 1.829 | 4.27 | 3.013 | 122 | 6 | 148 | |
| IO4− | 1.781 | 4.243 | 3.009 | 123 | 8 | 148 | |
| MnO4− | 1.630 | 4.095 | 3.031 | 120 | 8 | 2610 | 149 |
| ReO4− | 1.735 | 4.197 | 3.01 | 122 | 8 | 2592 | 149 |
| SO32− | 1.53 | 3.68 | 2.878 | 109 | 9 | 150 | |
| SO42− | 1.495 | 3.61 | 2.85 | 108 | 12 | 2477 | 2 and 151 |
| S2O32− | 1.479 | 3.622 | 2.854 | 109 | 12 | 152 | |
| SeO32− | 1.709 | 3.87 | 2.85 | 114 | 6–9 | 2478 | 153 |
| SeO42− | 1.657 | 3.94 | 2.85 | 120 | 8 | 2480 | 153 |
| CrO42− | 1.660 | 3.955 | 2.85 | 120 | 8 | 2472 | 149 |
| MoO42− | 1.775 | 4.010 | 2.85 | 118 | 8 | 2472 | 149 |
| WO42− | 1.797 | 4.024 | 2.85 | 118 | 8 | 2472 | 149 |
| H2PO4− | 1.527 | 3.711 | 2.85 | 111 | 12 | 2478 | 154 and 155 |
| HPO42− | 1.531 | 154 and 155 | |||||
| PO43− | 1.533 | 2434 | 154 and 155 | ||||
| H2AsO3− | 1.785 | 4.096 | 2.85 | 122 | 6 | 156 | |
| AsO33− | 1.785 | 156 | |||||
| H2AsO4− | 1.707 | 156 | |||||
| HAsO42− | 1.704 | 156 | |||||
| AsO43− | 1.706 | 3.929 | 2.85 | 121 | 8 | 156 | |
| HVO42− | 1.738 | 3.754 | 2.85 | 123 | 8 | 149 | |
| Cr2O72− | 1.616 + 1.806 | 149 | |||||
An interesting observation is the difference in the X–O⋯OI angle of oxoanions as this angle also determines the number of water molecules binding to each oxygen atom in the oxoanion. The oxochloro, oxosulfur and oxophosphosphorus anions have X–O⋯OI angles close to the tetrahedral angle, 109.5°, indicating that three water molecules bind to each oxygen, while for oxobromo, oxoiodo, oxoselenium, oxoarsenic and oxometallate anions, the X-O⋯OI angle is close to 120°, indicating that only two water molecules bind to each anion oxygen, Table 4.
The X–O bond distance in all oxoanions studied, except the phosphate ion, increases by ca. 0.02 Å on hydration in aqueous solution in comparison to the X–O bond distance in the solid state. This shows that the hydration reduces the charge density of the oxoanion oxygens, and that this effect is independent of the total charge of the anion, Table 6. This lengthening of the X–O bond distance can also be expected for oxoanions such as nitrate and nitrite although there are no accurate structure determinations in aqueous solution due to the weak scattering power of nitrogen for X-rays.
Relationship between ionic diffusion coefficient and size of hydrated ions in aqueous solution
The larger the ion, the slower the expected diffusion rate in aqueous solution. Reported ionic diffusion coefficients are summarized in Table 6 together with structural information for hydrated metal ions and anions, and the estimated van der Waals radii. This has been calculated assuming a spherical model for all ions except for the linearly hydrated silver ion, where a cylindrical form has been applied. The relationship between ionic diffusion coefficient and size of hydrated ions in aqueous solution is shown in Fig. 8. The monovalent anions, monovalent metal ions, except sodium, and most divalent metal ions display a linear relationship with the assumed volume the hydrated ion. However, the volume of the aluminum(III), chromium(III) and iron(III) ions seems to be too large with three full hydration shells, but too small with two. This indicates that fractions of a third hydration shell are present around these ions, Fig. 8, as also indicated by the LAXS studies, vide supra. The volumes of the calcium(II), strontium(II), barium(II) and lead(II) ions are slightly larger than expected from the ionic diffusion coefficient-hydrated volume relationship, Fig. 8. This may indicate that the second hydration shells are not complete or that the water molecules in the second hydration shell are less stiff. This is supported by the increasing deviation from the linear relationship with increasing size/decreasing charge density of these ions, Fig. 8. The volumes of the divalent anions seem to be underestimated, indicating the presence of portion of a second hydration shell even though they have not been detected in LAXS studies. The ν(O–D) stretching frequencies of the first hydration shell being lower than in bulk water may indicate the possibility of portion of a second hydration shell, vide supra. The ionic diffusion coefficient-hydrated volume relationship strongly indicate that the sodium ion has two hydration shells even though neither the LAXS nor the DDIR studies indicate this (Table 5). The hydrated silver ion fits well into the size-ionic diffusion coefficient relationship assuming a cylindrical form.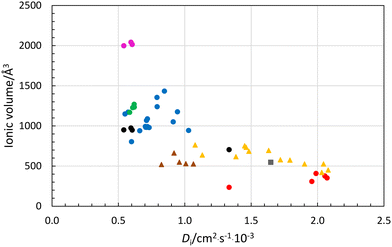 | ||
| Fig. 8 Relationship between ionic diffusion coefficient, Di, and volume of hydrated ion, Å3, using experimental structure data in aqueous solution from Table 6; red circles-monovalent metal ions assuming one hydration shell, blue circles-divalent metal ions assuming two hydration shells, green circles-lanthanum(III) and lanthanoid(III) ions assuming two hydration shells, cerice circles-trivalent metal ions assuming three hydration shells, black circles-trivalent metal ions and sodium ion assuming two hydration shells, yellow triangles-monovalent anions with one hydration shell, and brown triangles-divalent anions assuming one hydration shell. | ||
| d(M–O)(s) | d(M–O)(aq) | Diff. | Ref. | |
|---|---|---|---|---|
| ClO2− | 1.573 | 1.591 | 0.018 | 148 |
| ClO3− | 1.474 | 1.501 | 0.027 | 148 |
| ClO4− | 1.430 | 1.453 | 0.023 | 2, 62 and 148 |
| BrO3− | 1.652 | 1.671 | 0.019 | 148 |
| IO3− | 1.807 | 1.829 | 0.022 | 148 |
| IO4− | 1.756 | 1.781 | 0.025 | 148 |
| MnO4− | 1.611 | 1.630 | 0.019 | 149 |
| ReO4− | 1.710 | 1.735 | 0.025 | 149 |
| SO32− | 1.524 | 1.53 | 0.008 | 150 |
| SO42− | 1.475 | 1.495 | 0.020 | 151 |
| SeO32− | 1.692 | 1.709 | 0.017 | 153 |
| SO32− | 1.635 | 1.657 | 0.022 | 153 |
| S2O32− | 1.470 + 2.000 | 1.479 + 2.020 | 0.009 + 0.020 | 152 |
| CrO42− | 1.643 | 1.660 | 0.017 | 149 |
| Cr2O72− | 1.608 + 1.782 | 1.616 + 1.806 | 0.012 + 0.024 | 149 |
| MoO42− | 1.756 | 1.775 | 0.019 | 149 |
| WO42− | 1.776 | 1.797 | 0.021 | 149 |
| PO43− | 1.540 | 1.531 | −0.009 | 154 and 155 |
| AsO43− | 1.678 | 1.704 | 0.026 | 156 |
| VO43− | 1.720 | 1.738 | 0.018 | 149 |
| Ion |
D
i![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
d(M–O) | d(OI⋯OII) | d(M–(OI–H)⋯OII) | ∠(M–OI⋯OII) | R | V |
|---|---|---|---|---|---|---|---|
| One hydration shell ions | |||||||
| Na+ | 1.334 | 2.43 | 3.83 | 235 | |||
| Na+ | 1.334 | 2.43 | 2.95c | 4.67c | 120c | 5.52c | 704c |
| K+ | 1.957 | 2.79 | 4.19 | 308 | |||
| Rb+ | 2.072 | 2.98 | 4.38 | 352 | |||
| Cs+ | 2.056 | 2.43 | 4.48 | 377 | |||
| Tl+ | 1.989 | 2.8 + 3.2 | 4.6 | 407 | |||
| Two hydration shell ions | |||||||
| Li+ | 1.029 | 1.95 | 2.85c | 4.0 | 114.1 | 6.09 | 943 |
| Be2+ | 0.599 | 1.62 | 2.785c | 3.7 | 111.5 | 5.97 | 803 |
| Mg2+ | 0.706 | 2.069 | 2.785 | 4.08 | 113.4 | 6.17 | 983 |
| Ca2+ | 0.792 | 2.46 | 2.785 | 4.58 | 121.0 | 6.66 | 1239 |
| Sr2+ | 0.791 | 2.64 | 2.785 | 4.78 | 123.0 | 6.86 | 1355 |
| Ba2+ | 0.847 | 2.82 | 2.785 | 4.90 | 121.4 | 6.99 | 1433 |
| Mn2+ | 0.712 | 2.174 | 2.783 | 4.26 | 116.7 | 6.34 | 1068 |
| Fe2+ | 0.719 | 2.124 | 2.785 | 4.091 | 112.2 | 6.19 | 991 |
| Co2+ | 0.732 | 2.090 | 2.788 | 4.07 | 112.4 | 6.16 | 980 |
| Ni2+ | 0.661 | 2.057 | 2.787 | 3.981 | 109.6 | 6.08 | 940 |
| Cu2+ | 0.714 | 1.965 | 2.787 | 4.094 | 117.9 | 6.17 | 982 |
| Ag+ | 1.648 | 2.33 | 2.785 | 4.90 | 125.6 | 6.72; 3.6b | 547 |
| Zn2+ | 0.703 | 2.091 | 2.790 | 4.10 | 113.5 | 6.19 | 993 |
| Cd2+ | 0.719 | 2.302 | 2.785 | 4.284 | 114.4 | 6.38 | 1088 |
| Hg2+ | 0.913 | 2.33 | 2.785 | 4.198 | 109.6 | 6.31 | 1051 |
| Pb2+ | 0.945 | 2.53 | 2.785 | 4.44 | 113.2 | 6.55 | 1176 |
| Sc3+ | 0.574 | 2.174 | 2.752 | 4.276 | 120.0 | 6.35 | 1171 |
| Y3+ | 0.550 | 2.365 | 2.725 | 4.41 | 119.9 | 6.50 | 1149 |
| La3+ | 0.619 | 2.527 | 2.785 | 4.63 | 121.2 | 6.72 | 1269 |
| Ce3+ | 0.620 | 2.516 | 2.785 | 4.56 | 119.9 | 6.65 | 1234 |
| Sm3+ | 0.608 | 2.43 | 2.785 | 4.56 | 121.0 | 6.64 | 1227 |
| Er3+ | 0.585 | 2.36 | 2.785 | 4.45 | 120.1 | 6.54 | 1169 |
| Al3+ | 0.541 | 1.891 | 2.725 | 5.52 | 119.9 | 6.10c | 950c |
| Cr3+ | 0.595 | 1.966 | 2.725 | 5.58 | 120.0 | 6.15c | 974c |
| Fe3+ | 0.604 | 1.999 | 2.746 | 5.51 | 114.5 | 6.10c | 949c |
| Three hydration shell ions | |||||||
| Al3+ | 0.541 | 1.891 | 2.725 | 5.52 | 119.9 | 7.81 | 1999 |
| Cr3+ | 0.595 | 1.966 | 2.725 | 5.58 | 120.0 | 7.88 | 2042 |
| Fe3+ | 0.604 | 1.999 | 2.746 | 5.51 | 114.5 | 7.83 | 2014 |
| Ion | D i | d(X–O) | d(X⋯OI) | d(O–(H)⋯OI) | d(X–(H)⋯OI) | R | V |
|---|---|---|---|---|---|---|---|
| a Ref. 157. b Length and radius of an applied cylindric model. c Denotes assumed value. | |||||||
| Cl− | 2.032 | 3.25 | 4.65 | 421 | |||
| Br− | 2.080 | 3.36 | 4.76 | 452 | |||
| I− | 2.045 | 3.61 | 5.01 | 527 | |||
| NO3− | 1.902 | 1.28 | 3.02 | 3.61 | 5.01 | 527 | |
| ClO2− | 1.382 | 1.591 | 3.040 | 3.881 | 5.281 | 617 | |
| ClO3− | 1.72 | 1.501 | 3.021 | 3.770 | 5.170 | 579 | |
| ClO4− | 1.792 | 1.453 | 3.046 | 3.757 | 5.157 | 574 | |
| BrO3− | 1.483 | 1.671 | 2.987 | 4.068 | 5.468 | 685 | |
| IO3− | 1.078 | 1.829 | 3.013 | 4.270 | 5.670 | 764 | |
| IO4− | 1.451 | 1.781 | 3.009 | 4.243 | 5.643 | 753 | |
| MnO4− | 1.632 | 1.630 | 3.031 | 4.095 | 5.495 | 695 | |
| ReO4− | 1.462 | 1.735 | 3.010 | 4.197 | 5.597 | 734 | |
| CrO42− | 1.132 | 1.660 | 2.85 | 3.940 | 5.340 | 637 | |
| WO42− | 0.991 | 1.775 | 2.85 | 4.010 | 5.410 | 663 | |
| SO32− | 0.959 | 1.53 | 2.878 | 3.68 | 5.080 | 549 | |
| SO42− | 1.065 | 1.495 | 2.85 | 3.61 | 5.010 | 527 | |
| SeO42− | 1.008 | 1.657 | 2.85 | 3.622 | 5.022 | 531 | |
| PO43− | 0.824 | 1.537 | 2.815 | 3.588 | 4.988 | 520 | |
Conclusions
The good correlation between calculated volume of the hydrated metal ions from the LAXS measurements and the ionic diffusion coefficients metal ions and anions at infinite dilution, summarized in Table 6 and Fig. 8, show that the hydrate structures studied in relatively concentrated solution are also representative for dilute solutions. It is possible that the outer hydration shells of both metal ions and inorganic anions becomes more well-defined in dilute solution where the competition of the available water molecules is very limited.The results presented in this paper describe the arrangement of water molecules around metal ions and inorganic anions in aqueous solution. This information must be obtained in aqueous solution as at crystallization hydrate bonds weaker than hydrogen bonds in the aqueous bulk will transfer to the remaining aqueous bulk for bonding energy reasons. This can be partly overcome by crystallization at low temperatures as shown by Voigt and coworkers.5–7 However, the magnesium(II) and calcium(II) ions have only portions a second hydration shell in the solid state,5,6 and the aluminium(III) ion is completely lacking a third hydration shell.7 It is important to stress that hydrated metal ions and inorganic anions interact with significantly more water in aqueous solution than the amount observed in solid state structures. This is especially important at the analysis of reaction mechanisms and transport properties involving metal ions and anions in aqueous solution.
The alkali metal ions, except lithium, and the thallium(I) ion are weakly hydrated in aqueous solution and form only a single hydration shell with different physico-chemical properties to those of bulk water. However, the ionic diffusion coefficient-volume relationship supports the presence of two hydration shells around the sodium ion, Fig. 8. The lithium, silver(I), metal(II), scandium(III), yttrium(III), lanthanum(III) and lanthanoid(III) ions, and most likely the actinoid(III) ions, form two hydration shells with the second hydration shell water molecules having similar physico-chemical properties to those of the first hydration shell of the alkali and thallium(I) ions. The trivalent transition metal ions and the thorium(IV) and uranium(IV) ions form two well-defined hydration shells and portions of a third hydration shell. The M–O bond distances are not significantly affected by the presence of a second hydration shell, and M–O bond distances observed in solid compounds can be transferred to aqueous solution. On the other hand, the X–O bond distance in oxoanions, except phosphate, increase by ca. 0.02 Å in aqueous solution due to the hydration in comparison to the X–O bond distances in solid compounds where the anion is not bound to a metal ion other than the alkali metal ions. The monovalent anions have a single hydration shell, while the divalent anions form in addition portions of a second hydration shell, evidently in the lower ionic diffusion coefficients than expected for the size with a single hydration shell. The trivalent anions may have two hydration shells as experimentally observed for the vanadate ion.
Data availability
All experimental data are available upon request to author at E-mail: ingmar.persson@slu.se.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The support from the Department of Molecular Sciences, Swedish University of Agricultural Sciences, Uppsala, is gratefully acknowledged. Dr Lars Eriksson, Department of Materials and Environmental Chemistry, Stockholm University, is acknowledged for plotting the figure used in Fig. 1a and as TOC.References
- I. Persson, M. Sandström, H. Yokoyama and M. Chaudhry, Structures of the Solvated Barium and Strontium Ions in Aqueous, Dimethyl Sulfoxide and Pyridine Solution, and Crystal Structure of Strontium and Barium Hydroxide Octahydrate, Z. Naturforsch., A: Phys. Sci., 1995, 50, 21–37 CrossRef CAS.
- J. Stangret and T. Gampe, Ionic Hydration Behaviour Derived From Infrared Spectra in HDO, J. Phys. Chem. A, 2002, 106, 5393–5402 CrossRef CAS.
- A. Patra, S. Roy, S. Saha, D. K. Palit and J. A. Mondal, Observation of Extremely Weakly Interacting OH (∼3600 cm−1) in the Vicinity of High Charge Density Metal Ions (Mz+; z = 1,2,3): A Structural Heterogeneity in the Extended Hydration Shell, J. Phys. Chem. C, 2020, 124, 3028–3036 CrossRef CAS.
- Y. Marcus, Effect of Ions on the Structure of Water: Structure Making and Breaking, Chem. Rev., 2009, 109, 1346–1370 CrossRef CAS PubMed , and references therein.
- E. Hennings, H. Schmidt and W. Voigt, Crystal Structures of Hydrated of Simple Inorganic Salts. I. Water-rich Magnesium Halides MgCl2·8H2O, MgCl2·12H2O, MgBr2·6H2O, MgBr2·9H2O, MgI2·8H2O and MgI2·9H2O, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2013, 69, 1292–1300 CrossRef CAS PubMed.
- E. Hennings, H. Schmidt and W. Voigt, Crystal Structures of Hydrated of Simple Inorganic Salts. II. Water-rich Calcium Bromide and Iodide Hydrates: CaBr2·9H2O, CaI2·8H2O, CaI2·7H2O and CaI2·6.5H2O, Acta Crystallogr., Sect. C: Struct. Chem., 2014, 70, 876–881 CrossRef CAS PubMed.
- E. Hennings, H. Schmidt and W. Voigt, Crystal Structures of Hydrated of Simple Inorganic Salts. III. Water-rich Aluminium Halide Hydrates: AlCl3·15H2O, AlBr3·15H2O, AlI3·17H2O and AlBr3·9H2O, Acta Crystallogr., Sect. C: Struct. Chem., 2014, 70, 882–888 CrossRef PubMed.
- G. Johansson and M. Sandström, Computer-programs for Analysis of Data on X-ray-Diffraction by Liquids, Chem. Scr., 1973, 4, 195–198 CAS.
- C. M. V. Stålhandske, I. Persson, M. Sandström and E. Kamienska-Piotrowicz, A Large Angle Scattering and Vibrational Spectroscopic Study of the Solvated Zinc, Cadmium and Mercury(II) Ions in N,N-Dimethylthioformamide Solution, Inorg. Chem., 1997, 36, 3174–3182 CrossRef PubMed.
- International Tables for X-ray Crystallography, Kynoch Press, Birmingham, U.K., 1974, vol. 4 Search PubMed.
- D. T. Cromer, Compton Scattering Factors for Aspherical Free Atoms, J. Chem. Phys., 1969, 50, 4857–4859 CrossRef CAS.
- D. T. Cromer and J. B. Mann, Compton Scattering Factors for Spherically Symmetric Free Atoms, J. Chem. Phys., 1967, 47, 1892–1893 CrossRef CAS.
- H. A. Levy, M. D. Danford and A. H. Narten, Data Collection and Evaluation with an X-ray Diffractometer Designed for the Study of Liquid Structure, Technical Report ORNL-3960, Oak Ridge National Laboratory, Oak Ridge, TN, 1966 Search PubMed.
- M. Molund and I. Persson, STEPLR - A Program for Refinements of Data on X-Ray Scattering by Liquids, Chem. Scr., 1985, 25, 197–197 CAS.
- H. Ohtaki and T. Radnai, Structure and Synamics of Hydrated Ions, Chem. Rev., 1993, 93, 1157–1204 CrossRef CAS . and references therein.
- P. R. Smirnov and V. N. Trostin, Structure of the Nearest Surrounding of the Li+ Ion in Aqueous Solutions of Their Salts, Russ. J. Gen. Chem., 2006, 76, 175–182 CrossRef CAS , and references therein.
- P. R. Smirnov, Structure of the Li+ Ion Close Environment in Various Solvents, Russ. J. Gen. Chem., 2019, 89, 2443–2452 CrossRef CAS , and references therein.
- Y. Kameda, T. Miyazaki, T. Otomo, Y. Amo and T. Usuki, Neutron Diffraction Study on the Structure of Aqueous LiNO3 Solutions, J. Solution Chem., 2014, 43, 1588–1600 CrossRef CAS.
- Y. Kameda, T. Miyazaki, T. Otomo, Y. Amo, T. Usuki, K. Ikeda and T. Otomo, Neutron Diffraction Study on the Structure of Hydrated Li+ in Dilute Aqueous Solutions, J. Phys. Chem., 2018, 122, 1695–1701 CrossRef CAS PubMed.
- W. Rudolph, M. H. Brooker and C. C. Pye, Hydration of Lithium Ion in Aqueous Solution, J. Phys. Chem., 1995, 99, 3793–3797 CrossRef CAS.
- J. Mähler and I. Persson, A Study of the Hydration of the Alkali Metal Ions in Aqueous Solution, Inorg. Chem., 2012, 51, 425–438 CrossRef PubMed.
- P. D'Angelo and I. Persson, On the Structure of the Hydrated and Dimethylsulfoxide Solvated Rubidium Ions in Solution, Inorg. Chem., 2004, 43, 3543–3549 CrossRef PubMed.
- I. Persson, Structures of hydrated metal ions in solid state and aqueous solution, Liquids, 2022, 2, 210–242 CrossRef CAS , and references therein.
- T. Yamaguchi, H. Ohtaki, E. Spohr, G. Pálinkás, K. Heinzinger and M. M. Probst, Molecular Dynamics and X-ray Diffraction Study of Aqueous Beryllium(II) Chloride Solutions, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1986, 41, 1175–1185 Search PubMed.
- P. E. Mason, S. Ansell, G. W. Neilson and J. W. Brady, Be2+ Hydration in Concentrated Aqueous Solutions of BeCl2, J. Phys. Chem. B, 2008, 112, 1935–1939 CrossRef CAS PubMed.
- P. R. Smirnov and V. N. Trostin, Structural Patrameters of Hydration of Be2+ and Mg2+ in Aqueous Solution of Their Salts, Russ. J. Gen. Chem., 2008, 78, 1643–1649 CrossRef CAS , and references therein.
- A. D'Incal, T. S. Hofer, B. R. Randolf and B. M. Rode, Be(II) in Aqueous Solution - An Extended ab initio QM/MM MD Study, Phys. Chem. Chem. Phys., 2006, 8, 2841–2847 RSC.
- C. C. Pye, An Ab Initio Study of Beryllium(II) Hydration, J. Mol. Struct.: THEOCHEM, 2009, 913, 210–214 CrossRef CAS.
- J. M. Martínez, R. R. Pappalardo and E. Sánchez Marcos, First-Principles Ion-Water Interaction Potentials for Highly Charged Monatomic Cations. Computer Simulations of Al3+,Mg2+, and Be2+ in Water, J. Am. Chem. Soc., 1999, 121, 3175–3184 CrossRef.
- M. Pavlov, P. E. M. Siegbahn and M. Sandström, Hydration of Beryllium, Magnesium, Calcium, and Zinc Ions Using Density Functional Theory, J. Phys. Chem. A, 1998, 102, 219–228 CrossRef CAS.
- W. W. Rudolph, D. Fischer, D. Irmer and C. C. Pye, Hydration of Beryllium(II) in Aqueous Solutions of Common Inorganic Salts. A Combined Vibrational Spectroscopic and ab initio Molecular Orbital Study, Dalton Trans., 2009, 6513–6527 RSC.
- D. Di Tommaso and N. H. de Leeuw, Structure and Dynamics of the Hydrated Magnesium Ion and of the Solvated Magnesium Carbonates: Insights From First Principles Simulations, Phys. Chem. Chem. Phys., 2010, 12, 894–901 RSC.
- A. Bhattacharjee, A. B. Pribil, B. R. Randolf, B. M. Rode and T. S. Hofer, Hydration of Mg2+ and Its Influence on the Water Hydrogen Bonding Network via ab initio QMCF MD, Chem. Phys. Lett., 2012, 536, 39–44 CrossRef CAS.
- B. Das and A. Chandra, Ab Initio Molecular Dynamics Study of Aqueous Solutions of Magnesium and Calcium Nitrates: Hydration Shell Structure, Dynamics and Vibrational Echo Spectroscopy, J. Phys. Chem. B, 2022, 126, 528–544 CrossRef CAS PubMed.
- Y. Wang, G. Wang, D. T. Bowron, F. Zhu, A. C. Hannon, Y. Zhou, X. Liud and G. Shi, Unveiling the Structure of Aqueous Magnesium Nitrate Solutions by Combining X-ray Diffraction and Theoretical Calculations, Phys. Chem. Chem. Phys., 2022, 24, 22939–22949 RSC.
- F. Jalilehvand, D. Spångberg, P. Lindqvist-Reis, K. Hermansson, I. Persson and M. Sandström, The Hydration of the Calcium Ion. An EXAFS, Large Angle X-Ray Scattering and Molecular Dynamics Simulation Study, J. Am. Chem. Soc., 2001, 123, 431–441 CrossRef CAS PubMed.
- T. Yamaguchi, S. Hayasshi and H. Ohtaki, X-ray Diffraction Study of Calcium(II) Chloride Hydrate Melts: CaCl2·RH2O (R = 4.0, 5.6, 6.0, and 8.6), Inorg. Chem., 1989, 28, 2434–2439 CrossRef CAS.
- R. Caminiti, A. Musinu, G. Paschina and G. Pinna, X-ray Diffraction Study of Aqueous SrCl2 Solutions, J. Appl. Crystallogr., 1982, 15, 482–487 CrossRef CAS.
- R. R. Pappalardo, J. M. Martínez, D. Z. Caralampio and E. Sánchez Marcos, Hydration of Heavy Alkaline-Earth Cations Studied by Molecular Dynamics Simulations and X-ray Absorption Spectroscopy, Inorg. Chem., 2021, 60, 13578–13587 CrossRef CAS PubMed.
- V. Migliorati, A. Caruso and P. D'Angelo, Unraveling the Hydration Properties of the Ba2+ Aqua Ion: the Interplay of Quantum Mechanics, Molecular Dynamics, and EXAFS Spectroscopy, Inorg. Chem., 2019, 58, 14551–14559 CrossRef CAS PubMed.
- P. Lindqvist-Reis, I. Persson and M. Sandström, The Hydration of the Scandium(III) Ion in Aqueous Solution and Crystalline Hydrates Studied by XAFS Spectroscopy, Large Angle X-Ray Scattering and Crystallography, Dalton Trans., 2006, 3868–3878 RSC.
- P. Lindqvist-Reis, K. Lamble, S. Pattanaik, M. Sandström and I. Persson, Hydration of the Yttrium(III) Ion in Aqueous Solution. An X-ray Diffraction and XAFS Structural Study, J. Phys. Chem. B, 2000, 104, 402–408 CrossRef CAS.
- J. Näslund, P. Lindqvist-Reis, I. Persson and M. Sandström, Steric Effects Control the Structure of the Solvated Lanthanum(III) Ion in Aqueous, Dimethyl Sulfoxide, and N,N’-Dimethylpropyleneurea Solution. An EXAFS and Large-Angle X-ray Scattering Study, Inorg. Chem., 2000, 39, 4006–4011 CrossRef PubMed.
- I. Persson, P. D'Angelo, S. De Panfilis, M. Sandström and L. Eriksson, Hydration of the Lanthanoid(III) Ions in Aqueous Solution and Crystalline Hydrates Studied by EXAFS Spectroscopy and Crystallography. The Myth of the “Gadolinium Break”, Chem. – Eur. J., 2008, 14, 3056–3066 CrossRef CAS PubMed.
- G. Johansson and H. Wakita, X-ray Investigation of the Coordination and Complex Formation of Lanthanoid Ions in Aqueous Perchlorate and Selenate Solutions, Inorg. Chem., 1985, 26, 3047–3052 CrossRef.
- R. Caminiti, P. Cucca and A. D'Andrea, Hydration Phenomena in a Concentrated Aqueous Solution of Ce(NO3)3 - X-ray Diffraction and Raman Spectroscopy, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1983, 38, 533–539 Search PubMed.
- N. Torapava, I. Persson, L. Eriksson and D. Lundberg, Hydration and Hydrolysis of Thorium(IV) in Aqueous Solution and the Structures of Two Crystalline Thorium(IV) Hydrates, Inorg. Chem., 2009, 48, 11712–11723 CrossRef CAS PubMed.
- G. Johansson, M. Magini and H. Ohtaki, Coordination Around Thorium(IV) in Aqueous Perchlorate, Chloride and Nitrate Solutions, J. Solution Chem., 1991, 20, 775–792 CrossRef CAS.
- R. E. Wilson, S. Skanthakumar, P. C. Burns and L. Soderholm, Structure of the Homoleptic Thorium(IV) Aqua Ion [Th(H2O)10]Br4, Angew. Chem., Int. Ed., 2007, 46, 8043–8045 CrossRef CAS PubMed.
- R. Spezia, C. Beuchat, R. Vuilleumier, P. D'Angelo and L. Gagliardi, Unravelling the Hydration Structure of ThX4 (X = Br, Cl) Water Solutions by Molecular Dynamics Simulations and X-ray Absorption Spectroscopy, J. Phys. Chem. B, 2012, 116, 6465–6475 CrossRef CAS PubMed.
- N. Torapava, D. Lundberg and I. Persson, A Coordination Chemistry Study of Solvated Thorium(IV) Ions in Two Oxygen-Donor Solvents, Eur. J. Inorg. Chem., 2011, 5273–5278 CrossRef CAS.
- S. Pocev and G. Johansson, An X-ray Investigation of the Coordination of the Hydrolysis of the Uranium(IV) Ion in Aqueous Perchlorate Solutions, Acta Chem. Scand., 1973, 27, 2146–2160 CrossRef CAS.
- W. Bol and T. Welzen, The interpretation of X-ray diffraction by aqueous solutions of aluminum(III) nitrate and chromium(III) nitrate, Chem. Phys. Lett., 1977, 49, 189–192 CrossRef CAS.
- R. Caminiti, G. Licheri, G. Piccaluga, G. Pinna and T. Radnai, Order phenomena in aqueous AlCl3 solutions, J. Chem. Phys., 1979, 71, 2473–2476 CrossRef CAS.
- R. Caminiti and T. Radnai, X-ray Diffraction Study of a Concentrated Al(NO3)3 Solution, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1980, 35, 1368–1372 Search PubMed.
- V. E. Barlette, L. C. G. Freitas, P. H. Guadagnini and C. A. A. Bertran, Hydration properties of Al3+ ion using empirical ion-water potential by Monte Carlo simulation, J. Braz. Chem. Soc., 2008, 19, 101–110 CrossRef CAS.
- W. W. Rudolph, R. Mason and C. C. Pye, Aluminium(III) hydration in aqueous solution. A Raman spectroscopic investigation and an ab initio molecular orbital study of aluminium(III) water clusters, Phys. Chem. Chem. Phys., 2000, 2, 5030–5040 RSC.
- P. Lindqvist-Reis, S. Díaz-Moreno, A. Munoz-Páez, S. Pattanaik, I. Persson and M. Sandström, On the Structure of Hydrates Gallium, Indium and Chromium(III) Ions in Aqueous Solutions. A Large Angle X-ray Scattering and EXAFS Study, Inorg. Chem., 1998, 37, 6675–6683 CrossRef CAS PubMed.
- J. Glaser and G. Johansson, On the structures of the Hydrated Thallium(III) Ion and it Bromide Complexes in Aqueous Solution, Acta Chem. Scand., Ser. A, 1982, 36, 125–135 CrossRef.
- I. Persson, F. Jalilehvand and M. Sandström, Structure of the Solvated Thallium(I) Ion in Aqueous, Dimethyl Sulfoxide, N,N′-Dimethylpopyleneurea and N,N-Dimethylthioformamide Solution, Inorg. Chem., 2002, 41, 192–197 CrossRef CAS PubMed.
- I. Persson, P. D'Angelo and D. Lundberg, Hydrated and Solvated Tin(II) Ions in Solution and the Solid State, and a Coordination Chemistry Overview of the d10s2, Chem. – Eur. J., 2016, 22, 18583–18592 CrossRef CAS PubMed.
- I. Persson, K. Lyczko, D. Lundberg, L. Eriksson and A. Płaczek, A Coordination Chemistry Study of Hydrated and Solvated Lead(II) Ions in Solution and Solid State, Inorg. Chem., 2011, 50, 1058–1072 CrossRef CAS PubMed.
- E. Hennings, H. Schmidt, M. Köhler and W. Voigt, Crystal Structure of Tin(II) Perchlorate Trihydrate, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2014, 70, 474–476 CrossRef CAS PubMed.
- J. Näslund, I. Persson and M. Sandström, Solvation of the Bismuth(III) Ion by Water, Dimethyl Sulfoxide, N,N’-Dimethylpropyleneurea, and N,N-Dimethylthioformamide. An EXAFS, Large-Angle X-ray Scattering, and Crystallographic Structural Study, Inorg. Chem., 2000, 39, 4012–4021 CrossRef PubMed.
- R. Caminiti, G. Licheri, G. Piccaluga and G. Pinna, Hydration water–external water interactions around Cr3+ ions, J. Chem. Phys., 1977, 69, 1–4 CrossRef.
- A. Muñoz-Páez, R. R. Pappalardo and E. Sánchez Marcos, Determination of the Second Hydration Shell of Cr3+ and Zn2+ in Aqueous Solutions by Extended X-ray Absorption Fine Structure, J. Am. Chem. Soc., 1995, 117, 11710–11720 CrossRef.
- P. J. Merkling, A. Muñoz-Páez and E. Sánchez Marcos, Exploring the Capabilities of X-ray Absorption Spectroscopy for Determining the Structure of Electrolyte Solutions: Computed Spectra for Cr3+or Rh3+in Water Based on Molecular Dynamics, J. Am. Chem. Soc., 2002, 124, 10911–10920 CrossRef CAS PubMed.
- Y. Tajiri, N. Ichihashi, T. Mibuchi and H. Wakita, An X-Ray Diffraction Investigation of the Coordination Structure of Mn(II) Ions in Highly Concentrated Aqueous MnBr2 and MnCl2 Solutions, Bull. Chem. Soc. Jpn., 1986, 59, 1155–1159 CrossRef CAS.
- R. Caminiti, G. Cucca and T. Pintori, Hydration and ion-pairing in concentrated aqueous Mn(NO3)2 solutions. An X-ray and Raman Spectroscopy Study, Chem. Phys., 1984, 88, 155–161 CrossRef CAS.
- R. Caminiti, G. Marongiu and G. Paschina, A Comparative X-ray Diffraction Study of Aqueous MnSO4 and Crystals of MnSO4·5H2O, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1982, 37, 581–586 Search PubMed.
- G. Licheri, G. Paschina, G. Piccaluga and G. Pinna, X–ray Diffraction Study of Aqueous Solutions of NiSO4 and MnSO4, J. Chem. Phys., 1984, 81, 6059–6063 CrossRef.
- H. Konieczna, D. Lundberg and I. Persson, Solvation and Coordination Chemistry of Manganese(II) Ion in Solvents with Solvation Properties. A Transfer Thermodynamic, Complex Formation, EXAFS Spectroscopic and Crystallographic Study, Polyhedron, 2021, 195, 114961 CrossRef CAS.
- C. F. Schwenk, H. H. Loeffler and B. M. Rode, Structure and Dynamics of Metal Ions in Solution: QM/MM Molecular Dynamics Simulations of Mn2+ and V2+, J. Am. Chem. Soc., 2003, 125, 1618–1624 CrossRef CAS PubMed.
- W. W. Rudolph and G. Irmer, Hydration and Speciation Studies of Mn2+ in Aqueous Solution with Simple Monovalent Anions (ClO4−, NO3−, Cl−, Br−), Dalton Trans., 2013, 42, 14460–14472 RSC.
- E. Kálmán, T. Radnai, G. Pálinkás, F. Hajdu and A. Vértes, Hydration of Iron(II) Ion in Aqueous Solutions, Electrochim. Acta, 1988, 33, 1223–1228 CrossRef.
- G. Herdman and G. W. Neilson, Ferrous Fe(II) Hydration in a 1 Molal Heavy Water Solution of Iron Chloride, J. Phys.: Condens.Matter, 1992, 4, 649–653 CrossRef CAS.
- D. Lundberg, A.-S. Ullström, P. D'Angelo and I. Persson, A Structural Study of the Hydrated and the Dimethylsulfoxide, N,N′-dimethylpropyleneurea, and N,N-dimethylthioformamide Solvated Iron(II) and Iron(III) Ions in Solution and Solid State, Inorg. Chim. Acta, 2007, 360, 1809–1818 CrossRef CAS.
- A. M. Mohammed, Hydration Structure and Water Exchange Dynamics of Fe(II) Ion in Aqueous Solution, Bull. Chem. Soc. Ethiop., 2010, 24, 239–250 CrossRef CAS.
- S. T. Moin, T. S. Hofer, A. B. Pribil, B. R. Randolph and B. M. Rode, A Quantum Mechanical Charge Field Molecular Dynamics Study of Fe2+ and Fe3+ Ions in Aqueous Solutions, Inorg. Chem., 2010, 49, 5101–5106 CrossRef CAS PubMed.
- R. Caminiti and M. Magini, An X-ray diffraction study on the first and the second hydration shell of the Fe(III) ion in nitrate solutions, Chem. Phys. Lett., 1979, 61, 40–44 CrossRef CAS.
- G. Herdman and G. W. Neilson, Ferric (Fe(III) Coordination in Concentrated Aqueous Electyrolyte Solutions, J. Phys.: Condens.Matter, 1992, 4, 627–638 CrossRef CAS.
- R. Caminiti and T. Radnai, X–ray Diffraction Study of Ferric Chloride Solutions and Hydrated Melt. Analysis of the Iron(III)–Chloride Complexes Formation, J. Chem. Phys., 1979, 71, 4255–4262 CrossRef.
- G. Piccaluga, X-ray Diffraction Study of the Coordination of Fe(III) in a Highly Hydrolyzed Solution of Iron(III) Chloride, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1982, 37, 154–157 Search PubMed.
- M. Magini and R. Caminiti, Structure of Highly Concentrated Iron(III) Salt Solutions, J. Inorg. Nucl. Chem., 1977, 39, 91–94 CrossRef CAS.
- R. Caminiti and M. Magini, An X-ray Diffraction Study on the First and the Second Hydration Shell of the Fe(III) in Nitrate Solutions, Chem. Phys. Lett., 1979, 61, 40–44 CrossRef CAS.
- M. Magini, An X-ray Investigation on the Structure of Iron(III) Perchlorate Solutions, J. Inorg. Nucl. Chem., 1977, 40, 43–48 CrossRef.
- A. M. Mohammed, Structure of Co(III) and Fe(III) Transition Metal Ions in Aqueous Solution, Bull. Chem. Soc. Ethiop., 2006, 20, 121–131 CAS.
- A. Musinu, G. Paschina, G. Piccaluga and M. Magini, X–ray Diffraction Study of CoCl2−LiCl Aqueous Solutions, J. Chem. Phys., 1984, 80, 2772–2776 CrossRef CAS.
- M. Magini and G. Giubileo, Solute Structuring in Concentrated Aqueous Co(II) and Ni(II) Perchlorate Soutions, Gazz. Chim. Ital., 1981, 111, 449–454 CAS.
- M. Magini, Hydration and Complex Formation Study on Concentrated MCl2 solutions [M=Co(II), Ni(II), Cu(II)] by X–ray Diffraction Technique, J. Chem. Phys., 1981, 74, 2523–2529 CrossRef CAS.
- A. Corrias, A. Musinu and G. Pinna, EXAFS and X-ray Diffraction Study of a Mixed NiCl2/CoCl2 Solution, Chem. Phys. Lett., 1985, 120, 295–300 CrossRef CAS.
- P. D'Angelo, V. Barone, G. Chillemi, N. Sanna, W. Meyer-Klauke and N. V. Pavel, Hydrogen and Higher Shell Contributions in Zn2+, Ni2+, and Co2+ Aqueous Solutions: An X-ray Absorption Fine Structure and Molecular Dynamics Study, J. Am. Chem. Soc., 2002, 124, 1958–1967 CrossRef PubMed.
- R. Spezia, M. Duvail, P. Vitorge, T. Catailler, J. Tortajada, G. Chillemi, P. D'Angelo and M. P. Gaigeot, A coupled Car-Parinello Molecular Dynamics and EXAFS Data Analysis Investigation of Aqueous Co2+, J. Phys. Chem. A, 2006, 110, 13081–13088 CrossRef CAS PubMed.
- P. D'angelo, M. Benfatto, S. Della Longa and N. V. Nicolae, Combined XANES and EXAFS Analysis of Co2+, Ni2+ and Zn2+ Aqueous Solutions, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 064209 CrossRef.
- D. Z. Caralampio, B. Reeves, M. R. Beccia, J. M. Martínez, R. R. Pappalardo, C. den Auwer and E. Sánchez, Revisiting the Cobalt(II) hydration from Molecular Dynamics and X-ray Absorption Spectroscopy, Mol. Phys., 2019, 117, 3320–3328 CrossRef CAS.
- M. C. Read and M. Sandström, Second-Sphere Hydration of Rhodium(III) and Chromium(III) in Aqueous Solution. A Large Angle X-ray Scattering and EXAFS Study, Acta Chem. Scand., 1992, 46, 1177–1182 CrossRef CAS.
- R. Caminiti and P. Cucca, An X-ray Diffraction Study of Rh(III) Coordination in a Dilute Aqueous Solution of Rh(ClO4)3, Chem. Phys. Lett., 1984, 108, 51–57 CrossRef CAS.
- R. Caminiti, D. Atzei, P. Cucca, F. Squintu and G. Bongiovanni, Coordination of Rhodium(III) in Dilute Aqueous Solutions in Presence of Chloride Anion, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1985, 40, 1319–1328 Search PubMed.
- R. Caminiti, D. Atzei, P. Cucca, A. Anedda and G. Bongiovanni, Structure of Rhodium(III) Nitrate Aqueous Solutions - An Investigation by X-ray Diffraction and Raman Spectroscopy, J. Chem. Phys., 1986, 90, 238–243 CrossRef CAS.
- R. Caminiti, On Nickel–Sulfate Contacts and SO42−–H2O Interactions in Aqueous Solutions, J. Chem. Phys., 1986, 84, 3336–3338 CrossRef CAS.
- R. Caminiti and P. Cucca, X-ray Diffraction Study on a NiBr2 Aqueous Solution. Experimental Evidence of the Ni(II)-Br contacts, J. Chem. Phys., 1982, 89, 110–114 CAS.
- E. Kálmán, I. Serke, G. Pálinkás, G. Johansson, G. Kabisch, M. Maeda and H. Ohtaki, Potentiometric and Calorimetric Studies on the Formation of Ethylenediamine Complexes of Nickel(II) Ion in Water and Dioxane-Water Mixtures, Z. Naturforsch., A: Phys., Phys. Chem., Kosmophys., 1983, 38, 225–230 Search PubMed.
- R. Caminiti, Nickel and Cadmium Phosphates in Aqueous Solution - Cation Anion Complex Formation and Phosphate-H2O Interactions, J. Chem. Phys., 1982, 77, 5682–5686 CrossRef CAS.
- R. Caminiti, G. Paschina and G. Piccaluga, Ni–Cl Bonding in Concentrated Ni(II) Aqueous Solutions at High Cl−/Ni2+ Ratios. An X–ray Diffraction Investigation, J. Chem. Phys., 1982, 76, 1116–1121 CrossRef.
- J. R. Newsome, G. W. Neilson, J. E. Enderbyand and M. Sandström, Ni2+ Hydration in Perchlorate and Chloride Solutions, Chem. Phys. Lett., 1981, 82, 399–401 CrossRef CAS.
- B. Beagley, A. Eriksson, J. Lindgren, I. Persson, L. G. M. Pettersson, M. Sandström, U. Wahlgren and E. W. White, A Computational and Experimental Study on the Jahn-Teller Effect in the Hydrated Copper(II) Ion. Comparisons with Hydrated Nickel(II) Ions in Aqueous Solution and Solid Tutton's Salts, J. Phys.: Condens.Matter, 1989, 1, 2395–2408 CrossRef CAS.
- O. Kristiansson, I. Persson, D. Bobicz and D. Xu, A Structural Study of the Hydrated and the Dimethylsulfoxide, N,N′-Dimethylpropyleneurea, Acetonitrile, Pyridine and N,N-Dimethylthioformamide Solvated Nickel(II) Ion Solution and Solid State, Inorg. Chim. Acta, 2003, 344, 15–27 CrossRef CAS.
- P. D'Angelo, O. M. Roscioni, G. Chillemi, S. Della Longa and M. Benfatto, Detection of Second Hydration Shells in Ionic Solutions by XANES: Computed Spectra for Ni2+ in Water Based on Molecular Dynamics, J. Am. Chem. Soc., 2006, 128, 1853–1858 CrossRef PubMed.
- I. Persson, D. Lundberg, É. G. Bajnoczi, K. Klementiev, J. Just and K. G. V. Sigfridsson Clauss, EXAFS Study on the Coordination Chemistry of the Solvated Copper(II) Ion in a Series of Oxygen Donor Solvents, Inorg. Chem., 2020, 59, 9538–9550 CrossRef CAS PubMed , and references therein.
- P. Frank, M. Benfatto and M. Qayyum, [Cu(aq)]2+ is Structurally Plastic and the Axially Elongated Octahedron Goes Missing, J. Chem. Phys., 2018, 148, 204302 CrossRef PubMed.
- I. Persson, P. Persson, M. Sandström and A.-S. Ullström, Structure of Jahn-Teller Distorted Solvated Copper(II) Ions in Solution, and in Solids with Apparently Regular Octahedral Coordination Geometry, J. Chem. Soc., Dalton Trans., 2002, 1256–1265 RSC.
- P. S. Salmon and G. W. Neilson, The Coordination of Cu(II) in a Concentrated Copper Nitrate Solution, J. Phys.: Condens.Matter, 1989, 1, 5291–5295 CrossRef CAS.
- H. Ohtaki and M. Maeda, X-ray Diffraction Study of Hydrated Copper(II) in a Copper(II) Perchlorate Solution, Bull. Chem. Soc. Jpn., 1974, 47, 2197–2199 CrossRef.
- M. Magini, Coordination of copper(II). Evidence of the Jahn-Teller Effect in Aqueous Perchlorate Solutions, Inorg. Chem., 1982, 21, 1535–1538 CrossRef CAS.
- M. Nomura and T. Yamaguchi, Concentration Dependence of Extended X-ray Absorption Fine Structure and X-ray Absorption Near-Edge Structure of Copper(II) Perchlorate Aqueous Solution - Comparison of Solute Structure in Liquid and Glassy States, J. Phys. Chem., 1988, 92, 6157–6160 CrossRef CAS.
- G. Licheri, A. Musinu, G. Paschina, G. Piccaluga and A. F. Sedda, Coordination of Cu(II) in Cu(NO3)2 Aqueous Solutions, J. Chem. Phys., 1984, 80, 5308–5311 CrossRef CAS.
- A. Musinu, G. Paschina, G. Piccaluga and M. Magini, Coordination of Copper(II) in Aqueous CuSO4 Solution, Inorg. Chem., 1983, 22, 1184–1187 CrossRef CAS.
- W. Bol, G. J. A. Gerrits and C. L. van Panthaleon van Eck, The Hydration of Divalent Cations in Aqueous Solution. An X-ray Investigation with Isomorphous Replacement, J. Appl. Crystallogr., 1970, 3, 486–492 CrossRef CAS.
- M. Busato, A. Melchior, V. Migliorati, A. Colella, I. Persson, G. Mancini, D. Veclaniz and P. D'Angelo, The Elusive Coordination of the Ag+ Ion in Aqueous Solution: Evidence for a Linear Structure, Inorg. Chem., 2020, 59, 10291–10302 CrossRef PubMed.
- D. H. Powell, P. M. N. Gullidge, G. W. Neilson and M. C. Bellissent-Funel, Zn2+ Hydration and Complexation in Aqueous Electrolyte Solutions, Mol. Phys., 1990, 71, 1107–1116 CrossRef CAS.
- S. P. Dagnall, D. N. Hague and A. D. C. Towl, X-ray Diffraction Study of Aqueous Zinc(II) Nitrate, J. Chem. Soc., Faraday Trans. 2, 1982, 78, 2161–2167 RSC.
- H. Wakita, G. Johansson, P. L. Goggin and H. Ohtaki, Structure Determination of Zinc Iodide Complexes Formed in Aqueous Solution, J. Solution Chem., 1991, 20, 643–668 CrossRef CAS.
- M. Xu, T. Zhu and J. Z. H. Zhang, Molecular Dynamics Simulation of Zinc Ion in Water with an ab Initio Based Neural Network Potential, J. Phys. Chem., 2019, 123, 6587–6595 CrossRef CAS PubMed.
- V. Migliorati, A. Zitolo, G. Chillemi and P. D'Angelo, Influence of the Second Coordination Shell on the XANES Spectra of the Zn2+ Ion in Water and Methanol, ChemPlusChem, 2012, 77, 234–239 CrossRef CAS.
- M. Q. Fatmi, T. S. Hofer, B. R. Randolf and B. M. Rode, An Extended ab initio QM/MM MD Approach to Structure and Dynamics of Zn(II) in Aqueous Solution, J. Chem. Phys., 2005, 123, 054514 CrossRef PubMed.
- G. Chillemi, V. Barone, P. D'Angelo, G. Mancini, I. Persson and N. Sanna, Computational Evidence for a Variable First Coordination Shell of the Cadmium(II) Ion in Aqueous Solution, J. Phys. Chem. B, 2005, 109, 9186–9193 CrossRef CAS PubMed.
- R. Caminiti and G. Johansson, On the Structures of Cadmium Sulfate Complexes in Aqueous Solution, Acta Chem. Scand., Ser. A, 1981, 35, 373–381 CrossRef.
- R. Caminiti, P. Cucca and T. Radnai, Investigation on the Structure of Cadmium Nitrate Aqueous Solutions by X-ray Diffraction and Raman Spectroscopy, J. Phys. Chem., 1984, 88, 2382–2386 CrossRef CAS.
- G. Johansson and M. Sandström, The crystal structure of hexaaquamercury(II) perchlorate, [Hg(H2O)6](ClO4)2, Acta Chem. Scand., Ser. A, 1978, 32, 109–113 CrossRef.
- I. Persson, L. Eriksson, P. Lindqvist-Reis, P. Persson and M. Sandström, An EXAFS Spectroscopic, Large-Angle X-Ray Scattering, and Crystallographic Study of Hexahydrated, Dimethyl Sulfoxide and Pyridine 1-Oxide Hexasolvated Mercury(II) Ions, Chem. – Eur. J., 2008, 14, 6687–6696 CrossRef CAS PubMed.
- M. Sandström and I. Persson, Crystal and Molecular Structure of Hexakis(dimethylsulfoxide)mercury(II) Perchlorate, [Hg((CH3)2SO)6](ClO4)2, Acta Chem. Scand., Ser. A, 1978, 32, 95–100 CrossRef.
- G. Chillemi, G. Mancini, N. Sanna, V. Barone, S. Della Longa, M. Benfatto, N. V. Pavel and P. D'Angelo, Evidence for Sevenfold Coordination in the First Coordination Shell of Hg(II) aqua Ion, J. Am. Chem. Soc., 2007, 129, 5430–5436 CrossRef CAS PubMed.
- G. Mancini, N. Sanna, V. Barone, V. Migliorati, P. D'angelo and G. Chillemi, Structural and Dynamical Properties of the Hg2+ Aqua Ion: A Molecular Dynamics Study, J. Phys. Chem. B, 2008, 112, 4694–4702 CrossRef CAS PubMed.
- V. Migliorati, M. Busato and P. D'Angelo, Solvation Structure of the Hg(NO3)2 and Hg(TfO)2 Salts in Dilute Aqueous and Methanol Solutions: An Insight Into the Hg2+ Coordination Chemistry, J. Mol. Liq., 2022, 363, 119801 CrossRef CAS.
- J. Rosdahl, I. Persson, L. Kloo and K. Ståhl, On the Solvation of the Mercury(I) Ion. A Structural, Vibration Spectroscopic and Quantum Chemical Study, Inorg. Chim. Acta, 2004, 357, 2624–2634 CrossRef CAS.
- T. S. Hofer, B. R. Randolph and B. M. Rode, The Hydration of the Mercury(I)-Dimer - A Quantum Mechanical Charge Field Molecular Dynamics Study, Chem. Phys., 2008, 349, 210–218 CrossRef CAS.
- J. Krakowiak, D. Lundberg and I. Persson, A Coordination Chemistry Study of Hydrated and Solvated Cationic Vanadium Ions in Oxidation States +III, +IV, and +V in Solution and Solid State, Inorg. Chem., 2012, 51, 9598–9609 CrossRef CAS PubMed.
- S. J. Edwards, D. T. Bowron and R. J. Baker, Insights into the solution structure of the hydrated uranyl ion from neutron scattering and EXAFS experiments, Dalton Trans., 2022, 51, 13631–13635 RSC.
- M. Åberg, D. Feri, J. Glaser and I. Grenthe, Structure of the Hydrated Dioxouranium(VI) Ion in Aqueous Solution. An X-ray Diffraction and proton NMR Study, Inorg. Chem., 1983, 22, 3986–3989 CrossRef.
- S. Pérez-Conesa, F. Torrico, J. M. Martínez, R. R. Pappalardo and E. Sánchez Marcos, A hydrated ion model of [UO2]2+ in water: Structure, dynamics, and spectroscopy from classical molecular dynamics, J. Chem. Phys., 2016, 145, 224502 CrossRef PubMed.
- A. H. Narten and S. Lindenbaum, Diffraction Pattern and Structure of the System Tetra–n–butylammonium Fluoride–Water at 25 °C, J. Chem. Phys., 1969, 51, 1108–1114 CrossRef CAS.
- C.-G. Zhan and D. A. Dixon, Hydration of the Fluoride Anion: Structures and Absolute Hydration Free Energy from First-Principles Electronic Structure Calculations, J. Phys. Chem. A, 2004, 108, 2020–2029 CrossRef CAS.
- T. S. Hofer, Solvation Structure and Ion–Solvent Hydrogen Bonding of Hydrated Fluoride, Chloride and Bromide—A Comparative QM/MM MD Simulation Study, Liquids, 2022, 2, 445–464 CrossRef CAS.
- P. D'Angelo, V. Migliorati and L. Guidoni, Hydration Properties of the Bromide Aqua Ion: the Interplay of First Principle and Classical Molecular Dynamics, and X-ray Absorption Spectroscopy, Inorg. Chem., 2010, 49, 4224–4231 CrossRef PubMed.
- V. T. Pham, I. Tavernelli, C. J. Milne, R. M. van der Veen, P. D'Angelo, C. Bressler and M. Chergui, The solvent shell structure of aqueous iodide: X-ray absorption spectroscopy and classical, hybrid QM/MM and full quantum molecular dynamics simulations, Chem. Phys., 2010, 371, 24–29 CrossRef CAS.
- R. Caminiti, G. Licheri, G. Piccaluga and G. Pinna, NO3-H2O Interactions in aqueous-solutions, J. Chem. Phys., 1978, 68, 1967–1970 CrossRef CAS.
- L. Eklund, Hydration of Oxo Anions. A Combined Computational and Experimental Structure and Dynamics Study in Aqueous Solutions, doctoral thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2014 (available at https://pub.epsilon.slu.se/11192/) Search PubMed.
- L. Eklund, T. S. Hofer and I. Persson, I. Structure and water exchange of hydrated oxo halo ions in aqueous solution using QMCF MD simulation, large angle X-ray scattering and EXAFS, Dalton Trans., 2015, 44, 1816–1828 RSC.
- M. Śmiechowski and I. Persson, Hydration of Oxometallate Ions in Aqueous Solution, Inorg. Chem., 2020, 59, 8231–8239 CrossRef PubMed.
- L. Eklund, T. S. Hofer, A. B. Pribil, B. M. Rode and I. Persson, On the Structure and Dynamics of the Hydrated Sulfite Ion in Aqueous Solution – An ab initio QMCF MD Simulation and Large Angle X-ray Scattering Study, Dalton Trans., 2012, 41, 5209–5216 RSC.
- V. Vchirawongkwin, B. M. Rode and I. Persson, Structure and Dynamics of Sulfate Ion in Aqueous Solution - An ab initio QMCF MD Simulation and Large Angle X-Ray Scattering Study, J. Phys. Chem. B, 2007, 111, 4150–4155 CrossRef CAS PubMed.
- L. Eklund, T. S. Hofer, A. K. H. Weiss, A. O. Tirler and I. Persson, Detailed structure elucidation of the hydrated thiosulfate ion using QMCF MD simulation and large angle X-ray scattering in aqueous solution, Dalton Trans., 2014, 43, 12711–12720 RSC.
- L. Eklund and I. Persson, Structure and Hydrogen Bonding of the Hydrated Selenite and Selenate Ions in Aqueous Solution, Dalton Trans., 2014, 43, 6315–6321 RSC.
- I. Persson, M. Trublet and W. Klysubun, Structure Determination of Phosphoric Acid and Phosphate Ions in Aqueous Solution Using EXAFS Spectroscopy and Large Angle X-ray Scattering, J. Phys. Chem. A, 2018, 122, 7413–7420 CrossRef CAS PubMed.
- M. Śmiechowski, E. Gojło and J. Stangret, Systematic Study of Hydration Patterns of Phosphoric(V) Acid and Its Mono-, Di-, and Tripotassium Salts in Aqueous Solution, J. Phys. Chem. B, 2009, 113, 7650–7661 CrossRef PubMed.
- J. Mähler and I. Persson, Structure and Hydrogen Bonding of Arsenic Oxyacid Species in Aqueous Solution, Dalton Trans., 2013, 42, 1364–1377 RSC.
- P. Vanýsek, Ionic conductivity and diffusion at infinite dilution, in Handbook of Chemistry and Physics, ed. W. M. Haynes, CRC Press, Taylor Francis Group, Boca Raton, 96th edn, 2015–2016, pp. 5-76–5-78 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fitted radial distribution and intensity functions from large angle X-ray scattering studies of aqueous solutions of magnesium(II), aluminium(III), manganese(II), iron(II), iron(III), cobalt(II), nickel(II), copper(II) and zinc(II) perchlorate, and summaries of reported crystal structures containing hydrated metal ions and oxoanions in solid state. See DOI: https://doi.org/10.1039/d4dt01449a |
| This journal is © The Royal Society of Chemistry 2024 |