Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Role of Fe decoration on the oxygen evolving state of Co3O4 nanocatalysts†
Felix T.
Haase
a,
Eduardo
Ortega
 a,
Sascha
Saddeler
c,
Franz-Philipp
Schmidt
b,
Daniel
Cruz
b,
Fabian
Scholten
a,
Martina
Rüscher
a,
Andrea
Martini
a,
Hyo Sang
Jeon
a,
Antonia
Herzog
a,
Uta
Hejral
a,
Sascha
Saddeler
c,
Franz-Philipp
Schmidt
b,
Daniel
Cruz
b,
Fabian
Scholten
a,
Martina
Rüscher
a,
Andrea
Martini
a,
Hyo Sang
Jeon
a,
Antonia
Herzog
a,
Uta
Hejral
 a,
Earl M.
Davis
a,
Earl M.
Davis
 a,
Janis
Timoshenko
a,
Axel
Knop-Gericke
bd,
Thomas
Lunkenbein
a,
Janis
Timoshenko
a,
Axel
Knop-Gericke
bd,
Thomas
Lunkenbein
 b,
Stephan
Schulz
b,
Stephan
Schulz
 c,
Arno
Bergmann
c,
Arno
Bergmann
 *a and
Beatriz
Roldan Cuenya
*a and
Beatriz
Roldan Cuenya
 *a
*a
aDepartment of Interface Science, Fritz Haber Institute of the Max Planck Society, Berlin, Germany. E-mail: abergmann@fhi-berlin.mpg.de; roldan@fhi-berlin.mpg.de
bDepartment of Inorganic Chemistry, Fritz Haber Institute of the Max Planck Society, Berlin, Germany
cInstitute for Inorganic Chemistry and Center for Nanointegration Duisburg-Essen [CENIDE], University of Duisburg-Essen, Essen, Germany
dMax-Planck-Institut für Chemische Energiekonversion, Stiftstrasse 34-36, 45470 Mülheim, Germany
First published on 30th January 2024
Abstract
The production of green hydrogen through alkaline water electrolysis is the key technology for the future carbon-neutral industry. Nanocrystalline Co3O4 catalysts are highly promising electrocatalysts for the oxygen evolution reaction and their activity strongly benefits from Fe surface decoration. However, limited knowledge of decisive catalyst motifs at the atomic level during oxygen evolution prevents their knowledge-driven optimization. Here, we employ a variety of operando spectroscopic methods to unveil how Fe decoration increases the catalytic activity of Co3O4 nanocatalysts as well as steer the (near-surface) active state formation. Our study shows a link of the termination-dependent Fe decoration to the activity enhancement and a significantly stronger Co3O4 near-surface (structural) adaptation under the reaction conditions. The near-surface Fe– and Co–O species accumulate an oxidative charge and undergo a reversible bond contraction during the catalytic process. Moreover, our work demonstrates the importance of low coordination surface sites on the Co3O4 host to ensure an efficient Fe-induced activity enhancement, providing another puzzle piece to facilitate optimized catalyst design.
Broader contextEnvironmentally friendly technologies to convert electrical energy from renewable power sources and produce hydrogen are essential to facilitate the industrial and societal transformation towards carbon neutrality. Here, alkaline water electrolysis can play a major role but, currently, the efficiency of green hydrogen production is largely limited by the overpotential of the anodic oxygen evolution reaction (OER) and thus, more active and stable electrocatalysts are needed. For the alkaline OER, 3d transition metal oxide electrocatalysts such as Co3O4 and Ni(OH)2 are usually applied as electrocatalysts and it has been shown that the catalytic activity can be boosted by Fe addition. However, it is still unclear how the Fe interacts with the surface of the (crystalline) host catalyst and how it steers the formation of the oxygen-evolving catalyst state and thus increases the catalytic activity. This information is essential to facilitate the knowledge-driven catalyst design for large scale H2 production. Herein, we studied a series of Co3O4 nanocatalysts that we decorated with Fe electrolyte species in a controlled way to understand the structural, compositional and morphological roles of Fe on the catalytic OER activity and the active state formation. We were able to link the preferred Fe location to the shape of the Co3O4 nanocatalyst and the increase in OER activity. By using a variety of advanced bulk- and surface-sensitive operando methods, we revealed the essential differences in the oxygen-evolving state of Fe-decorated and Fe-free Co3O4 providing an important puzzle piece for a comprehensive understanding of the OER. Our results also highlight the necessity to consider the nanoscale heterogeneity of industrially relevant electrocatalysts to understand and design high-performing materials for the alkaline water electrolysis. |
Introduction
Hydrogen is regarded as the key feedstock and fuel in the carbon emission-free industry making its production an integral step towards clean energy conversion. It is therefore crucial to promote the production and application of so-called green hydrogen obtained from water electrolysis. During water electrolysis, the anodic oxygen evolution reaction (OER) is regarded as the bottleneck because of the sluggish kinetics involved in the 4-electron mechanism.1,2 Crystalline Co3O4 spinel catalysts are promising low-cost and stable anode materials for alkaline water electrolysis with high OER activity and stability.3–8 In addition, Co3O4-based electrocatalysts have shown superior performance in anion exchange membrane electrolyzers compared to liquid electrolyte state-of-the-art NiFeOx catalysts due to their better conductivity and suppressed degradation.9An elegant approach to enhance the OER performance of Co- and Ni-oxides, demonstrated by Corrigan,10,11 is to employ surface Fe decoration, e.g. from electrolyte impurities. Although this finding has attracted substantial attention in the last decade,12–15 the current state of knowledge does not provide a coherent picture of the catalytic role and active state properties of the Fe sites in the OER. Generating a unified picture of the catalytic role of Fe would enable a purely knowledge-driven catalyst optimization. On the one hand, it has been shown that the Fe3+ ions incorporated into MOOH (M = Co, Ni) matrices remain in a 3+ valence state16,17 and enhance the transition of Ni2+ to Ni4+ sites.18,19 On the other hand, it was shown that Fe3+ gets oxidized during the OER,20–22 which supposedly hampers the oxidation of Ni2+ to Ni3+ sites.23 Additionally, Fe may also benefit from a stabilizing, conductive host17,24,25 or might be found embedded in the crystalline structure of the OER active site.16,26 Thus, the chemical state and electronic structure of the oxygen-evolving Fe-containing near-surface is unclear. In this regard, it is important to note that revealing the oxidation of a transition metal M (=Co, Ni, Fe) with a valence state beyond M3+ requires careful analysis, as simultaneously electrophilic oxygen species, potentially crucial for the O–O bond formation, can form.15,21,27,28
So far, the interaction of solvated Fe ions with the surface of Co-based oxides has been studied mostly on amorphous CoOx(OH)y materials and the interaction with crystalline Co3O4 catalysts remains relatively unknown. Specifically, the reversible formation of the CoOx(OH)y adaption layer on Co3O4 during the OER8,29 could facilitate Fe incorporation into the near-surface spinel lattice, thus leading to OER activity enhancement.30–34 In previous studies on Co3−xFexO4 spinels, we correlated higher OER activity with a more pronounced Co oxidation and CoOx(OH)y adaption layer formation with a Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe surface ratio of 3
Fe surface ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.35,36 For epitaxial Co3−xFexO4(111) thin films, Fe-containing Co1+δFe2−δO4(111) are more active than the bare Co3O4(111), and evolve towards the Co-richer surface termination upon the OER.37 However, the location as well as the electronic structure and chemical state of the Fe species interacting with the Co3O4 host remain ambiguous. Identifying the preferred (most stable) Fe decoration sites on Co3O4 as well as their (electronic) structure during oxygen evolution is critical to generate a unified picture of the Co–Fe catalytically active surface.
1.35,36 For epitaxial Co3−xFexO4(111) thin films, Fe-containing Co1+δFe2−δO4(111) are more active than the bare Co3O4(111), and evolve towards the Co-richer surface termination upon the OER.37 However, the location as well as the electronic structure and chemical state of the Fe species interacting with the Co3O4 host remain ambiguous. Identifying the preferred (most stable) Fe decoration sites on Co3O4 as well as their (electronic) structure during oxygen evolution is critical to generate a unified picture of the Co–Fe catalytically active surface.
In this study, we tracked the incorporation of cationic Fe species from the electrolyte onto Co3O4 nanostructures. After Fe deposition, we found an affinity of Fe with Co3O4(100) surface sites using scanning transmission electron microscopy (STEM) and energy dispersive X-ray (EDX) mapping. Moreover, our findings suggest a saturating Fe concentration on specific surface sites, which we can correlate with the OER enhancement. Surprisingly, our operando investigations show subtle differences in the Co chemical state between the Fe-free and Fe-containing active catalyst's states. In the latter case, we identified combined accumulation of oxidative charges at the Fe, Co, and O sites during the OER, suggesting that the Fe–O also participates in redox electrochemistry as well as OER catalysis. Thus, our study provides new insights into the nature of Fe deposition on Co3O4 sites and the OER-active Fe–O sites.
Results and discussion
Co3O4 spinel oxide nanoparticle (NP) catalysts were synthesized following a solvothermal route from Co(NO3)2 using oleylamine as the capping agent and consecutive calcination at 300 °C.38 The X-ray photoelectron spectroscopy (XPS) data of the O and C1s peak regions showed the removal of organic ligands, Fig. S1 (ESI†). Fig. 1(a) shows a conglomerate of well-defined cubically shaped Co3O4 NPs alongside smaller, rather spherical non-cubic NPs. The particle sizes range between 15 and 50 nm and on average of 20–30 nm, see also Fig. S2 (ESI†). The cubic Co3O4 NPs expose primarily a (100) termination, while the rounded corners and non-cubic NPs derive likely from (110) and (111) and higher indexed orientations.38–40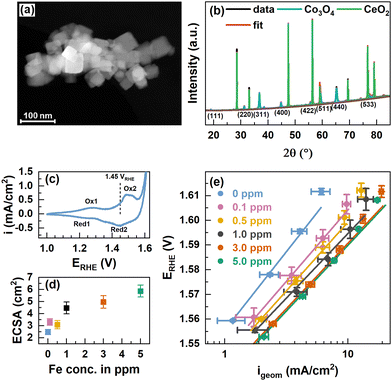 | ||
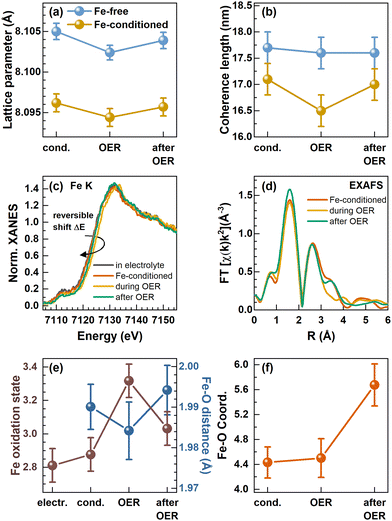
| Fig. 1 (a) Scanning transmission electron microscopy (STEM) annular dark-field (ADF) image of Co3O4 NPs. The scale bar corresponds to 100 nm. (b) XRD patterns of Co3O4 NPs and CeO2 NIST SRM674b standard with fits from Rietveld refinement. The fit parameters are shown in Table S1 (ESI†). (c) Cyclic voltammograms with 100 mV s−1 in purified 0.1 M KOH and indicated oxidation and reduction features. (d) Electrochemically active surface area (ECSA) extracted from cyclic voltammograms in the range of 0.95–1.05 VRHE in Fe-free 0.1 M KOH with different scan rates and linear regression of cathodic and anodic charging currents. The measured capacitance was normalized using a specific capacitance of 0.040 mF cm−2 which is suggested for metal oxides.42 Error bars reflect the standard deviations from 3 individual measurements. (e) Tafel plots from potential step experiments in Fe-free 0.1 M KOH with 20 mV potential steps applied for 2 minutes each. Measurements were performed after conditioning at 1.45 VRHE for 1 h in Fe-free, and 0.1, 0.5, 1.0, 3.0, and 5.0 ppm Fe-containing 0.1 M KOH. Tafel slopes are 65.0 ± 0.3, 57.4 ± 0.6, 55.6 ± 0.4, 52 ± 3, 53.8 ± 0.9 and 53 ± 1 mV dec−1, respectively. | ||
We recorded powder X-ray diffractograms of Co3O4 which confirm the phase purity, and we determined a total crystallinity of 85.8% by using a CeO2 reference (NIST SRM674b), Fig. 1(b). The Bragg peaks solely correspond to the Co3O4 spinel as well as the CeO2 and using Rietveld refinement we determined a volume-averaged crystallite size of ∼32 nm and a lattice parameter of ∼8.087 Å which agrees well with Co3O4, Table S1 (ESI†). The surface chemical state of the as-prepared Co3O4 NPs agrees well in the presence of a mixed Co2+/3+ valence state as the Co 2p XPS regions shown in Fig. S3 (ESI†) strongly resemble that of Co3O4 presented in the literature.29,41 Depth-dependent XPS analysis using kinetic energies of photoelectrons of ∼550 eV and ∼200 eV corresponding to information depths of ∼10 and ∼5 Å, respectively, suggest a Co2+-richer termination layer due to the slightly larger Co2+ satellite contribution as denoted in Table S2 (ESI†).
To vary the degree of the Fe surface decoration of the Co3O4 NPs, cationic Fe was anodically deposited at 1.45 VRHE for 1 h from Fe3+(NO3)3 in Fe-purified 0.1 M KOH. The potential of 1.45 VRHE was chosen to reflect non-catalytic conditions below the OER onset potential (∼1.55 VRHE) and between the two major Co oxide redox transitions – Co2+/Co3+ oxidation at 1.25 VRHE and the Co3+/Co3+δ oxidation at 1.52 VRHE, Fig. 1(c). Thereby, we aimed to ensure the morphological integrity of the NCs in addition to facilitating the interaction of the deposited Fe species with the formed CoOx(OH)y surface structure and thus to the formation of an Fe-containing Co3O4 surface and potentially subsurface regions. The Fe is expected to be deposited on the Co3+–O surface sites, i.e. the sites formed from the initially tetrahedrally coordinated Co2+ sites of the Co3O4 near-surface. Upon conditioning, the electrochemical reduction of the Fe-containing CoOx(OH)y near-surface will lead to the incorporation of the Fe species into the near-surface spinel lattice. We have to note the possibility that the selective oxidation of Co2+ sites could induce a selective deposition of Fe on Co3O4, contrasting Fe deposition on the more strongly oxidized CoOx(OH)y at higher electrode potentials during the OER. The Fe content in the electrolyte was varied between 0 and 5 ppm Fe and two exemplary chronoamperometry profiles of the conditioning with and without Fe are shown in Fig. S4 (ESI†). Measurements in the Fe-purified electrolyte after Fe conditioning show an increase of the electrochemically active surface area (ECSA) following the Fe concentration in the electrolyte, Fig. 1(d).
Revisiting the Pourbaix diagram of Fe and earlier works shows the instability of Fe oxides under OER conditions43 and thus, we studied the irreversible changes in the structure, morphology and composition of our materials after the catalytic testing in the Fe-free electrolyte. To quantify the catalytic improvement introduced by the Fe decoration of the surface of the CO3O4 NPs, we performed potential step experiments and Tafel analysis, Fig. 1(e). Overall, the current density in the whole potential regime increases with the Fe concentration and shows a ∼3-fold increase at 1.58 V from ∼2 mA cm−2 for the Fe-free Co3O4 to ∼6 mA cm−2 for the Co3O4 conditioned in 5 ppm Fe. Although there is a substantial increase in OER activity, it is apparently less pronounced for the crystalline Co3O4 NPs compared to electrodeposited CoOx(OH)y catalysts.17,43 Notably, the current density of the NPs is not stable at higher potentials and the current decrease is more pronounced for lower Fe concentrations, which suggests an OER activity-stabilizing effect of the Fe decoration. The Tafel slopes decrease with increasing Fe concentrations until a minimum of 52–54 mV dec−1 is reached for ≥1 ppm Fe suggesting the enhanced OER kinetics in the Fe-decorated case. Notably, the Fe conditioning does not have a pronounced influence on the Co-related redox transitions, Fig. S5 (ESI†). This finding is in agreement with earlier works on Co3−xFexO444,45 but contrasts the previously seen pronounced anodic shift with Fe incorporation into Co/Ni-Ox(OH)y catalysts.17,46
The presence of well-defined cubic as well as non-cubic NPs allows the investigation of the preferred location for Fe decoration on Co3O4 both before and after catalysis in the Fe-purified electrolyte. Thus, STEM-high angle annular dark-field (STEM-HAADF) with EDX mapping was performed, Fig. 2 and Fig. S6–S9 (ESI†). This shows that the conditioning with and without the addition of Fe as well as the subsequent OER catalysis do not alter the morphology of the NPs substantially. Only a slight increase in the roughness of the NP surface compared to the as-prepared state can be seen. High resolution (HR)-STEM measurements of the NPs allowed resolving Fe moieties on the surface after conditioning in Fe-free and 5.0 ppm Fe electrolytes, Fig. S10 and S11 (ESI†). In both cases, the cubic NPs retain their morphology exposing (100) facets and neither on (100) nor on (110)/(111) facets were we able to resolve FeOx NP formation. By tilting individual cubically shaped NPs and performing EDX line scans, we show that Fe is distributed on the (100) facets, Fig. S12 and S13 (ESI†). In particular, this analysis shows that the Fe accumulates in the upper ∼2 nm of Co3O4 and does not penetrate into the bulk of the nanocubes. Thus, we can conclude that the Fe decoration leads to the formation of a thin layer of Fe-containing Co3O4. Similar findings were reported previously while studying the interaction of Fe impurities in the electrolyte with Ni oxide NPs during the OER. In particular, using EC-TEM, we unveiled the initial formation of a NiFe-LDH skin layer.47 Nonetheless, further electrochemical cycling in the presence of Fe did not lead to additional Fe incorporation into the subsurface/bulk of the NiO, but to its accumulation on the surface as Fe oxide NPs. In the case of the Fe-conditioned Co3O4, STEM-EDX mapping after the OER in the Fe-purified electrolyte at 1.6 VRHE for 30 min proves the stability of the Fe decoration under OER conditions, Fig. 2 and Fig. S8 and S9 (ESI†). Additionally, we cannot rule out the partial irreversible formation of hydroxylated Fe/Co sites in the near-surface exclusively based on the ex situ spectro-microscopy analysis conducted here (TEM-EDX). Recently, we have shown using quasi in situ XPS that the Co3O4 surface appears hydroxylated after an extended exposure to OER conditions and that the OH/Lattice-O ratio is equivalent to a sub-nm hydroxide film. These measurements were however carried out after the OER, a sample rinse in water, and a subsequent sample transfer to the analysis chamber without air exposure.37
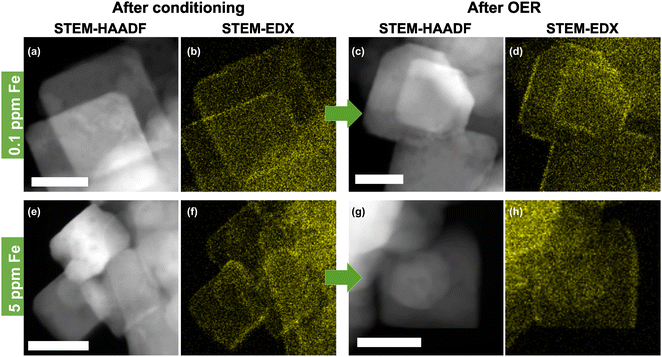 | ||
| Fig. 2 Scanning transmission electron microscopy-high angle annular dark-field (STEM-HAADF) images and corresponding energy dispersive X-ray spectroscopy (EDX) Fe-Kα color maps of Co3O4 catalysts after conditioning for 1 h at 1.45 VRHE in 0.1 M KOH and after the OER in Fe-purified 0.1 M KOH for 30 min at 1.6 VRHE. (a) and (b) Conditioning with 0.1 ppm Fe and (c) and (d) after the OER. (e) and (f) Conditioning with 5.0 ppm Fe and (g) and (h) after the oxygen evolution reaction (OER). Further images of all samples are shown in Fig. S6–S9 (ESI†). The scale bar is 20 nm. | ||
To quantify the surface Fe concentration before and after the OER on the Co3O4 NPs, we determined the Co and Fe EDX signals at the edges and the center of the cubic and the rounded particles, Fig. S6–S9 (ESI†), and performed the XPS analysis to reveal the changes in the chemical state and the average Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio on the surface. Notably, at the energies employed, the probing volume of STEM-based EDX (thickness of ∼4 nm) is substantially larger than that of XPS (thickness of 1 nm at 550 eV kinetic energy) and thus, the obtained data cannot be compared on a quantitative basis. Thus, the lower Fe/Co ratios obtained by STEM-EDX simply reveal less Fe-rich (or Fe-absent) regions in the core of the cobalt oxide particles that are not probed via the more surface-sensitive XPS analysis. We found that the surface-average Fe
Co ratio on the surface. Notably, at the energies employed, the probing volume of STEM-based EDX (thickness of ∼4 nm) is substantially larger than that of XPS (thickness of 1 nm at 550 eV kinetic energy) and thus, the obtained data cannot be compared on a quantitative basis. Thus, the lower Fe/Co ratios obtained by STEM-EDX simply reveal less Fe-rich (or Fe-absent) regions in the core of the cobalt oxide particles that are not probed via the more surface-sensitive XPS analysis. We found that the surface-average Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio increases with the Fe concentration during the conditioning from 9.5 at% at 0.1 ppm to ∼21 at% Fe for 3 and 5 ppm Fe, Fig. 3(a). Independent of the information depth, the XPS profiles after the OER resemble the same Co spinel features, with primarily Fe3+ and a similar Fe
Co ratio increases with the Fe concentration during the conditioning from 9.5 at% at 0.1 ppm to ∼21 at% Fe for 3 and 5 ppm Fe, Fig. 3(a). Independent of the information depth, the XPS profiles after the OER resemble the same Co spinel features, with primarily Fe3+ and a similar Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio as that measured after the 5 ppm conditioning, Fig. S15 (ESI†). Therefore, the NP surface remains Co3O4-like after the OER, and the cationic Fe species incorporated into the top layers of the spinel NP. Depth-dependent compositional analysis shows 22 at% Fe and 27 at% for 1 nm and 0.5 nm information depths, respectively, upon conditioning, Fig. S14 (ESI†). Notably, this depth analysis does not exceed the thickness of the Fe-containing layer determined by STEM-EDX as shown in Fig. S12 (ESI†). Therefore, it is understandable that the depth-dependent compositional analysis data obtained from XPS do not vary strongly between 0.5 and 1 nm probe depths. Thus, we can use the TEM-EDX data to conclude that the Fe did not migrate into the bulk of the Co3O4 NPs after conditioning, but remained in the top surface layers.
Co ratio as that measured after the 5 ppm conditioning, Fig. S15 (ESI†). Therefore, the NP surface remains Co3O4-like after the OER, and the cationic Fe species incorporated into the top layers of the spinel NP. Depth-dependent compositional analysis shows 22 at% Fe and 27 at% for 1 nm and 0.5 nm information depths, respectively, upon conditioning, Fig. S14 (ESI†). Notably, this depth analysis does not exceed the thickness of the Fe-containing layer determined by STEM-EDX as shown in Fig. S12 (ESI†). Therefore, it is understandable that the depth-dependent compositional analysis data obtained from XPS do not vary strongly between 0.5 and 1 nm probe depths. Thus, we can use the TEM-EDX data to conclude that the Fe did not migrate into the bulk of the Co3O4 NPs after conditioning, but remained in the top surface layers.
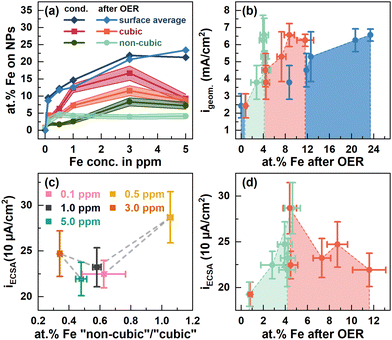 | ||
| Fig. 3 (a) at% Fe at the surface of cubic (red) and non-cubic Co3O4 NPs (green) from STEM-EDX measurements, Fig. S6–S9 (ESI†), Tables S3 and S4 (ESI†), and the sample averaged at% Fe from X-ray photoelectron spectroscopy (blue) after conditioning in different ppm Fe and after OER measurements displayed in Fig. 2. ECSA-normalized specific current densities at 1.58 VRHE from Fig. 1 dependent on (c) the at% Fe at non-cubic/cubic site ratios after the OER and (d) the cubic and non-cubic at% Fe concentrations. | ||
After the OER, the sample-averaged Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio exhibits only a small decrease of up to 1 at% Fe, which suggests a generally stable Fe configuration. Independent of the information depth, the XPS profiles after the OER resemble the same Co spinel features with primarily Fe3+ and a similar Fe
Co ratio exhibits only a small decrease of up to 1 at% Fe, which suggests a generally stable Fe configuration. Independent of the information depth, the XPS profiles after the OER resemble the same Co spinel features with primarily Fe3+ and a similar Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio after the 5 ppm conditioning, Fig. S15 (ESI†). Therefore, the NP surface remains Co3O4-like after the OER with the cationic Fe species incorporated into the near-surface.
Co ratio after the 5 ppm conditioning, Fig. S15 (ESI†). Therefore, the NP surface remains Co3O4-like after the OER with the cationic Fe species incorporated into the near-surface.
The semi-quantitative EDX analysis confirms that after conditioning at lower Fe concentrations the Fe accumulates preferably on the (100) facets. In the regime from 0.1 to 1 ppm Fe, the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio increases from 3 to 12 at%, while almost no Fe is found on the near-surface of non-cubic particles (2–2.6 at% Fe). On the (100) facets, the highest Fe
Co ratio increases from 3 to 12 at%, while almost no Fe is found on the near-surface of non-cubic particles (2–2.6 at% Fe). On the (100) facets, the highest Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio (16.7 at%) is present after the 3 ppm conditioning but the Fe
Co ratio (16.7 at%) is present after the 3 ppm conditioning but the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio is lower (9.3 at%) after 5 ppm conditioning. On the non-cubic NPs, we identified a qualitatively identical behavior though with a lower Fe
Co ratio is lower (9.3 at%) after 5 ppm conditioning. On the non-cubic NPs, we identified a qualitatively identical behavior though with a lower Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio of maximally 8 at% Fe.
Co ratio of maximally 8 at% Fe.
Upon the OER, the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios on the near-surface of cubic NPs decreased substantially, except for the Co3O4 conditioned with 5 ppm Fe. Concurrently, the Fe
Co ratios on the near-surface of cubic NPs decreased substantially, except for the Co3O4 conditioned with 5 ppm Fe. Concurrently, the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio on the non-cubic NPs converged to 4 at%, independent of the initial Fe
Co ratio on the non-cubic NPs converged to 4 at%, independent of the initial Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio thereon and thus, the Fe
Co ratio thereon and thus, the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio on the non-cubic NP increased for the Co3O4 conditioned with 1 ppm Fe and below. Since the sample-averaged Fe ions did not leach from the Co3O4 near-surface as shown by XPS, the Fe ions partially rearranged within the NP ensemble upon the OER in the Fe-free electrolyte. This process is in agreement with the discussed dissolution–redeposition cycles of Fe (and Co) ions from and to the CoOx(OH)y reaction zone.25 Furthermore, the rearrangement suggests that the tendency of Fe deposition under OER conditions shifts slightly towards the non-cubic NPs with a most stable Fe
Co ratio on the non-cubic NP increased for the Co3O4 conditioned with 1 ppm Fe and below. Since the sample-averaged Fe ions did not leach from the Co3O4 near-surface as shown by XPS, the Fe ions partially rearranged within the NP ensemble upon the OER in the Fe-free electrolyte. This process is in agreement with the discussed dissolution–redeposition cycles of Fe (and Co) ions from and to the CoOx(OH)y reaction zone.25 Furthermore, the rearrangement suggests that the tendency of Fe deposition under OER conditions shifts slightly towards the non-cubic NPs with a most stable Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio of 4 at% as compared to the cubic NPs. However, the cubic NPs still seem to be preferred for the Fe incorporation as its Fe
Co ratio of 4 at% as compared to the cubic NPs. However, the cubic NPs still seem to be preferred for the Fe incorporation as its Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio is higher also after the OER. Notably, the obtained Fe
Co ratio is higher also after the OER. Notably, the obtained Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio after the OER agrees well with the obtained optimum for the highest catalytic activity as it has been reported for Fe–metal oxyhydroxides.25 Intriguingly, values of 3
Co ratio after the OER agrees well with the obtained optimum for the highest catalytic activity as it has been reported for Fe–metal oxyhydroxides.25 Intriguingly, values of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 3.5
1 and 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 have been reported as the optimal Fe
1 have been reported as the optimal Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios for OER activity in mixed Co3−xFexO4 spinel catalysts.35,36,48
Co ratios for OER activity in mixed Co3−xFexO4 spinel catalysts.35,36,48
Evidently, there is a clear influence of the Fe decoration on the catalytic activity and here, different interactions of the Fe with cubic and non-cubic NPs are expected to play a crucial role. Thus, we linked the apparent and specific OER activity via the geometric and ECSA-normalized current density at 1.58 VRHE to the surface-average Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio as well as more specifically the Fe content on the non-cubic to cubic NPs after the OER, Fig. 3(b)–(d). The apparent OER activity monotonously increases with the surface-average Fe ratio, Fig. 3(b). This behavior can be linked to the increase in the electrochemical surface area and thus, likely the number of active sites with the increasing surface-averaged Fe
Co ratio as well as more specifically the Fe content on the non-cubic to cubic NPs after the OER, Fig. 3(b)–(d). The apparent OER activity monotonously increases with the surface-average Fe ratio, Fig. 3(b). This behavior can be linked to the increase in the electrochemical surface area and thus, likely the number of active sites with the increasing surface-averaged Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio, Fig. 1d. Similarly, the apparent OER activity increases with the Fe content on the non-cubic and cubic NPs up to ∼4 and 8 at%, respectively, Fig. 3(b). In contrast, the specific OER activity is with 287 μA cm−2 highest for ∼4 at% Fe on the non-cubic and cubic NPs after the OER, Fig. 3(c) and (d). For higher Fe contents and thus, more pronounced decoration of the cubic NPs, a specific activity of ∼220–250 μA cm−2 irrespective of the Fe content is only slightly higher compared to that of the Fe-free Co3O4 (∼200 μA cm−2). Thus, we conclude a larger fraction of Fe on non-cubic NPs after the OER enhances the specific OER activity stronger compared to Fe sites on the cubic NPs, Fig. 3(c). Overall, the Fe-induced enhancement of the apparent OER activity can be mostly attributed to an Fe-induced increase of the electrochemical surface area. However, we unveiled that the addition of Fe enhances the specific OER activity of the Co3O4 catalyst only for low Fe amounts and rather on non-cubic NPs. The Fe adsorption affinity remains facet- and morphology-dependent on Co3O4, which turns out to be the limiting characteristic for further OER-activity increases. It is unclear whether a stronger Fe decoration of non-cubic NPs with its apparently higher specific activity would lead to a stronger enhancement of the apparent OER activity. Furthermore, we expect that higher overall Fe decoration could lead to decreasing OER activity following the volcano like activity profile of the Fe
Co ratio, Fig. 1d. Similarly, the apparent OER activity increases with the Fe content on the non-cubic and cubic NPs up to ∼4 and 8 at%, respectively, Fig. 3(b). In contrast, the specific OER activity is with 287 μA cm−2 highest for ∼4 at% Fe on the non-cubic and cubic NPs after the OER, Fig. 3(c) and (d). For higher Fe contents and thus, more pronounced decoration of the cubic NPs, a specific activity of ∼220–250 μA cm−2 irrespective of the Fe content is only slightly higher compared to that of the Fe-free Co3O4 (∼200 μA cm−2). Thus, we conclude a larger fraction of Fe on non-cubic NPs after the OER enhances the specific OER activity stronger compared to Fe sites on the cubic NPs, Fig. 3(c). Overall, the Fe-induced enhancement of the apparent OER activity can be mostly attributed to an Fe-induced increase of the electrochemical surface area. However, we unveiled that the addition of Fe enhances the specific OER activity of the Co3O4 catalyst only for low Fe amounts and rather on non-cubic NPs. The Fe adsorption affinity remains facet- and morphology-dependent on Co3O4, which turns out to be the limiting characteristic for further OER-activity increases. It is unclear whether a stronger Fe decoration of non-cubic NPs with its apparently higher specific activity would lead to a stronger enhancement of the apparent OER activity. Furthermore, we expect that higher overall Fe decoration could lead to decreasing OER activity following the volcano like activity profile of the Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio.17,35,36,43
Co ratio.17,35,36,43
To fully understand the catalytic role of the cationic Fe species, the properties of the working catalysts have been identified. On the crystalline Co3O4, the formation of an X-ray amorphous CoOx(OH)y skin-layer or the reaction zone during the OER is well known,8,29,49,50 but active state properties in the presence of Fe surface decoration are not yet fully understood. We therefore applied a variety of bulk- and surface-sensitive operando methods to gain comprehensive insight into the Fe-free and Fe-decorated Co3O4 NPs during the OER. Prior to the OER measurements in the Fe-free electrolyte, all catalysts were conditioned in either 5 ppm Fe-containing 0.1 M KOH or the same electrolyte without Fe. Subsequently, the OER active state was probed by chronoamperometry for 30 minutes at 1.6 VRHE and measurements after the OER at 1 VRHE.
We followed the structural transformation of the Co3O4 and the CoOx(OH)y reaction zone formation by operando high-energy XRD for Fe-free and 5 ppm Fe conditioned catalysts, Fig. 4(a) and (b). Rietveld refinement showed that the Co3O4 spinel adapts to the OER conditions as the lattice contracts reversibly during the OER, which has been previously attributed to strain at the Co3O4–CoOx(OH)y interface.8,29,49,50 Under the Fe-free conditions, we did not detect any pronounced change in the Co3O4 coherence length during the OER, likely due to the comparably large domain size limiting the sensitivity of XRD. However, the electrochemical conditioning in the presence of Fe leads to an irreversible decrease of the Co3O4 lattice parameter as well as the structural coherence length under non-catalytic conditions. This change can be explained based on the irreversible formation of the Co3−xFexOx(OH)y near-surface region during the Fe conditioning which deviates at least in the outermost 0.5 nm structurally from the Co3O4 bulk of the NPs. We also emphasize the agreement between the lower structural coherence length and the increased electrochemical surface area, which suggests a smaller crystallite/particle size. However, we did not detect a significant decrease in the size of the cubic or non-cubic Co3O4 NPs upon Fe conditioning. Thus, the Co3−xFexOx(OH)y near-surface region is likely solely responsible for the increase in the ECSA.
Importantly, we show that the coherence length of the Fe-decorated Co3O4 clearly and reversibly decreases during the OER. This finding indicates the more pronounced formation of a Co3−xFexOx(OH)y near-surface layer, which agrees well with the obtained link between the catalytic activity and the formation of an oxyhydroxide reaction zone.
We did not see the formation of new phases besides the spinel phase like CoOx(OH)y-related Bragg peaks, Fig. S16 (ESI†), suggesting that only the near-surface of the NPs transform, which likely comprise the active sites for the OER. We have to emphasize here that this reversible transformation does not exclude a partial, irreversible hydroxylation of the (Fe-containing) Co3O4 near-surface after the reaction, as it has been found for Co3−xFexO4 thin film model surfaces after exposure to OER conditions.37
To resolve the changes in the local structure and oxidation state during the active state formation, we complementarily employed operando metal K-edge X-ray absorption spectroscopy (XAS).16,28,29,51 Similar to the operando HE-XRD insights, Co K-edge XAS cannot resolve the changes of the NP near-surface under OER conditions likely due to the low surface–volume ratio of the relatively large 15–50 nm Co3O4 catalysts, Fig. S17 (ESI†). Under all conditions investigated, the Co K-edge X-ray absorption near edge structure (XANES) thus clearly resembles that of the Co3O4 spinel.
In contrast, the Fe K-edge XANES spectra recorded for the Fe-conditioned Co3O4 show clear changes under different electrochemical conditions, Fig. 4(c), and the overall shape of the absorption edge clearly resembles the one of the Fe3+ references, Fig. S18 (ESI†). However, the Fe K-edge position shifts reversibly to higher energies during the OER, which suggests a reversible oxidation of the Fe–O species in the working catalyst. Following the calibration of the edge shift with the formal Fe oxidation state, we determined a formal Fe oxidation state during the OER of ∼+3.3. In addition to this shift, the Fe K pre-edge feature at ∼7112 eV decreases during the OER and further after the OER, which suggests irreversible changes in the Fe–O coordination symmetry towards octahedral during catalysis.52,53 We followed the Fe content under electrochemical conditions by determining the edge step of the non-normalized Fe K-edge XANES which increases during the conditioning while it remains constant during the OER in the Fe-free electrolyte, Fig. S19 (ESI†). This again shows that Fe remains deposited on the Co3O4 NPs under electrochemical conditions as well as during the OER, in contrast to the Fe dissolution during the OER which has been reported for other Co-oxide catalysts.25,43
To track the structural changes in the local atomic environment, extended X-ray absorption fine structure (EXAFS) analysis at the Fe K-edge and Co K-edge was performed, Fig. 4(d), Fig. S16 (ESI†), Co3O4 spinel structure model,35,36 Fig. S20–S22 and Tables S7–S9 (ESI†). Similar to the XANES data, no changes were visible at the Co K-edge with and without Fe decoration due to the low surface-to-volume ratio. At the Fe K-edge, we analyzed the first Fe–O coordination shell and retrieved the Fe–O bond distance which exhibits a reversible contraction during the OER, Fig. 4(e). We have to note that the obtained contraction of the Fe–O distance does not exceed strongly the uncertainty of our fitting. However, in combination with the clear shift of the Fe K-edge, we can conclude that the Fe–O bond contracts reversibly during the OER. Unfortunately, the limited data quality for these Fe-diluted samples does not allow solid quantitative statements on the extent of the Fe–O bond contraction to be provided. Nonetheless, the fact that there is an Fe–O bond contraction suggests the reversible Fe–O oxidation and thus, an accumulation of oxidative charges in the Fe or O ligand environment, as we previously unveiled for the Co–O bond in size-selected 1–5 nm CoOx(OH)y NPs during the OER.28 In particular, both the Fe–O bond contraction and the Fe oxidation suggest that Fe sites participate in the redox electrochemistry and thus potentially the OER mechanisms. The Fe–O coordination number was 4.4 ± 0.2 during the conditioning and did not change during the OER. This coordination number is lower than the expected 6 nearest neighbors for octahedrally coordinated, non-spinel, FeOx compounds. However, since the XANES spectra still suggest octahedral coordination for Fe species, we attribute this low coordination number to strong structural disorder, resulting in broad, asymmetric bond-length distributions that cannot be treated adequately by simple conventional EXAFS fitting approaches.28,54,55 The increase of the Fe–O coordination number after the OER to 5.6 ± 0.3 suggests the integration of the cationic Fe into more ordered structures, such as the Co3O4 spinel structure, and would match the Fe K pre-edge feature at ∼7112 eV.
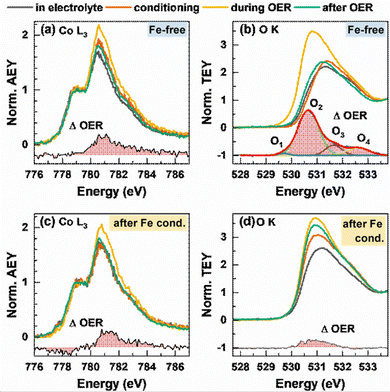
We further studied the near-surface adaptation using in situ Auger electron yield (AEY) near-edge X-ray absorption fine structure (NEXAFS) at the Co L-edge and in situ total electron yield (TEY) NEXAFS at the O K-edge, Fig. 5. The Co L3-edge spectra show negligible changes between the as-prepared and the conditioned state, but reversible changes during the OER. Here, the Co L3-edge spectra were normalized to the feature at 778.5 eV and thus to the amount of tetrahedrally coordinated Co2+.56
Correspondingly, the peak maximum at 780.5 eV represents octahedrally coordinated Co3+ and we can track the near-surface CoOx(OH)y adaption layer that forms reversibly during the OER via changes in the Co3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co2+ ratio. During the OER, the Co3+ ratio increases from 1.7 to 2.0 with Fe and 2.2 without Fe conditioning, which suggest a stronger near-surface oxidation in the Fe-free case and that the presence of Fe could suppress the oxidation of the Co sites.
Co2+ ratio. During the OER, the Co3+ ratio increases from 1.7 to 2.0 with Fe and 2.2 without Fe conditioning, which suggest a stronger near-surface oxidation in the Fe-free case and that the presence of Fe could suppress the oxidation of the Co sites.
The reversible CoOx(OH)y near-surface and Co3+δ formation is also visible through the appearance of a shoulder at 782–783 eV.28,29,35,56 In the case of the Fe-decorated Co3O4, a pre-edge feature at 777.8 eV is more pronounced under non-catalytic conditions, which corresponds to octahedral (high spin) Co2+ species. This feature is less strong or absent on the Fe-free Co3O4 suggesting a lower density of low coordination Co2+ sites predominantly present in X-ray amorphous CoOx(OH)y.56 Interestingly, this feature decreases reversibly during the OER, which in turn suggests a reversible oxidation of the low coordination Co2+ sites to Co3+δ sites. We note the link between the enhanced OER activity and the presence of these low coordination Co sites in the presence of Fe.
Recent works highlighted the importance of near-surface oxygen chemistry in OER electrocatalysis27,28,57 and therefore, we investigated the apparent changes via in situ O K-edge NEXAFS, Fig. 5(b) and (d). The features of the pre-edge region at 530–533 eV are caused by octahedrally coordinated Co3+/Fe3+ μ3-O/OH-M3+ sites at 531 eV and tetrahedral Co/Fe sites at 532 eV.56 The Fe surface decoration leads to a more pronounced feature at 531 eV, which suggests a strong contribution of octahedrally coordinated Fe3+ species to the overall signal after conditioning. During the OER, reversible changes in the O K pre-edge region and in the difference spectrum are present for the Fe-free and Fe-decorated Co3O4. Thus, the reversible increase of the μ3-O/OH-M3+ signal during the OER is more pronounced under the Fe-free conditions. Notably, the differential spectrum suggests small amounts of electrophilic oxygen species O1 at 529.5 eV as well as other species at 530.6, 532 and 533 eV (O2 + O3 + O4) in the Fe-free case.27,58,59 In the case of the Fe-conditioned Co3O4, there is a pronounced contribution of Fe oxide species but interestingly no clear indication for the presence of electrophilic oxygen. Thus, we note that the higher catalytic activity is accompanied by the absence of electrophilic oxygen as well as the presence of redox-active and oxidized Fe3+ and high spin Co2+ surface species. Also, the lower extent of oxidation from the Co perspective during the OER of the more active Fe-decorated Co3O4 NP fits to our previous findings in which sub-5 nm CoOx(OH)y NPs oxidize less than expected and exhibit a significantly higher specific activity.28
Combining the herein presented results allows us to outline how the surface Fe3+ decoration enhances the OER over the crystalline Co3O4 nanocatalysts. Clearly, the cationic Fe preferably deposits on the (100) facets of the Co3O4 NPs as compared to low coordination sites of non-cubic Co3O4 NPs while the surface-averaged Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio saturates at higher Fe coverages (towards 20%). Under these conditions, non-cubic Co3O4 NP sites are decorated and a slight tendency towards Fe domain formation can be seen. After the OER in the Fe-free electrolyte, the cationic Fe species remain on the surface as shown by XPS, Fig. 3. STEM-EDX analysis suggests that for the low Fe
Co ratio saturates at higher Fe coverages (towards 20%). Under these conditions, non-cubic Co3O4 NP sites are decorated and a slight tendency towards Fe domain formation can be seen. After the OER in the Fe-free electrolyte, the cationic Fe species remain on the surface as shown by XPS, Fig. 3. STEM-EDX analysis suggests that for the low Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios the Fe species appear to redistribute from the (100) facets towards non-cubic Co3O4, Fig. 3. For these samples, we determined the highest specific activity. This Fe redistribution is in agreement with the concept of dynamic active sites in which inter alia cationic Fe species undergo continuous dissolution redeposition cycles within the nanoparticles ensemble. Considering the discrepancy in electrode potential between the conditions of Fe deposition and the OER, our findings suggest that the affinity of Fe deposition on the non-cubic NPs is further enhanced with the increasing electrode potential compared to the cubic NPs. We have to note that any direct proof of the Fe redistribution is challenging and would require additional in-depth electrochemical electron microscopy investigations.
Co ratios the Fe species appear to redistribute from the (100) facets towards non-cubic Co3O4, Fig. 3. For these samples, we determined the highest specific activity. This Fe redistribution is in agreement with the concept of dynamic active sites in which inter alia cationic Fe species undergo continuous dissolution redeposition cycles within the nanoparticles ensemble. Considering the discrepancy in electrode potential between the conditions of Fe deposition and the OER, our findings suggest that the affinity of Fe deposition on the non-cubic NPs is further enhanced with the increasing electrode potential compared to the cubic NPs. We have to note that any direct proof of the Fe redistribution is challenging and would require additional in-depth electrochemical electron microscopy investigations.
We could correlate the most active surface-averaged Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratios with reports in the literature,17,35,36,60 although the current increase of the Fe-conditioned crystalline Co3O4 NPs is not as pronounced as that of the electrodeposited CoOx(OH)y catalysts. This characteristic could result from the lower fraction of non-cubic NPs with a higher density of low coordination sites, which exhibit a lower saturation level for Fe decoration but yielded in the highest specific OER activity. Notably, the obtained Fe decorations are not high enough to obtain decreasing catalytic activities of the Co3O4 but are stable on the surface even after the OER in the Fe-free electrolyte, contrasting previous reports.25,43 We found a clear correlation that a higher Fe surface coverage facilitates the apparent OER activity. In particular, cationic Fe species on Co3O4(100) facets are beneficial and show a higher saturation level for Fe decoration and thereby overall play a substantial role for the OER activity.61 Here, the primary reason for the increased activity is the increase in the electrochemical surface area which can be explained by the overall lower Co3O4 coherence length and potentially, the stronger structural flexibility of the Co–Fe (oxyhydr)oxide near-surface. Notably, the cationic surface Fe species incorporate into the near-surface spinel lattice which has been reported as the preferred structure under non-catalytic conditions and can be seen by considering the evolution of the Co L-edge and the Fe–O coordination numbers.35,56 Supposedly, this recrystallization of Co and Fe sites as a spinel structure is facilitated by the formation of (metal-)MOx(OH)y sites during the OER required as a host to integrate Fe.
Co ratios with reports in the literature,17,35,36,60 although the current increase of the Fe-conditioned crystalline Co3O4 NPs is not as pronounced as that of the electrodeposited CoOx(OH)y catalysts. This characteristic could result from the lower fraction of non-cubic NPs with a higher density of low coordination sites, which exhibit a lower saturation level for Fe decoration but yielded in the highest specific OER activity. Notably, the obtained Fe decorations are not high enough to obtain decreasing catalytic activities of the Co3O4 but are stable on the surface even after the OER in the Fe-free electrolyte, contrasting previous reports.25,43 We found a clear correlation that a higher Fe surface coverage facilitates the apparent OER activity. In particular, cationic Fe species on Co3O4(100) facets are beneficial and show a higher saturation level for Fe decoration and thereby overall play a substantial role for the OER activity.61 Here, the primary reason for the increased activity is the increase in the electrochemical surface area which can be explained by the overall lower Co3O4 coherence length and potentially, the stronger structural flexibility of the Co–Fe (oxyhydr)oxide near-surface. Notably, the cationic surface Fe species incorporate into the near-surface spinel lattice which has been reported as the preferred structure under non-catalytic conditions and can be seen by considering the evolution of the Co L-edge and the Fe–O coordination numbers.35,56 Supposedly, this recrystallization of Co and Fe sites as a spinel structure is facilitated by the formation of (metal-)MOx(OH)y sites during the OER required as a host to integrate Fe.
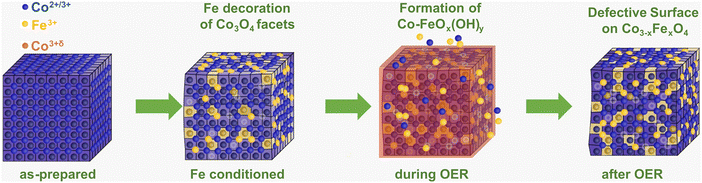
Importantly, our operando and in situ investigations revealed slight but important differences in the oxygen evolving state of Co3O4 induced by the presence of near-surface Fe, whose incorporation is schematically shown in Fig. 6. Independent of the Fe decoration, the Co3O4 NPs oxidize only slightly on the surface as seen in the operando surface-sensitive NEXAFS spectroscopy but is also reflected in the contraction of the bulk spinel lattice during the OER identified by operando HE-XRD. Without Fe decoration, there was no detectable near-surface structural transformation but in contrast, the near-surface clearly transforms in the presence of Fe as seen in the Co3O4 coherence length, suggesting the enhanced formation of a Co–Fe (oxyhydr)oxide. Furthermore, the extent of Co–O near-surface oxidation of the more active Fe-decorated Co3O4 is less pronounced compared to the Fe-free case. While the oxygen near-surface chemistry changes during the OER significantly less strongly, suggesting an apparently lower density of electrophilic oxygen O1; this is partially compensated by the oxidation of low coordination Co2+ sites and the cationic Fe species during the OER. Thus, we conclude that the presence of low coordination and thus, defect-attributed Co2+ as well as redox-active Fe sites lead to a less oxidized state (from the Co and O perspective) during the OER which seems to be linked to the higher catalytic activity. Previously, reducible and/or edge sites in Co(oxyhydr)oxides have been attributed to be crucial (or even responsible) for the OER turnover while exhibiting a lower average Co oxidation state.28,56 Similar to the case of NiFe-based OER catalysts, the Fe species seem to decrease the extent of oxidation of the host oxide and increase the density of highly active defect sites. Thus, we conclude that the two-fold influence of the Fe on the OER electrocatalysis over Co-based electrocatalysts is responsible for the OER improvement. At low Fe contents, the specific activity benefits mainly from the electronic interaction between Co and Fe in particular on non-cubic NPs. At higher Fe contents, the Fe incorporation into a Co–Fe (oxyhydr)oxide near-surface leads to a higher active site density dominating the increase in apparent OER activity.
So far, it has been unclear whether the Fe ions in the near-surface of Co- and Ni-based electrocatalysts are redox-active and whether they participate in the OER mechanism. Our findings suggest that the Fe ions in the Co3−xFexO4 near-surface undergo a redox transition and accumulate oxidative charges during the OER. Furthermore, the (homogeneous) incorporation of the Fe ions into the Co3−xFexO4 near-surface as well as the apparent changes in the near-surface electronic structure with a substantially increased conductivity suggest the collective response of the Co, Fe, and O ions on the applied potential. Concurrently, the redox electrochemistry does not suggest pronounced changes in the thermodynamics of the surface oxidation leading to a shift of the barrier for Fe oxidation. Thus, the near-surface Fe ions likely participate in the redox processes during the OER while they improve the catalytic activity.
It is however important to note that the intrinsic heterogeneity of the investigated Co3O4 electrocatalysts does not allow us to precisely disentangle the catalytic role of e.g. the cubic and non-cubic NPs. Recent works suggest that there is a facet dependence of the Co3O4-catalyzed OER, with a substantially more active CoOx(OH)y near-surface formed on the Co3O4(100) as compared to that formed on multi-facetted yet crystalline Co3O4 NPs.61 In our case, we focused on revealing trends in the atomic scale interaction between Fe electrolyte species and the CoOx(OH)y near-surface during the OER and their catalytic role. To unambiguously disentangle the catalytic role of cationic Fe species on the Co3O4 surface, in-depth investigations of model surfaces such as epitaxial Co3O4 thin films decorated with controlled amounts of Fe are still critically needed. Furthermore, the possible distinct incorporation, distribution and stability of the Fe species on Co oxide particles with different initial structures (e.g. cubic vs. octahedral or spherical NPs) should be the subject of future studies, if possible, at the single nanoparticle level as demonstrated in the past for other material systems based on particle-on-a-stick experiments.62–64
Conclusions
In this work, we investigated the Fe decoration of Co3O4 spinel nanoparticles from Fe electrolyte species in detail with ex situ, in situ and operando methods and identified its catalytic role and its influence on the OER active state. We were able to locate the Fe domains predominantly on the (100) facets of cubic NPs. Combining depth-profiling XPS and STEM-EDX, our present findings suggest that Co- and Fe-containing oxide near-surfaces is formed which may result from the formation of CoOx(OH)y during the OER. Notably, we could identify favorable synergies of Fe with non-cubic NPs, although the interaction of Fe with cubic NPs remains the dominant contribution of the OER improvement.With in situ and operando methods, we identified both Co and Fe participating in the redox electrochemistry and thus, presumably in the OER although the addition of Fe to the near-surface may hamper the accumulation of Co-related oxidative charges during the OER. Here, we emphasize the lower affinity to electrophilic O-ligands in the presence of Fe accompanied with a higher density of low-coordinated, redox-active Co3+ sites and a stronger structural flexibility leading to a more pronounced reaction zone formation. Thus, the near-surface cationic Fe facilitates the OER catalysis in a variety of ways as it irreversibly provides additional reaction centers due to its incorporation into the CoOx(OH)y near-surface during the OER and enhances the OER activity but does not remain solely responsible for the catalysis.
Finally, in this work, we demonstrate how in situ and operando methods can be used to understand the challenging processes of the formation of active catalyst structures under reaction conditions and extract principles for optimized catalyst design.
Methods
Synthesis of Co3O4 nanoparticles
The synthesis of Co3O4 was published previously following a solvothermal route.38 A mixture of oleylamine and ethanol was added dropwise to a 1 mmol solution of Co(NO3)2·6H2O in EtOH which was stirred rigorously. The mixture was heated in an electrical furnace for 15 h at 180 °C and after precipitation the mixture was centrifuged and washed with EtOH. Finally, the precipitate was dried in air and calcined at 300 °C in air for 1 h. The catalyst ink was prepared with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2O and EtOH and no binder like Nafion© was used. The mixture was sonicated for 30 minutes and drop-cast on a glassy carbon electrode.
1 mixture of H2O and EtOH and no binder like Nafion© was used. The mixture was sonicated for 30 minutes and drop-cast on a glassy carbon electrode.
X-ray diffractometry
In-house X-ray diffractometry (XRD) was performed with a Bruker D8 Advance diffractometer equipped with a Cu X-ray source in the Bragg–Brentano configuration and variable primary divergence slit. The detector was an energy-dispersive position-sensitive LynxEye XE-T detector (Bruker-AXS). The Rietveld refinement was performed for the Co3O4 spinel and the CeO2 structure taking into account the zero error, sample displacement and the instrumental broadening as determined from the NIST CeO2 reference.Electrochemistry and catalytic testing for the OER
The OER activity was determined in a three-electrode setup connected to a SP-300 potentiostat (Biologic) and the electrochemistry was performed in a PTFE cell (Pine Research). Prior to each measurement, the electrochemical cell was cleaned with 30% aqueous HNO3 solution and rinsed with water to remove metal impurities. Glassware was entirely avoided for all experiments, including in situ and operando experiments. The catalytic testing was performed in 0.1 M KOH (99.999%, Sigma Aldrich) which was freshly purified with Co(OH)2 and checked with inductively coupled plasma mass spectrometry (ICP-MS, iCAP RQ, ThermoFisher Scientific) for metal impurities.17,65 If conditioning with Fe preceded the catalytic testing, Fe(NO3)3·9H2O was added from an aqueous stock solution with known concentrations to purify 0.1 M KOH. After conditioning, the electrochemical cell and all contaminated parts were cleaned with nitric acid. Prior to each measurement, the electrolyte was saturated with N2. All electrochemical measurements were performed with a rotating disk electrode (RDE, Pine Research) with glassy carbon as the working electrode. The glassy carbon electrode had a diameter of 5 mm with a geometric electrode surface area of 0.196 cm2 and was incorporated into a PEEK shaft. The catalyst ink was drop-casted on the electrode tip and dried with Ar for a loading of 50 μg cm−2 and the rotation speed was set to 1200 rpm. A graphite rod (Pine Research) with a Zirfon® membrane (Agfa) separated PTFE compartment was used as the counter electrode. The reference electrode was a single junction Hg/HgO electrode (Pine Research) and was calibrated on a daily basis in Fe-free 0.1 M KOH with a commercial reversible hydrogen electrode (HydroFlex®, Gaskatel).All applied electrochemical potentials are provided versus the reversible hydrogen electrode (RHE) and are corrected for the solution resistance which was determined from Nyquist plots from potentio-electrochemical impedance spectroscopy (PEIS) at 1 VRHE. The conditioning step of all experiments consisted of 1 h chronoamperometry (CA) at 1.45 VRHE in 0.1 M KOH without Fe or with 0.1, 0.5, 1.0, 3.0 or 5.0 ppm Fe. After the conditioning, the sample was transferred into an Fe-free purified electrolyte for the catalytical testing. The measurements consisted of 2 min CAs from 1.5 to 1.64 VRHE with 20 mV steps and linear sweeps with a 5 mV s−1 scan rate from one potential to the next higher one in-between.
For in situ and operando measurements, the catalytical testing was performed as chronoamperometry at 1.6 VRHE for 30 minutes to provide sufficient scanning times. Measurements in the electrolyte before and after catalysis were usually performed at 1 VRHE if not stated differently.
Scanning transmission electron microscopy and energy-dispersive X-ray spectroscopy
STEM-EDX was conducted using a ThermoFisher Talos F200x spectrometer at 200 kV. The focused electron beam was scanned over a specific region and EDX spectra were recorded with a 4-quadrant detector (Super-X detection system, ThermoFisher). Thereby, multiple frames were collected to integrate the EDX spectra per frame to enhance the quality of the EDX signal. After the background subtraction, the Fe K and Co K peaks were quantified by Brown-Powell ionization cross sections (Velox 2.13, ThermoFisher Scientific). High resolution STEM images were acquired using a probe-corrected JEM-ARM 200F (JEOL, Japan) equipped with a cold field emission gun (CFEG) operated at 200 kV. The HAADF signal was collected from an electron probe with a 14.2 mrad convergence semi-angle and a 120–430 mrad collection semi-angle. Image acquisition and manipulation were performed with the DigitalMicrograph software v3.43 (Gatan, USA).X-ray photoelectron spectroscopy
Ex situ X-ray photoelectron spectroscopy (XPS) was performed as-prepared, after conditioning and after catalytic probing. The samples were drop-cast on glassy carbon electrodes.For laboratory-based XPS measurements, a non-monochromatic Mg anode with 1253.6 eV operated at 250 W was used. The detector was equipped with a hemispherical electron analyzer (Phoibos 100, SPECS GmbH) where a pass energy of 15 eV was applied. The X-ray source and the electron analyzer were aligned in a 54.7° angle.
XPS measurements with constant kinetic energy of photoelectrons were conducted at the ISISS end-station of the BESSY II synchrotron facility of HZB in Berlin. The excitation energy was adjusted for kinetic energies of ∼550 and ∼200 eV. The measured XPS peak areas were quantified with the photon illumination and the photoionization cross-section.66 Further information regarding the XPS fitting is provided in Supplementary Note 1 (ESI†).
In situ soft X-ray absorption spectroscopy
In situ X-ray absorption spectroscopy (XAS) at the Co L-edge and O K-edge was conducted at the ISISS endstation of the BESSY II synchrotron facility of HZB in Berlin (Proposal No. 222-11340-CR). The samples were spray-coated on FAS-membranes (Fumasep FAS-30, Fumatech) and covered with graphene for conductivity. The AEY signals at the Co L-edge were normalized after the linear background subtraction of the pre-edge region. The O K-edge spectra were corrected with a linear background subtraction and normalization on non-lattice-oxygen features. A SP-300 potentiostat was used for the electrochemical measurements. For an increased stability of the membrane with graphene, the sample was introduced into the vacuum system while flowing the electrolyte and applying 1 VRHE during the pumping process.In situ high-energy X-ray diffraction
In situ high-energy XRD was performed at the ID31 beamline at European Synchrotron Research Facility (ESRF) in Grenoble, France. The photon energy was set to 67 keV and a Dectris Pilatus3 X CdTe 2 M detector was used. The working distance was aligned with a CeO2 standard (National Institute of Standards and Technology, Standard Material 674b). The samples were prepared on highly oriented pyrolytic graphite (HOPG) and mounted in a home-made PEEK cell suitable for grazing incidence measurements. A Pt wire was used as the counter electrode and Ag/AgCl (3.4 M KCl, eDAQ ET072) as the reference electrode and all electrodes were connected to a SP-300 potentiostat. Rietveld refinement was performed with the Topas software package.Operando hard X-ray absorption spectroscopy
Operando XAS at the Co and Fe K-edges was carried out at the CryoEXAFS endstation at the KMC-3 beamline of BESSY II synchrotron radiation facility of HZB in Berlin. The XAS measurements were conducted in a self-made single-compartment PEEK cell. The samples were prepared on 10 μm thin glassy carbon electrodes (SIGRADUR®, HTW) and mounted on an orifice of the PEEK cell, fixed and sealed using a Kapton window and penetrable for incoming X-rays and emitted fluorescence signals. The counter electrode was a Pt mesh and the reference electrode was a leak free Ag/AgCl electrode. All measurements were performed in fluorescence mode. The KMC-3 beamline was equipped with a 13-element fluorescence yield detector.Energy alignment and data normalization were carried out with the software Athena.67 Quantitative EXAFS analysis was carried out with conventional least-square fitting as implemented in the FEFFIT8.5 code68,69 including complex exchange–correlation Hedin-Lundqvist potential. At the Co K-edge, the first three M–O, Co–M1 and Co–M2 paths were fitted based on a spinel crystal structure. The amplitudes were calculated following the published procedure and are based on the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of octahedral to tetrahedral sites.35,36 At the Fe K-edge, the first M–O path was fitted assuming an octahedral Fe–O coordination. Fitting parameters are provided in Tables S7–S9 (ESI†) and spectra in Fourier transformed k-space in note 2 (ESI†).
1 ratio of octahedral to tetrahedral sites.35,36 At the Fe K-edge, the first M–O path was fitted assuming an octahedral Fe–O coordination. Fitting parameters are provided in Tables S7–S9 (ESI†) and spectra in Fourier transformed k-space in note 2 (ESI†).
Author contributions
FTH, AB and BRC co-wrote the paper. BRC and AB designed and supervised the study. SS performed sample synthesis. FTH designed and performed all electrochemical and catalytic measurements and performed the XPS characterization and data analysis. EO and FPS performed the TEM experiments and data analysis. FTH and AB designed the operando experiments. FTH analyzed the XAS and NEXAFS data. AB analyzed the (HE-)XRD data. FTH, AB, JT, DC, FS, MR, AM, HSJ, AH, UH, and EMD performed synchrotron experiments. All authors contributed to the discussion and editing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – 388390466 – TRR 247, subprojects A4, B06, and C03 as well as project no. 406944504-SPP 2080. Additional support from the European Research Council under grant ERC-OPERANDOCAT (ERC-725915) and the German Federal Ministry for Education and Research (BMBF) under the grant Catlab (03EW0015B) were also obtained. Felix T. Haase, Clara Rettenmaier and Antonia Herzog acknowledge support by the IMPRS Elementary Processes in Physical Chemistry. We thank the Helmholtz-Zentrum Berlin für Materialien und Energie for the allocation of synchrotron radiation beamtime and Dr Götz Schuck and PD Dr Michael Haumann for the support at the KMC-3 beamline. We thank Dr Michael Hävecker for the Support at the ISISS beamline. The operando HE-XRD experiments were performed at the beamline ID31 at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We are grateful to Dr Jakub Drnec at the ESRF for providing assistance in using this beamline. Furthermore, the authors would like to thank Dr See Wee Chee, Dr Lichen Bai and Dr Thomas Götsch for stimulating discussions. Open Access funding provided by the Max Planck Society.References
- M. Yu, E. Budiyanto and H. Tüysüz, Angew. Chem., Int. Ed., 2022, 61, e202103824 CrossRef CAS PubMed.
- S. Wang, A. Lu and C.-J. Zhong, Nano Convergence, 2021, 8, 4 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
- H.-Y. Wang, S.-F. Hung, H.-Y. Chen, T.-S. Chan, H. M. Chen and B. Liu, J. Am. Chem. Soc., 2016, 138, 36–39 CrossRef CAS PubMed.
- Y. J. Sa, K. Kwon, J. Y. Cheon, F. Kleitz and S. H. Joo, J. Mater. Chem. A, 2013, 1, 9992–10001 RSC.
- X. Xiao, X. Liu, H. Zhao, D. Chen, F. Liu, J. Xiang, Z. Hu and Y. Li, Adv. Mater., 2012, 24, 5762–5766 CrossRef CAS PubMed.
- A. J. Esswein, M. J. McMurdo, P. N. Ross, A. T. Bell and T. D. Tilley, J. Phys. Chem. C, 2009, 113, 15068–15072 CrossRef CAS.
- C.-W. Tung, Y.-Y. Hsu, Y.-P. Shen, Y. Zheng, T.-S. Chan, H.-S. Sheu, Y.-C. Cheng and H. M. Chen, Nat. Commun., 2015, 6, 8106 CrossRef CAS PubMed.
- D. Xu, M. B. Stevens, M. R. Cosby, S. Z. Oener, A. M. Smith, L. J. Enman, K. E. Ayers, C. B. Capuano, J. N. Renner, N. Danilovic, Y. Li, H. Wang, Q. Zhang and S. W. Boettcher, ACS Catal., 2019, 9, 7–15 CrossRef CAS.
- D. A. Corrigan and R. M. Bendert, J. Electrochem. Soc., 1989, 136, 723 CrossRef CAS.
- D. A. Corrigan, J. Electrochem. Soc., 1987, 134, 377–384 CrossRef CAS.
- M. S. Burke, S. Zou, L. J. Enman, J. E. Kellon, C. A. Gabor, E. Pledger and S. W. Boettcher, J. Phys. Chem. Lett., 2015, 6, 3737–3742 CrossRef CAS PubMed.
- L. Trotochaud, J. K. Ranney, K. N. Williams and S. W. Boettcher, J. Am. Chem. Soc., 2012, 134, 17253–17261 CrossRef CAS PubMed.
- F. Dionigi and P. Strasser, Adv. Energy Mater., 2016, 6, 1600621 CrossRef.
- G. Wartner, D. Hein, A. Bergmann, R. Wendt, B. Roldan Cuenya and R. Seidel, J. Mater. Chem. A, 2023, 11, 8066–8080 RSC.
- D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M.-J. Cheng, D. Sokaras, T.-C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Nørskov, A. Nilsson and A. T. Bell, J. Am. Chem. Soc., 2015, 137, 1305–1313 CrossRef CAS PubMed.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- N. Li, D. K. Bediako, R. G. Hadt, D. Hayes, T. J. Kempa, F. von Cube, D. C. Bell, L. X. Chen and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 1486–1491 CrossRef CAS PubMed.
- D. González-Flores, K. Klingan, P. Chernev, S. Loos, M. R. Mohammadi, C. Pasquini, P. Kubella, I. Zaharieva, R. D. Smith and H. Dau, Sustainable Energy Fuels, 2018, 2, 1986–1994 RSC.
- J. Y. C. Chen, L. Dang, H. Liang, W. Bi, J. B. Gerken, S. Jin, E. E. Alp and S. S. Stahl, J. Am. Chem. Soc., 2015, 137, 15090–15093 CrossRef CAS PubMed.
- H. Xiao, H. Shin and W. A. Goddard, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5872–5877 CrossRef CAS PubMed.
- J. Timoshenko, F. T. Haase, S. Saddeler, M. Rüscher, H. S. Jeon, A. Herzog, U. Hejral, A. Bergmann, S. Schulz and B. Roldan Cuenya, J. Am. Chem. Soc., 2023, 145, 4065–4080 CrossRef CAS PubMed.
- M. Görlin, J. Ferreira de Araújo, H. Schmies, D. Bernsmeier, S. Dresp, M. Gliech, Z. Jusys, P. Chernev, R. Kraehnert, H. Dau and P. Strasser, J. Am. Chem. Soc., 2017, 139, 2070–2082 CrossRef PubMed.
- S. Zou, M. S. Burke, M. G. Kast, J. Fan, N. Danilovic and S. W. Boettcher, Chem. Mater., 2015, 27, 8011–8020 CrossRef CAS.
- D. Y. Chung, P. P. Lopes, P. Farinazzo Bergamo Dias Martins, H. He, T. Kawaguchi, P. Zapol, H. You, D. Tripkovic, D. Strmcnik, Y. Zhu, S. Seifert, S. Lee, V. R. Stamenkovic and N. M. Markovic, Nat. Energy, 2020, 5, 222–230 CrossRef.
- Z. Sun, A. Curto, J. Rodríguez-Fernández, Z. Wang, A. Parikh, J. Fester, M. Dong, A. Vojvodic and J. V. Lauritsen, ACS Nano, 2021, 15, 18226–18236 CrossRef CAS PubMed.
- D. Drevon, M. Görlin, P. Chernev, L. Xi, H. Dau and K. M. Lange, Sci. Rep., 2019, 9, 1–11 CrossRef CAS PubMed.
- F. T. Haase, A. Bergmann, T. E. Jones, J. Timoshenko, A. Herzog, H. S. Jeon, C. Rettenmaier and B. Roldan Cuenya, Nat. Energy, 2022, 7, 765–773 CrossRef CAS.
- A. Bergmann, E. Martinez-Moreno, D. Teschner, P. Chernev, M. Gliech, J. F. de Araújo, T. Reier, H. Dau and P. Strasser, Nat. Commun., 2015, 6, 8625 CrossRef CAS PubMed.
- Y. Liu, Y. Ying, L. Fei, Y. Liu, Q. Hu, G. Zhang, S. Y. Pang, W. Lu, C. L. Mak and X. Luo, J. Am. Chem. Soc., 2019, 141, 8136–8145 CrossRef CAS PubMed.
- E. Budiyanto, M. Yu, M. Chen, S. DeBeer, O. Rüdiger and H. Tüysüz, ACS Appl. Energy Mater., 2020, 3, 8583–8594 CrossRef CAS.
- Y. Gong, W. Ding, Z. Li, R. Su, X. Zhang, J. Wang, J. Zhou, Z. Wang, Y. Gao and S. Li, ACS Catal., 2018, 8, 4082–4090 CrossRef CAS.
- Y. Peng, H. Hajiyani and R. Pentcheva, ACS Catal., 2021, 11, 5601–5613 CrossRef CAS.
- X. T. Wang, T. Ouyang, L. Wang, J. H. Zhong, T. Ma and Z. Q. Liu, Angew. Chem., 2019, 131, 13425–13430 CrossRef.
- F. T. Haase, A. Rabe, F.-P. Schmidt, A. Herzog, H. S. Jeon, W. Frandsen, P. V. Narangoda, I. Spanos, K. Friedel Ortega, J. Timoshenko, T. Lunkenbein, M. Behrens, A. Bergmann, R. Schlögl and B. Roldan Cuenya, J. Am. Chem. Soc., 2022, 144, 12007–12019 CrossRef CAS PubMed.
- S. Saddeler, G. Bendt, S. Salamon, F. T. Haase, J. Landers, J. Timoshenko, C. Rettenmaier, H. S. Jeon, A. Bergmann, H. Wende, B. Roldan Cuenya and S. Schulz, J. Mater. Chem. A, 2021, 9, 25381–25390 RSC.
- E. M. Davis, A. Bergmann, C. Zhan, H. Kuhlenbeck and B. Roldan Cuenya, Nat. Commun., 2023, 14, 4791 CrossRef CAS PubMed.
- S. Saddeler, U. Hagemann and S. Schulz, Inorg. Chem., 2020, 59, 10013–10024 CrossRef CAS PubMed.
- F. Zasada, W. Piskorz, P. Stelmachowski, A. Kotarba, J.-F. Paul, T. Płociński, K. J. Kurzydłowski and Z. Sojka, J. Phys. Chem. C, 2011, 115, 6423–6432 CrossRef CAS.
- J. Bae, D. Shin, H. Jeong, C. Choe, Y. Choi, J. W. Han and H. Lee, ACS Catal., 2021, 11, 11066–11074 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. Lau, A. R. Gerson and R. S. C. Smart, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- C. C. L. McCrory, S. Jung, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2013, 135, 16977–16987 CrossRef CAS PubMed.
- T. Zhang, M. R. Nellist, L. J. Enman, J. Xiang and S. W. Boettcher, ChemSusChem, 2019, 12, 2015–2021 CrossRef CAS PubMed.
- N. K. Singh, M. K. Yadav, R. Parihar and I. Kumar, Int. J. Electrochem. Sci., 2020, 15, 6605–6619 CrossRef CAS.
- D. Guragain, S. Karna, J. Choi, R. Bhattarai, T. P. Poudel, R. K. Gupta, X. Shen and S. R. Mishra, Processes, 2021, 9, 2176 CrossRef CAS.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed.
- F. Yang, M. Lopez Luna, F. T. Haase, D. Escalera-López, A. Yoon, M. Rüscher, C. Rettenmaier, H. S. Jeon, E. Ortega, J. Timoshenko, A. Bergmann, S. W. Chee and B. Roldan Cuenya, J. Am. Chem. Soc., 2023, 145, 21465–21474 CrossRef CAS PubMed.
- W. Xiang, N. Yang, X. Li, J. Linnemann, U. Hagemann, O. Ruediger, M. Heidelmann, T. Falk, M. Aramini, S. DeBeer, M. Muhler, K. Tschulik and T. Li, Nat. Commun., 2022, 13, 179 CrossRef CAS PubMed.
- T. Wiegmann, I. Pacheco, F. Reikowski, J. Stettner, C. Qiu, M. Bouvier, M. Bertram, F. Faisal, O. Brummel, J. R. Libuda, J. Drnec, P. Allongue, F. Maroun and O. M. Magnussen, ACS Catal., 2022, 12, 3256–3268 CrossRef CAS PubMed.
- F. Reikowski, F. Maroun, I. Pacheco, T. Wiegmann, P. Allongue, J. Stettner and O. M. Magnussen, ACS Catal., 2019, 9, 3811–3821 CrossRef CAS.
- L. Bai, C.-S. Hsu, D. T. L. Alexander, H. M. Chen and X. Hu, Nat. Energy, 2021, 6, 1054–1066 CrossRef CAS.
- P.-E. Petit, F. Farges, M. Wilke and V. A. Solé, J. Synchrotron Radiat., 2001, 8, 952–954 CrossRef CAS PubMed.
- M. Wilke, F. o Farges, P.-E. Petit, G. E. Brown, Jr. and F. o Martin, Am. Mineral., 2001, 86, 714–730 CrossRef CAS.
- L. Gong, X. Y. E. Chng, Y. Du, S. Xi and B. S. Yeo, ACS Catal., 2018, 8, 807–814 CrossRef CAS.
- J. Nai, H. Yin, T. You, L. Zheng, J. Zhang, P. Wang, Z. Jin, Y. Tian, J. Liu, Z. Tang and L. Guo, Adv. Energy Mater., 2015, 5, 1401880 CrossRef.
- A. Bergmann, T. E. Jones, E. M. Moreno, D. Teschner, P. Chernev, M. Gliech, T. Reier, H. Dau and P. Strasser, Nat. Catal., 2018, 1, 711 CrossRef CAS.
- H. N. Nong, L. J. Falling, A. Bergmann, M. Klingenhof, H. P. Tran, C. Spöri, R. Mom, J. Timoshenko, G. Zichittella, A. Knop-Gericke, S. Piccinin, J. Pérez-Ramírez, B. Roldan Cuenya, R. Schlögl, P. Strasser, D. Teschner and T. E. Jones, Nature, 2020, 587, 408–413 CrossRef CAS PubMed.
- T. Götsch, N. Köpfle, M. Grünbacher, J. Bernardi, E. A. Carbonio, M. Hävecker, A. Knop-Gericke, M. F. Bekheet, L. Schlicker, A. Doran, A. Gurlo, A. Franz, B. Klötzer and S. Penner, Phys. Chem. Chem. Phys., 2019, 21, 3781–3794 RSC.
- Z. Shen, Y. Zhuang, W. Li, X. Huang, F. E. Oropeza, E. J. M. Hensen, J. P. Hofmann, M. Cui, A. Tadich, D. Qi, J. Cheng, J. Li and K. H. L. Zhang, J. Mater. Chem. A, 2020, 8, 4407–4415 RSC.
- I. Spanos, J. Masa, A. Zeradjanin and R. Schlögl, Catal. Lett., 2021, 151, 1843–1856 CrossRef CAS.
- Z. Liu, H. M. A. Amin, Y. Peng, M. Corva, R. Pentcheva and K. Tschulik, Adv. Funct. Mater., 2023, 33, 2210945 CrossRef CAS.
- H. B. Aiyappa, P. Wilde, T. Quast, J. Masa, C. Andronescu, Y.-T. Chen, M. Muhler, R. A. Fischer and W. Schuhmann, Angew. Chem., Int. Ed., 2019, 58, 8927–8931 CrossRef CAS PubMed.
- T. Quast, H. B. Aiyappa, S. Saddeler, P. Wilde, Y.-T. Chen, S. Schulz and W. Schuhmann, Angew. Chem., Int. Ed., 2021, 60, 3576–3580 CrossRef CAS PubMed.
- X. Lu, M. Li, Y. Peng, X. Xi, M. Li, Q. Chen and A. Dong, J. Am. Chem. Soc., 2021, 143, 16925–16929 CrossRef CAS PubMed.
- L. Bai, C.-S. Hsu, D. T. Alexander, H. M. Chen and X. Hu, J. Am. Chem. Soc., 2019, 141, 14190–14199 CrossRef CAS PubMed.
- J. Yeh and I. Lindau, At. Data Nucl. Data Tables, 1985, 32, 1–155 CrossRef CAS.
- B. Ravel and M. Newville, J. Synchrotron Radiat., 2005, 12, 537–541 CrossRef CAS PubMed.
- M. Newville, J. Synchrotron Radiat., 2001, 8, 322–324 CrossRef CAS PubMed.
- A. Ankudinov, B. Ravel, J. Rehr and S. Conradson, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, 7565 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee02809g |
| This journal is © The Royal Society of Chemistry 2024 |