Effect of microbially available phosphorous removal on Legionella spp. in multi-storey residential dwellings in Latvia†
Marta
Zemīte
 *abd,
Daina
Pūle
bc,
Olga
Kiriļina-Gūtmane
c,
Laima
Ķimse
c,
Mārtiņš
Strods
a,
Jurǵis
Zemītis
d,
Linda
Mežule
a,
Olga
Valciņa
c and
Tālis
Juhna
a
*abd,
Daina
Pūle
bc,
Olga
Kiriļina-Gūtmane
c,
Laima
Ķimse
c,
Mārtiņš
Strods
a,
Jurǵis
Zemītis
d,
Linda
Mežule
a,
Olga
Valciņa
c and
Tālis
Juhna
a
aWater Research and Environmental Biotechnology Laboratory, Riga Technical University, Riga, Latvia. E-mail: Marta.Zemite@rtu.lv
bDepartment of Water Engineering and Technology, Riga Technical University, Riga, Latvia
cInstitute of Food Safety, Animal Health and Environment “BIOR”, Riga, Latvia
dDepartment of Heat Engineering and Technology, Riga Technical University, Riga, Latvia
First published on 9th November 2023
Abstract
Phosphorus is one of the major nutrients that regulates microbial growth in water systems, containing a fraction that is easily utilized for bacterial processes known as microbially available phosphorus (MAP). However, its effect on opportunistic pathogens such as Legionella bacteria is unclear. In this case study, the impact of MAP on Legionella spp. was investigated. A point-of-use (POU) ferric hydroxide sorption filter was used to reduce MAP levels in the internal drinking water supply (DWS) of a multistorey residential building in Riga, Latvia. The study included two distinct domestic hot water (DHW) heat exchanger setpoints: initially set at 57 °C for 12 weeks, and then lowered to 48 °C due to a decision to reduce energy consumption. Prior to the startup of the POU device, the internal DWS underwent centralised chemical flushing and hydrogen peroxide disinfection, which proved insufficient for long-term Legionella control. The POU device successfully removed around 70% of MAP to a concentration of 3.6 μg l−1 (SD 1.5 μg l−1), nevertheless, Legionella pneumophila serogroups 1, 2, and 3 were identified. During the initial high-temperature period, similar concentrations of L. pneumophila were detected in both buildings, regardless of the presence of the POU device. However, at the lower setpoint, Legionella concentrations increased by more than tenfold in the MAP-limited environment. This was attributed to the opportunistic pathogen's higher growth rate compared to native bacteria experiencing a nutrient deficiency. In summary, reducing phosphorus levels alone is insufficient for effective control of Legionella bacteria and additional strategies are needed to address the complexities involved in Legionella control within DWS systems.
Water impactLegionnaires' disease persists as a preventable health threat in Europe. The study reveals that merely reducing growth-promoting nutrients, like phosphorus, is insufficient in effectively mitigating Legionella outbreaks or sporadic cases. Outdated biostability concepts, which focus solely on removing limiting nutrients, should be revised to consider ecosystems' carrying capacity for better prevention strategies. |
1. Introduction
In recent years, numerous water companies, especially in Denmark, the Netherlands, Germany, Switzerland, and parts of Belgium, have been pursuing the objective of enhancing the biological stability of drinking water by reducing the level of growth-limiting substrate.1 While this approach has demonstrated a degree of success in diminishing the formation of biofilms within internal piping networks, it has proven inadequate in effectively managing the proliferation of opportunistic pathogens found in premises plumbing, such as Legionella bacteria.2 This limitation arises from the fact that the organic carbon typically acts as the limiting substrate (electron donor), commonly quantified as assimilable organic carbon (AOC) or biodegradable organic carbon (BDOC), which, even reduced to low levels, can still be released from plastic pipes (PE or PVC-P) and soft hoses,3 which are commonly utilized in household showers.Another approach involves the reduction of microbially available phosphorus (MAP), which refers to the portion of phosphorus readily utilizable by bacteria. Furthermore, in Boreal regions, where water exhibits comparatively high concentrations of biodegradable organics, phosphorus (P) has been identified as the primary element governing bacterial growth in biofilms within drinking water distribution systems.4 MAP reduction has been successfully used in the mitigation of membrane biofouling,5 however, the impact of phosphorus on Legionella bacteria remains inconclusive.
Legionella spp. can be present in water environments in various forms, including as free-living bacteria, in a culturable state, viable-but-nonculturable (VBNC) state, or residing intracellularly within protozoa such as amoebae, where it can survive, proliferate, and consequently pose a potential risk.6 Therefore, the presence of nutrients, including P, can directly affect the growth of this opportunistic pathogen or indirectly influence it through the relevant microbiome. While in vitro studies have demonstrated that P can stimulate the growth of Legionella bacteria,7 the situation in real-world scenarios may differ due to factors such as the presence of competing native biota, the formation of multilayer biofilms, the ability of P to accumulate on iron plumbing and other contributing factors. Hence, the objective of this study was to investigate the impact of limiting microbially available phosphorus for Legionella control within a complex full-scale setting, specifically within multi-story residential buildings in Latvia.
Additionally, the study evaluated the impact of flushing and disinfection of the centralized internal water supply network on Legionella counts. The full-scale experiments were conducted over a period of five months, from July to December 2022, in two buildings that were commissioned in 1976. It is worth noting that in mid-October, coinciding with the implementation of energy-saving measures during the energy crisis, the temperature in the domestic hot water (DHW) networks was lowered.
2. Materials and methods
2.1. Pilot site description
Multistorey residential buildings were selected as a study site since in Latvia higher levels of Legionella have been observed in apartment buildings than in public buildings.8 The pilot site comprised two five-story residential buildings located within proximity, about 100 meters from each other, and they shared similar characteristics. One building was equipped with a sorption filter, aiming to eliminate MAP from the inflowing water. Thus, the building was referred to as the “POU-device building”. The other building was utilized as a reference, denoted as the “Reference building”.Prior to the test period, preliminary sampling confirmed the presence of cultivable Legionella bacteria in several water outflow points within both buildings. The buildings were commissioned in 1976. In the year 2000, the communal internal water pipelines were replaced with polypropylene pipes.
Both buildings received their drinking water from municipal water supply networks. They were located in a part of Riga where the municipal water source either underwent biological iron and manganese removal followed by post-chlorination (at the artificially recharged groundwater plant “Baltezers”) or received no specific treatment beyond chlorine disinfection (at the “Zaķumuiža” and “Remberģi” groundwater plants). Each of these plants provided water with distinctive electrical conductivity values (Table S1†), with “Baltezers” providing water with a range of 646–779 μS cm−1, while “Remberģi” and “Zaķumuiža” had less distinctive ranges of 302–356 μS cm−1 and 250–338 μS cm−1, respectively.
Hot water was prepared within plate heat exchangers in individual heating substations located in the basements of each building. The DHW temperature at the exit of the plate heat exchanger was initially set to 57 °C until mid-October 2022. Subsequently, changes were made in response to energy-saving measures in the autumn of 2022, and a new setpoint of 48 °C was adjusted for Monday through Friday during nighttime hours (from 23:00 to 7:00), while the temperature was maintained at 52 °C during the remaining hours. On weekends, the temperature was set back to 57 °C at the outflow of the DHW heat exchanger.
2.2. Internal network chemical flushing and disinfection
In order to eliminate the existing scale deposits in the hot water network and to disinfect the internal piping in both hot and cold water systems before the start of a test period, the internal water networks in both buildings underwent a centralised process of chemical flushing and disinfection. This procedure was aimed to provide more comparable test starting conditions among the two buildings.A specialized company carried out the purification process. Initially, to eliminate scale deposits, the DHW network was purified using a phosphate-free reagent that contained formic acid as its active component (ALBILEX®-KALK-EX, Germany). Subsequently, a disinfectant (ALBILEX®-SUPER-des, Germany), comprising a mixture of hydrogen peroxide and silver ions, was added to the inlet cold water, providing the disinfection of both the cold and hot drinking water systems.
To facilitate the disinfection of pipeline branches, specialists made attempts to visit each apartment, ensuring that the necessary concentrations of reagents were present in the residential water outflow points. Overall, the specialists were able to access and confirm the necessary disinfectant concentrations at water outflow points in 51% of all apartments in the POU-device building, while the Reference building achieved a slightly higher percentage of 68%.
2.3. Point-of-use treatment unit
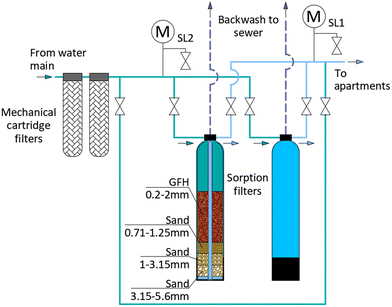
To enable the removal of MAP from the internal drinking water supply, a point-of-use (POU) ferric hydroxide sorption filter (Fig. 1) was installed on the water main inside the basement of one of the buildings. To ensure the safety of the drinking water supply, the POU filter was connected during the centralised disinfection phase, thus undergoing disinfection of filter materials. The unit comprised two 145 litre filter housings, each filled with 60 kg of granular ferric hydroxide (GFH) sorption media (GEH 102, GEH Wasserchemie, Germany), and sand support layers of three different grain sizes (0.71–1.25 mm, 1.00–3.15 mm, and 3.15–5.6 mm, around 17 litres per layer for each fraction).GFH is mostly used in drinking water installations for arsenic removal and similar materials are employed in wastewater installations for phosphate reduction. GFH consists primarily of β-FeOOH and Fe(OH)3, and it contains approximately 58% (± 10%) dry solids with an iron content of 600 g kg−1 (± 10%). The particle size ranges from 0.2–2.0 mm, and the backwashed bulk density is 1150 kg m−3 (± 10%), while the specific surface area is approximately 300 m2 g−1.
To notice the occurrence of filter clogging, the pressure was monitored before and after the unit. During the study period, there was no significant clogging or pressure build-up observed. Therefore, no filter backwashing to clean or maintain its performance was performed.
2.4. Sampling
The sampling was conducted over a duration of five months, commencing from the middle of July until the middle of December 2022. The first set of samples was collected on the day following the disinfection of the internal drinking water network. The frequency of sampling was higher at the beginning of the study and shortly after the reduction of DHW temperature, which was implemented to conserve energy. Fig. 2 illustrates a schematic of the sampling locations, providing a visual representation of where the samples were taken during the study.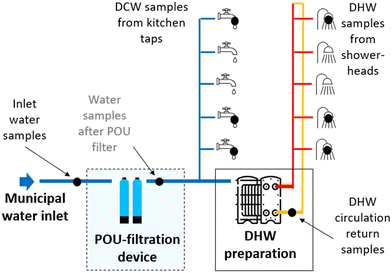 | ||
| Fig. 2 Schematic of the sampling locations (marked with black dots). DCW – domestic cold water, DHW – domestic hot water. | ||
The inlet water samples were collected from the cold-water mains close to the entry points of the buildings. The DHW samples were collected from two different locations: circulation return samples were taken from the heating substation before entering the heat exchanger, and showerhead hot water samples were collected from the apartments. For the domestic cold water (DCW) samples, water was collected from the kitchen taps in the apartments. In general, all samples were mainly collected on the same day, with only rare exceptions due to the availability of inhabitants.
Residents were given instructions to collect the first-draw samples directly from the showerheads and kitchen taps early in the morning, before using the shower. The individual responsible for sample collection ensured that the sample was taken before anyone in the apartment, including other residents, had used any water on the corresponding day. Although there was no fixed time interval, typically, there was a period of nighttime stagnation in the apartment pipes. Autoclavable polypropylene two-litre bottles were provided to them on the day before sampling. These bottles were washed with phosphate-free detergent and heat-sterilized (121 °C, 20 min) to ensure sample integrity. Identical bottles were used for collecting samples from heating substations and water inlets, which were performed by the researcher on the same day as the apartment sample collection.
The selection of apartments for sampling was based on their location, preferably ground and top floors, and the willingness of the inhabitants to participate in the sampling process. All residents involved in the study used hot water prepared inside the building's heating substation by a centralised DHW heat exchanger. There was variability in shower usage habits, ranging from several times per day to once every few days, depending on the number of residents in each apartment and their habits.
During the sampling period, the number of apartments included in the study varied due to the varying involvement of inhabitants. In total, the POU-device building had three 5th-floor apartments, one 2nd-floor apartment, and one 1st-floor apartment, while the Reference building had two 5th-floor apartments and one 1st-floor apartment. The water inlet mains, POU filtration device, and heating substations were all located at the basement level.
2.5. Physical and chemical analyses
Further, the samples were transferred to the laboratory, where electrical conductivity and pH were measured with HQ40D Portable Multi Meter (HACH, ASV) with corresponding sensors attached.
| Parameter | Value |
|---|---|
| Plasma mode | Normal, robust |
| RF power, kW | 1.30 |
| Sampling depth, mm | 8.0 |
| Carrier gas flow, l min−1 | 0.6 |
| Dilution gas flow, l min−1 | 0.4 |
| Spray chamber temperature, °C | 2.0 |
| Extraction lens l, V | 0 |
| Kinetic energy discrimination, V | 3 |
The nine-point calibration curves (eight standards and one blank) were constructed for each of the metal ions and the correlation coefficients were >0.99 before starting the sample analysis. Daily analyses of quality control materials (with concentrations of 2 μg l−1 and 50 μg l−1) were used for monitoring the repeatability and accuracy, as well as determining the method uncertainties. The calculation of the individual expanded method uncertainties for the individual chemical elements was provided using data from interlaboratory test results based on control reference materials.
To ensure the accuracy of the obtained results, blanks and controls with various TOC concentrations were analysed alongside each series of samples.
Next, the samples were amended with 4 mg C per litre from a sodium acetate (CH3COONa) stock solution, as well as 250 μg N l−1, 10 μg Mg l−1, 27 μg Ca l−1, 53 μg K l−1 and 40 μg Na l−1 from a salts stock solution, containing 42.7 g l−1 NH4NO3, 6.1 g l−1 MgSO4·7H2O, 5.9 g l−1 CaCl2·2H2O, 6.1 g l−1 KCl and 6.1 g l−1 NaCl in demineralised water. A natural bacterial consortium from mineral water (Evian, Danone, France) was used as an inoculum at a concentration of 103 intact cells per millilitre of sample.
Further, the amended and inoculated samples were incubated at 30 °C with continuous shaking at 150 RPM for five days. After incubation, the bacterial cell enumeration (see further) was performed as total cell count (TCC) and was measured by flow cytometry (CyFlow® Cube 6, Sysmex, Germany).
The MAP analyses were performed in triplicates for each sample, and the average TCC value was used to convert it to MAP concentration. In cases where the TCC values varied by more than 30% logarithmic difference in a sample triplicate, the specific sub-sample value was excluded from the calculation of the sample mean.
For the conversion of cell count to the corresponding MAP concentration, a locally determined linear standard curve (TCC = 3 × 106 MAP) was used, with a Pearson correlation of 0.973 (p < 0.001, n = 59). Before the conversion, an average TCC value for 0 μg MAP per litre was subtracted from all sample mean values to eliminate a background influence.
2.6. Microbiological analysis
In brief, one litre of water sample was filtered and concentrated using a 0.45 μm polyamide membrane filter (Millipore, Molsheim, France). The filter membranes were resuspended in 5 ml of sterile distilled water, then shaken for two minutes (Vortex Genius, IKA, Staufen, Germany), and kept at room temperature for 10 min. Three 0.1 ml aliquots (untreated, heat treated, acid-treated) were spread on buffered charcoal yeast extract agar (BCYE, OXOID, Basingstoke, UK) and glycine vancomycin polymyxin B cycloheximide agar (GVPC, Oxoid, Basingstoke, UK). Plates were incubated at 36 °C for 10 days and examined every day starting from day three. At least three characteristic colonies from each GVPC plate were subcultured on buffered charcoal extract agar medium (BCYE, OXOID, Basingstoke, UK) and buffered charcoal extract agar medium without L-cysteine (BCYE–Cys, OXOID, Basingstoke, UK), and incubated for at least 48 h at 36 °C. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI–TOF MS, Bruker, Bremen, Germany) was used for the identification of suspected Legionella colonies. An agglutination test (Thermo Fisher Scientific, Bred, Netherlands) was performed for the confirmation of L. pneumophila and detection of L. pneumophila serogroups was done with Latex reagents (Pro-Lab Diagnostics, Richmond Hill, Canada). Colonies from all plates were counted and confirmed, and the estimated count of Legionella was expressed as CFU l−1 of Legionella species and serogroup.
For TCC sample staining, a working fluorophore stock of SYBR Green I nucleic acid stain in DMSO (Sigma-Aldrich, USA) was diluted to 100× concentration with 10 mM TRIS buffer (pH 8). Within the sample, an additional 100× dilution of the working fluorophore stock was used for staining. For ICC/DCC sample staining, a working stock additionally contained 0.6 mM propidium iodide. The staining was conducted in a dark environment in a thermo-block at 35 °C for 10 min (TCC) or 15 min (ICC/DCC).
2.7. Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics software (version 23). A 2-tailed significance with α = 0.05 was used throughout the study.To test assumptions for normal distribution, Shapiro–Wilk's test of normality was generally used. However, for online data concerning electrical conductivity, the Kolmogorov–Smirnov test of normality was used instead. Throughout the study, mean (standard deviation) values are presented for normally distributed data or otherwise, median (min–max) is used.13 Levene's test was utilized to assess the homogeneity of variances. No outliers were excluded from the analysis.
Further, to determine statistical significance, either an independent sample t-test was used for normally distributed data with homogenous variances, or the non-parametric Mann–Whitney U test was employed for data that did not meet necessary assumptions. The mean ranks of the groups were compared in the Mann–Whitney U test.
The standard curve with corresponding coefficients was assessed in MS Excel, using a scatter plot with a linear trendline. Additionally, for parametric correlation analysis, bivariate correlation with the Pearson coefficient was conducted using IBM SPSS software.
3. Results and discussion
3.1. Effect of granular ferric hydroxide point-of-use filtration device on the inlet water parameters
To reduce the concentration of microbially available phosphorus (MAP) in the incoming water, in such a way as to limit the availability of vital nutrients for the proliferation of Legionella spp., the GFH sorption filter was installed on the municipal cold-water main inside one of the five-storey residential buildings.Due to the proximity of the buildings, the received water was expected to be of similar characteristics. However, the Reference building on average received water with lower electrical conductivity (EC), smaller calcium, magnesium, and total organic carbon (TOC) concentrations, smaller damaged cells concentration and higher copper concentration if compared to the POU-device building (Table 2). However, it is essential to note that these samples were collected as grab samples, which means they might not fully represent the average daily composition of the incoming water.
| Parameter | POU-device building | Reference building | P-valuea |
|---|---|---|---|
| Physical and chemical parameters | |||
| a Values stated only when p < 0.05; 2-tailed significance used for normally distributed data or exact significance value for non-parametric independent samples t-test. b Total cell count = intact cell count + damaged cell count.*Mean (standard deviation) values are presented for normally distributed data or otherwise, median (min–max) is used, with p-values in case of significantly different values between buildings. The number of samples = 12 for each building. | |||
| Temperature, °C | 10.78* (2.04) | 10.66* (2.28) | |
| pH | 7.93* (0.14) | 8.04* (0.14) | |
| Electrical conductivity, μS cm−1 | 386.0 (344–521) | 306.75* (30.00) | < 0.001 |
| Ca, mg l−1 | 38.35* (3.17) | 35.12* (3.22) | 0.021 |
| Mg, mg l−1 | 11.69* (1.26) | 9.74* (1.03) | < 0.001 |
| Mn, mg l−1 | 0.021* (0.005) | 0.023 (0.010–0.307) | |
| Cu, μg l−1 | 2.05 (0–210) | 6.2 (0–187) | 0.014 |
| Zn, mg l−1 | 0.006 (0–0.090) | 0.012 (0–0.249) | |
| Fe, mg l−1 | 0.094* (0.033) | 0.123 (0.042–0.652) | |
| Pb, μg l−1 | 0 (0–3.6) | 0.685 (0–17.4) | |
| Main microbial nutrients | |||
|---|---|---|---|
| Total organic carbon (TOC), mg l−1 | 2.24 (1.47–4.96) | 1.31 (0.96–5.76) | 0.007 |
| Microbially available phosphorus (MAP), μg l−1 | 12.17* (2.11) | 10.29* (4.23) | |
| Microbiological parameters | |||
|---|---|---|---|
| Total cell count, cells per mlb | 2.53 × 105* (5.77 × 104) | 2.19 × 105* (7.22 × 104) | |
| Damaged cell count, cells per ml | 1.19 × 105* (2.83 × 104) | 6.83 × 104 (5.77 × 104–1.14 × 105) | 0.003 |
| Intact cell count (ICC), cells per ml | 1.33 × 105* (4.54 × 104) | 1.39 × 105* (6.54 × 104) | |
| Low nucleic acid content cells, % ICC | 44* (4.8) | 46* (8.9) | |
| High nucleic acid content cells, % ICC | 56* (4.8) | 54* (8.9) | |
| L. pneumophila, CFU l−1 | 0 (0–400) | 0 (0–350) | |
To gain a wider overview of potential inconsistencies in inlet water characteristics, real-time measurements of EC were taken in the water main and logged on the server every 10 minutes. Additionally, water flow was automatically recorded every 20 minutes based on the water inlet meter data.
The analyses of EC values revealed significant differences in inlet water among both buildings when analysed for the whole sampling period (p < 0.001 with n = 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 247 for each building). The median EC values were 199 (156–477) μS cm−1 for the Reference building, and 266 (165–514) μS cm−1 for the POU-device building. These inconsistencies reflected drinking water supplied from different groundwater pumping stations that interchanged throughout the day (Table S1†). However, it was not possible to determine specific water sources by solely EC data, as the values for groundwater pumping plants “Zaķumuiža” and “Remberģi” had partly overlapping values, while generally higher EC values coming from plant “Baltezers” were diluted within a distribution network and became less distinctive in the inlet water EC patterns. Regarding the water volume, the POU-device building consumed 250 m3 more water during the study duration than the Reference building (Fig. S1†), while the consumption pattern was similar for both buildings (Fig. S2†).
247 for each building). The median EC values were 199 (156–477) μS cm−1 for the Reference building, and 266 (165–514) μS cm−1 for the POU-device building. These inconsistencies reflected drinking water supplied from different groundwater pumping stations that interchanged throughout the day (Table S1†). However, it was not possible to determine specific water sources by solely EC data, as the values for groundwater pumping plants “Zaķumuiža” and “Remberģi” had partly overlapping values, while generally higher EC values coming from plant “Baltezers” were diluted within a distribution network and became less distinctive in the inlet water EC patterns. Regarding the water volume, the POU-device building consumed 250 m3 more water during the study duration than the Reference building (Fig. S1†), while the consumption pattern was similar for both buildings (Fig. S2†).
The POU sorption filter in general did not affect inlet water parameters with the exception towards a reduction in MAP (p < 0.001) and Fe (p = 0.010) concentrations. Before the filtration, the average inflow MAP concentration was 10.3 μg l−1 (SD 4.2 μg l−1) for the Reference building and 12.2 μg l−1 (SD 2.1 μg l−1) for the POU-device building (Table 2). Following the passage through the sorption filter, the reduction level gradually decreased during the first month of operation, but the filter still provided a reasonably stable reduction ranging from about 60–80% (Fig. 3). The MAP concentration after filtration on average decreased to 3.6 μg l−1 (SD 1.5 μg l−1), resulting in an average reduction of 70% in MAP concentration. This reduction level was consistent with previous tests conducted with ferric oxides-coated biomass carriers.14
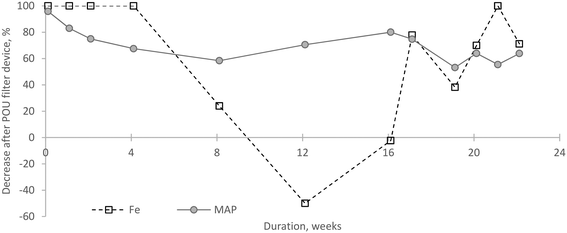 | ||
| Fig. 3 Microbially available phosphorus (MAP) and iron reduction levels in inlet water by point-of-use filtration device. | ||
The median iron concentration was 0.094 mg l−1 (SD 0.033 μg l−1), which decreased to 0.026 mg l−1 after filtration, with the highest filter outflow concentration of 0.134 mg l−1 during week 16. In general, it remained stable during the first month of operation. On average, the POU device reduced Fe concentration by around 60%, effectively removing it almost entirely during the first month. However, iron could have been desorbed from the POU device during week 12, transferring around 0.129 mg l−1 of iron to the internal water supply system, and the sorption device did not have an impact on its levels in week 16.
One of the reasons for iron release might be fluctuations in the pH of incoming water. On average, the pH was around 8.1 during the first eight weeks and the value slightly decreased (to an average of 7.8) during weeks 12–22. Another cause of desorption could be associated with potential changes in the hydraulic regime due to shifts in the water consumption pattern.
To mitigate biofilm formation, a suggested MAP concentration as low as 0.3 μg P per l was suggested.5 However, the implemented solution in the full-scale operation only achieved a reduction to 3.6 μg l−1 (SD 1.5 μg l−1), which is higher than the recommended value. Achieving such a high degree of removal would require more complex methods, such as coagulation,14 which may not be suitable for domestic use due to the need for experienced personnel and the generation of significant sludge byproducts. Additionally, frequent filter backwashes would increase household water demand.
3.2. Changes in microbially available phosphorus within internal drinking water networks
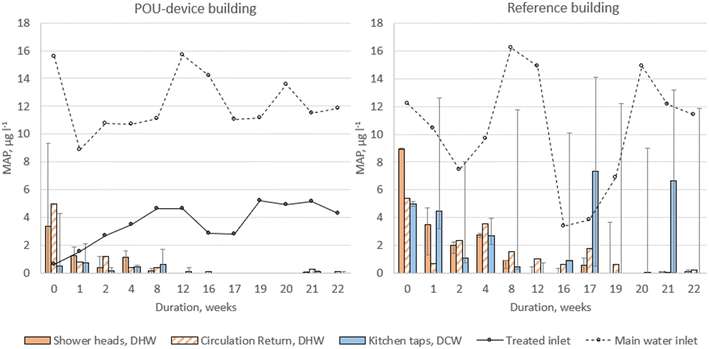
MAP concentration was analysed in samples collected from both the hot and cold internal drinking water networks (Fig. 4). The water entering DWS in the Reference building contained an average of 10.29 μg l−1 (SD 4.23 μg l−1) of MAP, while in the POU-device building, it was 3.56 μg l−1 (SD 1.49 μg l−1) after passing the sorption filter. During the first week, MAP values higher than inflow were observed in some sampling locations in the POU-device building, reaching up to 4.28 μg l−1 for DCW and up to 9.31 μg l−1 for DHW samples, showing that water exchange within the internal network continued on the next day after the start-up of MAP sorption filter. In the following weeks, the measured concentrations did not exceed 2.1 μg l−1 for DCW and 1.87 μg l−1 for DHW samples.In the Reference building, the MAP concentrations in the first-week samples were up to 5.15 μg l−1 for DCW and up to 9.01 μg l−1 for DHW samples. In the subsequent weeks, the maximal concentrations for the cold-water samples varied from 0.75 to 14.14 μg l−1, while DHW samples had a maximal concentration of 4.67 μg l−1 throughout all weeks.
As expected, the Reference building generally had higher MAP values than the POU device building, with statistical significance observed for DHW showerhead samples (p = 0.012) and DCW tap samples (p < 0.001) (Table S2†). However, for DHW circulation return samples, significant differences between buildings were only evident after the temperature change (weeks 16–22) with p = 0.001. Moreover, for every sampling time interval (weeks 0–22, 0–12 and 16–22), MAP concentrations between each building's DHW sampling location types (showerheads or circulation return pipelines) were similar, with p-values greater than 0.15 (data not shown).
Surprisingly, even in the Reference building without an additional treatment device, a decrease in MAP concentration was observed. T-tests indicated significantly lower MAP concentrations in all sampling locations when compared to the inflow samples (p < 0.001 for DHW samples and p = 0.001 for DCW samples, when analysed for the entire sampling period). It is possible that a portion of MAP was adsorbed by the system, either on system elements or by supporting biofilm growth, leading to non-detectable levels. This trend was observed in both buildings, more prominently in the POU-device building with its initially lower MAP concentration, where MAP gradually decreased and reached non-detectable levels after three months. This suggests that the pipes in the buildings, with their high surface-to-water ratio, serve as reservoirs for bacterial nutrients, providing a niche for biofilm growth and becoming “hot spots” for opportunistic bacterial growth. Another part of MAP could have been removed through association with possible deposits after water heating. Although the DCW system in the Reference building showed more variation in MAP levels, it did not exhibit significant differences in concentrations when compared to the DHW system (showerhead + circulation return samples taken together) throughout the entire sampling time (p = 0.057).
3.3. Legionella bacteria counts and impact of drinking water networks chemical flushing and disinfection
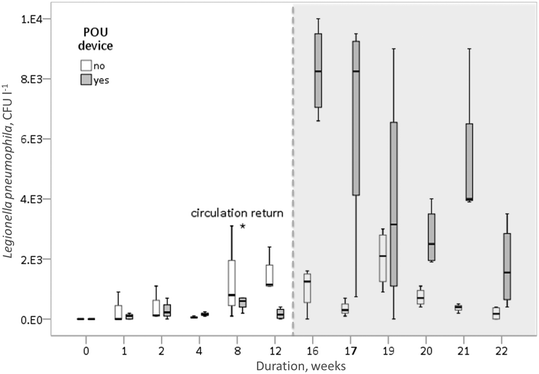
Cultivable Legionella numbers in the internal plumbing system were determined in samples taken from both buildings during high (samples from weeks 0–12) and reduced (samples from weeks 16–22) water temperature periods. The DHW temperature setpoint was lowered with the start of a heating season (week 14).During weeks 0–12 (regular temperature setting), the Reference building contained 1.5 × 102 (0–3.0 × 103) CFU l−1 in showerhead samples and 75 (0–1.2 × 103) CFU l−1 in circulation return samples. Meanwhile, the POU-device building contained 1.5 × 102 (0–7.0 × 102) CFU l−1 in the showerhead and 1.3 × 102 (0–3.1 × 103) CFU l−1 in circulation return samples. Furthermore, during weeks 16–22 (lowered temperature setpoint), the Reference building contained 5.5 × 102 (0–3.0 × 103) CFU l−1 in the showerhead and 9.0 × 102 (SD 1.0 × 103) CFU l−1 in circulation return samples, while the POU-device building contained 4.3 × 103 (SD 3.2 × 103) CFU l−1 in the showerhead and 6.0 × 103 (SD 3.8 × 103) CFU l−1 in circulation return samples. The samples collected from the water inlets in most cases did not show cultivable Legionella spp., with the maximum detected values in the inlet water reaching 3.5 × 102 CFU l−1 for the Reference building and 4.0 × 102 CFU l−1 for the POU-device building.
In general, there was no significant difference in Legionella CFUs in the DHW system between both buildings (with and without MAP removal) during the first 12 weeks (p = 0.124), when operating at a normal temperature setting. However, starting from week 16, when the DHW temperature setting was modified to save energy, significantly more Legionella was quantified in the samples collected from the POU-device building (p < 0.001). After the change in temperature regime, Legionella CFUs remained relatively stable in the Reference building (Fig. 5), while they increased over an order of magnitude higher for the POU-device building during weeks 16–22.
The question of why the growth was faster in the system with low MAP could be answered by considering the newly proposed “biostability theory” for drinking water systems.15 The theory is based on ecosystem evolution dynamics and it suggests that to reduce the risks of opportunistic pathogen growth, it is necessary to consider not only the concentration of limiting nutrients but also the presence of competing native bacteria. To become biostable, drinking water should contain a relatively high number of bacteria compared to the number of growth-limiting factors available.
During the first period, the temperature inside the DHW system, measured at the entry into the heat exchanger, averaged 46.3 °C in the Reference building and 47.6 °C in the POU-device building, while the DHW heat exchanger setpoint was 57 °C. After the temperature decrease, it averaged 42.1 °C and 44.1 °C, respectively, with the lowest heating setting at 48 °C. Ji et al.16 observed a significant change in the bulk microbiota at a temperature threshold of around 51 °C, indicating a transition from a mesophilic to a thermophilic environment. By lowering the DHW temperature setpoint, Legionella bacteria found more favourable conditions to quickly overtake the newly formed environmental niche and outcompete “beneficial” bacteria, as has been demonstrated for other pathogens in laboratory conditions.17 This resulted in high outflows of Legionella from the shower hoses. Although the temperature of DHW circulation return grab samples was on average 2 °C higher in the reduced-MAP system, it became more susceptible to favouring unwanted bacterial growth when the previous microbial balance was disturbed.
Interestingly, despite significantly cooler water after overnight stagnation in the apartment showerheads when compared to the DHW circulation system (p < 0.001 for both buildings) (Tables S3 and S4†), the analysis did not reveal significant differences in Legionella counts between circulation return and showerhead DHW samples (p = 0.409 and p = 0.304). However, these conditions favoured the proliferation of other microorganisms, resulting in a 38–48% increased intact cell count when analysed for the entire sampling time (p = 0.001 for both buildings). Previous studies by Lautenschlager et al.18 showed that water stagnation at the tap level can lead to a cell increase at the rate of 0.22 h−1, sustained by available substrate (in their case for 12 hours). These studies16,18 also observed shifts in the microbial community between these environments. Even an overnight stagnation of 8 hours was found to cause distinctive changes.16 The microbial community within one water system (cold or hot) is not uniform; it varies at the point of entry and point of use.19 This highlights that the internal water supply is a system comprising several distinct environments or niches. Each point within the system, differing in at least a 4 °C temperature range, could sustain a different microbial structure.20
Similar to temperature-induced niches, nutrient composition also plays a crucial role in shaping microbial communities.21–23 It affects the production of microbial products like exopolysaccharides (EPS),21 which, in turn, influence biofilm structure. Phosphate addition can impact both bacterial and fungal biofilm communities,22,23 leading to a decrease in bacterial abundance, while supporting the opposite for fungi.22 This addition may also affect functional traits,23 potentially inducing a “luxury P-uptake” mechanism. This mechanism could enhance phosphate uptake in microorganisms that were subjected to P-starvation within a MAP-limited system. Changes in P levels can also affect specific organisms. Douterelo et al.22 found that P addition influences such genera as Pseudomonas, Paenibacillus, Massilia, and Acinetobacter, which are linked to phosphate solubilization and accumulation. They also observed that P dosing favoured functional traits related to virions, thylakoids, and extrachromosomal DNA, particularly in Gram-negative bacteria. This may enhance their adhesion capabilities, and facilitate DNA transfer between cells, potentially boosting cell antibiotic resistance or adaptive metabolic pathways. They also noted a decrease in functional traits associated with quorum sensing, methanogenesis, beta-galactosidase, and vitamin binding. Consequently, the increase in Legionella numbers in the planktonic phase under P-reduced conditions might be linked to decreased adhesion capabilities. However, it should be noted that the aforementioned studies focus on phosphate dosing mainly in the context of metal dissolution prevention. The concentrations of P in such studies are much higher (up to 2 mg l−1), so there might be other processes governing changes in microbial communities. In our case, instead of shifting the balance towards a nutrient-rich environment, we aimed to induce nutrient-starved conditions, which could result in distinct community analysis patterns.
Another significant factor contributing to the increase in Legionella counts might be the periodic heat treatment of DHW during night-time (a rise from 48 °C to 52 °C) and especially during weekends (a rise to 57 °C). Legionella pneumophila is known to exhibit necrotrophic growth on heat-sterilized bacteria.24 In P-limited conditions, irregular heat treatment could have released nutrients from the drinking water biota and induced P-cycling. Interestingly, while there was no observed difference in bacterial counts between both buildings during the first period of constant temperature (Tables S5 and S6†), the P-limited building had 105 or 110% more intact cells (p = 0.002) in DHW circulation return samples (Table S6†), with a 12% greater shift towards high nucleic acid content cells (p = 0.026), which could indicate active growth. However, there were no significant differences in intact cell count between the buildings' showerhead samples (Table S5†), even after the change in temperature regime (p = 0.081). Both systems had significantly more damaged cells during the changed temperature regime (p = 0.025 for showerhead and p = 0.006), with an average increase of 39% or 3.3 × 104 cells per ml for showerheads and 77% or 5.7 × 104 cells per ml for circulation return samples.
In addition to temperature setting modifications, several other factors might have contributed to the rapid regrowth of Legionella. Various parameters, such as total chlorine concentration, pH, P, SO42− and Mg could be associated with most of the variation in the bulk water microbiome.25 During this period, the POU-device building, compared to the eference building, received water with a 55% or 0.76 mg l−1 higher total organic carbon content (p = 0.003), 9% or 3.2 mg l−1 more calcium (p = 0.009), and 17% or 1.58 mg l−1 more magnesium (p = 0.040). Furthermore, it received a 90% or 7.54 × 104 cells per ml greater inflow of intact cells (p = 0.041), with around 10% higher shift towards high nucleic acid content bacterial cells (p = 0.004). However, the POU-device building had 55% or 0.02 mg l−1 smaller manganese concentration (p = 0.026), 100% or 6.20 μg l−1 less copper (p = 0.015), and 70% or 0.11 mg l−1 less iron (p = 0.015) in the inflow water (Table S7†).
Regarding centralised internal network chemical flushing and disinfection of a building, it proved to be an ineffective strategy for long-term Legionella control. This measure ensured no cultivable Legionella spp. on the next day after the disinfection procedure. However, after one week, there was an average value of 2.3 × 102 CFU l−1 with a maximum of 9.0 × 102 CFU l−1 in the DHW samples of the Reference building, and an average value of 90 CFU l−1 with a maximum of 2.0 × 102 CFU l−1 in the POU-device building. In general, Legionella spp. were detected in 36% of the samples after the first week. Furthermore, just two weeks after disinfection, the maximum value reached 1.1 × 103 CFU l−1 in the Reference building, and the average value exceeded 1.0 × 103 CFU l−1 after two months. In the POU-device building, the first samples with concentrations exceeding 1.0 × 103 CFU l−1 were taken after two months. Overall, the effectiveness of this procedure lasted for around two months. However, afterwards, Legionella reached the value of 1 × 103 CFU l−1 in several sampling points, especially in the Reference building, exceeding the suggested risk assessment value stated in the EU directive on the quality of water intended for human consumption.13
3.4. Characteristics of Legionella species
No other Legionella species other than Legionella pneumophila were detected. The serotyping analysis identified its relation to the serogroups (SG) 1, 2 and 3. During the whole sampling campaign, the most prevalent serogroup was L. pneumophila SG 2, which was detected in 51% of samples in the POU-device building and 40% of samples in the Reference building (Table 3). SG 3 followed with presence in 24% and 22% of samples, respectively. SG 1 was found in 2% of samples in the POU-device building and 18% of samples in the Reference building.| Sampling site | n | Frequency of L. pneumophila | |||
|---|---|---|---|---|---|
| SG 1 | SG 2 | SG 3 | Not found | ||
| POU-device building | 51 | 1 (2%) | 26 (51%) | 12 (24%) | 12 (24%) |
| Reference building | 45 | 8 (18%) | 18 (40%) | 10 (22%) | 9 (20%) |
Temporally, SG 2 or SG 3 were detected one week after the internal network water supply disinfection (Table 4). L. pneumophila SG 1 was detected in samples after 3 months in the POU-device building, and after 1 month in the Reference building.
| Duration, weeks | POU-device building | Reference building | ||||||
|---|---|---|---|---|---|---|---|---|
| Samples tested/Legionella-positive samples | Serogroup | Samples tested/Legionella-positive samples | Serogroup | |||||
| SG 1 | SG 2 | SG 3 | SG 1 | SG 2 | SG 3 | |||
| 0 | 6/0 | — | — | — | 3/0 | — | — | — |
| 1 | 5/3 | — | 3 | — | 4/1 | — | — | 1 |
| 2 | 4/2 | — | 2 | — | 4/4 | — | 3 | 1 |
| 4 | 4/4 | — | 3 | 1 | 4/4 | 2 | — | 2 |
| 8 | 5/5 | — | 3 | 2 | 4/4 | 2 | — | 2 |
| 12 | 4/3 | 1 | 1 | 1 | 4/4 | — | 4 | — |
| 16 | 4/4 | — | 4 | — | 4/3 | — | — | 3 |
| 17 | 4/4 | — | 3 | 1 | 3/3 | 1 | 2 | — |
| 19 | 4/3 | — | 1 | 2 | 4/4 | — | 3 | 1 |
| 20 | 4/4 | — | 1 | 3 | 4/4 | 3 | 1 | — |
| 21 | 3/3 | — | 2 | 1 | 3/3 | — | 3 | — |
| 22 | 4/4 | — | 3 | 1 | 4/2 | — | 2 | — |
| Total | 51/39 | 1 (3%) | 26 (26%) | 12 (31%) | 45/36 | 8 (22%) | 18 (50%) | 10 (28%) |
Overall, the only clear distinction observed was the higher frequency and earlier appearance of L. pneumophila (Lp) SG 1 in the Reference building. This serogroup is linked to more than 90% of community-acquired Legionnaires' disease cases.26 Hence, investigating nutrient limitation to target the most harmful Legionella spp. requires further attention. Interestingly, although the total Legionella counts increased with the MAP sorption filter after changes in the temperature (samples from weeks 16–22), Legionella remained mainly non-Lp1 serogroups, which accounted for only 2% of DHW samples. In contrast, Lp-1 accounted for nearly one-fifth of DHW samples in the Reference building.
4. Conclusions
The introduction of a point-of-use granular ferric hydroxide sorption filter reduced MAP to 3.6 μg l−1 (SD 1.5 μg l−1) and did not significantly affect Legionella counts when a constant DHW preparation setpoint of 57 °C was maintained. However, it may have influenced the identified species, leading to a reduction in the frequency of Legionella pneumophila serogroup 1.Once the DHW preparation switched to a dynamic regime with varying setpoints between 48, 52, and 57 °C, Legionella counts increased more than tenfold in the MAP-limited building. However, this increase did not greatly impact the building without additional MAP removal. The temperature decrease provided optimal conditions for sustaining Legionella growth, allowing it to outcompete nutrient-starved microorganisms. This condition was amplified by periodic heat disinfection at 57 °C, inducing P-cycling in the P-starved system, further promoting rapid Legionella growth.
The findings suggest that simply lowering phosphorus levels to low values is insufficient to control Legionella growth, especially when the system faces disturbances in its established environment over the long term. Thus, the current concept of biostability should be updated by considering not only the level of nutrients but also the competition of opportunistic pathogens and native bacteria in water systems. It is crucial to maintain conditions favourable for the proliferation of “beneficial” microbiota, which can outcompete the unwanted bacteria. Although the creation of a selective environment to eliminate the growth of opportunistic premise plumbing pathogens remains a challenge, this could be a promising strategy to reduce Legionella in water systems.
Future research would benefit from microbial community analyses and interaction studies in disturbed environments, such as those governed by changes in nutrient availability and temperature disturbances. Additionally, the use of molecular methods would give valuable insight into opportunistic pathogen proliferation pathways.
Author contributions
Conceptualization: T. J.; formal analysis: M. Z.; investigation: M. Z., O. K.-G., L. Ķ., M. S., and J. Z.; methodology: M. Z., D. P., and T. J.; resources: D. P., L. M., O. V., and T. J.; visualization: M. Z.; writing – original draft: M. Z., T. J.; writing – review & editing: M. Z., D. P., O. K.-G., L. Ķ., M. S., J. Z., L. M., O. V., T. J.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Daina Pūle and Marta Zemīte would like to acknowledge the support received from Riga Technical University doctoral research grant no. DOK.BIF/19. Marta Zemīte expresses gratitude to Anton Rayan Priyasad Perera Weerasuriya Arachchige for his valuable assistance in preparing the POU device, Paula Luīze Biteniece for her involvement in early sample analyses, Ralfs Vēvers and Kishore Babu Ragi for their valuable help with sample collection and distribution, Jānis Krafts for providing consultation on pipeline chemical flushing and disinfection, and Sergejs Sidorko for his assistance with sampling place location, POU-treatment equipment acquisition, and provision of materials.References
- P. W. J. J. van der Wielen, A. Brouwer-Hanzens, R. Italiaander and W. A. M. Hijnen, Initiating guidance values for novel biological stability parameters in drinking water to control regrowth in the distribution system, Sci. Total Environ., 2023, 871 DOI:10.1016/j.scitotenv.2023.161930.
- K. L. G. Learbuch, M. C. Lut, G. Liu, H. Smidt and P. W. J. J. van der Wielen, Legionella growth potential of drinking water produced by a reverse osmosis pilot plant, Water Res., 2019, 157, 55–63, DOI:10.1016/j.watres.2019.03.037.
- C. R. Proctor, M. Gächter, S. Kötzsch, F. Rölli, R. Sigrist, J. C. Walser and F. Hammes, Biofilms in shower hoses – choice of pipe material influences bacterial growth and communities, Environ. Sci.: Water Res. Technol., 2016, 2, 670–682, 10.1039/C6EW00016A.
- M. J. Lehtola, T. Juhna, I. T. Miettinen, T. Vartiainen and P. J. Martikainen, Formation of biofilms in drinking water distribution networks, a case study in two cities in Finland and Latvia, J. Ind. Microbiol. Biotechnol., 2004, 31(11), 489–494, DOI:10.1007/s10295-004-0173-2.
- J. S. Vrouwenvelder, F. Beyer, K. Dahmani, N. Hasan, G. Galjaard, J. C. Kruithof and M. C. M. Van Loosdrecht, Phosphate limitation to control biofouling, Water Res., 2010, 44(11), 3454–3466, DOI:10.1016/j.watres.2010.03.026.
- A. K. T. Kirschner, Determination of viable legionellae in engineered water systems: Do we find what we are looking for?, Water Res., 2016, 93, 276–288, DOI:10.1016/j.watres.2016.02.016.
- G. Jereb, I. Eržen, M. Oder and B. Poljšak, Phosphate drinking water softeners promote Legionella growth, J. Water Health, 2022, 20(7), 1084–1090, DOI:10.2166/wh.2022.055.
- O. Valciņa, D. Pūle, A. Mališevs, J. Trofimova and S. Makarova, Co-Occurrence of Free-Living Amoeba and Legionella in Drinking Water Supply Systems, Med., 2019, 55(8), 492, DOI:10.3390/medicina55080492.
- M. J. Lehtola, I. T. Miettinen, T. Vartiainen and P. J. Martikainen, A new sensitive bioassay for determination of microbially available phosphorus in water, Appl. Environ. Microbiol., 1999, 65(5), 2032–2034, DOI:10.1128/aem.65.5.2032-2034.1999.
- G. Wen, Q. Deng, T.-L. Huang and J. Ma, An improved method for determining microbially available phosphorus in drinking water, Water Sci. Technol.: Water Supply, 2016, 16(4), 1149–1158, DOI:10.2166/ws.2016.036.
- European Committee for Standardization, EN ISO 11731:2017 Water quality – Enumeration of Legionella, Brussels, Belgium, 2017 Search PubMed.
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the quality of water intended for human consumption (recast), Official Journal of the European Union, 2020, vol. L 435, pp. 1–62, https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020L2184 Search PubMed.
- T. A. Lang and D. G. Altman, Basic statistical reporting for articles published in biomedical journals: the “Statistical Analyses and Methods in the Published Literature” or the SAMPL Guidelines, International Journal of Nursing Studies, 2015, 52(1), 5–9, DOI:10.1016/j.ijnurstu.2014.09.006.
- M. Frolova, K. Tihomirova, L. Mežule, J. Rubulis, K. Gruškeviča and T. Juhna, Evaluation of pre-Treatment technologies for phosphorous removal from drinking water to mitigate membrane biofouling, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 251 DOI:10.1088/1757-899X/251/1/012127.
- J. Favere, R. G. Barbosa, T. Sleutels, W. Verstraete, B. De Gusseme and N. Boon, Safeguarding the microbial water quality from source to tap, npj Clean Water, 2021, 4 DOI:10.1038/s41545-021-00118-1.
- P. Ji, W. J. Rhoads, M. A. Edwards and A. Pruden, Impact of water heater temperature setting and water use frequency on the building plumbing microbiome, ISME J., 2017, 11, 1318–1330, DOI:10.1038/ismej.2017.14.
- M. Vital, D. Stucki, T. Egli and F. Hammes, Evaluating the growth potential of pathogenic bacteria in water, Appl. Environ. Microbiol., 2010, 76(19), 6477–6484, DOI:10.1128/AEM.00794-10.
- K. Lautenschlager, N. Boon, Y. Wang, T. Egli and F. Hammes, Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition, Water Res., 2010, 44(17), 4868–4877, DOI:10.1016/j.watres.2010.07.032.
- B. Meyer, M. Pannekens, A. R. Soares, L. Timmermann, A. J. Probst, M. Hippelein, B. Bendinger and A. Nocker, Bacterial populations in different parts of domestic drinking water systems are distinct and adapted to the given ambient temperatures, Front. Water, 2023, 5 DOI:10.3389/frwa.2023.1119951.
- C. R. Proctor, D. Dai, M. A. Edwards and A. Pruden, Interactive effects of temperature, organic carbon, and pipe material on microbiota composition and Legionella pneumophila in hot water plumbing systems, Microbiome, 2017, 5(1), 130, DOI:10.1186/s40168-017-0348-5.
- W. Fang, J. Y. Hu and S. L. Ong, Influence of phosphorus on biofilm formation in model drinking water distribution systems, J. Appl. Microbiol., 2009, 106(4), 1328–1335, DOI:10.1111/j.1365-2672.2008.04099.x.
- I. Douterelo, B. E. Dutilh, C. Calero, E. Rosales, K. Martin and S. Husband, Impact of phosphate dosing on the microbial ecology of drinking water distribution systems: Fieldwork studies in chlorinated networks, Water Res., 2020, 187 DOI:10.1016/j.watres.2020.116416.
- E. Rosales, G. Del Olmo, C. Calero Preciado and I. Douterelo, Phosphate Dosing in Drinking Water Distribution Systems Promotes Changes in Biofilm Structure and Functional Genetic Diversity, Front. Microbiol., 2020, 11 DOI:10.3389/fmicb.2020.599091.
- R. Temmerman, H. Vervaeren, B. Noseda, N. Boon and W. Verstraete, Necrotrophic growth of Legionella pneumophila, Appl. Environ. Microbiol., 2006, 72(6), 4323–4328, DOI:10.1128/AEM.00070-06.
- P. Ji, J. Parks, M. A. Edwards and A. Pruden, Impact of water chemistry, pipe material and stagnation on the building plumbing microbiome, PLoS One, 2015, 10(10) DOI:10.1371/journal.pone.0141087.
- J. Beauté, D. Plachouras, S. Sandin, J. Giesecke and P. Sparén, Healthcare-Associated Legionnaires’ Disease, Europe, 2008-2017, Emerging Infect. Dis., 2020, 26(10), 2309–2318, DOI:10.3201/eid2610.181889.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ew00588g |
| This journal is © The Royal Society of Chemistry 2024 |