Open Access Article
Open Access ArticleLead ion (Pb2+) electrochemical sensors based on novel Schiff base ligands
Zahra
Akbari
a,
Khouloud
Abid
ab,
Daniela
Iannazzo
a,
Morteza
Montazerozohori
c,
Enza
Fazio
d,
Fortunato
Neri
d,
Carmelo
Corsaro
 d and
Giovanni
Neri
d and
Giovanni
Neri
 *a
*a
aDepartment of Engineering, University of Messina, I-98166 Messina, Italy. E-mail: sanazakbari701@yahoo.com; khouloud.abid.etud@fss.usf.tn; diannazzo@unime.it; gneri@unime.it
bCNR IPCF Istituto per i Processi Chimico-Fisici, viale F. Stagno D'Alcontres 37, Messina, Italy
cDepartment of Chemistry, Yasouj University, Yasouj, Iran. E-mail: mmzohory@yahoo.com
dDepartment of Mathematical and Computational Sciences, Physics Science and Earth Science, University of Messina, Viale F. Stagno d'Alcontres 31, I-98166 Messina, Italy
First published on 13th September 2024
Abstract
In this study, a novel bidentate Schiff base ligand, namely (1E,1′E,2E,2′E)-N,N′-(butane-1,4-diyl)bis(3-(2-methoxyphenyl)prop-2-en-1-imine) (L1), and a tetradentate Schiff base ligand, namely N1,N2-bis(2-(((1E,2E)-3-(4-(dimethylamino)phenyl)allylidene)amino)ethyl)ethane-1,2-diamine (L2), were successfully synthesized through a simple procedure. The synthesized Schiff base ligands were characterized by scanning electron microscopy (SEM) analysis, attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), nuclear magnetic resonance (NMR) and ultraviolet-visible (UV-vis) spectroscopy. Moreover, the thermal behavior was studied through thermogravimetric (TG)/differential thermogravimetric (DTG)/differential thermal (DT) analyses under a nitrogen atmosphere. Subsequently, the features and performances of the synthesized ligands (L1 and L2) as electrochemical sensors for the detection of heavy metal ions (HMIs) have been investigated. A different behavior was noticed using these two ligands, with L1 being the best candidate for developing a modified screen-printed carbon electrode (L1/SPCE) electrochemical Pb2+ sensor. To improve further the performances, gold nanoparticles (AuNPs) were deposited by an electrochemical process on the L1/SPCE platform. The developed AuNPs-L1/SPCE sensor displayed enhanced lead ion sensing with a high sensitivity of 56.78 μA μM−1 cm−2 and a detection limit of 0.298 μM. This novel sensor demonstrated promising performances for the detection of Pb2+ ions in real seawater with no sample treatment.
Water impactA new electrochemical sensor was developed by combining AuNPs with a Schiff base ligand. This generated a synergistic effect that was exploited for improving the electrochemical sensing performances for the detection of Pb2+ in the sub-micromolar range. The feasibility of the proposed platform for practical applications in the environmental field was confirmed by determining Pb(II) in seawater. |
Introduction
Schiff bases are unique ligands that play an essential role in the development of coordination chemistry. They are easily synthesized and can be readily modified both electronically and sterically.1 Schiff bases form an outstanding class of ligands due to their unique properties such as stability under different conditions, diversity of donor sites, synthetic flexibility, and formation of a wide range of complexes in various coordination geometries. Schiff base ligands based on the number of donor sites are classified as monodentate, bidentate, tridentate, and so on. Various bidentate and tetradentate Schiff base ligands have been prepared from condensation reactions of different aldehydes and amines.2–6 Also, Schiff bases having tetradentate ligands are known to exhibit good fluorescence properties and high thermodynamic and kinetic stability.7–10 Schiff bases emerge as attracting ligands for binding with heavy metal ions (HMIs). Various studies have shown good affinity of the nitrogen atom in the azomethine group for metal ions. The mechanism of electrochemical sensors for monitoring metal ions is based on the adsorption of metal ions from solution to the electrode surface by complexation with the ligand immobilized on the working electrode.11 Hence, the choices of materials to modify the working electrode are of great importance, and Schiff bases are reported to be an excellent choice for fabricating metal ion sensors due to their good selectivity. Furthermore, their capability for the accumulation of metal ions at the electrode surface results in the voltammetric determination of low detection limits.12–16Environmental pollution by heavy metal ions is a serious and complex problem.17–20 Among various HMIs, lead ions (Pb2+) are one of the most serious environmental contaminants because of their high toxicity, persistence, and tendency to accumulate in living organisms.21,22 The detection and monitoring of Pb2+ in water environments, especially seawater, are crucial for environmental protection and public health.23 Therefore, it is desirable to develop simple, selective, sensitive, and efficient methods for the determination of Pb2+ ion trace levels in environmental samples. In the past few years, plenty of detection methods have been established for the detection of Pb2+, including spectrophotometric methods, atomic absorption and emission spectroscopies, and mass spectrometry (MS).24–27 However, most of these techniques are either time-consuming, involving multiple sample manipulations, or highly expensive for most analytical laboratories.
To bypass these limitations, electrochemical sensors have been widely used in the determination of various substances due to their ease of use, low cost, high sensitivity, and desirable selectivity in their responses.28–31 Ahmed et al.32 proposed an electrochemical sensor by functionalizing the working electrode with a freshly synthesized Schiff base, 4-((2-hydroxy-5-((4-nitrophenyl)diazenyl)benzylidene)amino)benzoic acid (HDBA) and used it for simultaneous determination of lead (Pb2+), copper (Cu2+) and cadmium (Cd2+) ions in a buffer solution of 0.1 M Tris-HCl. Interestingly, the authors reported that the addition of Pb2+ to a solution containing Cd2+ has an insignificant effect on the Cd2+ peak current which implies that this Schiff base endows extra selectivity to the modified electrode and avoids mutual interference during simultaneous determination of the heavy metals. In the study of Kumar Saren et al.,33 the Katiragum–Arginine Schiff base material is used as a sensor–adsorbent for Pb2+ in an aqueous solution. The sensing of Pb2+ is analyzed by the electrochemical method with a detection limit of 0.146 μM. The Schiff base/glassy carbon electrode (KGDR/GCE) is highly selective towards Pb2+ ions in comparison to other environmentally relevant ions as well as in water samples. Chandra et al.34 developed a new Pb2+ PVC membrane sensor based on Schiff base thiophene-2-aldehyde thiosemicarbazone (TATS) as an ionophore, tetraphenylborate (NaTPB) in the form of an anion excluder and dioctyl phthalate (DOP) as a plasticizer. The sensor exhibits good selectivity to a wide variety of alkali, alkaline earth, and other metal ions and works over a pH range of 2.4–8.0. A highly selective graphene/Schiff base fluorescent chemosensor for iron ions was developed by modification of functionalized graphene oxide with a naphthaldehyde–diaminobenzophenone Schiff base.35 The detection limit of this sensor for Fe2+ and Fe3+ was found to be 0.277 × 10−5 mol L−1 and 0.337 × 10−5 mol L−1, respectively. The lower detection limit of this sensor for Fe2+ and Fe3+ ions proved its application in the sensing phenomenon and showed its sensitivity.
In this work, two Schiff bases named L1 and L2 were successfully synthesized through a simple procedure. Using several techniques, their morphological, optical and electrochemical features were studied. These two ligands have been then used to prepare two electrodes for the determination of Pb2+ ions in PBS at pH = 9 using the square wave voltammetry (SWV) technique. The sensitivity increased through the electrochemical deposition of AuNPs on the surface of the modified electrode and the obtained detection limit is equal to 0.298 μM.
Results and discussion
L1 and L2 ligand characterization
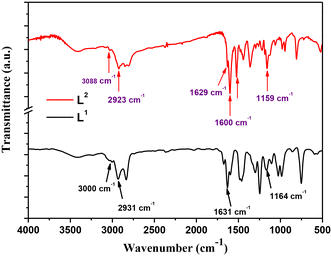
The Schiff base ligands (L1) and (L2) synthesized according to a previously reported procedure36 have been characterized by several techniques for elucidating and confirming their chemical structure. FTIR spectra within the wavenumber range of 4000–400 cm−1 are reported in Fig. 1. As noticed, the spectrum of Schiff base ligand L1 (black line) shows the characteristic stretching frequency of the azomethine group at 1631 cm−1.2 Moreover, the absorption bands appearing at 3000 cm−1, 2931 cm−1, and 1164 cm−1 are attributed to the vibrations of ν(C–H) alkene, ν(C–H) aliphatic, and ν(C–N) bonds in the ligand (L1), respectively.Regarding the L2 Schiff base ligand (red line), at 1600 cm−1 and 1629 cm−1, the symmetric and asymmetric stretching frequencies of the azomethine (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) group are observed, respectively. Additionally, the absorption bands appearing at 3088 cm−1, 2923 cm−1, and 1159 cm−1 are assigned to the stretching vibrations of ν(C–H) alkene, ν(C–H) aliphatic, and ν(C–N) bonds, respectively. Furthermore, a stretching vibration is noticed at 3215 cm−1 which corresponds to the ν(N–H) bond.
N) group are observed, respectively. Additionally, the absorption bands appearing at 3088 cm−1, 2923 cm−1, and 1159 cm−1 are assigned to the stretching vibrations of ν(C–H) alkene, ν(C–H) aliphatic, and ν(C–N) bonds, respectively. Furthermore, a stretching vibration is noticed at 3215 cm−1 which corresponds to the ν(N–H) bond.
The molecular structure of the prepared Schiff base ligands was demonstrated through 1H NMR and 13C NMR analyses in deuterated DMSO solution, and the obtained spectral data are depicted in Fig. 2.
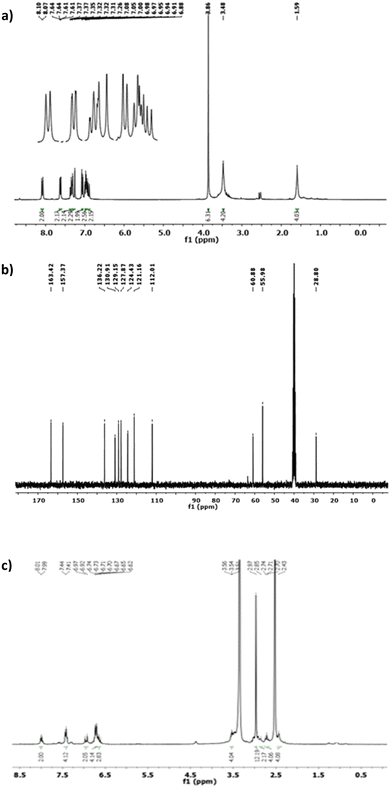 | ||
| Fig. 2 The 1H NMR (a) and 13C NMR spectra (b) of the Schiff base ligand L1. (c) 1H NMR spectrum of L2. | ||
As shown in Fig. 2a, among the significant proton signals in the 1H NMR spectrum, the azomethine proton signal in the L1 ligand (HCC′) appears as a doublet at 8.08 ppm. The aromatic hydrogens (Hff′, Hgg′, Hhh′, and Hii′) exhibit signals as a doublet of the doublet, triplet, doublet of triplet, and doublet at 7.62, 6.98, 7.35, and 7.06 ppm, respectively.
In the 13C NMR spectrum of ligand L1, the carbon signals corresponding to aromatic, aliphatic, and olefinic carbons are observed within the ranges of 121.16–157.37 ppm, 28.8–55.96 ppm, and 112.01–136.22 ppm, respectively (see Fig. 2b). The distinctive carbon signal in the bidentate ligand (L1) is attributed to the azomethine carbon peak, which appears at 163.42 ppm. Regarding the 1H NMR spectrum of ligand L2 reported in Fig. 2c, the olefinic hydrogens (Hdd′ and Hee′) are observed as a doublet of doublet and a doublet, centered at 6.92 ppm and 7.28 ppm, respectively. Two broad singlet signals at 1.59 ppm and 3.45 ppm are assigned to the aliphatic hydrogens of Haa′ and Hbb′, respectively.
Photoluminescence (PL) and ultraviolet-visible (UV-vis) absorbance spectra were used to investigate the optical properties of both L1 and L2 ligands, as shown in Fig. 3.
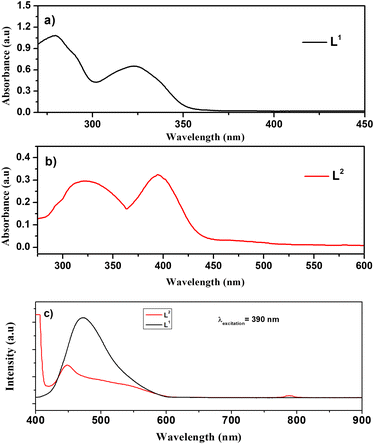 | ||
| Fig. 3 UV-visible spectra of (a) L1 and (b) L2 ligands. (c) PL spectra of the Schiff base ligands; L1 (black line) and L2 (red line). | ||
The UV-vis spectra of ligand L1 (black line) and ligand L2 (red line) are collected at room temperature in the range of 200–800 nm. In Fig. 3a, two distinct UV-vis intra-ligand transition bands were observed exhibiting maximum absorption (λmax) values at 280 nm and 325 nm, respectively. The first one is attributed to the π → π* electronic transition involving olefinic bonds and aromatic rings.36 On the other hand, the 325 nm band was assigned to the n → π*/π → π* transitions associated with iminic bonds.36
Fig. 3b shows the UV-vis optical absorbance spectra of ligand L2, which exhibited two characteristic intra-ligand transitions centred at the λmax of 322 nm and 399 nm. The band observed at 322 nm corresponds to π → π* electronic transitions involving olefinic bonds and aromatic rings, whereas the band observed at 399 nm is attributed to n → π*/π → π* transitions of iminic bonds.
Fig. 3c shows the PL spectra of both L1 (black line) and L2 (red line) ligands collected under 390 nm excitation at room temperature. Herein, the spectra of both Schiff base ligands are dominated by a characteristic emission peak of the L1 ligand centred at about 450 nm in agreement with G. Wu, et al.37 Moreover, a shoulder is observed at 550 nm for ligand L2; also, the second harmonic at 780 nm can be observed.
Modified-SPCE characterization
The L1 and L2 ligands were then used to fabricate modified-SPCEs, as reported in the Experimental section. Fig. 4 displays the SEM analysis of the working electrode surface of the bare and L1 and L2 ligand-modified SPCEs. The images show clearly that the working electrode surface is almost covered by a continuous film of the L1 and L2 ligands. In the case of the L1 film, a homogeneous distribution of small structures with a particle-like shape is also observed which is absent on the L2 film.The high resolution image in Fig. 5a shows the porous structure of the L1 ligand modified-SPCE. Fig. 5b also shows the surface of the AuNPs-L1/SPCE working electrode. The image highlights the presence of uniformly distributed AuNPs seen as white round nanoparticles having a mean size lower than 30 nm.
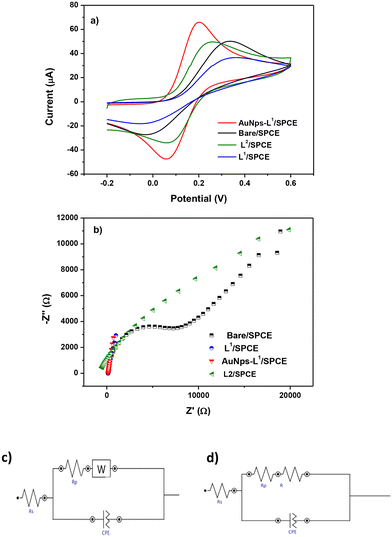
To investigate the electrochemical characteristics of the bare SPCE and other modified SPCEs (L1/SPCE, L2/SPCE, and AuNPs-L1/SPCE), cyclic voltammetry (CV) and EIS tests were performed in 0.1 mol L−1 KCl solution containing 5 mM Fe(CN)6. CV analysis in the presence of the model ferrocyanide/ferricyanide redox couple provides valuable information about the involved redox process. Comparative CV analysis reported in Fig. 6a indicated that the redox couple process feasibility follows the order AuNPs-L1/SPCE > SPCE = L1/SPCE > L2/SPCE, which can be due to the improved microstructural and electrical characteristics (e.g., surface area, metal particle presence, hydrophilicity, porosity, electron transfer resistance) introduced by modifying the bare electrode. EIS is a tool for investigating the electrode interface properties in terms of impedance changes, over a wide range of applied frequency. This technique can provide helpful indication on the electrical characteristics of the electrode–solution interface. The Nyquist plot of each electrode is presented in Fig. 6b, showing a semi-circle and a linear portion.38–40 Using the Nova software, the obtained Nyquist plots were fitted through the equivalent circuits in Fig. 6c and d. The Randles parameters, i.e. the charge transfer resistance (RCT) and electrolyte resistance (Rs), were calculated and are compared in Table 1.
| Sensor | Randles parameters | |
|---|---|---|
| R CT (Ω) | R s (Ω) | |
| Bare/SPCE | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 692 692 |
156 |
| L2/SPCE | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 199 199 |
179 |
| L1/SPCE | 83 | 133 |
| AuNPs-L1/SPCE | 490 | 1221 |
The order of RCT established: L1/SPCE < AuNPs-L1/SPCE < SPCE < L2/SPCE displays a different trend compared to one based on CV data, suggesting that the electron transfer resistance is not the only parameter taken into account for the electrochemical characteristics of the electrodes.
At last, as demonstrated by the combined data of CV in ferrocyanide and EIS analysis, the AuNPs-L1/SPCE sensor displays the best electrochemical properties as evidenced by the values of the two key parameters i.e. high Ipa and low RCT, respectively.
Electroanalytical performance toward Pb2+: parameter optimization
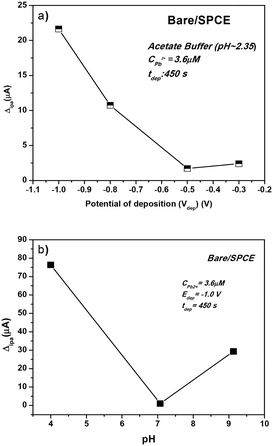
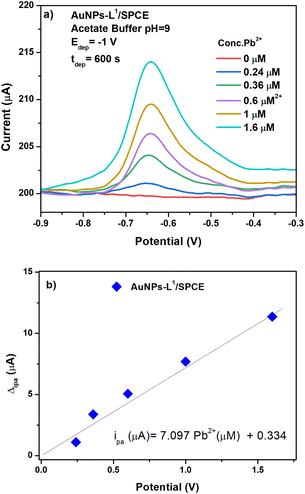
The determination of Pb2+ ions by SWASV was carried out with the investigated sensors. To obtain better sensitivity, the operating conditions of SWASV, i.e. pH, deposition potential (Vdep), and deposition time (tdep), were first optimized (Fig. 7).The effect of Vdep, from −0.2 V to −1.0 V, in acetate buffer solution (pH = 2.35) containing 3.6 μM of Pb2+ on the anodic current peak is reported in Fig. 7a. Decreasing the deposition potential up to −1.0 V clearly increases the Pb2+ peak current. H2 bubble production was noted at a lower potential than −1.0 V, which could be a potential destabilizing factor for the film adhesion on the working electrode sensor. Therefore, the deposition potential value of −1.0 V was selected.
The pH of the acetate buffer solution should be also optimized to have a better electroanalytical response in the Pb2+ determination. To achieve this, three different media were chosen: acidic (pH = 4), neutral (pH = 7), and basic (pH = 9). The effect of pH on the determination of the Pb(II) ions is presented in Fig. 7b. At pH 4.0, the peak current reached the maximum for Pb2+ while a poorer result was obtained in the neutral medium. In the basic medium, the Pb2+ ions are monitored with an intensity 3 times higher than that obtained at pH = 7, but almost 5 times lower than that noticed at acidic pH. However, since the target of this work is the detection of heavy metals in seawater, the value of pH = 9 is selected.
For heavy metal ion determination at low concentrations, generally a long deposition time (tdep) is selected which ensures the deposition of more lead ions on the working electrode surface. In this work, we select a deposition time of 600 s. To sum up, the parameters selected for the successive Pb2+ analysis tests were: tdep = 600 s, Vdep = −1.0 V, and pH = 9.
Electroanalytical performance toward Pb2+ on L1/L2-based modified SPCEs
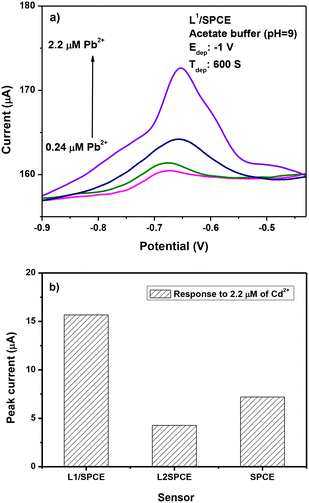
The results of SWASV tests for the determination of lead ions are reported in Fig. 8. The typical current variation registered during the SWASV test in the potential range between −0.9 and −0.4 V is illustrated in Fig. 8a. From the current variation versus the addition of an increasing quantity of lead ions in the calibration curves, it appears that the modified L1/SPCE electrode provides the highest sensitivity to Pb2+ compared to bare SPCE and L2/SPCE.The best performance of L1/SPCE, compared to bare SPCE, may be attributed to the presence of bidentate C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups in the chemical structure of the L1 ligand (see Scheme 1a), which acts favorably for the coordination of Pb2+.
N groups in the chemical structure of the L1 ligand (see Scheme 1a), which acts favorably for the coordination of Pb2+.
Accordingly, due to the lack of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups in the L2 ligand (Scheme 1b), the L2/SPCE sensor displays very low sensitivity. The limit of detection (LoD), defined as LoD = 3.3 × SD/m,40,41 where SD and m are the standard deviation of the blank and the slope of the calibration graph, respectively, obtained with the L1/SPCE sensor is equal to 0.446 μM.
N groups in the L2 ligand (Scheme 1b), the L2/SPCE sensor displays very low sensitivity. The limit of detection (LoD), defined as LoD = 3.3 × SD/m,40,41 where SD and m are the standard deviation of the blank and the slope of the calibration graph, respectively, obtained with the L1/SPCE sensor is equal to 0.446 μM.
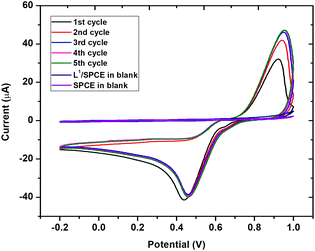
It is well known that the presence of noble metals can help in increasing the performances of the electrode material. For this, we further modified L1/SPCE through the electrochemical deposition of Au nanoparticles as described in the Experimental section. The successful electrodeposition of AuNPs on L1/SPCE was confirmed by the appearance of the characteristic oxidation and reduction peaks of gold observed at 0.9 V and 0.4 V, respectively (see Fig. 9).
The AuNP-L1/SPCE sensor was then tested for the determination of Pb2+ (see Fig. 10a). The addition of AuNPs on the surface of the L1/SPCE working electrode introduces an improvement in the electrooxidation performance toward Pb2+ ions, resulting in an almost double response with respect to that of the pristine L1/SPCE sensor.
From the calibration curve of AuNPs-L1/SPCE (see Fig. 10b), the sensitivity has been calculated as ipa (μA) = 7.097CPb2+ (μM) + 0.334. The computed sensitivity of the sensor being 56.776 μA μM−1 cm−2 is higher than those reported for the other SPCEs (Fig. 8b). The calculated LoD value, at S/N ratio = 3, was 0.298 μM.
A scan rate variation study was performed to determine the controlling mechanism on the surface of AuNPs-L1/SPCE. Tests were carried out in 5 mM Fe(CN)6 in 0.1 mol L−1 KCl solution in the potential range [−0.2 V; 0.6 V] at different scan rates ranging from 0.02 V s−1 to 0.3 V s−1 (Fig. 11a). The increase of the scan rate leads to an increase in the anodic and cathodic current peaks labelled as ipa and ipc, respectively. As depicted in Fig. 11b, this increase is linear to the scan rate square root, with the regression equation ipa (μA) = 164.805V1/2 + 4.146 and R2 = 0.98, proving that the electrooxidation on AuNP-L1/SPCE is diffusion controlled. Based on this data, the electrochemically active surface area (EASA) is equal to 4.87 e−2 cm2 and is determined using the following Randles–Sevcik formula:
| Ipa (in A) = 2.69 × 105AD1/2n3/2ν1/2C. |
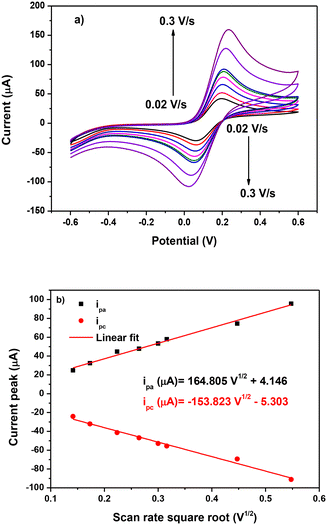 | ||
| Fig. 11 (a) Scan rate variation study on AuNPs-L1/SPCE and (b) linearity of the ip current peak vs. scan rate square root. | ||
To study the selectivity of the proposed modified electrode, various HMIs such as Pb2+, Cu2+, and Cd2+ were tested under the same conditions (acetate buffer and pH = 9). Fig. 12a demonstrates that no oxidation peak is obtained in the blank sample. When adding 1 μM of Pb2+, the expected oxidation peak is observed at −0.5 V. After adding 1 μM of Cu2+ and Cd2+, a peak at −0.6 V is observed related to cadmium ions, whereas the peak intensity of Pb2+ is almost unchanged with respect to the expected current variation in the presence of Pb ions only, indicating that the tested interferents do not influence Pb ion determination.
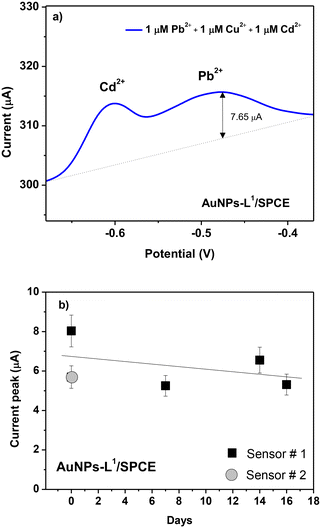 | ||
| Fig. 12 (a) Selectivity test of the various HMI ions (Pb2+, Cu2+, and Cd2+) at 1 μM in seawater and (b) reproducibility test of two AuNPs-L1/SPCE sensors and signal stability test up to 16 days. | ||
Another important parameter for the sensor is also the stability feature. To study it, at 3-day time intervals up to 16 days, the SWAV technique is carried out on AuNPs-L1/SPCE toward Pb2+ ions using the same conditions (Fig. 12b). Only a slight decrease of the current variation after about 2 weeks of operation can be observed, likely due to the poisoning of some sensitive sites by lead ions.
Real sample test on AuNPs-L1/SPCE
As described in the Introduction section, the major goal of this investigation is the determination of Pb2+ in seawater. Thus, it is crucial to check the efficiency of the L1-based SPCE sensor in a real seawater sample, collected from Messina, Italy. The sample, having a pH = 8.05, was used for the analysis as sampled and without any pre-treatment. The SWASV result is reported in Fig. 13. As expected, an increase in the current peak is observed when increasing the Pb2+ concentration. Based on the calibration curve previously determined, recovery rates determined by the spike-and-recovery experiment with 0.24, 0.36, and 0.6 μM of Pb2+ are found in the range of 98.3–111.3%, demonstrating the promising performances of the modified sensor for the detection of Pb2+ ions in real seawater with no sample treatment. Indeed, our electrochemical analysis pointed out that the concentration of Pb2+ naturally present in the seawater sample is negligible, as certified by independent analysis performed by means of atomic absorption spectroscopy.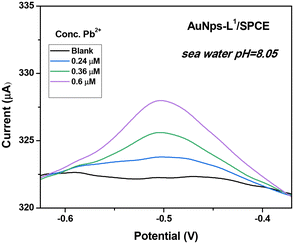 | ||
| Fig. 13 Square-wave anodic stripping voltammograms (SWASVs) in seawater. Conditions: tdep = 600 s and Vdep = −1 V. | ||
Experimental
Reagents
The starting materials used were procured from Merck and Aldrich companies and used without any further purification unless otherwise stated. Lead nitrate was used to prepare the aqueous stock solution of the target heavy metals. Acetate buffer was prepared by combining acetic acid (0.1 M) and NaOH to achieve the desired pH. Double-distilled water was employed in all experimental procedures.Apparatus
The ATR-FTIR spectra of the Schiff base ligands were recorded at a range of 4000–400 cm−1 employing a JASCO-680 model spectrometer. A spectrometer (JASCO-V570 model) was used for scanning the UV-visible spectra in a range of 200–800 nm. SEM analyses were carried out by means of a Zeiss (Gemini II model) microscope working at an acceleration voltage of 10 kV and at a working distance of 27 mm. 1H NMR and 13C NMR spectra were recorded using a Bruker DPX FT/NMR-400 spectrometer in the presence of tetramethylsilane (Me4Si) as a standard and deuterated dimethyl sulfoxide (DMSO-d6) as a solvent. The decomposition temperature or melting point and molar conductivity were measured using KRUSS SPIN melting point meters and a Metrohm-712 conductometer, respectively. A Perkin-Elmer Pyris model was used for the investigation of the thermal behavior of the compounds under a N2 gas atmosphere within the temperature limit of 25–900 °C with a heating rate of 20 °C min−1.Electrochemical measurements were conducted through a commercially available screen-printed carbon electrode (SPCE) obtained from Metrohm-DropSens. The SPCEs consisted of a flat substrate with a carbon working electrode (4 mm in diameter, geometric area of 0.1257 cm2), a silver pseudo reference electrode, and a carbon auxiliary electrode. Square wave anodic stripping voltammetry was performed using a DropSens μStat 400 Potentiostat, which was controlled using Dropview 8400 software for data acquisition.
Synthesis of Schiff base ligands L1 and L2
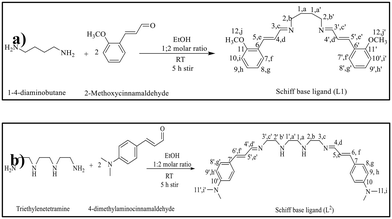
The L1 and L2 Schiff base ligands were prepared by following the same procedure used in our previous investigations.36 Briefly, an ethanolic solution of 1,4-diaminobutane (0.044 g, 0.5 mmol) is dropwise added to 2-methoxycinnamaldehyde (0.161 g, 1 mmol) at 25 °C for 5 h (Scheme 1a). The purity is checked by thin-layer chromatography (TLC) and the reduction of the solvent volume allowed us to obtain yellow crystals of ligand L1. The precipitate was collected, filtered, and washed with a small amount of ethanol and dried at room temperature.The synthesis of the L2 Schiff base ligand was carried out through the dropwise addition of an ethanolic solution of triethylenetetramine (0.073 g, 0.5 mmol) to an ethanolic solution of 4-dimethylaminocinnamaldehyde (0.175 g, 1 mmol) at 25 °C, (Scheme 1b). The reaction was carried out by stirring for five hours, and the purity of the synthesized Schiff base ligand (L2) was confirmed using thin-layer chromatography (TLC). Upon reducing the solvent volume, a yellow precipitate of ligand L2 formed. The precipitate was collected, filtered, and washed with a small amount of ethanol for further purification. Finally, ligand L2 was dried at room temperature.42 The prepared ligand was characterized using Fourier-transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) spectroscopic techniques.
Preparation of modified electrodes
The bare screen-printed carbon electrodes (SPCEs) were used as received without any treatment performed before the use. To perform the modification of the working electrode, 1.0 mg of each of the ligand sample (L1 and L2) was ultrasonically dispersed in 0.5 mL of dimethyl sulfoxide (DMSO), and 2 μL of this suspension was dropped directly onto the surface of the carbon working electrode and dried at room temperature until further use.AuNPs-L1/SPCE is prepared through electrodeposition of AuNPs on the surface of the WE of L1/SPCE. Herein, the modified electrode is immersed in 5 mL of HAuCl4− solution, and 5 cycles of cyclic voltammetry (CV) are carried out in the potential range [−0.2 V; 1 V] at a 0.05 V s−1 scan rate. The success of the electrodeposition is proved by the presence of Au oxidation and reduction peaks at 0.9 V and 0.4 V.
Electroanalytical analysis
Electrochemical studies were performed in 5 mL of acetate buffer solution to conduct square wave anodic stripping voltammetry (SWASV) measurements. Essential instrumental parameters, including pH and deposition potential/time (Edep/tdep), were optimized and determined to be pH = 9, −1 V, and 600 s, respectively. Following the preconcentration step, square wave voltammetry (SWV) was carried out in the voltage range of −1.5 V to 0 V to obtain the analytical signal corresponding to the concentration of the heavy metal ions.Conclusions
In this work, a new sensor based on a bidentate (1E,1′E,2E,2′E)-N,N′-(butane-1,4-diyl)bis(3-(2-methoxyphenyl)prop-2-en-1-imine) Schiff-base ligand was developed for electrochemistry-based detection of Pb2+ in seawater. By combining AuNPs with this Schiff base ligand, a synergy was created leading to improved electrochemical sensing performances. Finally, the feasibility of the proposed platform for practical applications in the environmental field was confirmed by determining Pb(II) in a seawater sample.Data availability
The datasets generated and/or analysed in the current study are available from the authors upon reasonable request.Author contributions
Z. Akbari: formal analysis and writing – editing. K. Abid, E. Fazio, C. Corsaro, and F. Neri: formal analysis and writing – review & editing. D. Iannazzo: project administration. M. Montazerozo: project administration. G. Neri: conceptualization and methodology.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the European Union (NextGeneration EU) through the MUR-PNRR project SAMOTHRACE (No. ECS00000022).References
- B. Liu, J. Chai, S. Feng and B. Yang, Structure, photochemistry and magnetic properties of tetrahydrogenated Schiff base chromium(III) complexes, Spectrochim. Acta, Part A, 2015, 140, 437–443 CrossRef CAS PubMed
.
- Z. Akbari, M. Montazerozohori, S. Joohari, P. Hayati, N. Micale and M. Cristani,
et al., Mono and binuclear cadmium complexes: X-ray crystal structures, Hirshfeld surface analysis and antimicrobial/antioxidant studies, Inorg. Chem. Commun., 2023, 158, 111513 CrossRef CAS
.
- F. N. Ejiah, M. O. Rofiu, O. A. Oloba-Whenu and T. M. Fasina, Schiff bases as analytical tools: synthesis, chemo-sensor, and computational studies of 2-aminophenol Schiff bases, Mater. Adv., 2023, 4(10), 2308–2321 RSC
.
- Z. Akbari, M. Montazerozohori, S. J. Hoseini and R. Naghiha, Ultrasonic assisted preparation of some new zinc complexes of a new tetradentate Schiff base ligand: thermal analyses data, antimicrobial and DNA cleavage potential, J. Phys. Org. Chem., 2021, 34(5), e4180 CrossRef CAS
.
- Z. Akbari, M. Montazerozohori, S. J. Hoseini, R. Naghiha, P. Hayati and G. Bruno,
et al., Synthesis, crystal structure, Hirshfeld surface analyses, antimicrobial activity, and thermal behavior of some novel nanostructure hexa-coordinated Cd(II) complexes: Precursors for CdO nanostructure, Appl. Organomet. Chem., 2021, 35(5), e6181 CrossRef CAS
.
- Z. Akbari, C. Stagno, N. Iraci, T. Efferth, E. A. Omer and A. Piperno,
et al., Biological evaluation, DFT, MEP, HOMO-LUMO analysis and ensemble docking studies of Zn(II) complexes of bidentate and tetradentate Schiff base ligands as antileukemia agents, J. Mol. Struct., 2024, 1301, 137400 CrossRef CAS
.
- Y. W. Choi, J. J. Lee, E. Nam, M. H. Lim and C. Kim, A fluorescent chemosensor for Al3+ based on julolidine and tryptophan moieties, Tetrahedron, 2016, 72(16), 1998–2005 CrossRef CAS
.
- Z. Liu, H. Xu, L. Sheng, S. Chen, D. Huang and J. Liu, A highly selective colorimetric and fluorescent chemosensor for Al(III) based-on simple naphthol in aqueous solution, Spectrochim. Acta, Part A, 2016, 157, 6–10 CrossRef CAS PubMed
.
- H. M. Park, B. N. Oh, J. H. Kim, W. Qiong, I. H. Hwang and K. D. Jung,
et al., Fluorescent chemosensor based-on naphthol–quinoline for selective detection of aluminum ions, Tetrahedron Lett., 2011, 52(43), 5581–5584 CrossRef CAS
.
- A. Barba-Bon, A. M. Costero, S. Gil, M. Parra, J. Soto and R. Martínez-Máñez,
et al., A new selective fluorogenic probe for trivalent cations, Chem. Commun., 2012, 48(24), 3000 RSC
.
- A. Salmanipour and M. A. Taher, An electrochemical sensor for stripping analysis of Pb(II) based on multiwalled carbon nanotube functionalized with 5-Br-PADAP, J. Solid State Electrochem., 2011, 15(11–12), 2695–2702 CrossRef CAS
.
- D. A. Atwood, Cationic group 13 complexes, Coord. Chem. Rev., 1998, 176(1), 407–430 CrossRef CAS
.
- Z. Akbari, M. Montazerozohori, G. Bruno, K. Moulaee and G. Neri, Development of a novel electrochemical nitrite sensor based on Zn-Schiff base complexes, Appl. Organomet. Chem., 2022, 36(4), e6610 CrossRef CAS
.
- A. Afkhami, H. Bagheri, H. Khoshsafar, M. Saber-Tehrani, M. Tabatabaee and A. Shirzadmehr, Simultaneous trace-levels determination of Hg(II) and Pb(II) ions in various samples using a modified carbon paste electrode based on multi-walled carbon nanotubes and a new synthesized Schiff base, Anal. Chim. Acta, 2012, 746, 98–106 CrossRef CAS PubMed
.
- A. Afkhami, H. Ghaedi, T. Madrakian and M. Rezaeivala, Highly sensitive simultaneous electrochemical determination of trace amounts of Pb(II) and Cd(II) using a carbon paste electrode modified with multi-walled carbon nanotubes and a newly synthesized Schiff base, Electrochim. Acta, 2013, 89, 377–386 CrossRef CAS
.
- A. Afkhami, F. Soltani-Felehgari, T. Madrakian, H. Ghaedi and M. Rezaeivala, Fabrication and application of a new modified electrochemical sensor
using nano-silica and a newly synthesized Schiff base for simultaneous determination of Cd2+, Cu2+ and Hg2+ ions in water and some foodstuff samples, Anal. Chim. Acta, 2013, 771, 21–30 CrossRef CAS PubMed
.
- A. García-Miranda Ferrari, P. Carrington, S. J. Rowley-Neale and C. E. Banks, Recent advances in portable heavy metal electrochemical sensing platforms, Environ. Sci.: Water Res. Technol., 2020, 6(10), 2676–2690 RSC
.
- A. Aravind, M. Sebastian and B. Mathew, Green synthesized unmodified silver nanoparticles as a multi-sensor for Cr(III) ions, Environ. Sci.: Water Res. Technol., 2018, 4(10), 1531–1542 RSC
.
- A. G. M. Ferrari, P. Carrington, S. J. Rowley-Neale and C. E. Banks, Recent advances in portable heavy metal electrochemical sensing platforms, Environ. Sci.: Water Res. Technol., 2020, 6(10), 2676–2690 RSC
.
- M. S. Collin, S. K. Venkatraman, N. Vijayakumar, V. Kanimozhi, S. M. Arbaaz and R. G. S. Stacey,
et al., Bioaccumulation of lead (Pb) and its effects on human: A review, J. Hazard. Mater. Adv., 2022, 7, 100094 CrossRef CAS
.
- S. Pourbeyram, S. Fathalipour, B. Rashidzadeh, H. Firuzmand and B. Rahimi, Simultaneous determination of Cd and Pb in the environment using a pencil graphite electrode modified with polyaniline/graphene oxide nanocomposite, Environ. Sci.: Water Res. Technol., 2023, 9(12), 3355–3365 RSC
.
- X. Liu, Y. Yao, Y. Ying and J. Ping, Recent advances in nanomaterial-enabled screen-printed electrochemical sensors for heavy metal detection, TrAC, Trends Anal. Chem., 2019, 115, 187–202 CrossRef CAS
.
- D. Wu, Y. Hu, H. Cheng and X. Ye, Detection Techniques for Lead Ions in Water: A Review, Molecules, 2023, 28(8), 3601 CrossRef CAS PubMed
.
- V. Bressi, Z. Akbari, M. Montazerozohori, A. Ferlazzo, D. Iannazzo and C. Espro,
et al., On the Electroanalytical Detection of Zn Ions by a Novel Schiff Base Ligand-SPCE Sensor, Sensors, 2022, 22(3), 900 CrossRef CAS PubMed
.
- P. B. Tchounwou, C. G. Yedjou, A. K. Patlolla and D. J. Sutton, Heavy metal toxicity and the environment, Exper. Suppl., 2012, 101, 133–164 CrossRef PubMed
.
- B. Bansod, T. Kumar, R. Thakur, S. Rana and I. Singh, A review on various electrochemical techniques for heavy metal ions detection with different sensing platforms, Biosens. Bioelectron., 2017, 94, 443–455 CrossRef CAS PubMed
.
- M. Kumar Goshisht, G. Kumar Patra and N. Tripathi, Fluorescent Schiff base sensors as a versatile tool for metal ion detection: strategies, mechanistic insights, and applications, Mater. Adv., 2022, 3(6), 2612–2669 RSC
.
- J. Acharya, U. Kumar and P. M. Rafi, Removal of Heavy Metal Ions from Wastewater by Chemically Modified Agricultural Waste Material as Potential Adsorbent-A Review, Int. J. Curr. Eng. Technol., 2018, 28, 526–530 Search PubMed
.
- S. Afroze and T. K. Sen, A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents, Water, Air, Soil Pollut., 2018, 229(7), 225 CrossRef
.
- Q. Ding, C. Li, H. Wang, C. Xu and H. Kuang, Electrochemical detection of heavy metal ions in water, Chem. Commun., 2021, 57(59), 7215–7231 RSC
.
- C. Z. Zhang, B. Chen, Y. Bai and J. Xie, A new functionalized reduced graphene oxide adsorbent for removing heavy metal ions in water via coordination and ion exchange, Sep. Sci. Technol., 2018, 53(18), 2896–2905 CrossRef CAS
.
- A. S. Ahmed, M. B. I. Mohamed, M. A. Bedair, A. A. El-Zomrawy and M. F. Bakr, A new Schiff base-fabricated pencil lead electrode for the efficient detection of copper, lead, and cadmium ions in aqueous media, RSC Adv., 2023, 13(23), 15651–15666 RSC
.
- R. Kumar Saren, S. Banerjee, B. Mondal, S. Senapati and T. Tripathy, An electrochemical sensor–adsorbent for lead (Pb 2+) ions in an aqueous environment based on Katiragum–Arginine Schiff
base, New J. Chem., 2022, 46(41), 19740–19750 RSC
.
- C. Mohan, K. Sharma and S. Chandra, Lead (II)-Selective Potentiometric Sensor Based on Thiophene-2-Aldehyde Thiosemicarbazone (TATS) Schiff Base in PVC Matrix, Anal. Bioanal. Electrochem., 2022, 14(9), 860–870 CAS
.
- M. Rashid, R. Kouser, F. Arjmand and S. Tabassum, New graphene oxide-loaded probe as a highly selective fluorescent chemosensor for the detection of iron ions in water samples using optical methods, Opt. Mater., 2023, 142, 114077 CrossRef CAS
.
- Z. Akbari, M. Montazerozohori, R. Naghiha, P. Hayati, N. Micale and M. Cristani,
et al., Some new antimicrobial/antioxidant nanostructure zinc complexes: Synthesis, crystal structure, Hirshfeld surface analyses and thermal behavior, Results Chem., 2022, 4, 100636 CrossRef CAS
.
- G. Wu, M. Li, J. Zhu, K. W. Chiu Lai, Q. Tong and F. Lu, A highly sensitive and selective turn-on fluorescent probe for Pb( ii ) ions based on a coumarin–quinoline platform, RSC Adv., 2016, 6(103), 100696–100699 RSC
.
- B. A. Mei, O. Munteshari, J. Lau, B. Dunn and L. Pilon, Physical Interpretations of Nyquist Plots for EDLC Electrodes and Devices, J. Phys. Chem. C, 2018, 122, 194–206 CrossRef CAS
.
- H. S. Magar, R. Y. A. Hassan and A. Mulchandani, Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications, Sensors, 2021, 21(19), 6578 CrossRef CAS PubMed
.
- M. Khan, K. Abid, A. Ferlazzo, V. Bressi, C. Espro and M. Hussain,
et al., A Sensitive and Selective Non-Enzymatic Dopamine Sensor Based on Nanostructured Co3O4–Fe2O3 Heterojunctions, Chemosensors, 2023, 11(7), 379 CrossRef CAS
.
- K. Abid, A. Foti, A. Khaskhoussi, C. Celesti, C. D'Andrea and P. Polykretis,
et al., A study of Screen-Printed Electrodes Modified with MoSe2 and AuNPs-MoSe2 Nanosheets for Dopamine Sensing, Electrochim. Acta, 2023, 143371 Search PubMed
.
- Z. Akbari, M. Montazerozohori, S. J. Hoseini, R. Naghiha, P. Hayati and G. Bruno,
et al., Synthesis, crystal structure, Hirshfeld surface analyses, antimicrobial activity, and thermal behavior of some novel nanostructure hexa-coordinated Cd(II) complexes: Precursors for CdO nanostructure, Appl. Organomet. Chem., 2021, 35(5), e6181 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |