Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insects as a sustainable source of emerging proteins and their processing to obtain bioactive compounds: an updated review†
Francielle Miranda
de Matos
 *,
Gabriela Boscariol
Rasera
and
Ruann Janser Soares
de Castro
*,
Gabriela Boscariol
Rasera
and
Ruann Janser Soares
de Castro
 *
*
Department of Food Science and Nutrition, School of Food Engineering, University of Campinas, Rua Monteiro Lobato, 80, Campinas-SP, Brazil. E-mail: franciellemirandadematos@gmail.com; ruann@unicamp.br
First published on 7th November 2023
Abstract
Insects have been considered alternative foods, mainly as sources of protein. The inclusion of insects in the human diet can help meet the growing demand for food, whether consumed directly or as ingredients in other formulations. In recent years, there has been great interest in using insect proteins as a substrate to obtain bioactive peptides. This review provides an overview of obtaining bioactive peptides, addressing the advantages of using insects as a protein substrate, as well as the challenges associated with their use. Techniques for simulating gastrointestinal digestion, microbial fermentation and application of commercial enzymes were described as suitable methods for obtaining peptides. The principles of antioxidant, antidiabetic and antihypertensive properties have been elucidated. Considering an alternative use of peptides as ingredients in other food formulations, possible changes in their bioactivities were reported. This could result from the interaction of peptides with phenolic compounds and their involvement in the Maillard reaction. Finally, allergenic and regulatory aspects were discussed as the main challenges in using insects as a hydrolysis substrate.
Sustainability spotlightEdible insects are rich in proteins and obtained by more sustainable processes compared to conventional livestock. Insect production has low greenhouse gas emissions, little water, and space are required, and food waste can be used as animal feed. In this context, addressing the use of insects as a substrate for obtaining bioactive compounds is an incentive to use them in food, mainly as an alternative source of protein. The consequences of this practice are aligned with some of the UN's Sustainable Development Goals., such: ensure food security, adopt practices of more sustainable production processes, reduce of environmental damage, and improve the management of water resources. |
1. Introduction
Bioactive peptides are low molecular weight protein fragments that cause significant physiological effects in living organisms. These physiological properties encompass antiobesity, antihypertensive, antithrombotic, antioxidant, hypocholesterolemic, antimicrobial, opioid, cytomodulatory, and immunomodulatory activities.1 Due to their diverse functions, high bioavailability, minimal or no toxic effects, and efficacy even at low concentrations, bioactive peptides have received significant attention.2 Although some peptides occur naturally in isolation, many are hidden within the intact structure of protein molecules. As a result, several food proteins, including those derived from milk, eggs, soy, fish, and meat, have been extensively studied and identified as potential sources of bioactive peptides.1Insects are rich in proteins and their cultivation offers convincing environmental and economic advantages when compared to systems used to obtain traditional protein sources, such as meat and plants.3 Although insect consumption is a common practice for more than two billion people, it has not yet become widespread worldwide.4 Some strategies to improve acceptance are the use of insects as pastes, powders, and protein concentrates or isolates, which can also be incorporated as ingredients or fortifying agents in food products. Considering this, edible insects have also been widely explored as a protein substrate to obtain bioactive peptides.5
Antioxidant, antidiabetic and antihypertensive properties have been the most investigated bioactivities in insect protein hydrolysates.6 Published works mention the use of various methods, including the application of commercial enzymes,7 simulated digestion,8 fermentation,9 and the adoption of new processing technologies, such as ultrasound10 and microwave,11 to release or produce these peptides. The most commonly employed insects as protein substrates to obtain bioactive peptides include mealworms,12 house crickets,13 the silkworm14 and black fly soldier.15
In this review article, the advantages of the use of edible insects as a substrate for enzymatic hydrolysis to obtain bioactive peptides were approached. This section describes the main methods employed to obtain bioactive peptides and explores emerging processing technologies. Additionally, it describes several bioactive properties and their potential mechanisms of action. In the context of using these compounds as ingredients in other formulations, a discussion about the potential interactions of bioactive peptides with phenolic compounds and their involvement in the formation of Maillard reaction products was presented. These interactions can potentially lead to modifications in the structure and properties of bioactive peptides. Lastly, the challenges associated with using insects as a substrate, including allergenic and regulatory factors were addressed.
2. Edible insects
By 2050, the world population is expected to be approximately 10 billion people, and there may be an increase of more than 50% in the demand for food, mainly those of animal origin.16 Although agro-livestock production is sufficient to meet current demand, this practice is considered one of the main threats to the environment, being responsible for problems such as deforestation, loss of biodiversity, eutrophication of water bodies, and overexploitation of natural resources. Therefore, the adoption of sustainable food systems is essential to guarantee the food supply for future generations.17In the last years, the use of insects in human food has been extensively studied, as they are rich in proteins and obtained by more sustainable processes compared to breeding systems to obtain conventional proteins (e.g., poultry, swine, and cattle).18 In conventional livestock, there is usually low feed conversion efficiency, and enteric fermentation in ruminants, as well as manure, are responsible for the emission of polluting gases such as methane. On the other hand, insect production has low greenhouse gas emissions, little water and space is required, and food waste can be used as animal feed.19 This last characteristic allows the use of insects as a bioconversion tool in insect biorefineries, which also represents an economic advantage. In this system, insects convert organic matter into a value-added source of proteins and fats, and produce other beneficial products, including biofertilizers, biodiesel, biopolymers, enzymes, animal feeds and edible foods.20
Due to cultural habits, insects are already used as food in many regions, mainly in countries of Africa, Southeast Asia, and Central America. There are over 1 million known insect species, of which about 2111 are traditionally consumed in over 100 countries.21 Worldwide, the most consumed insects are mealworms (31%); caterpillars (17%), wasps, bees, and ants (15%); and crickets and grasshoppers (14%).22 Besides having an average protein content that varies from 30% to 65% on a dry basis, insects are sources of lipids, carbohydrates, vitamins, and minerals, such as iron, zinc, and calcium.23,24
The digestibility of insect proteins can vary from 76 to 96%, being lower than the digestibility of eggs (95%) and beef (98%), however, superior to the digestibility of vegetable proteins (13–60%).24,25 Regarding the amino acids present in the protein fraction, 35 to 50% of these are essential. The content of unsaturated fatty acids can represent more than 70% of the lipid fraction, with oleic, linoleic, and palmitic acids the most abundant in insects. Despite this, these composition characteristics of insects may vary according to the species, stage of development, the diet of insects, and environmental factors related to breeding.26
Although the consumption of insects has numerous advantages, acceptance by consumers is the biggest obstacle to use in the human diet. The lack of familiarity with insects as food (neophobia) is one of the factors associated with rejection, especially in Western countries, where people do not consider insects to be edible and suitable for consumption.27 One of the strategies to improve the acceptance of edible insects is to use them as ingredients in other formulations, modifying visual appearance, and thus obtaining more attractive and value-added products.22
Recently, many insect-based products have been introduced into the food market. Companies such as Circle Harvest (Australia) and Future Food (England), in addition to selling dehydrated insects, have used insect flour in the preparation of pasta, corn snacks, granola, and marshmallows.28,29 In Brazil, the startup Hakkuna has carried out the artisanal production of protein bars based on cricket flour (Gryllus assimilis), while haute cuisine chefs have used insects in the preparation of sophisticated recipes, such as rack of lamb with ant farofa, spring rolls stuffed with vegetables and crickets, fried rice with mealworms, and cricket covered in Belgian chocolate.30
Concerning academic research, it is possible to find works that study the characterization of food products that have had insects added to their formulation, such as bakery products,31 emulsified meat products,32 and fermented foods.33 On the other hand, insects have been extensively studied as a protein substrate in enzymatic hydrolysis processes, in order to obtain products with biological properties of interest: the bioactive peptides.34,35
3. Bioactive peptides of insect proteins
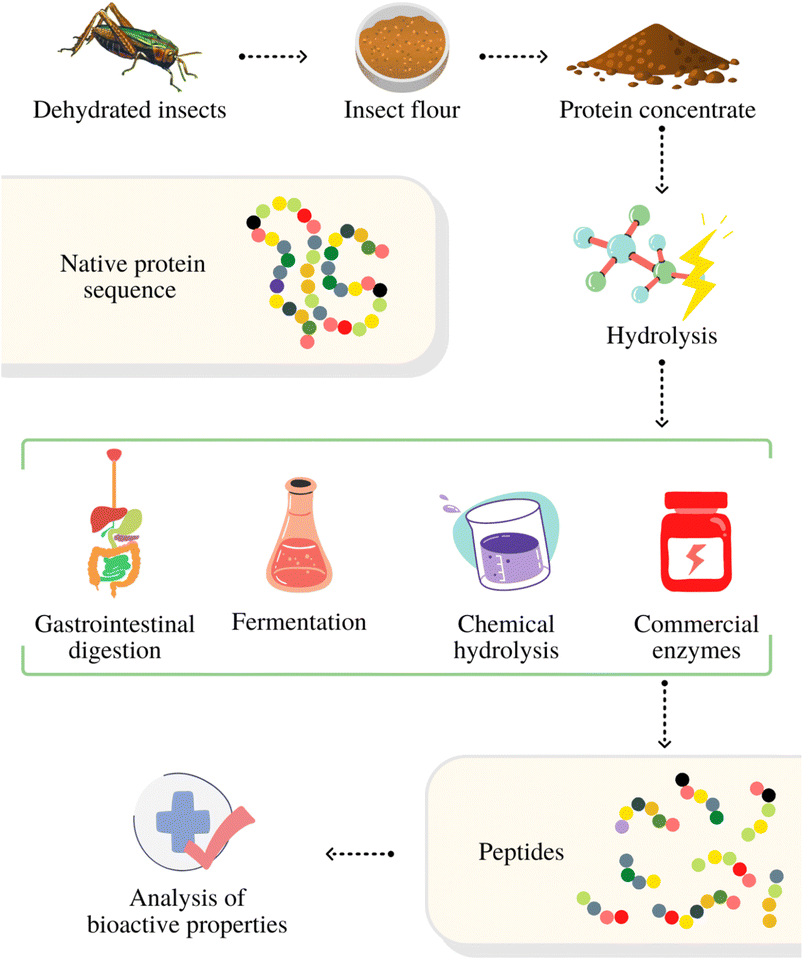
Bioactive peptides are protein fragments containing between 2 and 20 amino acids that positively modulate physiological functions.36 As part of the native protein sequence, these peptides are initially inactive and their bioactive potential is harnessed upon release.37 Hydrolysis of proteins to release these peptides can occur during the digestion process, but can also be obtained through fermentation, use of commercial enzymes or chemical hydrolysis. However, chemical hydrolysis is generally considered unsuitable due to its nonspecific cleavage, low yields, and generation of chemical waste.36,38,39Fig. 1 provides an overview of the various processes for producing bioactive peptides from insect proteins.Among the various possible bioactivities, antioxidant, antidiabetic and antihypertensive properties are the best characterized in peptides.3 These properties depend on the size of the peptides, the composition of the amino acids and their sequence in the protein structure, with hydrophobicity and amino acid charge being important factors.40Table 1 provides examples of various treatments that have been employed to obtain bioactive peptides from insect proteins. ESI Table S1† contains an extensive list of research using the same approach.
| Insect specie | Treatment | Enzymes | Activity | References |
|---|---|---|---|---|
| Spodoptera littoralis | Hydrolysis | Thermolysin | Antioxidant, ACE inhibition | 41 |
| Alcalase | ||||
| Simulated gastrointestinal digestion | Pepsin | |||
| Trypsin | ||||
| α-Chymotrypsin | ||||
| Digestion with mucosal peptidases | Mucosal peptidases | |||
| Hermetia illucens | Sonication | — | Antioxidant | 42 |
| Hydrolysis | Alkaline protease (Novozymes) | |||
| Gryllodes sigillatus | Hydrolysis | Alcalase | DPP-IV inhibition, ACE inhibition | 11 |
| Conventional heating | — | |||
| Microwave radiation | — | |||
| Tenebrio molitor | Ultrasound | — | α-Glucosidase inhibition | 10 |
| Hydrolysis | Trypsin | |||
| Alcalase | ||||
| Tenebrio molitor, Schistocerca gregaria, Gryllodes sigillatus | Heat treatment | — | ACE inhibition, pancreatic lipase inhibition, α-glucosidase inhibition | 8 |
| Simulated gastrointestinal digestion | Pepsin | |||
| Pancreatin | ||||
| Antheraea assamensis | Hydrolysis | Alcalase | ACE inhibition, DPP-IV inhibition, antioxidant | 43 |
| Flavourzyme | ||||
| Thermolysin | ||||
| Papain | ||||
| Simulated gastrointestinal digestion | Pepsin | |||
| Trypsin | ||||
| α-Chymotrypsin | ||||
| Gryllus bimaculatus | Fermentation with Leuconostoc mesenteroides, Lactobacillus plantarum and Bacillus amyloliquefaciens strains | — | Antioxidant | 44 |
| Gryllus assimilis | Hydrolysis | Flavourzyme | α-Amylase inhibition, α-glucosidase inhibition, ACE inhibition | 45 |
| Alcalase | ||||
| Neutrase |
3.1 Methods for obtaining bioactive peptides
Although gastrointestinal digestion of proteins in vivo is possible, assessment of released bioactive peptides requires removal of intestinal contents from living organisms that have been fed a protein diet, making it a rarely used approach.47 Instead, in vitro digestion models have been developed to simulate digestion, offering a more practical alternative.49 The methodologies used are based on human physiology and are often more economical, faster and do not require approval from ethics committees when compared to carrying out clinical tests.50 These tests involve replicating the conditions of the gastrointestinal tract in terms of pH, temperature, presence of enzymes and bile salts.49
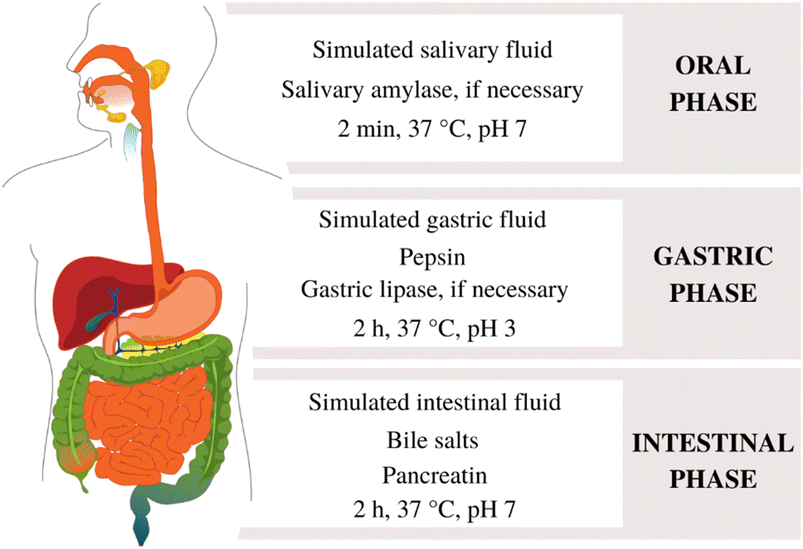
Obtaining digestive enzymes from the human body and microvilli from the intestinal epithelium is a challenging and expensive process. As an economical alternative, the enzymes and bile salts used in in vitro digestion are typically sourced from the digestive systems of other mammals. This includes bile bovine, porcine gastric mucosa pepsin and porcine pancreatin.51 Porcine pancreatin is an extract from the pancreas, comprising several enzymes, including proteases, lipases and amylases. In Fig. 2, the digestion process is simulated accordingly with the INFOGEST protocol is illustrated.52 During the intestinal phase, the protease activity of pancreatin can be replaced by the enzymes trypsin and chymotrypsin.52
In a study carried out by Zielińska et al.,53 peptides with antioxidant activities were obtained through in vitro gastrointestinal digestion of five species of edible insects (Blaptica dubia, Gromphadorhina portentosa, Locusta migratoria, Zophobas morio and Amphiacusta annulipes). The digestion process involved simulating the oral phase using a salivary solution with pH 6.75 and the enzyme α-amylase. For simulated gastric digestion, samples were hydrolyzed with pepsin at pH 2.5, and for simulated intestinal juice, the solution was adjusted to pH 7 with the addition of pancreatin and bile extract solutions. After digestion, the peptide concentration increased in all samples. The hydrolysates obtained after digestion of A. annulipes exhibited greater antiradical activity against DPPH (with an IC50 value of 19.1 μg mL−1), greater capacity to chelate Fe2+ (58.82%) and greater reducing power value (Abs700 nm = 0.652).
The two main fermentation systems used are submerged fermentation and solid state fermentation, with microorganisms naturally present in the substrate or introduced as a starter culture. Submerged fermentation is suitable for microorganisms that thrive in a nutrient-rich medium with plenty of free-flowing water, such as bacteria. In this method, fermentation occurs in a liquid medium, which facilitates the purification of the generated peptides and the measurement of process parameters.56 On the other hand, solid state fermentation is more appropriate for the cultivation of fungi and microorganisms that require less water. This technique offers advantages such as lower risk of contamination, greater productivity, simplified processing, reduced energy needs and less wastewater production.57
Among bacteria, lactic acid bacteria are highly valuable for obtaining bioactive peptides. In addition to their safety profile, with several strains recognized as ‘generally recognized as safe’ (GRAS), lactic acid bacteria have an efficient proteolytic system characterized by the presence of cell membrane-associated proteinases and intracellular peptidases.58Aspergillus oryzae and Kluyveromyces marxianus are examples of fungi and yeasts used in the generation of bioactive peptides. Although their proteolytic systems are not as elaborate as those of lactic acid bacteria, they are still capable of cleaving peptide bonds and generating bioactive peptides through limited hydrolysis.59
In a study carried out by Cruz et al.,60 peptides with antioxidant properties were obtained from a protein concentrate from black cricket (Gryllus assimilis), which served as substrate for a submerged fermentation process conducted by the filamentous fungus Aspergillus tubingensis. During the initial 24 hours of fermentation, a reduction in antioxidant properties was observed, which was attributed to the use of released peptides as a source of nitrogen. However, the most favorable properties were achieved after 72 and 96 hours of fermentation, with DPPH, ABTS radical inhibition activities and ferric reducing antioxidant power reaching levels of 290.79 μmol TE per g, 862.82 μmol TE per g and 1020.11 μmol TE per g, respectively.
Mealworm and locust flours were subjected to fermentation using strains of Lactococcus lactis (NRRL B-50571 and NRRL B-50572) to evaluate the production of peptides with antioxidant and antihypertensive properties. For the fermentation process, mealworm and locust flours were dispersed in a buffer solution (0.5% w/v) at pH 7, containing 3.5% dextrose. Each flour was individually inoculated (3%) with strains of Lactococcus lactis. In general, the highest antioxidant activity was observed in grasshopper fractions fermented with Lactococcus lactis NRRL B-50572. After 24 hours of fermentation, there were increases of about 55%, 34% and 190% in antioxidant activities measured by ABTS, DPPH and ORAC methods, respectively. This fraction was also evaluated for ACE inhibition activity, showing an inhibition of 23.47% with an IC50 of 0.97 mg mL−1.9
Although proteolytic enzymes can be obtained from animal and plant sources, their use presents certain limitations, especially with regard to extraction. Therefore, it is more common to use microbial enzymes, obtained through fermentation. Hydrolysis can be carried out under traditional batch conditions, using immobilized enzymes, or with ultrafiltration membranes.59 Throughout these processes, the cleavage of peptide bonds leads to an increase in charge density and a decrease in the molecular mass of the substrate proteins, thus increasing the solubility of the resulting products. By centrifuging the solutions, the precipitate (non-hydrolyzed proteins) is separated from the supernatant (which contains soluble peptides).35
As seen in Table 1, many studies combine the use of commercial proteases with other technological processes. In a study conducted by Mintah et al.,42 proteins from Hermetia illucens larvae was pretreated and hydrolyzed using alkaline protease under swept frequency ultrasound, resulting in hydrolysates with greater solubility and enhanced antioxidant properties. According to Rivero-Pino et al.,10 ultrasonic treatment is capable of modifying the native structure of proteins, breaking interactions between amino acids and exposing hydrophobic residues. On the other hand, Hall et al.11 examined the effects of microwave radiation on the hydrolysis of cricket (Gryllodes sigillatus) proteins with the protease Alcalase. The hydrolysates showed superior DPP-IV and ACE inhibitory activity compared to samples pretreated with conventional heating. Furthermore, microwave treatment generated samples with lower reactivity to tropomyosin-IgG. These effects are believed to be the result of simultaneous thermal and non-thermal interactions during the radiation process.
3.2 Bioactive properties
The antioxidant properties of peptides are linked to their ability to inactivate reactive oxygen species, scavenge free radicals, chelate pro-oxidative transition metals and reduce hydroperoxides.63 In biological systems, these reactive oxygen species are produced during metabolic processes and play essential roles in protecting cells against infections and regulating intercellular signaling pathways.64 However, its excessive accumulation in cells can lead to aging and pose a risk for the development of cardiovascular problems, diabetes and cancer. Consequently, there is significant interest in obtaining antioxidant peptides, whether for use as nutraceuticals or for industrial purposes as inhibitors of lipid peroxidation in foods.64According to the literature, low molecular weight peptides (<3 kDa) and those containing hydrophobic amino acids tend to have superior antioxidant properties.65 This is attributed to the increased exposure of amino acid side groups for interaction with free radicals, while hydrophobic amino acids readily donate electrons for free radical scavenging.66
Bioactive peptides with antidiabetic properties exert their effects mainly by regulating important enzymes in carbohydrate metabolism.67 Enzymes such as α-amylase and α-glucosidase participate in the conversion of carbohydrates into absorbable sugars. On the other hand, glucagon-like peptide 1 (GLP-1) slows gastric emptying and stimulates insulin release, but is degraded by the enzyme dipeptidyl peptidase-IV (DPP-IV). Inhibition of these digestive enzymes and DPP-IV can reduce postprandial hyperglycemia, contributing to the treatment of individuals with type II diabetes mellitus.68
The production of bioactive peptides with antihypertensive properties has been extensively explored.69 This biological effect is associated with the ability of specific amino acid sequences to inhibit the action of the angiotensin-converting enzyme (ACE). ACE plays a crucial role in regulating blood pressure through the renin-angiotensin and bradykinin pathways. Its main function is to convert angiotensin I (an inactive decapeptide) into angiotensin II, a vasoconstrictor octapeptide. ACE can also cleave bradykinin, which has vasodilatory properties.70 ACE inhibition attenuates the increase in blood pressure it promotes.
Although peptides possess several other properties, those mentioned above are the most extensively investigated in insect protein hydrolysates.
3.3 Potential changes in bioactivities during processing and digestion
The chemical structure, composition and amino acid sequence of peptides play a fundamental role in their bioactive properties. These characteristics allow peptides to modulate the activity of enzymes, inhibiting or activating them, stabilizing or eliminating free radicals and establishing various interactions. Consequently, when incorporating peptides as ingredients in food formulations, it is essential to consider their interactions with other compounds, which can lead to changes in previously identified bioactive properties. For example, the interaction between peptides and phenolic compounds can result in different products, potentially modifying their properties and digestibility.71,72Likewise, in the presence of reducing sugars and at elevated temperatures, peptides can participate in the Maillard reaction, which can have both negative effects, such as the reduction of their biological properties, and positive effects, such as the production of melanoidins, which have it has been reported to have beneficial functions.73 Several modifications can occur before peptides reach their site of action and carry out their physiological activities.74
In this sense, the bioaccessibility of bioactive peptides can be assessed by simulating gastrointestinal digestion, allowing us to understand how the action of digestive enzymes modifies the structure and properties of peptides when administered orally.75
Due to the properties mentioned, as well as many others (anti-ulcer, anti-inflammatory, anti-allergic, antimicrobial, analgesic activities), the beneficial effects provided by phenolic compounds are mainly due to their interactions with proteins. These interactions involve digestive enzymes, salivary proteins, plasma proteins, or proteins involved in the development of disease.79 According to scientific research, these interactions can occur through hydrogen bonds, hydrophobic and ionic interactions and van der Waals forces. The nature of these interactions depends on the structural characteristics of polyphenols and proteins, as well as their concentrations, proportions and environmental conditions, including pH, ionic strength and temperature.79,80Fig. 3 illustrates noncovalent interactions between proteins and phenolic compounds.
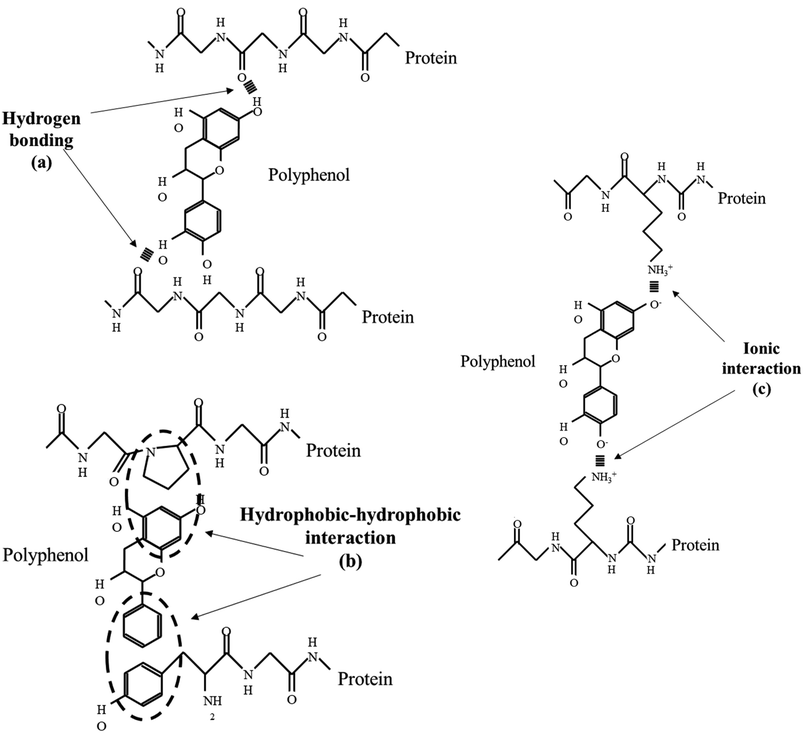 | ||
| Fig. 3 Non-covalent interactions between proteins and phenolic compounds. Figure adapted from ref. 81 with permission from Elsevier, copyright 2019. | ||
Recognizing that the interaction between proteins and phenolics can result in diverse outcomes, including inhibition or denaturation of enzymes, protection against degradation of phenolic compounds, restriction of their bioactive action, changes in metabolism, absorption and transport in the bloodstream, the formation of complex aggregates and modifications of protein structures,79 it becomes apparent that interactions between phenolics and peptides could similarly impact the bioactive properties of these compounds. Although such studies are relatively scarce, some have explored this approach.
Sardine (Sardinella sindensis) protein hydrolysates demonstrated improved antioxidant properties when combined with a green pistachio shell extract. According to the authors, the interaction with phenolic compounds may have exposed hydrophobic amino acids in the hydrolysate, increasing its ability to scavenge DPPH radicals. When evaluating the antidiabetic properties of the samples, the study found that the interaction between the hydrolysate and phenolic compounds led to a reduction in the inhibition activities of the enzymes α-glucosidase and α-amylase. It is suggested that this interaction may have limited the chemical groups that were previously available for interaction with digestive enzymes.71
In a study conducted by Su et al.,82 the interaction between walnut phenolic compounds and walnut protein hydrolysates resulted in a reduction in tryptophan fluorescence intensity. This reduction indicated that phenolic compounds can bind to regions hydrophobic properties of the peptides, leading to the extinction of tryptophan. The study found that as the phenolic-to-peptide ratio increased (ranging from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 120 to 1
120 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18), there was a gradual increase in the average particle size. However, when this ratio exceeded 1
18), there was a gradual increase in the average particle size. However, when this ratio exceeded 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 (increasing from 1
18 (increasing from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to 1
12 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6), there was a significant reduction in the average particle size, suggesting a potential saturation of the peptide binding sites and the formation of compact peptide-phenolic complexes. Regarding the scavenging activity of the DPPH radical of the hydrolysates, this increased with the greater proportion of phenolics. However, this property was lower than the activity of phenolics alone, indicating a negative impact of the interaction on the activity of phenolics. On the other hand, for the Fe2+ chelating capacity, the interaction between the compounds, up to a ratio of 1
6), there was a significant reduction in the average particle size, suggesting a potential saturation of the peptide binding sites and the formation of compact peptide-phenolic complexes. Regarding the scavenging activity of the DPPH radical of the hydrolysates, this increased with the greater proportion of phenolics. However, this property was lower than the activity of phenolics alone, indicating a negative impact of the interaction on the activity of phenolics. On the other hand, for the Fe2+ chelating capacity, the interaction between the compounds, up to a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12, had a synergistic effect.
12, had a synergistic effect.
When evaluating the effect of the interaction between hydroxycinnamic acids (HCAs) from green coffee extract and whey protein hydrolysates (WPH), egg ovalbumin (EOH) and soy protein (SPH), it was observed that heating of hydrolysates at 90 °C with HCAs resulted in the formation of interaction products. These products exhibited greater scavenging capacity for DPPH˙ and OH˙ radicals than those hydrolyzed alone. However, these properties were even more pronounced when the interaction occurred with HCAs in the form of β-cyclodextrin inclusion complexes.83 This study suggests the use of encapsulation of bioactive compounds to incorporate them into fortified foods, aiming to prevent undesirable interactions and changes during processing and storage.
In summary, the increase in the bioactive properties of peptides when complexed with phenolics may result from synergistic effects between these compounds. However, changes resulting from complexation can also decrease the bioactivity of the final products, since critical structures necessary for biological activities may become unavailable for reaction.84 Considering the use of insect proteins to obtain bioactive peptides and their application as ingredients in food formulations, it is intriguing to evaluate the interaction of these bioactive peptides with phenolic compounds. Although this approach has already been applied to other protein hydrolysates, it remains an emerging field and has yet to be explored extensively in the context of insect protein hydrolysates.
The reaction between reducing sugars and proteins is induced mainly by increasing temperature, but the rate of reaction is also influenced by water activity, pH, type of sugars and amino acids involved, ratio between compounds, among other factors.86 The next three stages of the reaction include the formation of the Schiff base products, the Amadori reaction products and the irreversible advanced glycation products (melanoidins), which have a brown color and can lead to changes in the sensory, functional and biological properties of foods.87
Considering that the Maillard reaction is of great importance in the development of color, flavor and odor of food products, specifically those that are baked, roasted or grilled,88 many studies have already been carried out with the aim of evaluating the influence of reaction conditions and the interaction between specific amino acids and sugars on the development of these sensory properties.89 As food proteins are also recognized for their functional properties, the effects of the Maillard reaction on the solubility, thermal stability,86 emulsifying and foaming capacity90 of food proteins and their derivatives have also been investigated.91
As for the biological properties, the most described for the products of the Maillard reaction are the antioxidant and antimicrobial properties.92 The antioxidant activity of these products is mainly associated with compounds derived from the Amadori reaction and melanoidins, which are able to scavenge free radicals, chelate metal ions and act as reducing agents, characteristics associated with the presence of hydroxyl groups and the ability to donate electrons.86
For the antimicrobial properties, melanoidins tend to chelate the magnesium ion (Mg2+) of the bacterial cell membrane, causing its rupture.92 Furthermore, these compounds tend to chelate iron from the growth medium and the siderophore-Fe3+ complex produced by bacteria, leaving this ion unavailable for use by microorganisms. Finally, in the most advanced stages of the Maillard reaction, hydrogen peroxide is generated and can act as an antimicrobial agent.93 Other properties already associated with the Maillard reaction products include antihypertensive, antitumor, anti-cariogenic, anti-inflammatory, and anti-osteoporosis activities, besides to act regulating intestinal flora and improving immunity.91,94
When considering the bioactivities acquired by the hydrolysis of protein substrates and the structural changes resulting from the Maillard reaction, there has been a great interest in evaluating the effects of the latter on the bioactive properties of peptides. In the work performed by Zhang et al.,95 Maillard products were obtained by reacting galactose with isolated and hydrolyzed whey protein, through heat treatment at 95 °C for up to 4 h. The highest antioxidant properties were obtained for the hydrolyzed sample submitted to the Maillard reaction, and its reducing power was 9 times higher than the activity of the only hydrolyzed sample. Casein hydrolysates also had their ACE-inhibiting properties increased by approximately 30% after a 2 h reaction at 110 °C with xylose.96
The bioactive properties of many other protein hydrolysates, such as vegetable seed hydrolysates,97 exotic meats,98 fish99,100 and cereals,101 were evaluated after induction of the Maillard reaction. In general, these studies involve the production of hydrolysates using isolated or combined proteases, the Maillard reaction, which has variables such as the type of reducing sugars used, the relationship between hydrolysate and saccharides, time and temperature conditions, as well as the evaluation of the effects of the reaction. These effects include changes in bioactive and techno-functional properties, the formation of intermediates and dark compounds, changes sensory and identification of Maillard reaction products. A list of studies from the last five years that investigated the impact of the Maillard reaction on the bioactivity of hydrolysates can be found in the ESI (Table S2†). Although the positive effects of the Maillard reaction on the bioactivities of peptides are reported, few works deal with the identification of the complexes/products formed and their association with the bioactive properties. Additionally, there are few studies that approach of negative effects associated with advanced glycation products.94
In a study performed by Grossmann et al.,102 proteins from crickets (Acheta domesticus) and mealworms (Tenebrio molitor) were hydrolyzed using the proteases Flavourzyme and Protease A “Amano.” The hydrolysates were then subjected to heating at 98 °C for 30 minutes in the presence of xylose (1% w/v). Subsequently, the hydrolysates were evaluated for their flavor potential, including the identification of odor-active molecules through gas chromatography-olfactometry. To date, this is the only study that combined the production of protein hydrolysates from insects with the formation of Maillard reaction products. However, it's worth noting that this study did not assess the formation of bioactive compounds. In this regard, exploring the formation of bioactive compounds in such a context could be an important area for further research.
The bioaccessibility of bioactive peptides could be determined by in vitro digestion. After that, it is also possible to verify the bioavailability of the bioactive compounds, that is, to quantify the compounds that were absorbed and reached the systemic circulation, being available to perform their bioactive properties in the target tissues.104 In the human body, the absorption of the digested compounds occurs in the intestinal epithelium, thus, the in vitro evaluation of the bioavailability of compounds can be performed by dialysis, by centrifugation followed by filtration, or using cell models (Caco-2 or co-cultures of Caco-2/HT29-MTX).105
In a study of Nongonierma et al.,106 protein hydrolysates from tropical crickets (Gryllodes sigillatus) were obtained through enzymatic hydrolysis using the enzyme Protamex®. Process parameters studied included temperature, time and enzyme: substrate ratio. The hydrolysate with the greatest ability to inhibit the enzyme dipeptidyl peptidase IV (DPP-IV) had an IC50 of 0.47 mg mL−1, while the IC50 of the unhydrolyzed protein isolate was 3.57 mg mL−1. After submitting both samples to simulated digestion, it was found that the hydrolysate retained 68% of its inhibition activity (IC50 = 0.71 mg mL−1), which was even higher than the inhibition activity of the control sample (IC50 = 0.78 mg mL−1), which had a significant increase in its activity.
In general, digestion tends to increase the degree of hydrolysis of the pretreated samples. When there is an improvement in the bioactivities, it could be due to the release of fragments with a structure more favorable for the property of interest.107 If the digestion reduces the bioactivities, it is supposed that more bioactive peptides are degraded than new ones are formed (can reach extensive peptide cleavage), being the results expressed in terms of activity retention.14 An aspect that helps to understand these modifications promoted by digestion is to consider the specificity of the digestive enzymes, as the position of specific amino acids is essential for some bioactive properties.41
4. Challenges associated with insect-based production
Although numerous studies focus on obtaining bioactive peptides from insect proteins, there are challenges related to the use of this substrate, particularly safety and regulatory aspects. A significant safety concern associated with insect consumption is the potential for allergic responses.108 Insects belong to the class Hexapoda (Insecta), a subphylum of the Arthropoda. The taxonomic similarities between insects and other arthropods, such as crustaceans and mites, help explain the occurrence of allergic reactions. The main allergen responsible for this cross-allergenicity is tropomyosin, a myofibrillar protein.107 Other allergens that may also be present in insects include arginine kinase, sarcoplasmic calcium-binding protein, myosin light chain, troponin C, sarcoplasmic endoreticulum calcium ATPase, hemocyanin, and phospholipase.109In addition to enzymatic hydrolysis, applied in the production of bioactive peptides, several other technological processes have been used with insects as ingredients in food products.108 Some studies have evaluated the effects of processes such as heating, microwaves, enzymatic hydrolysis and acid–alkali treatment as strategies to reduce food allergenicity.11,110 In a study performed by De Marchi et al.,111 the tropomyosin allergen present in crickets (Acheta domesticus) remained stable after heat treatment at 180 °C for 10 minutes and remained immunoreactive after simulated digestion. In another study, frozen Tenebrio molitor larvae were subjected to different treatments. Freeze-dried or cooked mealworm proteins induced allergic responses in crustacean-allergic patients, but the allergens did not show immunoreactivity after frying for 5 minutes at 180 °C.112 A study conducted by Hall et al.107 demonstrated that hydrolysis of crickets (Gryllodes sigillatus) with Alcalase altered the IgE binding characteristics of shrimp-allergic human sera to tropomyosin, with samples exhibiting 60–85% DH showing no reactivity. However, despite the different approaches applied, the information available on the allergenicity of edible insects is still limited and there is no consensus regarding the positive or negative effects caused by treatments.109
In terms of regulation, the European scenario has been at the forefront in recent years and shows promise in the use of insects in human food. The European Food Safety Authority (EFSA) is responsible for assessing the safety of using insects in food for human consumption. In January 2021, the institution issued a positive opinion on the yellow mealworm (Tenebrio molitor), marking the first approval of an insect as food by the European Commission.113,114 Subsequently, in November 2021, the European Commission authorized the use of the migratory grasshopper (Locusta migratoria) as a new food, followed by the approval of domestic crickets in February 2022 and the use of mealworm (Alphitobius diaperinus larvae) in April 2022.115
Despite the progress made in the field, the regulatory process for insects as food faces several challenges, including the vast diversity of insect species, variations in breeding, processing and use, and safety concerns associated with their consumption by humans or animals.116 However, even in the absence of comprehensive legislation, insect-based products are already used in many countries, both in traditional dishes and as artisanal products.117 In the European Union, certain insects currently under evaluation, such as the tropical cricket (Gryllodes sigillatus), the black soldier fly (Hermetia illucens) and the European bee (Apis mellifera), may continue to be traded until EFSA issues its opinion on its safety.118
5. Conclusion
This review showed that insects are a viable alternative source of proteins, which can be used to produce peptides with several biological properties. The diversity of insect species, the life stages for use, and the diversity of techniques and enzymes available for hydrolysis provide a wide scope for generating different peptide structures with multiple functionalities. However, this diversity, while presenting opportunities for new research, also poses challenges such as: few species of insects are approved for human consumption, data on allergenic factors are limited and the effects of different treatments on the potential allergenicity generated by insects' consumption are controversial.When considering the incorporation of protein hydrolysates into food formulations, the review provided relevant evidence that these compounds can interact with other ingredients, potentially altering the biological potential of the peptides. These interactions may lead to synergistic effect between compounds, the formation of new bioactive compounds, or even the reduction of peptide properties. Some of these interactions, such as those between peptide-phenolics and the formation of Maillard reaction products, have received limited attention and could emerge as a significant approach in the study of bioactive peptides.
Author contributions
Francielle Miranda de Matos: conceptualization, writing – original draft, preparation, visualization, investigation. Gabriela Boscariol Rasera: conceptualization, writing – original draft, preparation, visualization, investigation. Ruann Janser Soares de Castro: conceptualization, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Council for Scientific and Technological Development – Brazil (CNPq) [Process Numbers 311150/2020-9 and 403769/2021-3]; and the Coordination of Improvement of Higher Education Personnel – Brazil (CAPES) [Finance Code 001].References
- L. Mora, M. C. Aristoy and F. Toldrá, Encycl. Food Chem., 2019, 381–389 CrossRef CAS
.
- M. Akbarian, A. Khani, S. Eghbalpour and V. N. Uversky, Int. J. Mol. Sci., 2022, 23, 1445 CrossRef CAS
.
- C. S. S. Teixeira, C. Villa, J. Costa, I. M. P. L. V. O. Ferreira and I. Mafra, Foods, 2023, 12, 2026 CrossRef CAS PubMed
.
- R. Ordoñez-Araque and E. Egas-Montenegro, International Journal of Gastronomy and Food Science, 2021, 23, 100304 CrossRef
, Contents..
- H. C. D. Tuhumury, IOP Conference Series: Earth and Environmental Science, 2021, 883, 012029 Search PubMed
.
- Z. Ma, M. Mondor, F. Goycoolea Valencia and A. J. Hernández-Álvarez, Food Funct., 2023, 14, 8129–8156 RSC
.
- S. Yoon, N. A. K. Wong, M. Chae and J.-H. Auh, Foods, 2019, 8, 563 CrossRef CAS
.
- E. Zielińska, M. Karaś, B. Baraniak and A. Jakubczyk, Eur. Food Res. Technol., 2020, 246, 1361–1369 CrossRef
.
- A. Mendoza-Salazar, L. Santiago-López, M. J. Torres-Llanez, A. Hernández-Mendoza, B. Vallejo-Cordoba, A. M. Liceaga and A. F. González-Córdova, Fermentation, 2021, 7, 153 CrossRef CAS
.
- F. Rivero-Pino, F. J. Espejo-Carpio, R. Pérez-Gálvez, A. Guadix and E. M. Guadix, Food Bioprod. Process., 2020, 123, 217–224 CrossRef CAS
.
- F. Hall and A. Liceaga, J. Funct. Foods, 2020, 64, 103634 CrossRef CAS
.
- F. R. Pino, R. P. Gálvez, F. J. E. Carpio and E. M. Guadix, Food Funct., 2020, 11, 4376–4386 RSC
.
- F. Hall, L. Reddivari and A. M. Liceaga, Nutrients, 2020, 12, 1–16 CrossRef PubMed
.
- Y. Liu, S. Wan, J. Liu, Y. Zou and S. Liao, J. Food Process. Preserv., 2017, 41, e13081 CrossRef
.
- M. Firmansyah and M. Y. Abduh, Heliyon, 2019, 5, e02005 CrossRef
.
-
T. Searchinger, R. Waite, C. Hanson and J. Ranganathan, Creating a sustainable food future: a menu of solutions to feed nearly 10 billion people by 2050, 2019 Search PubMed
.
- N. Ramankutty, Z. Mehrabi, K. Waha, L. Jarvis, C. Kremen, M. Herrero and L. H. Rieseberg, Annu. Rev. Plant Biol., 2018, 69, 789–815 CrossRef CAS PubMed
.
- L. Nissen, S. P. Samaei, E. Babini and A. Gianotti, Food Chem., 2020, 333, 127410 CrossRef CAS
.
- M. Kulma, L. Kouřimská, D. Homolková, M. Božik, V. Plachý and V. Vrabec, J. Food Compos. Anal., 2020, 92, 103570 CrossRef CAS
.
- P. E. Kee, Y. S. Cheng, J. S. Chang, H. S. Yim, J. C. Y. Tan, S. S. Lam, J. C. W. Lan, H. S. Ng and K. S. Khoo, Environ. Res., 2023, 221, 115284 CrossRef CAS PubMed
.
-
Y. Jongema, List of Edible Insects of the World, Wageningen, The Netherlands, 2017 Search PubMed
.
- S. Imathiu, NFS J., 2020, 18, 1–11 CrossRef
.
- J. Frigerio, G. Agostinetto, A. Galimberti, F. De Mattia, M. Labra and A. Bruno, Food Res. Int., 2020, 137, 109426 CrossRef CAS
.
- L. J. Montiel-Aguilar, J. A. Torres-Castillo, R. Rodríguez-Servin, A. B. López-Flores, V. E. Aguirre-Arzola, G. Méndez-Zamora and S. R. Sinagawa-García, J. Asia-Pac. Entomol., 2020, 23, 756–761 CrossRef
.
- L. Kouřimská and A. Adámková, NFS J., 2016, 4, 22–26 CrossRef
.
- R. T. Gahukar, Global Food Security, 2020, 25, 100348 CrossRef
.
- C. H. d. F. Domingues, J. A. R. Borges, C. F. Ruviaro, D. G. F. Guidolin and J. R. M. Carrijo, PLoS One, 2020, 15, e0224059 CrossRef CAS
.
- Circle Harvest, Shop, https://www.circleharvest.com.au/, accessed 13 June 2023.
- Future Food, Shop, https://www.futurefoodshop.com/, accessed 13 June 2023.
- L. Monteiro, Insetos na alimentação: A espera de autorização, https://www.em.com.br/app/noticia/bem-viver/2021/02/14/interna_bem_viver,1236860/insetos-na-alimentacao-a-espera-de-autorizacao.shtml, accessed 13 June 2023.
- E. Zielińska, U. Pankiewicz and M. Sujka, Antioxidants, 2021, 10, 1–17 Search PubMed
.
- H.-W. Kim, D. Setyabrata, Y. J. Lee, O. G. Jones and Y. H. B. Kim, Innovative Food Sci. Emerging Technol., 2016, 38, 116–123 CrossRef CAS
.
- H.-Y. Seong and M. Kim, LWT, 2021, 150, 111948 CrossRef CAS
.
- B. A. Acosta-Estrada, A. Reyes, C. M. Rosell, D. Rodrigo and C. C. Ibarra-Herrera, Front. Nutr., 2021, 8, 687712 CrossRef
.
- F. M. de Matos and R. J. S. de Castro, Braz. J. Food Technol., 2021, 24, e2020044 CrossRef CAS
.
- A. Kose, Turkish Journal of Fisheries and Aquatic Sciences, 2021, 21, 615–625 Search PubMed
.
- A. Majid, M. Lakshmikanth, N. K. Lokanath and C. G. Poornima Priyadarshini, Food Chem., 2022, 369, 130888 CrossRef CAS
.
- R. E. Aluko, Annu. Rev. Food Sci. Technol., 2015, 6, 235–262 CrossRef CAS
.
- J. Bechaux, P. Gatellier, J. F. Le Page, Y. Drillet and V. Sante-Lhoutellier, Food Funct., 2019, 10, 6244–6266 RSC
.
- A. G. Garzón, F. F. Veras, A. Brandelli and S. R. Drago, LWT, 2021, 153, 112414 CrossRef
.
- L. Vercruysse, G. Smagghe, T. Beckers and J. Van Camp, Food Chem., 2009, 114, 38–43 CrossRef CAS
.
- B. K. Mintah, R. He, M. Dabbour, J. Xiang, A. A. Agyekum and H. Ma, Ultrason. Sonochem., 2019, 58, 104676 CrossRef CAS
.
- P. Sarkar, G. Valacchi and R. K. Duary, Eur. Food Res. Technol., 2022, 248, 357–379 CrossRef CAS
.
- H.-Y. Seong and M. Kim, LWT, 2021, 150, 111948 CrossRef CAS
.
- F. M. de Matos, J. T. J. G. de Lacerda, G. Zanetti and R. J. S. de Castro, Biocatal. Agric. Biotechnol., 2022, 39, 102276 CrossRef CAS
.
- D. Dupont, Curr. Opin. Food Sci., 2017, 16, 53–58 CrossRef
.
-
R. E. Aluko, Functional Foods and Nutraceuticals, Springer, New York, 2012 Search PubMed
.
- S. Fernández-Tomé and B. Hernández-Ledesma, Mol. Nutr. Food Res., 2020, 64, 2000401 CrossRef
.
-
D. Jones, S. Caballero and G. Davidov-Pardo, in Advances in Food and Nutrition Research, ed. L.-T. Lim and M. Rogers, Academic Press, 2019, vol. 88, pp. 235–273 Search PubMed
.
-
D. I. Santos, J. M. A. Saraiva, A. A. Vicente and M. Moldão-Martins, in Innovative Thermal and Non-Thermal Processing, Bioaccessibility and Bioavailability of Nutrients and Bioactive Compounds, ed. F. J. Barba, J. M. A. Saraiva, G. Cravotto and J. M. Lorenzo, Elsevier, 2019, pp. 23–54 Search PubMed
.
-
J. Singh, T. Berg, A. Hardacre and M. J. Boland, in Food Structures, Digestion and Health, ed. M. Boland, M. Golding and H. Singh, Elsevier, 2014, pp. 223–242 Search PubMed
.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, Nat. Protoc., 2019, 14, 991–1014 CrossRef CAS
.
- E. Zielińska, M. Karaś and A. Jakubczyk, Int. J. Food Sci. Technol., 2017, 52, 306–312 CrossRef
.
- P. G. Manfredini, V. A. F. Cavanhi, J. A. V. Costa and L. M. Colla, Biocatal. Biotransform., 2021, 39, 360–377 CrossRef CAS
.
- D. Rizwan, F. A. Masoodi, S. M. Wani and S. A. Mir, Food Prod., Process. Nutr., 2023, 5, 53 CrossRef CAS
.
- J. Suriya, S. Bharathiraja, M. Krishnan, P. Manivasagan and S. K. Kim, Adv. Food Nutr. Res., 2016, 79, 161–177 CAS
.
- R. J. S. de Castro and H. H. Sato, Waste Biomass Valorization, 2015, 6, 1085–1093 CrossRef
.
-
A. Gennari, F. Leonhardt, G. B. Paludo, D. N. Lehn, G. Renard, G. Volpato and C. F. Volken De Souza, in Microbial Bioreactors for Industrial Molecules, Wiley, 2023, pp. 237–260 Search PubMed
.
- D. E. Cruz-Casas, C. N. Aguilar, J. A. Ascacio-Valdés, R. Rodríguez-Herrera, M. L. Chávez-González and A. C. Flores-Gallegos, Food Chem.: Mol. Sci., 2021, 3, 100047 CAS
.
- L. N. da Cruz, L. d. O. Rocha and R. J. S. de Castro, Food and Humanity, 2023, 1, 1018–1026 CrossRef
.
- S. K. Ulug, F. Jahandideh and J. Wu, Trends Food Sci. Technol., 2021, 108, 27–39 CrossRef CAS
.
- R. J. S. De Castro and H. H. Sato, Food Res. Int., 2015, 74, 185–198 CrossRef PubMed
.
- A. C. da C. Rocha, C. J. de Andrade and D. de Oliveira, Trends Food Sci. Technol., 2021, 116, 480–491 CrossRef
.
- S. A. Tadesse and S. A. Emire, Heliyon, 2020, 6, e04765 CrossRef PubMed
.
- X.-R. Yang, L. Zhang, D.-G. Ding, C.-F. Chi, B. Wang and J.-C. Huo, Mar. Drugs, 2019, 17, 1–18 Search PubMed
.
- F. M. de Matos, P. K. Novelli and R. J. S. de Castro, J. Food Sci., 2021, 86, 571–578 CrossRef CAS PubMed
.
- B. A. Kehinde and P. Sharma, Crit. Rev. Food Sci. Nutr., 2020, 60, 322–340 CrossRef CAS PubMed
.
- J. Yan, J. Zhao, R. Yang and W. Zhao, Int. J. Food Sci. Technol., 2019, 1–11 Search PubMed
.
- Z. F. Bhat, S. Kumar and H. F. Bhat, Crit. Rev. Food Sci. Nutr., 2017, 57, 566–578 CrossRef CAS PubMed
.
- M. Z. Durak and N. A. Turan, American Journal of Biomedical Science and Research, 2020, 7, 191–195 CrossRef
.
- R. A. Sarteshnizi, M. A. Sahari, H. A. Gavlighi, J. M. Regenstein, M. Nikoo and C. C. Udenigwe, LWT, 2021, 142, 111019 CrossRef
.
- Q. Zhao, X. Yu, C. Zhou, A. E. G. A. Yagoub and H. Ma, Lwt, 2020, 124, 109192 CrossRef CAS
.
- K. Arihara, I. Yokoyama and M. Ohata, Meat Sci., 2021, 180, 108561 CrossRef CAS PubMed
.
- E. S. do Nascimento, K. Anaya, J. M. C. de Oliveira, J. T. J. G. de Lacerda, M. E. Miller, M. Dias, M. A. Mendes, J. d. A. L. Pallone, C. W. Arns, M. A. Juliano, T. S. Gadelha, M. T. B. Pacheco and C. A. d. A. Gadelha, Food Res. Int., 2021, 143, 110286 CrossRef PubMed
.
- I. D. Putra, Y. Marsono and R. Indrati, Nutr. Food Sci., 2021, 51, 244–254 CrossRef
.
- D. d. P. Farias, F. F. de Araújo, I. A. Neri-Numa and G. M. Pastore, Food Res. Int., 2021, 145, 110383 CrossRef PubMed
.
-
P. Saranraj, S. S. Behera and R. C. Ray, in Innovations in Traditional Foods, Elsevier, 2019, pp. 159–191 Search PubMed
.
- L. Chen, L. Wang, G. Shu and J. Li, J. Agric. Food Chem., 2021, 69, 5305 Search PubMed
.
- N. P. Ulrih, Crit. Rev. Food Sci. Nutr., 2017, 57, 2144–2161 CrossRef PubMed
.
- F. Chamizo-González, B. Gordillo and F. J. Heredia, Food Chem., 2021, 347, 129014 CrossRef PubMed
.
- T. H. Quan, S. Benjakul, T. Sae-leaw, A. K. Balange and S. Maqsood, Trends Food Sci. Technol., 2019, 91, 507–517 CrossRef CAS
.
- S. Su, Y. Wan, S. Guo, C. Zhang, T. Zhang and M. Liang, Int. J. Food Sci. Technol., 2018, 53, 508–515 CrossRef CAS
.
- G. Budryn, D. Zaczyńska and D. Rachwał-Rosiak, J. Food Process. Preserv., 2017, 41, e12908 CrossRef
.
- A. Hernández-Jabalera, I. Cortés-Giraldo, G. Dávila-Ortíz, J. Vioque, M. Alaiz, J. Girón-Calle, C. Megías and C. Jiménez-Martínez, Food Chem., 2015, 178, 346–357 CrossRef PubMed
.
-
R. V. Hedegaard and L. H. Skibsted, in Handbook of Food Powders: Processes and Properties, ed. B. Bhandari, N. Bansal, M. Zhang and P. Schuck, Woodhead Publishing, 2013, pp. 409–434 Search PubMed
.
- H. Kchaou, N. Benbettaieb, M. Jridi, M. Nasri and F. Debeaufort, Food Hydrocolloids, 2019, 97, 105196 CrossRef CAS
.
- H. Kchaou, N. Benbettaïeb, M. Jridi, O. Abdelhedi, T. Karbowiak, C.-H. Brachais, M.-L. Léonard, F. Debeaufort and M. Nasri, Food Hydrocolloids, 2018, 83, 326–339 CrossRef CAS
.
-
J. A. Rufián-Henares and S. Pastoriza, in Encyclopedia of Food and Health, ed. B. Caballero, P. M. Finglas and F. Toldrá, Academic Press, 2016, pp. 593–600 Search PubMed
.
- J. Liu, M. Liu, C. He, H. Song and F. Chen, LWT–Food Sci. Technol., 2015, 64, 316–325 CrossRef CAS
.
- K. Chen, Q. Yang, H. Hong, L. Feng, J. Liu and Y. Luo, Food Chem., 2020, 333, 127489 CrossRef CAS PubMed
.
- M. Nooshkam, M. Varidi and D. K. Verma, Food Res. Int., 2020, 131, 109003 CrossRef CAS PubMed
.
- R. R. Naik, Y. Wang and C. Selomulya, Crit. Rev. Food Sci. Nutr., 2021, 1–26 CAS
.
- J. A. Rufián-Henares and S. P. De La Cueva, J. Agric. Food Chem., 2009, 57, 432–438 CrossRef PubMed
.
- J. Ding, Z. Huang, M. Yang, Y. Huang, S. Ma and Y. Fang, Food Sci., 2023, 44, 305–318 Search PubMed
.
- X. Zhang, X. Li, L. Liu, L. Wang, A. F. Massounga Bora and L. Du, Int. Dairy J., 2020, 102, 104584 CrossRef CAS
.
- X. Hong, J. Meng and R. R. Lu, J. Sci. Food Agric., 2014, 95, 66–71 CrossRef PubMed
.
- Z. J. Ni, X. Liu, B. Xia, L. T. Hu, K. Thakur and Z. J. Wei, Food Chem.: X, 2021, 11, 100127 CAS
.
- Y. Li, D. Hu, J. Huang and S. Wang, Food Chem. Toxicol., 2021, 155, 112376 CrossRef CAS PubMed
.
- Y. Liang, K. Wang, Q. Yang, L. Zhang, C. Shi, S. Tavakoli, Y. Tan, Y. Luo and H. Hong, LWT, 2022, 158, 113104 CrossRef CAS
.
- T. Zhao, Q. Zhang, S. Wang, C. Qiu, Y. Liu, G. Su and M. Zhao, LWT–Food Sci. Technol., 2018, 97, 245–253 CrossRef CAS
.
- T. Feng, Y. Zhou, X. Wang, X. Wang and S. Xia, Food Biosci., 2021, 41, 100951 CrossRef CAS
.
- K. K. Grossmann, M. Merz, D. Appel, M. M. De Araujo and L. Fischer, Food Chem., 2021, 364, 130336 CrossRef CAS PubMed
.
- C. Dima, E. Assadpour, S. Dima and S. M. Jafari, Compr. Rev. Food Sci. Food Saf., 2020, 19, 2862–2884 CrossRef PubMed
.
- S. Ojha, A. E. D. Bekhit, T. Grune and O. K. Schlüter, Curr. Opin. Food Sci., 2021, 41, 240–248 CrossRef CAS
.
-
O. L. Ramos, R. N. Pereira, L. S. Simões, D. A. Madalena, R. M. Rodrigues, J. A. Teixeira and A. A. Vicente, in Biopolymer Nanostructures for Food Encapsulation Purposes, ed. S. M. Jafari, Academic Press, 2019, vol. 1, pp. 69–100 Search PubMed
.
- A. B. Nongonierma, C. Lamoureux and R. J. FitzGerald, Food Funct., 2018, 9, 407–416 RSC
.
- F. Hall, P. E. Johnson and A. Liceaga, Food Chem., 2018, 262, 39–47 CrossRef CAS PubMed
.
- L. De Marchi, A. Wangorsch and G. Zoccatelli, Curr. Allergy Asthma Rep., 2021, 21, 35 CrossRef CAS PubMed
.
- A. Cappelli, E. Cini, C. Lorini, N. Oliva and G. Bonaccorsi, Food Control, 2020, 108, 106877 CrossRef CAS
.
- W. He, K. He, F. Sun, L. Mu, S. Liao, Q. Li, J. Yi, Z. Liu and X. Wu, Food Chem., 2021, 343, 128461 CrossRef CAS PubMed
.
- L. De Marchi, F. Mainente, M. Leonardi, S. Scheurer, A. Wangorsch, V. Mahler, R. Pilolli, D. Sorio and G. Zoccatelli, Food Chem., 2021, 359, 129878 CrossRef CAS PubMed
.
- S. van Broekhoven, S. Bastiaan-Net, N. W. De Jong and H. J. Wichers, Food Chem., 2016, 196, 1075–1083 CrossRef CAS PubMed
.
- A. van Huis, B. A. Rumpold, H. J. van der Fels-Klerx and J. K. Tomberlin, Journal of Insects as Food and Feed, 2021, 7, 935–948 Search PubMed
.
-
European Food Safety Authority (EFSA), Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283, 2021, vol. 19 Search PubMed
.
- N. F. and F. A. (NDA) EFSA Panel on Nutrition, D. Turck, T. Bohn, J. Castenmiller, S. De Henauw, K. I. Hirsch-Ernst, A. Maciuk, I. Mangelsdorf, H. J. McArdle, A. Naska, C. Pelaez, K. Pentieva, A. Siani, F. Thies, S. Tsabouri, M. Vinceti, F. Cubadda, T. Frenzel, M. Heinonen, R. Marchelli, M. Neuhäuser-Berthold, M. Poulsen, M. Prieto Maradona, J. R. Schlatter, H. van Loveren, E. Ververis and H. K. Knutsen, EFSA J., 2022, 20, e07325 Search PubMed
.
-
G. Precup, E. Ververis, D. Azzollini, F. Rivero-Pino, P. Zakidou and A. Germini, in Novel Foods and Edible Insects in the European Union, Springer International Publishing, 2022, pp. 123–146 Search PubMed
.
- F. Cymbaluk, Vamos todos comer insetos?, https://www.uol/noticias/especiais/insetos-comestiveis.htm#vamos-todos-comer-insetos, accessed 26 October 2023.
- A. Lähteenmäki-Uutela, S. B. Marimuthu and N. Meijer, Journal of Insects as Food and Feed, 2021, 7, 849–856 Search PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fb00097d |
| This journal is © The Royal Society of Chemistry 2024 |