Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recovery of anthocyanins from Eugenia spp. fruit peels: a comparison between heat- and ultrasound-assisted extraction†
Bianca R.
Albuquerque
 abc,
José
Pinela
abc,
José
Pinela
 *ab,
Carla
Pereira
*ab,
Carla
Pereira
 ab,
Filipa
Mandim
ab,
Filipa
Mandim
 ab,
Sandrina
Heleno
ab,
Sandrina
Heleno
 ab,
M. Beatriz P. P.
Oliveira
ab,
M. Beatriz P. P.
Oliveira
 c and
Lillian
Barros
c and
Lillian
Barros
 ab
ab
aCentro de Investigação de Montanha (CIMO), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal. E-mail: jpinela@ipb.pt
bLaboratório Associado para a Sustentabilidade e Tecnologia em Regiões de Montanha (SusTEC), Instituto Politécnico de Bragança, Campus de Santa Apolónia, 5300-253 Bragança, Portugal
cREQUIMTE/LAQV, Department of Chemical Sciences, Faculty of Pharmacy, University of Porto, Rua Jorge Viterbo Ferreira 228, 4050-313 Porto, Portugal
First published on 30th October 2023
Abstract
Natural colorants have gained increased popularity among consumers and food producers due to their reputation as safer and healthier alternatives to commonly used artificial analogues. These natural pigments can be obtained from by-products resulting from food processing, such as the fruit peels of the Brazilian species Eugenia brasiliensis and Eugenia involucrata, thus contributing to the valorisation and circularity of these undervalued raw materials. Therefore, since these fruit peels present anthocyanin concentrations that justify their exploitation, this study aimed to optimize and compare the recovery of these pigments from these plant by-products using heat- and ultrasound-assisted extraction (HAE and UAE, respectively) methods. For process optimization, a central composite rotatable design coupled with response surface methodology was implemented, considering time, ethanol/water ratio, and temperature (for HAE) or ultrasonic power (for UAE) as relevant independent variables. While UAE resulted in higher extraction yields (40–42%, w/w), HAE led to higher anthocyanin contents (18 mg g−1 from E. involucrata and 323 mg g−1 from E. brasiliensis). Furthermore, the HAE global optimum involved only 2 min of processing. Both theoretical models were experimentally validated by applying the model-predicted extraction conditions, and the obtained anthocyanin-rich extracts were analysed for colour and in vitro bioactive properties. In general, the extraction method did not greatly affect the colour or the antimicrobial and cytotoxic activities of the extracts. However, only E. brasiliensis extracts showed cytotoxicity on human tumour cell lines, which also stood out for their antioxidant activity, possibly due to the higher anthocyanin content. Thus, Eugenia spp. fruit peels could be an alternative renewable source of natural food colourants with bioactive properties. Nonetheless, since E. brasiliensis extracts displayed moderate toxicity towards normal cells, the toxicity threshold should be further investigated to ensure the safe exploitation of this raw material as a possible source of natural food colourants.
Sustainability spotlightEugenia brasiliensis and Eugenia involucrata fruit peels, by-products of industrial fruit processing, were explored as sustainable sources of natural anthocyanin-based food colorants. The study compared two extraction methods to determine yield and efficiency. Ultrasound-assisted extraction demonstrated higher extract yields, while heat-assisted extraction produced extracts with higher anthocyanin content in a shorter processing time. The extracts displayed antioxidant activity and cytotoxicity against tumor cells, especially E. brasiliensis extract. This research promotes the transformation of fruit peels into bioactive colorants, reducing waste and advancing sustainable technologies. It aligns with UN SDGs 12 (responsible consumption and production), 9 (industry and innovation), 3 (health and well-being), 2 (zero hunger), and 13 (climate action) by supporting resource efficiency, healthier food choices, and climate-friendly alternatives. |
1. Introduction
The use of natural colorants has become increasingly popular over the last few decades as they are regarded as safer and healthier options than the widely used artificial colorants. As a result, many food and beverage manufacturing companies have started looking for natural colorants to replace some artificial additives in their products.1–3 Among the natural pigments widespread in nature, anthocyanins play a crucial role as food colorants because they are responsible for a wide range of colours (pink, red, purple, blue, and black) in fruits, flowers, leaves, etc. depending on the pH. In addition to their colouring capacity, anthocyanins are known for their health-promoting effects provided by their antioxidant, anti-inflammatory, and antitumor properties. They are also thought to help improve heart health and cognitive function and reduce the risk of obesity when included in a regular diet.4–6 Since anthocyanins are considered safe for human consumption, they are regulated as a natural additive by the Codex Alimentarius Commission and have the European Union E code E163. Thus, food and beverage manufacturers can use anthocyanins to colour foodstuffs as long as they meet the established specifications.3,4Among the most common sources of anthocyanins, red-purple berries such as grapes, blueberries, blackberries, cranberries, and purple corn stand out.7,8 These pigments can also be found in grape pomace and winery by-products, among other plant waste from the agri-food sector.7 Fortunately, there is a growing trend towards the recovery of anthocyanins from food waste and by-products, as well as the use of green solvents and more efficient and energy-saving technologies. These future-oriented approaches promote the sustainable production of natural colorants in a scenario of resource-use efficiency and circularity.7,9 However, there are no specific guidelines to standardize the extraction method to be used in the production of extracts/fractions of target compounds. This is because the extraction conditions are highly dependent on the intrinsic nature of the raw material to be upcycled, as well as the extraction method and solvent type to be used. Different plant materials may require a different processing temperature, time, ultrasonic power, pressure, solvent type and proportion, or other process intensification factors (or independent variables) to produce suitable extraction yields. The optimum processing conditions are those that optimize the balance between factors combined in the experimental design, thus maximizing the target compound yield. In addition to the process efficiency and final product quality, the associated costs and environmental impact can also be minimized.10,11
Fruit by-products from Myrtaceae species, such as Eugenia brasiliensis Lam. and E. involucrata DC., two Brazilian berries commonly known as “grumixama” and “Cereja-do-Rio-Grande”, respectively, can be interesting sources of natural colorants. Some previous research has shown that high concentrations of anthocyanins can be found in the fruit peels of E. brasiliensis12–15 and E. involucrate.16 Therefore, this study aimed to determine the best extraction conditions to recover anthocyanins from the peels of these two fruits, which are discarded after processing. For this, heat-assisted extraction (HAE) and ultrasound-assisted extraction (UAE) methods were investigated by combining three relevant independent variables of each method in a central composite rotatable design (CCRD) coupled with response surface methodology (RSM) for process optimization. In addition to the experimental validation of the predictive models, the colour and bioactivity of anthocyanin-rich extracts obtained under optimized conditions were evaluated using the CIELAB colour space and in vitro bioassays, respectively.
2. Materials and methods
2.1. Plant material
Eugenia brasiliensis and Eugenia involucrata biowaste from industrial manufacturing of frozen pulp was supplied by a local producer from Paraibuna, São Paulo, southeast Brazil, in December 2020. For both samples, the peel was manually separated from the seeds, frozen at −20 °C and lyophilized (LyoQuest -55 Plus, Azbil Telstar) until complete dryness. The obtained dry peel samples were reduced to a fine powder and stored in the dark at −20 °C until analysis.2.2. Anthocyanin extraction optimization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 688x − 42
688x − 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 802; r2 = 0.9969); limits of detection and quantification of 0.59 μg mL−1 and 1.81 μg mL−1, respectively. The total anthocyanin content (TAC) used as a response variable for process optimization resulted from the sum of all quantified compounds (Tables S1 and S2†).
802; r2 = 0.9969); limits of detection and quantification of 0.59 μg mL−1 and 1.81 μg mL−1, respectively. The total anthocyanin content (TAC) used as a response variable for process optimization resulted from the sum of all quantified compounds (Tables S1 and S2†).
2.3. Experimental validation of models and colour and bioactivity assessment of the anthocyanin-rich extracts
2.4. Statistical analysis
Colour and bioactivity analyses were performed in triplicate and the results were shown as mean ± standard deviation. Statistical differences between two samples (extracts) were determined by a Student's t-test (α = 95%) using R software, version 4.0.3. For antioxidant activity, significant differences (p < 0.05) between three samples (extracts and positive control) were evaluated by an analysis of variance (ANOVA) and discriminated by a Tukey's honestly significant difference (HSD) test.3. Results and discussion
3.1. Experimental data for process optimization
The results obtained with the HAE and UAE methods under the 20-run CCRD for the two Eugenia species are shown in Tables 1–4. For the fruit peel of E. brasiliensis (Tables 1 and 2), the extraction yield obtained by HAE ranged from 24.42 to 40.08% (w/w), while the UAE yielded from 12.82 to 40.01% (w/w). The highest HAE yield was achieved with run 12, which used the highest tested temperature, whereas with UAE, the highest response value was obtained with run 2, which employed a long processing time and low power and ethanol concentrations. Furthermore, the ethanol percentage induced distinct effects depending on the applied extraction method. With HAE, the lowest amount of extract was obtained at the lowest ethanol percentage (run 13); conversely, for UAE, the lowest yield was reached at the highest ethanol percentage (run 14). Still, the second worst HAE yield was observed with run 14.| Run | Experimental domain (natural and coded values) | Experimental and model-predictive responses | |||||
|---|---|---|---|---|---|---|---|
| Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | ||||||
| t (min) | T (°C) | S (% ethanol) | Experimental | Predicted | Experimental | Predicted | |
| a t: time; T: temperature; S: solvent; TAC: total anthocyanin content; E: extract. | |||||||
| 1 | 20 (−1) | 34 (−1) | 20 (−1) | 30.61 ± 0.49 | 29.98 | 139.42 ± 2.40 | 113.19 |
| 2 | 72 (1) | 34 (−1) | 20 (−1) | 29.83 ± 0.05 | 29.98 | 169.16 ± 0.86 | 183.37 |
| 3 | 20 (−1) | 76 (1) | 20 (−1) | 33.83 ± 0.50 | 33.53 | 153.14 ± 3.96 | 167.45 |
| 4 | 72 (1) | 76 (1) | 20 (−1) | 32.73 ± 0.03 | 33.53 | 59.41 ± 5.71 | 81.36 |
| 5 | 20 (−1) | 34 (−1) | 80 (1) | 34.03 ± 0.38 | 33.53 | 218.74 ± 5.93 | 208.29 |
| 6 | 72 (1) | 34 (−1) | 80 (1) | 36.16 ± 0.60 | 33.53 | 297.78 ± 10.43 | 278.47 |
| 7 | 20 (−1) | 76 (1) | 80 (1) | 37.7 ± 3.37 | 36.90 | 285.79 ± 4.96 | 262.55 |
| 8 | 72 (1) | 76 (1) | 80 (1) | 36.74 ± 0.01 | 36.90 | 179.76 ± 0.78 | 176.46 |
| 9 | 2 (−1.68) | 55 (0) | 50 (0) | 34.37 ± 1.43 | 37.03 | 212.35 ± 1.08 | 216.78 |
| 10 | 90 (1.68) | 55 (0) | 50 (0) | 37.65 ± 0.51 | 37.03 | 236.55 ± 0.42 | 203.41 |
| 11 | 46 (0) | 20 (−1.68) | 50 (0) | 32.43 ± 0.34 | 34.05 | 190.02 ± 0.54 | 230.17 |
| 12 | 46 (0) | 90 (1.68) | 50 (0) | 40.08 ± 0.25 | 40.02 | 178.14 ± 0.37 | 190.02 |
| 13 | 46 (0) | 55 (0) | 0 (−1.68) | 24.42 ± 0.33 | 24.04 | 72.99 ± 0.37 | 56.01 |
| 14 | 46 (0) | 55 (0) | 100 (1.68) | 28.23 ± 0.44 | 29.71 | 186.22 ± 0.10 | 215.95 |
| 15 | 46 (0) | 55 (0) | 50 (0) | 37.24 ± 0.66 | 37.03 | 204.87 ± 0.59 | 210.10 |
| 16 | 46 (0) | 55 (0) | 50 (0) | 37.43 ± 0.30 | 37.03 | 190.08 ± 4.72 | 210.10 |
| 17 | 46 (0) | 55 (0) | 50 (0) | 34.35 ± 1.25 | 37.03 | 224.87 ± 5.78 | 210.10 |
| 18 | 46 (0) | 55 (0) | 50 (0) | 36.01 ± 0.97 | 37.03 | 223.78 ± 4.82 | 210.10 |
| 19 | 46 (0) | 55 (0) | 50 (0) | 35.87 ± 1.85 | 37.03 | 214.79 ± 1.74 | 210.10 |
| 20 | 46 (0) | 55 (0) | 50 (0) | 36.02 ± 1.25 | 37.03 | 202.21 ± 9.05 | 210.10 |
| Run | Experimental domain (natural and coded values) | Experimental and model-predictive responses | |||||
|---|---|---|---|---|---|---|---|
| Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | ||||||
| t (min) | P (W) | S (% ethanol) | Experimental | Predicted | Experimental | Predicted | |
| a t: time; P: power; S: solvent; TAC: total anthocyanin content; E: extract. | |||||||
| 1 | 11 (−1) | 105 (−1) | 20 (−1) | 19.16 ± 0.25 | 19.79 | 75.92 ± 1.87 | 79.40 |
| 2 | 36 (1) | 105 (−1) | 20 (−1) | 40.01 ± 0.83 | 38.77 | 50.68 ± 0.36 | 79.40 |
| 3 | 11 (−1) | 400 (1) | 20 (−1) | 19.82 ± 0.30 | 19.73 | 190.39 ± 4.82 | 211.27 |
| 4 | 36 (1) | 400 (1) | 20 (−1) | 36.04 ± 1.05 | 38.70 | 230.63 ± 3.52 | 211.27 |
| 5 | 11 (−1) | 105 (−1) | 80 (1) | 12.82 ± 0.34 | 11.18 | 190.36 ± 2.68 | 182.63 |
| 6 | 36 (1) | 105 (−1) | 80 (1) | 13.35 ± 0.54 | 12.67 | 189.35 ± 2.13 | 182.63 |
| 7 | 11 (−1) | 400 (1) | 80 (1) | 30.64 ± 1.06 | 31.10 | 230.03 ± 2.07 | 200.78 |
| 8 | 36 (1) | 400 (1) | 80 (1) | 32.19 ± 1.09 | 32.59 | 216.65 ± 1.03 | 200.78 |
| 9 | 2 (−1.68) | 253 (0) | 50 (0) | 21.09 ± 0.46 | 21.52 | 235.08 ± 11.53 | 237.59 |
| 10 | 45 (1.68) | 253 (0) | 50 (0) | 39.34 ± 0.27 | 38.72 | 223.55 ± 0.27 | 237.59 |
| 11 | 25 (0) | 5 (−1.68) | 50 (0) | 18.03 ± 0.24 | 19.38 | 138.04 ± 14.25 | 124.37 |
| 12 | 25 (0) | 500 (1.68) | 50 (0) | 38.51 ± 0.03 | 36.53 | 227.72 ± 0.55 | 250.53 |
| 13 | 25 (0) | 253 (0) | 0 (−1.68) | 27.32 ± 0.39 | 27.32 | 76.54 ± 2.57 | 53.38 |
| 14 | 25 (0) | 253 (0) | 100 (1.68) | 12.91 ± 0.75 | 13.84 | 99.04 ± 0.86 | 131.36 |
| 15 | 25 (0) | 253 (0) | 50 (0) | 36.45 ± 0.23 | 35.05 | 269.84 ± 9.33 | 237.59 |
| 16 | 25 (0) | 253 (0) | 50 (0) | 35.6 ± 0.55 | 35.05 | 227.85 ± 9.73 | 237.59 |
| 17 | 25 (0) | 253 (0) | 50 (0) | 36.78 ± 0.48 | 35.05 | 253.96 ± 0.44 | 237.59 |
| 18 | 25 (0) | 253 (0) | 50 (0) | 34.71 ± 0.67 | 35.05 | 233.75 ± 3.84 | 237.59 |
| 19 | 25 (0) | 253 (0) | 50 (0) | 35.98 ± 0.19 | 35.05 | 230.48 ± 0.28 | 237.59 |
| 20 | 25 (0) | 253 (0) | 50 (0) | 30.77 ± 0.63 | 35.05 | 225.6 ± 2.16 | 237.59 |
Regarding the HAE of anthocyanins from E. brasiliensis fruit peel, the results presented in Table 1 show that there was a high relationship between the processing conditions and the recovery rate of these pigments, since TAC ranged from 59.41 to 297.78 mg per g E. The lowest process efficiency was observed with run 4, with the combination of long extraction time, high temperature, and low ethanol percentage. On the other hand, the best recovery was obtained with run 6, which involved long time, low temperature, and a high ethanol percentage. For UAE (Table 2), the lowest TAC (50.68 mg per g E) was reached with run 2, which combined a low power and ethanol percentage and a longer extraction time. The best response value (269.84 mg per g E) was obtained with the centre point of the design (run 15).
Based on the data in Table 3, the extraction yields obtained from E. involucrate fruit peel using HAE ranged from 12.26% to 35.53% (w/w), values that were achieved with the axial points 14, which had the highest ethanol percentage, and 10, where the longest extraction time was applied. In Table 4, a greater variance was observed with UAE, as the results ranged from 9.31 to 38.28% (w/w). In both methods, the higher percentage of ethanol seemed to negatively affect the extraction yield. On the other hand, ethanol may be more selective than water in some cases, as other compounds naturally present in the plant material, such as free sugars, proteins, and other hydrosoluble compounds, may not be extracted as effectively with this organic solvent.10,28
| Run | Experimental domain (natural and coded values) | Experimental and model-predictive responses | |||||
|---|---|---|---|---|---|---|---|
| Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | ||||||
| t (min) | T (°C) | S (% ethanol) | Experimental | Predicted | Experimental | Predicted | |
| a t: time; T: temperature; S: solvent; TAC: total anthocyanin content; E: extract. *Experimental points are not considered in the optimization process. | |||||||
| 1 | 20 (−1) | 34 (−1) | 20 (−1) | 27.08 ± 0.61 | 26.30 | 17.56 ± 0.09 | 19.66 |
| 2 | 72 (1) | 34 (−1) | 20 (−1) | 33.12 ± 0.67 | 33.26 | 12.32 ± 0.45 | 10.83 |
| 3 | 20 (−1) | 76 (1) | 20 (−1) | 27.84 ± 0.56 | 28.23 | 20.29 ± 0.13 | 18.53 |
| 4 | 72 (1) | 76 (1) | 20 (−1) | 28.31 ± 0.20 | 29.64 | 8.85 ± 0.26 | 9.70 |
| 5 | 20 (−1) | 34 (−1) | 80 (1) | 25.75 ± 0.18 | 25.46 | 4.20 ± 0.11 | 4.97 |
| 6 | 72 (1) | 34 (−1) | 80 (1) | 32.43 ± 0.21 | 32.43 | 3.22 ± 0.01 | 3.42 |
| 7 | 20 (−1) | 76 (1) | 80 (1) | 32.27 ± 1.38 | 33.18 | 4.16 ± 0.08 | 3.84 |
| 8 | 72 (1) | 76 (1) | 80 (1) | 33.42 ± 0.34 | 34.59 | 3.01 ± 0.05 | 2.28 |
| 9 | 2 (−1.68) | 55 (0) | 50 (0) | 27.05 ± 0.48 | 26.86 | 27.14 ± 1.46 | 26.62 |
| 10 | 90 (1.68) | 55 (0) | 50 (0) | 35.53 ± 0.43 | 33.91 | 17.24 ± 0.52 | 17.89 |
| 11 | 46 (0) | 20 (−1.68) | 50 (0) | 27.00 ± 0.29 | 28.66 | 18.73 ± 0.81 | 17.74 |
| 12 | 46 (0) | 90 (1.68) | 50 (0) | 33.26 ± 0.69 | 32.11 | 14.72 ± 1.49 | 15.84 |
| 13 | 46 (0) | 55 (0) | 0 (−1.68) | 28.23 ± 0.5 | 28.65 | 0.23 ± 0.01 | −1.12 |
| 14* | 46 (0) | 55 (0) | 100 (1.68) | 12.26 ± 0.55 | 19.27 | 27.38 ± 0.22 | 1.83 |
| 15 | 46 (0) | 55 (0) | 50 (0) | 29.42 ± 0.78 | 30.39 | 25.39 ± 0.29 | 25.39 |
| 16 | 46 (0) | 55 (0) | 50 (0) | 30.85 ± 0.61 | 30.39 | 25.77 ± 0.74 | 25.77 |
| 17 | 46 (0) | 55 (0) | 50 (0) | 30.93 ± 0.58 | 30.39 | 23.91 ± 0.64 | 23.91 |
| 18 | 46 (0) | 55 (0) | 50 (0) | 32.19 ± 0.28 | 30.39 | 24.09 ± 0.50 | 24.09 |
| 19 | 46 (0) | 55 (0) | 50 (0) | 31.35 ± 1.02 | 30.39 | 23.60 ± 1.36 | 23.60 |
| 20 | 46 (0) | 55 (0) | 50 (0) | 29.57 ± 0.86 | 30.39 | 24.68 ± 1.22 | 24.68 |
| Run | Experimental domain (natural and coded values) | Experimental and predictive responses | |||||
|---|---|---|---|---|---|---|---|
| Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | ||||||
| t (min) | P (W) | S (% ethanol) | Experimental | Predicted | Experimental | Predicted | |
| a t: time; P: power; S: solvent; TAC: total anthocyanin content; E: extract. *Experimental points are not considered in the optimization process. | |||||||
| 1 | 11 (−1) | 105 (−1) | 20 (−1) | 31.31 ± 0.47 | 29.57 | 6.14 ± 0.59 | 8.65 |
| 2 | 36 (1) | 105 (−1) | 20 (−1) | 34.93 ± 0.32 | 34.65 | 4.89 ± 0.32 | 5.57 |
| 3 | 11 (−1) | 400 (1) | 20 (−1) | 34.44 ± 0.91 | 32.5 | 12.3 ± 0.16 | 13.30 |
| 4 | 36 (1) | 400 (1) | 20 (−1) | 38.28 ± 0.46 | 37.58 | 5.91 ± 0.40 | 10.21 |
| 5 | 11 (−1) | 105 (−1) | 80 (1) | 27.88 ± 0.64 | 26.22 | 1.11 ± 0.01 | 2.82 |
| 6 | 36 (1) | 105 (−1) | 80 (1) | 32.14 ± 0.98 | 31.30 | 1.31 ± 0.05 | −0.26 |
| 7 | 11 (−1) | 400 (1) | 80 (1) | 29.58 ± 0.64 | 29.15 | 6.19 ± 0.03 | 7.46 |
| 8 | 36 (1) | 400 (1) | 80 (1) | 37.18 ± 0.61 | 34.23 | 3.63 ± 0.07 | 4.38 |
| 9 | 2 (−1.68) | 253 (0) | 50 (0) | 22.46 ± 0.98 | 22.58 | 17.33 ± 0.20 | 17.33 |
| 10 | 45 (1.68) | 253 (0) | 50 (0) | 31.6 ± 0.32 | 31.13 | 10.76 ± 0.24 | 12.14 |
| 11 | 25 (0) | 5 (−1.68) | 50 (0) | 28.53 ± 0.98 | 29.94 | 8.96 ± 0.85 | 10.83 |
| 12 | 25 (0) | 500 (1.68) | 50 (0) | 32.56 ± 0.49 | 34.87 | 19.16 ± 0.66 | 18.64 |
| 13 | 25 (0) | 253 (0) | 0 (−1.68) | 36.89 ± 0.95 | 38.39 | 0.15 ± 0.01 | −3.61 |
| 14* | 25 (0) | 253 (0) | 100 (1.68) | 9.31 ± 0.15 | 19.44 | 4.02 ± 0.16 | −0.15 |
| 15 | 25 (0) | 253 (0) | 50 (0) | 28.2 ± 0.98 | 26.85 | 13.27 ± 2.44 | 14.47 |
| 16 | 25 (0) | 253 (0) | 50 (0) | 24.68 ± 0.37 | 26.85 | 17.87 ± 0.98 | 14.47 |
| 17 | 25 (0) | 253 (0) | 50 (0) | 25.73 ± 0.82 | 26.85 | 17.84 ± 1.53 | 14.47 |
| 18 | 25 (0) | 253 (0) | 50 (0) | 23.15 ± 0.78 | 26.85 | 18.35 ± 0.11 | 14.47 |
| 19 | 25 (0) | 253 (0) | 50 (0) | 26.01 ± 0.61 | 26.85 | 16.05 ± 0.79 | 14.47 |
| 20 | 25 (0) | 253 (0) | 50 (0) | 29.91 ± 0.96 | 26.85 | 14.67 ± 0.85 | 14.47 |
The recovery of anthocyanins from the E. involucrata fruit peel varied greatly depending on the experimental conditions (Tables 3 and 4). With the HAE runs, the lowest TAC recovery (0.23 mg per g E) was achieved with run 13, which used only water as solvent. On the other hand, the maximum value (27.38 mg per g E) was reached using 100% ethanol. Regarding the UAE responses, the combination of medium time and ultrasonic power with 0% ethanol (run 13) was also the least efficient (0.15 mg per g E), while the combination of a medium time and ethanol percentage with the highest power applied allowed maximum recovery (19.16 mg per g E) with this non-conventional extraction method. Anthocyanins are typically recognized as water-soluble compounds. However, some studies have demonstrated that it is possible to enhance the solubility of anthocyanins by utilizing aqueous organic solvent mixtures within a specific composition range. This approach can yield better results compared to using pure water as the solvent.29
3.2. Mathematical models and statistical fitting information
According to the statistical parameters shown in Table 3, all models were significant (p < 0.001) and did not show a significant lack-of-fit (p ≥ 0.0702). In addition, the values of R2 and R2 adj were equal to or greater than 0.8800 and 0.8137, respectively. The models' adequate precision, or the accuracy with which the model can predict the outcome of a given data set, was greater than 4, showing that the models can accurately predict the outcome in most cases.30 Analysing the regression coefficients of the models built to describe the anthocyanin extraction process from the E. brasiliensis fruit peel (Table 3), it was possible to conclude that the amount of crude extract recovered (Y1) by HAE does not depend on the processing time (p > 0.05), while temperature and ethanol percentage had positive linear effects (b2 = 1.8 and b3 = 1.7) on the response, and solvent also had a marked quadratic effect (b33 = 3.6). On the other hand, the UAE yield was affected by processing time, mainly through linear effects (b1 = 5.1), as well as by the tested temperature and ethanol percentage. For temperature, the linear effect (b2 = 5.0) predominated over the quadratic one (b22 = −2.7), while the quadratic term (b2 = −5.3) marked the effects of the percentage, which interacted with the other two variables (b13 = −3.4 and b23 = 4.0), thus justifying the use of RSM. Regarding TAC (Y2), only the solvent has significant linear and quadratic effects in the HAE process (Table 5). Furthermore, the time × temperature interaction (b12 = 39.7) was highly significant, which made it necessary to keep the non-significant linear terms of these two independent variables in the regression model to ensure its hierarchy. A different trend was observed for the UAE process, in which time was not significant (p > 0.05) but power and solvent affected TAC through linear, quadratic, and interaction effects.| E. brasiliensis fruit peel | E. involucrata fruit peel | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HAE | UAE | HAE | UAE | ||||||
| Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | Y 1: yield (%, w/w) | Y 2: TAC (mg per g E) | ||
| a TAC: total anthocyanin content; E: extract; ns: not significant. Significance levels determined by ANOVA: *p-value <0.05; ** p-value <0.001. | |||||||||
| Regression coefficients | |||||||||
| Intercept | b 0 | 36.2 ± 1.0 | 210.1 ± 14.2 | 35.0 ± 2.0** | 237.6 ± 16.1** | 30.5 ± 0.7 | 24.6 ± 1 | 26.7 ± 1.5 | 14.74 ± 1.1 |
| Linear effects | b 1 | ns | −4.0 ± 13.5ns | 5.1 ± 1.32** | ns | 1.7 ± 0.8** | −2.6 ± 0.9** | 2.5 ± 1.2** | −1.5 ± 1.5* |
| b 2 | 1.8 ± 0.9** | −11.9 ± 13.5ns | 5.0 ± 1.3** | 37.5 ± 12.6** | 1.0 ± 0.8* | −0.57 ± 0.91ns | 1.5 ± 1.2* | 2.3 ± 1.5* | |
| b 3 | 1.7 ± 0.9** | −47.5 ± 13.4** | −3.1 ± 1.3** | 23.2 ± 12.6** | 1.1 ± 0.9* | −5.5 ± 1.1** | −1.0 ± 1.5ns | −2.1 ± 1.8* | |
| Quadratic effects | b 11 | ns | ns | −1.7 ± 1.3* | ns | ns | −0.81 ± 0.78* | ns | ns |
| b 22 | ns | ns | −2.7 ± 1.3** | −17.7 ± 12.2** | ns | −2.7 ± 0.9** | 1.8 ± 1.1* | ns | |
| b 33 | −3.6 ± 0.9** | 26.2 ± 13.0** | −5.3 ± 1.3** | −51.3 ± 12.2** | ns | −11.9 ± 1.1** | 3.8 ± 1.5** | −8.2 ± 1.9** | |
| Interaction effects | b 12 | ns | −39.7 ± 17.6** | ns | ns | −1.4 ± 1.1* | ns | ns | ns |
| b 13 | ns | ns | −3.4 ± 1.7** | ns | 1.4 ± 1.1* | 1.8 ± 1.2* | ns | ns | |
| b 23 | ns | ns | 4.0 ± 1.1** | −28.4 ± 16.4** | ns | ns | ns | ns | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||
| Statistical parameters | |||||||||
| Model (p-value) | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |
| Lack-of-fit (p-value) | 0.2009 | 0.0702 | 0.5084 | 0.1680 | 0.3759 | 0.1072 | 0.8062 | 0.5609 | |
| R 2 | 0.9241 | 0.8800 | 0.9655 | 0.9128 | 0.8668 | 0.9879 | 0.8654 | 0.8830 | |
| R 2 adj | 0.9099 | 0.8372 | 0.9404 | 0.8890 | 0.8156 | 0.9803 | 0.8137 | 0.8496 | |
| Adequate precision | 30.2935 | 17.4970 | 18.1128 | 16.6016 | 13.7753 | 31.3101 | 14.7267 | 17.1098 | |
Regarding E. involucrata, the mathematical models that translate the HAE effects on the extraction yield (Y1) showed a positive linear effect for all independent variables and a positive time × ethanol percentage interaction (b13 = 1.4) (Table 5). On the other hand, the temperature × ethanol percentage interaction (b12 = −1.4) negatively impacted the process. The model translating the extraction yield obtained by UAE was composed of significant effects of time (b1 = 2.5) and solvent (b33 = 3.8). The ultrasonic power also influenced the process positively with its linear (b2 = 1.5) and quadratic (b22 = 1.8) effects. The TACs (Y2) obtained using both extraction methods were negatively impacted by time (b1 = −1.5) and ethanol percentage (b3 = −2.1 and b33 = −8.2). In addition, while the HAE temperature had a negative effect (b22 = −2.7) on TAC, the UAE power had a positive effect (b2 = 2.3).
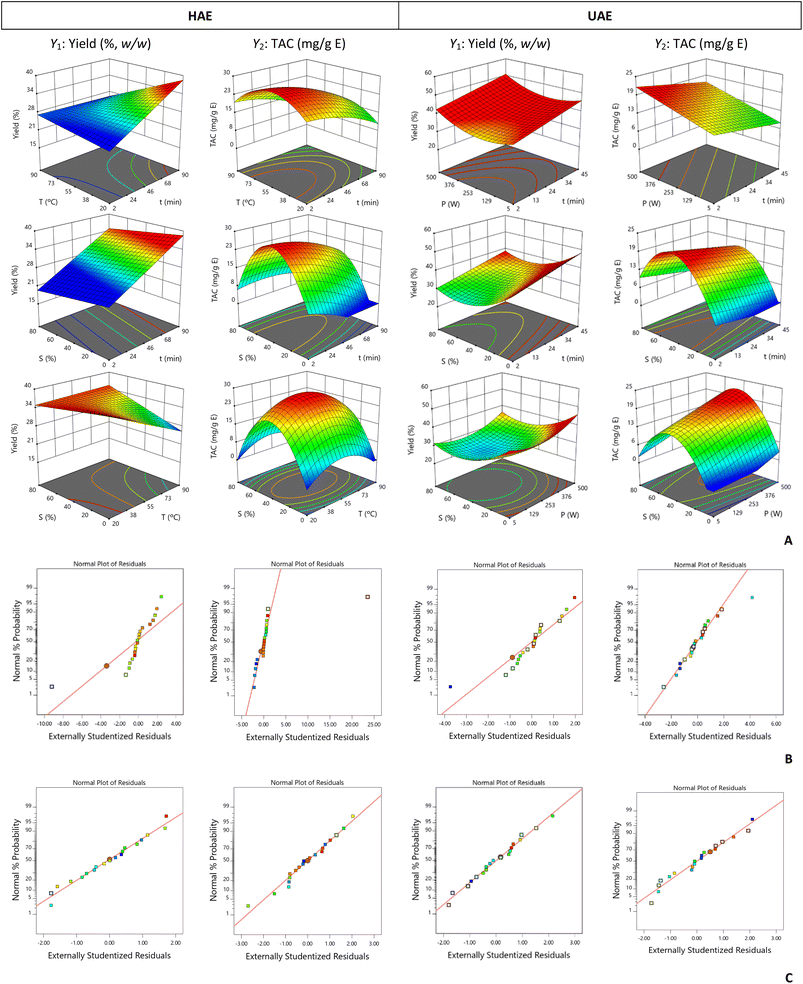
The experimental data obtained with the 20 runs of the CCRD matrix were fitted to a second-order mathematical model. Any noise that might have been associated with these data was assessed using standardized residual plots. As shown in the plots in Fig. 1B, the experimental values obtained for E. brasiliensis followed a normal distribution. However, for E. involucrata fruit peel, an outlier was detected in the four plots generated (Fig. 2B), which correspond to run 14 involving 100% ethanol. Thus, to reduce noise during the optimization process, the responses corresponding to run 14 were not considered for the models' construction (Fig. 2C). The model coefficients obtained after removing this outlier and considering only the significant terms (p < 0.05) and those necessary for the hierarchy are presented in Table 5.
3.3. Individual optimal conditions for extraction
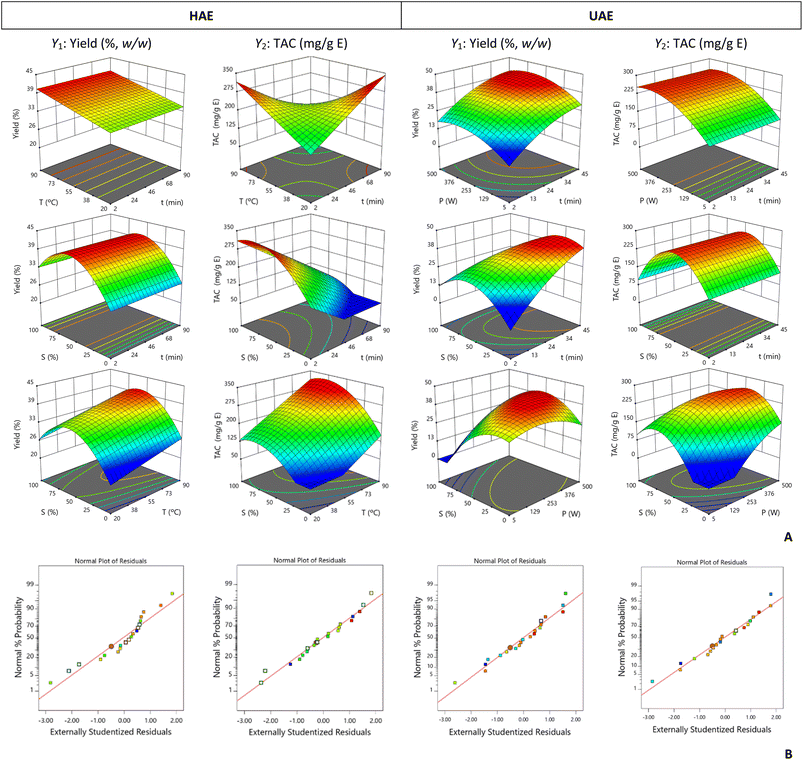
The combined effects of the independent variables extraction yield (Y1) and TAC (Y2) obtained by each extraction method for E. brasiliensis and E. involucrata fruit peels are visually illustrated in Fig. 1A and 2A, respectively.For E. brasiliensis fruit peel submitted to HAE, the highest extract yield was obtained with a temperature above 55 °C and an average ethanol percentage. The variation in processing time did not interfere with the process efficiency (Fig. 1A). For TAC, an adequate balance between processing time and temperature was necessary, since in the evaluated design space, combinations of high temperature and short extraction time or low temperature and a long extraction time could benefit the process. Regarding the solvent, the highest recovery rate of anthocyanins occurred at medium–high ethanol percentages. In general, better response values could be obtained with the combination of low time, high temperature, and a medium ethanol percentage. For the UAE process, the yield was maximized by applying a long extraction time (>34 min). Due to its interaction with solvent, a low ethanol percentage was needed, and due to its interaction with power, more than 253 W were required. The recovery of anthocyanins from E. brasiliensis fruit peel by this technique was independent of the extraction time, and greater responses were achieved with increasing power and a medium ethanol percentage. The optimal conditions for each response variable and each extraction method are presented below:
For HAE:
- Y1: t = 2.2 min, T = 90 °C, and S = 57% ethanol (v/v), yielding 40 ± 1% (w/w);
- Y2: t = 2 min, T = 90 °C, and S = 76% ethanol (v/v), yielding 329 ± 59 mg TAC per g E;
For UAE:
- Y1: t = 43 min, P = 338 W, and S = 35% ethanol (v/v), yielding 42 ± 2% (w/w);
- Y2: t = 2 min, P = 400 W, and S = 48% ethanol (v/v), yielding 257 ± 20 mg TAC per g E.
Although an extract yield of 42% (w/w) was achieved with the UAE method, a yield very close to this one was obtained by HAE with a much shorter processing time. The HAE method also allowed a higher TAC to be obtained. Some reactions intrinsic to the extraction process may have affected this result, e.g., the mass transfer may have been promoted by the increase in temperature and/or the cavitation phenomenon caused by ultrasonic waves could have potentiated anthocyanin degradation.31
According to the 3D plots shown in Fig. 2A when using E. involucrata fruit peel as an alternative source of anthocyanins, the extraction yield (Y1) obtained by HAE could benefit from a long extraction time combined with low temperature and a high percentage of ethanol. For UAE, the optimal conditions of time and solvent were different from those observed for HAE. In this case, Y1 benefited from increased time and a low ethanol percentage and extreme power values. For both extraction methods, the recovery of anthocyanins required a short processing time and a medium ethanol percentage. In HAE, increasing the temperature to mean values facilitated the recovery of these compounds; however, higher temperatures will have caused their degradation, as well as longer processing. In UAE, high power enabled better TAC recovery. The optimal conditions determined for obtaining TAC from the E. involucrata fruit peel are presented below:
For HAE:
- Y1: t = 90 min, T = 20 °C, and S = 0% ethanol (v/v), yielding 38 ± 4% (w/w);
- Y2: t = 7 min, T = 53 °C, and S = 40% ethanol (v/v), yielding 28 ± 2 mg TAC per g E.
For UAE:
- Y1: t = 13 min, P = 478 W, and S = 0% ethanol (v/v), yielding 44 ± 2% (w/w);
- Y2: t = 2 min, P = 500 W, and S = 45% ethanol (v/v), yielding 21 ± 2 mg TAC per g E.
As observed for the E. brasiliensis fruit peel, UAE was more effective in obtaining a higher extraction yield. However, HAE allowed the production of an extract more concentrated in anthocyanins. These findings show that UAE results in a greater quantity of different solutes recovered from the samples. This may be due to the ultrasonic wave capacity to increase the pressure inside plant cells, leading to their rupture and subsequent release of solutes into the extraction solvent. Therefore, this intensification factor promotes the breakdown of plant cell walls, thus improving mass transfer.32,33 However, when dealing with polyphenols, more specifically anthocyanins, the use of high ultrasonic power can increase the extraction yield up to a certain level and then lead to their degradation after that. This is partly due to the production of a significant number of OH radicals through the known cavitation effects, which trigger oxidation mechanisms.29,32,33
Although anthocyanins are thermosensitive compounds, the optimal conditions determined for HAE indicate that higher temperatures may be associated with better recovery of these compounds. This phenomenon has also been reported for the extraction of anthocyanins from various other natural sources. This may be due to a balance between time and temperature, as high temperatures increase the mass transfer rate when combined with the minimum extraction time required to not cause degradation during processing. Moreover, an increase in temperature can lead to the inactivation of enzymes, such as polyphenol oxidase and peroxidase, which are naturally present in plant tissues and can potentially degrade anthocyanins. Hence, an adequate balance between these two process factors is pivotal to achieve successful anthocyanin recovery.34
3.4. Global optimal conditions for extraction
In this study, both response variables were optimized simultaneously to obtain the highest possible amounts of both extract weight and anthocyanins. For E. brasiliensis fruit peel, the global optimum conditions were t = 2 min, T = 90 °C, and S = 63% by HAE, allowing a yield of 40 ± 1% (w/w) and 323 ± 59 mg TAC per g E; and t = 40 min, P = 394 W, and S = 45% by UAE, leading to a yield of 42 ± 2% (w/w) and 257 ± 22 mg TAC per g E. For E. involucrata fruit peel, the optimum HAE conditions were t = 2 min, T = 86 °C, and S = 51%, and yielded 32 ± 1% (w/w) and 18 ± 1 mg TAC per g E; and for UAE they were t = 45 min, P = 500 W, and S = 38%, which led to a yield of 40 ± 2% and 16 ± 3 mg TAC per g E.To determine which extraction technique could be more effective to produce Eugenia spp. extracts with higher content of anthocyanins, the two methods optimized in this study were compared. For both raw materials, the optimal conditions determined for UAE led to very close extraction yields (Y1), although HAE involved less time than UAE (2 vs. 40 min), which needs to be considered as an important factor when choosing the extraction method. Regarding the total anthocyanin content (Y2), HAE showed greater efficiency than UAE, allowing a more concentrated anthocyanin extract to be obtained in a short processing time. These results are comparable to those previously described for the recovery of anthocyanins from Arbutus unedo fruit, as the optimized HAE method also involved a shorter processing time compared to UAE.35 However, in a study carried out using red cabbage (Brassica oleracea L.) as a source of anthocyanins, the performance of a conventional solid–liquid extraction method was compared with that of UAE in an ultrasonic bath (37 Hz). Both methods involved the same time (5–75 min), temperature (40–80 °C), and ethanol concentration (5–75%) and the optimal conditions were comparable (75 min at 40 °C with 42.4% ethanol). However, the anthocyanin concentration obtained when applying ultrasound was slightly higher, possibly due to the cavitation effect that may have facilitated mass transfer phenomena.36
Although this study demonstrated that HAE outperforms UAE in terms of efficiency, the method was based on sonication with a titanium probe and examined three extraction variables, but only one related to the ultrasound equipment (i.e., power). However, in addition to the type of equipment used (extraction with a probe or in an ultrasonic bath), other variables besides power can be controlled depending on the equipment, such as frequency, amplitude, and pulsation cycle.33,36 In the same way, other independent variables such as the solid/liquid ratio, stirring rate, and pH value, among others, could also be included in HAE and UAE experimental designs.
3.5. Experimental validation of the predictive models
To evaluate the predictive capacity of the theoretical models, the global HAE and UAE conditions were experimentally tested to obtain anthocyanin-rich extracts, which were tested for their bioactive properties in vitro. The post-analysis verification carried out using Design-Expert software showed that the experimental results obtained for extraction yield and TAC were in good agreement with the model-predicted values (α = 0.05). For E. brasiliensis fruit peel, the extract weight (Y1) produced by the HAE and UAE processes was 38 ± 1% and 37 ± 1%, respectively. These values were similarly close to the predicted values in both cases. Furthermore, UAE yielded 229 ± 2 mg of anthocyanin/g extract (Y2), while each gram of extract obtained by HAE contained 268 ± 1 mg of anthocyanins. In the case of E. involucrata fruit peel, the extraction yields (Y1) produced by HAE and UAE were 38% and 33 ± 1%, respectively. In both cases, the result was reasonably close to the predicted one. Furthermore, each gram of extract obtained by HAE contained 20 ± 1 mg of anthocyanins (Y2), while 16.8 ± 0.5 mg was achieved by UAE. Both experimental responses fit within the confidence interval of the model-predicted values.3.6. Colour of the anthocyanin-rich extracts
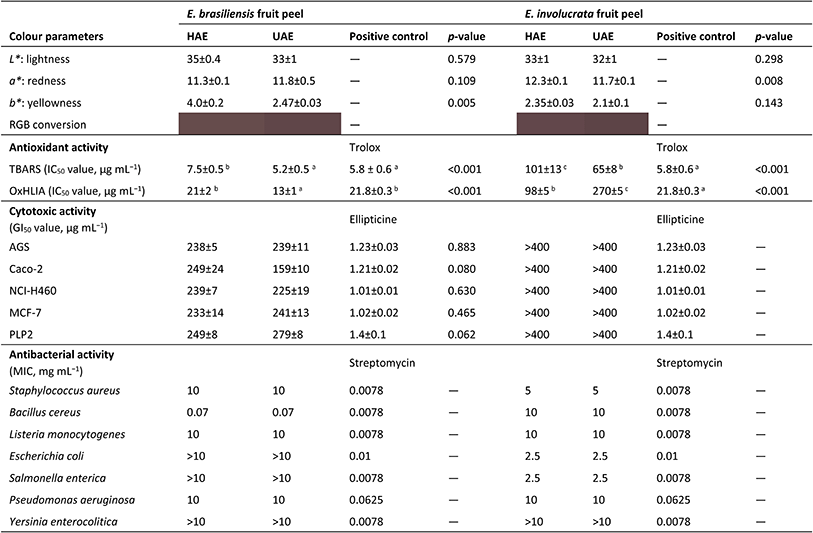
Table 6 shows the colour measured in the lyophilized fruit peel extracts of E. brasiliensis and E. involucrata obtained under global optimum conditions of HAE and UAE. According to the t-student test, the colour of E. brasiliensis fruit peel extracts differed (p < 0.01) only regarding the blueness-yellowness (b*), as the extract obtained by UAE showed less tendency to yellow. Both extracts were characterized by moderate to low lightness (L*), tending towards redness (a*). The colour of E. involucrata fruit peel extracts was similar. However, the a* value differed statistically (p < 0.005) and the HAE-produced extract was redder. These findings differ from those obtained for the colour of purple corn pericarp extracts. The increase in temperature up to 55 °C caused the formation of brown or colourless pigments, weakening the intensity of the red colour of the extracts.32 However, the low pH used in our extractions may have positively affected the anthocyanin stability, preventing its breakdown and consequent colour loss.37| a For OxHLIA, the IC50 values were calculated for a 60 min Δt. For colour parameters and cytotoxic activity, a Student's t-test was applied to assess statistical differences (p < 0.05) between samples (extracts), while for antioxidant activity, statistical differences (p < 0.05) between samples (extract and trolox) were assessed by an ANOVA and are indicated by a different letter. MIC: minimal inhibitory concentration. |
|---|
|
3.7. Bioactivity of the anthocyanin-rich extracts
The bioactive potential of the anthocyanin-rich extracts was evaluated in vitro and the results are presented in Table 6. For the E. brasiliensis fruit peel extracts, those obtained by UAE showed higher antioxidant activity than those obtained by HAE in both TBARS and OxHLIA assays. Interestingly, the UAE obtained extract showed better activity to inhibit oxidative haemolysis than the positive control, trolox, and its potential to inhibit lipid peroxidation was comparable to that of this synthetic antioxidant. This result showed that the highest concentration of anthocyanins is not entirely related to the highest antioxidative activity. In turn, the results achieved for the anthocyanin-rich extracts of E. involucrata fruit peel showed some disparity, with the TBARS assay highlighting the UAE-produced extract with nearly twice the antioxidant activity obtained by HAE. As shown in Table 6, all extracts showed an anti-haemolytic effect, mainly the E. brasiliensis fruit peel extract obtained by UAE. Regarding E. involucrata fruit peel extracts, the one obtained by HAE showed a better anti-haemolytic activity. It is important to note that the anthocyanin concentration alone does not determine the antioxidant activity of the extracts, as other compounds not analysed in this study may contribute to the observed differences. In fact, previous studies suggest that E. brasiliensis fruits contain other bioactive compounds such as phenolic acids, ellagic acid, quercetin derivatives, and β-cryptoxanthin, which display antioxidant activity.14,15 The E. involucrata fruits also present different bioactive molecules, such as gallic acid, catechin, proanthocyanidins, myricetin, quercetin, and kaempferol derivatives.38,39Regarding the cytotoxic potential of the samples, only E. brasiliensis fruit peel extracts were able to inhibit the growth of tumour cells at the tested concentrations (Table 6). In addition, there were no significant differences (p > 0.05) between the anthocyanin-rich extracts obtained by HAE and UAE in the tested cell lines. Eugenia brasiliensis fruit peel extracts also had a certain level of toxicity toward normal PLP2 cells. However, the GI50 values obtained for this primary cell culture were higher than those for tumour cell lines (except for the HAE extract in Caco-2, which had the same result). However, the threshold of toxicity should be further investigated using additional methodologies. On the other hand, E. involucrata fruit peel extracts did not show cytotoxicity on normal cells at the maximum concentration tested. Moreover, no extract was able to inhibit the formation of nitric oxide at the tested concentrations.
The antibacterial activity of the anthocyanin-rich extracts was evaluated against eight foodborne microorganisms and the results are shown in Table 6. The extraction method did not affect the antimicrobial activity. On the other hand, while E. brasiliensis fruit peel extracts showed greater inhibitory activity against B. cereus (with a MIC of 0.07 mg mL−1), E. involucrata fruit peel extracts were more active against E. coli and S. enterica (at 2.5 mg mL−1) and S. aureus (at 5 mg mL−1). Therefore, in addition to their colouring capacity, these anthocyanin-rich extracts may have a preservative effect when added to food products.
4. Conclusion
The anthocyanin recovery from E. brasiliensis and E. involucrata fruit peels by HAE and UAE was optimized using RSM. The UAE resulted in higher extract yields from both raw materials. However, HAE allowed a greater recovery of anthocyanins. The results obtained for the global optimum extraction conditions showed that HAE can be more efficient than UAE, as the produced extracts were more concentrated in anthocyanins (323 vs. 257 mg TAC per g E for E. brasiliensis and 18 vs. 16 mg/TAC g E for E. involucrata) and could be obtained more quickly (2 vs. 40 min for E. brasiliensis and 2 vs. 45 min for E. involucrata). Although these results suggest that HAE can lead to better recovery of anthocyanins from Eugenia fruit peels, it may be interesting to test other extraction technologies and evaluate their techno-economic viability at the industrial level.The tested extraction methods did not greatly affect the colour of the extract (although E. involucrata HAE extracts were slightly redder and E. brasiliensis HAE extracts were slightly yellowish) or the antimicrobial and cytotoxic activities of the extracts. However, only E. brasiliensis extracts showed cytotoxic effects on the human tumour cell lines selected for this study. These also stood out for their in vitro antioxidant activity, possibly due to the higher anthocyanin content. These results highlighted the underexplored potential of Eugenia spp. peel as an alternative source of natural colorants with bioactive properties. However, future studies are important to highlight the need for additional research focused on the extract stability when exposed to different factors (e.g., moisture, temperature, and radiation), as well as when incorporated into certain food matrices.
Author contributions
Bianca R. Albuquerque: methodology, investigation, formal analysis, experimental design, data analysis, writing – original draft. José Pinela: experimental design, methodology, data analysis, validation, writing – review & editing. Carla Pereira: methodology, data analysis; Filipa Mandim: formal analysis, data analysis. Sandrina Heleno: methodology, data analysis, validation. M. Beatriz P. P. Oliveira: conceptualization, supervision, writing – review & editing. Lillian Barros: conceptualization, supervision, project administration, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES (PIDDAC) to CIMO (UIDB/00690/2020 and UIDP/00690/2020) and SusTEC (LA/P/0007/2020). National funding by FCT, P. I., through the scientific employment program-contract for the contracts of J. Pinela (CEECIND/01011/2018), C. Pereira (CEEC Institutional), S. Heleno (CEEC Institutional), and L. Barros (CEEC Institutional), and the research grants of B. R. Albuquerque (SFRH/BD/136370/2018 and COVID/BD/152908/2022) and F. Mandim (SFRH/BD/146614/2019) are acknowledged.References
- S. Ghosh, T. Sarkar, R. Chakraborty, M. A. Shariati and J. Simal-Gandara, Crit. Rev. Food Sci. Nutr., 2022, 6, 1–45 CrossRef PubMed.
- S. Jurić, M. Jurić, Ż. Król-Kilińska, K. Vlahoviček-Kahlina, M. Vinceković, V. Dragović-Uzelac and F. Donsì, Food Rev. Int., 2022, 38, 1735–1790 CrossRef.
- A. Rodríguez-Mena, L. A. Ochoa-Martínez, S. M. González-Herrera, O. M. Rutiaga-Quiñones, R. F. González-Laredo and B. Olmedilla-Alonso, Food Chem., 2023, 398, 133908 CrossRef PubMed.
- J. S. Câmara, M. Locatelli, J. A. M. Pereira, H. Oliveira, M. Arlorio, I. Fernandes, R. Perestrelo, V. Freitas and M. Bordiga, Nutrients, 2022, 14, 5133 CrossRef.
- P. Li, D. Feng, D. Yang, X. Li, J. Sun, G. Wang, L. Tian, X. Jiang and W. Bai, Trends Food Sci. Technol., 2021, 117, 205–217 CrossRef CAS.
- Q. Qi, M. Chu, X. Yu, Y. Xie, Y. Li, Y. Du, X. Liu, Z. Zhang, J. Shi and N. Yan, Food Rev. Int., 2022, 1–29 Search PubMed.
- P. R. Venskutonis, in Valorization of Fruit Processing By-Products, Elsevier, 2020, pp. 95–125 Search PubMed.
- I. D. Boateng, A. Mustapha, C. R. Daubert, L. Kuehnel, R. Kumar, S. Flint-Garcia, J. Agliata, Q. Li, C. Wan and P. Somavat, Food Bioprocess Technol., 2023, 16, 2668–2691 CrossRef CAS.
- C. Campalani, E. Amadio, S. Zanini, S. Dall'Acqua, M. Panozzo, S. Ferrari, G. De Nadai, S. Francescato, M. Selva and A. Perosa, J. CO2 Util., 2020, 41, 101259 CrossRef CAS.
- S. Farooq, M. A. Shah, M. W. Siddiqui, B. N. Dar, S. A. Mir and A. Ali, J. Food Meas. Charact., 2020, 14, 3508–3519 CrossRef.
- E. Gil-Martín, T. Forbes-Hernández, A. Romero, D. Cianciosi, F. Giampieri and M. Battino, Food Chem., 2022, 378, 131918 CrossRef PubMed.
- G. Flores, K. Dastmalchi, S. Paulino, K. Whalen, A. J. Dabo, K. A. Reynertson, R. F. Foronjy, J. M. D'Armiento and E. J. Kennelly, Food Chem., 2012, 134, 1256–1262 CrossRef CAS.
- A. P. D. F. Machado, A. L. D. Pereira, G. F. Barbero and J. Martínez, Food Chem., 2017, 231, 1–10 CrossRef CAS.
- L. S. M. Nascimento, M. C. P. A. Santiago, E. M. M. Oliveira, R. G. Borguini, E. C. O. Braga, V. C. Martins, S. Pacheco, M. C. Souza and R. L. O. Gogoy, Nutr. Food Technol., 2017, 3, 3 Search PubMed.
- L. d. L. Teixeira, F. C. Bertoldi, F. M. Lajolo and N. M. A. Hassimotto, J. Agric. Food Chem., 2015, 63, 5417–5427 CrossRef CAS PubMed.
- J. R. Girardelo, E. L. Munari, J. C. S. Dallorsoleta, G. Cechinel, A. L. F. Goetten, L. R. Sales, F. H. Reginatto, V. C. Chaves, F. A. Smaniotto, S. Somacal, T. Emanuelli, J. C. Benech, C. Soldi, E. Winter and G. M. M. Conterato, Food Res. Int., 2020, 137, 109615 CrossRef CAS.
- B. R. Albuquerque, J. Pinela, L. Barros, M. B. P. P. Oliveira and I. C. F. R. Ferreira, Food Chem., 2020, 316, 126364 CrossRef CAS PubMed.
- B. R. Albuquerque, J. Pinela, M. I. Dias, C. Pereira, J. Petrović, M. Soković, R. C. Calhelha, M. B. P. P. Oliveira, I. C. F. R. Ferreira and L. Barros, Food Res. Int., 2023, 165, 112574 CrossRef CAS PubMed.
- R. Rocha, J. Pinela, R. M. V. Abreu, M. Añibarro-Ortega, T. C. S. P. Pires, A. L. Saldanha, M. J. Alves, A. Nogueira, I. C. F. R. Ferreira and L. Barros, Processes, 2020, 8, 1447 CrossRef CAS.
- G. A. Gonçalves, A. A. Soares, R. C. G. Correa, L. Barros, C. W. I. Haminiuk, R. M. Peralta, I. C. F. R. Ferreira and A. Bracht, J. Funct. Foods, 2017, 33, 408–418 CrossRef.
- B. R. Albuquerque, T. C. Finimundy, J. Pinela, T. Pires, F. Mandim, J. Vaz, R. Corrêa, B. Oliveira and L. Barros, Food Funct., 2023, 14, 3994–4003 RSC.
- B. R. Albuquerque, T. C. Finimundy, J. Pinela, T. C. S. P. Pires, F. Mandim, J. Vaz, R. C. G. Corrêa, M. B. P. P. Oliveira and L. Barros, Food Funct., 2023, 14, 3994–4005 RSC.
- J. Kainthola, A. S. Kalamdhad and V. V. Goud, Renewable Energy, 2020, 149, 1352–1359 CrossRef CAS.
- R. C. G. Corrêa, A. H. P. de Souza, R. C. Calhelha, L. Barros, J. Glamoclija, M. Sokovic, R. M. Peralta, A. Bracht and I. C. F. R. Ferreira, Food Funct., 2015, 6, 2155–2164 RSC.
- L. Lockowandt, J. Pinela, C. L. Roriz, C. Pereira, R. M. V. Abreu, R. C. Calhelha, M. J. Alves, L. Barros, M. Bredol and I. C. F. R. Ferreira, Ind. Crops Prod., 2019, 128, 496–503 CrossRef CAS.
- F. Mandim, M. I. Dias, J. Pinela, P. Barracosa, M. Ivanov, D. Stojković, M. Soković, C. Santos-Buelga, L. Barros and I. C. F. R. Ferreira, Food Chem., 2020, 323, 126838 CrossRef CAS PubMed.
- T. C. S. P. Pires, M. I. Dias, L. Barros, M. J. Alves, M. B. P. P. Oliveira, C. Santos-Buelga and I. C. F. R. Ferreira, Food Chem., 2018, 240, 701–706 CrossRef CAS.
- T. Lefebvre, E. Destandau and E. Lesellier, J. Chromatogr. A, 2021, 1635, 461770 CrossRef CAS PubMed.
- A. G. Tarone, E. K. Silva, H. D. F. Q. Barros, C. B. B. Cazarin and M. R. M. Junior, Food Res. Int., 2021, 140, 110048 CrossRef PubMed.
- A. R. Silva, J. Pinela, P. A. García, I. C. F. R. Ferreira and L. Barros, Sep. Purif. Technol., 2021, 276, 119358 CrossRef CAS.
- M. Bonfigli, E. Godoy, M. A. Reinheimer and N. J. Scenna, J. Food Eng., 2017, 207, 56–72 CrossRef CAS.
- I. D. Boateng, R. Kumar, C. R. Daubert, S. Flint-Garcia, A. Mustapha, L. Kuehnel, J. Agliata, Q. Li, C. Wan and P. Somavat, Ultrason. Sonochem., 2023, 95, 106418 CrossRef CAS.
- C. S. Dzah, Y. Duan, H. Zhang, C. Wen, J. Zhang, G. Chen and H. Ma, Food Biosci., 2020, 35, 100547 CrossRef CAS.
- G. Ijod, F. N. Musa, F. Anwar, N. Suleiman, N. M. Adzahan and E. M. Azman, J. Food Process. Preserv., 2022, 46, e17255 CAS.
- C. J. López, C. Caleja, M. A. Prieto, M. F. Barreiro, L. Barros and I. C. F. R. Ferreira, Food Chem., 2018, 264, 81–91 CrossRef.
- A. Demirdöven, K. Özdoğan and K. Erdoğan-Tokatlı, J. Food Biochem., 2015, 39, 491–500 CrossRef.
- I. D. Boateng, A. Mustapha, L. Kuehnel, C. R. Daubert, R. Kumar, J. Agliata, S. Flint-Garcia, C. Wan and P. Somavat, Ind. Crops Prod., 2023, 200, 116871 CrossRef CAS.
- G. Mannino, G. Serio, A. Asteggiano, N. Gatti, C. M. Bertea, C. Medana and C. Gentile, Antioxidants, 2022, 11, 1769 CrossRef CAS.
- A. E. Nicácio, E. M. Rotta, J. S. Boeing, É. O. Barizão, E. Kimura, J. V. Visentainer and L. Maldaner, Food Anal. Methods, 2017, 10, 2718–2728 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fb00115f |
| This journal is © The Royal Society of Chemistry 2024 |