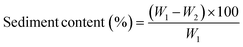Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Storage stability, nutritional profiling and consumer acceptability of a milk-sorghum-based breakfast smoothie
Rekha
Rani†
 *a,
Latha
Sabikhi
b and
Sathish Kumar
M. H.
c
*a,
Latha
Sabikhi
b and
Sathish Kumar
M. H.
c
aDepartment of Dairy Technology, ICAR-National Dairy Research Institute, Karnal, Haryana, India. E-mail: verma.rekha@gmail.com; Tel: +91-8005321813
bPrincipal Scientist and Head, Dairy Technology Division, ICAR-National Dairy Research Institute, Karnal, Haryana, India
cScientist, Dairy Technology Section, ICAR-National Dairy Research Institute, SRS, Adugodi, Hosur Road, PIN-560030, Bengaluru, Karnataka, India
First published on 11th March 2024
Abstract
This study was conducted to estimate the storage stability of a milk-sorghum-based functional breakfast smoothie (MSBS). The product was packed in 200 mL glass bottles and stored at 4 °C and 30 °C. The milk-sorghum-based smoothie was suitable for consumption for up to 75 days and 60 days at 4 °C and 30 °C, respectively; afterwards, the product lost its aroma and flavour. This product is nutritionally rich in dietary fibre (1.68%), calcium (560.46 ppm), iron (28.12 ppm), and vitamin A (679.3 IU/L); low in fat (0.65%) with a low calorific value (89.09 kcal per 100 g) and free of any synthetic preservatives and colour. The consumer acceptance study revealed that ∼88% consumers liked this product, 33% were willing to consume it daily and 89% were ready to purchase it at a price of Rs. 20 per 200 mL bottle. It is a convenient grab-and-go option for all age groups.
Sustainability spotlightBreakfast is the most important meal of the day and thus people who skip breakfast are disproportionately likely to have problems with concentration, metabolism and weight. The aim of this study was to formulate a breakfast smoothie based on germinated sorghum flour together with a fruit (mango), vegetable (carrot), honey and milk sources to provide an adequate amount of minerals, vitamins and dietary fiber. Sorghum is the fifth leading crop in the world and milk is described as nature's nearly perfect food. This smoothie can serve as a good breakfast replacement for those who skip breakfast owing to the lack of time in the morning. It can be consumed easily and conveniently while on the go and will provide all essential nutrients in one's diet. |
1 Introduction
Cereal-based breakfast is a widely popular morning meal and is a source of nutrients and fibers for people of all age groups. In this case, sorghum has attracted more scientific attention than kodo (Paspalum scrobiculatum) and finger millet in terms of its protein content, structure and higher production volume.1 Sorghum is a potential sustainable crop in the context of food security and climatic change. Moreover, it is less susceptible to changes in the weather, thus sustaining high productivity while providing food security in the tropics and a source of nutrition for humans, animals, and industrial manufacturing. Temperature rise and changing precipitation patterns affect crop water needs, which reduce crop potential and yields, while increasing the cost of water availability across the agricultural landscape. Sorghum is a multipurpose crop that serves as both a major feed crop and a staple food for humans. It can adapt to a wide range of agronomic and environmental conditions, including salinity, limited precipitation and insufficient irrigation water supply.31 Horticultural produce, i.e. fruits and vegetables, are indispensable in the human diet. Fruit- and vegetable-based smoothies are alcohol-free beverages, which are generally prepared using fruits, vegetables or both without straining the blend, followed by mixing with crushed ice for immediate consumption. Dairy-based smoothies contain milk and dairy products, such as yoghurt and ice-cream, and have a consistency similar to milk shakes. Smoothies are exceptional and appropriate candidates that can encourage the everyday intake of vegetables, fruit and sometimes milk and milk products if added during their preparation.2 With the increasing awareness about healthy food habits and lifestyles, consumers are now switching to smoothies that contain fruit pulp, vegetables, milk, yoghurt and ice-cream instead of those that contain only fruit and fruit juice.3 As per a study conducted by the U.S. Department of Agriculture (USDA) in the period 2007–2010 on children aged 1–18 years, 60–93% children do not meet fruit and vegetable intake recommendations.29,30 It is evident from the current consumption trends that people are becoming increasingly interested in natural, nutrient-dense, and healthful foods. Generally, energy-containing drinks/beverages except soup are considered less satiating than foods, including their near equivalent semi-solid or solid foods.43 Companies that produce natural fruit and vegetable (F&V)-based drinks (smoothies and juices) have experienced rapid growth, given that these products are seen as a useful means of consuming F&V nutrients and bioactives and meeting the needs of even the pickiest natural product customers.4 Presently, in the market, various breakfast options are available such as MTR instant poha, upma, Quaker oats, but they require preparation, and other breakfast options such as in India samosa, kachori, bread, burgers, and buns, and in other countries, also unhealthy or high-calorie options lack nutrition, where juices and cold drinks are also available as instant ready-to-drink options. However, to date, a combination of milk, cereal, fruit, and vegetables, which is high in minerals and vitamins and low in fat and calories and a grab-and-go option is not available anywhere. Thus, the launch of this product (which is a combination of all the above-mentioned ingredients) in the market will provide a healthy, nutritious and convenient breakfast option and it can also be taken together with a meal. Currently, smoothies have been endorsed as a health-conscious beverage option for wellness. They can be consumed either as a main meal or as a wholesome snack between meals, depending on their serving size, leading to an increase in its market share in the beverage sector.Storage stability is very important from a marketing perspective for food products. It contributes to ensuring regulatory compliance by providing evidence of product stability and safety under specific conditions. However, the short shelf life of “natural” (untreated) beverages is mostly caused by microbial growth-related spoilage.36 Despite often being extremely acidic products (pH < 4.6), certain microbes that are resistant to acid can endure and proliferate. Mild heat treatment followed by refrigeration storage is preferred to obtain a longer shelf-life and maintain freshness. The heat treatment should be as minimum as possible to protect the sensory and nutritional quality of the product. To inactivate spoilage-causing enzymes and microorganisms in fruit purees and juices, temperatures in the range of 80 °C to 95 °C are employed for heat treatment at the commercial level.5 The link between storage studies and shelf life is crucial in understanding the quality and safety of various products, especially perishable foods. Shelf life refers to the duration for which a product remains safe to consume or meets its intended quality standards under specified storage conditions.
Rani et al.6 optimized a sorghum-incorporated milk-based smoothie using dairy and plant-based ingredients such as germinated sorghum flour, milk, carrot juice, mango pulp, sugar and pectin. Milk, as the dairy ingredient, is a calcium-enriched product and an important mineral for bone health. Sorghum (Sorghum bicolor (L.)) is a major cereal crop, which is grown in the tropical semi-arid regions of Asia and Africa, mainly due to its resistance to drought. Currently, sorghum ranks third among the major food grain crops of India, after wheat and rice. It is a reliable energy source, has complex carbohydrates, and is also rich in vitamins (riboflavin, niacin and pyridoxine), minerals (calcium, phosphorus, iron and zinc) and fiber. It can be an alternative food for people allergic to gluten and is a rich source of phytochemicals. Honey has a characteristic flavour and sweetness and is preferred over sugar and other sweeteners. Furthermore, it acts as an anti-microbial substance due to its low water activity (0.6). Carrots (Daucus carota L.) are a good source of β-carotene, the precursor of vitamin A. Thus, they are considered important for combating vitamin A deficiency diseases. Carotenoid colouring matter serves as a powerful antioxidant for safeguarding the body from free radicals, providing defense from oxidative damage to cells, and also stimulating the immune function. Cereals or millets and legumes are not edible in raw form and processing is required for denaturing their proteins, gelatinizing starch, and enhancing their sensory qualities. The most often used traditional and modern processing techniques include milling, soaking, malting, sprouting, fermentation, heating, roller drying and extrusion cooking. All these techniques are known to improve the palatability and digestibility of food, reduce anti-nutritional compounds and convert complex grain components into readily accessible simple compounds, which are easily digestible and nutritionally beneficial. Therefore, germinated and gelatinized flour was used in the present investigation.
The prospect of an increased market for the consumption of fresh-like fruit smoothies would require precise data related to their shelf life, which includes retaining the fresh-like fruit flavour and colour and preventing off flavor, phase separation and product spoilage due to microorganisms. Product behavior during storage at ambient temperature is important, particularly for tropical countries. Therefore, a shelf life study was conducted to assess the sensory, physico-chemical and microbial quality changes in the milk-sorghum-based smoothie (MSBS) throughout storage at 4 °C and 30 °C. This can give a clear idea about the shelf life of the product.
2 Materials and methods
2.1 Procurement of materials
Cow milk was obtained from the Experimental Dairy of National Dairy Research Institute (NDRI), Karnal, India and standardized to 8.5% solids-not-fat (SNF) and 3.0% fat by adding skim milk separated from the milk used for the experiment. Feed variety of sorghum grains (Sorghum bicolor PC-9), Totapuri variety of mango pulp, food-grade crystalline sugar, honey (Dabur Pvt. Ltd, 80% total solids), and carrot juice (Cargill India Pvt. Ltd, 70.8° Brix) were all bought from the local market in Karnal, India. High methoxy pectin (CPKelco) was procured from Huber India Ltd, Mumbai, India. Citric acid anhydrous (SD-Fine Chem. Ltd, Mumbai), tri-sodium citrate (HiMedia Pvt. Ltd, Mumbai) and alphonso mango flavour (International Flavours and Fragrances India Pvt. Ltd, Chennai) were used for the preparation of the product.2.2 Preparation of breakfast smoothie using sorghum
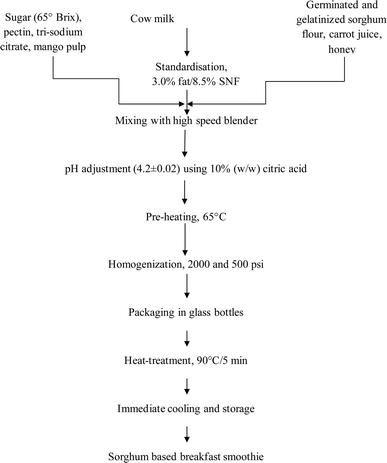
The breakfast smoothie was prepared by incorporating sorghum using the process shown in Fig. 1.2.3 Physico-chemical analysis of MSBS
MSBS was analysed for moisture, protein content and pH using the method described by FSSAI.7 The smoothie was also analysed for crude fat, ash and carbohydrates according to the method given by AOAC8 and for titratable acidity, the cheese method of acidity measurement was used for product estimation, with a minor change. In a 200 mL capacity conical flask, approximately 2 g of the product was weighed and 20 mL of distilled water (65 °C) was added after thorough shaking to achieve homogeneous mixing. Following the addition of 10 mL of sodium hydroxide (0.1 N) and 1 mL of phenolphthalein indicator (0.5%), a titration against 0.1 N HCl was performed while stirring continuously until the complete disappearance of the pink colour. The product acidity was calculated in terms of % lactic acid.The tyrosine value was assessed by determining the absorbance at 650 nm according to the method by Hull,9 free fatty acids according to Deeth and Fitz-Gerald10 and the browning index of the product was calculated by determining its optical density at 420 nm according to Ranganna.11 The water activity of the smoothie was measured using an AquaLab (Model Series 3 TE) provided by Decagon Devices, WA, USA at 25 °C, and the colour indices of the smoothie were examined using the reflectance spectroscopy technique using a reflectance metre, Colorflex® (Hunter lab, Reston, Virginia, USA). A phytic acid estimation kit (Megazyme International Ireland Limited12) was used to measure phytic acid and dietary fibre, using the Megazyme kit.13 The calcium and iron content of the smoothie were estimated using atomic absorption spectrophotometry according to AOAC.8
According to De-Vries and Silvera's14 method, reverse-phase HPLC was used to evaluate the vitamin A content in the smoothie, and the parameters used were as follows: mobile phase having acetonitrile, methanol and chloroform in the ratio of (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) with the flow rate of 1 mL min−1; PDA detector – λmax 325: max 325 nm; analytical column: Symmetry C18, 5 μm (internal pore size) (4.6 × 250 mm dimensions and particle size: 10 nm) and water column temperature: 40 °C. A 20 mL centrifuge tube was used and 2 mL of the product was taken after 1 mL of 2.5% ammonium hydroxide, 1 mL of aqueous potassium hydroxide (60%, w/v) and 100 μL of alcoholic pyrogallol (30%, w/v) were added. The mixture was flushed with ultrapure nitrogen gas before being saponified at 70 °C per 30 min in a water bath, and then cooled to room temperature using an ice bath, and after that vortexed.
4) with the flow rate of 1 mL min−1; PDA detector – λmax 325: max 325 nm; analytical column: Symmetry C18, 5 μm (internal pore size) (4.6 × 250 mm dimensions and particle size: 10 nm) and water column temperature: 40 °C. A 20 mL centrifuge tube was used and 2 mL of the product was taken after 1 mL of 2.5% ammonium hydroxide, 1 mL of aqueous potassium hydroxide (60%, w/v) and 100 μL of alcoholic pyrogallol (30%, w/v) were added. The mixture was flushed with ultrapure nitrogen gas before being saponified at 70 °C per 30 min in a water bath, and then cooled to room temperature using an ice bath, and after that vortexed.
2 mL hexane and a few drops of ethanol added to the above-mentioned mixture and the contents were vortexed. The mixture was centrifuged at 350 × G for 10 min. Using a pipette, the upper hexane layer was transferred to another centrifuge tube. All the extracts were transferred to centrifuge tubes after this extraction stage was carried out twice, each time with the addition of 2 mL of hexane. Under nitrogen, the obtained extracts were dried. Then, 1 mL mobile phase, was added, vortexed and collected in an Eppendorf tube and centrifuged at 430 × G for 10 min at 0 °C in a refrigerated centrifuge. Before use, the mobile phase was filtered using 0.22 m membrane filters and degassed. The sample was passed through a 0.22 μm syringe filter for filtration, and 20 μL of the filtrate was injected into an HPLC system after being deaerated with a vacuum pump and an ultrasonic cleaner. At a wavelength of 325 nm, the area of the vitamin A peak was recorded. The vitamin A content in the smoothie was calculated using a regular retinol solution.
Using a Viscostar Plus Viscometer having a TL-7 spindle and 1–1 system, the viscosity of the product was calculated at 20 °C and expressed as cP.
The sediment content in the milk-sorghum based smoothie was analyzed using the method reported by Rani et al.6 Briefly, 20 g of sample was placed in a centrifuge tube and centrifuged using a Hermle Refrigerated Benchtop Centrifuge at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 × G for 20 min at 20 °C. The sediment % was also calculated using the following formula:
700 × G for 20 min at 20 °C. The sediment % was also calculated using the following formula:
Using the centrifugation process, the whey separation in the product was identified.
The centrifugation method was used for milk-sorghum based smoothie whey separation. In a 15 mL graduated centrifuge tube, 10 mL of the sample was taken and centrifuged at 430 × G for 10 min. The separated whey was noted in mL and expressed as mL per 10 mL.
2.4 Sensory evaluation of MSBS
An expert panel of ten judges used a 9-point hedonic scale to assess the sensory attributes of the product, as reported by Stone et al.15 The panellists assessed the smoothie based on its flavour, consistency, sweetness, colour and appearance and overall acceptability.2.5 Microbial analysis of MSBS
The developed samples were assessed for yeast and mould count, total viable count and coliform count on zero day of sample using the procedure described by Indian Standards, IS: 7889.162.6 Storage study of MSBS
The samples of MSBS were analysed for relevant parameters at 15 days intervals from day 0 up to day 75 of storage. The physico-chemical parameters monitored during storage were sedimentation, wheying off, viscosity, pH, acidity, FFA, tyrosine value and browning index. The sensory evaluation of the product was done at a temperature of 20 °C. Microbiological analysis was carried out for total viable count TVC, coliforms and yeasts and molds.2.7 Statistical analysis
The statistical analysis was done using a “completely randomised design” with an equal number of observations using the average value of each parameter under study, which was acquired from duplicate samples of seven replications (two treatments) of the study.The statistical model of Steel and Torrie17 was used, as follows:
| Yij = μ + Ti + Eij |
2.8 Consumer study
A questionnaire was prepared to collect information regarding the age, gender, occupation and marital status of the consumers; employment status of their spouse, if married; necessity of a convenient breakfast option; readiness about consumption of a product that would fulfil nutritional requirement in a breakfast; consideration of the product as a breakfast option; frequency of consumption of the product (alternatively, weekly, twice in a week or occasionally); degree of liking of product (excellent, very good, good and fair) and willingness to purchase the product at given price.3 Results and discussion
3.1 Chemical composition and nutritional content of MSBS
The chemical composition, water activity and colour indices, i.e. yellowness (b*), lightness (L*) and redness (a*) of MSBS are shown in Table 1, while the nutritional parameters are presented in Table 3. A serving of the smoothie (325 mL) provides 15.09% and 9.75% of the daily requirement of total carbohydrate according to the US Food and Drug Administration (USFDA)32 and ICMR,33 respectively. It also provides approximately 50% iron, 14% calcium and 22% dietary fibre of daily requirement. A serving of MSBS also provides around 290 kcal (Table 2). It also meets the standards prescribed by the Food Safety and Standards Authority of India (FSSAI)-2011 under the ‘Proprietary food’ category. The carbohydrate content affects the viscosity, where increased carbohydrates in a formulation interact with the other ingredients, enhancing their thickening or gelling properties. The sorghum starch content increases the viscosity with an increase in temperature, forming a more viscous solution when dissolved in water, absorbing water, swelling and forming a gel-like structure and increasing the viscosity. This is because a gelatinized flour mixture was used in the preparation of the product. Vitamins can degrade over time due to various factors (exposure to light, oxygen, temperature, moisture and humidity, pH level, packaging material, chemical interactions, and storage period) and the degradation process can be influenced by the environmental conditions, storage methods, and exposure to certain elements. Vitamin C (ascorbic acid) and vitamin B2 (riboflavin) are sensitive to light, vitamins such as vitamin A, vitamin C and vitamin E are particularly susceptible to oxidation, and vitamins may interact with other ingredients in a product or with environmental factors, leading to chemical changes and degradation. Thus, to minimize the degradation of vitamins, it is essential to store supplements in a cool, dry place away from direct sunlight, moisture, and temperature extremes. For the analysis of sedimentation, the product was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 × G (high speed 10
700 × G (high speed 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm) for 20 min at 20 °C considering the transportation of the product for selling, otherwise during storage, visible sedimentation was not observed, and a similar case applies to the whey separation procedure. When we applied the whey separation process by storage for 24 h, no visible whey separation was observed. Furthermore, no visible browning was observed in the product during the entire storage period, and according to the browning index method, the values were very low.
000 rpm) for 20 min at 20 °C considering the transportation of the product for selling, otherwise during storage, visible sedimentation was not observed, and a similar case applies to the whey separation procedure. When we applied the whey separation process by storage for 24 h, no visible whey separation was observed. Furthermore, no visible browning was observed in the product during the entire storage period, and according to the browning index method, the values were very low.
| Parameter | Value |
|---|---|
| Total solids, % by mass | 23.46 ± 0.57 |
| Fat, % by mass | 0.65 ± 0.22 |
| Protein, % by mass | 0.87 ± 0.25 |
| Ash, % by mass | 0.31 ± 0.11 |
| Carbohydrate, % by mass | 13.93 ± 0.44 |
| Dietary fibre, % by mass | 1.68 ± 0.15 |
| Phytic acid, mg/100 g | 14.67 ± 1.80 |
| Water activity (aw) | 0.977 ± 0.002 |
| Lightness (L*) | 63.94 ± 1.01 |
| Redness (a*) | 11.99 ± 0.42 |
| Yellowness (b*) | 36.25 ± 0.65 |
| Parameter | Amount per servingb | % Daily valuec | Calories per servingd | Calories per 100 mL | |
|---|---|---|---|---|---|
| USFDA | ICMR | ||||
| a NP Not prescribed. *Daily values are based on 2000 kilocalorie diet for USFDA and 2730 kilocalorie for Indian RDA. b Average of triplicate analysis (mean ± SE). c % daily values are calculated as per USFDA (2013) and ICMR (2010) guidelines. d Calorie was calculated as per fat-9 kcal g−1, carbohydrates-4 kcal g−1, and protein-4 kcal g−1. | |||||
| Fat, g | 2.11 | 3.25 | 3.52 | 19.01 | 5.85 |
| Total carbohydrate, g | 45.27 | 15.09 | 9.75 | 181.09 | 55.72 |
| Dietary fibre, g | 5.46 | 21.84 | NP | ||
| Calcium, mg | 182.22 | 14.02 | 30.37 | ||
| Iron, mg | 9.13 | 50.73 | 51.72 | ||
| Vitamin A, IU | 220.77 | 7.36 | 12.27 | ||
| Total calories | 289.54 | 89.09 | |||
| Attribute | Temp/Days | 0 | 15 | 30 | 45 | 60 | 75 | Average | CD (0.05) |
|---|---|---|---|---|---|---|---|---|---|
| a a, b, and c indicate significant (P < 0.05) change at a particular temperature at 15 days interval, x and y in column indicate significant (P < 0.05) change in temperature during storage, p, q, r, and s in row indicate significant (P < 0.05) change in days at both temperatures. | |||||||||
| Sedimentation, % | 4 °C | 40.76 ± 0.59 | 40.71 ± 0.80 | 39.30 ± 0.79 | 38.58 ± 0.57 | 36.57 ± 0.66 | 34.70 ± 0.39 | 38.43x | T-0.81 |
| 30 °C | 40.76 ± 0.59 | 39.71 ± 0.37 | 37.45 ± 0.84 | 37.17 ± 0.40 | 36.05 ± 1.09 | 34.25 ± 0.90 | 37.56y | D-1.41 | |
| Average | 40.76p | 40.21p | 38.37q | 37.87q | 36.31r | 34.48s | TxD-NS | ||
| Wheying off, mL/10 mL | 4 °C | 1.27 ± 0.12a | 1.27 ± 0.09a | 1.53 ± 0.09a | 1.77 ± 0.09b | 2.07 ± 0.15c | 2.13 ± 0.09c | 1.67x | T-0.12 |
| 30 °C | 1.27 ± 0.12a | 1.53 ± 0.09a | 1.63 ± 0.09b | 1.87 ± 0.07b | 2.33 ± 0.09c | 2.87 ± 0.09d | 1.92y | D-0.20 | |
| Average | 1.27p | 1.40p | 1.58q | 1.82r | 2.20s | 2.50t | TxD-0.35 | ||
| Viscosity, cP | 4 °C | 410.50 ± 9.31a | 403.17 ± 9.88a | 402.53 ± 9.90a | 392.97 ± 9.24a | 376.80 ± 9.92a | 372.33 ± 10.02b | 393.05x | T-11.55 |
| 30 °C | 410.50 ± 9.31a | 374.17 ± 9.22b | 368.87 ± 9.59b | 354.57 ± 9.52b | 333.57 ± 10.78c | 292.50 ± 9.54d | 355.69y | D-20.01 | |
| Average | 410.50p | 388.67q | 385.70q | 373.77q | 355.18r | 332.42s | TxD-34.66 | ||
| pH | 4 °C | 4.22 ± 0.016a | 4.22 ± 0.016a | 4.21 ± 0.018a | 4.17 ± 0.019a | 4.04 ± 0.018b | 3.99 ± 0.020c | 4.140x | T-0.02 |
| 30 °C | 4.22 ± 0.016a | 4.21 ± 0.014a | 4.17 ± 0.020a | 4.09 ± 0.018b | 3.95 ± 0.020c | 3.84 ± 0.019c | 4.080y | D-0.04 | |
| Average | 4.220p | 4.215p | 4.190p | 4.130q | 3.995r | 3.915r | TxD-0.06 | ||
| Acidity, % LA | 4 °C | 0.262 ± 0.013 | 0.276 ± 0.012 | 0.289 ± 0.012 | 0.318 ± 0.014 | 0.349 ± 0.018 | 0.367 ± 0.011 | 0.310x | T-0.016 |
| 30 °C | 0.262 ± 0.013 | 0.288 ± 0.017 | 0.308 ± 0.012 | 0.340 ± 0.013 | 0.385 ± 0.014 | 0.407 ± 0.014 | 0.332y | D-0.028 | |
| Average | 0.262p | 0.282p | 0.298p | 0.329q | 0.367r | 0.387r | TxD-NS | ||
| FFA, μeq g−1 | 4 °C | 4.84 ± 0.18 | 5.05 ± 0.22 | 5.09 ± 0.10 | 5.37 ± 0.34 | 6.04 ± 0.09 | 6.22 ± 0.40 | 5.44x | T-0.29 |
| 30 °C | 4.81 ± 0.18 | 5.20 ± 0.17 | 5.39 ± 0.35 | 6.38 ± 0.21 | 6.36 ± 0.21 | 6.73 ± 0.34 | 5.81y | D-0.51 | |
| Average | 4.83p | 5.12p | 5.24p | 5.88q | 6.20q | 6.48r | TxD-NS | ||
| Tyrosine value, μg g−1 | 4 °C | 28.69 ± 1.91 | 38.14 ± 1.13 | 45.46 ± 1.86 | 46.33 ± 1.98 | 46.82 ± 0.95 | 47.29 ± 0.98 | 42.12x | T-1.59 |
| 30 °C | 28.69 ± 1.91 | 41.25 ± 0.80 | 49.11 ± 0.77 | 50.15 ± 0.68 | 52.36 ± 1.10 | 52.36 ± 0.84 | 45.65y | D-2.76 | |
| Average | 28.69p | 39.70q | 47.29r | 48.24r | 49.59r | 49.82r | TxD-NS | ||
| Browning index | 4 °C | 0.025 ± 0.005 | 0.028 ± 0.005 | 0.042 ± 0.007 | 0.055 ± 0.005 | 0.075 ± 0.007 | 0.082 ± 0.006 | 0.051x | T-0.007 |
| 30 °C | 0.025 ± 0.005 | 0.033 ± 0.006 | 0.057 ± 0.005 | 0.066 ± 0.007 | 0.085 ± 0.006 | 0.087 ± 0.006 | 0.059y | D-0.012 | |
| Average | 0.025p | 0.030p | 0.049q | 0.060q | 0.080r | 0.084r | TxD-NS | ||
3.2 Storage studies
The smoothie was heated at 90 °C per 5 min to extend its shelf life. This high-heat treatment can kill pathogens and control mesophilic spoilage drastically, though not completely.3 The extent of physico-chemical changes depend on the temperature and storage duration. The higher the temperature, the faster the deterioration in the quality of the product and the shorter the shelf life. Subjecting high-moisture foods to thermal processing ensures their extended shelf life. The next section discusses the changes in the physico-chemical, sensory and microbiological properties of the product.Sedimentation is the result of the settling of insoluble sorghum flour particles at the bottom of the product. The temperature (T) and days (D) of storage significantly (P < 0.05) affected sedimentation, while their interaction (TxD) did not influence it. Sedimentation was higher during the initial period of storage and it was reduced gradually with progress of storage. High methoxy (HM) pectin is frequently used to stabilize acidified milk beverages (AMD), preventing the sedimentation issue caused by the flocculation of milk proteins, and therefore enhancing and preserving the desirable qualities of dairy products.35 In AMD, the pH is reduced by adding a chemical acidulant such as citric acid or incorporation of lactic acid bacteria. A stabilizing agent must be added to milk dispersions to prevent their phase separation and wheying-off because casein (CSN) micelles agglomerate at pH < 4.6.40 The pectin molecules in acidic milk beverages can interact with CSNs via calcium ions to stop them from aggregating, sedimenting, and ultimately separating serum through steric and ionic stabilization.39 If HM pectin contains blocks of non-esterified anhydrogalacturonic acids, it can thicken or form soft gels in the presence of calcium ions.37 The adsorption of pectin occurs at or below pH 5.0 in diluted acidified milk systems through electrostatic interactions. Below pH 5.0, pectin can stop CSN particles from aggregating and sedimenting through steric and electrostatic stabilization. In steric stabilization, a portion of pectin adsorbs on the surface of the casein aggregates and another portion extends from it as loops and hanging tails. It is considered that the entropy of the pectin chain sticking together contributes to the stability by the introduction of steric repulsion between the casein aggregates. It has also been found that a multilayer of pectin surrounds the casein aggregates, with the inner layer adhering to the aggregates more firmly than the outer layer. Tromp et al.41 demonstrated that high pectin concentrations are required to create a weak gel network and maintain long-term stability by keeping CSN aggregates from sedimentation. Both steric and electrostatic interactions have the potential to prevent the suspended CSN particles from colliding. Another theory also suggests that the presence of a weak pectin gel network prevents casein aggregates from sedimentation and results in long-term stability. The proposed weak gel is thought to be strong enough to defy gravity and prevent the suspended casein particles from colliding during the serum phase.42 The decline in sedimentation may be due to the breakdown of starch to simpler compounds such as mono-, di- and oligosaccharides, which are soluble in water and reduce sedimentation.18 The lower sedimentation at a higher temperature can be attributed to the higher activity of enzymes responsible for starch breakdown. Abedi et al.19 noted a similar pattern in mango and jackfruit smoothies. Gad38 used pectin in a yoghurt drink to modify its viscosity, physical properties and sedimentation. The viscosity changed due to the stabilizer concentration and adhesion between the particles. A concentration of 0.35% pectin and mild homogenisation of approx. 50 bar were found best to establish equilibrium between the tendency to sediment and repulsion, stabilizing the casein particles with the minimum sediment % and high viscosity.
Wheying-off gives an unattractive appearance by the separation of translucent liquid on the surface of the product. Wheying-off in the smoothie was significantly (P < 0.05) increased by the temperature (T) and days (D) of storage as well as their interaction effect. The first significant increase was observed on the 30th day of storage. However, this was not evident during the sensory evaluation given that it was not visible by the naked eye but was recorded after centrifugation at 430 × G for 10 min. Less wheying-off was observed due to the intense heat-treatment (90 °C per 5 min) applied to the smoothie during its preparation and the use of pectin as a stabilizer. Pectin had two effects on the creation of the structure, as follows: (1) on the capacity of the matrix to allow air inclusion and (2) on its elasticity and storage modulus. Pectin prevents the dehydration of casein during the heat treatment and does not lose its integrity after being homogenised.20 The increase in whey separation during storage can be attributed to the increased breakdown of protein and starch by the enzymes produced due to microbial action, which results in the production of simpler compounds that cannot retain moisture and reduction in viscosity upon storage.
Viscosity influences mouthfeel and ultimately, the flavour perception of the product, influencing the acceptability by consumers. Several other physico-chemical changes occurring during storage have an impact on the viscosity. The temperature (T) and days (D) of storage and their interaction significantly (P < 0.05) influenced the viscosity of MSBS. The viscosity was reduced with an increase in the number of days of storage. A greater reduction in viscosity was noted at a higher storage temperature. A significant (P < 0.05) reduction in viscosity was noticed on the 75th day at 4 °C, while at 30 °C, a significant (P < 0.05) reduction was observed on the 15th day only. Storage for 75 days resulted in the viscosity reduction of 9.30% and 28.75% at 4 °C and 30 °C, respectively. The decrease in viscosity can be ascribed to the breakdown of starch into simpler compounds such as mono-, di- and oligosaccharides by the enzymes produced by spoilage microorganisms, as reported by Cronk et al.18 A decline in pH indicates changes in the molecular charges of protein due to the loss of the free amino group of lysine because of Maillard reaction. The pH of the smoothie was significantly (P < 0.05) influenced by temperature (T) and days (D) of storage as well as their interaction effect (TxD). Its pH decreased with the progress of storage and with an increase in temperature. The reduction in pH can also be ascribed to microbial growth, in addition to the residual activity of pectin methyl esterase, which causes pectin to be converted into dimethyl ester, liberating acidic pectin.21 Li et al.22 observed a decrease in pH from 4.84 to 4.74 after 15 days at 4 °C in a banana smoothie. The acidity of the smoothie was significantly (P < 0.05) affected by the temperature (T) and days (D) of storage but not by their interaction. The acidity increased with the days of storage and increase in temperature. The acidity increased from 0.262% LA to 0.367% LA and 0.407% LA at 4 °C and 30 °C. On the 45th day of storage, the first significant (P < 0.05) increase in acidity content was noted. The increase in acidity is the result of the conversion of lactose to lactic acid by microbial action. The increase in acidity can be correlated with the decrease in pH. The presence of free fatty acids (FFA) in a food product gives an indication of its extent of lipolysis. The amount of FFA in MSBS increased progressively during its storage. The temperature (T) and (D) days of storage significantly (P < 0.05) influenced the FFA content in MSBS but their interaction had no effect. The increase in FFA content was 28.51% and 39.05% at 4 °C and 30 °C, respectively. The FFA content in the smoothie increased due to the release of free fatty acids by microbial lipase. The increase in FFA content can be correlated with the decline in the flavour score of MSBS. In the current study, although the FFA content increased, the sensory panel did not criticize any of the stored samples for rancid flavour. This is because the added alphonso mango flavour may have masked it very well.
The tyrosine value is the measure of proteolysis in a food product. The tyrosine value increased significantly (P < 0.05) with temperature (T) and days (D) of storage, while their interaction effect did not exert any impact on it. The first significant (P < 0.05) effect was noticed on the 15th day of storage but the rate of increase in tyrosine value decreased in the later stage of storage and it was not perceived by the experts during the sensory evaluation of the product. The increase in the tyrosine value can be ascribed to the protein breakdown by proteolytic enzymes produced by spoilage microorganisms. The increase in browning can be also attributed to free tyrosine available, which may participate in browning reactions. The browning index of MSBS was significantly (P < 0.05) influenced by temperature (T) and days (D) of storage but their interaction effect did not impact it. The first significant (P < 0.05) increase was observed on the 30th day of storage. The difference in browning index was much less, which was 0.057 and 0.052 at 4 °C and 30 °C, respectively, and not highly indicated by the sensory colour and appearance score. The maximum browning occurs between aw 0.60 and 0.85, and the browning rate increase with an increase in pH, reaching the peak at about 10.34 This product had a value of 0.98 aw, and during storage, the pH decreased, and thus the change in the browning index was very low. The increased browning index can be due to the formation of brown pigmented products owing to Maillard reactions and increase in hydroxyl-methyl furfural (HMF) content during storage. The greater increase in browning index at a higher temperature is due to the rapid development of Maillard reactions and the increase in browning intensity was more pronounced at higher temperatures. A greater reduction in the colour and appearance score was also noticed at a higher temperature during storage. To preserve the quality of a mixed fruit and vegetable smoothie, extend its shelf life, and ensure its safety, various combinations of natural antimicrobials, including nisin, natamycin, citric acid, and green tea extract (GTE) were studied by Nieva et al.4 and they found that treatment with 12.5 mg kg−1 nisin, 200 mg kg−1 natamycin, and citric acid (up to pH 3.5) could achieve a shelf-life extension of 14 days and a product with great nutritional and microbiological quality until 28 days of storage at 5 °C. However, in our product, we did not add any add any preservative to enhance its shelf life; instead we used natural ingredients and found better shelf stability with ingredient effects only. The developed smoothie is also free from any artificial colour.
| Attribute | Temp/Days | 0 | 15 | 30 | 45 | 60 | 75 | Average | CD (0.05) |
|---|---|---|---|---|---|---|---|---|---|
| a TVC = Total viable count. a, b, and c indicate significant (P < 0.05) change at a particular temperature during 15 days interval x and y in column indicate significant (P < 0.05) change in temperature during storage, p, q, r, and s in row indicate significant (P < 0.05) change in days at both temperatures. | |||||||||
| Flavour | 4 °C | 7.79 ± 0.14 | 7.64 ± 0.45 | 7.61 ± 0.41 | 7.36 ± 0.44 | 6.54 ± 0.57 | 6.29 ± 0.57 | 7.21 | T-NS |
| 30 °C | 7.79 ± 0.14 | 7.43 ± 0.35 | 7.14 ± 0.59 | 6.79 ± 0.36 | 6.27 ± 0.43 | 5.43 ± 0.52 | 6.81 | D-0.90 | |
| Average | 7.79p | 7.54p | 7.38p | 7.08p | 6.41q | 6.79q | TxD-NS | ||
| Consistency | 4 °C | 7.79 ± 0.15 | 7.68 ± 0.18 | 7.57 ± 0.17 | 7.57 ± 0.16 | 7.31 ± 0.31 | 6.29 ± 0.51 | 7.37 | T-NS |
| 30 °C | 7.79 ± 0.15 | 7.57 ± 0.20 | 7.14 ± 0.24 | 7.14 ± 0.21 | 6.87 ± 0.45 | 5.57 ± 0.49 | 7.01 | D-0.95 | |
| Average | 7.79p | 7.63p | 7.36p | 7.36p | 7.09p | 5.86q | TxD-NS | ||
| Sweetness | 4 °C | 7.71 ± 0.15 | 7.71 ± 0.18 | 7.57 ± 0.17 | 7.50 ± 0.19 | 7.19 ± 0.27 | 6.64 ± 0.39 | 7.39 | T-NS |
| 30 °C | 7.71 ± 0.15 | 7.57 ± 0.20 | 7.36 ± 0.18 | 7.29 ± 0.15 | 6.93 ± 0.32 | 6.46 ± 0.21 | 7.22 | D-0.70 | |
| Average | 7.71p | 7.64p | 7.47p | 7.40p | 7.06p | 6.55q | TxD-NS | ||
| Colour and appearance | 4 °C | 7.93 ± 0.07 | 7.86 ± 0.18 | 7.79 ± 0.21 | 7.71 ± 0.30 | 7.57 ± 0.38 | 6.79 ± 0.11 | 7.61 | T-NS |
| 30 °C | 7.93 ± 0.07 | 7.57 ± 0.23 | 7.57 ± 0.22 | 7.46 ± 0.19 | 7.36 ± 0.32 | 6.79 ± 0.34 | 7.45 | D-NS | |
| Average | 7.93 | 7.71 | 7.68 | 7.59 | 7.46 | 7.25 | TxD-NS | ||
| Overall acceptability | 4 °C | 7.80 ± 0.90 | 7.72 ± 0.13 | 7.63 ± 0.13 | 7.57 ± 0.13 | 7.10 ± 0.25 | 6.46 ± 0.41 | 7.38 | T-NS |
| 30 °C | 7.80 ± 0.90 | 7.54 ± 0.15 | 7.23 ± 0.24 | 7.23 ± 0.12 | 6.59 ± 0.23 | 6.06 ± 0.34 | 7.08 | D-0.68 | |
| Average | 7.80p | 7.63p | 7.43p | 7.40p | 6.85q | 6.26q | TxD-NS | ||
| TVC, cfu g−1 | 4 °C | 22.00 ± 3.06a | 23.00 ± 3.79a | 43.33 ± 2.03a | 113.33 ± 18.56a | 153.33 ± 3.33a | 213.33 ± 32.83b | 94.72x | T-53.85 |
| 30 °C | 22.00 ± 3.06a | 47.33 ± 5.45a | 115.67 ± 2.60a | 173.33 ± 39.30a | 486.67 ± 18.56c | 533.33 ± 35.30c | 229.72y | D-93.27 | |
| Average | 22.00p | 35.17p | 79.50p | 143.33q | 320.00r | 373.33r | TxD-161.55 | ||
The flavour of the smoothie was significantly (P < 0.05) reduced with the days of storage, while the temperature of storage and the temperature and days of storage interaction effect did not impact flavour. A significant (P < 0.05) reduction in flavour score was noticed on the 60th day of storage. The flavour score was 5.43 on the 75th day at 30 °C, which is designated as neither like nor dislike according to the hedonic scale, when the sensory panellist remarked that product lost its aroma and flavour because the sorghum cereal off-flavour developed in product. The sensory evaluation judges also commented on the development of a stale flavour in the product. A negative correlation in the flavour score with FFA content, tyrosine value and acidity was observed. Yadav et al.23 reported a significant decrease in flavour score with an increase in the storage period from 7.50 to 5.20 within 20 days of refrigeration storage for a whey-based banaba herbal (Menta arvensis) beverage.
Consistency explains the physical nature of the smoothie (thickness or viscosity, smoothness or lumpiness, and ease of pouring). Days of storage had a significant (P 0.05) impact on the consistency score. The consistency score was reduced by 19.26% and 28.49% at 4 °C and 30 °C, respectively, on the 75th day of storage compared to the initial score. However, up to 60 days of storage, the consistency changes were not significant, followed by a quick reduction, with the decline being sharper at 30 °C. The decrease in consistency score can be correlated with the decrease in the viscosity of the product and breakdown of carbohydrates and starch to simpler compounds that are soluble in water. During storage, microorganisms can produce proteolytic and amylolytic enzymes, resulting in a significant decrease in consistency. Hussain et al.24 observed a decrease in the consistency score during storage at 5 ± 1 °C in probiotic lassi added with Aloe barbadensis miller juice.
Sweetness plays an essential role in the consumer acceptance of a product. The sweetness score was significantly (P < 0.05) reduced with the days of storage (D), while the temperature of storage (T) and their interaction (TxD) did not influence it. The average reduction in the sweetness score was 13.88% and 16.21% at both the temperatures, i.e. 4 °C and 30 °C, respectively (Table 4). A significant (P < 0.05) decline was observed in the sweetness scores after storage for 75 days. The reduction in sweetness score reduced the overall acceptability of the product.
Colour and appearance are the first attributes to be noticed during sensory evaluation. The colour and appearance score of the smoothie remained unaffected by temperature (T) and days (D) of storage, as well as their interaction. Although there was an increase in the browning index and whey separation, their impact on product appearance and colour was not significant because the difference or change in browning index (0.052 to 0.057) and whey separation was very low (0.86 mL to 1.60 mL).
Overall acceptability is the combined impression of the different sensory attributes discussed above. The overall acceptability of the smoothie significantly (P < 0.05) decreased with days of storage (D), while the temperature of storage (T) and interaction between temperature and days of storage did not influence it. The first significant reduction in overall acceptability was observed on the 60th day of storage. The reduction in sensory scores was faster during the last phase of storage period and at ambient temperature. Two varieties of red vegetable smoothies using carrots, broccoli, tomato and pepper had a shelf life of 28 days at 5 °C, whereas the smoothie heat processed at 80 °C per 3 min was stable for up to 40 days at 20 °C and 58 days at 5 °C. The shelf life of red fresh vegetable smoothies was significantly extended by a mild thermal treatment and low-temperature storage.2 The untreated (UT) and mildly heat-treated (HT) at 90 °C for 45 s smoothies were kept at 5 °C, 15 °C, and 25 °C and studied for changes in their sensory, microbiological, and bio-active quality. The heat-treated samples showed a higher shelf stability of overall acceptability due to their strong correlation (hierarchical clustering) with flavor. The smoothie shelf-life (UT/HT) based on sensory quality data was 18/55 days at 5 °C, 4.5/12 days at 15 °C, and 2.4/5.8 days at 25 °C.25
3.3 Consumer acceptance study for sorghum-based breakfast smoothie
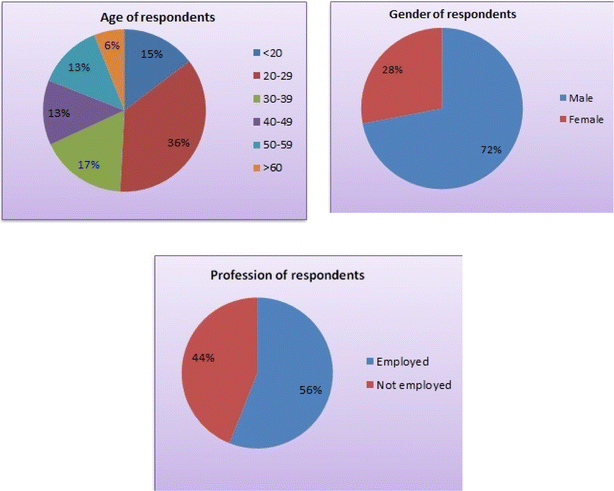
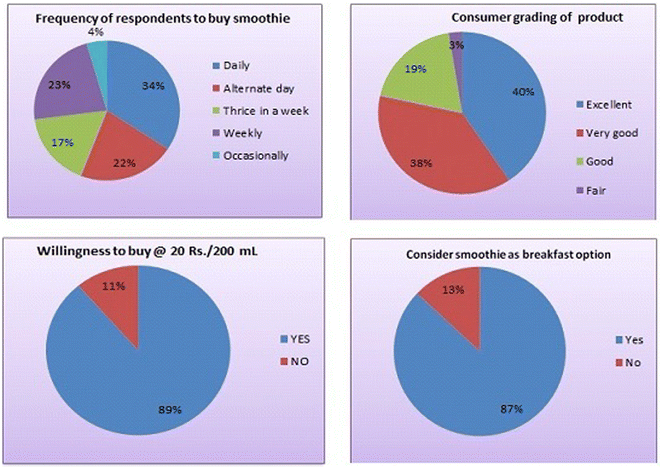
Testing consumer acceptance is a critical step in the product development process. It provides insights into market viability, user preferences, reducing risk, helps in brand image and optimizing market strategies, economic consideration and potential challenges, enabling businesses to make informed decisions and increase the likelihood of a successful product launch in society. The consumer acceptance study for the sorghum-based breakfast smoothie was carried out to check its potential as a well-accepted product in society given that it provides information regarding the preference of consumers and improvements and modifications to be done in the formulation, if required.28 The consumer acceptance study for MSBS was carried out at the NDRI, Karnal, Haryana. This study included the response of consumers towards the product as a ready-to-consume breakfast option, as well as their willingness to purchase the product at specified price, i.e. Rs. 20 per 200 mL. The profile of respondents for the smoothie is presented in Fig. 2. A total of 116 respondents, 72.41% men and 27.59% women, were given the MSBS. The consumers were from different age groups (from below 20 to above 60) and segments (students, employees, business persons and homemakers). The maximum number of consumers was from the age group of 20–29 (36.21%), while the minimum number of consumers crossed the age of 60 (6.03%). The consumer responses are presented in Fig. 2 and 3. Approximately 88% consumers liked the product in terms of colour and appearance, taste, flavour, sweetness, smoothness, easy to consume, and attractive in colour without the addition of artificial colour because the product obtained good colour from the mango pulp. Alternatively, ∼41% rated it excellent, ∼38%, very good and ∼19%, good. The remaining rated it fair. None of the consumers reported it poor. They found it a good option as a nutritive breakfast compared to other available breakfast items such as bread, milk, oats, paratha and others because the smoothie was nutritive, tasty, attractive in colour and appearance and low in calories. Consumers liked the concept of mixing ingredients such as milk, cereal, fruits, vegetables and honey and making a homogeneous product packed in bottles. Consumers also stated that generally they do not consume sorghum but mixed in this form, its taste is good. About 33% of the consumers was willing to consume it daily, while only 4.31% respondents would consume it occasionally and 89% were ready to pay Rs. 20 for a bottle of 200 mL because it is a cheaper option compared to other breakfast available in market together with having good nutritional ingredients and ready to consume. This product was found to be a good option for those with both spouses working, people staying alone either due to their job or study or those lacking time to prepare breakfast in the morning.4 Conclusion
The developed smoothie is a wholesome breakfast option containing ingredients from different food groups and it is low in fat and calories. The addition of sorghum flour helped to increase the iron and fibre content, mango and carrot increased the vitamin A content and the calcium content is obtained from milk. The developed product is a good source of iron (28.12 ppm) and dietary fibre (1.68%), as well as a moderate source of 560.46 ppm calcium and 679.3 IU/L vitamin A. The addition of mango pulp masked the cereal flavour and taste and improved the flavour, colour and attractiveness of the product, while homogenization improved the smoothness of the product and reduced the grittiness of the flour added in the product. The milk-sorghum-based smoothie was acceptable for 75 days and 60 days at storage temperatures of 4 °C and 30 °C, respectively, before losing its flavor and aroma. Higher temperatures were also associated with a decrease in viscosity, which was reflected in the overall acceptability score and consistency score of the product. The physico-chemical characteristics of the product declined, with a greater temperature accelerating the decline. Alternatively, with an increase in the storage duration, the wheying off, acidity, FFA, tyrosine value, browning index, and microbiological counts increased, where the rate in the increase was larger at higher temperatures. The product is minimally processed and has a respectable shelf life from a marketing point of view. It also fulfill microbial requirements according to the European Regulation Commission (2005) and FSSAI (2011) up to the end of storage. From a microbial point of view, the product is acceptable according to all standards. Thus, it can be concluded that the developed smoothie in the current study has a fresh-like flavour that is highly acceptable among consumers. However, a limitation of the product is that it developed a cereal flavour during longer storage. From an industrial point of view and continuous production, the germinated sorghum flour has a low shelf life, and thus further treatment such as roasting and microwave treatment can be done to enhance its shelf life as a raw material for making the product.Future research directions
To further enhance the shelf life of the smoothie, it can be aseptically packed and the bio-availability of its minerals and vitamins can be assessed by an animal bio-assay. To reduce the cereal-based flavour, germinated sorghum flour can be added by the micro-encapsulation method and the shelf life can be enhanced compared to that currently achieved. Suitable probiotics and prebiotics can be blended for the sake of healthy longevity, which can also provide a healthy approach for marginal groups.Authors agreement
All authors are agree for this research work.Data availability
The corresponding author will give the information supporting this work upon reasonable request.Conflicts of interest
There are no conflicts of interest for the authors.Acknowledgements
The authors are indebted to NDRI, Karnal for giving financial assistance and research facilities. The authors are also obliged to M/s. Huber India Pvt. Ltd, Mumbai for providing pectin sample.References
- P. Singh, R. Singh, V. Bhadauria and H. Singh, The functionality and extraction of protein from sorghum, finger millet, and Kodo millet: a review, Int. J. Food Sci. Technol., 2023, 59, 1, DOI:10.1111/ijfs.16747.
- N. Castillejo, G. B. Martínez-Hernández, P. A. Gómez, F. Artés and F. Artés-Hernández, Red fresh vegetables smoothies with extended shelf life as an innovative source of health-promoting compounds, J. Food Sci. Technol., 2016, 53, 1475–1486 CrossRef CAS PubMed.
- C. Zacconi, S. Giosuè, M. Marudelli and G. Scolari, Microbiological quality and safety of smoothies treated in different pressure–temperature domains: effects on indigenous fruit microbiota and Listeria monocytogenes and their survival during storage, Eur. Food Res. Technol., 2015, 241, 317–328 CrossRef CAS.
- S. G. Nieva, R. J. Jagus, M. V. Agüero and M. V. Fernandez, Fruit and vegetable smoothies preservation with natural antimicrobials for the assurance of safety and quality, LWT, 2022, 154, 112663, DOI:10.1016/j.lwt.2021.112663.
- R. Di Cagno, G. Minervini, C. G. Rizzello, M. De Angelis and M. Gobbetti, Effect of lactic acid fermentation on antioxidant, texture, colour and sensory properties of red and green smoothies, Food Microbiol., 2011, 28, 1062–1071 CrossRef CAS PubMed.
- R. Rani, M. H. K. Sathish and L. Sabikhi, Process optimization for a ready-to-serve breakfast smoothie from a composite milk-sorghum base, Int. J. Dairy Technol., 2016, 6, 1–15 CrossRef.
- FSSAI in Manual of Methods of Analysis of Foods. Milk and Milk Products, Ministry of health and family welfare, Government of India, New Delhi, 2015, pp. 59–88 Search PubMed.
- AOAC, Official methods of analysis, The association of official analytical chemists AOAC, 481, North Frederick Avenue Gaithersburg, Maryland, USA, 18th edn, 2005 Search PubMed.
- M. E. Hull, Studies on milk proteins II. Colorimetric determination of the partial hydrolysis of the proteins in milk, J. Dairy Sci., 1947, 30, 881–884 CrossRef CAS.
- H. C. Deeth and C. H. Fitz-Gerald, Lipolysis in dairy products: A Review, Australian J, Dairy Technol., 1976, 31, 53–64 CAS.
- S. Ranganna, Handbook of Analysis and Quality Control for Fruit and Vegetable Products, Tata McGraw-Hill Publishing Co. Ltd, New Delhi, 2nd edn, 2005, pp. 87–96 Search PubMed.
- Megazyme International Ireland Limited, Phytic Acid (Phytase) Total Phosphorus Kit (K-Phyt12/12), 2007 Search PubMed.
- Megazyme, Available Carbohydrates and Dietary Fibre Assay Kit (K-ACHDF 11/08), International Ireland Limited, 2006 Search PubMed.
- J. De-Vries and K. Silvera, Determination of Vitamins (Retinol) and E (alpha-Tocopherol) in Foods by Liquid Chromatography: Collaborative Study, J. AOAC Int., 2002, 85, 424–434 CrossRef CAS PubMed.
- H. Stone, H. Sidel, S. Oliver and R. C. Singlehon, Sensory evaluation by quantitative descriptive analysis, Food Technol., 1980, 28, 24–26 Search PubMed.
- IS: 7889, Yogurt – Enumeration of characteristic microorganisms – Colony-count technique at 37 degrees C, Indian Standards Institution, Manak Bhavan, New Delhi, 2003 Search PubMed.
- R. G. D. Steel and J. H. Torrie, Principles of Experimental Design in Principles and Procedure of Statistics – A Biometrical Approach, Mc-Graw Hill, Columbus, The United States, 2nd edn, 1980, pp. 137–167 Search PubMed.
- T. C. Cronk, K. H. Steinkraus, L. R. Hackler and L. R. Mattick, Indonesian tape ketan fermentation, Appl. Environ. Microbiol., 1977, 33, 1067–1073 CrossRef CAS PubMed.
- F. Abedi, A. M. Sani and H. Karazhiyan, Effect of some hydrocolloids blend on viscosity and sensory properties of raspberry juice-milk, J. Food Sci. Technol., 2014, 51, 2246–2250, DOI:10.1007/s13197-012-0705-0.
- S. J. E Snel, K. Otto, M. Schlangen, M. Beyrer and A. J. V. D. Goot, Type of pectin determines structuring potential of soy proteins into meat analogue applications, Food Hydrocolloids, 2024, 146(Part B), 109262, DOI:10.1016/j.foodhyd.2023.109262.
- F. Micheli, Pectin methylesterases: cell wall enzymes with important roles in plant physiology, Trends Plant Sci., 2001, 6, 414–419 CrossRef CAS PubMed.
- R. Li, Y. Wang, S. Wang and X. Liao, Comparative study of changes in microbiological quality and physicochemical properties of N2-infused and N2-degassed banana smoothies after high pressure processing, Food Bioprocess Technol., 2015, 8, 333–342 CrossRef CAS.
- R. B. Yadav, B. S. Yadav and N. Kalia, Development and storage studies on whey-based banaba herbal (Mentaarvensis) beverage, Am. J. Food Technol., 2010, 5, 121–129 CrossRef CAS.
- S. K. Hussain, G. R. Patil, V. Yadav and R. R. B. Singh, Effect of storage on sensory quality, pH, wheying off and probiotic count of lassi supplemented with Aloe barbadensis Miller juice, Indian J. Dairy Sci., 2015, 68, 105–110 Search PubMed.
- G. A. González-Tejedor, G. B. Martínez-Hernández, A. Garre, J. A. Egea, P. S. Fernández and F. Artés-Hernández, Quality Changes and Shelf-Life Prediction of a Fresh Fruit and Vegetable Purple Smoothie, Food Bioprocess Technol., 2017, 10(3–4), 1892–1904, DOI:10.1007/s11947-017-1965-5.
- A. Hurtado, A. Picouet, P. Picouet, A. Jofré, M. D. Guàrdia, J. M. Ros and S. Bañón, Application of high pressure processing for obtaining “Fresh-Like” fruit smoothies, Food Bioprocess Technol., 2015, 8, 2470–2482 CrossRef CAS.
- European Commission Regulation (EC) No 2073/2005 (OJ L338, P1, 22/12/2005) of 15 November 2005 on Microbiological Criteria for Foodstuffs Search PubMed.
- I. D. Choi, R. D. Phillips and A. V. A. Resurreccion, Consumer-Based Optimization of a Third-Generation Product Made from Peanut and Rice Flour, J. Food Sci., 2007, 72, 443–449 Search PubMed.
- S. A. Kim, L. V. Moore, D. Galuska, A. P. Wright, D. Harris, L. M. Grummer-Strawn and D. G. Rhodes, Vital signs: Fruit and vegetable intake among children: United States, 2003-2010, Morb. Mortal. Wkly. Rep., 2014, 63, 671–676 Search PubMed.
- B. S. Daylen Bates and J. Price, Impact of Fruit Smoothies on Adolescent Fruit Consumption at School, Health Educ. Behav., 2015, 1–6, DOI:10.1177/1090198114561514.
- Md. Saddam Hossain, Md. Nahidul Islam, Md. Mamunur Rahman, M. G. Mostofa and Md. A. R. Khan, Sorghum: A prospective crop for climatic vulnerability, food and nutritional security, J. Agric. Food Res., 2022, 8, 100300 Search PubMed.
- USDA Food and Nutrition Service, Team Nutrition, 2013, https://www.fns.usda.gov/tn/team-nutrition Search PubMed.
- Indian Council of Medical Research (ICMR), Nutrient Requirements and Recommended Dietary Allowances for Indians, 2010, https://icmr.nic.in/final/rda-2010.pdf Search PubMed.
- K. Erminawati, T. Yanto, R. Setyowati and P. Haryanti, Effect of pH and temperature on browning intensity of coconut sugar and its antioxidant activity, Food Res., 2018, 1–7 Search PubMed.
- S. Jensen, C. Rolin and R. Ipsen, Stabilisation of acidified skimmed milk with HM pectin, Food Hydrocolloids, 2010, 24, 291–299 CrossRef CAS.
- A. Bevilacqua, L. Petruzzi, M. Perricone, B. Speranza, D. Campaniello, M. Sinigaglia and M. S. Corbo, Non-thermal technologies for fruit and vegetable juices and beverages: Overview and advances, Compr. Rev. Food Sci. Food Saf., 2018, 17, 2–62 CrossRef PubMed.
- S. Jensen, C. Rolin and R. Ipsen, Stabilisation of acidified skimmed milk with HM pectin, Food Hydrocolloids, 2010, 24, 291–299 CrossRef CAS.
- A. S. Gad, Effect of pectin concentration and homogenisation pressure on viscosity and sediment percentage of plain youghurt, J. Agric. Sci., 2004, 29(2), 741–747 Search PubMed.
- H. Joudaki, M. Mousavi, M. Safari, S. H. Razavi, Z. Emam-Djomeh and S. M. T. Gharibzahedi, Scrutinizing the different pectin types on stability of an Iranian traditional drink “Doogh”, Int. J. Biol. Macromol., 2013, 60, 375–382 CrossRef CAS PubMed.
- J. Liu, N. Akihiro and M. Corredig, J. Agric. Food Chem., 2006, 54, 6241–6246 CrossRef CAS PubMed.
- R. H. Tromp, C. G. de Kruif, M. van Eijk and C. Rolin, Food Hydrocolloids, 2004, 18, 565–572 CrossRef CAS.
- S. Jensen, C. Rolin and R. Ipsen, Stabilisation of acidified skimmed milk with HM pectin, Food Hydrocolloids, 2010, 24, 291–299 CrossRef CAS.
- P. J. Rogers and R. Shahrokni, A Comparison of the Satiety Effects of a Fruit Smoothie, Its Fresh Fruit Equivalent and Other Drinks, Nutrients, 2018, 10, 431 CrossRef PubMed.
Footnote |
| † Assistant Professor (Dairy Chemistry), College of Dairy and Food Technology, Agriculture University, Jodhpur-342304, Rajasthan, India. |
| This journal is © The Royal Society of Chemistry 2024 |