Maternal inulin alleviates high-fat diet-induced lipid disorder in offspring by epigenetically modulating hypothalamus feeding circuit-related genes
Qian
Zhang
 ,
Xinhua
Xiao
,
Xinhua
Xiao
 *,
Jia
Zheng
,
Ming
Li
,
Miao
Yu
,
Fan
Ping
and
Tong
Wang
*,
Jia
Zheng
,
Ming
Li
,
Miao
Yu
,
Fan
Ping
and
Tong
Wang
Key Laboratory of Endocrinology, Ministry of Health, Department of Endocrinology, Peking Union Medical College Hospital, Peking Union Medical College, Chinese Academy of Medical Sciences, Beijing, 100730, China. E-mail: xiaoxh2014@vip.163.com; Fax: +86 10 69155073; Tel: +86 10 69155073
First published on 4th December 2023
Abstract
Increasing evidence supports the existence of fetal-originated adult diseases. Recent research indicates that the intrauterine environment affects the fetal hypothalamic energy intake center. Inulin is a probiotic that can moderate metabolic disorders, but whether maternal inulin intervention confers long-term metabolic benefits to lipid metabolism in offspring in their adult lives and the mechanism involved are unknown. Here, we used a maternal overnutrition model that was induced by excess energy intake before and during pregnancy and lactation and maternal inulin intervention was performed during pregnancy and lactation. The hypothalamic genome methylation in offspring was analyzed using a methylation array. The results showed that maternal inulin treatment modified the maternal high-fat diet (HFD)-induced increases in body weight, adipose tissue weight, and serum insulin and leptin levels and decreases in serum adiponectin levels. Maternal inulin intervention regulated the impairments in hypothalamic leptin resistance, induced the methylation of Socs3, Npy, and Il6, and inhibited the methylation of Lepr in the hypothalamus of offspring. In conclusion, maternal inulin intervention modifies offspring lipid metabolism, and the underlying mechanism involves the methylation of genes in the hypothalamus feeding circuit.
Introduction
Obesity has become an urgent health problem worldwide and affects not only wealthy countries but also low- and middle-income countries. Currently, more than one-third of adults are overweight1 and being overweight or obese is an independent risk factor for many diseases, such as diabetes,2 nonalcoholic fatty liver disease,3 cardiovascular disease,4 cancers,5 and COVID-19.6 Maternal overnutrition and obesity have many adverse effects, such as preeclampsia,7 cesarean section, increased duration of maternal and neonatal hospital stay, maternal hemorrhage, infant mortality,8 and stillbirth.9 In addition, maternal overnutrition and obesity are related to higher birthweight and neonatal fat mass.9,10 Moreover, increasing evidence reveals that maternal overnutrition and obesity might lead to offspring obesity later in life, and higher birthweight is a risk factor.11–14 The high prevalence of high birthweight and maternal overnutrition and obesity has led to concerns about their links with later life obesity.15 A record linkage cohort study revealed a 35% increase in all-cause mortality in the offspring of obese mothers,16 even after adjusting for other confounding factors. Thus, effective early intervention is needed to avoid metabolic disorders in offspring later in life.Inulin is a type of fructooligosaccharide that is mainly derived from vegetables, such as chicory.17 This molecule is the energy source of Bifidobacteria in the gut. Currently, inulin is used for the treatment of some diseases, such as allergic disease18 and colon cancer.19 In addition, inulin exerts beneficial effects on body weight reduction and insulin sensitivity moderation in obese mice and subjects.20–22 Moreover, maternal inulin supplementation during gestation can mitigate food allergies.23 Importantly, our research found that maternal inulin intervention modifies glucose metabolism in offspring.24,25
The hypothalamus is the central regulator of food intake and nutrient availability26 and influences feeding behavior through the feeding circuit.27 The arcuate nucleus of the hypothalamus (ARH) contains two opposing types of neuropeptidergic fibers, anorexigenic pro-opiomelanocortin (POMC) neurons and orexigenic agouti-related peptide (AgRP) neurons, which produce α-melanocortin-stimulating hormone (αMSH) and AgRP, respectively.28,29 As an adipocyte-derived hormone, leptin and its receptor are regulators of this neurotransmission signaling.30 Maternal overnutrition and obesity cause hypothalamic feeding circuit structure and function disorders in offspring.31 However, the underlying mechanism has not been fully elucidated.
Accumulating data suggest that epigenetic changes are the key mechanisms linking maternal adverse environmental factors and offspring metabolic diseases.32,33 Various epigenetic regulatory mechanisms, such as DNA methylation, provide a plausible link between nutritional status changes early in development and the susceptibility to developing metabolic diseases later in life.34 Hypermethylation of gene promoter regions may inhibit gene expression, whereas hypomethylation may activate gene expression. Our previous study showed that a maternal high-fat diet (HFD) can program brown adipose DNA methylation and lipid metabolism in male offspring.35
The hypothalamus is highly dynamic and sensitive and can undergo adaptive changes in response to nutritional and environmental stimuli. Early maternal inulin intervention improves glucose tolerance and insulin resistance in offspring.25 However, the effect of maternal inulin on the energy metabolism of offspring has been less studied. The purpose of the present study was to determine whether maternal inulin treatment has a positive impact on the metabolism of offspring whose mothers are fed a HFD during gestation and lactation. This study utilized a mouse model to examine metabolic parameters and the hypothalamic gene DNA methylation status in adulthood.
Materials and methods
Animal treatments and diets
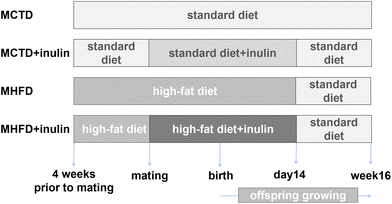
All animal procedures were approved by the Animal Care and Use Committee of the Peking Union Medical Hospital (XHDW-2015-0051), and conformed to the Guide for the Care and Use of Laboratory Animals. Five-week-old C57BL/6J mice (13.7 ± 0.7 g) were provided by the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China). Throughout the experimental procedure, the mice were maintained in an SPF animal facility at 22 °C under a 12 h light/12 h dark cycle and were provided free access to food. Female mice were randomly divided into a control group fed a standard control diet (CTD, 16% kcal fat, 64% kcal carbohydrate, 20% kcal protein, D11112201, Research Diets, New Brunswick, NJ, USA, n = 16) and an HFD group fed an HFD diet (45% kcal fat, 35% kcal carbohydrate, 20% kcal protein, D12451, Research Diets, New Brunswick, NJ, USA, n = 16). All male mice were fed the standard diet. After four weeks on their corresponding diet, the female mice were mated with the males (2 females![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 male). After examination of the females revealed a vaginal plug, the maternal control diet (MCTD) group and the maternal HFD (MHFD) group were subgrouped into the MCTD, MCTD + inulin, MHFD, and MHFD + inulin groups (n = 8 per group). Inulin was dissolved in water and then added to the rodent diet mixture to create a custom rodent diet (10 wt/wt%, Vilof™ Soluble Dietary Fiber, HAHEAL Medical Inc., Qingdao, China).36,37 This diet and treatment protocol was maintained during the following gestation period and lactation days 0 to 14. To avoid the confounding factors of pup mouse pellet diet intake after 14 days of age, all dams were switched to a standard diet at that time. At birth, the pups were balanced to 8 per dam. The pups were weaned at 3 weeks of age and then fed a standard diet until 16 weeks of age (Fig. 1). The dams were weighed prior to the onset of diet initiation and then on a weekly basis prior to and through gestation and lactation. The offspring were weighed at birth, weaning and 16 weeks of age. The food intake of each dam was recorded weekly prior to and through gestation and lactation. The food intake of each offspring was recorded during postnatal day (PND) 21 to 28, and week 15 to 16 of age per mice. The daily food intake was calculated as weekly food intake divided by 7. According to the rodent diet formula, energy intake was calculated as the food intake multiplied by 3.76 kcal g−1 (CTD) or 4.7 kcal g−1 (HFD). The offspring body length was determined by measuring the nasal-to-anal distance at weaning and 16 weeks of age. After an overdose injection of pentobarbital sodium (150 mg kg−1), dams (n = 8 per group) were sacrificed at weaning, and male offspring (n = 8 per group, from different litters) were sacrificed at 16 weeks of age. This study focused on male offspring to avoid the interference of female hormone levels and the estrus cycle. According to the mouse brain atlas,38 the hypothalamus was immediately isolated from 16-week-old offspring brains, frozen in liquid nitrogen and stored at −80 °C. The subcutaneous adipose tissue (SAT, inguinal adipose tissue) and visceral adipose tissue (VAT, perirenal adipose tissue) were removed and weighed after the dams (at weaning) and pups (at 16 weeks of age) were euthanized.
1 male). After examination of the females revealed a vaginal plug, the maternal control diet (MCTD) group and the maternal HFD (MHFD) group were subgrouped into the MCTD, MCTD + inulin, MHFD, and MHFD + inulin groups (n = 8 per group). Inulin was dissolved in water and then added to the rodent diet mixture to create a custom rodent diet (10 wt/wt%, Vilof™ Soluble Dietary Fiber, HAHEAL Medical Inc., Qingdao, China).36,37 This diet and treatment protocol was maintained during the following gestation period and lactation days 0 to 14. To avoid the confounding factors of pup mouse pellet diet intake after 14 days of age, all dams were switched to a standard diet at that time. At birth, the pups were balanced to 8 per dam. The pups were weaned at 3 weeks of age and then fed a standard diet until 16 weeks of age (Fig. 1). The dams were weighed prior to the onset of diet initiation and then on a weekly basis prior to and through gestation and lactation. The offspring were weighed at birth, weaning and 16 weeks of age. The food intake of each dam was recorded weekly prior to and through gestation and lactation. The food intake of each offspring was recorded during postnatal day (PND) 21 to 28, and week 15 to 16 of age per mice. The daily food intake was calculated as weekly food intake divided by 7. According to the rodent diet formula, energy intake was calculated as the food intake multiplied by 3.76 kcal g−1 (CTD) or 4.7 kcal g−1 (HFD). The offspring body length was determined by measuring the nasal-to-anal distance at weaning and 16 weeks of age. After an overdose injection of pentobarbital sodium (150 mg kg−1), dams (n = 8 per group) were sacrificed at weaning, and male offspring (n = 8 per group, from different litters) were sacrificed at 16 weeks of age. This study focused on male offspring to avoid the interference of female hormone levels and the estrus cycle. According to the mouse brain atlas,38 the hypothalamus was immediately isolated from 16-week-old offspring brains, frozen in liquid nitrogen and stored at −80 °C. The subcutaneous adipose tissue (SAT, inguinal adipose tissue) and visceral adipose tissue (VAT, perirenal adipose tissue) were removed and weighed after the dams (at weaning) and pups (at 16 weeks of age) were euthanized.
Serum biochemical measurements
At 16 weeks of age, offspring blood samples were collected from the intra-orbital retrobulbar plexus after 12 h of fasting. The serum triglyceride (TG) and total cholesterol (TC) levels were determined by an enzymatic colorimetric method using a Cabas 8000 instrument with a commercial kit (Roche Diagnostics GmbH, Mannheim, Germany). The serum insulin, leptin and adiponectin levels were detected using an enzyme-linked immunosorbent assay (ELISA) kit (Millipore, Billerica, MA, USA).Adipose tissue TG content
According to the Bligh and Dyer method, adipose tissue was extracted with chloroform and methanol.39 After centrifugation, the contents of TG were determined by an enzymatic colorimetric method using a Cabas 8000 instrument with a commercial kit (Roche Diagnostics GmbH, Mannheim, Germany).DNA preparation and DNA methylation microarray
Genomic DNA (gDNA) was isolated from hypothalamus tissues using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) and fragmented to 200 to 1000 bp. One microgram of sonicated gDNA was immunoprecipitated with 1 μL of mouse monoclonal anti-5-methylcytosine antibody (Diagenode, Liege, Belgium). Cy3 and Cy5 were used to label the total input and immunoprecipitated DNA, respectively. DNA was then hybridized to the Arraystar Mouse RefSeq Promoter Methylation Array 4 × 180 K (Arraystar Inc., Rockville, MD, USA), which contains 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 327 gene promoter regions (from approximately −1300 to +500 of the transcriptional starting sites (TSSs)) completely covered by ∼180
327 gene promoter regions (from approximately −1300 to +500 of the transcriptional starting sites (TSSs)) completely covered by ∼180![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 probes with a spacing of approximately 205 bp. Scanning was performed with the Agilent Scanner G2505C (Agilent Technologies, Waldbronn, Germany).
000 probes with a spacing of approximately 205 bp. Scanning was performed with the Agilent Scanner G2505C (Agilent Technologies, Waldbronn, Germany).
Data normalization and analysis
The raw DNA methylation microarray data were median centered, quantile normalized, and linearly smoothed with the Bioconductor packages Ringo, limma, and MEDME (https://www.Bioconductor.org). Normalized log2-ratio data were analyzed with NimbleScan v2.5 (Roche NimbleGen Inc., Madison, WI, USA). A normalized peak M value (peak M value = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2MeDIP/Input) change ≥ 0 represented the different enrichment peaks (DEPs). Based on the CpG density, CpG ratio and length of the CpG-rich region, the promoters were subdivided into three classes: high (HCP), low (LCP), and intermediate (ICP) CpG density.
2MeDIP/Input) change ≥ 0 represented the different enrichment peaks (DEPs). Based on the CpG density, CpG ratio and length of the CpG-rich region, the promoters were subdivided into three classes: high (HCP), low (LCP), and intermediate (ICP) CpG density.
Pathway and bioinformatics analyses of the array results
Kyoto Encyclopedia of Gene and Genomes (KEGG) pathway and Gene Ontology (GO) analyses of the DEPs were performed using the Database for Annotation, Visualization, and Integrated Discovery (DAVID) website (https://david.ncifcrf.gov/).40MeDIP qPCR
Genomic DNA was isolated from the hypothalamus and then fragmented into 200–800 bp fragments. One microgram of the DNA fragments was incubated in boiling water for 10 min. After the addition of anti-5-methylcytosine (5 mC) antibodies (Zymo Research, Irvine, CA), the mixture was rotated at 4 °C overnight. Some DNA fragments (20 ng μL−1) were retained as the input. The rest of the DNA was immunoprecipitated with the DNA–antibody complexes, captured with protein A/G PLUS-agarose (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and digested with proteinase K (Beyotime Biotechnology, Shanghai). The released methylated DNA fragments in inputs and controls were then purified by phenol–chloroform extraction and subjected to real-time PCR.41,42 Relative enrichment was calculated using the percent input method, in which each immunoprecipitation was adjusted to the input and compared to the control for the assessment of significant 5 mC enrichment. CpG island prediction and primer design were performed using EMBOSS Cpgplot online software (https://www.ebi.ac.uk/Tools/seqstats/emboss_cpgplot/).43 The primers used for MeDIP qPCR are listed in Table 1.| Genes | GenBank ID | Forward primer | Reverse primer | Production size (bp) |
|---|---|---|---|---|
| Socs3, suppressor of cytokine signaling 3; Npy, neuropeptide Y; Il6, interleukin 6; Lepr, leptin receptor. | ||||
| Socs3 | NM_007707 | CGACATTCCTTCTCAGGTTTG | AGAATAAGAGGTTGTGGGGCT | 103 |
| Npy | NM_023456 | CTTAGATTCAAACTTCCAGGGG | GAGCAGATTAAAAAACCAACACC | 142 |
| Il6 | NM_001314054 | TGGAGACAGGTGGACAGAAAAC | TAACCCCTCCAATGCTCAAGT | 112 |
| Lepr | NM_001122899 | CACTTTCCTTGCGATTATTACTGC | AGGGATGCCTGGGCTCTATG | 218 |
RNA isolation and quantitative real-time PCR (qPCR) analysis
RNA from the hypothalamus was extracted using TRIzol and then reverse transcribed using a TaKaRa RT kit (TaKaRa, Shiga, Japan). A Viia7 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) was used to perform qPCR under the standard thermal cycling conditions. The relative RNA expression levels were calculated using the comparative Ct method (2−ΔΔCt) and standardized to Gapdh. The primers for qPCR are listed in Table 2.| Genes | GenBank ID | Forward primer | Reverse primer | Production size (bp) |
|---|---|---|---|---|
| Socs3, suppressor of cytokine signaling 3; Npy, neuropeptide Y; Il6, interleukin 6; Lepr, leptin receptor. | ||||
| Socs3 | NM_007707 | TGTCGGAAGACTGTCAACGG | CGTTGGGGCTGGATTTTTG | 191 |
| Npy | NM_023456 | TGGACTGACCCTCGCTCTAT | TAGTGTCGCAGAGCGGAGTA | 135 |
| Il6 | NM_001314054 | GACAAAGCCAGAGTCCTTCAGA | TGTGACTCCAGCTTATCTCTTGG | 76 |
| Lepr | NM_001122899 | CCTGGGCACAAGGACTGAAT | TGGACTGTTGGGAAGTTGGT | 101 |
Statistical analysis
The data are presented as the means ± SEMs and were analyzed using the statistical program GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA). The data were normally distributed, and all compared groups had the same variance. One-way ANOVA followed by Tukey's post hoc test was used to analyze the differences among the groups. We analyzed the data from eight samples per group, obtained from mice of different litters. P < 0.05 was considered to indicate significant differences.Results
Food intake, energy intake and body weight during pregnancy and lactation
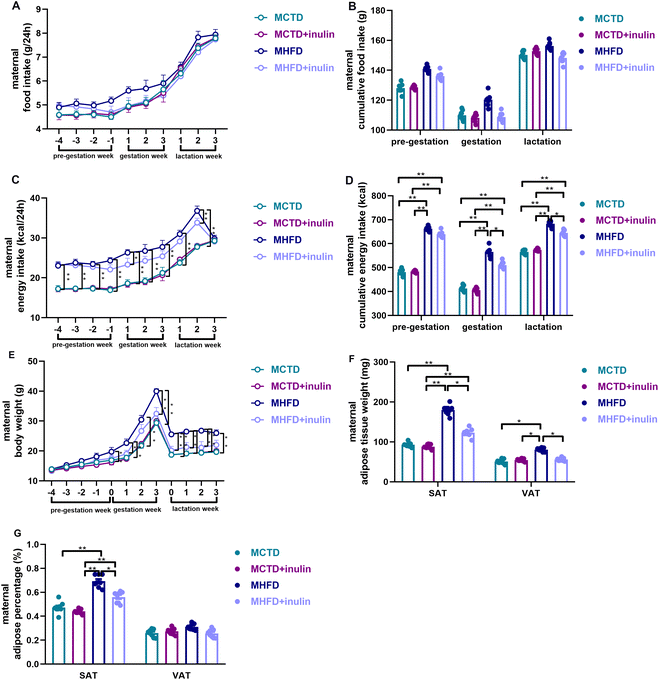
Although the food intake did not significantly differ among the groups (P > 0.05, Fig. 2A and B), the MHFD dams had a higher energy intake than the MCTD dams during pregnancy and lactation (P < 0.01, Fig. 2C and D). Inulin treatment reduced the energy intake of HFD-fed dams during pregnancy and lactation (P < 0.05 or 0.01, Fig. 2C and D). During the gestational and lactation period, the MHFD dams had a greater body weight and SAT weight and percentage than the MCTD dams (P < 0.01, Fig. 2E–G). The MHFD + inulin dams had a lower body weight and SAT weight and percentage than the MHFD dams (P < 0.05 or 0.01, Fig. 2E–G). Inulin treatment reduced the VAT weight (P < 0.05, Fig. 2F), but not the VAT percentage in the dams (P > 0.05, Fig. 2G).Maternal inulin intervention reduced the energy intake and body weight of male adult offspring
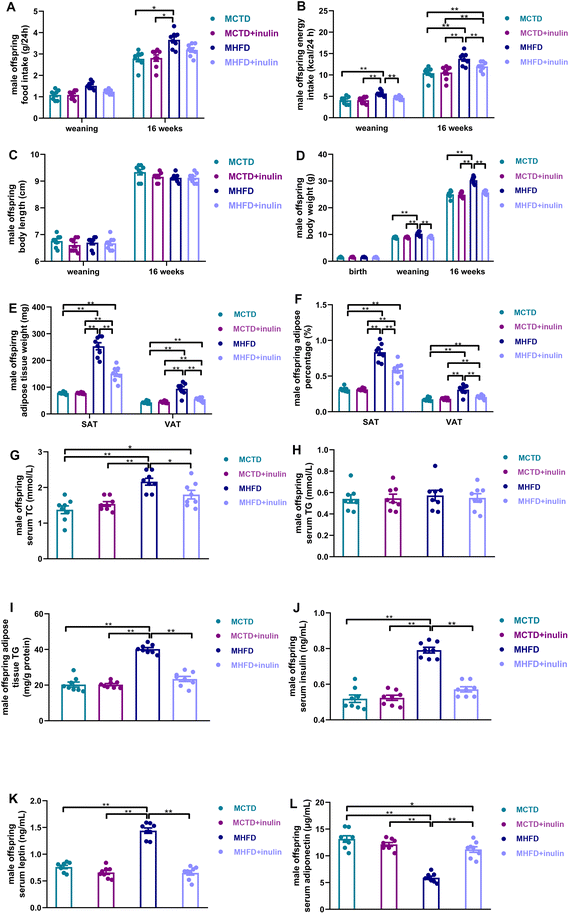
Sixteen-week-old offspring from the MHFD dams had a greater food intake than those from the MCTD dams (P < 0.05, Fig. 3A). Higher energy intake was observed in the male offspring from the MHFD dams than in those from the MCTD dams at 3 and 16 weeks of age (P < 0.01, Fig. 3B). Maternal inulin intervention reduced the energy intake of male offspring (P < 0.01, Fig. 3B). No significant difference in body length was found among the four groups at weaning and 16 weeks age (P > 0.05, Fig. 3C). Additionally, the birth weight did not significantly differ among the four groups (P > 0.05, Fig. 3D). At 3 and 16 weeks of age, the male offspring from the MHFD dams showed higher body weights than those from the MCTD dams (P < 0.01, Fig. 3D). Maternal inulin intervention reduced the male offspring body weight at weaning and 16 weeks of age (P < 0.01, Fig. 3D). At 16 weeks of age, the SAT and VAT weight and percentage of the MHFD group were higher than those of the MCTD group (P < 0.01, Fig. 3E and F). Maternal inulin intervention reduced the SAT and VAT weight and percentage of male offspring at 16 weeks of age (P < 0.01, Fig. 3E and F).Maternal inulin intervention alleviated lipid metabolic disorders in male adult offspring
The serum TC levels were significantly increased in male offspring from the MHFD dams (P < 0.01, Fig. 3G). Maternal inulin intervention reduced the serum TC levels in male offspring (P < 0.05, Fig. 3G). No significant differences in the serum TG levels were found among the four groups (P > 0.05, Fig. 3H). However, maternal inulin intervention significantly reduced the adipose tissue TG content (P < 0.01, Fig. 3I). Maternal HFD led to the elevation of the serum insulin and leptin levels and reduction of the serum adiponectin levels in male offspring (P < 0.01, Fig. 3J, K, and L). Maternal inulin intervention regulated the serum insulin, leptin, and adiponectin levels in male offspring (P < 0.01, Fig. 3J, K and L).DNA methylation analysis of offspring hypothalamus
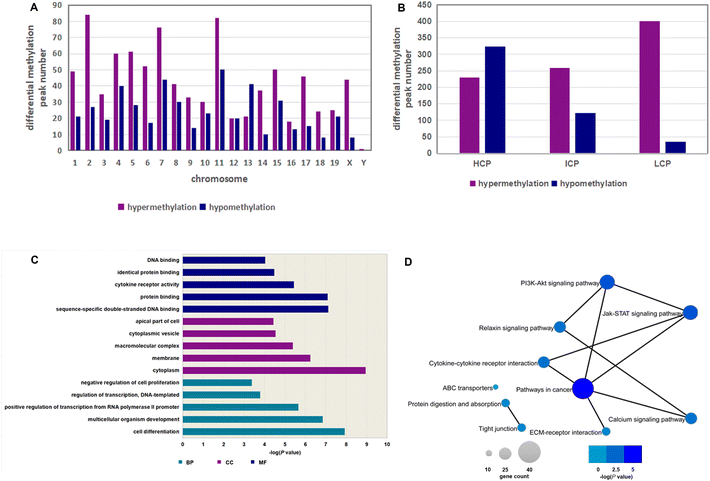
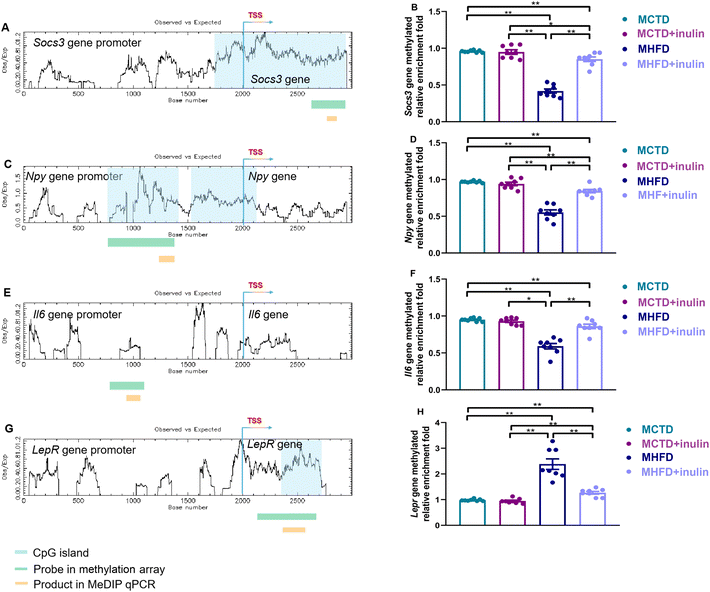
To investigate the gene methylation changes in the hypothalamus of offspring caused by maternal inulin intervention, we performed a promoter methylation array. The original data generated from the DNA methylation array are available from the NCBI Gene Expression Omnibus (GEO) database under the accession number GSE218748 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE218748). The MHFD + inulin group had 1369 differentially methylated peaks compared to the MHFD group. Of these, the majority (64.9%) were hypermethylated, and 35.1% were hypomethylated. The differentially methylated peaks were found on 21 chromosomes, mainly on chromosomes 1, 2, 4, 5, 7, 8, 11, and 15 (Fig. 4A). Most of the hypermethylated peaks resided in LCPs, whereas most hypomethylated peaks resided in HCPs (Fig. 4B).Differentially methylated gene function analysis
The identified differentially methylated genes were significantly associated with “cell differentiation”, “multicellular organism development”, “positive regulation of transcription from RNA polymerase II promoter”, “regulation of transcription, DNA-templated”, and “negative regulation of cell proliferation” in the biological process category (Fig. 4C, Table 3). The top 10 terms in the KEGG pathway were “calcium signaling pathway”, “cytokine–cytokine receptor interaction”, “ABC transporters”, “protein digestion and absorption”, “PI3K-Akt signaling pathway”, “tight junction”, “ECM-receptor interaction”, “relaxin signaling pathway”, “pathways in cancer”, and “JAK-STAT signaling pathway” (Fig. 4D, Table 4).| Category | Term ID | Term name | Count | P value |
|---|---|---|---|---|
| BP, biological processes; CC, cellular components; MF, molecular function. | ||||
| BP | GO:0030154 | Cell differentiation | 91 | 1.19 × 10−8 |
| BP | GO:0007275 | Multicellular organism development | 92 | 1.43 × 10−7 |
| BP | GO:0045944 | Positive regulation of transcription from RNA polymerase II promoter | 95 | 2.23 × 10−6 |
| BP | GO:0006355 | Regulation of transcription, DNA-templated | 88 | 1.66 × 10−4 |
| BP | GO:0008285 | Negative regulation of cell proliferation | 38 | 4.21 × 10−4 |
| CC | GO:0005737 | Cytoplasm | 433 | 1.13 × 10−9 |
| CC | GO:0016020 | Membrane | 387 | 5.81 × 10−7 |
| CC | GO:0032991 | Macromolecular complex | 75 | 4.16 × 10−6 |
| CC | GO:0031410 | Cytoplasmic vesicle | 69 | 2.89 × 10−5 |
| CC | GO:0045177 | Apical part of cells | 20 | 3.73 × 10−5 |
| MF | GO:1990837 | Sequence-specific double-stranded DNA binding | 58 | 7.57 × 10−8 |
| MF | GO:0005515 | Protein binding | 336 | 8.13 × 10−8 |
| MF | GO:0004896 | Cytokine receptor activity | 14 | 3.71 × 10−6 |
| MF | GO:0042802 | Identical protein binding | 136 | 3.33 × 10−5 |
| MF | GO:0003677 | DNA binding | 126 | 9.41 × 10−5 |
| Term ID | Term name | Count | Fold enrichment | P value | Genes |
|---|---|---|---|---|---|
| mmu04020 | Calcium signaling pathway | 28 | 2.428 | 3.01 × 10−5 | CHRM3, ATP2A1, MST1R, HTR4, FGF1, ADCY7, CACNA1H, HTR7, EDNRB, ERBB3, PHKG1, DRD5, NTRK1, NTRK2, AVPR1B, NOS3, SPHK1, TNNC2, TRHR2, VEGFA, P2RX7, FGF15, PLCB4, P2RX1, ORAI2, GNAS, SLC25A31, PLCD4 |
| mmu04060 | Cytokine–cytokine receptor interaction | 30 | 2.139 | 1.50 × 10−4 | ACVRL1, CNTFR, CSF1, MPL, CSF2RA, IL27RA, ACVR1C, LEPR, TNFRSF8, CCL19, CCL21A, IL11RA2, GDF11, IL15RA, IFNGR1, IL19, TNFRSF10B, IL17RB, EPOR, IL17RA, TGFBR2, IL22RA1, CSF2RB2, IL6, CXCL12, FASL, IL2RB, GM13305, GM13306, TNFRSF21 |
| mmu02010 | ABC transporters | 10 | 4.003 | 7.19 × 10−4 | ABCD4, ABCC3, ABCG8, ABCB7, ABCC9, ABCA7, ABCB11, ABCC12, ABCG1, ABCA15 |
| mmu04974 | Protein digestion and absorption | 14 | 2.698 | 0.00190 | KCNK5, COL18A1, SLC6A19, CELA3B, COL24A1, COL12A1, ATP1A4, ATP1A2, COL1A1, DPP4, SLC7A9, COL4A4, COL4A3, COL9A2 |
| mmu04151 | PI3K-Akt signaling pathway | 31 | 1.797 | 0.00207 | TNXB, CSF1, FLT3, LAMC3, LAMA3, FGF1, PPP2CA, STK11, GNGT2, YWHAQ, ERBB3, CREB3L1, NTRK1, NTRK2, HSP90AA1, VWF, NOS3, ITGA1, EPOR, VEGFA, COL1A1, IL6, FGF15, TCL1, FASL, COL4A4, COL4A3, IL2RB, COL9A2, PIK3AP1, TLR2 |
| mmu04530 | Tight junction | 18 | 2.244 | 0.00265 | JUN, TUBA3B, MYL12B, RUNX1, PPP2CA, CLDN11, CLDN10, TIAM1, STK11, PARD6B, PARD6A, CLDN9, PARD3, CLDN19, CLDN18, ARHGEF2, SYNPO, TJP3 |
| mmu04512 | ECM–receptor interaction | 12 | 2.838 | 0.00308 | COL1A1, GP9, TNXB, VWF, LAMC3, COL4A4, ITGA1, LAMA3, COL4A3, COL9A2, FREM1, HSPG2 |
| mmu04926 | Relaxin signaling pathway | 15 | 2.420 | 0.00344 | JUN, SHC3, NOS3, MMP9, ADCY7, VEGFA, TGFBR2, COL1A1, PLCB4, EDNRB, GNGT2, CREB3L1, COL4A4, COL4A3, GNAS |
| mmu05200 | Pathways in cancer | 41 | 1.572 | 0.00411 | HDAC2, FLT3, LAMC3, LAMA3, TCF7, FGF1, CSF2RA, ADCY7, DLL4, SHH, EDNRB, GNGT2, RXRG, EGLN1, NTRK1, IL15RA, JUN, HSP90AA1, GADD45B, IFNGR1, TRAF2, KIF7, MMP9, EPOR, PML, VEGFA, RUNX1, TGFBR2, CSF2RB2, CCNA1, IL6, FGF15, CXCL12, RAD51, PLCB4, FASL, COL4A4, COL4A3, IL2RB, CYCT, GNAS |
| mmu04630 | JAK-STAT signaling pathway | 17 | 2.106 | 0.00667 | CNTFR, IL15RA, IL11RA2, IFNGR1, MPL, IL19, CSF2RA, EPOR, IL27RA, IL22RA1, CSF2RB2, SOCS3, IL6, IL2RB, LEPR, PTPN6, GM13305 |
Maternal inulin intervention regulated hypothalamic gene methylation
As shown in Fig. 4D, the JAK-STAT signaling pathway in the hypothalamus of the offspring was affected by maternal inulin intervention. Thus, the methylation of four genes in this pathway was selected for verification by MeDIP qPCR. We identified CpG islands in the promoters and transcriptional start sites (TSSs) of the suppressor of cytokine signaling 3 (Socs3, Fig. 5A), neuropeptide Y (Npy, Fig. 5C), interleukin 6 (Il6, Fig. 5E), and leptin receptor (Lepr, Fig. 5G). The MHFD group had lower methylation levels in the Socs3, Npy, and Il6 promoters and a higher methylation level in the Lepr promoter compared with the MCTD group (P < 0.01, Fig. 5B, D, F, and H). Maternal inulin regulated these hypothalamus methylation disorders (P < 0.01, Fig. 5B, D, F, and H).Maternal inulin intervention regulated hypothalamus feeding circuit-related gene expression
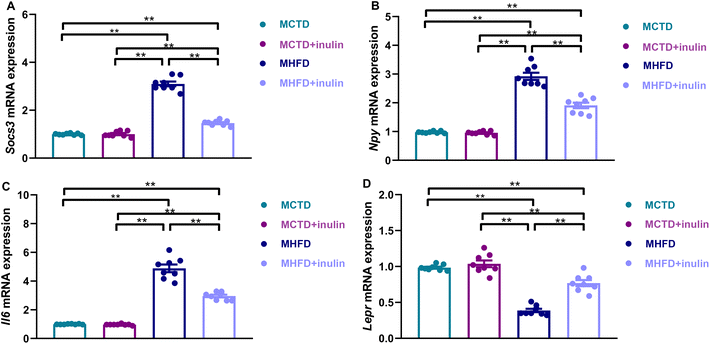
The MHFD group had an increased Socs3 (Fig. 6A), Npy (Fig. 6B), and Il6 (Fig. 6C) expression and reduced Lepr expression (P < 0.01, Fig. 6D) compared with the MCTD group. Maternal inulin intervention moderated Socs3, Npy, Il6, and Lepr expression (P < 0.01, Fig. 6A–D).Discussion
In this study, HFD feeding during pregnancy and lactation increased the energy intake, body weight, and SAT weight and percentage in the dams. Inulin intervention during pregnancy and lactation reduced the energy intake, body weight, and SAT weight and percentage of the HFD dams. Zhou et al. provided evidence showing that inulin attenuated the HFD-induced body weight gain in a pregnant sow model.44 Moreover, Miao et al. found that inulin-type fructans reduced the body weight and fat accumulation during pregnancy.45 These results were in good agreement with previous studies conducted with nonpregnant individuals.46,47In the current study, maternal HFD did not alter the offspring body length at weaning and 16 of weeks of age or birth weight, but influenced the offspring energy intake and body weight both at weaning and adulthood. Children born from obese mothers who gained inadequate weight had lower BMI Z scores at some percentiles of the BMI Z score distribution.48 Moreover, we found that offspring from the HFD dams had a higher adipose weight. The offspring of the HFD dams had a greater postnatal body weight and a higher total body adiposity.49 A meta-analysis revealed that maternal HFD feeding induced adipocyte hypertrophy in offspring, independent of the offspring diet.50 The serum TC, adipose tissue TG, insulin, and leptin levels were higher and the serum adiponectin levels were lower in the offspring in the MHFD group. The pups from the HFD-fed dams had higher levels of TC than those from chow-fed dams.51 Offspring from obese dams displayed persistent hyperleptinemia from postnatal day 12, day 21 to month 9.52 Twenty-eight-day-old offspring from dams fed with an HFD during pregnancy and lactation showed insulin resistance.53 Gregoraszczuk et al. found that the plasma adiponectin levels of offspring from HFD-fed dams are lower than those of offspring from standard diet-fed dam rats.54
Maternal inulin intervention during gestation and lactation reduced the energy intake, body weight, adipose tissue ratio, serum TC and adipose tissue TG levels of the offspring in adult life. We also found moderation of the serum insulin, leptin and adiponectin levels of male offspring from the inulin-treated HFD-fed dams. Desbuards et al. did not find a difference in the serum leptin levels between the offspring from the prebiotic-supplemented group and the offspring from the control diet-fed dam mice.55 The reason for the different results may be because the observation time used in the study conducted by Desbuards et al. was at weaning.
DNA methylation is an important epigenetic mechanism in mammalian gene expression regulation. Because it is relatively stable, DNA methylation can be inherited by offspring DNA by replication.56 Differences in the methylation status of gene promoter regions may change due to environmental variation, and these differences thus affect biological processes.57,58 These methylation changes can inhibit gene expression by preventing the binding of transcription factors.59 Once methylation occurs in early life, these patterns may be stably maintained throughout the whole lifespan.60 Increasing evidence has revealed that DNA methylation is an important link between the maternal metabolic status and the long-term health of offspring.61
To understand the biological processes that may be regulated by maternal HFD and inulin intervention, we performed a DNA methylation array of the hypothalamus of offspring. Eight hundred and eighty-nine hypermethylated regions and 480 hypomethylated regions were found in the MHFD + inulin offspring compared with the MHFD offspring. GO and KEGG enrichment analyses revealed that pathways were enriched in the term cytokine–cytokine receptor interaction. Moreover, our results revealed that maternal inulin intervention activated the methylation of Il6 in the hypothalamus. Il6 expression was decreased in male offspring from the MHFD + inulin dams. Obesity has been considered low-grade chronic inflammation, including peripheral inflammation and central nervous system (CNS) inflammation.62 The hypothalamus is the most sensitive region exposed to HFD.63,64 The hypothalamic inflammatory response induced by a HFD may lead to insulin and leptin resistance and excess food intake.65,66 Hypothalamic inflammatory signaling is active within 1 to 3 days after HFD intake, which is sooner than the occurrence of peripheral inflammation.67 The inflammatory response in the hypothalamus to a HFD is mediated by Toll-like receptors (TLRs) and nuclear factor kappa-β (NF-κβ), and inflammatory cytokines, such as interleukin (IL)-1β and IL-6, are then activated.68 Our results showed that maternal inulin intervention moderated offspring CNS inflammation through DNA methylation patterns.
In the MHFD + inulin group, we also observed hypermethylation and low gene expression of Socs3. SOCS3 is a member of the SOCS family which is a negative regulator of cytokine signaling.69 In obesity, chronic Janus kinase (JAK)-signal transducer and activator of transcription (STAT3)-SOCS3 signaling in the CNS is activated by leptin resistance.70 Pedroso et al. reported that SOCS3 expression is higher in the hypothalamus of obese mice.71 More interestingly, ameliorated leptin sensitivity and resistance to HFD are observed in Socs3-haploinsufficient mice.72 In children, SOCS3 gene polymorphisms are significantly associated with obesity.73 Moreover, SOCS3 methylation is inversely correlated with the BMI in children, adolescents and adults and is thought to mediate the effect of the early life environment on adult metabolism.74 Zhu et al. provided evidence showing that maternal Socs3 knockdown attenuates the obesity phenotype and metabolic disorder in offspring.75
Our results showed that the MHFD offspring exhibited high serum leptin levels. However, hypermethylation and lower expression of the hypothalamic Lepr gene were observed in the offspring from the MHFD dams compared with the offspring from the control dams. These results indicate that maternal HFD leads to leptin resistance in male offspring. Male offspring mice from the MHFD + inulin dams had lower Lepr gene methylation levels, and higher Lepr expression. Moreover, the serum leptin levels were lower in male offspring from the MHFD + inulin dams. Leptin is secreted from adipose tissue and regulates appetite through the leptin receptor in the hypothalamus. Obese subjects have high levels of circulating leptin and damaged leptin effects in the hypothalamus, which is called leptin resistance.76 Maternal obesity programs leptin signaling impairment in offspring at birth.77 Leptin is a major regulator of food intake and energy homeostasis in animals.78 Leptin receptors are mainly expressed in the hypothalamus. In the hypothalamus, leptin binds to its receptor to trigger a series of chemical signals that reduce hunger. Thus, the hypomethylation and high expression of Lepr in the MHFD + inulin group observed in our study may explain the reduction in food intake in male offspring from dams provided maternal inulin intervention.
In addition, we found that maternal inulin intervention activated Npy methylation and inhibited its expression in the hypothalamus of male offspring. Neuropeptides released from the hypothalamus are regulated by nutrients and peripheral signals, such as leptin.79 Hypothalamic NPY neurons are considered key regulators between maternal under- and overnutrition and the risk of obesity in offspring.80–82 Maternal animal models with undernutrition during pregnancy lead to a reduction in NPY expression in the hypothalamus of offspring.83–86 Plagemann et al.87 demonstrated that neonatal overfed rats exhibit reduced NPY methylation in the hypothalamus. The mRNA expression levels of orexigenic Npy were significantly increased in the offspring mice of obese mothers.88 A maternal HFD, high-sugar diet increased NPY expression in offspring in adulthood.89 Song et al. found that maternal exercise decreased the hypothalamic content of NPY in female offspring at postnatal day 21. However, these studies did not find altered expression of hypothalamic NPY in 15-week-old female offspring rats.90 These different results may be due to the sex differences in the programming of food preference in offspring from HFD-fed dams.91,92 In our study, at 16 weeks of age, male offspring in the MHFD + inulin group exhibited a lower energy intake than those in the MHFD group. Hypermethylation and lower expression of Npy may partly explain the appetite inhibition observed in male offspring from the dams provided maternal inulin intervention. Together with our results, these results indicate that maternal inulin intervention activates Npy and Socs3 methylation and inhibits Lepr methylation in the hypothalamus of offspring. This result shows that the methylation of feeding circuit-related genes in the hypothalamus is a key mechanism linking maternal inulin intervention with food intake reduction and lipid metabolic benefits in male offspring.
Our results are limited by several factors. First, in this study, only male offspring were investigated. Further studies should be performed to explore the effect of maternal inulin intervention in both male and female offspring. Additionally, inulin treatment reduced the dam body weight and may also reduce the production of milk. As such, the milk amount should be measured in further studies.
Conclusion
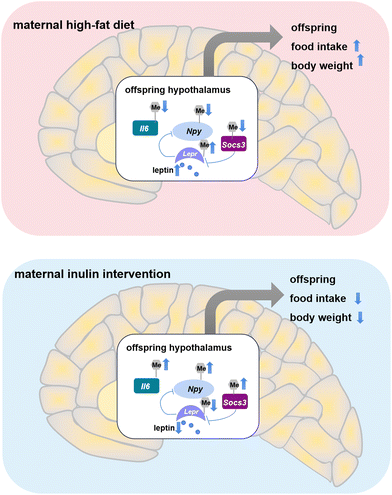
In summary, early maternal inulin intervention during pregnancy and lactation ameliorates lipid metabolic disorders in offspring induced by maternal HFD in adulthood. This moderation is mediated by epigenetic programming of the hypothalamus food intake circuit in adult offspring. Although some studies focused on the effect of the genes involved in the feeding circuit pathway, the changes were only observed at birth and weaning. Importantly, this study is the first to reveal that DNA methylation of Socs3, Npy, Il6 and Lepr is a key mechanism for the link between early maternal intervention and offspring metabolism benefits (Fig. 7). These novel findings highlight DNA methylation in the CNS as a potential target of inulin treatment.Author contributions
XHX: conceptualization and funding acquisition; QZ, JZ, and TW: investigation; MY, ML, and FP: data curation; QZ: writing – original draft; all authors: writing – review and editing.Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China (No. 81870545, 82170854, 81870579, 81570715, 81170736), the Beijing Natural Science Foundation (7202163), the Beijing Municipal Science & Technology Commission (Z201100005520011), the National Key Research and Development Program of China (2021YFC2501700, 2016YFA0101002, 2018YFC2001100), the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-019), the Scientific Activities Foundation for Selected Returned Overseas Professionals of Human Resources and Social Security Ministry, the Beijing Dongcheng District Outstanding Talent Funding Project (2019DCT-M-05), and the CAMS Innovation Fund for Medical Sciences (CIFMS2021-1-I2M-002, CIFMS2017-I2M-1-008).References
- C. L. Ogden, M. D. Carroll, B. K. Kit and K. M. Flegal, Prevalence of childhood and adult obesity in the United States, 2011-2012, J. Am. Med. Assoc., 2014, 311, 806–814 CrossRef CAS PubMed.
- M. Aras, B. G. Tchang and J. Pape, Obesity and Diabetes, Nurs. Clin. North Am., 2021, 56, 527–541 CrossRef.
- L. Li, D. W. Liu, H. Y. Yan, Z. Y. Wang, S. H. Zhao and B. Wang, Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies, Obes. Rev., 2016, 17, 510–519 CrossRef CAS PubMed.
- B. Zhou, Y. Wu, J. Yang, Y. Li, H. Zhang and L. Zhao, Overweight is an independent risk factor for cardiovascular disease in Chinese populations, Obes. Rev., 2002, 3, 147–156 CrossRef.
- X. Liu, W. Ju, C. Huo, S. Zhang, X. Wang and K. Huang, Overweight and Obesity as Independent Factors for Increased Risk of Hepatocellular Cancer-Related Mortality: A Meta-Analysis, J. Am. Coll. Nutr., 2021, 40, 287–293 CrossRef.
- D. K. Longmore, J. E. Miller, S. Bekkering, C. Saner, E. Mifsud, Y. Zhu, R. Saffery, A. Nichol, G. Colditz, K. R. Short and D. P. Burgner, Diabetes and Overweight/Obesity Are Independent, Nonadditive Risk Factors for In-Hospital Severity of COVID-19: An International, Multicenter Retrospective Meta-analysis, Diabetes Care, 2021, 44, 1281–1290 CrossRef CAS PubMed.
- T. E. O'Brien, J. G. Ray and W. S. Chan, Maternal body mass index and the risk of preeclampsia: a systematic overview, Epidemiology, 2003, 14, 368–374 CrossRef.
- S. Johansson, E. Villamor, M. Altman, A. K. Bonamy, F. Granath and S. Cnattingius, Maternal overweight and obesity in early pregnancy and risk of infant mortality: a population based cohort study in Sweden, BMJ), 2014, 349, g6572 CrossRef.
- N. Heslehurst, H. Simpson, L. J. Ells, J. Rankin, J. Wilkinson, R. Lang, T. J. Brown and C. D. Summerbell, The impact of maternal BMI status on pregnancy outcomes with immediate short-term obstetric resource implications: a meta-analysis, Obes. Rev., 2008, 9, 635–683 CrossRef CAS.
- A. P. Starling, J. T. Brinton, D. H. Glueck, A. L. Shapiro, C. S. Harrod, A. M. Lynch, A. M. Siega-Riz and D. Dabelea, Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study, Am. J. Clin. Nutr., 2015, 101, 302–309 CrossRef CAS.
- G. C. Curhan, W. C. Willett, E. B. Rimm, D. Spiegelman, A. L. Ascherio and M. J. Stampfer, Birth weight and adult hypertension, diabetes mellitus, and obesity in US men, Circulation, 1996, 94, 3246–3250 CrossRef CAS.
- G. C. Curhan, G. M. Chertow, W. C. Willett, D. Spiegelman, G. A. Colditz, J. E. Manson, F. E. Speizer and M. J. Stampfer, Birth weight and adult hypertension and obesity in women, Circulation, 1996, 94, 1310–1315 CrossRef CAS.
- T. J. Parsons, C. Power, S. Logan and C. D. Summerbell, Childhood predictors of adult obesity: a systematic review, Int. J. Obes. Relat. Metab. Disord., 1999, 23(Suppl 8), S1–107 Search PubMed.
- L. Graversen, T. I. Sørensen, T. A. Gerds, L. Petersen, U. Sovio, M. Kaakinen, A. Sandbaek, J. Laitinen, A. Taanila, A. Pouta, M. R. Järvelin and C. Obel, Prediction of adolescent and adult adiposity outcomes from early life anthropometrics, Obesity, 2015, 23, 162–169 CrossRef PubMed.
- J. Yeh and J. Shelton, Reasons for increasing trends in large for gestational age births, Obstet. Gynecol., 2005, 105, 444 CrossRef ; author reply 444–445.
- R. M. Reynolds, K. M. Allan, E. A. Raja, S. Bhattacharya, G. McNeill, P. C. Hannaford, N. Sarwar, A. J. Lee, S. Bhattacharya and J. E. Norman, Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years, BMJ, 2013, 347, f4539 CrossRef PubMed.
- M. B. Roberfroid, Concepts in functional foods: the case of inulin and oligofructose, J. Nutr., 1999, 129, 1398s–1401s CrossRef CAS.
- A. Trompette, E. S. Gollwitzer, K. Yadava, A. K. Sichelstiel, N. Sprenger, C. Ngom-Bru, C. Blanchard, T. Junt, L. P. Nicod, N. L. Harris and B. J. Marsland, Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis, Nat. Med., 2014, 20, 159–166 CrossRef CAS PubMed.
- B. L. Pool-Zobel, Inulin-type fructans and reduction in colon cancer risk: review of experimental and human data, Br. J. Nutr., 2005, 93(Suppl 1), S73–S90 CrossRef CAS PubMed.
- K. Weitkunat, S. Schumann, D. Nickel, K. A. Kappo, K. J. Petzke, A. P. Kipp, M. Blaut and S. Klaus, Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity, Mol. Nutr. Food Res., 2016, 60, 2611–2621 CrossRef CAS PubMed.
- E. M. Dewulf, P. D. Cani, A. M. Neyrinck, S. Possemiers, A. Van Holle, G. G. Muccioli, L. Deldicque, L. B. Bindels, B. D. Pachikian, F. M. Sohet, E. Mignolet, M. Francaux, Y. Larondelle and N. M. Delzenne, Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice, J. Nutr. Biochem., 2011, 22, 712–722 CrossRef CAS.
- N. Salazar, E. M. Dewulf, A. M. Neyrinck, L. B. Bindels, P. D. Cani, J. Mahillon, W. M. de Vos, J. P. Thissen, M. Gueimonde, C. G. de Los Reyes-Gavilán and N. M. Delzenne, Inulin-type fructans modulate intestinal Bifidobacterium species populations and decrease fecal short-chain fatty acids in obese women, Clin. Nutr., 2015, 34, 501–507 CrossRef CAS PubMed.
- A. Selle, C. Brosseau, W. Dijk, A. Duval, G. Bouchaud, A. Rousseaux, A. Bruneau, C. Cherbuy, M. Mariadassou, V. Cariou, S. Barbarot and M. Bodinier, Prebiotic Supplementation During Gestation Induces a Tolerogenic Environment and a Protective Microbiota in Offspring Mitigating Food Allergy, Front. Immunol., 2021, 12, 745535 CrossRef CAS.
- Q. Zhang, X. Xiao, J. Zheng, M. Li, M. Yu, F. Ping, T. Wang and X. Wang, Maternal Inulin Supplementation Alters Hepatic DNA Methylation Profile and Improves Glucose Metabolism in Offspring Mice, Front. Physiol., 2020, 11, 70 CrossRef.
- Q. Zhang, X. Xiao, J. Zheng, M. Li, M. Yu, F. Ping, T. Wang and X. Wang, Influence of Maternal Inulin-Type Prebiotic Intervention on Glucose Metabolism and Gut Microbiota in the Offspring of C57BL Mice, Front. Endocrinol., 2019, 10, 675 CrossRef PubMed.
- M. W. Schwartz, S. C. Woods, D. Porte Jr., R. J. Seeley and D. G. Baskin, Central nervous system control of food intake, Nature, 2000, 404, 661–671 CrossRef CAS PubMed.
- I. Cakir and E. A. Nillni, Endoplasmic Reticulum Stress, the Hypothalamus, and Energy Balance, Trends Endocrinol. Metab., 2019, 30, 163–176 CrossRef CAS.
- S. G. Bouret, S. J. Draper and R. B. Simerly, Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice, J. Neurosci., 2004, 24, 2797–2805 CrossRef CAS PubMed.
- S. G. Bouret, S. J. Draper and R. B. Simerly, Trophic action of leptin on hypothalamic neurons that regulate feeding, Science, 2004, 304, 108–110 CrossRef CAS.
- K. Bouyer and R. B. Simerly, Neonatal leptin exposure specifies innervation of presympathetic hypothalamic neurons and improves the metabolic status of leptin-deficient mice, J. Neurosci., 2013, 33, 840–851 CrossRef CAS.
- L. M. Zeltser, Feeding circuit development and early-life influences on future feeding behaviour, Nat. Rev. Neurosci., 2018, 19, 302–316 CrossRef CAS PubMed.
- M. Franzago, F. Fraticelli, L. Stuppia and E. Vitacolonna, Nutrigenetics, epigenetics and gestational diabetes: consequences in mother and child, Epigenetics, 2019, 14, 215–235 CrossRef.
- D. Dabelea, The predisposition to obesity and diabetes in offspring of diabetic mothers, Diabetes Care, 2007, 30(Suppl 2), S169–S174 CrossRef.
- H. Zhu, B. Chen, Y. Cheng, Y. Zhou, Y. S. Yan, Q. Luo, Y. Jiang, J. Z. Sheng, G. L. Ding and H. F. Huang, Insulin Therapy for Gestational Diabetes Mellitus Does Not Fully Protect Offspring From Diet-Induced Metabolic Disorders, Diabetes, 2019, 68, 696–708 CrossRef CAS PubMed.
- Q. Zhang, X. Xiao, J. Zheng, M. Li, M. Yu, F. Ping, T. Wang and X. Wang, Maternal High-Fat Diet Disturbs the DNA Methylation Profile in the Brown Adipose Tissue of Offspring Mice, Front. Endocrinol., 2021, 12, 705827 CrossRef PubMed.
- R. Aoki, M. Onuki, K. Hattori, M. Ito, T. Yamada, K. Kamikado, Y. G. Kim, N. Nakamoto, I. Kimura, J. M. Clarke, T. Kanai and K. Hase, Commensal microbe-derived acetate suppresses NAFLD/NASH development via hepatic FFAR2 signalling in mice, Microbiome, 2021, 9, 188 CrossRef CAS.
- P. D. Cani, S. Possemiers, T. Van de Wiele, Y. Guiot, A. Everard, O. Rottier, L. Geurts, D. Naslain, A. Neyrinck, D. M. Lambert, G. G. Muccioli and N. M. Delzenne, Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability, Gut, 2009, 58, 1091–1103 CrossRef CAS.
- G. P. Keith and B. J. Franklin, The Mouse Brain in Stereotaxic Coordinates, Elsevier Academic Press, Amsterdam, Boston, 3rd edn, 2007 Search PubMed.
- E. G. Bligh and W. J. Dyer, A rapid method of total lipid extraction and purification, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS.
- B. T. Sherman, M. Hao, J. Qiu, X. Jiao, M. W. Baseler, H. C. Lane, T. Imamichi and W. Chang, DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update), Nucleic Acids Res., 2022, 50, W216–W221 CrossRef CAS PubMed.
- Y. T. Chen, Y. Hu, Q. Y. Yang, J. S. Son, X. D. Liu, J. M. de Avila, M. J. Zhu and M. Du, Excessive Glucocorticoids During Pregnancy Impair Fetal Brown Fat Development and Predispose Offspring to Metabolic Dysfunctions, Diabetes, 2020, 69, 1662–1674 CrossRef CAS.
- Y. T. Chen, Q. Y. Yang, Y. Hu, X. D. Liu, J. M. de Avila, M. J. Zhu, P. W. Nathanielsz and M. Du, Imprinted lncRNA Dio3os preprograms intergenerational brown fat development and obesity resistance, Nat. Commun., 2021, 12, 6845 CrossRef CAS.
- H. Liu, L. Zhang, Z. Niu, M. Zhou, C. Peng, X. Li, T. Deng, L. Shi, Y. Tan and G. Li, Promoter methylation inhibits BRD7 expression in human nasopharyngeal carcinoma cells, BMC Cancer, 2008, 8, 253 CrossRef PubMed.
- P. Zhou, Y. Zhao, P. Zhang, Y. Li, T. Gui, J. Wang, C. Jin, L. Che, J. Li, Y. Lin, S. Xu, B. Feng, Z. Fang and D. Wu, Microbial Mechanistic Insight into the Role of Inulin in Improving Maternal Health in a Pregnant Sow Model, Front. Microbiol., 2017, 8, 2242 CrossRef.
- M. Miao, Q. Wang, X. Wang, C. Fan, T. Luan, L. Yan, Y. Zhang, X. Zeng, Y. Dai and P. Li, The Protective Effects of Inulin-Type Fructans Against High-Fat/Sucrose Diet-Induced Gestational Diabetes Mice in Association With Gut Microbiota Regulation, Front. Microbiol., 2022, 13, 832151 CrossRef PubMed.
- M. Alligier, E. M. Dewulf, N. Salazar, A. Mairal, A. M. Neyrinck, P. D. Cani, D. Langin and N. M. Delzenne, Positive interaction between prebiotics and thiazolidinedione treatment on adiposity in diet-induced obese mice, Obesity, 2014, 22, 1653–1661 CrossRef CAS.
- N. L. Cluny, L. K. Eller, C. M. Keenan, R. A. Reimer and K. A. Sharkey, Interactive effects of oligofructose and obesity predisposition on gut hormones and microbiota in diet-induced obese rats, Obesity, 2015, 23, 769–778 CrossRef CAS PubMed.
- J. Liu, N. S. Boghossian, E. A. Frongillo, B. Cai, L. J. Hazlett and J. Liu, Associations of maternal gestational weight gain with the risk of offspring obesity and body mass index Z scores beyond the mean, Ann. Epidemiol., 2019, 32, 64–71 CrossRef PubMed.
- G. J. Howie, D. M. Sloboda, T. Kamal and M. H. Vickers, Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet, J. Physiol., 2009, 587, 905–915 CrossRef CAS PubMed.
- C. Saullo, L. L. D. Cruz, D. C. Damasceno, G. T. Volpato, Y. K. Sinzato, B. Karki, F. Q. Gallego and G. Vesentini, Effects of a maternal high-fat diet on adipose tissue in murine offspring: A systematic review and meta-analysis, Biochimie, 2022, 201, 18–32 CrossRef CAS PubMed.
- Y. Guo, Z. Wang, L. Chen, L. Tang, S. Wen, Y. Liu and J. Yuan, Diet induced maternal obesity affects offspring gut microbiota and persists into young adulthood, Food Funct., 2018, 9, 4317–4327 RSC.
- S. Lecoutre, F. Oger, C. Pourpe, L. Butruille, L. Marousez, A. Dickes-Coopman, C. Laborie, C. Guinez, J. Lesage, D. Vieau, C. Junien, D. Eberlé, A. Gabory, J. Eeckhoute and C. Breton, Maternal obesity programs increased leptin gene expression in rat male offspring via epigenetic modifications in a depot-specific manner, Mol. Metab., 2017, 6, 922–930 CrossRef CAS.
- A. M. Melo, R. O. Benatti, L. M. Ignacio-Souza, C. Okino, A. S. Torsoni, M. Milanski, L. A. Velloso and M. A. Torsoni, Hypothalamic endoplasmic reticulum stress and insulin resistance in offspring of mice dams fed high-fat diet during pregnancy and lactation, Metabolism, 2014, 63, 682–692 CrossRef CAS.
- E. Gregoraszczuk, M. Slupecka, J. Wolinski, A. Hejmej, B. Bilinska, E. Fiedor, N. Piwnicka and A. Rak, Maternal high-fat diet during pregnancy and lactation had gender difference effect on adiponectin in rat offspring, J. Physiol. Pharmacol., 2016, 67, 543–553 CAS.
- N. Desbuards, P. Gourbeyre, V. Haure-Mirande, D. Darmaun, M. Champ and M. Bodinier, Impact of perinatal prebiotic consumption on gestating mice and their offspring: a preliminary report, Br. J. Nutr., 2012, 107, 1245–1248 CrossRef CAS.
- X. Pan, D. Gong, D. N. Nguyen, X. Zhang, Q. Hu, H. Lu, M. Fredholm, P. T. Sangild and F. Gao, Early microbial colonization affects DNA methylation of genes related to intestinal immunity and metabolism in preterm pigs, DNA Res., 2018, 25, 287–296 CrossRef CAS.
- G. S. Prins, S. H. Ye, L. Birch, X. Zhang, A. Cheong, H. Lin, E. Calderon-Gierszal, J. Groen, W. Y. Hu, S. M. Ho and R. B. van Breemen, Prostate Cancer Risk and DNA Methylation Signatures in Aging Rats following Developmental BPA Exposure: A Dose-Response Analysis, Environ. Health Perspect., 2017, 125, 077007 CrossRef.
- J. Z. Maccani, D. C. Koestler, E. A. Houseman, D. A. Armstrong, C. J. Marsit and K. T. Kelsey, DNA methylation changes in the placenta are associated with fetal manganese exposure, Reprod. Toxicol., 2015, 57, 43–49 CrossRef CAS.
- D. Schübeler, Function and information content of DNA methylation, Nature, 2015, 517, 321–326 CrossRef.
- H. Liu, J. Wang, D. Mou, L. Che, Z. Fang, B. Feng, Y. Lin, S. Xu, J. Li and D. Wu, Maternal Methyl Donor Supplementation during Gestation Counteracts the Bisphenol A-Induced Impairment of Intestinal Morphology, Disaccharidase Activity, and Nutrient Transporters Gene Expression in Newborn and Weaning Pigs, Nutrients, 2017, 9, 423 CrossRef PubMed.
- K. A. Lillycrop and G. C. Burdge, Epigenetic mechanisms linking early nutrition to long term health, Best Pract. Res., Clin. Endocrinol. Metab., 2012, 26, 667–676 CrossRef.
- B. Misiak, J. Leszek and A. Kiejna, Metabolic syndrome, mild cognitive impairment and Alzheimer's disease–the emerging role of systemic low-grade inflammation and adiposity, Brain Res. Bull., 2012, 89, 144–149 CrossRef CAS.
- L. M. Williams, Hypothalamic dysfunction in obesity, Proc. Nutr. Soc., 2012, 71, 521–533 CrossRef CAS PubMed.
- J. P. Thaler, S. J. Guyenet, M. D. Dorfman, B. E. Wisse and M. W. Schwartz, Hypothalamic inflammation: marker or mechanism of obesity pathogenesis?, Diabetes, 2013, 62, 2629–2634 CrossRef CAS PubMed.
- C. T. De Souza, E. P. Araujo, S. Bordin, R. Ashimine, R. L. Zollner, A. C. Boschero, M. J. Saad and L. A. Velloso, Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus, Endocrinology, 2005, 146, 4192–4199 CrossRef CAS.
- J. B. Carvalheira, E. B. Ribeiro, E. P. Araújo, R. B. Guimarães, M. M. Telles, M. Torsoni, J. A. Gontijo, L. A. Velloso and M. J. Saad, Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats, Diabetologia, 2003, 46, 1629–1640 CrossRef CAS PubMed.
- J. P. Thaler, C. X. Yi, E. A. Schur, S. J. Guyenet, B. H. Hwang, M. O. Dietrich, X. Zhao, D. A. Sarruf, V. Izgur, K. R. Maravilla, H. T. Nguyen, J. D. Fischer, M. E. Matsen, B. E. Wisse, G. J. Morton, T. L. Horvath, D. G. Baskin, M. H. Tschöp and M. W. Schwartz, Obesity is associated with hypothalamic injury in rodents and humans, J. Clin. Invest., 2012, 122, 153–162 CrossRef CAS.
- M. Milanski, G. Degasperi, A. Coope, J. Morari, R. Denis, D. E. Cintra, D. M. Tsukumo, G. Anhe, M. E. Amaral, H. K. Takahashi, R. Curi, H. C. Oliveira, J. B. Carvalheira, S. Bordin, M. J. Saad and L. A. Velloso, Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity, J. Neurosci., 2009, 29, 359–370 CrossRef CAS.
- D. L. Krebs and D. J. Hilton, SOCS proteins: negative regulators of cytokine signaling, Stem Cells, 2001, 19, 378–387 CrossRef CAS PubMed.
- C. M. Wunderlich, N. Hövelmeyer and F. T. Wunderlich, Mechanisms of chronic JAK-STAT3-SOCS3 signaling in obesity, JAK-STAT, 2013, 2, e23878 CrossRef.
- J. A. Pedroso, D. C. Buonfiglio, L. I. Cardinali, I. C. Furigo, A. M. Ramos-Lobo, J. Tirapegui, C. F. Elias and J. Donato Jr., Inactivation of SOCS3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity, Mol. Metab., 2014, 3, 608–618 CrossRef CAS PubMed.
- J. K. Howard, B. J. Cave, L. J. Oksanen, I. Tzameli, C. Bjørbaek and J. S. Flier, Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3, Nat. Med., 2004, 10, 734–738 CrossRef CAS PubMed.
- M. Boyraz, E. Yeşilkaya, F. Ezgü, A. Bideci, H. Doğan, K. Ulucan and P. Cinaz, Effect of Cytokine Signaling 3 Gene Polymorphisms in Childhood Obesity, J. Clin. Res. Pediatr. Endocrinol., 2016, 8, 452–460 CrossRef PubMed.
- O. Ali, D. Cerjak, J. W. Kent Jr., R. James, J. Blangero, M. A. Carless and Y. Zhang, Methylation of SOCS3 is inversely associated with metabolic syndrome in an epigenome-wide association study of obesity, Epigenetics, 2016, 11, 699–707 CrossRef PubMed.
- F. Zhu, D. Zhang, F. Shen, K. Xu, X. Huang, J. Liu, J. Zhang and Y. Teng, Maternal Socs3 knockdown attenuates postnatal obesity caused by an early life environment of maternal obesity and intrauterine overnutrition in progeny mice, IUBMB Life, 2021, 73, 1210–1221 CrossRef CAS.
- J. A. B. Pedroso, A. M. Ramos-Lobo and J. Donato Jr., SOCS3 as a future target to treat metabolic disorders, Hormones, 2019, 18, 127–136 CrossRef PubMed.
- M. J. Morris and H. Chen, Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth, Int. J. Obes., 2009, 33, 115–122 CrossRef CAS.
- J. Wang, R. Liu, M. Hawkins, N. Barzilai and L. Rossetti, A nutrient-sensing pathway regulates leptin gene expression in muscle and fat, Nature, 1998, 393, 684–688 CrossRef CAS PubMed.
- M. López, S. Tovar, M. J. Vázquez, L. M. Williams and C. Diéguez, Peripheral tissue-brain interactions in the regulation of food intake, Proc. Nutr. Soc., 2007, 66, 131–155 CrossRef.
- T. M. Loftus, An adipocyte-central nervous system regulatory loop in the control of adipose homeostasis, Semin. Cell Dev. Biol., 1999, 10, 11–18 CrossRef CAS PubMed.
- T. Gali Ramamoorthy, G. Begum, E. Harno and A. White, Developmental programming of hypothalamic neuronal circuits: impact on energy balance control, Front. Neurosci., 2015, 9, 126 Search PubMed.
- S. Arora and Anubhuti, Role of neuropeptides in appetite regulation and obesity–a review, Neuropeptides, 2006, 40, 375–401 CrossRef CAS.
- P. L. Terroni, F. W. Anthony, M. A. Hanson and F. R. Cagampang, Expression of agouti-related peptide, neuropeptide Y, pro-opiomelanocortin and the leptin receptor isoforms in fetal mouse brain from pregnant dams on a protein-restricted diet, Mol. Brain Res., 2005, 140, 111–115 CrossRef CAS PubMed.
- A. Plagemann, T. Waas, T. Harder, F. Rittel, T. Ziska and W. Rohde, Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats, Neuropeptides, 2000, 34, 1–6 CrossRef CAS PubMed.
- S. Lee, E. J. Kwon, Y. A. You, J. E. Du, I. Jo and Y. J. Kim, Long-term effects of pro-opiomelanocortin methylation induced in food-restricted dams on metabolic phenotypes in male rat offspring, Obstet. Gynecol. Sci., 2020, 63, 239–250 CrossRef PubMed.
- B. Coupé, V. Amarger, I. Grit, A. Benani and P. Parnet, Nutritional programming affects hypothalamic organization and early response to leptin, Endocrinology, 2010, 151, 702–713 CrossRef.
- A. Plagemann, T. Harder, M. Brunn, A. Harder, K. Roepke, M. Wittrock-Staar, T. Ziska, K. Schellong, E. Rodekamp, K. Melchior and J. W. Dudenhausen, Hypothalamic proopiomelanocortin promoter methylation becomes altered by early overfeeding: an epigenetic model of obesity and the metabolic syndrome, J. Physiol., 2009, 587, 4963–4976 CrossRef CAS PubMed.
- L. Dearden, S. Buller, I. C. Furigo, D. S. Fernandez-Twinn and S. E. Ozanne, Maternal obesity causes fetal hypothalamic insulin resistance and disrupts development of hypothalamic feeding pathways, Mol. Metab., 2020, 42, 101079 CrossRef CAS PubMed.
- H. César, M. Nascimento Sertorio, A. Santamarina, E. Alves de Souza, L. Valles Mennitti, G. Jamar, A. Jucá, B. Picin Casagrande, D. Estadela and L. Pellegrini Pisani, The influence of parental high-fat high-sugar diet on the gut-brain axis in male offspring, Food Res. Int., 2022, 160, 111706 CrossRef PubMed.
- L. Song, J. Cui, N. Wang, R. Wang, J. Yan and B. Sun, Maternal exercise during gestation and lactation decreases high-fat diet preference by altering central reward system gene expression in adult female offspring from high-fat fed dams, Behav. Brain Res., 2020, 390, 112660 CrossRef CAS PubMed.
- C. P. Dias-Rocha, M. M. Almeida, E. M. Santana, J. C. B. Costa, J. G. Franco, C. C. Pazos-Moura and I. H. Trevenzoli, Maternal high-fat diet induces sex-specific endocannabinoid system changes in newborn rats and programs adiposity, energy expenditure and food preference in adulthood, J. Nutr. Biochem., 2018, 51, 56–68 CrossRef CAS PubMed.
- Z. Y. Ong and B. S. Muhlhausler, Consuming a low-fat diet from weaning to adulthood reverses the programming of food preferences in male, but not in female, offspring of ‘junk food’-fed rat dams, Acta Physiol., 2014, 210, 127–141 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |