The targeted development of collagen-active peptides based on composite enzyme hydrolysis: a study on the structure–activity relationship†
Xinnuo
Hu
 ab,
Yanjun
Yang
*ab,
Cuihua
Chang
*ab,
Junhua
Li
ab,
Yujie
Su
ab and
Luping
Gu
ab
ab,
Yanjun
Yang
*ab,
Cuihua
Chang
*ab,
Junhua
Li
ab,
Yujie
Su
ab and
Luping
Gu
ab
aSchool of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, PR China. E-mail: yangyj@jiangnan.edu.cn; chang.cuihua@jiangnan.edu.cn
bState Key Laboratory of Food Science and Technology, Jiangnan University, Wuxi, Jiangsu 214122, China
First published on 1st December 2023
Abstract
Fish collagen, derived from sustainable sources, offers a valuable substrate for generating peptides with diverse biofunctionalities. In this study, alkaline, papain, and ginger protease were used to enzymatically hydrolyze fish skin collagen. The peptide molecular weight distribution and sequence were measured using HPLC and ICP-MS-MS, with papain/alkaline protease (AP) and papain/alkaline/ginger protease (APG) hydrolyzed samples compared. As the results showed, the incorporation of ginger protease was useful for increasing the degree of hydrolysis, with the content of <400 Da peptides increasing from 49.82% to 58.56%. The identified peptide sequence in the APG sample had more proline at the C-terminal. The peptides were separated into two components (different in molecular weight) using gel column chromatography. The molecular weight distribution, amino acid composition, ACE inhibitory activity, and fibroblast proliferation activity of the collected components were measured. In comparison, the contents of proline and hydroxyproline in the larger peptides decreased obviously after combined hydrolysis by ginger protease, reflecting the formation of a peptide sequence of smaller molecular weight containing glycine and hydroxyproline. The combined hydrolysis of ginger protease was beneficial for the improvement of the ACE inhibitory activity of the sample. However, the fibroblast proliferation activity of AP was higher than that of APG, indicating that further hydrolysis by ginger protease may destroy the hydroxyproline at the end of the peptide sequence. This study proposed a creative directional hydrolysis method and provided practical guidance for the production of collagen peptides with enhanced functional activity.
1. Introduction
According to various estimates, waste products from fish processing can be up to 85% of the total processing capacity. A significant percentage of the waste products (about 30%) is skin, bones, and scales with a high collagen content.1 These resources put a lot of pressure on the environment if they are discarded and buried. However, they can be processed into valuable products.2 Edible aquatic animal-derived collagen has weaker structural and thermal stability than mammalian collagen, due to the lower content of amino acids, such as proline and hydroxyproline.3 However, edible aquatic animal-derived collagen is more easily hydrolyzed by enzymes than mammalian collagen and is more suitable for the preparation of bioactive peptides.4 Studies on fish collagen peptides have mainly been focused on their functional effect on skin health, such as diminishing inflammation, promoting fibroblast proliferation, and enhancing hyaluronic acid production in human skin.5 Recent research has demonstrated that biomaterials derived from fish skin have the potential to be used as scaffold materials owing to their good biodegradability and biocompatibility in human tissues.6 Furthermore, the biocompatibility and cell proliferation bioactivity of fish skin collagen can fit the standards of medical materials.7 Another biological function of fish collagen peptide is ACE inhibitory activity. As reported, tripeptides and tetrapeptides have been recommended as ideal candidates for seeking potential peptide additives in functional foods with satisfactory ACE binding.8 In addition, 380–920 Da fish collagen peptides are responsible for exhibiting ACE-inhibitory activity.9 Some research studies also describe the bone health-maintaining function of fish collagen peptides, such as accelerating bone fracture healing through the stimulation of osteoblast calcification or providing novel candidate implants for bone repair, and boosting sustainable utilization of bio-resources.10,11The degree of hydrolysis is closely related to the characteristics of collagen peptides, as the functionality and bioactivity of peptides rely on the size, amino acid type and sequence of the hydrolysates. The special triple helix structure of collagen leads to its indigestibility, while over-hydrolysis may release peptides with no functional or bioactive properties,12 indicating the importance of regulating the hydrolysis degree to maximize the functionality of peptides. A large number of recent studies have proposed that small molecular peptides obtained after collagen hydrolysis are more easily absorbed by the body and detected in the blood.13,14 Shoko et al. reported that ingestion of small molecular collagen peptides can improve the efficiency of peptide absorption into the blood.15 This suggests that active transporters, such as oligopeptide transporters or PepT1, might be involved in the permeation of small peptides across the intestinal epithelial layer.16,17 However, this does not mean the higher degree of hydrolysis would result in higher biological activity of collagen peptide. A medium degree of enzymatic hydrolysis would be appropriate for good antioxidant activity while small peptides are essential to obtain high ACE-inhibitory activity,18,19 A 5–10 kDa fraction of the collagen peptides obtained would be good tyrosinase and collagenase inhibitors with the best potential antiaging activity.20 Therefore, the relationship between the degree of hydrolysis and the function of collagen is complex, which needs further research work to explore potential collagen peptides with high physiological activity.
Each strand of collagen consists of the repeating sequence Gly-Xaa-Yaa, where Xaa and Yaa are often proline (Pro) residues. The post-translational hydroxylation of the proline residues would generate 4(R)-hydroxy-L-proline (4-Hyp) residues or 3(S)-hydroxy-L-proline (3-Hyp) residues.21 Enzyme selection is critical for collagen hydrolysis degree and activity. In this study, alkaline protease and papain were selected as the non-specific enzymes to hydrolyze collagen. Alkaline protease has been documented as the most cost-effective for hydrolyzing fish proteins, which can effectively degrade the triple-helix structure of collagen within a short time, breaking peptide bonds from nonterminal amino acids randomly and facilitating further protein hydrolysis.22 In addition, some researchers conducted peptide cutter analysis with BIOPEP, suggesting that papain can release the highest number of potential angiotensin converting enzyme (ACE)-inhibitory peptides from alpha collagen. However, it is difficult for non-specific enzymes to hydrolyze the proline C-terminal, limiting the increase in the hydrolysis degree and release of terminal proline.23,24 Therefore, in order to increase the hydrolysis degree of collagen, specific enzymes are required for peptide chains that are difficult to hydrolyze using non-specific enzymes. The ginger protease used in our research is a product containing a proline-specific endopeptidase, which can theoretically further increase the hydrolysis degree of collagen and obtain peptides ending in proline.
This study aims to explore the effect of compound enzymatic hydrolysis of papain, alkaline protease and ginger protease on the hydrolysis degree, peptide sequence distribution and functionality of fish skin collagen. The obtained hydrolysates were separated into two collections using TSK-gel PW column chromatography according to molecular weight, to evaluate the functional properties of collagen peptides with various lengths and sequences. The research is meaningful for the production of collagen peptides with higher yield and bio-activity.
2. Materials and methods
2.1 Materials and chemicals
Fish gelatin was purchased from Wuhan Lanabai Pharmaceutical Chemical Co., Ltd (Wuhan, China). Alcalase (1 × 105 U g−1) was purchased from Angel Yeast Co., Ltd (Yichang, China). Papain (2 × 105 U g−1) was purchased from Nanning Pangbo Biological Engineering Co., Ltd (Nanning, China). Ginger protease (2 × 105 U mL−1) was purchased from Royal DSM Co., Ltd (The Netherlands). Gly-Pro-Hyp standard was purchased from Shanghai Qiangyao Biological Technology Co., Ltd (Qingdao, China). Angiotensin converting enzyme and N-hippuryl-His-Leu hydrate were purchased from Sigma-Aldrich Trading Co., Ltd (Shanghai, China). Mouse embryonic fibroblast cells (NIH-3T3) were kindly provided by Stem Cell Bank, Chinese Academy of Sciences. CCK-8 kits were purchased from Beyotime Biotechnology Co., Ltd (Shanghai, China). All the other reagents used were of analytical grade.2.2 Collagen hydrolysate preparation
The purpose of the preparation was to study the functional activity of small molecule peptides. In order to achieve a high degree of hydrolysis, we selected the following processes: the fish collagen solution (5%, w/v) was treated with papain (3000 U g−1 protein) at pH 6.0 with an incubation time of 2 h at 55 °C, and then treated with alcalase (3000 U g−1 protein) at pH 10.0 with an incubation time of 2 h at 55 °C. Afterward, the sample was treated with ginger protease (3000 U g−1 protein) at pH 4.5 with an incubation time of 2 h at 55 °C. The obtained samples were recorded as AP. Another group of APGs was obtained by enzymatic hydrolysis: the same concentration of the substrate solution was treated under the reaction parameters of protease 3000 U g−1 protein, alcalase protease (pH 10.0), and papain protease (pH 6.0), and the incubation time was 2 h at 55 °C, while without ginger protease. The enzymatic hydrolysates obtained above were inactivated at 90 °C for 10 min. After centrifugation, the supernatant was collected for subsequent experiments.2.3 Peptide sequences
HPLC-ESI-MS/MS (MALDI SYNAPT MS, Waters, USA) was used to identify the structure of characteristic collagen peptide sequences. Instrument parameters were set based on our previous experiments. Then, MassLynx V4.1 was used to analyze the identified peptide sequences to further verify the sequences in collagen peptides. Analysis of collagen peptide required 100 mg of freeze-dried sample diluted to 20 mL with deionized water for testing (5 mg mL−1). After filtration using a 0.45 μm membrane, two kinds of samples were analyzed using ICP-MS-MS (NexION 350D, PerkinElmer, USA) combined with online HPLC. Instrument parameters: column: ESA III anion-exchange column (250 mm × 4.6 mm); mobile phase: 25 mmol L−1 ammonium citrate solution; pH 3.1; flow rate: 1 mL min−1; and injection volume: 20 μL.2.4 Sample separation
The selection of CPs was performed using the TSK G2000SW (300 × 7.5 mm) column on the HPLC instrument. Instrument parameters: mobile phase ratio: 39.95% water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60% acetonitrile
60% acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05% trichloroacetic acid (TCA); flow rate: 1 mL min−1; detection wavelength: 214 nm; and sample volume: 100 μL. The peak time duration of the sample was 5 min, and the sample that flowed out in 1–2 min was recorded as 1, such as AP-1 and APG-1, and the sample that flowed out in 2–5 min was recorded as 2, such as AP-2 and APG-2. Solid samples were obtained after concentration using a vacuum concentrator (CentriVap, Labconco, USA) and by drying with a freeze dryer.
0.05% trichloroacetic acid (TCA); flow rate: 1 mL min−1; detection wavelength: 214 nm; and sample volume: 100 μL. The peak time duration of the sample was 5 min, and the sample that flowed out in 1–2 min was recorded as 1, such as AP-1 and APG-1, and the sample that flowed out in 2–5 min was recorded as 2, such as AP-2 and APG-2. Solid samples were obtained after concentration using a vacuum concentrator (CentriVap, Labconco, USA) and by drying with a freeze dryer.
2.5 Molecular weight distribution
The determination of the molecular weight distribution of collagen peptides was performed using a TSK G2000SW (300 × 7.5 mm) column on the HPLC instrument. Instrument parameters: mobile phase ratio: 39.95% water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60% acetonitrile
60% acetonitrile![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05% trichloroacetic acid (TCA); flow rate: 1 mL min−1; detection wavelength: 214 nm; and sample volume: 10 μL. A molecular weight calibration curve was prepared from the average elution volume of the following standards: cytochrome C (12
0.05% trichloroacetic acid (TCA); flow rate: 1 mL min−1; detection wavelength: 214 nm; and sample volume: 10 μL. A molecular weight calibration curve was prepared from the average elution volume of the following standards: cytochrome C (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 384 Da), insulin from bovine pancreas (5733.49 Da), bacitracin (1422.69 Da), reduced L-glutathione (307.32 Da) and glycine (75.07 Da).
384 Da), insulin from bovine pancreas (5733.49 Da), bacitracin (1422.69 Da), reduced L-glutathione (307.32 Da) and glycine (75.07 Da).
2.6 Amino acid composition
Amino acid composition and mass fraction of collagen peptides were analyzed using HPLC (Agilent 1260, Agilent Technologies Inc., USA) according to the OPA pre-column derivatization method.25 Samples were hydrolyzed with acid in advance. Specifically, 1 mL of sample (10 mg mL−1) was first mixed with 7 mL of 6 mol L−1 HCl and 1 mL of 12 mol L−1 HCl. After filling with N2, the hydrolysis tube was sealed, and the mixture completely reacted at 120 °C for 22 h. Then, 4.8 mL of 10 mol L−1 NaOH was added for neutralization to stop the reaction, and the volume was held constant at 25 mL. Instrument parameters: column: Agilent Hypersil ODS (5 μm, 4.0 mm × 250 mm); mobile phase: A phase, 27.6 mmol L−1 sodium acetate/triethylamine/tetrahydrofuran (volume ratio: 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.11
0.11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5), and B phase, 80.9 mmol L−1 sodium acetate/methanol/acetonitrile (volume ratio: 1
2.5), and B phase, 80.9 mmol L−1 sodium acetate/methanol/acetonitrile (volume ratio: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2); gradient elution: 0–17 min 8% B phase–92% A phase, 17–21 min 50% B phase–50% A phase, 21–24 min 100% B phase, 24–25 min 100% A phase; injection volume: 5 μL; flow rate: 1.0 mL min−1; column temperature: 40 °C; detection wavelength: 338 nm (proline was 262 nm); and amino acid content was quantified using the external standard method.
2); gradient elution: 0–17 min 8% B phase–92% A phase, 17–21 min 50% B phase–50% A phase, 21–24 min 100% B phase, 24–25 min 100% A phase; injection volume: 5 μL; flow rate: 1.0 mL min−1; column temperature: 40 °C; detection wavelength: 338 nm (proline was 262 nm); and amino acid content was quantified using the external standard method.
2.7 ACE-inhibitory activity
The method used for determining ACE-inhibitory activity was that according to Cushman with slight modifications.26 In each assay, 100 μL of sodium borate buffer (0.1 M) and 20 μL of collagen peptides were mixed with an aliquot (200 μL) of a hippuryl-histidyl-leucine (Sigma-Aldrich) solution (5 mM hippuryl-histidyl-leucine in 0.1 M sodium borate buffer containing 0.3 M NaCl, pH 8.3) and incubated at 37 °C for 30 min. The reaction was activated by adding 40 μL of ACE solution (Sigma-Aldrich) (0.1 U mL−1, pH 8.3) and incubated in a water bath (37 °C) for 30 min. Then, 200 μL of 1 M HCl was added to terminate the reaction, after which 1.2 mL of ethyl acetate was added to the mixture to extract the released hippuric acid. After the samples were vigorously stirred for 10 s, they were centrifuged at 5000g for 5 min, then 0.8 mL of the organic phase was transferred to a fresh test tube. Ethyl acetate was evaporated to dryness in a drying oven. The residue was dissolved in 1.0 mL of deionized water, and absorbance was measured using a spectrophotometer at 228 nm. The following equation was used to calculate ACE-inhibitory activity, where A represents the absorbance with ACE but without the sample, B denotes the absorbance in the presence of ACE and the sample, while C is the absorbance without ACE and the sample. The ACE inhibition rate is calculated as follows: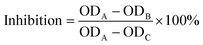 | (1) |
2.8 Cell culture and cell proliferative activity
NIH-3T3 fibroblasts were maintained in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS) and 1% penicillin/streptomycin until confluence, hereinafter referred to as complete medium. The cytotoxicity of NIH-3T3 preadipocytes was measured by a CCK-8 kit. The NIH-3T3 fibroblasts were placed in wells with 1 × 105 cells per well and incubated with Dulbecco's modified Eagle's medium for 24 h, and then incubated with collagen peptides of different concentrations (0, 6.25, 12.5, 25.0, 50.0, 100.0 and 200 μg mL−1) dissolved in basic culture for 24 h, which contained 4% fetal calf serum (FCS) and 1% penicillin/streptomycin until confluence was reached. The basic medium contained 4% newborn calf serum and 1% penicillin/streptomycin. After the reaction, the NIH-3T3 fibroblasts were washed with phosphate-buffered saline (PBS) three times. Subsequently, 10 μL of CCK-8 mixed with 90 μL of the complete medium solution was added and incubated for 1.5 h. Absorbance was measured at 450 nm.2.9 Statistical analysis
The results are expressed as the mean ± SD. Statistical analysis was calculated using SPSS version 25.0, and one-way analysis of variance (ANOVA) was conducted. The significant differences between samples were determined at p < 0.05 using Duncan's multiple range tests. The significant differences are represented by a, b, c, d, e, and f. Graphs were plotted using Origin Pro 2023 software (OriginLab Corporation, Northampton, MA, USA) and GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA).3. Results and discussion
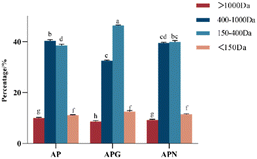
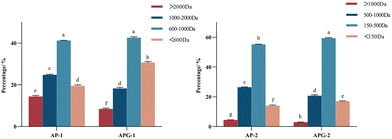
3.1 The molecular weight distribution of two or three enzyme hydrolyzed collagen peptides
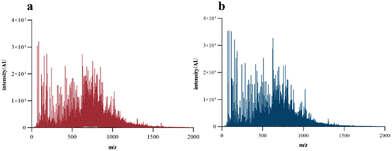
To intuitively compare the hydrolysis degree of fish collagen under the combined hydrolysis of different enzymes, the molecular weight distribution of the hydrolysate was determined using HPLC. In the experiments we did before, the molecular weight of the original gelatin molecule was around 15.0 kDa without small-sized peptides. As shown in Fig. 1, the AP sample, which showed a high content of peptide from 150 to 400 Da, greatly increased the hydrolysis degree of gelatin, probably because the alkaline protease rapidly degraded the triple helix structure of gelatin in the early stage,27 exposing more amino acid sites such as glycine, L-arginine and L-lysine for papain to act on.28 The gelatin hydrolysate obtained after hydrolysis with two enzymes (alcalase/papain) or three enzymes (alcalase/papain/ginger) was obviously different in its content of peptides below 1.0 kDa. After combining ginger protease, the content of peptide segments of 400 to 1000 Da decreased from 40.12% to 32.36%, and the peptide segments of 150 to 400 Da increased from 38.64% to 46.38%. We used neutral protease for comparison and found that the addition of neutral protease (APN) made a limited contribution to the degree of hydrolysis. These results indicated that ginger protease could further act on sites that alcalase and papain could not hydrolyze.MALDI-TOF-MS analyses were performed to accurately determine the MW profile and sequence of fish collagen peptides. As shown in Fig. 2, the MS spectrum of the collagen peptides obtained through the complex enzymes confirmed the results that the molecular weight of the prepared collagen peptides was basically below 1500 Da, and most of them were concentrated at 500–1000 Da. In addition, the APG sample showed an obviously larger content of small molecular sized peptides than the AP sample, which was consistent with the results in Fig. 1, indicating that ginger protease could further degrade high molecular weight peptides. After adding ginger protease, the abundance of peptides with m/z of 70.06, 116.07, 286.14, and 626.31 was significantly increased, indicating the generation of peptide fragments.
The peptide sequence could be identified using mass spectrometry to obtain the amino acid composition, which was directly related to the bio-activity of the collected peptides. By comparing the peptide sequences shown in Tables 1 and 2, it can be seen that after adding ginger protease, the proportion of peptide with proline at the C-terminal increased from 1.5% to 16.0%, indicating that ginger protease could specifically recognize and act on the carboxyl terminal of proline, which was actually difficult for alkaline protease and papain to hydrolyze.29 As reported, collagen peptides containing a proline or hydroxyproline residue at the C-terminal position had strong ACE-inhibitory activity.30 In addition, ginger protease was helpful for releasing more hydrophobic amino acids to the terminal, the quantity of which in the APG sample was 40, while in the AP sample it was 26. The existence of hydrophobic amino acids at the peptide terminals was also beneficial for improving ACE-inhibition activity.27 Therefore, it could be predicted that the combination with ginger protease to hydrolyze collagen would increase the ACE-inhibitory activity of collagen peptides.
| m/z | Peptide | m/z | Peptide | m/z | Peptide | m/z | Peptide | m/z | Peptide |
|---|---|---|---|---|---|---|---|---|---|
| 301.14 | GPAG | 373.2 | LLQ | 444.2 | SPGPS | 499.25 | GPTGPA | 558.26 | GEAGPQ |
| 301.17 | GPK | 388.19 | GSPAG | 455.23 | GPNPA | 501.22 | SGPAG | 559.21 | GEAGPE |
| 303.18 | AGR | 388.19 | SGPAG | 470.24 | LLGPA | 511.22 | GPLGPA | 563.24 | GEAGVM |
| 329.17 | GPR | 400.21 | GVGPA | 471.23 | GPLNA | 511.26 | GPPGPS | 571.25 | GPLGPM |
| 329.17 | GPVG | 402.21 | TGPQ | 471.23 | QVGPA | 513.25 | GPSGPV | 573.26 | DEGPVG |
| 329.18 | PGR | 405.16 | EAGE | 472.2 | GPLGE | 513.26 | GPVGPS | 586.25 | AGPGSPT |
| 331.16 | GPTG | 414.22 | AVGPA | 474.24 | LLGTA | 515.22 | DAGPVG | 592.2 | GDAGDTG |
| 343.15 | PGLG | 415.21 | LGPE | 476.18 | SPGSE | 516.25 | VLGDL | 629.29 | GDQGVPG |
| 343.18 | GPLG | 428.2 | SPGPA | 478.24 | PYGAA | 531.23 | DATGPA | 629.32 | DKAQAP |
| 343.18 | VGPA | 430.17 | GPAGE | 485.24 | GESGPQ | 531.24 | EGTGPA | 642.32 | DGVPGPT |
| 345.22 | LGR | 430.19 | EGPGA | 487.2 | GDAGPA | 543.24 | GPLGE | ||
| 372.18 | AGPQ | 430.19 | EGPQ | 487.21 | ADGAPG | 555.27 | GPPGPM | ||
| 372.19 | QGPA | 430.24 | LGGPS | 487.24 | SVGPQ | 557.28 | GVVGPE | ||
| 373.2 | ALGL | 431.21 | LLGE | 495.24 | GPPGPA | 558.26 | WQGPA |
| m/z | Peptide | m/z | Peptide | m/z | Peptide | m/z | Peptide | m/z | Peptide |
|---|---|---|---|---|---|---|---|---|---|
| 301.15 | GPAG | 373.2 | SPGL | 431.21 | LLGE | 497.25 | GPVGPA | 543.24 | SGPEGP |
| 301.17 | GPK | 373.22 | LLK | 440.25 | LPGGP | 499.23 | GPAGPT | 557.28 | GVVGPE |
| 301.19 | PGQ | 388.18 | GSPAG | 444.2 | SPGPS | 499.24 | GPTGPA | 558.26 | WQGPA |
| 320.14 | GPF | 398.17 | GPAGP | 444.22 | AEGPA | 501.23 | QEGPA | 558.26 | GEAGPQ |
| 329.17 | PGR | 399.22 | LLGP | 455.22 | NPAGP | 511.22 | GPLGPA | 559.24 | GEAGPE |
| 329.17 | GPVG | 400.19 | VGGPA | 455.23 | GPGQP | 511.23 | AGPLGP | 561.23 | TDTGPA |
| 331.16 | GPTG | 400.21 | GVGPA | 460.2 | AGPSE | 511.25 | PGPGPS | 563.24 | GEAGVM |
| 343.16 | PGLG | 405.17 | EAGE | 470.25 | LLGPA | 511.25 | GPGLPA | 573.24 | DEGPVG |
| 343.18 | VGPA | 414.19 | GPSGP | 471.22 | GPLNA | 513.25 | GPSGPV | 574.25 | EGSGPQ |
| 343.19 | GPLG | 414.21 | AVGPA | 471.24 | QVGPA | 513.26 | GPVGPS | 586.25 | AGPGSPT |
| 345.22 | SGPAG | 424.21 | GPPGP | 474.24 | LGTLA | 515.22 | VPGDAG | 587.25 | EGPEGV |
| 345.22 | LGR | 426.2 | GPVGP | 485.23 | SPGPQ | 515.24 | EGGPVG | 592.22 | GDAGDTG |
| 357.16 | PSGP | 428.2 | SPGPA | 485.23 | GPSGPA | 526.24 | GPAGPQ | 626.28 | DAGLPGP |
| 372.18 | GPAGA | 430.2 | GEGPA | 487.21 | DGAGPA | 531.23 | GETGPA | 629.29 | GDQGVPG |
| 372.22 | AGPK | 430.2 | EGPQ | 495.25 | GPPGPA | 543.23 | AGPLGE | 642.31 | DGPVGPT |
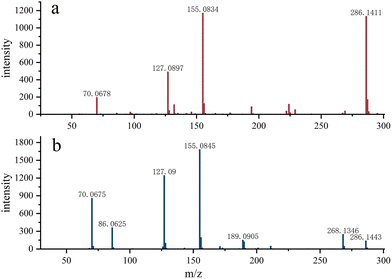
As shown in Fig. 3, Gly-Pro-Hyp was detected in the AP sample, while in the APG sample, no obvious fragments were detected and the signal intensity was low, suggesting that ginger protease cleaves the Pro site and thus the content of these polypeptides is greatly reduced in APG.
3.2 The composition of collagen peptide components separated via size exclusion chromatography
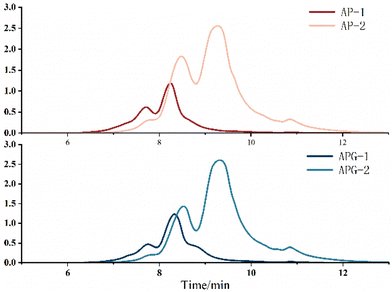
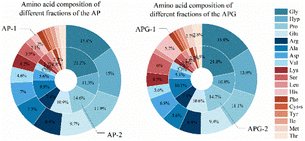
The molecular size distribution of the collected components was further analyzed using HPLC to verify the separation efficiency (Fig. 4). As shown in Fig. 5, in the AP-1 sample, peptide segments larger than 1000 Da accounted for about 39.2%, peptide segments below 1000 Da accounted for about 60.8%, and the content of free amino acids of 150 Da was less than 1%. The APG-1 sample was similar, but the proportion of peptides below 1000 Da was more, about 73.49%. In the AP-2 sample, the molecular weight was mainly below 500 Da, of which the proportion of peptides from 150 to 500 Da was about 55.2%, and the proportion of free amino acids of about 150 Da was about 13.6%. The APG-2 sample was similar, but the proportion of peptides from 150 to 500 Da was more, about 59.3%. Molecular size is one of the factors determining peptide bioavailability, that is, small molecule collagen peptides were absorbed into the blood rapidly and thereafter also transported into the tissues rapidly because its mean molecular weight is much smaller than that of conventional collagen peptides.31 From this aspect, it can be predicted that small molecule collagen peptides have higher biological activity. However, the high degree of hydrolysis also brings about the problem of increasing free amino acids, and the proportion of some free amino acids in small molecular polypeptides was close to 5–10%. Free amino acids may not be as easily absorbed because they may compete with each other for transport across the intestinal wall. Peptides, on the other hand, can be transported across the intestinal wall as intact molecules, which may increase their bioavailability.32 Therefore, whether the activity of the components AP-2 and APG-2 is higher than that of AP-1 and APG-1 is still unclear.The amino acid composition could reflect the distribution of amino acids in macromolecular and small molecular peptide segments. As shown in Fig. 6, fish collagen peptides are rich in glycine, proline, hydroxyproline, glutamic acid and alanine. Glycine and imino acids (proline and hydroxyproline) accounted for about 42% of the total amino acid content of fish collagen, which was similar to silver carp skin collagen peptides (containing 41.06% of glycine and imino acids).33 The analysis displayed in Fig. 6 reveals that in small molecular peptides, the content of glycine, proline, glutamic acid, alanine, leucine, and phenylalanine had increased compared to that of large molecular peptides, while the content of hydroxyproline, aspartic acid, lysine, histidine, cysteine, methionine, and tyrosine had decreased. Notably, the content of glycine in small molecular peptides was approximately 40% higher than that in large molecular peptides, and the content of proline was approximately 20% higher in small molecular peptides. Proline is highly abundant and more frequently occurs in antihypertensive peptides than in non-antihypertensive peptides.34 The presence of glycine (N-terminal) and alanine (C-terminal) may exert strong ACE-inhibitory activity according to the peptide sequence of waste protein hydrolysate.35
Conversely, the content of hydroxyproline in small molecular peptides had decreased by approximately 25% compared to that in large molecular peptides. These differences in peptide levels may be due to differences in enzyme cleavage sites, as some amino acids were more easily cleaved into smaller peptides, therefore resulting in higher levels of small molecular peptides.
The proportion of hydrophobic amino acids was 30.35% in the AP-1 sample and 34.71% in the AP-2 sample, while the proportion of the two in the APG sample was 24.65% and 34.68%, respectively. This result reflected the high content of hydrophobic amino acids in small molecule polypeptides, which can also be inferred from other studies. Some researchers used membranes to separate collagen peptides and the hydrophobic amino acids were rich in permeate fluid.36,37 However, the overall content of the hydrophobic amino acids decreased, which may be due to the omission of some free hydrophobic amino acids produced by excessive hydrolysis that flowed out during the separation process. Studies have shown that peptides have higher hydrophobicity values, and their IC50 values are also significantly lower (p < 0.05),38 which indicate stronger ACE-inhibitory activity. The common trend of AP and APG is that the content of glycine and proline in small peptides increased, and the content of hydroxyproline decreased, which may mean there were more enzyme cleavage sites near glycine and proline.
3.3 The functional properties of collected collagen peptide components
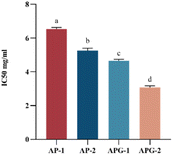
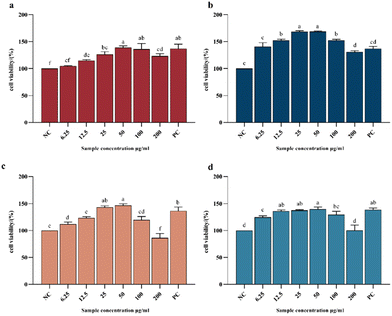
Fig. 7 shows the IC50 values of the ACE-inhibitory activity of different collagen peptide collections, with lower values reflecting higher ACE-inhibitory activity. The component with lower molecular weight (AP-2 and APG-2) showed higher ACE-inhibitory activity both in the AP and APG samples. For the AP sample, the IC50 value of component AP-2 was 19.9% lower than that of component AP-1, indicating significantly higher ACEinhibitory activity related to the relatively higher percentage of low-molecular-weight peptides.39 Several authors attribute the antihypertensive effectiveness of peptides to their structure, which is generally reported to be of low molecular weight and short length.40,41 Some scholars have explored the ACE-inhibitory activity of peptide fragments obtained from tomato processing by-products fermented using Bacillus subtilis and found that peptides with molecular weights ranging from 500 to 850 Da were very active in their ACE-inhibitory activity compared with those with molecular weights ranging from 850 to 3000 Da.42 This may be because small peptides can easily enter ACE active sites.43 It may also be due to the fact that component 2 contains more hydrophobic amino acids such as alanine, proline, valine, etc. (as shown in Fig. 6). Studies have shown that peptides have higher hydrophobicity values, and their IC50 values are also significantly lower.38 The molecular docking models illustrate that there were some amino acid residues in ACE, constituting a hydrophobic pocket, enveloping the screened bioactive peptides, indicating that the bioactive peptides have a hydrophobic effect with the amino acid residues in ACE, which may explain C-terminal tripeptides comprising hydrophobic amino acids including those binding to ACE.44 The APG-2 sample showed a 43.4% lower value than the AP-2 component, reflecting the relatively higher ACE inhibitory activity of ginger protease co-hydrolyzed peptide products. This may be due to the increased content of peptides with proline at the C-terminal. ACE possesses three major active pockets, S1 (consisting of residues Ala354, Glu384 and Tyr523), S1́ (Glu162) and S2́ (including residues Gln281, His253, Lys511, His513 and Tyr520) to where inhibitors can bind.45 The peptide PRP forms eight hydrogen bonds with residues of ACE while LPK only forms two hydrogen bonds.46 The common clinical antihypertensive drug captopril, [(2S)-1-(3-mercapto-2-methyl-1-oxo-propyl)-L-proline] is characterized by ending with proline.47Fig. 8 shows the CCK8 cell proliferation rates of different collagen peptide components at different concentrations. For all the collagen peptide collections, the viability rate of the NIH-3T3 cells showed a trend of first increasing and then decreasing, which was consistent with the findings of Hou.48 The viability of the cells treated with 50 μg mL−1 collagen peptide was higher than that of the positive control (PC) group, reflecting the strong protection effect of the collagen hydrolysate. Ma et al. also reported the function of collagen hydrolysate on the protection of human skin fibroblasts.49 The groups cultured with the AP-2 collagen peptide collection showed the highest activity in promoting fibroblast proliferation, due to the positive effect of tripeptides and dipeptides on cell proliferation, such as Gly-Pro-Hyp and Pro-Hyp,50,51 which can stimulate hyaluronic acid production in skin fibroblasts.50 For the APG sample, at low intake concentrations (6.25 μg mL−1 and 12.5 μg mL−1), the low molecular weight peptide component also showed obviously higher activity for the growth of fibroblasts. However, at an intake concentration increasing to 50 μg mL−1, the advantages of APG-2 over APG-1 are absent, which may be due to the antagonism of the negative effect of free amino acids on cell proliferation. As previously reported, the excessive hydrolysis of protein may induce the generation of free amino acids, which had a detrimental effect on fibroblast proliferation. When the sample containing free amino acids was taken above the tolerable upper intake level, oxidative pathways would be saturated, and metabolites from amino acid catabolic pathways started to accumulate, increasing the risk of adverse events.52
The activity of APG-1 was overall slightly higher than that of the AP-1 group, indicating that the addition of ginger protease in higher molecular weight peptides favored the proliferative activity of fibroblasts, which might be related to the overall reduction of molecular weight and the release of proline. However, APG-2 had a lower activity than AP-2, indicating that the free amino acid generation and the breakage of the characteristic peptide segments (Gly-Pro-Hyp and Pro-Hyp) limited the further enhancement of activity (Fig. 3). In conclusion, the fibroblast growth-promoting activity of collagen peptides is inevitably associated with molecular weight. However, whether triple enzyme hydrolysis has a positive effect on the enhancement of the fibroblast growth-promoting activity of collagen peptides needs to be further verified and analyzed.
4. Conclusion
The composite enzymatic hydrolysis showed significantly higher efficiency in increasing the hydrolysis degree of collagen and promoting the release of special peptide chains. Ginger protease could specifically act on the C-terminal of proline, increasing the content of peptide sequences ending with proline, which was beneficial for enhancing ACE-inhibitory activity. The collected two components of collagen peptide separated using gel column chromatography according to molecular weight showed great differences in terms of ACE-inhibitory activity and fibroblast proliferation activity. Overall, the smaller molecular weight collagen peptide segments, abundant in short peptides such as tripeptides, had greater ACE inhibition ability and proliferative activity of fibroblasts. This phenomenon may be attributed to the greater facility with which small peptide molecules access the active site of ACE and their enhanced susceptibility to cellular uptake by fibroblasts, which implies that the trifecta enzymatic cleavage employed in this study is beneficial for enhancing the activity of peptide samples. It should be noted that the combined hydrolysis of ginger protease was unhelpful for the increase in fibroblast proliferation activity, related to the destruction of the Gly-Pro-Hyp tripeptide structure. The research work revealed the effect of molecular weight and special sequence on the functional properties of collagen peptides, which is meaningful for the directional preparation of target functional peptides.Author contributions
Xinnuo Hu: conceptualization, methodology, investigation, formal analysis and writing – original draft. Yanjun Yang: conceptualization and funding acquisition. Cuihua Chang: supervision, methodology and writing – review & editing. Junhua Li: supervision. Yujie Su: supervision. Luping Gu: supervision.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (2022YFD2101004). The authors are thankful for the grants from the “Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province” industry development program, and appreciate support from the Natural Science Foundation of Jiangsu Province (no. BK20221071), and the China Postdoctoral Science Foundation (no. 2023M731335).References
- S. R. Derkach, N. G. Voron'ko, Y. A. Kuchina and D. S. Kolotova, Modified Fish Gelatin as an Alternative to Mammalian Gelatin in Modern Food Technologies, Polymers, 2020, 12, 3051–3061 CrossRef CAS PubMed.
- S. Xu, Y. Zhao, W. Song, C. Zhang, Q. Wang, R. Li, Y. Shen, S. Gong, M. Li and L. Sun, Improving the Sustainability of Processing By-Products: Extraction and Recent Biological Activities of Collagen Peptides, Foods, 2023, 12, 1965–1983 CrossRef CAS PubMed.
- Z. D. Xiang, Q. Xue, P. Gao, H. T. Yu, M. Z. Wu, Z. Z. Zhao, Y. N. Li, S. P. Wang, J. Y. Zhang and L. Dai, Antioxidant peptides from edible aquatic animals: Preparation method, mechanism of action, and structure-activity relationships, Food Chem., 2023, 404, 134701 CrossRef CAS PubMed.
- L. Y. Li, Y. Q. Zhao, Y. He, C. F. Chi and B. Wang, Physicochemical and Antioxidant Properties of Acid- and Pepsin-Soluble Collagens from the Scales of Miiuy Croaker (Miichthys Miiuy), Mar. Drugs, 2018, 16, 394–413 CrossRef CAS PubMed.
- A. Hakuta, Y. Yamaguchi, T. Okawa, S. Yamamoto, Y. Sakai and M. Aihara, Anti-inflammatory effect of collagen tripeptide in atopic dermatitis, J. Dermatol. Sci., 2017, 88, 357–364 CrossRef CAS PubMed.
- E. T. Aksun Tümerkan, L. D. Kozaci, A. K. Miri, S. Maharjan and B. Cecen, Sustainable aquatic waste and by-products processing: biomaterials in tissue engineering facts and gaps, Mater. Today Sustain., 2023, 23, 100445 CrossRef.
- X. Song, Z. Li, Y. Li and H. Hou, Typical structure, biocompatibility, and cell proliferation bioactivity of collagen from Tilapia and Pacific cod, Colloids Surf., B, 2022, 210, 112238 CrossRef CAS PubMed.
- P. Zhou, C. Yang, Y. Ren, C. Wang and F. Tian, What are the ideal properties for functional food peptides with antihypertensive effect? A computational peptidology approach, Food Chem., 2013, 141, 2967–2973 CrossRef CAS PubMed.
- S. Heffernan, L. Giblin and N. O'Brien, Assessment of the biological activity of fish muscle protein hydrolysates using in vitro model systems, Food Chem., 2021, 359, 129852 CrossRef CAS PubMed.
- T. Naoki, Y. Rumiko, S. Yasuo, Y. Yoshino and Y. Hideto, Promotion by Collagen Tripeptide of TypeI Collagen Gene Expression in Human Osteoblastic Cells and Fracture Healing of Rat Femur, Biosci., Biotechnol., Biochem., 2007, 71, 2680–2687 CrossRef PubMed.
- Q. Di, X. G. You, H. N. Wang, Y. X. Liu, Y. Shi, N. Wang, X. Zhang, C. Feng, Y. Liu, M. Kong, X. J. Cheng, S. C. Bi and X. G. Chen, Natural micropatterned fish scales combing direct osteogenesis and osteoimmunomodulatory functions for enhancing bone regeneration, Composites, Part B, 2023, 255, 110620 CrossRef.
- M. Tu, S. Cheng, W. Lu and M. Du, Advancement and prospects of bioinformatics analysis for studying bioactive peptides from food-derived protein: Sequence, structure, and functions, TrAC, Trends Anal. Chem., 2018, 105, 7–17 CrossRef CAS.
- H. Ohara, H. Matsumoto, K. Ito, K. Iwai and K. Sato, Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources, J. Agric. Food Chem., 2007, 55, 1532–1535 CrossRef CAS PubMed.
- M. Chae, C. Y. Moon, S.-H. Lim, Y. Yamashita, H. Yamada, M. Ide, C. W. Park, J. Roh and W. Kim, Oral Ingestion of AP Collagen Peptide Leads to Systemic Absorption of Gly-Pro-Hyp, Alleviating H2O2-Induced Dermal Fibroblast Aging, J. Med. Food, 2023, 26, 299–306 CrossRef CAS PubMed.
- Y. Shoko, D. Kisaburo, O. Masamichi, N. Noriaki and S. Yasuo, Absorption and Urinary Excretion of Peptides after Collagen Tripeptide Ingestion in Humans, Biol. Pharm. Bull., 2016, 39, 428–434 CrossRef PubMed.
- S. B. Sontakke, J.-H. Jung, Z. Piao and H. J. Chung, Orally Available Collagen Tripeptide: Enzymatic Stability, Intestinal Permeability, and Absorption of Gly-Pro-Hyp and Pro-Hyp, J. Agric. Food Chem., 2016, 64, 7127–7133 CrossRef CAS PubMed.
- S. Caira, G. Picariello, G. Renzone, S. Arena, A. D. Troise, S. de Pascale, V. Ciaravolo, G. Pinto, F. Addeo and A. Scaloni, Recent developments in peptidomics for the quali-quantitative analysis of food-derived peptides in human body fluids and tissues, Trends Food Sci. Technol., 2022, 126, 41–60 CrossRef CAS.
- K. L. Hernández-Ruiz, J. López-Cervantes, D. I. Sánchez-Machado, O. N. Campas-Baypoli, A. A. Quintero-Guerrero, M. de Lourdes Grijalva-Delgado and A. F. Chávez-Almanza, Collagen peptide fractions from tilapia (Oreochromis aureus Steindachner, 1864) scales: Chemical characterization and biological activity, Food Biosci., 2023, 53, 102658 CrossRef.
- Q. Liu, S. Shao, J. Bao, S. J. Shah, S. Yue, X. Luan, Q. Liu, L. Xing, Z. Shi, Z. Zhao and Z. Zhao, High accuracy prediction of dipeptide angiotensin-converting enzyme (ACE) inhibitory activity by improving the credibility of the 3D-quantitative structure-activity relationship (3D-QSAR) model database and investigating inhibition mechanism, Process Biochem., 2023, 131, 114–124 CrossRef CAS.
- S. H. Park and Y.-J. Jo, Static hydrothermal processing and fractionation for production of a collagen peptide with anti-oxidative and anti-aging properties, Process Biochem., 2019, 83, 176–182 CrossRef CAS.
- C. L. Jenkins, L. E. Bretscher, I. A. Guzei and R. T. Raines, Effect of 3-hydroxyproline residues on collagen stability, J. Am. Chem. Soc., 2003, 125, 6422–6427 CrossRef CAS PubMed.
- O. Abdelhedi and M. Nasri, Basic and recent advances in marine antihypertensive peptides: Production, structure-activity relationship and bioavailability, Trends Food Sci. Technol., 2019, 88, 543–557 CrossRef CAS.
- R. J. FitzGerald and H. Meisel, Milk protein-derived peptide inhibitors of angiotensin-I-converting enzyme, Br. J. Nutr., 2000, 84, 33–37 CrossRef PubMed.
- M. Bielecka, G. Cichosz and H. Czeczot, Antioxidant, antimicrobial and anticarcinogenic activities of bovine milk proteins and their hydrolysates - A review, Int. Dairy J., 2022, 127, 105208 CrossRef CAS.
- C. Cereser, J. Guichard, J. Drai, E. Bannier, I. Garcia, S. Boget and P. Parviz, Quantitation of reduced and total glutathione at the femtomole level by high-performance liquid chromatography with fluorescence detection: application to red blood cells and cultured fibroblasts, J. Chromatogr. B: Biomed. Sci. Appl., 2001, 752, 123–132 CrossRef CAS PubMed.
- D. W. Cushman and H. S. Cheung, Spectrophotometric assay and properties of the angiotensin-converting emzyme of rabbit lung, Biochem. Pharmacol., 1971, 20, 1637–1648 CrossRef CAS PubMed.
- S. Sun, Y. Gao, J. Chen and R. Liu, Identification and release kinetics of peptides from tilapia skin collagen during alcalase hydrolysis, Food Chem., 2022, 378, 132089 CrossRef CAS PubMed.
- H. Xiong, Application and research development of carica papaya papain, Sichuan Food Ferment., 2006, 6, 7–8 Search PubMed.
- W. Liu, W. Yang, X. Li, D. Qi, H. Chen, H. Liu, S. Yu, G. Wang and Y. Liu, Evaluating the Properties of Ginger Protease-Degraded Collagen Hydrolysate and Identifying the Cleavage Site of Ginger Protease by Using an Integrated Strategy and LC-MS Technology, Molecules, 2022, 27, 5001–5015 CrossRef CAS PubMed.
- Y. Zhang, K. Olsen, A. Grossi and J. Otte, Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides, Food Chem., 2013, 141, 2343–2354 CrossRef CAS PubMed.
- S. Yamamoto, F. Hayasaka, K. Deguchi, T. Okudera, T. Furusawa and Y. Sakai, Absorption and plasma kinetics of collagen tripeptide after peroral or intraperitoneal administration in rats, Biosci., Biotechnol., Biochem., 2015, 79, 2026–2033 CrossRef CAS PubMed.
- D. B. Silk, G. K. Grimble and R. G. Rees, Protein digestion and amino acid and peptide absorption, Proc. Nutr. Soc., 1985, 44, 63–72 CrossRef CAS PubMed.
- J. J. Huang, H. L. Li, G. Q. Xiong, J. Cai, T. Liao and X. Y. Zu, Extraction, identification and anti-photoaging activity evaluation of collagen peptides from silver carp (Hypophthalmichthys molitrix) skin, LWT – Food Sci. Technol., 2023, 173, 114384 CrossRef CAS.
- S. Lertampaiporn, A. Hongsthong, W. Wattanapornprom and C. Thammarongtham, Ensemble-AHTPpred: A robust ensemble machine learning model integrated with a new composite feature for identifying antihypertensive peptides, Front. Genet., 2022, 13, 883766 CrossRef CAS PubMed.
- N. H. Ishak, M. I. Shaik, N. K. Yellapu, N. K. Howell and N. M. Sarbon, Purification, characterization and molecular docking study of angiotensin-I converting enzyme (ACE) inhibitory peptide from shortfin scad (Decapterus macrosoma) protein hydrolysate, J. Food Sci. Technol., 2021, 58, 4567–4577 CrossRef CAS PubMed.
- L. Picot, R. Ravallec, M. Fouchereau-Péron, L. Vandanjon, P. Jaouen, M. Chaplain-Derouiniot, F. Guérard, A. Chabeaud, Y. Legal, O. M. Alvarez, J.-P. Bergé, J.-M. Piot, I. Batista, C. Pires, G. Thorkelsson, C. Delannoy, G. Jakobsen, I. Johansson and P. Bourseau, Impact of ultrafiltration and nanofiltration of an industrial fish protein hydrolysate on its bioactive properties, J. Sci. Food Agric., 2010, 90, 1819–1826 CAS.
- X. Y. Zu, Y. Q. Huang, Y. J. Zhao, G. Q. Xiong, T. Liao and H. L. Li, Peptide extraction from silver carp (Hypophthalmichthys molitrix) scales via enzymatic hydrolysis and membrane filtration, Ital. J. Food Sci., 2023, 35, 44–53 CAS.
- Z. Yin, R. Yan, Y. Jiang, S. Feng, H. Sun, J. Sun, D. Zhao, H. Li, B. Wang and N. Zhang, Identification of peptides in Qingke baijiu and evaluation of its angiotensin converting enzyme (ACE) inhibitory activity and stability, Food Chem., 2022, 395, 133551 CrossRef CAS PubMed.
- Y. Yang, G. Tao, P. Liu and J. Liu, Peptide with angiotensin I-converting enzyme inhibitory activity from hydrolyzed corn gluten meal, J. Agric. Food Chem., 2007, 55, 7891–7895 CrossRef CAS PubMed.
- B. Daza-Rodríguez, A. R. Martínez, A. M. Padilla and J. M. Lázaro, Food-derived Bioactive Peptides with Angiotensin-Converting Enzyme Inhibiting Effect: A Systematic Review, J. Pharmacol. Pharmacother., 2023, 14, 14–24 CrossRef.
- X. Li, C. Feng, H. Hong, Y. Zhang, Z. Luo, Q. Wang, Y. Luo and Y. Tan, Novel ACE inhibitory peptides derived from whey protein hydrolysates: Identification and molecular docking analysis, Food Biosci., 2022, 48, 101737 CrossRef CAS.
- A. Moayedi, L. Mora, M.-C. Aristoy, M. Hashemi, M. Safari and F. Toldrá, ACE-Inhibitory and Antioxidant Activities of Peptide Fragments Obtained from Tomato Processing By-Products Fermented Using Bacillus subtilis: Effect of Amino Acid Composition and Peptides Molecular Mass Distribution, Appl. Biochem. Biotechnol., 2017, 181, 48–64 CrossRef CAS PubMed.
- K. Lin, L. W. Zhang, X. Han and D. Y. Cheng, Novel angiotensin I-converting enzyme inhibitory peptides from protease hydrolysates of Qula casein: Quantitative structure-activity relationship modeling and molecular docking study, J. Funct. Foods, 2017, 32, 266–277 CrossRef CAS.
- G. Q. Wei, Q. Zhao, D. D. Wang, Y. Z. Fan, Y. N. Shi and A. X. Huang, Novel ACE inhibitory, antioxidant and α-glucosidase inhibitory peptides identified from fermented rubing cheese through peptidomic and molecular docking, LWT – Food Sci. Technol., 2022, 159, 113196 CrossRef CAS.
- M. Andújar-Sánchez, A. Cámara-Artigas and V. Jara-Pérez, A calorimetric study of the binding of lisinopril, enalaprilat and captopril to angiotensin-converting enzyme, Biophys. Chem., 2004, 111, 183–189 CrossRef PubMed.
- H. Liu, X. Cai, M. Huang, T. Wang, L. Li, H. Luo and Y. Lu, Dual bioactivity of angiotensin converting enzyme inhibition and antioxidant novel tripeptides from sipunculus nudus L. and their related mechanism analysis for antihypertention, Int. J. Pept. Res. Ther., 2023, 29, 3–29 CrossRef CAS.
- B. C. David, A. A. Steven, H. L. John, E. S. Jean, A. S. Partricia and N. M. Doris, Clinical experience with blockade of the renin-angiotensin- aldosterone system by an oral converting-enzyme inhibitor (SQ 14,225, Captopril) in hypertensive patients, Prog. Cardiovasc. Dis., 1978, 21, 195–206 CrossRef PubMed.
- C. Hou, Y. Lei, N. Li, M. Wei, S. Wang, S. U. Rahman, C. Bao, B. Bao, J. Elango and W. Wu, Collagen from Iris squid grafted with polyethylene glycol and collagen peptides promote the proliferation of fibroblast through PI3K/AKT and Ras/RAF/MAPK signaling pathways, Int. J. Biol. Macromol., 2023, 247, 125772 CrossRef CAS PubMed.
- Y. Q. Ma, J. G. Yan, Y. Wei, Y. G. Xu, R. X. Zhang and X. Y. Qin, Effects of Nahlsgen and collagen hydrolysate on protection and collagen synthesis in human skin fibroblasts, Food Ferment. Ind., 2022, 48, 86–91 Search PubMed.
- T. Okawa, Y. Yamaguchi, S. Takada, Y. Sakai, N. Numata, F. Nakamura, Y. Nagashima, Z. Ikezawa and M. Aihara, Oral administration of collagen tripeptide improves dryness and pruritus in the acetone-induced dry skin model, J. Dermatol. Sci., 2012, 66, 136–143 CrossRef CAS PubMed.
- Y. Shigemura, K. Iwai, F. Morimatsu, T. Iwamoto, T. Mori, C. Oda, T. Taira, E. Y. Park, Y. Nakamura and K. Sato, Effect of Prolyl-hydroxyproline (Pro-Hyp), a food-derived collagen peptide in human blood, on growth of fibroblasts from mouse skin, J. Agric. Food Chem., 2009, 57, 444–449 CrossRef CAS PubMed.
- R. Elango, Tolerable upper intake level for individual amino acids in humans: a narrative review of recent clinical studies, Adv. Nutr., 2023, 14, 885–894 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3fo04455f |
| This journal is © The Royal Society of Chemistry 2024 |