Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Supramolecular interaction-driven delignification of lignocellulose†
Juho Antti
Sirviö
 *a,
Idamaria
Romakkaniemi
b,
Juha
Ahola
*a,
Idamaria
Romakkaniemi
b,
Juha
Ahola
 b,
Svitlana
Filonenko
b,
Svitlana
Filonenko
 c,
Juha P.
Heiskanen
c,
Juha P.
Heiskanen
 d and
Ari
Ämmälä
d and
Ari
Ämmälä
 a
a
aFibre and Particle Engineering Research Unit, University of Oulu, P.O. Box 4300, Oulu, 90014, Finland. E-mail: juho.sirvio@oulu.fi
bChemical Process Engineering Research Unit, University of Oulu, P.O. Box 4300, Oulu, 90014, Finland
cMax Planck Institute of Colloids and Interfaces, Potsdam, 14424, Germany
dResearch Unit of Sustainable Chemistry, University of Oulu, P. O. Box 4300, Oulu, FI-90014, Finland
First published on 7th December 2023
Abstract
Lignocellulose has the great potential as a sustainable resource to replace fossil-based raw materials, however, properties, such as a complicated crosslinked structure, create a significant obstacle for utilization, as the isolation of lignocellulose components has hardly been achieved under mild conditions. Here, we demonstrate that the use of an aromatic hydrogen bond donor (thymol) creates a supramolecular interaction between the delignification medium and lignin, which is key to removing almost all the lignin from the softwood within minutes, at near-ambient temperatures. Strong support for supramolecular interactions was demonstrated via the formation of a room temperature liquid between two solids (lignin and thymol). The concept of supramolecular interaction between lignin and thymol will help elevate the feasibility of biobased materials across a wide range of applications.
1. Introduction
The complex structure of lignocellulose hinders or prevents its utilization in many applications.1 In fact, lignin is commonly considered an obstacle because it holds other compounds together to create a barrier for many outside influences, such as chemical attacks.2 For example, direct hydrolysis of a lignin-containing carbohydrate fraction results in a poor yield of monomeric units due to the inhibition of enzyme activity by lignin.3 Therefore, the fractionation of lignocellulose is a pivotal step in many traditional as well as new applications that aim to use renewable biomaterials to replace fossil-based resources.4,5 Historically, fractionation, or delignification, has focused on the isolation of carbohydrate fractions; but lately, lignin has also been recognized as a valuable resource6–8 and research has steered toward a method that can allow fractionation to be achieved in milder conditions. Several strategies, including lignin-protecting fractionation9,10 and the use of lignin-like or lignin-derived compounds,11 have elevated the potential of lignocellulose into applications that are beyond the capabilities of current industrial lignin. Despite the notable advantages of recent fractionation methods, their high temperature, long treatment times, and harsh reaction conditions still hamper their wider usability. Although methods based on hydrophobic and lignin-like chemicals have been introduced,11,12 the full potential of these concepts has not yet been revealed. An important key to lignin removal is to understand the chemical structure of lignin and to maximize the interaction between the solvent system and lignin.Here, we present a supramolecular interaction-driven delignification process using thymol as an aromatic, naturally occurring (it is present in high quantities in thyme oil), and biodegradable phenolic compound. Due to its aromatic structure, partial delocalization makes thymol a strong hydrogen bond donor via its phenolic group.13 Conversely, lignin, despite being the polymer of phenolic monomers, has a minor amount of free phenolic units.14 Instead, lignin has numerous ether bonds, which act as hydrogen bond acceptors, as well as aliphatic hydroxyl groups, which exhibit both hydrogen bond-acceptor and -donor properties (also, a few carbonyls present in lignin are hydrogen bond acceptors) (Fig. 1a). Therefore, we postulated that thymol can participate in both aromatic π–π stacking and hydrogen bonding with lignin, and that these noncovalent, supramolecular interactions can enable the removal of lignin from lignocellulose under acidic conditions. Methanesulfonic acid (MSA) was utilized as an acidic component to cleave lignin–lignin and lignin–carbohydrate chemical bonds. MSA has environmentally friendly characteristics, such as biodegradability and low volatility, and it can be produced from methanol or methane,15 making MSA a sustainable alternative to other oil-based sulfonic acids. MSA is also less reactive than sulfuric acid (ESI†). To demonstrate the high efficiency of this supramolecular interaction-based delignification, softwood (refined Norway spruce chips) was chosen as a raw material because it has been generally recognized as highly resistant to delignification/fractionation.16,17 Furthermore, nature and high lignin content notable hampers the enzymatic hydrolysis of softwood, when compared to other lignocellulose sources.18,19
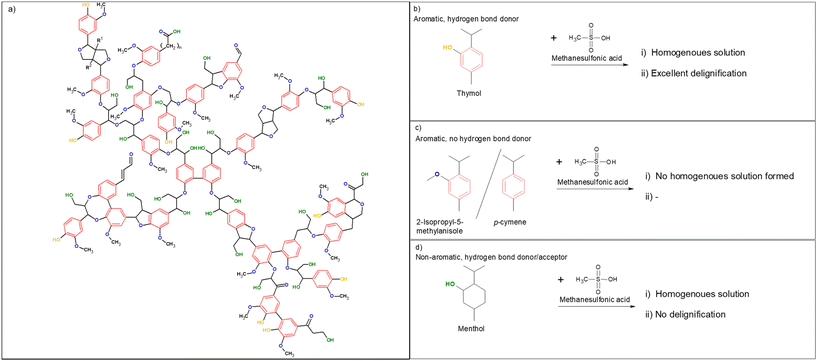 | ||
Fig. 1 (a) Chemical structure of lignin (milled spruce lignin, simplified from ref. 20) with aromatic structures presented in red, hydrogen bond acceptors in blue, hydrogen bond donors in orange, and hydrogen bond donor/acceptors in green, (b) thymol, (c) 2-isopropyl-5-methylanisole and p-cymene as nonhydrogen bonding analogs, and (d) menthol as nonaromatic analog of thymol, and (b–d) descriptions of their behavior (i) when mixed with MSA at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and (ii) in delignification. 2 and (ii) in delignification. | ||
2. Results and discussion
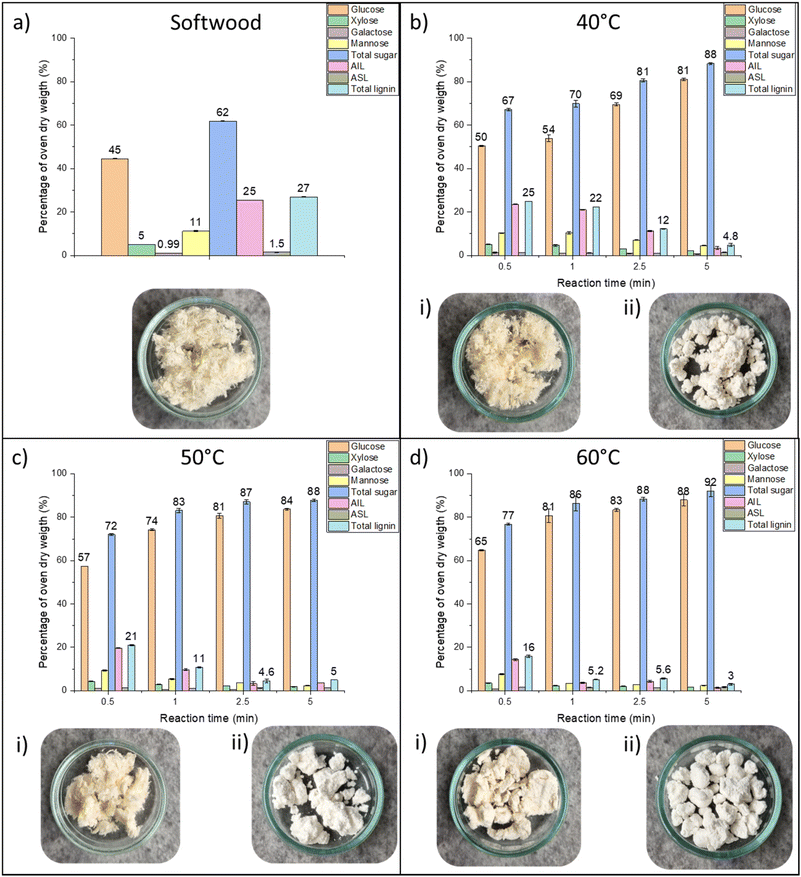
The delignification experiments were carried out at temperatures of 40, 50, and 60 °C with reaction times ranging from 0.5 to 5 min with a liquid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wood mass ratio of 10
wood mass ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Almost immediately after the addition of wood in the MSA–thymol mixture (with a molar ratio of 1
1. Almost immediately after the addition of wood in the MSA–thymol mixture (with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), the solution began to change color to an intensively deep purple. After the addition of ethanol, filtration, and washing with ethanol, the color disappeared, and bright products were obtained at all investigated temperatures (Fig. 2). The color of the solid fraction is in stark contrast to that of many previously reported methods, including commercial kraft cooking, where the color of the delignified biomass is often notably darker than that of the raw material. Importantly, in addition to the production of brightly colored solid residues, the delignification efficiency was found to be exceptionally high (Fig. 2), especially when considering the low temperature and extremely short treatment times. At a temperature of 40 °C, the delignification efficiency remained relatively low during the first minute (remaining lignin content over 20%); however, at 2.5 min, the lignin content dropped to 12%, and at 5 min, the residual lignin content was merely 4.8%. Approximately 5% residual lignin content was found at 2.5 min at 50. At, 60 °C, the reaction time decreased to 1 min for obtaining a similar lignin content. Furthermore, a minimal residual lignin content of 3% was obtained at 5 min at 60 °C. After delignification, solid fraction contained mainly glucose, indicating the high cellulose content.
2), the solution began to change color to an intensively deep purple. After the addition of ethanol, filtration, and washing with ethanol, the color disappeared, and bright products were obtained at all investigated temperatures (Fig. 2). The color of the solid fraction is in stark contrast to that of many previously reported methods, including commercial kraft cooking, where the color of the delignified biomass is often notably darker than that of the raw material. Importantly, in addition to the production of brightly colored solid residues, the delignification efficiency was found to be exceptionally high (Fig. 2), especially when considering the low temperature and extremely short treatment times. At a temperature of 40 °C, the delignification efficiency remained relatively low during the first minute (remaining lignin content over 20%); however, at 2.5 min, the lignin content dropped to 12%, and at 5 min, the residual lignin content was merely 4.8%. Approximately 5% residual lignin content was found at 2.5 min at 50. At, 60 °C, the reaction time decreased to 1 min for obtaining a similar lignin content. Furthermore, a minimal residual lignin content of 3% was obtained at 5 min at 60 °C. After delignification, solid fraction contained mainly glucose, indicating the high cellulose content.
After removal of the solid fraction from the delignification mixture, a sodium salt of MSA with high purity (Fig. S1†) was precipitated with aqueous NaOH, and the MSA could be recycled using electrodialysis.21 Thymol was recovered using column extraction with high purity (Fig. S2†) and could be directly reused. With column extraction, two lignin fractions (LF1 and LF2) with distinctively low molecular weights (Table S1†) were obtained (see ESI† for analysis of the two lignin fractions). The degrees of polymerization of the washed and dried solid fractions were found to be in the range of 312–216, similar to commercial microcrystalline cellulose (MCC). The industrial production of MCC mainly relies on the acid hydrolysis of bleached wood pulp, thus requiring multistep synthesis and large consumption of hazardous chemicals (halogen-based bleaching chemicals, particularly).22 MCC is a safe food additive (E-number E460)23 used in the cosmetic, pharmacy, and food industries and is a widely used starting material for various cellulose-based materials and chemicals,24 including nanomaterials25 (see ESI† for the production of nanocellulose from MSA–thymol-treated softwood).
The delignification results clearly demonstrated that by using the MSA–thymol mixture, a delignification that has historically been carried out at high temperature for several hours can be performed in minutes at near-ambient temperature. Table S2† lists various delignification/fractionation methods published in the literature for softwood and hardwood. Furthermore, selected previous delignification systems reported in the literature were tested with our raw material. Mixture of choline chloride-lactic acid has been recognized as one of the most typical DES capable to delignify lignocellulose biomass.26 Excellent delignification efficiency was also noted as remaining lignin content after choline chloride-lactic acid treatment were 6.8%, similar to those of MSA–thymol systems. However, choline chloride-lactic acid operated at notable higher temperature (120 °C) and longer reaction time (180 min). Furthermore, severe darkening of the solid residue was observed. Compared to DESs, acidic hydrotropes can usually be applied in delignification at lower temperatures and with shorter reaction times.12p-Toluenesulfonic acid was introduced as one of the first acidic hydrotropes for nearly complete dissolution of lignin from hardwood.27 However, notable lower delignification efficiency has been observed in case of softwood.28 Here, when delignification of refined softwood was done using 80% aqueous p-toluenesulfonic acid solution at 80 °C for 20 min, lignin content remained relatively high, around 18%, and similarly to choline chloride-lactic acid DES, solid residue was dark in color. The highest delignification efficiency in the studied reference systems was observed with an aqueous 4-hydroxybenzenesulfonic acid29 after 120 min at 60 °C, as the remaining lignin content was 5.9%. Furthermore, the aqueous 4-hydroxybenzenesulfonic acid was the only studied system aside the MSA–thymol to produce mildly colored solid residue. However, when the reaction time was decreased to 5 min, the remaining lignin content was 15%, being higher than what was obtained using MSA–thymol system at temperature of 40 °C after half shorter reaction time. Although studied reference systems represent a small fraction of various delignification systems reported in literature, they are amongst the most notable example of novel systems used biomass delignification. Therefore, MSA–thymol exhibits a clear advantage over traditional and emerging delignification methods, and to the best of our knowledge, MSA–thymol is the fastest high-efficiency delignification method (defined by a remaining lignin content ≤5%) demonstrated on any type of biomass, while operating at the lowest reported temperature. It is also noteworthy, that although phenolic pulping has been reported previously,30–32 these systems request high temperature and hours of treatment time, most likely due to the presence of large extent of competing hydrogen bond acceptor/donor (i.e., water). Therefore, MSA–thymol is unique method for treatment of lignocellulose biomass.
Conversion of cellulose to glucose was investigated to evaluate the enzymatic digestibility of wood before and after MSA–thymol treatment at 40, 50, and 60 °C for one minute. The glucose yield and hydrolysis efficiency notable increased from original wood by applying the delignification (Fig. 3). Although the sample prepared at 60 °C exhibited slightly lower hydrolysis rate during the first 36 h of enzymatic hydrolysis, the final hydrolysis efficiency order is by the increased temperature. The glucan hydrolysis efficiency of the samples obtained at 40, 50 and 60 °C increased by 30, 45 and 51%, respectively, when compared to the original wood sample, demonstrating that the very short MSA–thymol delignification treatment is suitable method to increase the glucose yield from softwood. Although the overall hydrolysis efficiency of MSA–thymol treated softwood cannot match with those reported for example for high temperature organosolv treated of softwood,16 or other lignocellulose sources,33 low temperature and extremely short reaction provides notable egologic and economic advantage over most of the previous method. Furthermore, MSA–thymol enzymatic hydrolysis efficiency of delignified softwood was notable higher compared to the other low-temperature (<100 °C) delignification methods reported in literature. For example, hydrolysis efficiency around 20% was reported for p-toluenesulfonic acid treated Masson pine at 80 °C,34 whereas hydrolysis efficiency around 10% was obtained by Lewis acid-based deep eutectic solvent delignification at 80 °C.35 Slightly higher hydrolysis rate (around 55%) compared to MSA–thymol delignified softwood was reported from Chinese fir after formic acid treatment at 90 °C.36 However, 240 times longer reaction time was utilized compared to the study presented here.
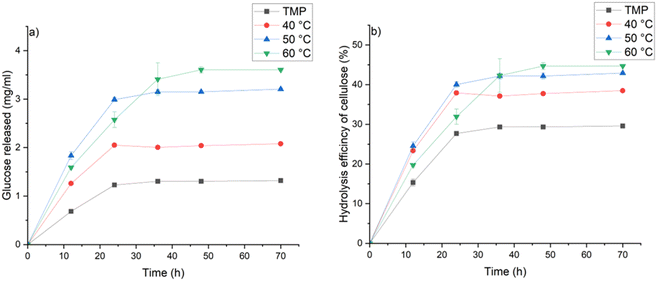 | ||
| Fig. 3 (a) Glucose releasing rate and (b) hydrolysis efficiency of original and after one minute treatment with MSA–thymol at various temperature at different enzymatic hydrolysis times. | ||
To demonstrate that the hydrogen bond-donating properties of phenolic compounds are the key feature of delignification using MSA–thymol mixtures, several reference experiments were conducted. Neither 2-isopropyl-5-methylanisole (a methyl ether of thymol that has no hydrogen bond donating property) nor p-cymene (a thymol analog without a hydroxyl group) (Fig. 1b) formed a clear liquid with MSA and were thus not suitable for delignification. More notably, when thymol was replaced with nonaromatic menthol (hexahydrothymol) (Fig. 1c), room temperature liquid was obtained, yet no removal of lignin or carbohydrate was observed. Menthol is an aliphatic alcohol, and its hydroxyl group can function both as a hydrogen bond acceptor and donor.13 Therefore, it can be assumed that the hydrogen bonding interaction between menthol and the hydrogen bond-accepting groups of lignin is weaker than that between thymol and lignin. Furthermore, because menthol lacks aromaticity, there is no π stacking between menthol and lignin. Due to the weak interactions, no removal of lignin occurred when menthol was used with MSA.
When water was used instead of thymol (molar ratio between MSA and water was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, water content of 18.02 wt%), the lignin content was observed to increase from 27 to 32 wt%, which was mainly due to the removal of carbohydrate (i.e., hemicelluloses). The inability of aqueous MSA to dissolve lignin indicates that MSA acts merely as an acid catalyst during the delignification, and does not function as a hydrotropic component for delignification, as previously shown with aromatic sulfonic acids.27,29,37 Furthermore, when water was used at similar mass ratio that in case of thymol (water content of 75.75 wt%), practically no reaction occurred as lignin and carbohydrate content remained in similar range compared to original softwood.
2, water content of 18.02 wt%), the lignin content was observed to increase from 27 to 32 wt%, which was mainly due to the removal of carbohydrate (i.e., hemicelluloses). The inability of aqueous MSA to dissolve lignin indicates that MSA acts merely as an acid catalyst during the delignification, and does not function as a hydrotropic component for delignification, as previously shown with aromatic sulfonic acids.27,29,37 Furthermore, when water was used at similar mass ratio that in case of thymol (water content of 75.75 wt%), practically no reaction occurred as lignin and carbohydrate content remained in similar range compared to original softwood.
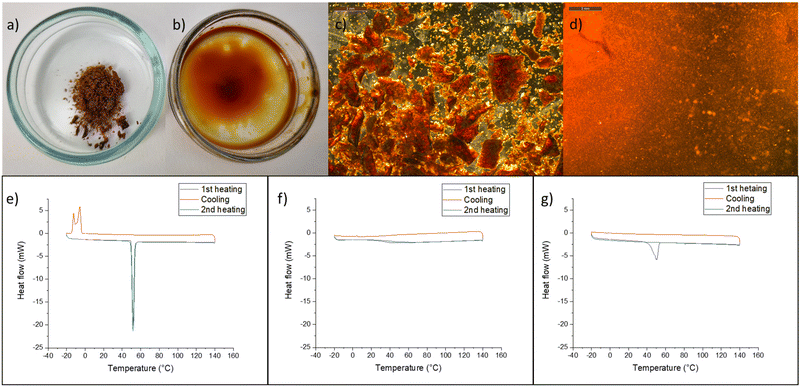
Further experiments were conducted to demonstrate the interaction between thymol and lignin. The isolated lignin (LF1) was solid at room temperature and did not melt when heated in an oven at 80 °C. However, when lignin was manually mixed with thymol at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and heated for approximately 30 min at 80 °C, solid particles disappeared and a viscous liquid was obtained. After cooling this liquid to room temperature, no precipitation of either component was observed, and the mixture was a homogeneous and strong gel-like material (Fig. 4). On the other hand, when isolated lignin was mixed with menthol and heated in an oven, melting of menthol was observed, the lignin remained as solid particles, and the mixture was as an uneven solid at room temperature. These observations with menthol are in line with its delignification results, as the mixture of menthol and MSA showed no removal of lignin.
2 and heated for approximately 30 min at 80 °C, solid particles disappeared and a viscous liquid was obtained. After cooling this liquid to room temperature, no precipitation of either component was observed, and the mixture was a homogeneous and strong gel-like material (Fig. 4). On the other hand, when isolated lignin was mixed with menthol and heated in an oven, melting of menthol was observed, the lignin remained as solid particles, and the mixture was as an uneven solid at room temperature. These observations with menthol are in line with its delignification results, as the mixture of menthol and MSA showed no removal of lignin.
To verify that the formation of a liquid between lignin and thymol was not exclusively related to the isolation method, two commercial lignins were also investigated: ethanolytic lignin oligomers are obtained by high-temperature organosolv-type fractionation,38 and kraft lignin is the most widely produced lignin and is obtained by sulfate cooking.39 When either ethanolytic lignin or kraft lignin was heated together with thymol at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 in an oven at 80 °C, they formed liquid with no visible solid particles. The kraft lignin–thymol mixture was a strong, gel-like material at room temperature, similar to the mixture of thymol and LF1. On the other hand, oligomeric lignin and thymol formed a mixture that stayed liquid, albeit highly viscous, even at room temperature (Fig. S3†). Ethanolytic lignin formed a homogenous mixture with thymol even at a mass ratio of 1
2 in an oven at 80 °C, they formed liquid with no visible solid particles. The kraft lignin–thymol mixture was a strong, gel-like material at room temperature, similar to the mixture of thymol and LF1. On the other hand, oligomeric lignin and thymol formed a mixture that stayed liquid, albeit highly viscous, even at room temperature (Fig. S3†). Ethanolytic lignin formed a homogenous mixture with thymol even at a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a condition where it was a homogeneous, hard gel at room temperature.
1, a condition where it was a homogeneous, hard gel at room temperature.
Differential scanning calorimetry (DSC) analysis showed that neither LF1, LF2, ethanolytic, nor kraft lignin exhibited a melting point in the range of −20–140 °C (Fig. 4f and Fig. S4†). On the other hand, the melting peak of pure thymol was observed at approximately 51 °C (49–51 °C in the literature40) (Fig. 4e), and when solid mixtures of thymol and LF1 were heated in DSC, thymol melting was observed at the same temperature during the first heating cycle (Fig. 4f). However, unlike pure thymol, no crystallization of the thymol–lignin mixture was observed when cooled to −20 °C, and during the second heating cycle, no melting peak was observed. The results from DSC analysis indicate that after the melting of thymol, a homogeneous mixture of lignin and thymol was formed, which showed no crystallization at −20 °C. The results with ethanolytic and kraft lignin are in line with those of isolated lignin fractions (Fig. S4†). Furthermore, a lower interaction between menthol and lignin was demonstrated with ethanolytic lignin (Fig. S4†).
It can be assumed that the formation of a liquid/gel between thymol and lignin is due to the strong interaction between the two components, preventing the crystallization of otherwise solid compounds. The interaction between lignin and thymol can result in the formation of a deep eutectic supramolecular polymer (DESP),41 previously shown with cyclodextrin (a macrocyclic oligosaccharide of glucose) and various hydrogen bond donors, such as carboxylic acids.42 DESP between cyclodextrin and carboxylic acid was assumed to form due to the strong hydrogen bonding interaction between these two components, similar to the formation of DES. When cyclodextrin and carboxylic acid were dissolved in water followed by evaporation of the solvent, hydrogen bonding interactions prevented the crystallization of individual compounds and a gel-like material was obtained.41 In the case of thymol and lignin, the melting of thymol, as well as the strong interaction between the two compounds, enables the formation of DESP even without an external solvent. Therefore, in addition to the demonstration of efficient biomass fractionation with the thymol-based system, we present a novel concept of lignin-based DESP. In addition to thymol, other aromatic alcohols, such as carvacrol (an isomer of thymol), phenol, and eugenol, formed liquid with lignin, and preliminary results showed that other phenolic compounds are also suitable for biomass fractionation (we note that eugenol was solidified with MSA, indicating that the usability of vinylic phenols is limited).
Based on the abovementioned observations, supramolecular interactions can be proposed as the driving force in delignification. The strong interaction between thymol and lignin allows the amphiphilic MSA to diffuse to the reaction site, resulting in the acid-catalyzed formation of carbocations on the lignin.43 In a thymol-rich environment, the carbocation is thymolated (similar to phenolation44), followed by the fragmentation (depolymerization) of lignin and the rapid dissolution of these lignin fragments due to supramolecular interactions. The fragmentation is backed by the low molecular weight of lignin fractions, and thymolation is verified with nuclear magnetic resonance analyses (see ESI†). Thymolation prevents the self-condensation of lignin, which is generally recognized as the main side reaction in delignification that can lead to mixture darkening and the poor removal of residual lignin.45 The lack of condensation during MSA–thymol delignification could explain the mild color of the cellulosic fraction still containing a small quantity of lignin (cf. brown color of unbleached kraft pulp).
3. Conclusion
This work demonstrates the existence of supramolecular interaction between lignin and phenolic compounds. The finding can provide a novel route and technological breakthrough in the development of selective fractionation chemistry. Here it was shown that this phenomenon can lead to a highly effective delignification of lignocelluloses to be used, for instance, in the production of monomeric or oligomeric platform chemicals, strongly supported by notable increase in enzymatic digestibility of softwood even after one minute treatment. Both used chemicals in this study (thymol and methanesulfonic acid) can be regarded as green compounds and their recovery with high purity demonstrated that they are recyclable, making concept presented here environmental feasible and sustainable alternative for treatment of lignocellulose biomass. The possibility of the use of near ambient temperature with exceptional short reaction times will increase economic feasibility due to lower operation and investment costs associated to low processing energy need and simplified process equipment. The low visible light and high UV radiation absorption could elevate the feasibility of lignin fraction in composite application. Furthermore, low molecular weight enables good solubility of lignin, which could allow lignin to be utilized in various applications. Furthermore, it can be envisioned that DESP between lignin and various aromatic alcohols could be used as water-tolerance adhesive or for example as bio-based wood preservative, due to the antimicrobial effect of phenolic compounds. As the chemical and physical structure of various lignocellulose sources can notable different from each other, in the future studies, delignification of biomass, such as hardwood and non-wood sources will be investigated.Author contributions
J. A. S. conceived of the presented idea, designed and carried out the experiments and data analysis, and wrote the manuscript; I. R., J. A., S. F., J. P. H. performed the analysis and helped with writing; A. Ä. contributed to the experimental design and writing of the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
We acknowledge Jani Österlund, Jarno Karvonen, Ville Karvonen, Konsta Rekinen for their assistance in material analysis.References
- D. Weidener, M. Dama, S. K. Dietrich, B. Ohrem, M. Pauly and W. Leitner, et al., Multiscale analysis of lignocellulose recalcitrance towards OrganoCat pretreatment and fractionation, Biotechnol. Biofuels, 2020, 13(1), 155 CrossRef CAS PubMed.
- K. E. L. Eriksson and H. Bermek, Lignin, Lignocellulose, Ligninase, in Encyclopedia of Microbiology, ed. M. Schaechter, Academic Press, Oxford, 3rd edn, 2009, pp. 373–384. Available from: https://www.sciencedirect.com/science/article/pii/B9780123739445001528 Search PubMed.
- A. C. dos Santos, E. Ximenes, Y. Kim and M. R. Ladisch, Lignin–Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass, Trends Biotechnol., 2019, 37(5), 518–531 CrossRef CAS PubMed.
- C. Wang, S. Yang, X. Song, Q. Pi, Q. Zhang and Q. Liu, et al., Novel Solvent Systems for Biomass Fractionation Based on Hydrogen-Bond Interaction: A Minireview, Adv. Sustainable Syst., 2020, 2000085 CrossRef CAS.
- J. S. M. Samec, Holistic Approach for Converting Biomass to Fuels, Chem, 2018, 4(6), 1199–1200 CAS.
- M. M. Abu-Omar, K. Barta, G. T. Beckham, J. S. Luterbacher, J. Ralph and R. Rinaldi, et al., Guidelines for performing lignin-first biorefining, Energy Environ. Sci., 2021, 14(1), 262–292 RSC.
- A. J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen and M. F. Davis, et al., Lignin Valorization: Improving Lignin Processing in the Biorefinery, Science, 2014, 344(6185), 1246843 CrossRef PubMed.
- L. Dong, L. Lin, X. Han, X. Si, X. Liu and Y. Guo, et al., Breaking the Limit of Lignin Monomer Production via Cleavage of Interunit Carbon–Carbon Linkages, Chem, 2019, 5(6), 1521–1536 CAS.
- L. Shuai, M. T. Amiri, Y. M. Questell-Santiago, F. Héroguel, Y. Li and H. Kim, et al., Formaldehyde stabilization facilitates lignin monomer production during biomass depolymerization, Science, 2016, 354(6310), 329–333 CrossRef CAS PubMed.
- Y. Liu, N. Deak, Z. Wang, H. Yu, L. Hameleers and E. Jurak, et al., Tunable and functional deep eutectic solvents for lignocellulose valorization, Nat. Commun., 2021, 12(1), 5424 CrossRef CAS PubMed.
- K. H. Kim, T. Dutta, J. Sun, B. Simmons and S. Singh, Biomass pretreatment using deep eutectic solvents from lignin derived phenols, Green Chem., 2018, 20(4), 809–815 RSC.
- J. Zhu, L. Chen and C. Cai, Acid Hydrotropic Fractionation of Lignocelluloses for Sustainable Biorefinery: Advantages, Opportunities, and Research Needs, ChemSusChem, 2021, 14(15), 3031–3046 CrossRef CAS PubMed.
- D. O. Abranches, M. A. R. Martins, L. P. Silva, N. Schaeffer, S. P. Pinho and J. A. P. Coutinho, Phenolic hydrogen bond donors in the formation of non-ionic deep eutectic solvents: the quest for type V DES, Chem. Commun., 2019, 55(69), 10253–10256 RSC.
- W. G. Glasser, About Making Lignin Great Again—Some Lessons From the Past, Front. Chem., 2019, 7, 565 CrossRef CAS PubMed.
- C. Díaz-Urrutia and T. Ott, Activation of methane to CH3+: A selective industrial route to methanesulfonic acid, Science, 2019, 363(6433), 1326–1329 CrossRef PubMed.
- A. A. Vaidya, K. D. Murton, D. A. Smith and G. Dedual, A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose, Biomass Convers. Biorefin., 2022, 12(11), 5427–5442 CrossRef CAS.
- S. V. den Bosch, W. Schutyser, R. Vanholme, T. Driessen, S. F. Koelewijn and T. Renders, et al., Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps, Energy Environ. Sci., 2015, 8(6), 1748–1763 RSC.
- F. Cotana, G. Cavalaglio, M. Gelosia, A. Nicolini, V. Coccia and A. Petrozzi, Production of Bioethanol in a Second Generation Prototype from Pine Wood Chips, Energy Procedia, 2014, 45, 42–51 CrossRef CAS.
- W. E. Mabee, D. J. Gregg, C. Arato, A. Berlin, R. Bura and N. Gilkes, et al., Updates on Softwood-to-Ethanol Process Development, in Twenty-Seventh Symposium on Biotechnology for Fuels and Chemicals, ed. J. D. McMillan, W. S. Adney, J. R. Mielenz and K. T. Klasson, Humana Press, Totowa, NJ, 2006, pp. 55–70 Search PubMed.
- M. Balakshin, E. Capanema, X. Zhu, I. Sulaeva, A. Potthast and T. Rosenau, et al., Spruce Milled Wood Lignin: Linear, Branched or Cross-linked?, Green Chem., 2020, 22, 3985–4001 RSC.
- H. Voss and R. Schneider, Liberation of organic sulfonic acids, US5221443A, 1993.
- R. Husgafvel, K. Vanhatalo, L. Rodriguez-Chiang, L. Linkosalmi and O. Dahl, Comparative global warming potential assessment of eight microcrystalline cellulose manufacturing systems, J. Cleaner Prod., 2016, 126, 620–629 CrossRef CAS.
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS), M. Younes, P. Aggett, F. Aguilar, R. Crebelli and A. Di Domenico, et al., Re-evaluation of celluloses E 460(i), E 460(ii), E 461, E 462, E 463, E 464, E 465, E 466, E 468 and E 469 as food additives, EFSA J., 2018, 16(1), e05047 Search PubMed.
- Y. Jing, Y. Guo, Q. Xia, X. Liu and Y. Wang, Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellulosic Biomass, Chem, 2019, 5(10), 2520–2546 CAS.
- T. Li, C. Chen, A. H. Brozena, J. Y. Zhu, L. Xu and C. Driemeier, et al., Developing fibrillated cellulose as a sustainable technological material, Nature, 2021, 590(7844), 47–56 CrossRef CAS PubMed.
- D. Smink, A. Juan, B. Schuur and S. R. A. Kersten, Understanding the Role of Choline Chloride in Deep Eutectic Solvents Used for Biomass Delignification, Ind. Eng. Chem. Res., 2019, 58(36), 16348–16357 CrossRef CAS.
- L. Chen, J. Dou, Q. Ma, N. Li, R. Wu and H. Bian, et al., Rapid and near-complete dissolution of wood lignin at ≤80 °C by a recyclable acid hydrotrope, Sci. Adv., 2017, 3(9), e1701735 CrossRef PubMed.
- P. Li, H. Ji, L. Shan, Y. Dong, Z. Long and Z. Zou, et al., Insights into delignification behavior using aqueous p-toluenesulfonic acid treatment: comparison with different biomass species, Cellulose, 2020, 27(17), 10345–10358 CrossRef CAS.
- D. He, Y. Wang, C. G. Yoo, Q. J. Chen and Q. Yang, The fractionation of woody biomass under mild conditions using bifunctional phenol-4-sulfonic acid as a catalyst and lignin solvent, Green Chem., 2020, 22(16), 5414–5422 RSC.
- Verfahren zur Gewinnung der das sogenannte Lignin bildenden Stoffe aus zellulosehaltigen Materialien [Internet]. AT83396B, 1921. Available from: https://patents.google.com/patent/AT83396B/de.
- A. Vega and M. Bao, Fractionation of lignocellulose materials with phenol and dilute HCl, Wood Sci. Technol., 1991, 25(6), 459–466 CAS.
- G. Wang, S. Qi, Y. Xia, A. M. Parvez, C. Si and Y. Ni, Mild One-Pot Lignocellulose Fractionation Based on Acid-Catalyzed Biphasic Water/Phenol System to Enhance Components’ Processability, ACS Sustainable Chem. Eng., 2020, 8(7), 2772–2782 CrossRef CAS.
- D. V. Sahayaraj, A. Lusi, A. J. Kohler, H. Bateni, H. Radhakrishnan and A. Saraeian, et al., An effective strategy to produce highly amenable cellulose and enhance lignin upgrading to aromatic and olefinic hydrocarbons, Energy Environ. Sci., 2023, 16(1), 97–112 RSC.
- H. Chen, B. Jiang, W. Wu and Y. Jin, Comparison of enzymatic saccharification and lignin structure of masson pine and poplar pretreated by p-Toluenesulfonic acid, Int. J. Biol. Macromol., 2020, 151, 861–869 CrossRef CAS PubMed.
- J. Zheng, L. Chen, X. Qiu, Y. Liu and Y. Qin, Structure investigation of light-colored lignin extracted by Lewis acid-based deep eutectic solvent from softwood, Bioresour. Technol., 2023, 385, 129458 CrossRef CAS PubMed.
- H. Qiao, Y. Wang, Z. Ma, M. Han, Z. Zheng and J. Ouyang, In-depth investigation of formic acid pretreatment for various biomasses: Chemical properties, structural features, and enzymatic hydrolysis, Bioresour. Technol., 2023, 374, 128747 CrossRef CAS PubMed.
- Q. Zhai, S. Han, C. Y. Hse, J. Jiang and J. Xu, 5-Sulfosalicylic acid as an acid hydrotrope for the rapid and green fractionation of woody biomass, Ind. Crops Prod., 2022, 177, 114435 CrossRef CAS.
- Vertoro [Internet]. [cited 2022 Nov 9]. Technology. Available from: https://vertoro.com/technology/.
- F. S. Chakar and A. J. Ragauskas, Review of current and future softwood kraft lignin process chemistry, Ind. Crops Prod., 2004, 20(2), 131–141 CrossRef CAS.
- PubChem. Thymol [Internet]. [cited 2022 Nov 9]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/6989.
- S. Wu, C. Cai, F. Li, Z. Tan and S. Dong, Deep Eutectic Supramolecular Polymers: Bulk Supramolecular Materials, Angew. Chem., Int. Ed., 2020, 59(29), 11871–11875 CrossRef CAS PubMed.
- P. Janicka, M. Kaykhaii, J. Płotka-Wasylka and J. Gębicki, Supramolecular deep eutectic solvents and their applications, Green Chem., 2022, 24(13), 5035–5045 RSC.
- K. Lundquist, R. Lundgren, J. Danielsen, A. Haaland and S. Svensson, Acid Degradation of Lignin. Part VII. The Cleavage of Ether Bonds, Acta Chem. Scand., 1972, 26, 2005–2023 CrossRef CAS.
- H.K. Ono and K. Sudo, Wood Adhesives from Phenolysis Lignin, in: Lignin. American Chemical Society, 1989, vol. 397, pp. 334–345. (ACS Symposium Series). Available from: DOI:10.1021/bk-1989-0397.ch025.
- G. Gellerstedt, A. Majtnerova and L. Zhang, Towards a new concept of lignin condensation in kraft pulping. Initial results, C. R. Biol., 2004, 327(9), 817–826 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and additional text; Fig. S1–S8; Tables S1–S2. See DOI: https://doi.org/10.1039/d3gc03857b |
| This journal is © The Royal Society of Chemistry 2024 |