Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Green aromatic aldehyde production from biomass via catalytic fractionation and ozonolysis†
Tianyu
Ren
 ,
Peidong
Li
,
Zhuo
He
,
Xinfeng
Pan
,
Yutao
Yang
,
Yuhe
Liao
,
Haiyong
Wang
,
Peidong
Li
,
Zhuo
He
,
Xinfeng
Pan
,
Yutao
Yang
,
Yuhe
Liao
,
Haiyong
Wang
 ,
Yanbin
Cui
* and
Chenguang
Wang
,
Yanbin
Cui
* and
Chenguang
Wang
 *
*
CAS Key Laboratory of Renewable Energy, Guangdong Provincial Key Laboratory of New and Renewable Energy Research and Development, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Tianhe District, Guangzhou 510640, China. E-mail: wangcg@ms.giec.ac.cn; cuiyb@ms.giec.ac.cn
First published on 15th November 2024
Abstract
Herein, we propose a catalytic fractionation–ozonolysis strategy for producing aromatic aldehydes from biomass. Native lignin is selectively depolymerized into ∼30 wt% 4-methoxypropenyl-guaiacol/syringol over MoO2 at 160–180 °C, followed by ozonolysis yielding 20 wt% vanillin and syringaldehyde. This strategy is free of base and well preserves carbohydrate pulp.
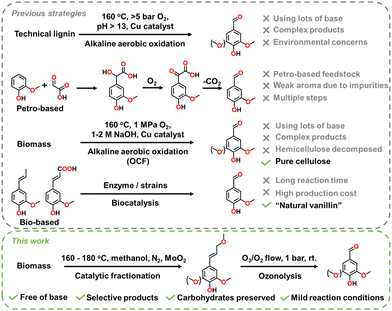
Aromatic aldehydes are a class of important compounds with broad applications in the flavoring, polymer, and pharmaceutical industries.1,2 Thanks to the functional carbonyl group, aromatic aldehydes also act as precursors for alcohols, acids, amines, etc., and building blocks of complex macromolecules. Sustainably producing aromatic aldehydes from bioresources is highly desirable.
One of the well-known aromatic aldehydes is vanillin, a famous flavoring additive and building block for versatile products with a worldwide production of around 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons per year.3–6 It is historically produced by aerobic oxidation of technical lignin in caustic solution.3,4,7 This process dominated the market in the middle of the last century (Scheme 1), but later, due to the environmental concerns of alkaline effluent and high production cost, it was largely replaced by petro-based processes using guaiacol and glyoxylic acid as the feedstocks (Scheme 1).3,4 However, efforts for improving the lignin-based vanillin process have continued. Recent studies reported a conversion strategy, termed oxidative catalytic fractionation (OCF), that realized a higher yield of aromatic aldehydes (including vanillin, syringaldehyde, and 4-hydroxybenzaldehyde) (15–40 wt%) via alkaline aerobic oxidation of lignocellulosic biomass instead of technical lignin, since native lignin is easier to depolymerize.8–10 However, the use of a high concentration of base (1–2 M NaOH) remains environmentally and cost-unfriendly. OCF can also be conducted in organic solvents without involving a base, although the yield of aromatic aldehydes is lower and the risk of explosive oxidation of organic solvents should be noted.11
000 tons per year.3–6 It is historically produced by aerobic oxidation of technical lignin in caustic solution.3,4,7 This process dominated the market in the middle of the last century (Scheme 1), but later, due to the environmental concerns of alkaline effluent and high production cost, it was largely replaced by petro-based processes using guaiacol and glyoxylic acid as the feedstocks (Scheme 1).3,4 However, efforts for improving the lignin-based vanillin process have continued. Recent studies reported a conversion strategy, termed oxidative catalytic fractionation (OCF), that realized a higher yield of aromatic aldehydes (including vanillin, syringaldehyde, and 4-hydroxybenzaldehyde) (15–40 wt%) via alkaline aerobic oxidation of lignocellulosic biomass instead of technical lignin, since native lignin is easier to depolymerize.8–10 However, the use of a high concentration of base (1–2 M NaOH) remains environmentally and cost-unfriendly. OCF can also be conducted in organic solvents without involving a base, although the yield of aromatic aldehydes is lower and the risk of explosive oxidation of organic solvents should be noted.11
Oxidizing lignin with other oxidants such as nitrobenzene, cupric oxide, and H2O2 also produces aromatic aldehydes.12,13 However, the methods consume large amounts of oxidants and the product compositions are complex. Oxidation via photo- or electro-catalysis, which are free of costly oxidants, has shown potential for depolymerizing lignin and producing aromatic aldehydes, although further studies on catalysts and mechanisms are required.14–16
Besides lignin, isoeugenol and ferulic acid are primary precursors for sustainable vanillin production. They are converted by fermentation or enzyme catalysis which mimic vanillin formation in natural vanilla (Scheme 1).5,6 Thus, although the feedstock and production are costly, the as-formed vanillin can be labeled as “natural vanillin” and sold at higher prices.5,6,17 Other conversion approaches involve oxidation using O2, H2O2, O3, or other oxidants which generates synthetic vanillin but is less profitable than the guaiacol and glyoxylic acid route.18–21
As introduced above, current production methods for sustainable aromatic aldehydes primarily involve oxidation of lignin and monophenols. However, those methods typically consume large amounts of costly reagents and deconstruct carbohydrate if biomass is used. To circumvent those drawbacks, herein, we report a potential green and sustainable production route for vanillin and related aromatic aldehydes from biomass (Scheme 1). In this route, biomass (i.e., native lignin) is selectively depolymerized into 4-methoxypropenyl-guaiacol and 4-methoxypropenyl-syringol (MP-G and MP-S) followed by ozonolysis forming aromatic aldehydes. The advantages of this route include being free of base, the absence of the risk of explosive hydrogen or oxidation of solvent, cost-effective biomass feedstock and ozonolysis process, highly selective products, and the recycling of the carbohydrate part of biomass.
For the first step, depolymerizing lignin into highly selective alkene (Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cβ)-functionalized monophenols is currently a challenge, with relevant studies rarely reported. This is because such structures are unstable and easy to polymerize.22,23 Therefore, by catalytically hydrogenating the alkenyl intermediates during depolymerization, 4-propyl- and 4-propanol-guaiacol/syringol (G/S) monomers can be efficiently yielded, which is the mechanism for the emerging reductive catalytic fractionation (RCF) conversion strategy.22,23 Very recently, Wang and Song's group reported an atomically dispersed Pd catalyst, realizing 40.7 wt% monomer yield with 62% selectivity to 4-propenyl-G/S (and 20% selectivity to 4-propyl-G/S).24 Two types of Mo-based catalysts, supported MoOx catalysts and Mo-based single-atom catalysts, were reported to be efficient for producing coniferyl and sinapyl alcohols and their methyl ethers (i.e., MP-G and MP-S), with a total yield and selectivity higher than 40 wt% and 90%, respectively.25,26
Cβ)-functionalized monophenols is currently a challenge, with relevant studies rarely reported. This is because such structures are unstable and easy to polymerize.22,23 Therefore, by catalytically hydrogenating the alkenyl intermediates during depolymerization, 4-propyl- and 4-propanol-guaiacol/syringol (G/S) monomers can be efficiently yielded, which is the mechanism for the emerging reductive catalytic fractionation (RCF) conversion strategy.22,23 Very recently, Wang and Song's group reported an atomically dispersed Pd catalyst, realizing 40.7 wt% monomer yield with 62% selectivity to 4-propenyl-G/S (and 20% selectivity to 4-propyl-G/S).24 Two types of Mo-based catalysts, supported MoOx catalysts and Mo-based single-atom catalysts, were reported to be efficient for producing coniferyl and sinapyl alcohols and their methyl ethers (i.e., MP-G and MP-S), with a total yield and selectivity higher than 40 wt% and 90%, respectively.25,26
Inspired by the works of Mo-based catalysts, we attempted to apply simple and commercial MoO2 as the catalyst. To our delight, MP-G and MP-S were highly selectively produced from poplar in methanol at 180 °C with MoO2 (Fig. 1a and b), without 4-propyl-G/S, 4-propenyl-G/S, and 4-propanol-G/S detected. The total yield and selectivity of MP-G and MP-S peaked at around 25 wt% and 75%, respectively, at 2 h. Compared with the traditional RCF process, this conversion system does not involve reductive function (i.e., reducing the Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Cβ bonds) and may therefore be termed catalytic fractionation (CF). The only notable by-product is methyl 4-hydroxybenzoate, which is a characteristic component in poplar lignin (Fig. 1b).27,28 Compared with the blank test, MoO2 evidently enhanced the monomer yield, demonstrating a promotional effect on lignin depolymerization. 10% MoO2 dosage realized ∼30 wt% total monomer yield, which is comparable to the typical RCF conversion of poplar using noble catalysts (e.g., Ru/C and Pd/C).27–29 Additionally, MoO3 showed a similar catalytic performance to MoO2. The screening of solvents shows that methanol is the most suitable (Fig. 1c and d). Ethanol, ethylene glycol, and cosolvents with water were reported to be efficient in other RCF-based processes,28–31 but turned out to be inefficient in CF. This is probably due to their poorer depolymerization ability at low reaction temperatures. 160 and 180 °C were found to be suitable for CF (Fig. 1e). At lower temperatures (140 and 160 °C), we found small amounts of coniferyl and sinapyl alcohols, while at 200 °C, the yields of MP-G and MP-S decreased probably due to repolymerization as alkene-functionalized phenols are unstable at high temperature. Furthermore, CF at a higher biomass dosage resulted in lower yields of MP-G and MP-S (Fig. S1†), probably due to poor stirring and mass transfer.
Cβ bonds) and may therefore be termed catalytic fractionation (CF). The only notable by-product is methyl 4-hydroxybenzoate, which is a characteristic component in poplar lignin (Fig. 1b).27,28 Compared with the blank test, MoO2 evidently enhanced the monomer yield, demonstrating a promotional effect on lignin depolymerization. 10% MoO2 dosage realized ∼30 wt% total monomer yield, which is comparable to the typical RCF conversion of poplar using noble catalysts (e.g., Ru/C and Pd/C).27–29 Additionally, MoO3 showed a similar catalytic performance to MoO2. The screening of solvents shows that methanol is the most suitable (Fig. 1c and d). Ethanol, ethylene glycol, and cosolvents with water were reported to be efficient in other RCF-based processes,28–31 but turned out to be inefficient in CF. This is probably due to their poorer depolymerization ability at low reaction temperatures. 160 and 180 °C were found to be suitable for CF (Fig. 1e). At lower temperatures (140 and 160 °C), we found small amounts of coniferyl and sinapyl alcohols, while at 200 °C, the yields of MP-G and MP-S decreased probably due to repolymerization as alkene-functionalized phenols are unstable at high temperature. Furthermore, CF at a higher biomass dosage resulted in lower yields of MP-G and MP-S (Fig. S1†), probably due to poor stirring and mass transfer.
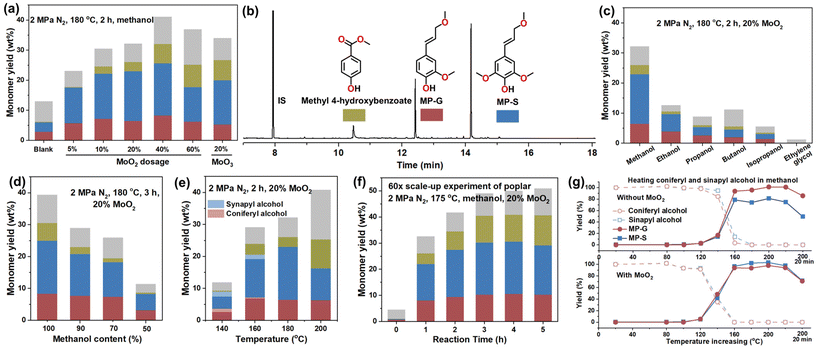 | ||
| Fig. 1 CF conversion of biomass. (a) Influence of MoO2 dosage; (b) GC-MS spectrum of the CF product (2 h in Fig. S2a†)(more GC spectra can be found in Fig. S21 and S22†); (c) influence of alcohol solvent; (d) influence of water as cosolvent; (e) influence of temperature; (f) 60× scale-up experiments; and (g) function of MoO2 in gradually heating coniferyl and sinapyl alcohols in methanol at a rate of 2 °C min−1. CF conditions: 300 mg of poplar powder, 10 mL of methanol or other solvents, 20% (60 mg) MoO2 or other dosages, 2 MPa N2, 500 rpm, and 2 or 3 h; for scale-up experiments, 18 g poplar sawdust, 600 mL of methanol, 3.6 g MoO2, 2.5 MPa N2, 175 °C, and 500 rpm. Note that the gray columns represent all by-products in the monomer region in the GC spectra, calculated by adding up all peaks of by-products within 10–20 min in the GC spectra (details can be found in Fig. S4, S21, and S22†). | ||
We further expanded the reaction size by a factor of 60 or 80 (Fig. 1f and S2†). After reaching the reaction temperature (175 °C), MP-G, MP-S, and methyl 4-hydroxybenzoate were gradually accumulated, achieving similar product distribution to the small experiments. Their yields became stable at 10.3, 19.6, and 9.4 wt%, respectively, after 3 h, indicating that the alkene-functional phenols are stable at 175 °C. Similarly, the conversion of birch sawdust produced 32 wt% MP-G and MP-S in total (Fig. S2b†). Comparatively, converting a softwood, pine, was less efficient (Fig. S2c†), which is similar to other RCF-based processes and related to the unique and stubborn softwood lignin. In the carbohydrate residual of poplar, we found that ∼94% cellulose and hemicellulose were well preserved (Table S1†), probably thanks to the low reaction temperature. By simply hydrolyzing the residual in diluted acid at 100 °C, we obtained 70% yield of xylose and xylulose (possibly from catalytic isomerization from xylose over residual MoO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32,33) from hemicellulose, while most cellulose remained intact as <5% glucose was yielded (Fig. S3†). Therefore, the results exhibit a potential CF based strategy for utilizing lignin, hemicellulose, and cellulose in sequence from biomass.
32,33) from hemicellulose, while most cellulose remained intact as <5% glucose was yielded (Fig. S3†). Therefore, the results exhibit a potential CF based strategy for utilizing lignin, hemicellulose, and cellulose in sequence from biomass.
The depolymerization mechanism is analogous to that of RCF due to their similar reaction conditions. It is widely reported that lignin can be fractionated into fragments and coniferyl and sinapyl alcohols under the solvolysis effect of hot methanol. The process is promoted via a mechanism of methylating Cα–OH into Cα–OMe.22,25,34 We detected minor amounts of coniferyl and sinapyl alcohols at low reaction temperature (Fig. 1e) and the beginning of the CF reaction (Fig. S21†), conforming to the mechanism and indicating that they are precursors of MP-G and MP-S. By heating coniferyl and sinapyl alcohols in methanol, we found that coniferyl alcohols were rapidly transformed into MP-G and MP-S when the temperature was ramped to 140–160 °C (Fig. 1g); comparatively, the reaction began at 120–140 °C when MoO2 was involved in the system. The results demonstrate that the methylation reaction can be catalytically promoted by MoO2 and is a noncatalytic process at >160 °C. Moreover, MP-G and MP-S were found to gradually decompose and polymerize at 200 °C.22,25 The above results indicate that 160–180 °C would be more suitable for accumulating MP-G and MP-S in CF considering their formation from lignin and decomposition. By analyzing the monomeric by-products of CF (Fig. S4†), we found that most components are monophenols with Cα![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and Cα–OMe structures. Their formation and the catalytic mechanism of MoO2 for promoting lignin depolymerization are unclear at the present stage, largely because the products are unstable and easy to condense especially at high concentration (Fig. S5 and Note S1†). Thus, the samples were unable to be thoroughly analyzed by techniques such as 2D NMR.
O and Cα–OMe structures. Their formation and the catalytic mechanism of MoO2 for promoting lignin depolymerization are unclear at the present stage, largely because the products are unstable and easy to condense especially at high concentration (Fig. S5 and Note S1†). Thus, the samples were unable to be thoroughly analyzed by techniques such as 2D NMR.
The above results of the CF system are inspiring. In recent years, several efficient lignin-first depolymerization strategies were developed to overcome the drawbacks of the emerging RCF process.24,28,35–38 As a variant of RCF, the CF process uses evidently lower temperature and a commercial non-noble catalyst, without using hydrogen and acid/base additives; it yields considerable highly selective alkene-functionalized monomers with carbohydrate pulp well preserved; therefore, it is promising for biomass valorization. Moreover, the application of a commercial catalyst (MoO2) is conducive to further investigation by other labs. At this stage, the preliminary investigation offers a simple approach for producing alkene-functionalized monomers, while a systematic study will follow after this work.
For the second step, we cleave the alkene moiety into the carbonyl group via ozonolysis. Ozonolysis is a classic organic conversion widely used for transforming alkenes into aldehydes and ketones,39,40 but it has rarely been reported for upgrading biomass-derived chemicals. In an ozonolysis reaction, alkenes are simply treated with an O3 (ozone)/O2 gas flow at normal temperature and pressure. Then, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds selectively cleave, generating carbonyl products typically with 70–90% yield.39,40 Therefore, considering the sustainable and cheap oxidant and mild reaction conditions, ozonolysis is an efficient and cost-effective tool for upgrading alkene-functionalized lignin monomers into valuable aromatic aldehydes. However, the major drawbacks of ozonolysis include the formation of energetic peroxides (could be explosive at high temperature and concentration) and overoxidation due to the strong oxidation ability of O3. The former issue is typically resolved by reduction with a stoichiometric amount of reducing agent, which inevitably increases the production cost;39,40 or by a simple catalytic decomposition approach using MnO2 as reported recently by us.41 As for the latter drawback, reaction conditions and pathway are unclear for lignin-derived phenols.
C double bonds selectively cleave, generating carbonyl products typically with 70–90% yield.39,40 Therefore, considering the sustainable and cheap oxidant and mild reaction conditions, ozonolysis is an efficient and cost-effective tool for upgrading alkene-functionalized lignin monomers into valuable aromatic aldehydes. However, the major drawbacks of ozonolysis include the formation of energetic peroxides (could be explosive at high temperature and concentration) and overoxidation due to the strong oxidation ability of O3. The former issue is typically resolved by reduction with a stoichiometric amount of reducing agent, which inevitably increases the production cost;39,40 or by a simple catalytic decomposition approach using MnO2 as reported recently by us.41 As for the latter drawback, reaction conditions and pathway are unclear for lignin-derived phenols.
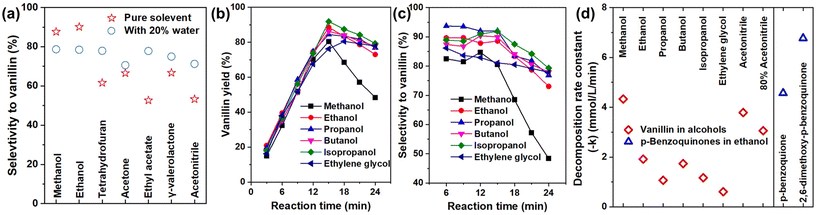
To address this issue and establish suitable conversion strategies for lignin-derived alkene-functionalized phenols (e.g., MP-G and MP-S), we intensively investigated the influence of solvent. Solvent is a crucial factor in ozonolysis, as it (particularly protic water and alcohols) directly reacts with ozonolysis intermediates (carbonyl oxides), forming different downstream intermediates with different reactivities, thereby significantly affecting the yield of the final products.18,39,42 We selected the conversion of a lignin monomer 4-propenyl-G to vanillin as the model ozonolysis reaction. Although this reaction was reported elsewhere, the vanillin yield was greatly different (Table S2†), indicating a high impact on reaction conditions and overoxidation. By screening the solvent (Fig. 2a), we found vanillin is the major product, without evident by-products detected in HPLC in all tests. The reaction is simple with conversion and vanillin yield increasing linearly until consumption of all 4-propenyl-G (Fig. 2b and c), so vanillin selectivity was compared. Pure alcohols including methanol and ethanol are more suitable for producing vanillin with a higher selectivity of around 90% (Fig. 2a). Comparatively, aprotic solvents exhibited a lower vanillin selectivity of around 60%, indicating a significant loss of the product due to overoxidation. Mixing the solvents with 20% water decreased the selectivity in alcohols, while increasing it in aprotic solvents (Fig. 2a). Among different alcohols (Fig. 2b and c), vanillin yield peaked at 80–92% at 15 min with the selectivity ranging from 83 to 93%, after which they decreased linearly. By oxidizing vanillin alone, we calculated the decomposition rates (Fig. 2d) and found that they were close to the rate of vanillin loss after ozonolysis of 4-propenyl-G (Fig. S6†). This demonstrates overoxidation of vanillin by O3 (rather than reaction with other components) after ozonolysis. Interestingly, the decomposition rate in methanol, the best solvent for CF, is more than twice that in other alcohols and also faster than in aprotic acetonitrile. Additionally, ozonolysis of other lignin-related alkene-functionalized monomers (coniferyl aldehyde, coniferyl alcohol, and methyl isoeugenol) efficiently yielded the corresponding aromatic aldehydes (Fig. S7†). In particular, converting methyl isoeugenol produced methyl vanillin with ∼100% selectivity, proving that methylation of Ar–OH effectively prevented overoxidation.
To further understand the influences of solvents on the ozonolysis of 4-propenyl-G and overoxidation, we repeated the reactions in deuterated solvents (methanol-d4, ethanol-d6, acetonitrile-d3, and 80% acetonitrile-d3–20% water) and analyzed the products using NMR (Fig. 3 and Fig. S8–S15†). The major peaks are assigned in Fig. 3. The results show that, during the ozonolysis of 4-propenyl-G, vanillin was formed as the major aromatic product in all solvents. The side chain of 4-propenyl-G was transformed into acetaldehyde and its solvated and peroxide forms (Fig. 3a). The hydroperoxyacetals of vanillin were probably formed in alcohols. It is unexpected that we did not detect the ozonide (1,2,4-trioxane) of vanillin in aprotic acetonitrile, which might be due to the involvement of trace water produced by complete overoxidation of 4-propenyl-G. Oxidizing vanillin in alcohols (especially methanol) triggered its addition with the solvent to form hemiacetals, which were subsequently transformed into a quinone methide (Fig. 3b).18,39,42 We speculate that the adducts could be more easily oxidized, which might be a possible reason for the high decomposition rate for vanillin in methanol (Fig. 2b–d). In all tests, we did not find evidence of the existence of vanillic acid, proving that the carbonyl group is relatively stable to O3 and the overoxidation begins with the oxidation of Ar-OH. The finding of quinone methide (Fig. 3b) and the fast decomposition rates of quinones (right columns in Fig. 2d) support this oxidation route. Thus, O3 oxidation is different from alkaline aerobic oxidation (as introduced above) which produces an aromatic acid and a wide range of small acids. Moreover, we did not find other significant by-products except for formic acid and formate (dashed box in Fig. 3b). This demonstrates the fast overoxidation of vanillin into C1 components, CO2, and H2O, which is the primary reason for the decrease of vanillin yield during the ozonolysis of 4-propenyl-G. The oxidation pathway of 4-propenyl-G is concluded and depicted in Fig. 3c.
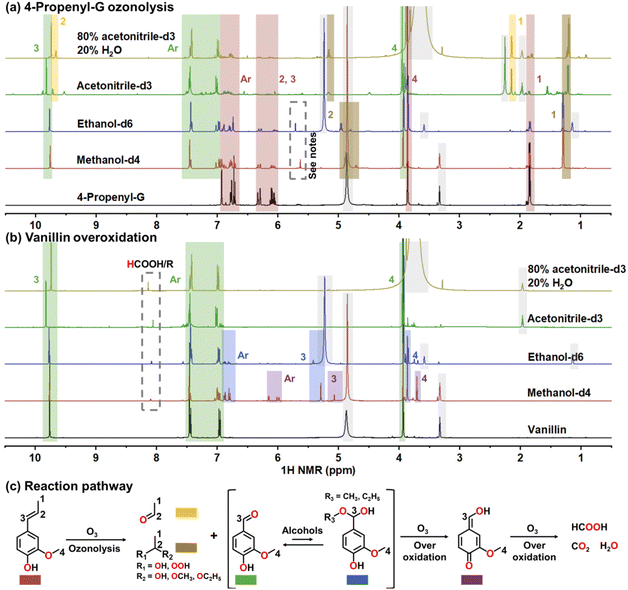 | ||
| Fig. 3 NMR study of solvent functions. 1H NMR spectra of (a) ozonolysis products of 4-propenyl-G and (b) decomposition products of vanillin in different deuterated solvents; and (c) scheme of the proposed ozonolysis and overoxidation pathway. Oxidation conditions: 0.1 mL of 4-propenyl-G or 0.1 g vanillin, 5 mL of deuterated solvent, 50 mL min−1 O3/O2 gas flow at an O3 concentration of 60 mg L−1, and 9 min. Note: the gray color indicates solvent and water peaks; the peaks in the dashed box in (a) are speculated to be Cα–H of vanillin hydroperoxyacetals. Spectra of reactions at 0–24 min can be found in Fig. S8–S15.† | ||
The above results and analysis show that, during and after the ozonolysis of 4-propenyl-G, vanillin is gradually oxidatively decomposed. This is inevitable due to the high oxidative capacity of O3 but can be alleviated by using alcohols as the solvent and controlling the input of O3. These findings are important as alcohols (particularly methanol) are the most suitable solvents for CF and other RCF-based processes. The product solution of CF is readily ozonolyzed without the need for tedious product work-up.
To investigate CF-ozonolysis cascade processes, poplar, birch, and pine were subjected to the CF process (Fig. 1e and S2†). The liquid products of poplar were rotary-evaporated and redissolved in ethanol. Afterward, the concentrated solutions were bubbled with an O3/O2 flow. As shown in Fig. 4a, the main alkene-functionalized monomers of hardwood, MP-G and MP-S, were rapidly and linearly converted into vanillin and syringaldehyde, respectively, within minutes. Their yields peaked at around 95% and 50%, respectively. The low yield and rapid decomposition of syringaldehyde (further proved in Fig. S16†) indicate that S units are more easily overoxidized by O3 than G units. Ozonolyzing sinapic acid also produces a low yield of syringaldehyde (Fig. S17†). Compared with the ozonolysis of the CF product in methanol (Fig. S18†), the use of ethanol instead of methanol mitigated the syringaldehyde decomposition (Fig. S19†), consistent with the solvent effect on vanillin decomposition (Fig. 2d). It is interestingly found that vanillin was not decomposed after consuming MP-G, which is discrepant with the model conversions (Fig. 2d and 3b). This is probably due to the fast consumption of O3 by components (e.g., syringyl components) that are easily oxidized and act as sacrificial agents. In addition to components with S units, lignin oligomers are probably prone to consumption due to overoxidation, as we observed that the solution evidently turned a lighter color during the overoxidation period (Fig. S20†). Moreover, it is also interesting to find that the ozonolysis of the product of pine yielded 160% of vanillin (Fig. 4b), based on the initial content of MP-G. We speculate that the additional vanillin may come from the ozonolysis of alkene-functionalized oligomers, as pine lignin was hard to depolymerize in the CF process (Fig. S2c†).
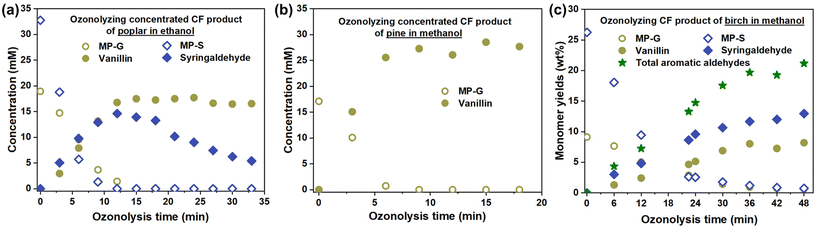 | ||
| Fig. 4 Ozonolysis of CF products of (a) poplar, (b) pine, and (c) birch. The CF products and conditions are exhibited in Fig. S2.† Ozonolysis conditions: for (a) and (b), 30 mL of concentrated substrate solution or (c) all liquid CF product, and 300 mL min−1 O3/O2 gas flow at a O3 concentration of 60 (30 for (c)) mg L−1. Detailed procedures can be found in ESI S2.3.† | ||
After the CF reaction, the liquid CF products can be immediately ozonolyzed (Fig. 4c). In this case, the ozone input was decreased to avoid overoxidation. Eventually, 7.8 wt% vanillin and 12.2 wt% syringaldehyde (20.0 wt% in total) were obtained. The detailed GC spectra of these CF-ozonolysis cascade processes are presented in Fig. S21 and S22,† which show that the by-products were from CF particularly after obtaining a high yield of alkene-functionalized phenols and ozonolysis did not generate new by-products. This also shows the high selectivity of aromatic aldehydes, which can be further improved by shortening the CF reaction time, and indicates an easier subsequent isolation procedure.
The above results demonstrate that vanillin and syringaldehyde can be produced via CF-ozonolysis cascade processes. Vanillin is not lost due to overoxidation. Despite the fast oxidative decomposition of syringyl components, it can be expected to alkylate the Ar–OH group of MP-G and MP-S prior to ozonolysis to prevent overoxidation (Fig. S7†). The as-formed veratraldehyde (methyl vanillin) and methyl syringaldehyde are also value-added and commercial for flavoring and organic synthesis purposes.43,44
In comparison with the traditional alkaline aerobic oxidation methods introduced before (Scheme 1), the yield of aromatic aldehydes in the CF-ozonolysis process is lower at the present stage, largely due to the decomposition of syringaldehyde. However, the preservation of hemicellulose, base-free condition, and more selective products are its advantages. In terms of the toxicity of the main chemicals used, the CF-ozonolysis process circumvents toxic and corrosive bases and acids (for neutralization after the alkaline oxidation reaction). The solvent methanol is ranked as recommended (after discussion) according to the CHEM21 selection guide of solvents.45 However, it should be noted that ozone is toxic to human health. Thus, when operated on a large scale, the off-gas from ozonolysis is suggested to be treated to catalytically decompose residual ozone and recover volatile methanol.46
Conclusions
We propose CF-ozonolysis cascade processes for producing aromatic aldehydes from lignocellulosic biomass. The native lignin is depolymerized via a CF process, a variant of RCF, to selectively form alkene-functionalized MP-G and MP-S with a total yield of over 30 wt% at 175 °C. Then, the liquid product is simply bubbled with an O3/O2 flow, affording 20 wt% vanillin and syringaldehyde from MP-G and MP-S, respectively. The vanillin yield is over 95% and is not lost due to overoxidation. Compared with the traditional alkaline aerobic oxidation approach, CF-ozonolysis processes neither use a base nor produce evident oxidative by-products. Moreover, as a lignin-first strategy, the CF process applies low-cost and commercial MoO2 as the catalyst and well preserves the carbohydrate. Therefore, CF-ozonolysis represents an advancement in sustainable production of aromatic aldehydes and biomass valorization.Author contributions
Conceptualization: T. Ren; investigation: T. Ren, P. Li, Z. He, and X. Pan; methodology, formal analysis, and visualization: T. Ren; resources, funding acquisition, and project administration: T. Ren, Y. Liao, H. Wang, Y. Cui, and C. Wang; writing – original draft: T. Ren; writing – review and editing: T. Ren, Y. Cui, and C. Wang.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the China Postdoctoral Science Foundation (2023M743509), the Guangdong Basic and Applied Basic Research Foundation (2024A1515010569), the International Science and Technology Innovation Cooperation Program of the National Key Research and Development Plan of China (2021YFE0114400), and the National Natural Science Foundation of China (52276220, 22408365).References
- N. Lee, Y. T. Kim and J. Lee, Polymers, 2021, 13, 364 CrossRef CAS PubMed.
- A. Olatunde, A. Mohammed, M. A. Ibrahim, N. Tajuddeen and M. N. Shuaibu, Eur. J. Med. Chem. Rep., 2022, 5, 100055 CAS.
- V. Tarabanko and N. Tarabanko, Int. J. Mol. Sci., 2017, 18, 2421 CrossRef PubMed.
- M. Fache, B. Boutevin and S. Caillol, ACS Sustainable Chem. Eng., 2015, 4, 35–46 CrossRef.
- G. A. Martău, L.-F. Călinoiu and D. C. Vodnar, Trends Food Sci. Technol., 2021, 109, 579–592 CrossRef.
- L. Xu, F. Liaqat, J. Sun, M. I. Khazi, R. Xie and D. Zhu, Renewable Sustainable Energy Rev., 2024, 189, 113905 CrossRef CAS.
- T. Ren, W. Qi, R. Su and Z. He, ChemCatChem, 2019, 11, 639–654 CrossRef CAS.
- Y. Zhu, Y. Liao, L. Lu, W. Lv, J. Liu, X. Song, J. Wu, L. Li, C. Wang, L. Ma and B. F. Sels, ACS Catal., 2023, 13, 7929–7941 CrossRef CAS.
- W. Schutyser, J. S. Kruger, A. M. Robinson, R. Katahira, D. G. Brandner, N. S. Cleveland, A. Mittal, D. J. Peterson, R. Meilan, Y. Román-Leshkov and G. T. Beckham, Green Chem., 2018, 20, 3828–3844 RSC.
- J. S. Kruger, R. J. Dreiling, D. G. Wilcox, A. J. Ringsby, K. L. Noon, C. K. Amador, D. G. Brandner, K. J. Ramirez, S. J. Haugen, B. C. Klein, R. Davis, R. J. Hanes, R. M. Happs, N. S. Cleveland, E. D. Christensen, J. Miscall and G. T. Beckham, Green Chem., 2022, 24, 8733–8741 RSC.
- H. Luo, E. P. Weeda, M. Alherech, C. W. Anson, S. D. Karlen, Y. Cui, C. E. Foster and S. S. Stahl, J. Am. Chem. Soc., 2021, 143, 15462–15470 CrossRef CAS PubMed.
- G. Yang, Z. Gong, X. Luo and L. Shuai, Front. Bioeng. Biotechnol., 2022, 10, 1002145 CrossRef PubMed.
- C. Qu, M. Kaneko, K. Kashimura, K. Tanaka, S. Ozawa and T. Watanabe, ACS Sustainable Chem. Eng., 2017, 5, 11551–11557 CrossRef CAS.
- P. D’Arrigo, L. A. M. Rossato, A. Strini and S. Serra, Molecules, 2024, 29, 442 CrossRef PubMed.
- P. Li, Y. Ouyang, G. Xiao, Y. Zhao, S. Sarina, J. Baeyens, H. Su and H.-Y. Zhu, Green Chem., 2021, 23, 7780–7789 RSC.
- J.-x. Song, H.-j. Zhang, M.-h. Niu, Y.-z. Guo and H.-m. Li, Ind. Crops Prod., 2024, 214, 118443 CrossRef CAS.
- M. Sabisch and D. Smith, Perfum. Flavor., 2012, 37, 38–41 Search PubMed.
- S. Buntasana, J. Hayashi, P. Saetung, P. Klumphu, T. Vilaivan and P. Padungros, J. Org. Chem., 2022, 87, 6525–6540 CrossRef CAS PubMed.
- A. I. Martín-Perales, D. Rodríguez-Padrón, A. M. Balu, S. Halloumi, I. Malpartida and R. Luque, Ind. Eng. Chem. Res., 2023, 62, 17545–17552 CrossRef.
- M. D. Marquez-Medina, P. Prinsen, H. Li, K. Shih, A. A. Romero and R. Luque, ChemSusChem, 2017, 11, 389–396 CrossRef PubMed.
- Y. Zhang, N. Hatami, N. S. Lange, E. Ronge, W. Schilling, C. Jooss and S. Das, Green Chem., 2020, 22, 4516–4522 RSC.
- S. Van den Bosch, T. Renders, S. Kennis, S. F. Koelewijn, G. Van den Bossche, T. Vangeel, A. Deneyer, D. Depuydt, C. M. Courtin, J. M. Thevelein, W. Schutyser and B. F. Sels, Green Chem., 2017, 19, 3313–3326 RSC.
- T. Renders, G. Van den Bossche, T. Vangeel, K. Van Aelst and B. Sels, Curr. Opin. Biotechnol, 2019, 56, 193–201 CrossRef CAS PubMed.
- S. Wang, X. Li, C. Fu, H. Li and G. Song, ACS Catal., 2024, 14, 3565–3574 CrossRef CAS.
- J. Sun, H. Li, L.-P. Xiao, X. Guo, Y. Fang, R.-C. Sun and G. Song, ACS Sustainable Chem. Eng., 2019, 7, 4666–4674 CrossRef CAS.
- G. Meng, W. Lan, L. Zhang, S. Wang, T. Zhang, S. Zhang, M. Xu, Y. Wang, J. Zhang, F. Yue, Y. Wu and D. Wang, J. Am. Chem. Soc., 2023, 145, 12884–12893 CrossRef CAS PubMed.
- E. M. Anderson, M. L. Stone, R. Katahira, M. Reed, W. Muchero, K. J. Ramirez, G. T. Beckham and Y. Roman-Leshkov, Nat. Commun., 2019, 10, 2033 CrossRef PubMed.
- T. Ren, S. You, Z. Zhang, Y. Wang, W. Qi, R. Su and Z. He, Green Chem., 2021, 23, 1648–1657 RSC.
- T. Renders, S. Van den Bosch, T. Vangeel, T. Ennaert, S.-F. Koelewijn, G. Van den Bossche, C. M. Courtin, W. Schutyser and B. F. Sels, ACS Sustainable Chem. Eng., 2016, 4, 6894–6904 CrossRef CAS.
- W. Schutyser, S. Van den Bosch, T. Renders, T. De Boe, S. F. Koelewijn, A. Dewaele, T. Ennaert, O. Verkinderen, B. Goderis, C. M. Courtin and B. F. Sels, Green Chem., 2015, 17, 5035–5045 RSC.
- L. Chen, A. P. van Muyden, X. Cui, Z. Fei, N. Yan, G. Laurenczy and P. J. Dyson, JACS Au, 2021, 1, 729–733 CrossRef CAS PubMed.
- N. K. Gupta, A. Fukuoka and K. Nakajima, ACS Catal., 2017, 7, 2430–2436 CrossRef CAS.
- I. Delidovich, ACS Catal., 2023, 13, 2250–2267 CrossRef CAS.
- Q. Wang, L.-P. Xiao, Y.-H. Lv, W.-Z. Yin, C.-J. Hou and R.-C. Sun, ACS Catal., 2022, 12, 11899–11909 CrossRef CAS.
- Z. Zhang, C. W. Lahive, J. G. M. Winkelman, K. Barta and P. J. Deuss, Green Chem., 2022, 24, 3193–3207 RSC.
- H. Zhou, X. Liu, Y. Guo and Y. Wang, JACS Au, 2023, 3, 1911–1917 CrossRef CAS PubMed.
- J. K. Kenny, S. R. Neefe, D. G. Brandner, M. L. Stone, R. M. Happs, I. Kumaniaev, W. P. Mounfield 3rd, A. E. Harman-Ware, K. M. Devos, T. H. t. Pendergast, J. W. Medlin, Y. Roman-Leshkov and G. T. Beckham, JACS Au, 2024, 4, 2173–2187 CrossRef CAS PubMed.
- Y. Li, Y. Yu, Y. Lou, S. Zeng, Y. Sun, Y. Liu and H. Yu, Angew. Chem., Int. Ed., 2023, 62, e202307116 CrossRef CAS PubMed.
- T. J. Fisher and P. H. Dussault, Tetrahedron, 2017, 73, 4233–4258 CrossRef CAS.
- Y. V. Myasoedova, I. S. Nazarov and G. Y. Ishmuratov, Russ. J. Org. Chem., 2019, 55, 47–73 CrossRef CAS.
- T. Ren, Y. Luo, X. Chen and C. Wang, ACS Sustainable Chem. Eng., 2024, 12, 12719–12725 CrossRef CAS.
- Z. Hassan, M. Stahlberger, N. Rosenbaum and S. Brase, Angew. Chem., Int. Ed., 2021, 60, 15138–15152 CrossRef CAS PubMed.
- M. Yang, J. Yi, F. Tu, C. Wei, Y. Lu, S. Wang, Z. Yang, L. Zhao and X. Jiang, Food Control, 2023, 154, 109986 CrossRef CAS.
- Y.-F. Ji, J.-A. Jiang, H.-W. Liu, D.-H. Liao and X.-Y. Wei, Synth. Commun., 2013, 43, 1517–1522 CrossRef CAS.
- D. Prat, A. Wells, J. Hayler, H. Sneddon, C. R. McElroy, S. Abou-Shehada and P. J. Dunn, Green Chem., 2016, 18, 288–296 RSC.
- X. Li, J. Ma and H. He, J. Environ. Sci., 2020, 94, 14–31 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc04199b |
| This journal is © The Royal Society of Chemistry 2024 |