Open Access Article
Open Access ArticleEfficient decomposition of a melamine–formaldehyde foam into melamine via selective disconnection of bonds†
Chaohui
Yang
abc,
Xinyu
Li
abc,
Hongyan
Li
abc,
Chizhou
Wang
abc,
Qianqian
Xing
abc,
Xiaoliang
Jia
abc,
Xiaojing
Cui
*d,
Xianglin
Hou
*abc and
Tiansheng
Deng
 *abc
*abc
aState Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, 27 South Taoyuan Road, Taiyuan, 030001, People's Republic of China. E-mail: dts117@sxicc.ac.cn; houxianglin@sxicc.ac.cn
bCenter of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing, 100049, People's Republic of China
cCAS Key Laboratory of Carbon Materials, Institute of Coal Chemistry, Chinese Academy of Sciences, 27 South Taoyuan Road, Taiyuan, 030001, People's Republic of China
dInstitute of Interface Chemistry and Engineering, Department of Chemistry and Chemical Engineering, Taiyuan Institute of Technology, Taiyuan, Shanxi 030008, People's Republic of China. E-mail: cxjtyut@126.com
First published on 18th November 2024
Abstract
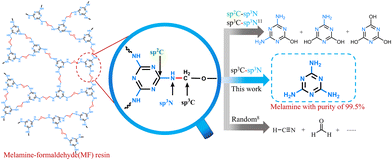
The precise disconnection of one type of chemical bond in polymers realizes high-yield recovery of valuable chemicals. In this study, we demonstrate a sustainable and efficient strategy to selectively cleave the specific sp3C–sp3N bond of a thermoset melamine–formaldehyde foam, by which valuable melamine of 99.5% purity was obtained with a yield of 95.3%.
The recovery of high value-added chemicals from waste plastics is of great significance for the plastic recycling industry and environmental management.1 Melamine formaldehyde (MF) resin is a thermoset resin made by the polymerization of melamine and formaldehyde, in which melamine are interlinked via dimethyl ether bonds (pH > 9), forming a reticular network (Fig. 1).2 MF resin has been widely applied in vehicle manufacturing, home decoration, daily cleaning products and other fields.3 The widespread production and utilization of MF resin inevitably causes the fast accumulation of the end-of-life MF resin waste, causing environmental protection problems.4 Developing an effective MF resin recovery method is crucial to address the issue. However, thermosetting MF resin has good thermal and chemical stability, making its degradation a challenge.5
 | ||
| Fig. 1 Structure of MF resin and products formed by the cleavage of different types of chemical bonds. | ||
Presently, the primary methods for recovering thermosetting resins include mechanical, thermal, and chemical processes.6 The process of mechanical recycling involves pulverizing scrap resin into powders of specified particle size and utilizing it directly as one of the feedstocks for composite materials or as a reinforcing agent for other resins.7 These simplistic treatments (crushing, cutting and grinding, etc.) of MF resin in the mechanical method difficult the fully utilization of the high-value units within the structure of MF resin. In the thermal recovery method, waste resins are commonly decomposed into low-molecular-weight oil or gas products at elevated temperatures.8 This process facilitates the irregular breaking of the chemical bonds within the resin, complexing product distributions and the subsequent product separation process. Additionally, the release of toxic gases is unavoidable in the thermal recycling process.9 Alternatively, the chemical recovery process involves the cleavage of the chemical bonds of the resin in a degradation system under relatively mild reaction conditions.10 It is ideal but challenging to develop an efficient and green degradation system, in which the specific chemical bonds in the resins can be selectively cleaved, yielding monomers that can be easily separated and recycled. Noticeably, the majority of chemical bonds existing in MF resin are the C–N bonds of different types, including the sp2C–sp2N bond in the 1,3,5-triazine (Tr) ring, the sp2C–sp3N bond on the Tr ring, and the neighbouring sp3C–sp3N bond (see Fig. 1). These C–N bonds have similar chemical activities. Hence, it remains a great challenge to selectively break one type of C–N bond without destroying the other types of C–N bonds.
H+ or OH− ions in acid or base catalysts can disconnect the C–N bonds in polymers. This bond disconnection is commonly non-selective due to the high catalytic activity of H+ or OH− ions. Wu et al.11 applied the acid or base recovery system, i.e., the methane sulfonic acid-tetrahydrofuran-10wt% H2O or 10wt% NaOH–H2O, to cleave the C–N bonds of MF resin. They found that either the acid or base system disconnected the sp2C–sp3N and sp3C–sp3N bonds of MF resin, producing a blend of ammelide and ammeline. If a catalytic system enables the precise cleavage of the sp3C–sp3N bond without influencing the other types of C–N bonds, the main products will be melamine instead of a complex mixture of ammelide and ammeline. In such a case, the product separation will be largely simplified. Surprisingly, we report herein NH3·H2O as a selective and efficient system to precisely cleave the sp3C–sp3N bond of melamine formaldehyde resin foam (MFF) with 100% selectivity to recover melamine of high purity (99.5%) with a high yield of 95.3% (entry 9, Table 1). Besides sound-absorbing MFF, other two types of commercial MFFs, namely the flame-retardant melamine compression foam and the cleaning-type melamine foam, can be efficiently degraded to melamine in the NH3–H2O system, showing high melamine yields of 94.8% and 93.1%, respectively (entries 27, 28, Table 1 and Fig. S1, ESI†). Compared with the previous MF resin degradation technology,11 in this work, NH3 was used as a catalyst, and it is a more ecologically environment-friendly substance (raw material for chemical fertilizer production). A high-purity melamine monomer was obtained instead of a hard-to-separate mixture of ammelide and ammeline. NH3 can be separated directly through volatilization, product melamine is insoluble in H2O and can be directly obtained, the post-treatment process is simple, compared with the post-treatment of products in previous work, and this work greatly reduces the consumption of energy and resources. Therefore, the NH3·H2O system degradation of MF resin is greener and more efficient (Table S1, ESI†).
| Entry | System | System mass/g | Temp/°C | Time/h |
R
d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b/% b/% |
Y
ma![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c/% c/% |
|---|---|---|---|---|---|---|
| a Reaction conditions: 10 ml Teflon-lined reactor, MFF: 0.15 g. b Degradation ratio = (m0 − m1)/m0, where m0 and m1 are the mass of original MFF and residue MFF after degradation, respectively. c Melamine yield = (m2 − m3)/(m0 × 0.65), where m2 is the mass of the product after drying to remove NH3·H2O after the reaction and m3 is the quality of the remaining insoluble solid after the dried product was dissolved in DMSO, and 0.65 in the equation represents the theoretical mass of melamine rings in 1 g of MFF. d Reaction pressure: 1.2–1.4 MPa, greater than the reaction pressure (0.4 MPa) in entry 9. e Reactant was melamine standard (0.15 g), no MFF. f EDA is ethylenediamine. g Reaction condition: 0.15 g flame-retardant-type MFF. h Reaction condition: 0.15 g cleaning-type MFF. | ||||||
| 1 | 27wt% NH3–H2O | 3.00 | 170 | 12 | 100 | 93.8 |
| 2 | 27wt% (NH4)2CO3–H2O | 3.00 | 170 | 12 | 100 | 90.3 |
| 3 | 27wt% Urea–H2O | 3.00 | 170 | 12 | 100 | 89.6 |
| 4 | 27wt% (NH4)2SO4–H2O | 3.00 | 170 | 12 | 100 | 67.1 |
| 5 | 27wt% NH4Cl–H2O | 3.00 | 170 | 12 | 100 | 27.8 |
| 6 | 27wt% NH3–H2O | 2.00 | 170 | 12 | 100 | 94.5 |
| 7 | 27wt% NH3–H2O | 1.00 | 170 | 12 | 100 | 95.1 |
| 8 | 27wt% NH3–H2O | 0.50 | 170 | 12 | 100 | 94.6 |
| 9 | 27wt% NH3–H2O | 0.15 | 170 | 12 | 100 | 95.3 |
| 10 | 27wt% NH3–H2O | 0.05 | 170 | 12 | 100 | 89.4 |
| 11 | 27wt% NH3–H2O | 0.15 | 170 | 4 | 100 | 65.4 |
| 12 | 27wt% NH3–H2O | 0.15 | 170 | 6 | 100 | 87.4 |
| 13 | 27wt% NH3–H2O | 0.15 | 170 | 8 | 100 | 89.9 |
| 14 | 27wt% NH3–H2O | 0.15 | 170 | 10 | 100 | 91.6 |
| 15 | 27wt% NH3–H2O | 0.15 | 160 | 12 | 100 | 84.9 |
| 16 | 27wt% NH3–H2O | 0.15 | 150 | 12 | 100 | 81.9 |
| 17 | 27wt% NH3–H2O | 0.15 | 140 | 12 | 92.6 | 63.7 |
| 18 | NaOH–H2O (pH = 13.5) | 3.00 | 170 | 12 | 2.7 | 0 |
| 19 | KOH–H2O (pH = 13.5) | 3.00 | 170 | 12 | 3.5 | 0 |
| 20 | 5wt% NaOH–H2O | 3.00 | 170 | 12 | 92.1 | 0 |
| 21 | 30wt% Na2CO3–H2O | 3.00 | 170 | 12 | 1.2 | 0 |
| 22 | 30wt% K2CO3–H2O | 3.00 | 170 | 12 | 1.9 | 0 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
NH3 (1 MPa) | — | 170 | 12 | 3.2 | 0 |
| 24 | H2O | 3.00 | 170 | 12 | 1.9 | 0 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e e |
27wt% NH3–H2O | 3.00 | 170 | 12 | 0 | >99 |
26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f f |
27wt% EDA–H2O | 3.00 | 170 | 12 | 100 | 92.1 |
27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) g g |
27wt% NH3–H2O | 0.15 | 170 | 12 | 100 | 94.8 |
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) h h |
27wt% NH3–H2O | 0.15 | 170 | 12 | 100 | 93.1 |
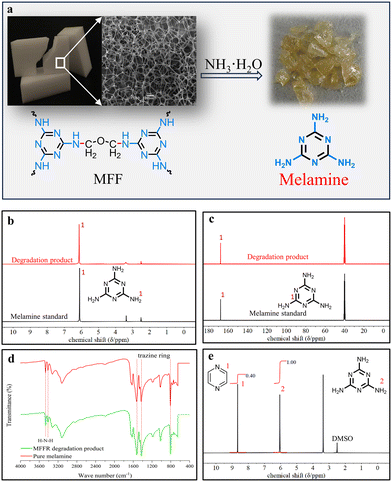
When degraded in the 27 wt% ammonia solution (NH3·H2O), the white foam-like MFF gradually disappeared and a yellow crystal product precipitated from the aqueous system after the degradation reaction (Fig. 2a). The crystal product was characterized by NMR and IR analysis. The 1H- and 13C-NMR spectra of product showed a single unipolar peak at chemical shifts of 6.13 ppm and 167.5 ppm, respectively, which was identical to that of the melamine standard (Fig. 2b and c). Besides, the IR spectrum of solid product was similar to that of the melamine standard, showing the peaks for the N–H bond on the NH2 group exterior to Tr (at 3467 cm−1 and 3413 cm−1) and for the triazine (Tr) ring (at 1528 cm−1, 1423 cm−1 and 809 cm−1). The IR analysis is in good agreement with the NMR result, identifying the solid crystal product as melamine (Fig. 2d). In addition, the side products in the degradation solution were analyzed by 1H- and 13C-NMR characterizations. As displayed in Fig. S3 and S4,† the side products were either volatile or unstable molecules such as methanol, methylamine, dimethyl ether, and diamine methyl ether, their content was very low in the degradation solution.
On the basis of 1H-NMR analysis, a high selectivity to melamine (99.5%) and a yield (93.8%) were obtained in 27 wt% NH3·H2O (entry 1, Table 1). Besides the product yield, the product purity is a key factor affecting the efficiency of whole degradation process. The purity of melamine product was determined by 1H-NMR quantitative analysis (Fig. 2e).12 The result showed that the melamine product had a purity as high as 99.5%. The elemental analysis showed that the content of C, N and H elements in the melamine product (28.57%C, 65.94%N and 4.83%H) was very close to those in the melamine standard (28.58%C, 66.55%N and 4.80%H) (Table S2, ESI†), further proving the melamine product of high purity. The high yield and purity of melamine will benefit the application of NH3·H2O in the industrial degradation of MFF.
Several types of aqueous ammonium salt solutions with the same mass concentration to NH3·H2O, were also employed for the degradation reaction (entries 2–5, Table 1). They all exhibited activity for the degradation reaction, resulting in the melamine yield ranging from 27.8% to 90.3%. These ammonium salts decomposed at the reaction temperature to produce NH3, as indicated by the pungent NH3 smell after reaction. These released NH3 enabled the decomposition of MFF. Noticeably, NH3·H2O showed a higher melamine yield than the ammonium salt solutions. NH3·H2O can release NH3 directly without the salt decomposition step, accelerating the reaction. Moreover, it did not have the anions existed in the ammonium salts, including CO32−, SO42−, and Cl−. In such case, the possible negative effect of these anions on the degradation reaction can be avoided.
Furthermore, the impact of reaction conditions on the degradation reaction was explored, including the NH3·H2O amount, the reaction time and reaction temperature. A reduction of NH3·H2O amount from 3.00 g to 0.15 g led to the same melamine selectivity and comparable melamine yield, whereas a further decrease in NH3·H2O amount to 0.05 g resulted in a declined degradation efficacy (entries 1, 6–10, Table 1). The highest melamine yield of 95.3% was reached with the NH3·H2O amount of 0.15 g. Hence, the effects of reaction time and temperature on the degradation reaction were studied with 0.15 g of NH3·H2O (entries 9, 11–14 and 15–17, Table 1). An increased reaction time and an elevated reaction temperature enhanced the MFF degradation reaction.
NH3·H2O (27wt%) was alkaline with a pH value of 13.5. To exclude the catalytic effect of alkaline OH− ions on the depolymerization of MFF, the solutions of NaOH–H2O and KOH–H2O that have the same pH values (13.5) as that of NH3·H2O (27wt%), were chosen for MFF degradation. MFF did not degrade in the above two solutions (entries 18 and 19, Table 1). In addition, 5wt% NaOH–H2O was applied to degrade MF resin under the same reaction conditions as that of NH3·H2O (entry 20, Table 1). The degradation rate reached 92.1%, and the products included ammelide and ammeline. Therefore, NH3 has a higher degradation activity and a narrower product distribution than NaOH. The Na2CO3–H2O (30 wt%) and K2CO3–H2O (30 wt%) solutions that have similar mass concentrations to that of NH3·H2O were also applied to recycle MFF under the identical condition, both of which were inert toward MFF degradation (entries 21 and 22, Table 1). NH3 or H2O alone was employed to react with MFF, and neither of them can degrade MFF. This result suggested that the degradation of MFF by NH3·H2O was achieved through the synergy of NH3 and H2O (entries 23 and 24, Table 1).
In order to further evaluate the greenness of the NH3·H2O system, we analyzed and compared the green metrics13 of the NH3·H2O system recovery of MF resin with the previous recovery technology. The detailed information is shown in Tables S3 and S4.† Environmental impact factors (EF) and process mass intensity (PMI) were calculated respectively. The results show that simple EF, complete EF, EF, Waste amount and PMI values of the NH3·H2O system (1.06, 2.27, 1.06, 0.66, and 3.27) are lower than those of the MSA–THF–H2O system (2.36, 8.92, 2.93, 1.70, and 9.92) and NaOH–H2O system (1.65, 11.23, 1.65, 1.03, 12.23). This proves the greenness and economy of NH3·H2O to degrade MF resin.
The possible reaction route was explored for the degradation of MF resin to melamine. Two routes were proposed. The first one involved the formation of melamine via a multi-step process, i.e., MF resin degraded into ammelide, ammeline and/or cyanic acid, followed by their transformation to melamine, while in the second one, MF resin degraded directly into melamine without the above intermediate(s). Ammelide, ammeline and cyanic acid were chosen as the model reactant and tested in 27 wt% NH3·H2O, respectively. No melamine was formed in all of the tests (Fig. S5, ESI†), which ruled out the first route as the reaction pathway. Additionally, the stability experiment showed that >99% of melamine was stable in NH3·H2O (entry 25, Table 1) with the structure unchanged (Fig. S6, ESI†). These results proved the second route as the reaction pathway, i.e., MF resin degraded directly into melamine.
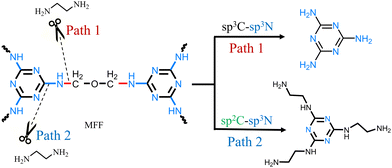
In the direct depolymerization route of MF resin, the nature of selective disconnection of C–N bond was elucidated via model reactions. Two possible paths existed for NH3·H2O to directly depolymerize MF resin into melamine (Fig. 3): path1 involved the selective breakage of the sp3C–sp3N bond (without breaking the sp2C–sp3N bond) to produce melamine, while in path 2, the ammonia molecule attacked the Tr ring, by which the sp2C–sp3N bond was cleaved to form melamine. It is noticed that in path 2, the cleavage of the sp2C–sp3N bond led to the leaving of a sp3N atom of MF resin, and this vacancy was replenished by a N atom of NH3. When NH3 is replaced by ethylenediamine (EDA), the replenishment of a N atom of EDA will lead to the formation of N2,N4,N6-tris(2-aminoethyl)-1,3,5-triazine-2,4,6-triamine, if path 2 is the reaction path. EDA was utilized as the model substitute for NH3 in the NH3·H2O solution to cleave the C–N bond of MF resin (entry 26, Table 1). The product was identified to be melamine (Fig. S7, in ESI†), which excluded path 2 as the reaction path. Hence, path 1 was the reaction pathway.
It is essential for NH3·H2O to interact with MF resin before it cleaves the C–N bond of MF resin. The interaction between NH3·H2O and MF resin was explored by 13C-NMR characterization, in order to understand how NH3·H2O initiate the cleavage of C–N bond. For a simple and accurate analysis, MF resin prepolymer that has the typical structure unit of MF resin was chosen as to model MF resin (Fig. S8, ESI†). MF resin prepolymer itself and its mixture with NH3·H2O were analyzed by 13C-NMR (Fig. S9, ESI†). MF resin prepolymer has two distinct types of C atoms, i.e., the sp2 C atoms (located at the Tr ring) and sp3 C atoms (located at the chains). Both sp2 C and sp3 C were neighbored by N atom(s). Notably, the presence of NH3·H2O caused a movement of chemical shift (δ) of the two types of C atoms towards the high δ direction, indicating the decrease in the electron cloud density of the two types of C atoms. This result implied that the C atoms were surrounded by more electron-withdrawing atoms, i.e., N and O atoms of NH3·H2O. It is possible that the N and O atoms of NH3 and H2O can associate with the H, O and N atoms of MF resin prepolymers via hydrogen bonds (O–H⋯N, N–H⋯N, and N–H⋯O, etc.) to form a stable six-ring structure (Fig. S10, ESI†). In this six-ring structure, the electron transfer from the C atoms of the MF resin prepolymer to the N and O atoms of NH3 and H2O was achieved via an inductive effect.
Additionally, 13C-NMR characterization was employed to probe the alterations in chemical bonds during the degradation of MF resin at a microscopic level (Fig. S11, ESI†), 0.15 g of MF resin was degraded in 3 g of NH3·H2O at 170 °C with different reaction periods (3 h, 6 h, and 9 h), and the resulting degradation solution was directly characterized by 13C-NMR. The degradation of MF resin became increasingly intensive as the reaction time progressed. At 3 h, Tr–NH2 groups and Tr–NH–CH2–O–CH2–NH–Tr groups were dominant in the degradation system, whereas Tr–NH–CH2–OH groups were scarce and few. As the reaction time prolonged, the content of Tr–NH2 groups increased progressively, accompanied by the significant diminishing of Tr–NH–CH2–O–CH2–NH–Tr groups and nearly complete consumption of Tr–NH–CH2–OH groups. Nevertheless, NMR evidenced that the concentration of Tr–NH2 groups was substantially higher than that of Tr–NH–CH2–O–CH2–NH–Tr groups at 9 h, indicating that the reaction mechanism involved the breakage of ether bonds and sp3C–sp3N bonds.
The degradation mechanism of MF resin was further detailed by density functional theory (DFT) on the Gaussian 16 platform (Fig. 4a).14 The model compound, R1, which possesses a comparable structure to MF resin, was developed using Gaussview 6.0.15 In the depolymerization process, NH3 and R1 interacted with each other to form two hydrogen bonds, generating the intermediate species 2 with a six-membered-ring structure, and this step had an energy of 12.25 kcal mol−1. One hydrogen (H) atom of NH3 attacked and then bound to the (O) oxygen atom of R1, meanwhile one H atom on the N atom of intermediate 2 interacted and then bounded to the N atom of NH3, leading to the formation of intermediates 3 and 4 through the transition state TS1. The Gibbs activation energy and Gibbs reaction energy for this step were 24.19 kcal mol−1 and 10.26 kcal mol−1, respectively. Subsequently, the additive reaction between the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of intermediate 3 and H2O produced intermediate 4, with an activation energy of 25.81 kcal mol−1. Thereafter, NH3 and intermediate 4 interacted with each other to produce intermediate 5 via hydrogen bonding, showing an activation energy of 4.66 kcal mol−1. Intermediate 5 subsequently cleaved its sp3C–sp3N bond to yield melamine via the transition state TS2 and H transfer. The Gibbs activation energy and Gibbs reaction energy for this step were 27.82 kcal mol−1 and −4.78 kcal mol−1, respectively. Briefly, the principal process of MF resin depolymerization, as shown in Fig. 4b, started from the formation of a six-membered-ring intermediate through the H-bond association between MF resin and NH3, followed by the breakage of ether bonds and sp3C–sp3N bonds via H transfer.
N bond of intermediate 3 and H2O produced intermediate 4, with an activation energy of 25.81 kcal mol−1. Thereafter, NH3 and intermediate 4 interacted with each other to produce intermediate 5 via hydrogen bonding, showing an activation energy of 4.66 kcal mol−1. Intermediate 5 subsequently cleaved its sp3C–sp3N bond to yield melamine via the transition state TS2 and H transfer. The Gibbs activation energy and Gibbs reaction energy for this step were 27.82 kcal mol−1 and −4.78 kcal mol−1, respectively. Briefly, the principal process of MF resin depolymerization, as shown in Fig. 4b, started from the formation of a six-membered-ring intermediate through the H-bond association between MF resin and NH3, followed by the breakage of ether bonds and sp3C–sp3N bonds via H transfer.
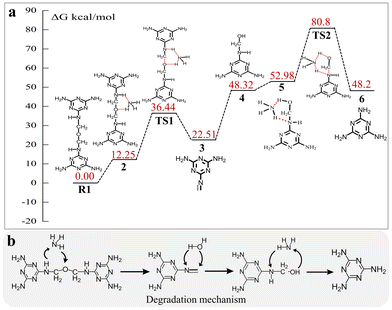 | ||
| Fig. 4 (a) The changes in the structure and energy in the process of NH3·H2O depolymerizing MF resin were DFT calculated using the Gaussian 16 platform. (b) Degradation mechanism of MF resin. | ||
Conclusions
This study introduces a novel approach to utilize NH3·H2O to greenly and efficiently degrade MF resin. The synergy effect of NH3 and H2O selectively cleaved the sp3C–sp3N bond within MF resin without damaging the sp2C–sp3N bonds of MF resin at 170 °C for 12 h, by which valuable melamine was recovered with a high yield of 95.3%. The recovered melamine was precipitated from the degradation solution as crystals with a high purity of 99.5%, which simplifies largely the following product separation. Compared with previous MF resin recovery systems, in this work, the catalyst NH3 is a more ecologically environment-friendly substance, melamine monomer insoluble in NH3·H2O was obtained directly, without tedious and resource-intensive post-processing, greatly reducing the consumption of resources and energy, so NH3·H2O system degradation MF resin is greener and more efficient. Through the comparison of EF of different systems, it is more intuitive to reflect the NH3·H2O system greenness and economy. A viable degradation mechanism was proposed and corroborated by combining NMR and density functional theory calculations. The ability of NH3·H2O to precisely cleave the specific C–N bond makes it a great candidate in the controllable recovery of high-value-added chemicals from MF resin and other polymers that contain different types of C–N bonds.Author contributions
Chaohui Yang: data curation, investigation, and writing – original draft. Xinyu Li: methodology and software. Hongyan Li: methodology and software. Chizhou Wang: methodology. Qianqian Xing: software. Xiaoliang Jia: methodology and software. Xiaojing Cui: writing – review & editing. Xianglin Hou: resources, funding acquisition, investigation, and project administration. Tiansheng Deng: conceptualization, resources, methodology, supervision, validation, project administration, and writing – review & editing.Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the autonomous research project of SKLCC (Grant No.: 2024BWZ007), Fundamental Research Program of Shanxi Province (No. 202303021211257 and No. 202303021212375) and the Fund of Shanxi Province patent Transformation Special Plan Project (No. 202306005).References
- (a) J. M. Garcia and M. L. Robertson, Science, 2017, 358, 870–872 CrossRef CAS PubMed; (b) T. El Darai, A. Ter-Halle, M. Blanzat, G. Despras, V. Sartor, G. Bordeau, A. Lattes, S. Franceschi, S. Cassel, N. Chouini-Lalanne, E. Perez, C. Déjugnat and J.-C. Garrigues, Green Chem., 2024, 26, 6857–6885 RSC; (c) A. Conroy, S. Halliwell and T. Reynolds, Composites, Part A, 2006, 37, 1216–1222 CrossRef.
- (a) H. Zhu and S.-A. Xu, RSC Adv., 2018, 8, 17879–17887 RSC; (b) Y.-Y. Pan, W.-M. Yin, R.-J. Meng, Y.-R. Guo, J.-G. Zhang and Q.-J. Pan, RSC Adv., 2021, 11, 24038–24043 Search PubMed; (c) B. Song, X. Zhu, W. Wang, L. Wang, X. Pei, X. Qian, L. Liu and Z. Xu, J. Ind. Eng. Chem., 2023, 119, 130–152 Search PubMed; (d) Y. Yang, Y. Deng, Z. Tong and C. Wang, J. Mater. Chem. A, 2014, 2, 9994–9999 RSC.
- (a) A. Khan, C. L. Y. Leon, D. A. David, A. Pacheeri, P. Sreeram, K. T. Mohammed Kenz, P. Raghavan and P. S. Owuor, Handbook of Thermosetting Foams, Aerogels, and Hydrogels, 2024, pp. 505–532, DOI:10.1016/b978-0-323-99452-1.00010-3; (b) S. Li, W. Mo, Y. Liu and Q. Wang, Chem. Eng. J., 2023, 454, 140133 Search PubMed; (c) D. J. Merline, S. Vukusic and A. A. Abdala, Polym. J., 2013, 45, 413–419 CrossRef CAS.
- (a) S. Chen and Y. H. Hu, Chem. Eng. J., 2024, 493, 152727 CrossRef CAS; (b) W. S. El-Sayed, A. F. El-Baz and A. M. Othman, Int. Biodeterior. Biodegrad., 2006, 57, 75–81 CrossRef CAS; (c) T. U. Nisa, W. A. Khokhar, U. Imran, S. A. Khokhar and N. Soomro, Chemosphere, 2024, 349, 140778 Search PubMed.
- (a) T. Q. Bui, L. J. Konwar, A. Samikannu, D. Nikjoo and J.-P. Mikkola, ACS Sustainable Chem. Eng., 2020, 8, 12852–12869 Search PubMed; (b) K. S. Kim, S. Y. Choi, T. W. Kim and M. J. Kang, ACS Sustainable Chem. Eng., 2024, 12, 7083–7091 CrossRef CAS; (c) Q. Si, F. Jin and J. Yang, New Chem. Mater., 2017, 45, 125–127 Search PubMed; (d) H. He, D. Meng and X. Chen, Appl. Chem. Ind., 2013, 42, 1065 CAS.
- (a) F. Ruan, J. Shi, Z. Xu and J. Xing, Text. Res. J., 2019, 40, 152–157 Search PubMed; (b) C. Wang, N. Zhang, S. Wu, W. Wang, P. Zhao, S. Jia, Y. Qi, X. Hou, X. Cui and T. Deng, Compos. Sci. Technol., 2024, 248, 110442 CrossRef CAS; (c) Y. Wang, X. Cui, Q. Yang, T. Deng, Y. Wang, Y. Yang, S. Jia, Z. Qin and X. Hou, Green Chem., 2015, 17, 4527–4532 RSC; (d) J. J. Jiang, G. L. Deng, X. Chen, X. Y. Gao, Q. Guo, C. M. Xu and L. C. Zhou, Compos. Sci. Technol., 2017, 151, 243–251 CrossRef CAS.
- (a) J. M. Soto, G. Blázquez, M. Calero, L. Quesada, V. Godoy and M. A. Martín-Lara, J. Cleaner Prod., 2018, 203, 777–787 Search PubMed; (b) J. Q. Pan, Z. F. Liu, G. F. Liu, S. W. Wang and H. H. Huang, J. Cent. South Univ. Technol., 2005, 12, 157–161 CrossRef; (c) P. He, Z. Wu, S. Pan, C. Chen and H. Li, Eng. Plast. Appl., 2013, 41, 42–45 CrossRef CAS.
- (a) W. Wang, N. Zhang, C. Wang, H. Li, S. Jia, Y. Qi, H. Fan, X. Cui, X. Hou and T. Deng, Green Chem., 2023, 25, 5213–5221 RSC; (b) Z. Czech and R. Pelech, J. Therm. Anal. Calorim., 2010, 101, 309–313 CrossRef CAS; (c) L. Y. Huang, Y. Shi, X. G. Jin, Z. Q. Wu, F. M. Li and S. Z. D. Cheng, Sci. China, Ser. B: Chem., 1999, 42, 316–325 CrossRef CAS.
- (a) Z. Czech and R. Pelech, Mater. Sci., 2009, 27, 851–856 CAS; (b) S. Dattilo, C. Puglisi, E. F. Mirabella, A. Spina, A. A. Scamporrino, D. C. Zampino, I. Blanco, G. Cicala, G. Ognibene, C. Di Mauro and F. Samperi, Polymers, 2020, 12, 1810 Search PubMed; (c) Z. Czech and R. Pelech, Prog. Org. Coat., 2010, 67, 72 CrossRef CAS.
- (a) N. Hu, L. Su, H. Li, N. Zhang, Y. Qi, H. Wang, X. Cui, X. Hou and T. Deng, Green Chem., 2024, 26, 9378–9387 RSC; (b) P. Zhao, X. Cui, C. Wang, X. Shao, H. Li, N. Zhang, X. Hou and T. Deng, Polym. Degrad. Stab., 2024, 225, 110774 CrossRef CAS; (c) Z. Tian, Q. Zhao, X. Liu, Y. Wang, J. Zhao, Y. Wang and X. Hou, ACS Sustainable Chem. Eng., 2023, 11, 11590–11600 CrossRef CAS; (d) N. Zhang, S. Wu, C. Wang, X. Cui, T. Zhao, L. Yuan, Y. Qi, X. Hou, H. Jin and T. Deng, Green Chem., 2022, 24, 7395–7402 RSC.
- (a) S. Wu, N. Zhang, S. Jia, C. Wang, Y. Wang, Y. Qi, H. Wang, X. Cui, X. Hou and T. Deng, Green Chem., 2021, 23, 7816–7824 RSC; (b) S. Wu, N. Zhang, C. Wang, X. Hou, J. Zhao, S. Jia, J. Zhao, X. Cui, H. Jin and T. Deng, Green Energy Environ., 2024, 9, 919–926 CrossRef CAS.
- H. Ma, C. M. Pedersen, Q. Zhao, Z. Lyu, H. Chang, Y. Qiao, X. Hou and Y. Wang, Anal. Chim. Acta, 2019, 1066, 21–27 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC; R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC; F. Roschangar, R. A. Sheldon and C. H. Senanayake, Green Chem., 2015, 17, 752–768 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16, Wallingford CT, 2016, https://gaussian.com/gaussian16/.
- R. Dennington, T. A. Keith and J. M. Millam, GaussView, Version 6, Semichem Inc., Shawnee Mission, KS, 2016, https://gaussian.com/gaussview6/.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4gc04481a |
| This journal is © The Royal Society of Chemistry 2024 |