Open Access Article
Open Access ArticleHighly efficient oil-fouling and foam removal achieved by surfactant mixed systems†
Zeyu
Zhao
ad,
Tengda
Wang
bc,
Jiling
Yue
a,
Yaxun
Fan
 *abc and
Yilin
Wang
*abc and
Yilin
Wang
 *abcd
*abcd
aBeijing National Laboratory for Molecular Sciences and Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: yxfan@iccas.ac.cn; yilinwang@iccas.ac.cn
bSuzhou Institute for Advanced Research, University of Science and Technology of China, Suzhou, Jiangsu 215123, P. R. China
cUniversity of Science and Technology of China, Hefei, Anhui 230026, P. R. China
dUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 28th September 2023
Abstract
Excessive usage of surfactants in daily life and industry and their undesirable high foamability have caused serious environmental pollution and economic loss. Improving cleaning efficiency and reducing foam stability concurrently is a delicate strategy but a challenging task. Herein, we mixed the most widely used surfactant sodium dodecyl sulfate (SDS) with cyclic amines (CnN, n = 6, 8, 12), by which the self-assembly ability of SDS at the air/water interface and in bulk is significantly enhanced, while spherical micelles, vesicles and wormlike micelles are formed at appropriate total surfactant concentration (CT) and molar fraction of SDS (XSDS). Especially around XSDS = 0.50 and above critical micellar concentration (CMC), the stronger self-assembly ability leads to a higher contact angle of machine oil on stainless-steel plates and lower oil–water interfacial tension in CnN/SDS solution, thus the oil-fouling removal efficiency of CnN/SDS solutions is remarkably improved. Meanwhile, the foamability and foam stability dramatically decline at smaller XSDS and slightly above CMC, attributed to the rapid molecular migration from liquid film of foams to the bulk between the films when the limited surfactant molecules in the films prefer to aggregate in bulk. As a result, C8N/SDS exhibits the best oil cleaning and lowest foaming simultaneously at low XSDS and just above the CMC. This study opens an efficient avenue to eliminate the contradiction between cleaning ability and foamability, thereby obtaining a high-efficiency and low-foam detergent.
Introduction
Amphiphilicity of surfactants endows them with unique surface activity and self-assembly ability in aqueous solution, thus they are widely applied in many fields, such as consumer household cleaning products,1–4 industrial cleaning,5–7 food industry,8,9 agriculture,10–14 drug delivery,15–18etc. Particularly as detergents, the global surfactant market turnover reached USD 42.1 billion in 2020 according to the data from Markets and Markets™.19 Moreover, the global cleaning products industry is projected to reach USD 61.6 billion by 2026.20 Excessive consumption of surfactants causes environment chemical exposure and huge economic loss. With regard to this issue, many researchers have devoted their efforts in developing a wide range of novel surfactants by introducing more functional groups,21–24 increasing the oligomerization degree,25–28 and adjusting the length of the spacer or alkyl chain29–31 so as to optimize the cleaning performance of surfactants and reduce their dosage by altering the static/dynamic surface tension,32–35 adsorption kinetics,36–39 aggregation ability,40–42etc. In parallel, mixing surfactants with additives (inorganic/organic salts, other surfactants, polymers, etc.) is also an effective way to adjust the physicochemical properties of surfactants at the surface/interface and in solution,43–49 while a large number of commercial additives offer much more optionality, convenience and practicability. Hence, introducing appropriate additives into commonly used surfactants is undoubtedly an economic and convenient approach to obtain highly efficient surfactant systems and achieve sustainable development.The generation of liquid foams is at the heart of numerous natural, technical, or scientific processes related to surfactants. In many cases, they have very useful properties for practical applications, such as cleaning, foam flotation, food processing and firefighting. However, foams are not always desirable. The unwanted foams can obstruct gas transport and render the process of interest ineffective with significant cost implications. Kister et al.50,51 made a survey on causes of malfunctions in industry, and they found that the existence of foam in surfactant solutions in distillation processes was a major reason for 900 investigated cases of column malfunctions. The accumulation of impurities that stabilize the foam on the sea also produces foams and destroys the normal ecological environment.52–54 Coincidently, those aforementioned physicochemical properties facilitating the cleaning efficiency, such as low surface tension, strong aggregation ability and fast adsorption kinetics, often result in the strong foamability and foam stability in surfactant solution.55–58 Therefore, the development of surfactant systems with high cleaning efficiency but adjustable foaming ability in different conditions encounters great technological hurdles although it is urgently desired in multiple fields.
The length and topological structure of alkyl chains play important roles in physicochemical properties of surfactants at surface/interface and in solution. Cyclic amines with bulky alkyl chains may form a larger hydrophobic domain in bulk but create looser arrangement at the air/water interface.59,60 Their special structure makes it greatly possible for cyclic amine-containing systems to achieve high cleaning efficiency and low foam concurrently. Bearing in mind the structural advantages, we designed mixed systems composed of cyclic amines with various sizes of the cycloalkane ring (CnN, n = 6, 8, 12) and widely used sodium dodecyl sulfate (SDS) at pH = 7.0. We found that the mixed solutions (CnN/SDS) show lower CMC and surface tension and form various assemblies at different molar fractions of SDS (XSDS) and total concentrations (CT), driven by the electrostatic and hydrophobic interactions between SDS and cyclic amines. With the balance of the self-assembly of surfactants at the liquid film of foam and in bulk between the films, the oil cleaning efficiency of C8N/SDS is much higher than that of the other two systems, whereas its foamability and foam stability are remarkably weakened. This work offers a feasible method for effectively removing oil with low foamability and foam stability at low surfactant concentrations, thereby potentially reducing surfactant usage and promoting sustainable development of environments.
Experimental section
Materials
Cyclohexylamine (C6N, >99.5%) was purchased from Energy Chemical, cyclooctylamine (C8N, >97%) was purchased from Acros Organics, cyclododecylamine (C12N, >99%) was purchased from Leyan Chemical, sodium dodecyl sulfate (SDS, >99%) was purchased from Sigma-Aldrich, and hydrochloric acid (HCl, 36%) was purchased from Beijing Chemical Works. 20# machine oil was purchased from Kunlun Lubricant Company. Deionized water (18.2 MΩ cm) from Milli-Q equipment was used in all the experiments.Surface tension measurement
The surface tension measurements of the CnN/SDS (n = 6, 8, 12) mixed systems at different molar fractions of SDS (XSDS) were carried out with a Wilhelmy plate method on the DCAT21 tensiometer (DataPhysics Co., Germany). The length and width of the plate were 19.90 and 0.20 mm, respectively. The standard error of surface tension is ∼0.03 mN m−1, and the test temperature was 25.00 ± 0.01 °C controlled by using a thermostat. Each measure was tested at least three times.Turbidity measurement
The turbidity values of the CnN/SDS (n = 6, 8, 12) mixtures at different CT and XSDS values, reported as 100 − % T, were measured at 450 nm using a Shimadzu UV-vis spectrophotometer (model UV-2800) with a water-circulating thermostat at 25.0 ± 0.01 °C. All the measured values were corrected by taking the turbidity of Milli-Q water as the controller.Dynamic light scattering (DLS)
The size distribution of CnN/SDS (n = 6, 8, 12) mixtures at CT = 20 mM with XSDS from 0 to 1.00 was measured using a Malvern ZetaSizer Nano ZS Instrument (ZEN3600, Malvern Instruments, Worcestershire, UK) at a scattering angle of 173° equipped with a 4 mW He–Ne laser (λ = 632.8 nm) and a thermosetting chamber at 25.0 ± 0.1 °C.Cryogenic transmission electron microscopy (cryo-TEM)
5 μL C6N/SDS solutions at XSDS = 0.10, C8N/SDS solutions at XSDS = 0.20, 0.60 and C12N/SDS solutions at XSDS = 0.80 (CT = 20 mM) were loaded onto a carbon-coated holey TEM grid. The excessive solution was sucked away by filter paper leaving a thin liquid film on the grid. After a few seconds, the grid was quickly vitrified by plunging into liquid ethane (cooled by liquid nitrogen) at −183 °C. The vitrified sample was transferred to a cryogenic sample holder and examined with a Themis 300 TEM (Thermo Fisher Scientific, America) at about −174 °C. The images were recorded on a Gatan charge-coupled device (CCD) camera in the minimal electron dose mode.Interfacial tension measurement
The interfacial tension between surfactant mixtures and machine oil was measured by a TX500™ Spinning Drop Interface Tensiometer (Model TX500C). 1 mL CnN/SDS (n = 6, 8, 12) mixtures at CT = 5 mM with XSDS from 0 to 1.00 were injected into a quartz glass tube as an external phase and 10 μL of machine oil was injected into liquid solution as an internal phase. Oil drop formations at different rotation rates were recorded by a CCD camera, and interfacial tension was calculated by measuring the length and width of the oil drop and using Vonnegut and Bashford–Adams fitting. All the measurements were tested at 25.0 ± 0.1 °C.Contact angle measurement
CnN/SDS (n = 6, 8, 12) mixture solutions at CT = 5 mM with XSDS from 0 to 1.00 were poured into a cuboid glass case. The stainless-steel plate was stuck to the bottom of a floating foam broad on the liquid solution. The machine oil droplet was squeezed out through a crooked needle and floated to the plate. The contact angle was recorded by a digital camera and fitted by Image J software. Each measure was tested at least three times.Cleaning measurement
Oil cleaning efficiency was determined by weighing the metage of the weight gap during cleaning progress. The weight of the stainless-steel plate was M0. Each 50 μL of machine oil was daubed onto stainless-steel plates evenly. Stainless-steel plates with oil were weighed as M1 and then soaked into 10 mL liquid solution at different ratios of SDS. After ultrasonic vibration in an ultrasonic cleaning tank with an ultrasonic power of 120 W at the temperature of 25 °C for 10 min, the oil on the stainless-steel plates was removed to different extent and weighed as M2. All the measures were tested at least three times. The oil cleaning efficiency was calculated by using the following equation,| Oil cleaning efficiency% = [(M1 − M2)/(M1 − M0)] × 100 |
Foaming and defoaming measurement
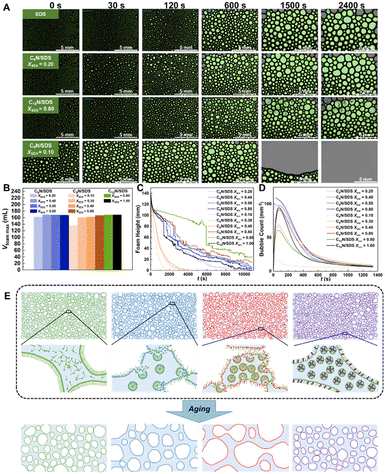
The foaming and defoaming ability of various surfactant mixtures were tested by two methods. One is the air blowing method using a dynamic foam analyzer DFA 100 (KRÜSS, Germany). N2 was continuously blown into 50 mL analyte liquor at the bottom of the container until the foam height did not increase, then the highest foam height was recorded as foaming ability. The state change of foams with time was recorded with a CCD camera, and bubble counts and half-time were recorded as defoaming ability. The other is the visual inspection method, i.e., shaking 10 mL CnN/SDS solutions of 5 mM at different XSDS or fixed XSDS = 0.10 with various CT for 30 s. Following that, we recorded the change in foam height with aging and obtained the equilibrium foam height after resting for 2 h. The relative foam height is termed as the ratio of the measured foam height to the vial height.Results and discussion
Self-assembly of CnN/SDS mixtures at the air–water interface
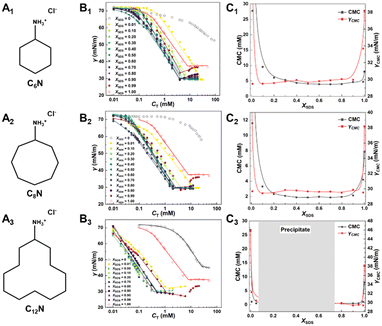
The surface activity and the onset of micellization of SDS with a series of cyclic amines (CnN, n = 6, 8, 12, Fig. 1A1–A3) were determined by surface tension measurements. Fig. 1B1–B3 show the surface tension curves for the CnN/SDS mixtures as a function of the total concentration (CT) at a fixed CnN/SDS molar fraction (XSDS = CSDS/(CSDS + CCnN), from 0.00 to 1.00). For comparison, the surface tension curves of pure SDS and CnN aqueous solution are also included. Correspondingly, all the CMC and the surface tension at CMC (γCMC) of C6N/SDS, C8N/SDS and C12N/SDS are summarized in Fig. 1C1–C3 to clearly show the influence of XSDS on CMC and γCMC.Obviously, the surface tension curves exhibit broadly similar profiles for all the CnN/SDS mixtures regardless of the sizes of the cycloalkane ring and the XSDS values. The CMC value of SDS without CnN derived from the breakpoint is 8.0 mM and the surface tension at CMC (γCMC) is 38 mN m−1, which are consistent with previously reported values. For CnN solution alone, the surface tension curves of C6N and C8N display no inflection point in the concentration range studied (<50 mM) and just show a slight decline to ∼55 mN m−1, whereas the CMC and γCMC values of C12N are 28 mM and 47 mN m−1, indicating that the three cyclic amines possess a very weak surface activity. As expected, the molecular arrangement at the air/water interface of cyclic amines with a bulky alkyl chain is much looser than that of the alkyl amine with the same carbon number, e.g., CMC and γCMC values of N-dodecylamine are 2.6 mM and 30 mN m−1 (Fig. S1†).
Upon mixing the two components, the CMC and γCMC values decrease significantly to lower than those of individual components, attributed to the strong electrostatic attraction between the cationic headgroup of protonated CnN and the anionic sulfate of SDS and the hydrophobic interaction of the alkyl ring of amines with the alkyl chain of SDS. Even at XSDS = 0.01, the CMC values have already reduced to 27.8, 11.6 and 1.1 mM for C6N/SDS, C8N/SDS and C12N/SDS mixtures, respectively, whereas the γCMC values declined to ∼30 mN m−1, nearly 8.0 mN m−1 lower than that of the pure SDS solution. When XSDS approaches 0.5 from 0 or 1.0, the CMC value is steadily decreased to the lowest one, i.e., 4.0, 2.0 and 0.4 mM for C6N/SDS, C8N/SDS and C12N/SDS, respectively, while the γCMC values are maintained at low values (∼30 mN m−1) and there is almost no further decrease with varying XSDS. Thus, the variation of CMC and γCMC values displays similar trends to XSDS changing from 0 to 1.0, i.e., decreasing first and then reaching a plateau and increasing rapidly again. It is worth noting that the surface tension curves of the mixed solutions at high XSDS show an upward end with increasing CT, which is attributed to the significant excess SDS, thus causing the increase in surface tension to reach the γCMC value of SDS itself. These results show that the binding affinity becomes stronger when the molar ratio of CnN and SDS is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the strong binding between the two components possibly induces the remarkable deformation or the transformation of the molecular configuration of CnN,60–62 thereby the molecular packing is significantly compacted at the air/water interface. However, the surface tension curves of the C12N/SDS mixtures at 0.20 < XSDS < 0.70 are excluded because the precipitation takes place in such a wide range of XSDS, which is different from the C6N/SDS and C8N/SDS mixtures with smaller cycloalkane rings. Despite these, we can still observe that the ability to reduce CMC is enhanced from C6N/SDS to C12N/SDS, demonstrating that the larger alkyl ring leads to the stronger hydrophobic interaction of the cyclic amines with SDS.
1, and the strong binding between the two components possibly induces the remarkable deformation or the transformation of the molecular configuration of CnN,60–62 thereby the molecular packing is significantly compacted at the air/water interface. However, the surface tension curves of the C12N/SDS mixtures at 0.20 < XSDS < 0.70 are excluded because the precipitation takes place in such a wide range of XSDS, which is different from the C6N/SDS and C8N/SDS mixtures with smaller cycloalkane rings. Despite these, we can still observe that the ability to reduce CMC is enhanced from C6N/SDS to C12N/SDS, demonstrating that the larger alkyl ring leads to the stronger hydrophobic interaction of the cyclic amines with SDS.
Aggregation behavior of CnN/SDS mixtures in bulk
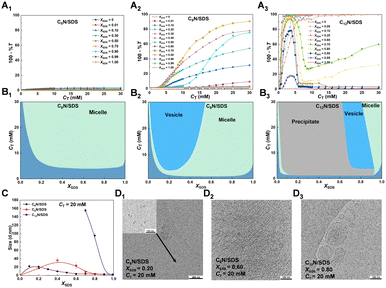
To further understand the impact of the cyclic amines with different sizes of the cycloalkane ring on the self-assembly of SDS in bulk, the aggregation behaviors of CnN/SDS mixtures were studied by turbidity measurement (Fig. 2A1–A3), dynamic light scattering (DLS, Fig. 2C) and cryogenic transmission electron microscopy (cryo-TEM, Fig. 2D1–D3). The different aggregate structures were observed with changing XSDS and CT as depicted in phase diagrams (Fig. 2B1–B3), which are derived from the turbidity curves of the CnN/SDS aqueous solution as a function of CT at various XSDS values.For C6N/SDS, the turbidity value remains extremely low no matter which value XSDS or CT is. Taking the situation at CT = 20 mM as a representative, the mean particle size remains below 20 nm. The broadly invariable turbidity and size as well as the lower CMC values (Fig. 1B1) demonstrate that the addition of C6N promotes the micellization of SDS, but only induces the formation of small spherical or rodlike micelles, which is confirmed by the cryo-TEM micrographs of C6N/SDS aggregates at XSDS = 0.20 and CT = 20 mM (Fig. S2†).
In contrast, the self-assembly behavior of SDS is significantly affected by C8N and C12N, and undergoes different processes at various XSDS and CT. For the C8N/SDS mixture, there is almost no change in the turbidity with CT as XSDS > 0.5, indicating that the small fraction of C8N has a strong ability to accelerate the micellization of SDS but not enough to affect the molecular packing and the aggregate structures. At XSDS > 0.5, the turbidity values increase with increasing CT, and the largest value appears at XSDS = 0.40. Fixing CT = 20 mM, a weakly bluish solution was observed, and the C8N/SDS mixed solution forms vesicles of a few tens of nanometers at XSDS = 0.20, while the solution becomes transparent and forms wormlike micelles with a few millimeters long at XSDS = 0.60, as evidenced by the results of DLS (Fig. 2C) and cryo-TEM (Fig. 2D1 and D2). Compared with the phase boundaries of C8N/SDS and C12N/SDS mixtures, they go through similar changing processes with increasing XSDS at the same CT, but the big differences take place at much lower XSDS and CT for the aggregate formation, the larger vesicles (>100 nm, Fig. 2D3) and the wider precipitate region over the CT range in C12N/SDS.
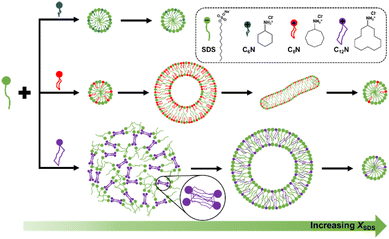
In summary, the addition of CnN only promotes the micellization of SDS for C6N, while leads to an aggregate transition from small micelles to vesicles and then to wormlike or spherical micelles at a fixed CT for C8N without precipitation and for C12N with a large range of precipitation. In all the three CnN/SDS mixtures, the electrostatic interaction between the protonated amine and sulfate groups and the hydrophobic interaction among the alkyl chains of CnN and SDS are the main driving forces for their self-assembly. However, taking a variety of conformations of cycloalkylamine in aqueous solution into account,60–62 we speculate that both the size and conformation of the cycloalkane ring of CnN may play a decisive role in the different aggregate formation and transition. Fig. 3 summarizes a simplified schematic diagram of aggregation behavior for the CnN/SDS mixture in bulk with changing in XSDS at a fixed CT.
The C6N molecule has the smallest cycloalkane ring. It is not long enough to enlarge the hydrophobic area in the aggregate relative to the SDS itself, thereby the C6N/SDS mixture only forms small micelles. In comparison, the C8N molecule with a larger cycloalkane ring can produce stronger hydrophobic interaction with SDS and form a larger hydrophobic area. As such, the spontaneous curvature of the C8N/SDS aggregates becomes slightly smaller, forming the wormlike micelles rich in SDS (XSDS > 0.5) and vesicles rich in C8N (XSDS < 0.5). As for C12N with a much larger cycloalkane ring, it forms precipitate with SDS in a large range, which could be attributed to the remarkably enhanced hydrophobic interaction by the unique C12N ring. It was reported that the conformers of cyclooctane can be classified into three families, including boat–chair, chair–chair or crown, and boat–boat, while cyclododecane shows a square topology and has at least 100 possible conformations.62 Thus, the large hydrocarbon ring is flexible enough to optimize the molecular conformation and nest together, in which case the ion pairs formed by C12N and SDS may be drawn closer with each other due to the tight entanglement between the C12N rings, thereby the precipitate with compact microstructure is formed, especially with the excessive C12N. For the same reason, a small amount of C12N can lead to a significant change in the molecular packing of C12N/SDS mixtures, thus the vesicles are formed at a large XSDS (e.g., 0.65 < XSDS < 0.85 at CT = 20 mM). Hence, the cycloalkylamines with different sizes of rings lead to diverse aggregate structures, and the large chair conformation of C12N displays the most obvious effect on the association with SDS.
Oil-fouling removal efficiency of CnN/SDS
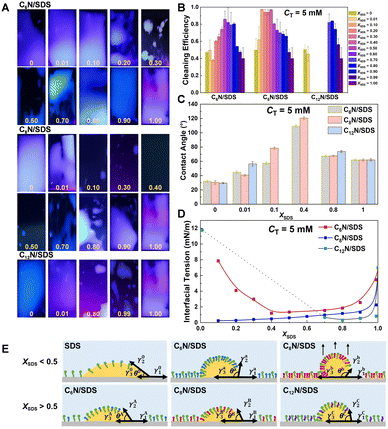
Surfactants that form aggregates with a strengthened hydrophobic domain and show strong ability to enhance surface activity should be beneficial to strip and solubilize the oil from substrates, so we selected CnN/SDS with CT = 5 mM, which is higher than their CMC but as low as possible, to test the oil-fouling removal efficiency. Stainless-steel plates covered with machine oil-fouling were immersed into the CnN/SDS solutions, and ultrasonically treated for 10 min, it was observed that the homogenous emulsion with machine oil was entrapped in the interior, or the machine oil was separated from the plate and suspended in the solution. After that, the stainless-steel plates were taken out from the solutions to evaluate the oil-fouling removal efficiency by inspection and weighting methods.With the visual approach to observe the oil-fouling efficiency, we took the photos of residual oil on stainless-steel plates under ultraviolet light (UV) based on the blue light character of oil-foiling under UV light (Fig. 4A). For both the C6N/SDS and C8N/SDS mixtures, the area of blue light on plates becomes smaller first and then enlarges again with the increase of XSDS, indicating that the optimum oil-fouling removal result appears around XSDS = 0.5. The C12N/SDS mixture follows the same changing pattern except for the precipitate region. In parallel, the quantitative evaluation by the weighting method is summarized in Fig. 4B, also indicating that the XSDS value for the highest oil-fouling removal efficiency is nearly 0.5. In addition, the C8N/SDS system shows the wider XSDS region (0.1 < XSDS < 0.5) to reach high cleaning efficiency and the efficiency is better than that of C6N/SDS. For C12N/SDS, in the region without precipitation at larger XSDS, it displays better cleaning performance than either C6N/SDS or C8N/SDS. That is, the CnN/SDS mixture with a larger cycloalkylamine and stronger aggregation ability exhibits higher oil-fouling removal efficiency.
To further understand the role of cycloalkylamine size in the oil-fouling removal efficiency of CnN/SDS, the cleaning process is investigated by oil–water interfacial tension and contact angles (θ) of oil on plate under solution (Fig. 4C and D). Normally, cleaning by surfactants is realized through the de-wetting mechanism,63 for which the contact angles of oil drops on substrates under surfactant solutions are very large. Herein, the contact angles of machine oil drops on the plates in the CnN/SDS solutions at the representative XSDS show a similar changing trend with the oil-fouling cleaning performance, i.e., good cleaning performance corresponds to a large contact angle. The oil-fouling tends to separate from the stainless-steel substrates at the medium XSDS (Fig. 4C). Therefore, the de-wetting occurs around XSDS = 0.5. Meanwhile, the interface tension between the oil and CnN/SDS solutions becomes smaller than that of either CnN or SDS, which is beneficial to the formation of O/W emulsion (Fig. 4D). And the O/W interfacial tension curves as a function of XSDS display a concave edge, showing a contrary changing trend of cleaning efficiency curves. It means that both the de-wetting and emulsion are enhanced at the medium XSDS, which facilitates the oil-fouling removal and impedes the separated oil to stick back on the substrates. Moreover, C8N/SDS shows 0.5 mN m−1 lower interfacial tension than C6N/SDS at XSDS < 0.50, whereas C12N/SDS shows the minimum interfacial tension at XSDS > 0.50. As a result, the oil-fouling is readily dispersed into the CnN/SDS solutions due to de-wetting and emulsion, and the highly efficient cleaning performance is obtained for C8N/SDS at XSDS < 0.50 and C12N/SDS at XSDS > 0.50.
Inspired by the Young's equation, we try to figure out the reasons for the various cleaning ability of CnN/SDS solutions in the view of equilibrium of forces. Herein, the interfacial tensions of solid/water, oil/water and solid/oil are defined as γ1, γ2 and γ3, respectively. The three parameters fit the equation, γ1 = γ2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + γ3 (Fig. 4E). The contact angle of liquid on solid is proportional to the interfacial tension and energy, so according to the contact angles of CnN/SDS (n = 6, 8, 12) solutions on the stainless-steel plates (Fig. S3†), the γ1 values for pure SDS (γ10), C6N/SDS (γ1a) and C8N/SDS (γ1b) at XSDS < 0.5 are in order of γ10 > γ1a ≈ γ1b, while for C6N/SDS (γ1A), C8N/SDS (γ1B) and C8N/SDS (γ1C) when XSDS > 0.5, the order is γ1A > γ1B > γ1C. Meanwhile, γ20 > γ2a > γ2b at XSDS < 0.5 and γ2A > γ2B > γ2C at XSDS > 0.5 are obtained from Fig. 4D. Combining with the order of contact angle of machine oil droplets on the plates in the CnN/SDS solution (Fig. 4C), i.e., θ0 < θa < θb at XSDS < 0.5 and θA < θB < θC at XSDS > 0.5, we can observe that γ30 ≤ γ3a < γ3b at XSDS < 0.5 and γ3A < γ2B < γ2C at XSDS > 0.5. The results mean that the oil droplet has a stronger trend to retract inward and escape from the plate in C8N/SDS at XSDS < 0.5 or in C12N/SDS at XSDS > 0.5. All the experimental results and theoretical derivations provide insights into the cleaning efficiency of CnN/SDS, from which the oil-foiling removal performance could be predicted by the oil/water interfacial tension and solid/liquid contact angle of oil droplets in surfactant solutions.
θ + γ3 (Fig. 4E). The contact angle of liquid on solid is proportional to the interfacial tension and energy, so according to the contact angles of CnN/SDS (n = 6, 8, 12) solutions on the stainless-steel plates (Fig. S3†), the γ1 values for pure SDS (γ10), C6N/SDS (γ1a) and C8N/SDS (γ1b) at XSDS < 0.5 are in order of γ10 > γ1a ≈ γ1b, while for C6N/SDS (γ1A), C8N/SDS (γ1B) and C8N/SDS (γ1C) when XSDS > 0.5, the order is γ1A > γ1B > γ1C. Meanwhile, γ20 > γ2a > γ2b at XSDS < 0.5 and γ2A > γ2B > γ2C at XSDS > 0.5 are obtained from Fig. 4D. Combining with the order of contact angle of machine oil droplets on the plates in the CnN/SDS solution (Fig. 4C), i.e., θ0 < θa < θb at XSDS < 0.5 and θA < θB < θC at XSDS > 0.5, we can observe that γ30 ≤ γ3a < γ3b at XSDS < 0.5 and γ3A < γ2B < γ2C at XSDS > 0.5. The results mean that the oil droplet has a stronger trend to retract inward and escape from the plate in C8N/SDS at XSDS < 0.5 or in C12N/SDS at XSDS > 0.5. All the experimental results and theoretical derivations provide insights into the cleaning efficiency of CnN/SDS, from which the oil-foiling removal performance could be predicted by the oil/water interfacial tension and solid/liquid contact angle of oil droplets in surfactant solutions.
Foaming and defoaming test
For surfactant applications in cosmetics, detergents, food, etc., strong foaming ability is normally needed. However, in industrial cleaning, petroleum refining and other large-scale applications, strong foamability and foam stability often lead to environmental hazards, wastewater treatment difficulty and economic loss. Therefore, surfactant systems with strong oil cleaning ability but weak foamability and foam stability are of importance in these fields.Herein, to intuitively observe the foaming and defoaming ability of CnN/SDS systems with great oil cleaning ability, we firstly shake 10 mL CnN/SDS solutions of 5 mM for 30 s and record the change in foam height with aging (Fig. S4†). In parallel, we gain the variation of foam volume (Vfoam max), foam state, foam height, and bubble counts with aging by using a dynamic foam analyzer (Fig. 5 and S5†). We compare foam state against aging for the C6N/SDS and C8N/SDS mixtures at fixed XSDS = 0.10 mM and CT = 5.0 mM with the visual inspection method (inset) and air blowing method using a dynamic foam analyzer (Fig. S6†), and apparently the visual and quantitative results are consistent with each other. CnN (n = 6, 8, 12) alone almost has no ability to form liquid foams. With the addition of CnN, the foamability characterized by the maximum foam volume (Vfoam max, Fig. 5B) gradually becomes weaker with decreasing XSDS, and the C8N/SDS system displays the smallest foam volume when XSDS < 0.50, especially at XSDS = 0.10. Although the values of the maximum foam volume do not show significant differences, the differences in the foamability and foam stability are manifested in the changes of the bubble size, the foam heights and bubble count with time. Obviously, the low foamability of C8N/SDS is further verified by the bigger bubbles formed at XSDS = 0.10 and 30 s (Fig. 5A and S5†), following which the bubbles begin to break at 1500 s when the foams in pure SDS, C6N/SDS (XSDS = 0.10, 0.40, 0.50, 0.80), C8N/SDS (XSDS = 0.30, 0.40, 0.80) and C12N/SDS (C12N/SDS = 0.80) are still very stable. Correspondingly, the C6N/SDS system shows the similar changing speed in foam height against aging compared with SDS, the C12N/SDS system defoams also quite slowly, but the foam height of C8N/SDS decreases 3–5 times faster than that of SDS itself (Fig. 5C). The bubble count of CnN/SDS also confirms that the C8N/SDS system shows the fastest defoaming speed (Fig. 5D). All the above results demonstrate that the C8N/SDS mixture at XSDS < 0.50 can effectively reduce the foamability and enhance the defoam ability, perfectly corresponding to the condition for the high oil-fouling removal efficiency.
We speculate that there are two factors affecting the foam stability and defoaming ability of CnN/SDS mixed systems: surface tension and dynamic molecular exchange between the bulk phase and air/water interface. The low surface tension manifests that the surfactant molecules prefer packing at the air/water interface, which contributes to the formation and stabilization of foams. However, all the CnN/SDS systems reduce the surface tension of SDS from 38 mN m−1 to 30 mN m−1, thus the foamability and foam stability should be improved in principle. Actually, only the C12N/SDS system obeys this prediction, while the C6N/SDS and C8N/SDS systems exhibit similar or even weaker foamability and foaming stability than SDS. We turn back to analyze the CT value we selected. In order to compare the foams, we selected the same CT (5 mM), which is below the CMC of SDS (∼8.0 mM) and C6N/SDS (∼6.0 mM), or much larger than that of C12N/SDS (∼0.40 mM), but only slightly higher than the CMC value of C8N/SDS (∼3.0 mM). In general, there are three different mechanisms governing the lifetime of foam: (i) foam drainage caused by gravity, (ii) coarsening caused by the transfer of gas between bubbles generated by the capillary pressure differences, and (iii) bubble coalescence caused by the rupture of liquid films between neighbouring bubbles.64 For the present systems, it is proposed that the dynamic molecular exchange between the bulk phase and air/water interface can accelerate the enlargement and destruction of bubbles, which can perfectly explain the anomalous phenomenon in the foam (Fig. 5E). Given that the selected CT is close to but below the CMC for SDS and C6N/SDS, the surfactant molecules are enough to promote the adsorption saturation at the air/water interface but still have no ability to form micelles, in which case the molecules locating in the liquid film are relatively stable, resulting in the low defoaming ability. However, this CT is much higher than the CMC of C12N/SDS, which indicates that the absorption saturation has been completely reached at the air/water interface, and the number of molecules in the C12N/SDS aqueous solution is large enough to form stable micelles, thereby the molecules at the air/water interface do not exhibit the tendency to enter the bulk solution, leading to the stronger foam stability. In comparison, the CT is slightly higher than the CMC value of C8N/SDS. In the foaming process of C8N/SDS, more surfactant molecules join the air/water interface, while the real surfactant concentration in bulk is just high enough for micellization. The unstable bubble films promote the fusion of neighbour bubbles, and the surfactant molecules prefer to return to the micelles in bulk solution from the bubble films, i.e., the air/water interface, leading to the enlargement and destruction of bubbles under this condition.
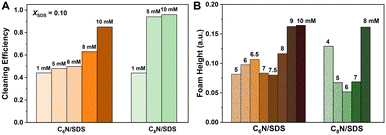
In brief, it is concluded that high oil cleaning efficiency and low foam stability of surfactant mixtures are achieved at an appropriate concentration, i.e., above CMC, but not too large at a proper XSDS. Inspired by the present results, we tested oil-fouling removal efficiency and foam height with the C6N/SDS and C8N/SDS mixtures after vortexing for 30 s and resting for 2 h at XSDS = 0.10 and various CT. For the given XSDS at 0.10, we found that the cleaning efficiency becomes higher with the increase of CT and is significantly enhanced especially above CMC. However, there is a minimum foam height just above CMC, after which the height becomes larger again (Fig. 6A and B, S7 and S8†). The CMC of C6N/SDS is larger than that of C8N/SDS, so the minimum appears at the larger concentration for C6N/SDS, i.e., at ∼7.5 mM for C6N/SDS and ∼6.0 mM for C8N/SDS. Combining with the continuous enhancement of cleaning efficiency and the minimum foam height above CMC, it can be speculated that selecting the concentration slightly above CMC at low XSDS should be beneficial to obtain a high-efficiency and low-foam cleaning system. Therefore, the concentration of 5 mM used above meets the requirements for C8N/SDS, resulting in good cleaning and deforming performance at XSDS < 0.50. Due to the weaker self-assembly ability of C6N/SDS at the air/water interface and in bulk, its performance in cleaning and defoaming efficiency cannot be better than that of C8N/SDS.
Conclusions
In this work, we designed mixed surfactant systems composed of SDS and CnN (n = 6, 8, 12) and studied their oil-fouling and foam removal ability. With addition of CnN, the surface activity and aggregation ability of SDS are significantly enhanced, and the degree of enhancement is increased with the larger size of the cycloalkane ring because the hydrophobic interaction becomes stronger. As a result, only spherical micelles form in C6N/SDS mixtures, while vesicles, spherical micelles and wormlike micelles form in the C8N/SDS and C12N/SDS mixtures. However, the flexible and adjustable cycloalkane ring of C12N leads to the changeable conformation transformation and facilitates entanglement with each other tightly, thereby precipitation takes place in a large concentration range in C12N/SDS. Except for the region of precipitate, the oil cleaning efficiency of CnN/SDS shows a similar changing trend against XSDS at a fixed CT and the optimum condition mainly appears around XSDS = 0.50. Meanwhile, the stronger aggregation ability induces the higher cleaning efficiency with increasing cycloalkane ring size and CT. As a result, the best cleaning performance is achieved by C8N/SDS in a broader XSDS region, especially at low XSDS. In parallel, the changing trend of foam stability is broadly consistent with cleaning efficiency against XSDS for a given CT, i.e., the strong defoaming ability appears at XSDS < 0.5, which is attributed to the low surface activity of CnN. But it is totally different with changing CT for a fixed XSDS, i.e., there is an obvious decline of foam ability slightly above CMC, which is driven by the migration of the limited molecules from the liquid film of foams to bulk between films for micellization. Due to the stronger self-assembly ability of C8N/SDS than C6N/SDS, the highly efficient foam removal is realized by C8N/SDS at smaller CT. It can be concluded that the highly efficient oil-fouling and foam removal can be achieved concurrently in a surfactant system mixing with an additive of relatively high solubilization and low surface activity like cyclic amines as we expected, which may form a larger hydrophobic domain in bulk through molecular conformation transition but create looser arrangement at the air/water interface because of bulky alkyl rings. This could also be realized in a single surfactant solution with such properties like star-shaped oligomeric surfactants. As such, the paradoxical self-assembly ability in bulk and at the air/water interface could induce a different changing tendency of cleaning and foaming performance against molar fraction or total concentration, providing a possible condition to achieve such a pair of contradictory properties. Thus, this work offers a simple approach obtain a high-efficiency and low-foam detergent with a low usage, meeting the requirements of some applications and supporting the sustainable development of the environment.Author contributions
Z. Z., Y. F., and Y. W. designed the research. Y. F. and Y. W. supervised the experimental work. Z. Z. performed most of the experiments. J. Y. performed cryo-TEM experiments on the CnN/SDS aggregates. T. W. and Z. Z. performed the experiments on cleaning measurements together. All authors discussed and contributed to the interpretation of the data. Z. Z. wrote the original manuscript, and Y. F. and Y. W. edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from the National Key R&D Program of China (2021YFA0716700), National Natural Science Foundation of China (21972149 and 21988102) and the Beijing National Laboratory for Molecular Sciences (BNLMS).Notes and references
- J. J. Scheibel, J. Surfactants Deterg., 2004, 7, 319–328 CrossRef CAS.
- J. J. Mueller and H. H. Wenk, Chimia, 2021, 75, 752–756 CrossRef CAS.
- L. Golsteijn, R. Menkveld, H. King, C. Schneider, D. Schowanek and S. Nissen, Environ. Sci. Eur., 2015, 27, 1–12 CrossRef CAS.
- M. Bernat, C. Pey, M. J. Bermejo, B. Nogues, J. Vilaret and N. Siscart, Riv. Ital. Sostanze Grasse, 2007, 84, 246–252 CAS.
- H. Lee, G. Amy, J. W. Cho, Y. M. Yoon, S. H. Moon and I. S. Kim, Water Res., 2001, 35, 3301–3308 CrossRef CAS PubMed.
- J. A. Howarter, K. L. Genson and J. P. Youngblood, ACS Appl. Mater. Interfaces, 2011, 3, 2022–2030 CrossRef CAS.
- T. Wang, Y. Si, S. Luo, Z. Dong and L. Jiang, Mater. Horiz., 2019, 6, 294–301 RSC.
- J. Weiss, E. A. Decker, D. J. McClements, K. Kristbergsson, T. Helgason and T. Awad, Food Biophys., 2008, 3, 146–154 CrossRef.
- I. Kralova and J. Sjoblom, J. Dispersion Sci. Technol., 2009, 30, 1363–1383 CrossRef CAS.
- J. Wang, Y. Fan, H. Wang, J. Yin, W. Tan, X. Li, Y. Shen and Y. L. Wang, Chem. Eng. J., 2022, 430, 132920 CrossRef CAS.
- D. P. Sachdev and S. S. Cameotra, Appl. Microbiol. Biotechnol., 2013, 97, 1005–1016 CrossRef CAS PubMed.
- P. Hu, J. An, M. M. Faulkner, H. Wu, Z. Li, X. Tian and J. P. Giraldo, ACS Nano, 2020, 14, 7970–7986 CrossRef CAS PubMed.
- I. S. Curtis and H. G. Nam, Transgenic Res., 2001, 10, 363–371 CrossRef CAS.
- B. Liu, Y. Fan, H. Li, W. Zhao, S. Luo, H. Wang, B. Guan, Q. Li, J. Yue, Z. Dong, Y. L. Wang and L. Jiang, Adv. Funct. Mater., 2021, 31, 2006606 CrossRef CAS.
- Y. Luo, Z. Teng, Y. Li and Q. Wang, Carbohydr. Polym., 2015, 122, 221–229 CrossRef CAS.
- Y. Singh, J. G. Meher, K. Raval, F. A. Khan, M. Chaurasia, N. K. Jain and M. K. Chourasia, J. Controlled Release, 2017, 252, 28–49 CrossRef CAS PubMed.
- C. H. Tsai, J. L. Vivero-Escoto, I. I. Slowing, I. Fang, B. G. Trewyn and V. S. Y. Lin, Biomaterials, 2011, 32, 6234–6244 CrossRef CAS PubMed.
- R. Guagliardo, J. Perez-Gil, S. De Smedt and K. Raemdonck, J. Controlled Release, 2018, 291, 116–126 CrossRef CAS.
- Surfactants market by type (anionic, non-ionic, cationic, and amphoteric), application (home care, personal care, industrial & institutional cleaning, textile, elastomers & plastics, agrochemicals, and food & beverage), region - global forecast to 2028, MARKETSANDMARKETS, 2023, https://www.marketsandmarkets.com/Market-Reports/biosurfactants-market-493.html.
- Industrial cleaning chemicals market by ingredient type (surfactants, solvents, chelating agents), product (gerernal & medical cleaning), application (manufacturing & commerical offices, healthcare, retail & food service), and region - global forecast to 2028, MARKETSANDMARKETS, 2023, https://www.marketsandmarkets.com/Market-Reports/industrial-institutional-cleaning-chemicals-market-52902227.html.
- T. Wang, Y. Han, S. Dai, J. Wang, B. Liu, M. Cao, B. Guan and Y. L. Wang, Nano Res., 2023, 16, 2551–2562 CrossRef CAS.
- B. O. Okesola and A. Mata, Chem. Soc. Rev., 2018, 47, 3721–3736 RSC.
- G. Ren, M. Wang, L. Wang, Z. Wang, Q. Chen, Z. Xu and D. Sun, Langmuir, 2018, 34, 5798–5806 CrossRef CAS PubMed.
- Q. Zeng, Q. Li, Y. Huang, Y. Lv, X. Liao and Q. Yang, J. Macromol. Sci., Part B: Phys., 2015, 54, 329–347 CrossRef CAS.
- Y. Fan, Y. Hou, J. Xiang, D. Yu, C. Wu, M. Tian, Y. Han and Y. L. Wang, Langmuir, 2011, 27, 10570–10579 CrossRef CAS PubMed.
- D. Yu, Y. Wang, J. Zhang, M. Tian, Y. Han and Y. L. Wang, J. Colloid Interface Sci., 2012, 381, 83–88 CrossRef CAS PubMed.
- L. Zhu, Y. Tang and Y. L. Wang, J. Surfactants Deterg., 2016, 19, 237–247 CrossRef CAS.
- R. Zana, Adv. Colloid Interface Sci., 2002, 97, 205–253 CrossRef CAS.
- D. Shao, G. Liu, H. Chen, C. Xu and J. Du, J. Surfactants Deterg., 2021, 24, 357–364 CrossRef CAS.
- M. Wang, Y. Wang, D. Yu, Y. Han and Y. L. Wang, Colloid Polym. Sci., 2013, 291, 1613–1621 CrossRef CAS.
- R. Zana, J. Colloid Interface Sci., 2002, 248, 203–220 CrossRef CAS PubMed.
- S. A. Onaizi, Eur. Biophys. J., 2018, 47, 631–640 CrossRef CAS PubMed.
- F. G. Valeeva, E. A. Vasilieva, G. A. Gaynanova, R. R. Kashapov, S. V. Zakharov, D. A. Kuryashov, S. S. Lukashenko, N. Y. Bashkirtseva and L. Y. Zakharova, J. Mol. Liq., 2015, 203, 104–110 CrossRef CAS.
- A. Bera, K. Ojha and A. Mandal, J. Surfactants Deterg., 2013, 16, 621–630 CrossRef CAS.
- J. Eastoe and J. S. Dalton, Adv. Colloid Interface Sci., 2000, 85, 103–144 CrossRef CAS PubMed.
- N. Genc, E. Durna and O. Kilicoglu, J. Water Chem. Technol., 2019, 41, 236–241 CrossRef.
- B. Riechers, F. Maes, E. Akoury, B. Semin, P. Gruner and J. C. Baret, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 11465–11470 CrossRef CAS.
- A. Bera, T. Kumar, K. Ojha and A. Mandal, Appl. Surf. Sci., 2013, 284, 87–99 CrossRef CAS.
- S. Paria and K. C. Khilar, Adv. Colloid Interface Sci., 2004, 110, 75–95 CrossRef CAS PubMed.
- D. Yu, Q. Zhang, C. Wu, Y. Wang, L. Peng, D. Zhang, Z. Li and Y. L. Wang, J. Phys. Chem. B, 2010, 114, 8934–8940 CrossRef CAS.
- M. Deng, M. Cao and Y. L. Wang, J. Phys. Chem. B, 2009, 113, 9436–9440 CrossRef CAS PubMed.
- M. L. Free, Corros. Sci., 2002, 44, 2865–2870 CrossRef CAS.
- Y. Chen, F. Qiao, Y. Fan, Y. Han and Y. L. Wang, Langmuir, 2017, 33, 2760–2769 CrossRef CAS PubMed.
- S. Luo, Y. Wang, M. Wang and Y. L. Wang, J. Surfactants Deterg., 2018, 21, 899–908 CrossRef CAS.
- Z. Chen, J. Penfold, P. Li, J. Doutch, Y. Fan and Y. L. Wang, Soft Matter, 2017, 13, 8980–8989 RSC.
- C. Zhou, D. Wang, M. Cao, Y. Chen, Z. Liu, C. Wu, H. Xu, S. Wang and Y. L. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 30811–30823 CrossRef CAS.
- J. Larsson, A. E. Leung, C. Lang, B. Wu, M. Wahlgren, T. Nylander, S. Ulvenlund and A. Sanchez-Fernandez, J. Colloid Interface Sci., 2021, 585, 178–183 CrossRef CAS.
- Q. Cui, J. Z. Liu, L. Yu, M. Z. Gao, L. T. Wang, W. Wang, X. H. Zhao, Y. J. Fu and J. C. Jiang, J. Cleaner Prod., 2020, 274, 122652 CrossRef CAS.
- A. Bhadani, A. Kafle, T. Ogura, M. Akamatsu, K. Sakai, H. Sakai and M. Abe, Curr. Opin. Colloid Interface Sci., 2020, 45, 124–135 CrossRef CAS.
- H. Z. Kister, Chem. Eng. Res. Des., 1997, 75, 563–589 CrossRef CAS.
- H. Z. Kister, Chem. Eng. Res. Des., 2003, 81, 5–26 CrossRef CAS.
- W. Dou, Z. Zhang, W. Huang, X. Wang, R. Zhang, Y. Wu, A. Sun, X. Shi and J. Chen, Chemosphere, 2022, 303, 135032 CrossRef CAS.
- L. Polasek, J. Bering, H. Kim, P. Neitlich, B. Pister, M. Terwilliger, K. Nicolato, C. Turner and T. Jones, Mar. Pollut. Bull., 2017, 117, 371–379 CrossRef CAS.
- K. Schilling and M. Zessner, Water Res., 2011, 45, 4355–4366 CrossRef CAS.
- D. Shao, G. Liu, H. Chen, C. Xu and J. Du, J. Surfactants Deterg., 2021, 24, 357–364 CrossRef CAS.
- P. Pal, I. Shittu, J. Oladunni and F. Banat, J. Nat. Gas Sci. Eng., 2020, 81, 103478 CrossRef CAS.
- Y. Sheng, X. Wu, S. Lu and C. Li, J. Surfactants Deterg., 2016, 19, 823–831 CrossRef CAS.
- Q. Liu, S. Zhang, D. Sun and J. Xu, Colloids Surf., A, 2010, 355, 151–157 CrossRef CAS.
- P. W. Pakes, T. C. Rounds and H. L. Strauss, J. Phys. Chem., 1981, 85, 2469–2475 CrossRef CAS.
- I. Kolossvary and W. C. Guida, J. Am. Chem. Soc., 1993, 115, 2107–2119 CrossRef CAS.
- Y. Wang, P. Kirsch, T. Lebl, A. M. Z. Slawin and D. O'Hagan, Beilstein J. Org. Chem., 2012, 8, 1271–1278 CrossRef CAS PubMed.
- E. J. Saavedra, S. A. Andujar, F. D. Suvire, M. A. Zamora, M. L. Freile and R. D. Enriz, Int. J. Quantum Chem., 2012, 112, 2382–2391 CrossRef CAS.
- B. Liu, T. Li, W. Y. Wang, L. M. C. Sagis, Q. P. Yuan, X. G. Lei, M. A. C. Stuart, D. Li, C. Bao and J. Bai, et al. , Nat. Sustain., 2020, 3, 448–458 CrossRef.
- I. Cantat, S. Cohen-Addad, F. Elias, F. Graner, R. Hohler, O. Pitois, F. Rouyer and A. Saint-Jalmes, Foams: structure and dynamics, Croydon: CPI Group (U.K.) Ltd, 2013 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S7. See DOI: https://doi.org/10.1039/d3lf00145h |
| This journal is © The Royal Society of Chemistry 2024 |