Open Access Article
Open Access ArticleAntimicrobial textiles based on photocrosslinked poly(ethylene-co-acrylic acid)†
Yimin
Zeng
 and
Michael O.
Wolf
and
Michael O.
Wolf
 *
*
Department of Chemistry, University of British Columbia, 2036 Main Mall, Vancouver, British Columbia V6T 1Z1, Canada. E-mail: mwolf@chem.ubc.ca
First published on 12th August 2024
Abstract
Photocrosslinking of a series of amine-containing poly (ethylene-co-acrylic acids) (PEAAs) on textile surfaces by reaction of photogenerated singlet oxygen at the amine groups to form imines is reported. The materials are hydrophilic and show antimicrobial activity. Modified PEAAs with 3 different lengths of side chains are synthesized to study the effect of varying side chain length on polymer photooxidative crosslinking and antimicrobial activity. Materials with longer side chains show elimination of E. coli and MRSA by contact lysis which is enhanced under irradiation with green light.
Introduction
Viral and bacterial infections can be transmitted by surface contamination.1 Bacterial infections are the second leading cause of death worldwide, affecting individuals in all age groups. In 2019, an estimated 7.7 million deaths were attributed to 33 bacterial pathogens, constituting 13.6% of all worldwide fatalities.2 Generally, disinfectants are used to sanitize surfaces to prevent infection by bacteria, viruses, and other pathogens. This labour-intensive process involves repeatedly wiping or spraying disinfectants to ensure continuous sanitization. In the process, volatile and toxic disinfectants can be released into the environment possibly resulting in ecological damage. To address this issue, modified surfaces that kill or inhibit the growth of microbes to reduce or eliminate disease transmission have been developed.3 Modification of textiles with coatings that have inherent antimicrobial properties that can prevent the spread of diseases is one approach that is being explored. By applying an antimicrobial coating to textiles, the spread of pathogens can be mitigated in health care and other high-risk settings, for example on hospital gowns, bedding, and clothing.4,5Antimicrobial surfaces can be prepared by functionalization with primary amine or phosphonium containing polymers, quaternary ammonium salts (QASs), or metal nanoparticles.6–10 Surfaces modified with these functionalities can act to kill bacteria through passive pathways, such as contact lysis or ion release, where the bacterial cell membranes are disrupted. Hydrophobic polymer coatings can also prevent moisture accumulation and reduce microbial adhesion on surfaces.11 Additionally, photosensitizers can be used to generate singlet oxygen (1O2, the first excited state of oxygen) or free radical ions that destroy microbial membranes, a process called antimicrobial photodynamic inactivation (aPDI).12–14 Coatings based on QASs and metal nanoparticles are passive while aPDI gives the possibility of active photosensitization utilizing light energy to destroy pathogens via singlet oxygen generation. Typically, the photosensitizer is excited to a singlet state with visible light and then undergoes intersystem crossing to a triplet state, followed by triplet–triplet energy transfer to ground state oxygen, forming 1O2.15,16
Our group recently developed dual-functional antimicrobial polymers, which employ primary amine-containing aminopropylmethylsiloxane (6–7 wt%)-dimethylsiloxane copolymers (PDMS-NH2) with a covalently attached photosensitizer, rose bengal (RB), that generate 1O2 on irradiation with green light.17 Others have trapped RB in the pores of organic polymers on cotton surfaces and demonstrated biocidal activity.18 Our PDMS-NH2 polymer is photocrosslinked by reaction with 1O2 at the amine groups to form imines. By forming covalent bonds between polymer chains a robust coating on textile surfaces results which prevents polymer leaching into the aqueous environment in real-world applications.11,19 Textiles can be modified by soaking with a solution of the polymer followed by photocrosslinking resulting in the formation of a durable antimicrobial coating.17,20
Photocrosslinking PDMS-NH2 results in a hydrophobic coating on textile substrates attributed to the presence of the alkyl side chains on the polysiloxane backbone. Although a hydrophobic polymer coating on fabric can reduce surface bacterial growth, there are disadvantages associated with the use of hydrophobic materials, such as poor durability in clothing applications.21 Moreover, silicones are not biodegradable or recyclable raising sustainability concerns. Here we report crosslinking of a hydrophilic carbon-based polymer, poly(ethylene-co-acrylic acid) (PEAA) that contains 15 wt% of carboxylic acid groups, and exploration of the antimicrobial properties of textile coatings based on this polymer. PEAA is widely applied in biosensor fabrication and in the food packaging industry due to its adhesive properties towards polar substrates.22,23 In 2017, Noh and co-workers reported an antimicrobial material consisting of QAS grafted onto PEAA.24 In the present work, both the benefits of PEAA as a carbon-based polymer and the dual-functional antimicrobial approach pioneered by our group are combined. Modified PEAAs with 3 different lengths of side chains are synthesized to study the effect of varying side chain length on polymer photooxidative crosslinking and antimicrobial activity. The modified PEAA-NH2 polymers, PEAA-3C-NH2, PEAA-6C-NH2, and PEAA-8C-NH2 have amine groups that have 3 carbon, 6 carbon, and 8 carbon spacers between the polymer backbone and amine group, respectively.
Results and discussion
PEAA-NH2 was synthesized by reacting PEAA with N,N-dicyclohexylcarbodiimide (DCC) and diamino alkanes with three different alkyl chain lengths (Scheme 1). Chain lengths were selected based on readily available diaminoalkanes, where the longer chain alkanes are typically more costly. 1,3-Diaminopropane, 1,6-diaminohexane and 1,8-diaminooctane were used to leave a terminal amino group available for photocrosslinking after formation of an amide link with one of the amino groups. PEAA-3C-NH2, PEAA-6C-NH2, and PEAA-8C-NH2 were prepared, in each case the product after coupling was isolated as a fine white powder, which precipitated with methanol and was washed by centrifugation. Cotton pieces are soaked in a hot DMSO solution of the polymer to coat the textile surface followed by crosslinking with light to create a robust coating.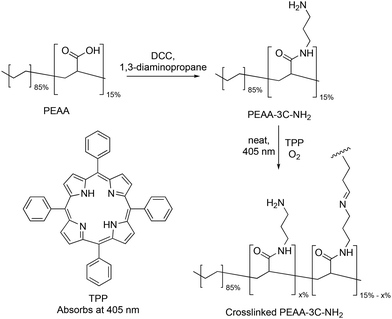 | ||
| Scheme 1 Synthesis of PEAA-3C-NH2. PEAA-6C-NH2 and PEAA-8C-NH2 are prepared the same way using 1,6-diaminohexane and 1,8-diaminooctane respectively. | ||
The PEAA-NH2 solution-treated cotton has both passive and active photosensitized functionality to kill microbes, through contact lysis and singlet oxygen formation. Crosslinking of PEAA-NH2 was carried out by 1O2 produced by tetraphenylporphyrin (TPP) co-adsorbed with the polymer on irradiation with a 36 W 405 nm LED bulb for 30 minutes. In a second step, polymer-coated cotton was washed with THF to remove remaining TPP and then soaked in a 0.1 wt% ethanol solution of RB to introduce the photosensitizer (Fig. 1). TPP is used to crosslink the polymer on the textile surface while RB is present to generate 1O2 to kill microbes under irradiation. TPP photosensitization is not initiated by the 530 nm green light used for the antimicrobial tests.
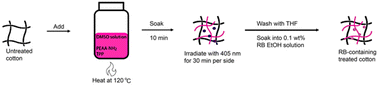 | ||
| Fig. 1 Scheme of textile treatment with solution soak and light irradiation resulting in RB-containing PEAA-NH2 solution-treated cotton. | ||
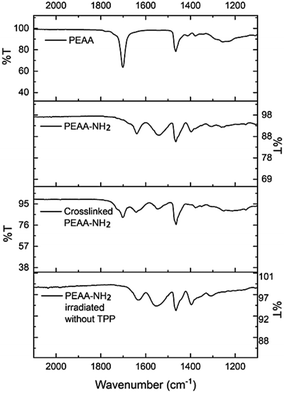
All three PEAA-NH2 derivatives are insoluble or very poorly soluble in most organic solvents and water, and only partially dissolve in dimethyl sulfoxide (DMSO) at 120 °C. Synthesis of PEAA-NH2 is confirmed by a shift in the α-H peak in the 1H NMR spectrum at 120 °C from 2.22 ppm in PEAA to 2.51 ppm in PEAA-NH2 due to the formation of the amide bond (Fig. S2–S7†). The NMR spectrum of PEAA-6C-NH2 could not be obtained due to the poor solubility of this polymer. In the FTIR spectrum, PEAA shows a carbonyl stretch due to the carboxylic acid groups at 1701 cm−1. After coupling, the characteristic carboxylic acid peak of PEAA is no longer present, and an amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch at 1639 cm−1 and an amine N–H bend at 1539 cm−1 appear, confirming the conversion from PEAA to PEAA-NH2. An imine C
O stretch at 1639 cm−1 and an amine N–H bend at 1539 cm−1 appear, confirming the conversion from PEAA to PEAA-NH2. An imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretch at 1690 cm−1 is observed in the crosslinked PEAA-NH2 along with the presence of amine and amide peaks (Fig. 2). The FTIR spectrum shows that not all amine groups are involved in crosslinking PEAA-NH2, thus the remaining free amine groups in the polymer can remain active for contact lysis. Crosslinking does not occur if PEAA-NH2 is irradiated without TPP, indicating the necessity of the photosensitizer to be present for crosslinking. Ninhydrin tests were performed to determine the percentage of amine groups remaining after crosslinking (Table S3†). The percentage of amine remaining after crosslinking is 62 ± 5% for PEAA-3C-NH2, 24 ± 2% for PEAA-6C-NH2, and 23 ± 4% for PEAA-8C-NH2. Based on this data, PEAA-3C-NH2 showed less crosslinking compare with the other two polymers.
N stretch at 1690 cm−1 is observed in the crosslinked PEAA-NH2 along with the presence of amine and amide peaks (Fig. 2). The FTIR spectrum shows that not all amine groups are involved in crosslinking PEAA-NH2, thus the remaining free amine groups in the polymer can remain active for contact lysis. Crosslinking does not occur if PEAA-NH2 is irradiated without TPP, indicating the necessity of the photosensitizer to be present for crosslinking. Ninhydrin tests were performed to determine the percentage of amine groups remaining after crosslinking (Table S3†). The percentage of amine remaining after crosslinking is 62 ± 5% for PEAA-3C-NH2, 24 ± 2% for PEAA-6C-NH2, and 23 ± 4% for PEAA-8C-NH2. Based on this data, PEAA-3C-NH2 showed less crosslinking compare with the other two polymers.
The average water contact angle of cotton treated with 1 wt% PDMS-NH2 is 134 ± 9°, while for all PEAA-NH2 samples water is absorbed, and a contact angle cannot be measured on the cotton surface (Fig. S10†). This indicates that while the 1 wt% PDMS-NH2 solution-treated cotton is hydrophobic, the PEAA-NH2 samples are very hydrophilic. Tensile tests (fabric stretching) were performed to probe if the physical properties of the modified fabrics are changed from prior to treatment. The load average of untreated plain cotton is 16 ± 2 N and 17 ± 2 N for 1 wt% PEAA-8C-NH2 solution-treated cotton (Table S2†). Thus, polymer treatment results in a minimal tensile strain change.
Antimicrobial tests were carried out with Escherichia coli (E. coli) and methicillin resistant Staphylococcus aureus (MRSA) with a concentration of 105 bacteria per mL. Identical sized pieces of coated cotton were used to compare the bacterial killing efficiency between PDMS-NH2 and the three PEAA-NH2 derivatives. To allow generation of 1O2 under irradiation, all coated fabrics were soaked in a 0.1 wt% RB solution for 3 min. The average loadings of RB on fabrics treated with PEAA-3C-NH2, PEAA-6C-NH2 and PEAA-8C-NH2 were determined by measuring the absorption of extracted RB at 560 nm (Table S4†). Unmodified cotton was also treated with RB under the same conditions for comparison. The average weight percentages (wt%) of RB on solution treated fabric were determined to be 3.7 ± 0.1% for plain cotton, 3.3 ± 0.2% for PEAA-3C-NH2 solution-treated cotton, 3.0 ± 0.2% for PEAA-6C-NH2 solution-treated cotton, and 3.3 ± 0.4% for PEAA-8C-NH2 solution-treated cotton. The PEAA coatings result in only a minor change in the RB loading relative to plain cotton.
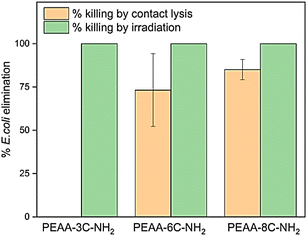
Contact lysis experiments were carried out by soaking the polymer solution-treated cotton sample in a bacterial culture solution in the dark. Photosensitization was conducted by irradiating a bacterial culture containing coated fabrics with a 15 W 530 nm green light. In both experiments, the bacterial cultures were assessed over 150 min to monitor bacterial growth under treatment. No reduction in the number of bacteria was observed when bacterial solutions or untreated textiles were irradiated with green light (Fig. S11†). Both 0.5 wt% PEAA-8C-NH2 and 0.5 wt% PDMS-NH2 solution-treated cotton behaved the same by gradually reducing the number of E. coli colony-forming units (CFUs) over 150 min (Fig. S12†). In this case, the 0.5 wt% PEAA-8C-NH2 solution-treated cotton showed an average of 85% of E. coli elimination and 30% killing of MRSA through contact lysis (Fig. 3 and Fig. S13†). All bacteria were killed under 10 min irradiation with green light. This indicates that changing the hydrophobicity of the polymer coating significantly does not change the dual-functional antimicrobial capability. The bacterial killing behaviour under irradiation is dominated by 1O2 produced by excitation of the RB. Identical treatment and antimicrobial assays were carried out with PEAA-3C-NH2 and PEAA-6C-NH2 in comparison to PEAA-8C-NH2 to study the impact of amine chain length on bacterial killing. All bacteria were killed with 10 min irradiation by PEAA-3C-NH2, PEAA-6C-NH2, and PEAA-8C-NH2. PEAA-6C-NH2 behaved the same as both PEAA-8C-NH2 and PDMS-NH2, while PEAA-3C-NH2 had no effect on bacteria present by contact lysis, even with a higher amount of amine groups remained after crosslinking (Fig. 4 and Table S1†). This can be rationalized by the short alkyl chain in PEAA-3C-NH2 which is less flexible for insertion into the bacteria cell membrane compared with the longer analogs. Although PDMS-NH2 also has amine groups that are 3 carbons away, the siloxane backbone in PDMS-NH2 may be more flexible than the carbon backbone in PEAA-3C-NH2.
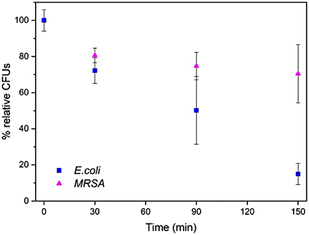 | ||
| Fig. 3 A comparison of contact lysis for E. coli and MRSA on 0.5 wt% PEAA-8C-NH2 solution-treated cotton over time. | ||
Conclusions
In summary, we report a series of new dual-functional organic antimicrobial polymer analogs to PDMS-NH2 to explore the effect of modifying the hydrophilicity of polymer coatings on fabrics while retaining its bacterial killing property. PEAA-3C-NH2, PEAA-6C-NH2, and PEAA-8C-NH2 can all be crosslinked under 405 nm light with an average of 36% amine remained after crosslinking. PEAA-6C-NH2 and PEAA-8C-NH2 behave the same as PDMS-NH2 in antimicrobial tests, where E. coli and MRSA were eliminated over 150 min by contact lysis and 10 min under irradiation of green light. The PEAA-NH2 treatment results in a hydrophilic coating on fabrics while not changing the tensile properties of the textile.Experimental section
General
PEAA and RB lactone (95%) were obtained from Sigma-Aldrich. DCC, 1,3-diaminopropane, 1,6-diaminohexane, and 1,8-diaminooctane were purchased from Thermo Fisher Scientific. TPP was purchased from STREM. Synthesis of PEAA-NH2 was carried out under nitrogen with properly dried glassware and solvents. 1H, HSQC and HMBC NMR experiments were carried out to obtain NMR peak assignments in the NMR lab at UBC. FTIR experiments were carried out in the Shared Instrument Facility (SIF) at UBC. Scheme 1 was followed to synthesize the desired products.PEAA-8C-NH2
0.5 g of PEAA was mixed with 0.1 g (0.48 mmol) of DCC and 0.8 g (5.5 mmol) of 1,8-diaminooctane. The neat reaction mixture was heated at 120 °C under N2 for 24 h. The desired amine containing PEAA-NH2 was precipitated with methanol and washed by centrifugation. The product was dried under vacuum. 1H NMR (120 °C, 400 MHz, DMSO): δ 2.51 (m, α-H, overlap with DMSO), δ 2.58 (t, amide-H2).PEAA-3C-NH2 and PEAA-6C-NH2 were prepared using the same procedure with excess 1,3-diaminopropane or 1,6-diaminohexane instead of 1,8-diaminooctane.
Polymer crosslinking
In 100 μL of DMSO solution, 50 mg of PEAA-NH2 was mixed with 0.1 mg (0.16 μmol) of TPP. The reaction mixture was irradiated with a 36 W 405 nm LED bulb for 30 min at 120 °C to crosslink the PEAA-NH2.Textile treatment
Cotton pieces were soaked in polymer solutions in DMSO at 120 °C with TPP for 10 min in the dark. DMSO was removed by soaking fabrics in stirring water. The fabrics were air dried and irradiated with a 36 W 405 nm LED bulb for 30 minutes per side. The light bulb was placed 9 cm above the samples. Once the polymer was coated onto cotton, residual TPP was removed by a THF wash. The treated cottons were soaked into a 0.1 wt% RB EtOH solution for 3 min and dried under vacuum.Loading of RB on treated fabrics
After treating coated fabrics with 0.1 wt% RB EtOH solution, RB was redissolved into 8 mL of EtOH by sonication. The absorption of redissolved RB was measured at 560 nm using a Cary 5000 UV/Vis spectrometer (Agilent Technologies Inc., Santa Clara).Antimicrobial tests
E. coli and MRSA (level 2, from the Biological Services Laboratory, UBC Chemistry) were used. Samples from the same batch of tensile tests were employed to ensure identical surface area. The cultures were prepared with a concentration of 105 bacteria per mL and 10 mL was transferred into Falcon tubes contain polymer-coated cotton. Samples were treated with or without irradiation for comparison. A 15 W 530 nm LED bulb was set 9 cm above the samples. The optical power at the center of the lamp was 13 mW cm−2 measured using a thermal power meter (Ophir 3A-P-SH). 20 μL of sample solution was taken at each time point and incubated on agar gel. Results were collected in triplicate.Hydrophobicity test
A drop of deionized water (10 μL) was placed on each fabric sample and measured from the horizon. Photos were taken with an iPhone XR and analysed by ImageJ.Tensile tests
Polymer coated cottons were cut into identical dog bone shape (10 mm in the course direction and 37 mm in the wale direction) by laser cutting. Tensile measurements on polymer-treated cottons were measured until the break point using an Instron 5980 instrument under a 2 kN load at 20 cm min−1 with a clamp distance of 30 mm. Results were averaged from ten samples for each comparison group.Ninhydrin test
50 mg of PEAA-NH2 was mixed with 0.1 mg (0.16 μmol) of TPP in 100 μL of DMSO. The reaction mixture was irradiated under identical conditions to those used to crosslink PEAA-NH2. For comparison, samples were also prepared the same way without TPP. A ninhydrin solution in ethanol (10 μL, 3.5 mg mL−1) was added to samples while heating. The absorption of ninhydrin after reaction was obtained using an Infinite M1000 Pro plate reader (Tecan Ltd, Morrisville) on a non-binding 96-well plate. Measurements were carried out in triplicate.Author contributions
The manuscript was written through contributions of all authors.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
The materials disclosed in this paper are included in a provisional patent that has been filed.Acknowledgements
We thank the Natural Sciences and Engineering Research Council (NSERC) of Canada for funding. We thank Dr Saeid Soltanian for assistance with the textile laser cutting. Antimicrobial testing was supported by Jessie (Jie) Chen. We thank Dr Maria Ezhova for assistance with the variable temperature NMR measurements and acknowledge Yihao Wang for assistance with the ninhydrin plate reader measurements. We thank Dr Saeid Kamal for assistance with the measurement of optical power.References
- H. Choi, P. Chatterjee, J. D. Coppin, J. A. Martel, M. Hwang, C. Jinadatha and V. K. Sharma, Environ. Chem. Lett., 2021, 19, 1935–1944 CrossRef CAS PubMed.
- T. Vos, S. S. Lim, C. Abbafati, K. M. Abbas, M. Abbasi and M. Abbasifard, et al. , Lancet, 2020, 396, 1204–1222 CrossRef PubMed.
- U. Mahanta, M. Khandelwal and A. S. Deshpande, J. Mater. Sci., 2021, 56, 17915–17941 CrossRef CAS PubMed.
- R. Gulati, S. Sharma and R. K. Sharma, Polym. Bull., 2022, 79, 5747–5771 CrossRef CAS PubMed.
- N. Karim, S. Afroj, K. Lloyd, L. C. Oaten, D. V. Andreeva, C. Carr, A. D. Farmery, I.-D. Kim and K. S. Novoselov, ACS Nano, 2020, 14, 12313–12340 CrossRef CAS PubMed.
- L. A. T. W. Asri, M. Crismaru, S. Roest, Y. Chen, O. Ivashenko, P. Rudolf, J. C. Tiller, H. C. van der Mei, T. J. A. Loontjens and H. J. Busscher, Adv. Funct. Mater., 2014, 24, 346–355 CrossRef CAS.
- S. E. Exley, L. C. Paslay, G. S. Sahukhal, B. A. Abel, T. D. Brown, C. L. McCormick, S. Heinhorst, V. Koul, V. Choudhary, M. O. Elasri and S. E. Morgan, Biomacromolecules, 2015, 16, 3845–3852 CrossRef CAS PubMed.
- N. C. Süer, C. Demir, N. A. Ünübol, Ö. Yalçın, T. Kocagöz and T. Eren, RSC Adv., 2016, 6, 86151–86157 RSC.
- F. Ghilini, D. E. Pissinis, A. Miñán, P. L. Schilardi and C. Diaz, ACS Biomater. Sci. Eng., 2019, 5, 4920–4936 CrossRef CAS PubMed.
- E. Sánchez-López, D. Gomes, G. Esteruelas, L. Bonilla, A. L. Lopez-Machado, R. Galindo, A. Cano, M. Espina, M. Ettcheto, A. Camins, A. M. Silva, A. Durazzo, A. Santini, M. L. Garcia and E. B. Souto, Nanomaterials, 2020, 10, 292 CrossRef PubMed.
- P. Singha, J. Pant, M. J. Goudie, C. D. Workman and H. Handa, Biomater. Sci., 2017, 5, 1246–1255 RSC.
- M. R. Hamblin, Curr. Opin. Microbiol., 2016, 33, 67–73 CrossRef CAS PubMed.
- Y. Li, J. Wang, Y. Yang, J. Shi, H. Zhang, X. Yao, W. Chen and X. Zhang, Mater. Sci. Eng., C, 2021, 118, 111447 CrossRef CAS PubMed.
- J. R. Kim and S. Michielsen, J. Appl. Polym. Sci., 2015, 132, 42114 CrossRef.
- M. Wainwright and K. B. Crossley, Int. Biodeterior. Biodegrad., 2004, 53, 119–126 CrossRef CAS.
- E. G. Alvarez, H. Wortham, R. Strekowski, C. Zetzsch and S. Gligorovski, Environ. Sci. Technol., 2012, 46, 1955–1963 CrossRef PubMed.
- T. Wright, D. Karis, S. C. Millik, T. Tomkovic, S. G. Hatzikiriakos, A. Nelson and M. O. Wolf, ACS Appl. Mater. Interfaces, 2021, 13, 22195–22203 CrossRef CAS PubMed.
- P. Tang, A. Y. El-Moghazy, B. Ji, N. Nitin and G. Sun, Mater. Adv., 2021, 2, 3569–3578 RSC.
- E. K. Riga, M. Vöhringer, V. T. Widyaya and K. Lienkamp, Macromol. Rapid Commun., 2017, 38, 1700216 CrossRef PubMed.
- T. Wright, M. Vlok, T. Shapira, A. D. Olmstead, F. Jean and M. O. Wolf, ACS Appl. Mater. Interfaces, 2022, 14, 49–56 CrossRef CAS PubMed.
- N. Cohen, A. Dotan, H. Dodiuk and S. Kenig, Mater. Manuf. Processes, 2015, 31, 1143–1155 CrossRef.
- B. Xiang, G. Sun, K. S. Lam and K. Xiao, J. Biomed. Mater. Res., Part A, 2010, 95A, 245–255 CrossRef CAS PubMed.
- M. F. Finlayson and B. A. Shah, J. Adhes. Sci. Technol., 1990, 4, 431–439 CrossRef CAS.
- H. Noh, J.-S. Yu, J. Ko, J. M. Kim and S.-G. Oh, Bull. Korean Chem. Soc., 2017, 38, 890–898 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supplemental NMR and FTIR data, contact angle measurements, antimicrobial and tensile tests are provided. See DOI: https://doi.org/10.1039/d4lp00145a |
| This journal is © The Royal Society of Chemistry 2024 |