Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Multifunctional MgAl LDH/Zn-MOF S-scheme heterojunction: efficient hydrogen production, methyl red removal, and CO2 adsorption†
Ihsan
Maseeh‡
a,
Farheen
Anwar‡
a,
Sadia
Aroob
a,
Tariq
Javed
b,
Ismat
Bibi
a,
Afaf
Almasoudi
c,
Ahmad
Raheel
d,
Muhammad Arshad
Javid
e,
Sónia A. C.
Carabineiro
 f and
Muhammad Babar
Taj
f and
Muhammad Babar
Taj
 *a
*a
aInstitute of Chemistry, The Islamia University of Bahawalpur, Bahawalpur 63100, Pakistan. E-mail: dr.taj@iub.edu.pk
bDepartment of Chemistry, University of Sahiwal, Sahiwal 57000, Pakistan
cChemistry Department, Faculty of Science, King Abdulaziz University, P.O. Box 42734, Jeddah, Saudi Arabia
dDepartment of Chemistry, Quaid-e-Azam University, Islamabad 44000, Pakistan
eInstitute of Physics, The Islamia University Bahawalpur, Bahawalpur 63100, Pakistan
fLAQV-REQUIMTE, Department of Chemistry, NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal
First published on 9th May 2024
Abstract
Metal–organic frameworks (MOFs) and layered double hydroxides (LDHs) are undoubtedly promising and valuable materials for developing advanced catalysts to achieve efficient hydrogen evolution. The unique structures, environmentally friendly nature, and high redox activities of these materials make them ideal for catalytic applications. In this study, a delicately constructed S-scheme heterojunction photocatalyst, denoted as MgAl LDH/Zn-MOF, was designed and synthesized through the in situ nucleation of Zn-MOF nanostructure on MgAl LDH nanosheets, based on their excellent electronic properties and opposite surface potential. The MgAl LDH/Zn-MOF photocatalyst exhibited enhanced photocatalytic hydrogen evolution activity (129 mmol g−1) compared to Zn-MOF and MgAl LDH alone. This was mainly due to the formation of the MgAl LDH/Zn-MOF S-scheme heterojunction, which effectively accelerated the recombination of several electrons (from the conduction band of Zn-MOF) and holes (from the valence band of MgAl LDH), thus preventing the recombination of other electrons (from the conduction band of MgAl LDH) and holes (from the valence band of Zn-MOF), which is a critical requirement for efficient hydrogen evolution. Further, the MgAl LDH/Zn-MOF has a high potential for methyl red removal (97% following the intraparticle diffusion model with a maximum R2 value of 0.996). The CO2 adsorption isotherms of the MgAl LDH/Zn-MOF revealed a gravimetric CO2 uptake capacity of 129.7 mg g−1 (at 298 K and 40 bar) and stable cyclic adsorption performance. These findings demonstrate the potential of MOFs and LDHs for developing advanced catalysts for efficient hydrogen evolution and highlight the importance of heterojunction design.
1. Introduction
As our world continues to grow and develop, more people strive for a better quality of life.1 This is a positive trend, but unfortunately, it has led to a significant increase in energy consumption. This overconsumption can potentially lead to a devastating energy crisis, which could have far-reaching consequences for our planet.1 Along with this issue, we face several other environmental challenges, such as pollution, ecological destruction, increased greenhouse gas emissions, deforestation, and waste production. Our dependence on fossil fuels worsens these challenges, and this is a significant obstacle we must overcome to secure a sustainable future.2 To address these issues, we must focus on developing clean and sustainable energy sources with the necessary adsorption capacity, selectivity, and regeneration capacity. That can reduce our dependence on fossil fuels and mitigate their negative environmental impact. It is a challenging task, that we must take on in order to build a brighter future for ourselves and generations to come.3 Sunlight is the most significant and abundant energy source, whether directly or indirectly used. Nevertheless, the conversion and utilization of solar energy have not yet reached optimal levels. However, researchers have found a promising solution to this challenge – the light-induced water-splitting process. It involves splitting water molecules into hydrogen and oxygen with the help of sunlight, resulting in the production of hydrogen. This gas is known for its high calorific value, environmental sustainability, and exceptional conversion efficiency. It is a clean and efficient energy source that holds immense potential to tackle the environmental and energy-related issues we face today.4 Hydrogen has been identified as a feasible replacement for fossil fuels in electricity generation and transportation. The transition to a “hydrogen economy” requires innovative techniques for producing H2, with photocatalytic water splitting and alcohol photo reforming using sunlight being the most promising methods.Currently, various types of semiconductors are being investigated, such as zinc oxide (ZnO), titanium dioxide (TiO2), cadmium sulfide (CdS), graphitic carbon nitride (g-C3N4), and copper oxide (CuO). Such materials can help us create better and more efficient ways to use sunlight to generate energy.5 Other examples are the layered double hydroxides (LDHs). These are inorganic compounds with distinct two-dimensional layered structures that are attracting significant research interest due to cost-effective and facile preparation, well-designed structure, biocompatibility, and their ability to accommodate various guest species, such as organic and inorganic materials, nanoparticles, functional and polymers, thus being versatile for various applications.6 These materials are typically designed using divalent and trivalent metal cations, although LDHs with monovalent and tetravalent cations are also reported. The general representation of an LDH is [M1−x2+ Mx3+ (OH)2]x+ [An−]x/n, where M2+ represents divalent metal cations, like Cu2+, Mg2+, Ni2+, Zn2+, etc. M3+ refers to trivalent metal cations, like Al3+, Fe3+, Ga3+, Mn3+, etc. An− is an inorganic or organic anion that neutralizes the charge, such as SO42−, Cl−, NO31−, etc. LDHs are cost-effective, abundant, and safe to use.7 They are an exceptional class of two-dimensional clay materials uniquely suited for various applications,8 such as energy storage, drug delivery, and photocatalysis, which uses sunlight to create chemical reactions.9 LDHs exhibit high stability and a basic surface, which is of utmost importance in adsorption, making them incredibly promising materials for CO2 adsorption.10 However, LDHs have some limitations, such as poor crystalline structure and low electrical conductivity. The aggregation of irreversible exfoliated nanosheets in LDH unequivocally hinders the migration of photo-excited electrons and holes, leading to the undeniable suppression of photocatalytic reactions.11,12 Different routes can be taken to improve LDHs performance by changing their structure and combining them with other materials. The formation of heterojunctions with other semiconductors is the most promising strategy for overcoming the shortcomings of the LDH photocatalyst in terms of charge separation and transfer efficiency.13
Metal–organic frameworks (MOFs) are highly porous materials with potential applications in catalysis, sensors, drug delivery, gas separation, and storage.14 They are formed by arranging organic ligands and metal atomic cluster structures.15 Different synthesis methods have been developed for MOFs, including ultrasonic synthesis and micro-emulsion technology.15 Polycarboxylate organic ligands are effective building blocks.16 In addition, MOFs are a type of coordination polymers with a crystal structure that offers potential porosity. This research field has rapidly developed in the past two decades.17 MOFs can immobilize active functional materials and manufacture highly controllable nanostructures as carriers, providing new materials for energy applications.18 A new photocatalyst, MgAl LDH/Zn-MOF, successfully designed and synthesized, is reported in this work. This material is a unique combination of Zn-based terephthalate frameworks with LDH (MgAl LDH), with excellent performance in photocatalytic hydrogen evolution due to its fast electron transfer. Moreover, it is also efficient in removing methyl red dye from water and CO2 from a gas stream.
2. Material and methods
2.1. Materials
The adsorbents used in this study were prepared from high-purity chemicals. The precursors used for the synthesis of the adsorbents included magnesium nitrate (Mg(NO3)2·6H2O, 99% purity, Sigma Aldrich), aluminum nitrate (Al(NO3)2·9H2O, 99% purity, Sigma Aldrich), sodium hydroxide (NaOH, 98% purity, Sigma Aldrich), zinc nitrate (Zn(NO3)2·6H2O, 99% purity, Sigma Aldrich), and benzene-1,4-dicarboxylic acid (C8H6O4, Sigma Aldrich). The dye used for adsorption was methyl red (C15H15N3O2, Sigma Aldrich). All chemicals were of high purity and were used without further treatment. The salt solutions were prepared using deionized water.2.2. Synthesis of MgAl LDH
The synthesis of MgAl LDH (molar ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was performed by co-precipitation. Firstly, a 20 mmol solution of Mg(NO3)2·6H2O and a 10 mmol solution of Al(NO3)2·9H2O were prepared in separate beakers using 40 ml of deionized water each. In a separate beaker, 10 mmol of benzene-1,4-dicarboxylic acid (BDC) was dissolved in 70 ml of deionized water, and then 10 mmol of sodium hydroxide was added to the solution. The aqueous solutions of Mg(NO3)2·6H2O and Al(NO3)2·9H2O were slowly added dropwise to the benzene-1,4-dicarboxylic acid (BDC) solution under continuous stirring for 18 h. The pH of the solution was maintained at 10 by adding NaOH solution dropwise. The resulting white precipitate (BDC intercalated MgAl LDHs) was filtered and washed with deionized water to remove impurities. The precipitate was dried in a vacuum desiccator at room temperature.
1) was performed by co-precipitation. Firstly, a 20 mmol solution of Mg(NO3)2·6H2O and a 10 mmol solution of Al(NO3)2·9H2O were prepared in separate beakers using 40 ml of deionized water each. In a separate beaker, 10 mmol of benzene-1,4-dicarboxylic acid (BDC) was dissolved in 70 ml of deionized water, and then 10 mmol of sodium hydroxide was added to the solution. The aqueous solutions of Mg(NO3)2·6H2O and Al(NO3)2·9H2O were slowly added dropwise to the benzene-1,4-dicarboxylic acid (BDC) solution under continuous stirring for 18 h. The pH of the solution was maintained at 10 by adding NaOH solution dropwise. The resulting white precipitate (BDC intercalated MgAl LDHs) was filtered and washed with deionized water to remove impurities. The precipitate was dried in a vacuum desiccator at room temperature.
2.3. Synthesis of MgAl LDH supported Zn-MOF nanocomposite
To obtain the MgAl-LDH/Zn-MOF nanocomposite (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), 3 g of MgAl LDH was dispersed in 50 ml of dimethylformamide. Then, a 8.99 mmol of Zn(NO3)2·6H2O solution was prepared and added to the above solution. The mixture obtained was refluxed at 120 °C for 1 hour. This led to the creation of highly dispersed Zn-MOFs nanostructure through in situ nucleation and growth on MgAl LDH nanosheets. The blue-colored solution was then filtered, washed, and dried at room temperature in a vacuum desiccator. Zn-MOF was also prepared using BDC19 according to the reported method for comparative study of H2 evolution.
3), 3 g of MgAl LDH was dispersed in 50 ml of dimethylformamide. Then, a 8.99 mmol of Zn(NO3)2·6H2O solution was prepared and added to the above solution. The mixture obtained was refluxed at 120 °C for 1 hour. This led to the creation of highly dispersed Zn-MOFs nanostructure through in situ nucleation and growth on MgAl LDH nanosheets. The blue-colored solution was then filtered, washed, and dried at room temperature in a vacuum desiccator. Zn-MOF was also prepared using BDC19 according to the reported method for comparative study of H2 evolution.
2.4. Characterization
The synthesized samples were subjected to various characterization techniques to determine their structural, surface, and morphological properties. The techniques used include X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-vis spectroscopy, fluorescence, Fourier transform infrared spectroscopy (FTIR), N2 adsorption at 77 K and zeta potential (PZC). The crystalline structure and size of the adsorbents were characterized by powder XRD on a Bruker D8 Advance instrument, using Cu-Kα radiation with a wavelength of 1.541 Å. The vibrational frequencies of the prepared composites were determined using FTIR spectroscopy (Tensor 27 FTIR) in the range of 400 to 4000 cm−1. The synthesis of MgAl LDH and MgAl-LDH/Zn-MOF was confirmed using a Cecil 7500 UV-vis spectrophotometer in the 200 to 800 nm range. The surface and morphological features of the composites were analyzed using SEM (MIRA-III TESCON). The pore size and specific surface area of MgAl-LDH/Zn-MOF were calculated by using surface area analyzer Gemini 2375, Shimadzu. To test the stability and surface charge of MgAl-LDH/Zn-MOF, a Zetasizer equipment (Nano-ZS, Malvern Instruments, UK) was used. Impedance spectroscopy (EIS) using electrochemical techniques was conducted by applying a 10 mV amplitude within a frequency range of 1 MHz to 1.0 Hz while keeping the potential constant at 200 mV (vs. Ag/AgCl). The working electrolyte used in the analysis was 0.1 M sodium sulfate dissolved in water. The electronic band structures were analyzed using ultraviolet photoemission spectroscopy (UPS) on a AXIS-Nova, Kratos Analytical Ltd; monochromatic (He I = 21.2 eV, Ag 3d5/2 < 100 meV). The high-pressure CO2 adsorption performance was analyzed on an IsoSORP adsorption analyzer (TA instruments, New Castle, DE, USA).2.5. Hydrogen production method
Experiments were conducted with precision to produce hydrogen through photocatalysis using a Pyrex reactor that was capable of being degassed and sampled. (The procedure used was similar to the one reported in the literature.20,21) The process involved the addition of 10 mg of catalytic material, 20 mg of Eosin Y (EY), and 20 ml (10% v/v) of triethanolamine (TEOA) into the reactor. Before exposing the solution to visible light, the excess gas was removed through vacuuming. The amount of hydrogen evolved was then analyzed using a gas chromatograph (Tianmei GC7900, with N2 as the carrier) at different time intervals, using gas headspace samples (0.5 ml) injected into the gas chromatograph. The H2 produced was quantified against an internal calibration curve. The rate of gas (H2) evolution was measured in units of mmol g−1 and mmol g−1 h−1 to enable confident comparison between MgAl LDH, Zn-MOF, and MgAl LDH/Zn-MOF photocatalysts under identical conditions. The experiment was repeated at least three times for each sample to ensure accuracy beyond doubt. The external quantum efficiency (EQE) under identical photocatalytic reaction conditions, was calculated using the following eqn (1).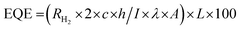 | (1) |
2.6. Methyl red adsorption studies
Studying dye adsorption under room temperature and atmospheric pressure is of significant interest.22–24 A series of adsorption experiments were conducted in dark conditions to evaluate the effectiveness of the prepared adsorbents for removing methyl red (MR) dye. In each experiment, 25 mg of the prepared adsorbent was added to 25 ml of 5 × 10−5 M MR dye solution, and the mixture was stirred at 298 K for different reaction times. At 20 min intervals, a small sample of the mixture was removed for UV-visible analysis.The adsorbent was separated from the mixture by centrifugation, and the remaining solution was analyzed using an ultraviolet-visible (UV-vis) spectrophotometer (Cecil 7500) to determine the concentration of MR dye at equilibrium (Ce) at 410 nm. After the adsorption reaction, the adsorbent was washed, filtered, and dried in an oven at 343 K.
The removal efficiency (R) and adsorption capacity (q) of the prepared adsorbents for MR dye can be calculated using the following eqn (2) and (3):25
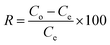 | (2) |
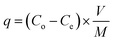 | (3) |
To examine the rate at which adsorption occurs, we analyzed experimental data using different models, such as pseudo-first-order kinetics, pseudo-second-order kinetics, liquid-film diffusion model, and intra-particle diffusion model. We also applied various isothermal models to the data, including the Langmuir, Freundlich, and Temkin models, to gain insight into the mechanism of dye removal. Additionally, we calculated the changes in Gibbs free energy (ΔGo, kJ mol−1), entropy (ΔSo, kJ mol−1 K−1), and enthalpy (ΔHo, kJ mol−1). (see ESI,† Section S1).
2.7. CO2 adsorption performance
Experiment was conducted to study the adsorption of CO2 using a previously reported procedure.26 The experiment involved degassing 0.5 g of adsorbent at 383 K under high vacuum for 4 h. The sample mass and volume were subsequently determined using a buoyancy test with He at 295 K. The CO2 adsorption–desorption isotherm of the MgAl LDH/Zn-MOF composite was then measured by dosing CO2 from a high vacuum to 40 bar, followed by a high vacuum with an equilibrium point every 10 bar. The equilibrium for each step was maintained until the deviation of mass was less than 0.1 mg per 10 min. The results of this experiment provided valuable insight into the adsorption behavior of CO2 and its potential applications in various industries.3. Results and discussion
3.1. PXRD analysis
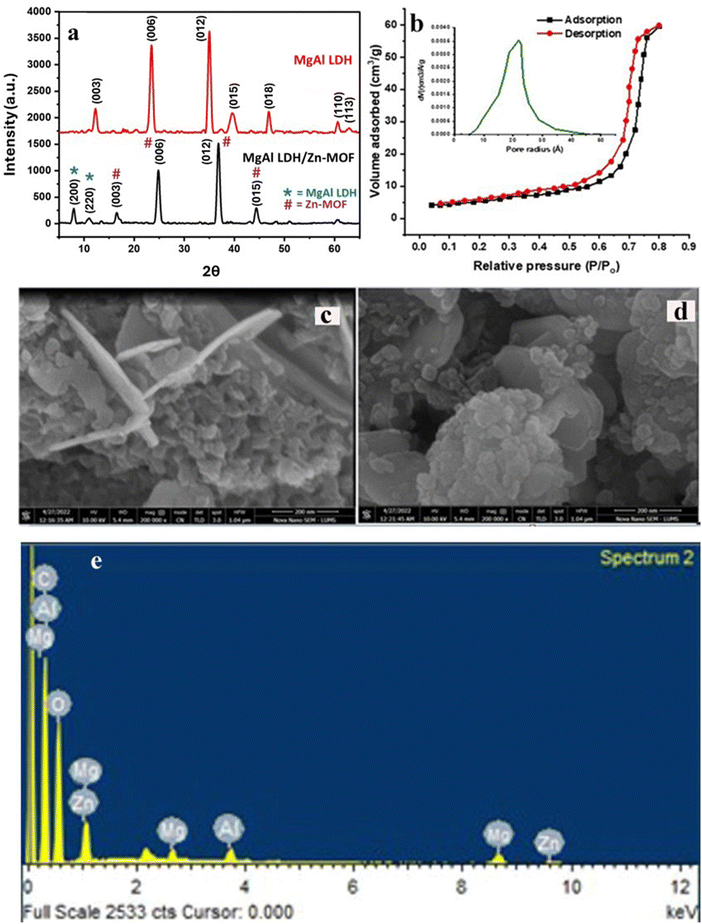
The phase structure and crystallinity of the synthesized nanocomposite were analyzed using XRD. Fig. 1(a) shows the MgAl LDH and MgAl-LDH/Zn-MOF nanocomposite diffractograms. The diffractogram of Zn-MOF (Fig. S2a, ESI†) shows 2θ values at 8.20, 10.52, and 13.28, which are assigned to the lattice planes of (200), (220), and (400), respectively, in agreement with the literature.27–29 The presence of BDC between the layer of LDH is confirmed by the 2θ shift of the characteristic peak (003)30 from 11.90° to 15.36° as shown in Fig. 1(a). This shifting could be attributed to the replacement of BDC ions from the interlayer region and coordinated to Zn(II) ions.30 Generally speaking, the shifting of 2θ is linked to the change in lattice parameters, microstrain and crystal size.The diffraction pattern of pure LDH exhibited characteristic peaks at 11.90°, 23.40°, 35.13°, 39.78°, 46.97°, 60.95°, and 62.90°, representing the (003), (006), (012), (015), (018), (110), and (113) crystal planes, respectively, which have been well indexed using a typical hexagonal MgAl-LDH (JCPDF 22-0452).31 Characteristic diffraction peaks representing the LDH were evidently displayed in the XRD spectrum of MgAl-LDH/Zn-MOF. Diffraction peaks at 2θ = 6.59°, 9.81°, 15.36°, 26.59°, 35.78°, and 43.64° were observed for MgAl-LDH/Zn-MOF, which were assigned to (200) (220), (003), (006), (012), and (015) lattice planes. The presence of major diffraction peaks of Zn-MOF at 6.59° and 9.81° are assigned to the lattice planes of (200) and (220), respectively.19,27 Interestingly, MgAl-LDH/Zn-MOF shows the shift in 2θ value to a higher angle, indicating the replacement of large BDC ions from the interlayer region.29 The crystallite size of MgAl LDH and MgAl LDH/Zn-MOF nanocomposite was estimated as 15 nm and 38.46 nm, respectively, using Debye–Scherrer's eqn (4),23 as follows.
D = k λ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (4) |
d = nλ/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (5) |
3.2. N2 adsorption analysis at 77 K
The effect of the increased surface area on the characteristics of nanocomposites is an area of growing importance for understanding, developing, and improving materials for various applications, especially in adsorption.32 The main objective of catalytic reactions is to increase the reaction rate while maintaining high product yield and selectivity.33 This goal can be achieved by developing a composite that provides many active sites for adsorption processes.The surface area and pore volume were examined using the multipoint Brunauer–Emmett–Teller (BET) method (Fig. 1(b)) to investigate the porosity of MgAl LDH/Zn-MOF. The average pore size for the nanocomposite was determined by the Dubinin–Astakhov (DA) plot, as shown in Fig. 1(b) (inset). The MgAl LDH/Zn-MOF composite was found to have a pore volume and an average pore radius of 0.045 cm3 g−1 and 20.7 Å, respectively.
3.3. Morphology
The average grain size of the obtained MgAl LDH and MgAl LDH/Zn-MOF was determined using SEM, as shown in Fig. 1(c) and (d). The images reveal a flat, non-uniform, plate-like structure of MgAl LDH sheets lying in layers, with Zn-MOF particles organized as spherical aggregates and arranged on the MgAl LDH sheet, with an average grain size of 34.2 nm. The SEM image of Zn-MOF is given in Fig. S2c (ESI†). It is evident from the EDS study (Fig. 1(e)) that the MgAl-LDH/Zn-MOF structure contains significant amounts of Mg, Al, Zn, and O. However, a notable presence of carbon is attributed to the BDC anions.3.4. FTIR spectral analysis
The FTIR spectra of the prepared samples, namely MgAl LDH and MgAl LDH/Zn-MOF, are presented in the ESI,† Fig. S1. The broad peak observed at 3459 cm−1 and 3520 cm−1 for MgAl LDH and MgAl LDH/Zn-MOF, respectively, corresponds to the stretching vibration of O–H due to the presence of water molecules. The sharp, weak peaks at 1559 cm−1 and 1572 cm−1 are attributed to O–H bending vibration and aromatic ring of BDC for MgAl LDH and MgAl LDH/Zn-MOF, respectively. The peaks 1050 cm−1, 1097 cm−1, and 1117 cm−1 presented the –C–OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–H groups of BDC. In addition, the vibrational peaks observed between 400 cm−1 to 800 cm−1 correspond to the stretching peaks of M–O–M and O–M–O vibrations, representing the Mg–O–Mg and Al–O–Al layers. The vibrational peak34–36 observed near 400 cm−1 confirms the presence of zinc in MgAl LDH/Zn-MOF, as shown in ESI† (Fig. S1). The FTIR spectrum of Zn-MOF (Fig. S1, ESI†) confirms the synthesis of this material.
O, and C–H groups of BDC. In addition, the vibrational peaks observed between 400 cm−1 to 800 cm−1 correspond to the stretching peaks of M–O–M and O–M–O vibrations, representing the Mg–O–Mg and Al–O–Al layers. The vibrational peak34–36 observed near 400 cm−1 confirms the presence of zinc in MgAl LDH/Zn-MOF, as shown in ESI† (Fig. S1). The FTIR spectrum of Zn-MOF (Fig. S1, ESI†) confirms the synthesis of this material.
3.5. Optical properties of the resulting samples
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the Zn-MOF. On the other hand, the electronic transitions of MgAl LDH/Zn-MOF are observed at higher wavelengths of 270 nm and 329 nm, which correspond to the π to π* and n to π* transitions of the BDC ligand. This red shift in the electronic transitions can be attributed to the decreased π electron density of Zn metal. The weak absorbance observed in the UV spectra of MgAl LDH/Zn-MOF can be attributed to the irregular arrangement of BDC anions. Moreover, the weak transition of C
O bonds in the Zn-MOF. On the other hand, the electronic transitions of MgAl LDH/Zn-MOF are observed at higher wavelengths of 270 nm and 329 nm, which correspond to the π to π* and n to π* transitions of the BDC ligand. This red shift in the electronic transitions can be attributed to the decreased π electron density of Zn metal. The weak absorbance observed in the UV spectra of MgAl LDH/Zn-MOF can be attributed to the irregular arrangement of BDC anions. Moreover, the weak transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O observed in the spectrum of MgAl LDH/Zn-MOF indicates the intercalation of BDC anions into zinc metal.30 The presence of a peak at ≈373 nm in the UV-vis spectrum (Fig. S2b, ESI†) of Zn-MOF confirms its synthesis.
O observed in the spectrum of MgAl LDH/Zn-MOF indicates the intercalation of BDC anions into zinc metal.30 The presence of a peak at ≈373 nm in the UV-vis spectrum (Fig. S2b, ESI†) of Zn-MOF confirms its synthesis.
| ∝hν = A(hν − Eg)n/2 | (6) |
3.6. Zeta potential and point of zero charge (PZC)
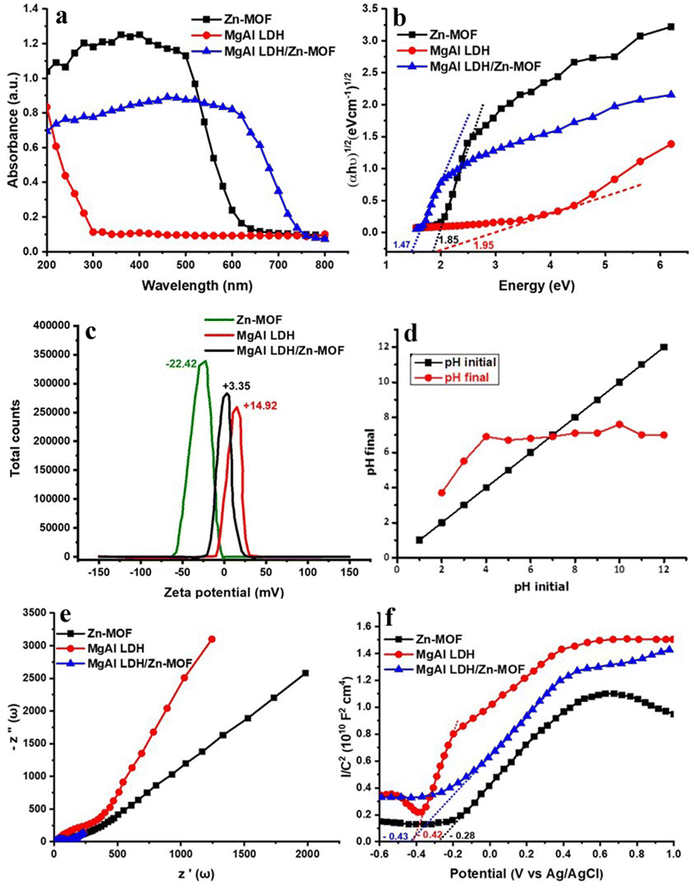
The stability of MgAl LDH/Zn-MOF was evaluated by measuring its zeta potential, which is displayed in Fig. 2(c). The zeta potential measures the electrostatic repulsion between particles and is indicative of their tendency to aggregate and overall stability.40 The average zeta potential of MgAl LDH was approximately 14.92 mV, while Zn-MOF had an average zeta potential of about −22.42 mV. On the other hand, the zeta potential of MgAl LDH/Zn-MOF was around 3.35 mV, which is intermediate between that of Zn-MOF and MgAl LDH. This phenomenon occurs due to the presence of opposite surface potentials between MgAl LDH and Zn-MOF, which strengthens the coupling force between them. Zn-MOF, being larger in size and having a negative surface zeta potential, indicating the formation of a new catalyst distinct from either MgAl LDH or Zn-MOF. This process enhances the proton absorption of MgAl LDH/Zn-MOF and promotes the efficiency of hydrogen evolution under visible light.The pH point of zero charges (pHpzc) is a measurement that reveals the adsorption capacity of an adsorbent and the type of its surface-active center. Essentially, the pHpzc value of an adsorbent indicates the pH at which the adsorbent surface has no net charge.41 When the number of positive and negative charges are equal, an adsorbent's surface reaches a zero point of charge (PZC), meaning it has no preference for attracting either positively or negatively charged substances. To determine the PZC, pH initial vs pH final plot is done and the point of intersection is identified, which is 7.1 in Fig. 2(d). This value is significant because it is the pH at which the adsorbent's surface carries no net charge, allowing it to adsorb/attract both positively and negatively charged substances equally.
3.7. Hydrogen evolution measurements
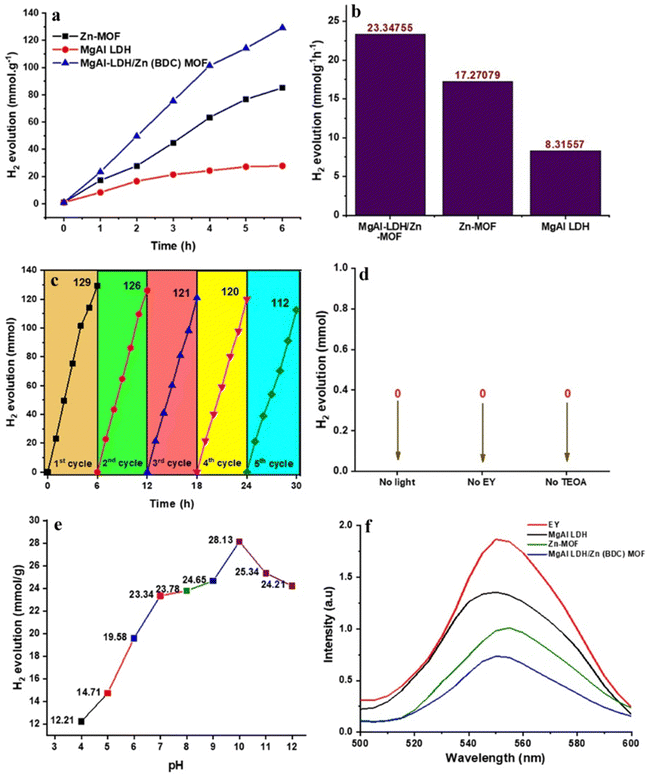
The present study compares the hydrogen evolution properties of two materials, namely magnesium–aluminum layered double hydroxide (MgAl LDH) and zinc-based metal–organic framework (Zn-MOF). The reaction occurs under visible light irradiation in a robust environment with a pH level of 10 (Fig. 3(a)–(f)). The photocatalytic hydrogen generation activity of both materials is low when used separately due to poor electron–hole pair separation and transfer efficiency under light. It has been discovered that when MgAl LDH and Zn-MOF are combined, they exhibit significantly better photocatalytic activity compared to when any of the materials is used alone. This suggests that there is a strong interaction between the two materials which leads to better hydrogen evolution. The yields for Zn-MOF and MgAl LDH are 85.07 mmol and 27.82 mmol, respectively. In contrast, the combined material MgAl LDH/Zn-MOF demonstrated hydrogen evolution activities that were 1.52 and 4.65 times higher than those of Zn-MOF and MgAl LDH alone, respectively. We have compared the effectiveness of MgAl LDH/Zn-MOF photocatalyst with various other MOF-based photocatalysts that have been previously reported in literature. The results of these comparisons are tabulated in ESI† (Table S1).The superior photocatalytic activity of MgAl LDH/Zn-MOF can be attributed to the rapid separation and migration of charge carriers induced by visible light. This is facilitated by the strong interaction between the two materials. Additionally, the incorporation of MgAl LDH improves the light absorption of Zn-MOF, while the incorporation of Zn-MOF helps to prevent the agglomeration of MgAl LDH nanolayers, thus assisting in electron migration during photocatalytic water-splitting reactions. These findings demonstrate that the MgAl LDH/Zn-MOF composite has promising potential for use as a photocatalyst in various applications, including hydrogen production and environmental remediation.
The combination of MgAl LDH and Zn-MOF creates an S-scheme heterojunction which enhances the separation efficiency of electron–hole pairs in space. This leads to a higher efficiency in generating photocatalytic hydrogen compared to using the materials individually. The hydrogen evolution mechanism section will provide detailed information on the specific migration process of light-excited electrons between MgAl LDH and Zn-MOF. To ensure the stability of MgAl LDH/Zn-MOF, EY was added in 5 mg increments in each cycle, to avoid any excessive shielding effects on the photocatalytic activity of hydrogen evolution (Fig. 3(c)). The experiment was conducted over five consecutive testing phases, where the photocatalyst was collected, washed, and dried after each cycle. The MgAl LDH/Zn-MOF demonstrated a consistent H2 production rate of 112 mmol g−1 even after five cycles, proving its good reusability. The research findings suggest that the combination of MgAl LDH and Zn-MOF results in a highly stable photocatalyst that can efficiently generate hydrogen through long-term photocatalysis. The study highlights the significant role of EY in the process of photocatalytic water-splitting to evolve hydrogen. Moreover, the hydrogen evolution activity of the catalyst was tested under different reaction conditions, which revealed its strong selectivity for hydrogen evolution systems. It was observed that the catalyst is unable to produce hydrogen in the absence of light, EY, or TEOA. These findings are illustrated in Fig. 3(d), which demonstrates the dependence of hydrogen evolution on the presence of light, EY, and TEOA. The pH of a solution affects hydrogen generation activity. To test this, 10% TEOA (sacrificial agent) was taken along with different acidity levels (using HCl/NaOH) in the solution. The results (Fig. 3(e)) show that the hydrogen production rate decreases in acidic solutions, and the best pH level for producing hydrogen is 10. If the pH level is too high, the hydrogen production rate also decreases due to defects in the photocatalyst surface. Additionally, when too many OH− ions are present, they can react with photogenerated H+ ions to produce water, slowing hydrogen production. The fluorescence spectra of three catalysts (MgAl LDH, Zn-MOF, and MgAl LDH/Zn-MOF) were examined to understand the separation and transfer of charge carriers in the MgAl LDH/Zn-MOF heterojunction catalyst (as shown in Fig. 3(f)). The fluorescence emission intensity of EY is an indicator of the recombination of electron–hole pairs. The results show that when the catalyst samples are introduced, there is a decrease in fluorescence emission intensity, indicating a reduction in the recombination of electron–hole pairs. It appears that there is a strong interaction between EY and the catalyst, resulting in the reduction of fluorescence when EY was exposed to light. The addition of MgAl LDH/Zn-MOF heterojunction catalyst results in the lowest fluorescence emission intensity. This is a sign of successful design and construction of the S-scheme heterojunction, which facilitated charge migration and effectively prevented the recombination of electron–hole pairs. As a result, the hydrogen evolution activity is significantly enhanced. In order to evaluate the effectiveness of the synthesized photocatalysts, it is necessary to determine their catalytic efficiency. The turnover number (TON)42 can be used as a metric for this purpose, and it can be calculated using eqn (7). The TON values of Zn-MOF, MgAl LDH, and MgAl LDH/Zn-MOF are 1.4, 4.3, and 6.5 respectively, while the rate of H2 production is 8.32, 17.27, and 23.34 mmol g−1 h−1 respectively (Fig. 3(b)).
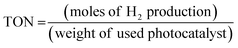 | (7) |
The results from zeta potential, fluorescence, and EIS studies support the superior hydrogen evolution performance of MgAl LDH/Zn-MOF catalysts. The Mott–Schottky curve indicates that Zn-MOF's flat band potential (Efb) is approximately −0.28 V versus Ag/AgCl, while MgAl LDH's is −0.42 V (Fig. 3(f)). All MgAl LDH, Zn-MOF, and MgAl LDH/Zn-MOF samples all exhibit positive slopes, indicating that they have n-type semiconductor characteristics.46,47 In addition, for n-type semiconductors, the conduction band potential (ECB) is typically negative48–50 by about 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V, compared to the Efb. Therefore, it can be confirmed that the ECB of Zn-MOF and MgAl LDH are −0.48
V, compared to the Efb. Therefore, it can be confirmed that the ECB of Zn-MOF and MgAl LDH are −0.48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V and −0.62
V and −0.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V, respectively. Valence band positions (EVB) were determined (eqn (8)) using UV-VIS/DRS (Fig. 3(b)) and M–S techniques (Fig. 3(f)), as mentioned in the literature.49,51 Notably, the M–S findings provided substantial evidence to clarify the electron transfer mechanism discussed in this study.51
V, respectively. Valence band positions (EVB) were determined (eqn (8)) using UV-VIS/DRS (Fig. 3(b)) and M–S techniques (Fig. 3(f)), as mentioned in the literature.49,51 Notably, the M–S findings provided substantial evidence to clarify the electron transfer mechanism discussed in this study.51
| EVB = ECB + Eg | (8) |
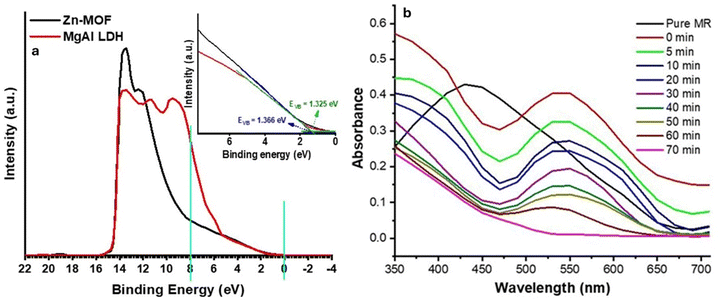
The terms ECB and EVB refer to the potential of the conduction and valence bands, respectively. The EVB values of Zn-MOF and MgAl LDH were calculated as 1.37 and 1.33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV, respectively. To confirm the absolute position of EVB for Zn-MOF and MgAl LDH, UPS spectra (Fig. 5(a)) were obtained. The low binding energy area (valence band region near the Fermi level) was observed to calculate the valence band position.52–54 The measured EVB values are 1.325 eV for Zn-MOF and 1.366 eV for MgAl LDH. These results agree with the above calculated EVB values.
eV, respectively. To confirm the absolute position of EVB for Zn-MOF and MgAl LDH, UPS spectra (Fig. 5(a)) were obtained. The low binding energy area (valence band region near the Fermi level) was observed to calculate the valence band position.52–54 The measured EVB values are 1.325 eV for Zn-MOF and 1.366 eV for MgAl LDH. These results agree with the above calculated EVB values.
It has been verified through charge analysis of the band that the formation of an intrinsic electric field (IEF) was facilitated at the interface by electron transfer of 0.04 eV from MgAl LDH to Zn-MOF, which resulted in the participation of photogenerated electrons in the hydrogen production process. The Zn-MOF/MgAl LDH heterojunction allows for the movement of electrons from VB to CB because both materials are UV-vis-responsive semiconductors.55 The irradiation facilitates this process (Fig. 4). Afterward, the excited electrons on the weakly reducing Zn-MOF combine with the excited holes on the weakly oxidizing MgAl LDH. The retention of photoexcited solid electrons and holes in the composite of MgAl LDH/Zn-MOF allows the creation of an S-scheme heterojunction.56 Through experimental results, the generation of IEF is demonstrated while also confirming the charge separation path of the S-scheme heterojunction.55
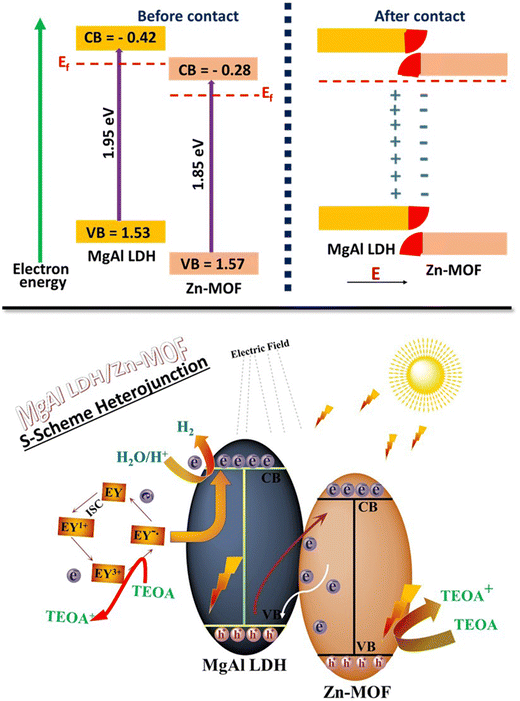 | ||
| Fig. 4 The internal mechanism of migration of electrons within the MgAl LDH/Zn-MOF heterojunction in the S-scheme. | ||
The transfer of charge in a heterojunction of S-scheme can be described in the following way.57,58
(1) Fig. 4 indicates that MgAl LDH has a higher CB and VB position and a more minor work function than Zn-MOF. When these two semiconductors are nearby, electrons in MgAl LDH diffuse spontaneously to Zn-MOF, creating an electron depletion layer and an electron accumulation layer near the interface of MgAl LDH and Zn-MOF, respectively. Electrons are transferred to Zn-MOF, causing it to be negatively charged, while MgAl LDH becomes positively charged. This generates an electric field from MgAl LDH to Zn-MOF, which facilitates the movement of photogenerated electrons from Zn-MOF to MgAl LDH.
(2) The alignment of Fermi energy is necessary when Zn-MOF and MgAl LDH come into contact. This alignment causes the Fermi levels of Zn-MOF to shift upwards and those of MgAl LDH to shift downward. As a result of the band-bending effect, the photogenerated electrons in the CB of Zn-MOF and holes in the VB of MgAl LDH tend to recombine at the interface region. This phenomenon can be easily understood by the analogy of water flowing downhill.
(3) The interface between the CB of Zn-MOF and the VB of MgAl LDH causes photogenerated electrons and holes to recombine due to Coulombic attraction. The internal electric field, band bending, and Coulombic attraction drive the recombination. During this process, unnecessary electrons and holes are removed. However, the potent photogenerated electrons in the CB of MgAl LDH and the holes in the VB of Zn-MOF are preserved for the purpose of photocatalytic reactions.59–61
3.8. Dye removal experiments
The present study displays the UV-visible spectra of a freshly prepared aqueous solution of MR. It is characterized by a dominant absorption band at 431 nm, as demonstrated in Fig. 5(b). When MgAl LDH/Zn-MOF is added to the solution, the peak shifts from 431 nm to 538 nm, resulting in a redshift of approximately 107 nm. Interestingly, the addition of MgAl LDH/Zn-MOF also changes the color of the solution from yellow to red and the pH from 6.7 to 5.0 (Fig. 6(a)). The decrease in intensity over time is due to the adsorption of MR with MgAl LDH/Zn-MOF. The absorption peak in the visible region is caused by the chromophore, which includes the azo linkage (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–). This is due to the n → π* transition that occurs because of the –N
N–). This is due to the n → π* transition that occurs because of the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– group. At least three adsorption experiments were conducted to ensure data accuracy and reproducibility. Blank experiments were also conducted in parallel and corrected as needed.56
N– group. At least three adsorption experiments were conducted to ensure data accuracy and reproducibility. Blank experiments were also conducted in parallel and corrected as needed.56
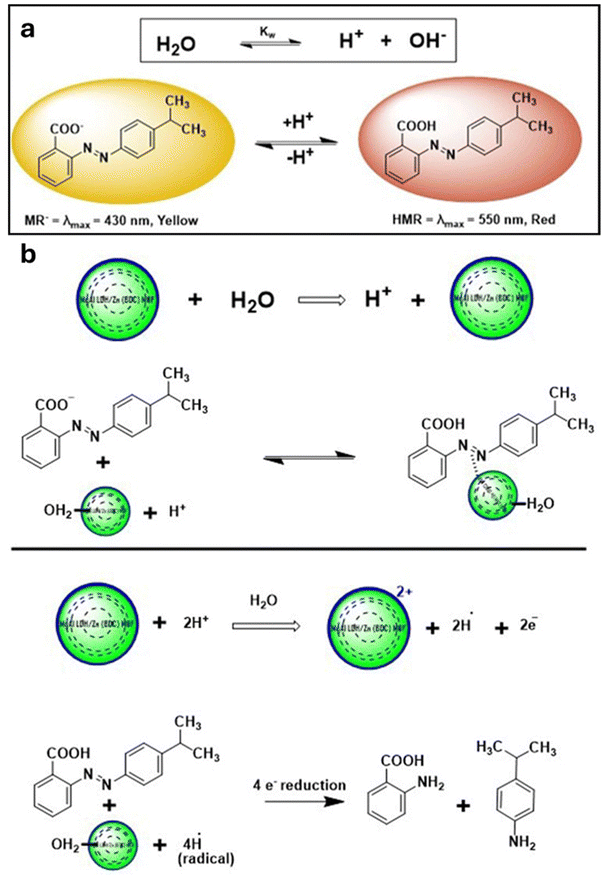 | ||
| Fig. 6 (a) At 25 °C, the presence of HMR and MR− species in water. (b) Degradation mechanism of MR by MgAl LDH/Zn-MOF. | ||
3.9. Factors affecting the adsorption process
3.10. Comparison with other studies
In ESI,† Table S2 shows a compilation of literature results regarding the adsorption of MR using different adsorbents. While many studies focus on the degradation of MR, it is noteworthy that our investigation revealed a maximum degradation efficiency of 98% using nanocomposites synthesized by the calcination method, which is associated with the significant drawback of lower yields. Considering the limitations of synthetic catalyst methods, degradation time, and degradation efficiency, MgAl LDH/Zn-MOF nanocomposites synthesis is a highly productive and demanding approach.3.11. Mechanism of MR removal
There are two possible mechanisms for removing MR, and we will discuss each of them below.(1) Adsorption is a process that involves the transfer of electrons between the adsorbent surface and the substance being adsorbed, resulting in the formation of a bond between the two.66 The strength of this bond between the adsorbent and the adsorbate can significantly impact the chemisorption energy required for the process. The adsorption process is inherently slow and requires equilibrium to be reached, typically resulting in a single layer of adsorbate on the surface of the adsorbent. An efficient adsorbent must exhibit high adsorption kinetics, resilience to various conditions such as high pressure and temperature and require minimal maintenance.67 Regarding MR dye, the molecules are adsorbed on the surface of the adsorbent and diffuse to the inner portion. The adsorption process depends on various factors, such as bonding type, surface interactions, and adsorbent nature. A variety of kinetic models, including pseudo-first-order kinetics, pseudo-second-order kinetics, liquid-film diffusion model, and intra-particle diffusion model, have been used to evaluate the mechanistic studies of the adsorption of MR dye on the surface of the hybrid composite that has been fabricated. Table S3 (ESI†) presents the values obtained from these kinetic models, which confirm that the current adsorption mechanism is chemisorption.68,69
(2) MR is used as a pH indicator, and it is crucial to thoroughly examine their reactive and predominant species. In aqueous solutions, the presence of –COOH and –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– groups leads to a balance between HMR and MR− species. Fig. 6(b) shows the Fenton-like reaction of MgAl LDH/Zn-MOF in an aqueous solution, which generates hydrogen ions. The adsorption of MR onto the surface of MgAl LDH/Zn-MOF was observed to cause a remarkable shift of λmax from 431 nm to 538 nm, which can be attributed to the protonation of the dye. This finding stands as the most significant outcome of the study, as depicted in Fig. 5(b). The reaction of MgAl LDH/Zn-MOF with H2O and/or H+ generates hydrogen radicals as shown in Fig. 6. According to Florence, the reduction of aromatic azo compounds is a four-electron process that results in the formation of corresponding aromatic amines via an unstable intermediate, hydrazobenzene (–NH–NH–), which is more labile than the parent –N
N– groups leads to a balance between HMR and MR− species. Fig. 6(b) shows the Fenton-like reaction of MgAl LDH/Zn-MOF in an aqueous solution, which generates hydrogen ions. The adsorption of MR onto the surface of MgAl LDH/Zn-MOF was observed to cause a remarkable shift of λmax from 431 nm to 538 nm, which can be attributed to the protonation of the dye. This finding stands as the most significant outcome of the study, as depicted in Fig. 5(b). The reaction of MgAl LDH/Zn-MOF with H2O and/or H+ generates hydrogen radicals as shown in Fig. 6. According to Florence, the reduction of aromatic azo compounds is a four-electron process that results in the formation of corresponding aromatic amines via an unstable intermediate, hydrazobenzene (–NH–NH–), which is more labile than the parent –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– compound.70 The multi-electron transfer oxidation–reduction mechanism (Fig. 6(b)) observed in the MgAl LDH/Zn-MOF complex is in complete agreement with the previous findings.71 The azo double bond (–N
N– compound.70 The multi-electron transfer oxidation–reduction mechanism (Fig. 6(b)) observed in the MgAl LDH/Zn-MOF complex is in complete agreement with the previous findings.71 The azo double bond (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) was successfully transformed into –NH2, resulting in a gradual decrease in the visible absorption peak of MR at 538 nm over time. Throughout the process, it is worth noting that MgAl LDH/Zn-MOF played a crucial dual role. Specifically, it was able to generate hydrogen radicals while simultaneously transferring these radicals to the MR via the electron relay effect. Fig. 6(b) mechanism strongly supports the proposals of Miller et al. regarding the catalytic reduction of water on the surface of a colloidal metal.72 Unfortunately, we did not find any degraded product on the surface of MgAl LDH/Zn-MOF.
N–) was successfully transformed into –NH2, resulting in a gradual decrease in the visible absorption peak of MR at 538 nm over time. Throughout the process, it is worth noting that MgAl LDH/Zn-MOF played a crucial dual role. Specifically, it was able to generate hydrogen radicals while simultaneously transferring these radicals to the MR via the electron relay effect. Fig. 6(b) mechanism strongly supports the proposals of Miller et al. regarding the catalytic reduction of water on the surface of a colloidal metal.72 Unfortunately, we did not find any degraded product on the surface of MgAl LDH/Zn-MOF.
3.12. Results for kinetics and adsorption studies
Various models, including pseudo-first-order kinetics, pseudo-second-order kinetics, liquid-film diffusion model, and intra-particle diffusion model, were used to study the adsorption kinetics and isotherms of MR dye on MgAl LDH/Zn-MOF. The experimental data were best fitted by the intra-particle diffusion model, which yielded an impressive R2 value of 0.996. Therefore, it can be confidently concluded that the intra-particle diffusion model is the most suitable to explain the adsorption behavior of MR dye on MgAl LDH/Zn-MOF (Table S3, ESI†). This suggests that the adsorption of MR dye molecules on the surface of the adsorbent and their diffusion into the inner pores are facilitated by electron transfer and the creation of bonding between them.The fitting of various kinetic models to the adsorption data is given in Fig. S5 (ESI†). In addition, different adsorption isotherms (Fig. S6, ESI†), including Langmuir, Freundlich, and Temkin, were evaluated to describe the adsorption behavior of MR dye on the prepared adsorbent. The Freundlich isotherm model best fits the experimental data, with an R2 value of 0.957 (Table S4, ESI†) and a higher adsorption capacity of 254.8 mg g−1 of MR dye on MgAl LDH/Zn-MOF. The straight line obtained for the isotherm models indicates that the adsorption behavior is consistent with monolayer adsorption.
3.13. Thermodynamic study of MR dye adsorption
The adsorption process is known to be dependent on temperature. In this study, the thermodynamic behavior of the adsorption of MR dye on MgAl LDH/Zn-MOF was also examined. The adsorption increased with increasing temperature from 303 K to 343 K, which was attributed to the increase in the value of KL. Table S5 (ESI†) presents the values of different thermodynamic parameters obtained from the study. A straight line was obtained by plotting the graph between ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL and 1/T, (Fig. S7a, ESI†). The values of ΔH° and ΔS° were deduced from the slope and intercept of the graph, respectively. It was concluded that the adsorption of MR dye was an endothermic, spontaneous process with enhanced randomness. The negative value of ΔG° indicated the thermal stability of the adsorption process. These results demonstrated that temperature played an essential role in the adsorption process and could be used to optimize the adsorption efficiency of MgAl LDH/Zn-MOF to remove MR dye.
KL and 1/T, (Fig. S7a, ESI†). The values of ΔH° and ΔS° were deduced from the slope and intercept of the graph, respectively. It was concluded that the adsorption of MR dye was an endothermic, spontaneous process with enhanced randomness. The negative value of ΔG° indicated the thermal stability of the adsorption process. These results demonstrated that temperature played an essential role in the adsorption process and could be used to optimize the adsorption efficiency of MgAl LDH/Zn-MOF to remove MR dye.
3.14. Regeneration performance and magnetic properties of MgAl LDH/Zn-MOF
In experimental applications, one of the most critical elements is the adsorbent capacity for reuse. Adsorption–desorption experiments were conducted to assess the reusability of MgAl-LDH/Zn MOF for MR dye removal. It is observed that the adsorption capacity of MR dye decreased slightly from 95.43% to 81.75% after five adsorption–desorption cycles (Fig. S7b, ESI†), indicating the adsorbent's reusability. However, the lower reduction rate after different cycles was due to incomplete desorption of MR dye from the adsorbent surface. Overall, the MgAl-LDH/Zn MOF exhibited a strong capacity for restoration and can be effectively used again to remove MR dye from contaminated water.The observed hysteresis loop characteristics (Fig. S7c, ESI†) demonstrate that the MgAl LDH/Zn-MOF exhibits exceptional magnetic properties and superior recyclability. Notably, the saturation magnetization rate of the catalyst is approximately 54.26 emu g−1, which represents a marked improvement over conventional heterogeneous catalysts that are difficult to recycle. The findings of this study suggest that the MgAl LDH/Zn-MOF is a promising catalyst with significant potential for industrial applications.
3.15. CO2 adsorption capacity of MgAl LDH/Zn-MOF
In ESI† (Fig. S8), a cyclic process of CO2 uptake and re-generation of the MgAl LDH/Zn-MOF is illustrated. The experiment was conducted at 298 K for 1 bar and 40 bar, for four cycles. The results indicate that the MgAl LDH/Zn-MOF composite exhibits excellent cyclic stability for CO2 separation. It has fast adsorption and desorption kinetics, making it a promising candidate for removing CO2 from gas streams. The CO2 uptake of MgAl LDH/Zn-MOF was 5.89 cm3 g−1 and 129.7 mg g−1, respectively, at 1 bar and 40 bars. These findings suggest that this composite can effectively remove CO2 from gas streams due to its reasonably high CO2 uptake. Table S6 (ESI†) summarizes the CO2 uptakes of both the adsorbents used in our study and those found in the literature. Our adsorbent (MgAl LDH/Zn-MOF) has shown exceptional performance in CO2 adsorption, which is a significant achievement in this field. This development will pave the way for even more promising research and advancements in this area.4. Conclusions
A new photocatalyst has been developed by combining two materials, Zn-MOF, and MgAl LDH, to form a special structure called MgAl LDH/Zn-MOF. This new structure effectively enhances electron and hole separation and promotes hydrogen evolution reaction under visible light. The light absorption performance of the Zn-MOF has been significantly enhanced by the addition of MgAl LDH. This photocatalyst is designed with a low steady-state fluorescence emission intensity and a high light-driven photocurrent response, which makes it highly effective in inhibiting the recombination of electron–hole pairs and significantly enhancing the photocatalytic hydrogen evolution reaction. To understand the exact mechanism of dye removal, various kinetic models and adsorption isotherms were studied. The results indicated that the intra-particle diffusion model and Freundlich isotherm model provided the most accurate fits to the experimental data, with maximum R2 values of 0.996 and 0.957, respectively. These findings demonstrate that the MgAl LDH/Zn-MOF composite is a promising adsorbent for the removal of MR dye from contaminated water. Additionally, the study evaluated the adsorbent's capacity for reuse and showed that it can be employed multiple times with only a slight reduction in MR dye removal efficiency. The affinity between MgAl LDH and Zn-MOF shows a considerable adsorption capacity for carbon dioxide (CO2). This strategic fusion of MOF architectures within LDH matrices presents a promising avenue for producing catalytic materials for an array of applications, such as air or liquid filtration, or medical applications.Data availability statement
All data are present within the manuscript.Author contributions
Ihsan Maseeh: writing – original draft, investigation; Farheen Anwar: writing – original draft, methodology, Sadia Aroob: writing – original draft; Tariq Javed: formal analysis; Ismat Bibi: formal analysis; Afaf Almasoudi: data curation; formal analysis; Ahmad Raheel: formal analysis. resources, investigation; Muhammad Arshad Javid: formal analysis; Sónia A. C. Carabineiro: visualization; writing – review & editing; Muhammad Babar Taj: writing – review & editing, project administration, supervision.Conflicts of interest
Authors declare no conflicts of interest.Acknowledgements
M. B. T. acknowledges support from HEC (Higher Education Commission) of Pakistan and thanks Dr Muhammad Imran (QAU) for help in the H2 evolution activity. A. A. acknowledges support from King Abdulaziz University, P. O. Box 42734, Jeddah, Saudi Arabia. S. A. C. C. acknowledges support from FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior, DOIs: 10.54499/LA/P/0008/2020, 10.54499/UIDP/50006/2020, 10.54499/UIDB/50006/2020 and the Scientific Employment Stimulus – Institutional Call (DOI: 10.54499/CEECINST/00102/2018/CP1567/CT0026).References
- I. Jonek-Kowalska and R. Wolniak, Smart Cities in Poland: Towards sustainability and a better quality of life?, Taylor & Francis, 2023 Search PubMed.
- V. B. Singh, S. Madhav, N. C. Pant and R. Shekhar, Weathering and Erosion Processes in the Natural Environment, John Wiley & Sons, 2024 Search PubMed.
- A. Kozhukhova, S. du Preez and D. Bessarabov, Int. J. Hydrogen Energy, 2024, 51, 1079–1096 CrossRef CAS.
- P. Hao, Z. Chen, Y. Yan, W. Shi and F. Guo, Sep. Purif. Technol., 2024, 330, 125302 CrossRef CAS.
- E. Fernandes, J. Gomes and R. C. Martins, Catalysts, 2022, 12, 1218 CrossRef CAS.
- G. Manasa and C. S. Rout, Mater. Adv., 2024 10.1039/D3MA00362K.
- E. M. Abd El-Monaem, H. M. Elshishini, S. S. Bakr, H. G. El-Aqapa, M. Hosny, G. Andaluri, G. M. El-Subruiti, A. M. Omer and A. S. Eltaweil, npj Clean Water, 2023, 6, 34 CrossRef CAS.
- M. E. Mahmoud, R. M. El-Sharkawy, E. A. Allam, G. M. Nabil, F. R. Louka, M. A. Salam and S. M. Elsayed, J. Water Proc. Engineering, 2024, 57, 104625 CrossRef.
- M. P. Jerome, F. A. Alahmad, M. T. Salem and M. Tahir, J. Environ. Chem. Eng., 2022, 10, 108151 CrossRef CAS.
- C. Li, J. Wang, L. Tong, Y. Wang, P. Zhang, M. Zhu and H. Dong, Coord. Chem. Rev., 2024, 502, 215623 CrossRef CAS.
- H. Lee, D. A. Reddy, Y. Kim, S. Y. Chun, R. Ma, D. P. Kumar, J. K. Song and T. K. Kim, ACS Sustainable Chem. Eng., 2018, 6, 16734–16743 CrossRef CAS.
- S. Das, S. Patnaik and K. Parida, Inorg. Chem. Front., 2019, 6, 94–109 RSC.
- A. Sherryna and M. Tahir, Int. J. Energy Res., 2022, 46, 2093–2140 CrossRef CAS.
- A. Felix Sahayaraj, H. Joy Prabu, J. Maniraj, M. Kannan, M. Bharathi, P. Diwahar and J. Salamon, J. Inorg. Organomet. Polym. Mater., 2023, 1–25 Search PubMed.
- G. A. Udourioh, M. Solomon, C. O. Matthews-Amune, E. Epelle, J. Okolie, V. E. Agbazue and U. Onyenze, React. Chem. Eng., 2023, 278–310 RSC.
- N. Yoshinari and T. Konno, Coord. Chem. Rev., 2023, 474, 214850 CrossRef CAS.
- A. I. Inamdar, S. Kamal, M. Usman, M.-H. Chiang and K.-L. Lu, Coord. Chem. Rev., 2024, 502, 215596 CrossRef CAS.
- W. Wang, M. Lin, W. Wang, Z. Shen and Z.-S. Wu, Bioact. Mater., 2024, 33, 279–310 CAS.
- A. S. Elsherbiny, A. Rady, R. M. Abdelhameed and A. H. Gemeay, Environ. Sci. Pollut. Res., 2023, 30, 106860 CrossRef CAS PubMed.
- R. R. Ikreedeegh, S. Tasleem and M. A. Hossen, Fuel, 2024, 360, 130561 CrossRef CAS.
- Z. Jin, Y. Li and Q. Ma, Trans. Tianjin Univ., 2021, 27, 127–138 CrossRef CAS.
- V. Şimşek, R. Z. Yarbay, V. Marttin and Ü. D. Gül, J. Cleaner Prod., 2023, 421, 138448 CrossRef.
- S. Aroob, M. B. Taj, S. Shabbir, M. Imran, R. H. Ahmad, S. Habib, A. Raheel, M. N. Akhtar, M. Ashfaq and M. Sillanpää, J. Environ. Chem. Eng., 2021, 9, 105590 CrossRef CAS.
- S. Aroob, S. A. Carabineiro, M. B. Taj, I. Bibi, A. Raheel, T. Javed, R. Yahya, W. Alelwani, F. Verpoort and K. Kamwilaisak, Catalysts, 2023, 13, 502 CrossRef CAS.
- L. Lu, J. Li, D. H. Ng, P. Yang, P. Song and M. Zuo, J. Ind. Eng. Chem., 2017, 46, 315–323 CrossRef CAS.
- S. G. Mtavangu, R. L. Machunda, B. van der Bruggen and K. N. J. S. R. Njau, Sci. Rep., 2022, 12, 15359 CrossRef CAS PubMed.
- Y. T. Dang, M.-H. D. Dang, N. X. D. Mai, L. H. T. Nguyen, T. B. Phan, H. V. Le and T. L. H. Doan, J. Sci.: Adv. Mater. Devices, 2020, 5, 560–565 CAS.
- A. S. Elsherbiny, A. Rady, R. M. Abdelhameed, A. H. J. E. S. Gemeay and P. Research, Environ. Sci. Pollut. Res., 2023, 30, 106860 CrossRef CAS PubMed.
- Z. Nazari, M. A. Taher and H. Fazelirad, RSC Adv., 2017, 7, 44890–44895 RSC.
- A. Chakraborty and H. Acharya, Colloid Interface Sci. Commun., 2018, 24, 35–39 CrossRef CAS.
- H. Sun and S.-J. Park, J. Ind. Eng. Chem., 2022, 111, 183–191 CrossRef CAS.
- C. I. Idumah and C. M. Obele, Surf. Interfaces, 2021, 22, 100879 CrossRef CAS.
- J. J. Villora-Picó, J. González-Arias, L. Pastor-Pérez, J. Odriozola and T. Reina, Environ. Res., 2024, 240, 117520 CrossRef PubMed.
- N. Iyi, Y. Ebina and T. Sasaki, J. Mater. Chem., 2011, 21, 8085–8095 RSC.
- F. Zhao, L. Fan, K. Xu, D. Hua, G. Zhan and S.-F. Zhou, J. CO2 Util., 2019, 33, 222–232 CrossRef CAS.
- B. S. Yadav and S. Dasgupta, Inorg. Chem. Commun., 2022, 137, 109203 CrossRef CAS.
- D. Umaru, H. Y. Hafeez, J. Mohammed, A. B. Suleiman, C. E. Ndikilar and Y. Zakariyya, Appl. Surf. Sci., 2023, 18, 100520 CrossRef.
- J. Lee, W. K. Chong, S. H. W. Kok, B. J. Ng, X. Y. Kong, S. P. Chai and L. L. Tan, Adv. Funct. Mater., 2023, 33, 2303430 CrossRef CAS.
- S. Dharani, S. Vadivel, L. Gnanasekaran and S. Rajendran, Fuel, 2023, 349, 128688 CrossRef CAS.
- R. Yan, S. Lin, W. Jiang, X. Yu, L. Zhang, W. Zhao and Q. Sui, Sci. Total Environ., 2023, 898, 165431 CrossRef CAS PubMed.
- E. Mosaffa, A. Banerjee and H. Ghafuri, Environ. Sci.: Water Res. Technol., 2023, 9, 2643–2663 RSC.
- W. Raza, K. Ahmad and H. J. N. Kim, Nanotechnology, 2021, 32, 495404 CrossRef CAS PubMed.
- F. Li, X. Yue, Y. Liao, L. Qiao, K. Lv and Q. J. N. C. Xiang, Nat. Commun., 2023, 14, 3901 CrossRef CAS PubMed.
- N. Kumar and H. J. R. A. Panda, RSC Adv., 2015, 5, 25676–25683 RSC.
- I. Ahmad, S. Shukrullah, H. Hussain, M. Y. Naz, M. Irfan, O. Alyahyawy and M. Al Thagafi, ACS Omega, 2023, 8, 26065–26078 CrossRef CAS PubMed.
- Z. Yan, W. Wang, L. Du, J. Zhu, D. L. Phillips and J. Xu, Appl. Catal., B, 2020, 275, 119151 CrossRef CAS.
- W. Zhang, C. Xu, E. Liu, J. Fan and X. J. A. S. S. Hu, Appl. Surf. Sci., 2020, 515, 146039 CrossRef CAS.
- X. Wang, X. Wang, J. Huang, S. Li, A. Meng and Z. J. N. C. Li, Nat. Commun., 2021, 12, 4112 CrossRef CAS PubMed.
- A. Meng, W. Tian, H. Yang, X. Wang, X. Wang and Z. Li, J. Hazard. Mater., 2021, 413, 125400 CrossRef CAS PubMed.
- L. Fan, S. Lei, H. M. K. Sari, L. Zhong, A. Kakimov, J. Wang, J. Chen, D. Liu, L. Huang and J. Hu, Nano Energy, 2020, 78, 105235 CrossRef CAS.
- Z. Jin, Y. Li and Q. Ma, Trans. Tianjin Univ., 2021, 27, 127–138 CrossRef CAS.
- Y.-J. Chen, M.-H. Li, J.-C.-A. Huang and P. Chen, Sci. Rep., 2018, 8, 7646 CrossRef PubMed.
- C. Maheu, L. Cardenas, E. Puzenat, P. Afanasiev and C. J. P. C. C. P. Geantet, Phys. Chem. Chem. Phys., 2018, 20, 25629–25637 RSC.
- A. Šutka, F.-K. Shieh, M. Kinka, L. Lapčinskis, C.-C. Chang, P. K. Lam, K. Pudzs and O. Verners, RSC Adv., 2023, 13, 41–46 RSC.
- W. Li, J.-J. Li, Z.-F. Liu, H.-Y. Ma, P.-F. Fang, R. Xiong and J.-H. Wei, Rare Met., 2024, 43, 533–542 CrossRef CAS.
- R. Jiang, D. Wu, G. Lu, Z. Yan, J. Liu, R. Zhou and M. Nkoom, J. Taiwan Inst. Chem. Eng., 2019, 96, 681–690 CrossRef CAS.
- P. Zhou, J. Yu and M. J. A. M. Jaroniec, Adv. Mater., 2014, 26, 4920–4935 CrossRef CAS PubMed.
- Q. Xu, L. Zhang, B. Cheng, J. Fan and J. Yu, Chem, 2020, 6, 1543–1559 CAS.
- F. He, A. Meng, B. Cheng, W. Ho and J. C. Yu, Chin. J. Catal., 2020, 41, 9–20 CrossRef CAS.
- J. Mu, F. Teng, H. Miao, Y. Wang and X. J. A. S. S. Hu, Appl. Surf. Sci., 2020, 501, 143974 CrossRef CAS.
- T. Hu, K. Dai, J. Zhang, G. Zhu and C. J. M. L. Liang, Mater. Lett., 2019, 257, 126740 CrossRef CAS.
- K. Filipović, M. Kostić, S. Najdanović, V. M. Radović, N. Velinov, D. Bojić and A. Bojić, Adv. Technol., 2023, 12, 75–83 CrossRef.
- S. Asghar, M. Roudgar-Amoli, A. Alizadeh and Z. Shariatinia, Water, Air, Soil Pollut., 2023, 234, 43 CrossRef CAS.
- M. A. Sachit and S. H. Kareem, Baghdad Sci. J., 2023, 1029 Search PubMed.
- N. Ahmad, F. S. Arsyad, I. Royani and A. Lesbani, Results Chem., 2023, 5, 100766 CrossRef CAS.
- B. N. S. Al-dhawi, S. R. M. Kutty, A. M. Alawag, N. M. Y. Almahbashi, F. A. H. Al-Towayti, A. Algamili, N. Aminu, A.-B. A. Al-Mekhlafi, A. H. Birniwa and A. H. Jagaba, Case Stud. Chem. Environ. Eng., 2023, 8, 100508 CrossRef CAS.
- O. P. Murphy, M. Vashishtha, P. Palanisamy and K. V. Kumar, ACS Omega, 2023, 8, 17407–17430 CrossRef CAS PubMed.
- X. Yang, C. Wang, B. Zhou and S. Cheng, J. Anal. Methods Chem., 2023, 2023, 9985984 Search PubMed.
- D. Brahma and H. Saikia, Inorganic and Nano-Metal Chemistry, 2023, 1–16 CrossRef.
- T. Florence, Aust. J. Chem., 1965, 18, 609–618 CrossRef CAS.
- Z. Zaheer, A.-A. Aisha and E. S. Aazam, J. Mol. Liq., 2019, 283, 287–298 CrossRef CAS.
- D. S. Miller, A. J. Bard, G. McLendon and J. Ferguson, J. Am. Chem. Soc., 1981, 103, 5336–5341 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma00038b |
| ‡ Both authors have equal contributions, so they are considered first authors. |
| This journal is © The Royal Society of Chemistry 2024 |