Open Access Article
Open Access ArticleStripping analysis of Pb2+ and Hg2+ in deveined shrimp and eggshells using a H2bpabza/MWCNT–modified graphite electrode
Kumar Sangeetha
Selvan
 *a,
Jayagopi
Gayathri
*a,
Jayagopi
Gayathri
 *b and
Sivakumar
Sivalingam
*b and
Sivakumar
Sivalingam
 b
b
aDepartment of Chemistry, Anna Adarsh College for Women, Anna Nagar, Chennai, Tamil Nadu 600040, India. E-mail: msc.sangi@gmail.com
bDepartment of Chemistry, Vel Tech Rangarajan Dr Sagunthala R & D Institute of Science and Technology, Avadi, Chennai, Tamil Nadu 600062, India. E-mail: drgayathrij@veltech.edu.in; drsivakumars@veltech.edu.in
First published on 30th August 2024
Abstract
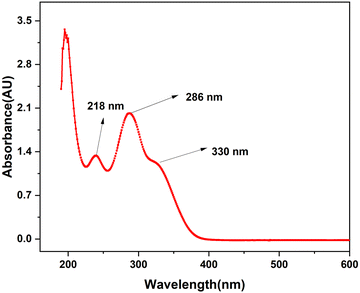
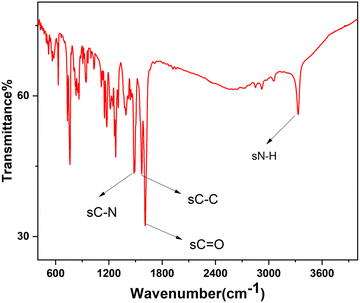
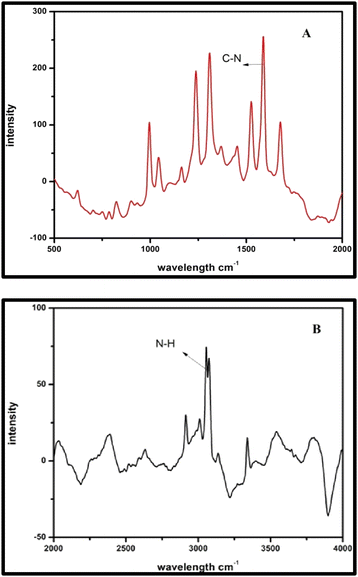
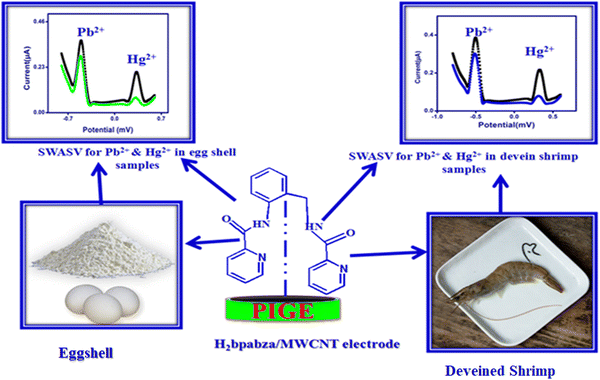
A novel synthesis was performed of asymmetrical carboxamide ligand N,N′-bis(2-pyridinecarboxamide)-2-aminobenzylamine (H2bpabza) derived from 2-pyridinecarboxylic acid and 2-aminobenzylamine. The N,N′-bis(2-pyridinecarboxamide)-2-aminobenzylamine (H2bpabza) ligand was confirmed by ultraviolet-Visible (UV-Vis), Fourier transform infrared (FT-IR), and Raman spectroscopy. The fabrication of N,N′-bis(2-pyridinecarboxamide)-2-aminobenzylamine (H2bpabza) embedded in a multi-walled carbon nanotube (MWCNT)-modified graphite electrode (GE) for use as an electrochemical sensor of Pb2+ and Hg2+ was demonstrated. The performance of the H2bpabza/MWCNT electrode and (Pb2+ and Hg2+–H2bpabza)/MWCNT was investigated by scanning electron microscopy (SEM) and square wave anodic stripping voltammetry (SWASV). In comparison to the MWCNT electrode, the H2bpabza/MWCNT electrode exhibited higher sensitivity and conductivity, as determined by cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). Stripping analysis and detailed experiments were conducted to establish the optimal parameters for deposition and stripping of metal ions, such as supporting electrolytes, pH, and accumulation time. The linear range was 2 to 140 μg L−1, with a detection limit of 0.1 μg L−1 for Pb2+ and 0.3 μg L−1 for Hg2+ (S/N = 3). The H2bpabza/MWCNT-modified GE showed excellent sensitivity, selectivity, stability, and reproducibility for the determination of Pb2+ and Hg2+. Ultimately, the H2bpabza/MWCNT-modified GE was used to demonstrate the electrochemical sensing of Pb2+ and Hg2+ in deveined shrimp and eggshells.
1. Introduction
It is well known that heavy metal ions (HMIs) such as those from lead and mercury are extremely hazardous environmental pollutants with toxic effects on living organisms.1–3 Trace amounts of lead and mercury enter the body primarily through inhalation and ingestion, and this damages many of the body's organ systems, e.g., the brain, lungs, and kidney.4,5 Although mercury is not an abundant chemical element in nature, it has become widespread as a result of its presence in many industrial and agricultural applications.6–8 Therefore, detection is important, and sensor performance has previously been investigated in the determination of lead and mercury in different food samples.9–11 Consequently, there has been considerable development of new methods for the detection of lead and mercury and at low or innocuous levels because of the potential for widespread practical applications for such technology.Traditionally, the presence of lead and mercury ions has been determined using spectroscopic methods such as inductively coupled plasma mass spectrometry (ICP-MS),12,13 inductively coupled plasma optical emission spectrometry (ICP-OES),14,15 atomic absorption spectrometry (AAS),16,17 and atomic fluorescence spectrometry (AFS).18–21 Although these methods have been developed for trace metal determination, unfortunately, these techniques require expensive instruments with high operating costs, and they are not suitable for on-site analysis.22 Among the different analytical methods, electrochemical techniques are an alternative to conventional spectroscopic techniques, and have been recognized as promising methods for trace and on-site analysis of toxic heavy metal ions due to their excellent sensitivity, portability, low cost, and suitability.23 Among all the electrochemical methods, anodic stripping voltammetry (ASV) is a powerful tool for detection of mercury and lead ions because it possesses high sensitivity and has the ability to simultaneously analyze several trace metal ions.24–27
There has been considerable academic and industrial research on multiwalled carbon nanotubes (MWCNTs) due to their excellent electrical properties, and when incorporated into graphite, high-performance conductivity and stability results. When graphite electrodes (GEs) are incorporated into sensor applications, they enhance the contact with the metal and improve the electron transfer charges to increase the sensitivity and selectivity of the electrode.
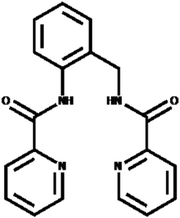
The important aspect in chemically modified electrodes (CMEs) is the choice of the modifier, which confers unique characteristics to the electrode surface.28–31 Asymmetric Schiff base ligand was chosen as the modifier in this work because it readily complexes with transition metal ions such as mercury, lead, and cadmium. Schiff base ligands play a key role as chelating agents in main group and transition metal coordination chemistry, due to the ease of synthesis, their role in biological systems, and diverse therapeutic activities.32–35 Carboxamide ligand was chosen as the modifier in this work because it readily complexes with transition metal ions such as lead and mercury. The structure of the ligand is given below in Scheme 1.
In this work, a carboxamide ligand was synthesized according to the protocol by Meghdadi et al.,36 which described a reaction between 2-pyridinecarboxylic acid and 2-aminobenzylamine, in tetrabutylammonium bromide medium with triphenyl phosphite as the activator. The MWCNT/ligand was coated on the electrode by the drop-casting method to fabricate a mercury-free electrode for the anodic stripping voltammetric determination of Pb2+ and Hg2+. In this study, the ligand was used for the first time as a sensing material on the surface of an electrode. This modified electrode was applied for the simultaneous determination of Pb2+ and Hg2+ in solution by square wave anodic stripping voltammetry (SWASV). The modified electrode displayed higher peak current responses for lead and cadmium as compared to the bare electrode. The modified platform exhibits satisfactory electrochemical stability and reusability.
2. Experimental
2.1. Materials and instruments
A solution of 0.1 M acetate buffer (ABS) was prepared using sodium acetate and acetic acid with double-distilled water (DDW). All reagents, 2-pyridinecarboxylic acid, 2-aminobenzylamine, tetrabutylammonium bromide (TBAB), and triphenyl phosphite (TPP) were purchased from Sigma-Aldrich and used without further purification. Stock solutions of lead acetate and cadmium acetate (1 mM) were prepared using DDW in standard flasks. Raman spectroscopy was performed using an inVia Raman microscope with a Raman-11i high-resolution confocal Raman microscope (Nanophoton, Japan), and UV-Visible spectroscopy was performed with a Cary 8453 UV-Vis diode array spectrophotometer. FTIR was carried out using a Cary 630 FTIR spectrometer. All electrochemical measurements were performed using a CHI 660B potentiostat, (CH Instruments, USA). A conventional three-electrode system was used that consisted of the Schiff base ligand/MWCNT-modified electrode as the working electrode (3-mm diameter), a platinum electrode as the auxiliary electrode, and an Ag/AgCl electrode saturated by KCl as the reference electrode. Solutions were mixed on a magnetic stirrer during purging with high-purity nitrogen, and were preconcentrated with a rotating PTFE stir bar.2.2. Synthesis of N,N′-bis(2-pyridinecarboxamide)-2-aminobenzylamine (H2bpabza) ligand
The preparation of carboxamide ligand was carried out as previously reported36 with slight modification. In brief, (10 mmol) 3.1 g triphenyl phosphite (TPP), (5 mmol) 1.61 g tetrabutylammonium bromide (TBAB), (10 mmol) 1.231 g 2-pyridinecarboxylic acid, and (5 mmol) 0.611 g 2-aminobenzylamine were placed in a 25-mL round bottom flask and heated in an oil bath. The reaction mixture was vigorously stirred for 20 min at 120 °C and then heated until a viscous and homogeneous solution was formed. The resulting viscous solution was cooled to room temperature, and then, 10 mL of methanol was added, and a clear solution was obtained by stirring. The solution was dried under vacuum for 24 h to obtain white crystals of the ligand.2.3. Fabrication of H2bpabza/MWCNT/PGE
Paraffin-impregnated graphite electrodes (PGEs) 4 cm in length with a diameter of 3 mm were used for modification. The PGEs were prepared as previously reported37 by immersing graphite rods into molten wax under vacuum until air bubbles ceased to evolve from the rods. After re-establishing atmospheric pressure, the graphite rods were removed before the solidification of the paraffin. The PGEs were then polished to a mirror-like finish by rubbing over the finest grade of emery paper, and the polished surface was washed thoroughly with DDW. Next, 5 μL (0.1 mg) of MWCNTs dispersed in 1 mL of ethanol was coated on the polished PGE surface and allowed to dry. Then, carboxamide ligand, which was dissolved in acetonitrile and 5 μL (1 mM) of carboxamide ligand, was drop-cast onto the MWCNT electrode surface, allowed to dry, and then washed with distilled water.2.4. Stripping voltammetry with the H2bpabza/MWCNT-modified electrode for Pb2+ and Hg2+ determination
The anodic stripping voltammetric determination of Pb2+ and Hg2+ was carried out in the following manner. The H2bpabza/MWCNT-modified electrode was immersed in a known amount of solution containing Pb2+ and Hg2+ present in 60 mL of 0.1 M acetate buffer solution (pH 4.5), and stirred for 180 s. Then, the modified electrode was removed, washed, and stored in fresh acetate buffer solution. Applying the negative potential of −0.8 V implies that the metal ions deposited on the electrode surface were reduced. The reduction of metal ion (Pb2+ and Hg2+) to metal (Pb0 and Hg0) on the electrode surface was determined by scanning the potential range from −0.8 V to 0.6 V, and the amplitude and the potential steps were 25 mV and 4 mV, respectively.2.5. Preparation of deveined shrimp and eggshells
Deveined shrimp (sample A) and eggshells (sample B) were collected from a fish market and egg shop in Chennai (India). Both samples were repeatedly washed with DDW, and then subsequently heated at 80 °C in an air oven for 1 hour. The dried samples of deveined shrimp and eggshells were separately powdered for further studies. 0.1 mM of Pb2+and Hg2+ was added to a freshly prepared stock solution of deveined shrimp and eggshell, and these samples were subsequently used to detect Pb2+and Hg2+ by SWASV.3. Results and discussion
3.1. Characterization of the H2bpabza ligand
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), which appear at 1616 cm−1 and 1636 cm−1. The strong absorption peak at 3155 cm−1 is due to the imine NH (bending) group, confirming the formation of asymmetric ligand. These observations are consistent with those described in an earlier report.36
N), which appear at 1616 cm−1 and 1636 cm−1. The strong absorption peak at 3155 cm−1 is due to the imine NH (bending) group, confirming the formation of asymmetric ligand. These observations are consistent with those described in an earlier report.36
3.2. SEM with EDAX
The surface morphologies of the PGEs (unmodified electrode), MWCNT, H2bpabza/MWCNT, and [Pb2+ and Hg2+–H2bpabza]/MWCNT (modified PGEs) were confirmed with scanning electron microscopy (SEM) and energy dispersive X-ray (EDAX) spectroscopy. The results of SEM with EDAX for different electrodes are shown in Fig. 4. The morphology of the PGE was observed as a homogeneous dispersed surface in Fig. 4(A), and the EDAX peak was noted as the presence of a carbon element in Fig. 4(E). The MWCNTs appear as needle-like structures (Fig. 4(B)), and the EDAX peak indicates the presence of carbon and oxygen (Fig. 4(F)). In Fig. 4(C), H2bpabza/MWCNT exhibits a cloudy fibre-like structure, and Fig. 4(G) shows that the EDAX of H2bpabza/MWCNT contains carbon, oxygen, and nitrogen peaks. The fibre-like structure of [Pb2+ and Hg2+–H2bpabza]/MWCNT is shown in Fig. 4(D), and in Fig. 4(H), the EDAX peak shows the presence of carbon, oxygen, nitrogen, lead, and mercury. It changes due to the chemical reactions leading to different SEM morphologies.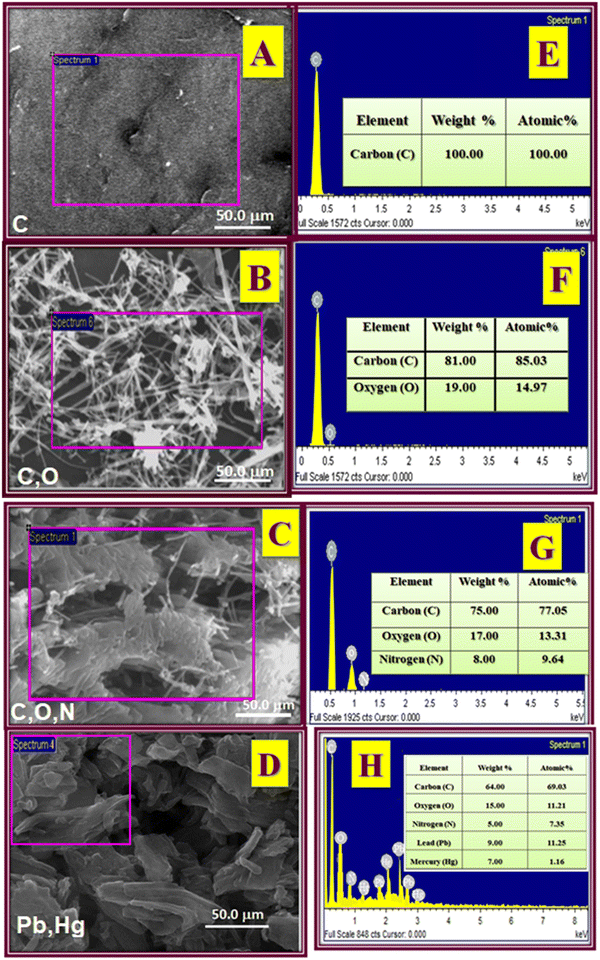 | ||
| Fig. 4 SEM with EDAX images of (A) and (E) PGE, (B) and (F) MWCNT, (C) and (G) H2bpabza/MWCNT, and (D) and (H) [Pb2+ and Hg2+–H2bpabza]/MWCNT electrodes. | ||
3.3. Cyclic voltammetry and electrochemical impedance spectroscopy
The elaborated H2bpabza/MWCNT, PGE, and MWCNT electrodes were electrochemically characterized using cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) in 5 mM of K4Fe[CN]2−/3− and 0.1 M acetate buffer solution (ABS) at a scan rate of 50 mV s−1. The cyclic voltammetry for the H2bpabza/MWCNT, PGE, and MWCNT electrodes is depicted in Fig. 5(A). The separation of the anodic and cathodic peak potential of ΔEp and also the ratio of peak current densities (Ipa/Ipc) between the anodic peak current (Ipa) and cathodic peak current (Ipc) values are evaluated in Table 2. After electrode modification, there was a clear tendency towards a reversible redox probe when MWCNTs were drop-cast with the H2bpabza ligand. Additionally, there was a poor redox peak for the PGE and MWCNT electrode.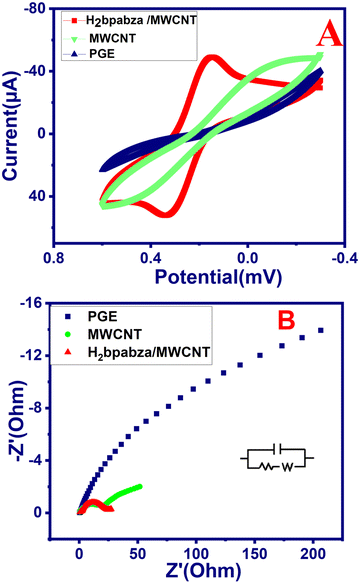 | ||
| Fig. 5 Electrochemical characterization for H2bpabza/MWCNT: (A) cyclic voltammetry (CV) and (B) EIS for Fe(CN)62−/3− containing 0.1 M acetate buffer, scan rate: 50 mV s−1. | ||
To increase our understanding regarding the information acquired from CV curves, EIS was carried out. The electron transfer capacity on the surface of the MWCNT electrode was examined using EIS before and after its modification. The Nyquist plot in Fig. 5(B) demonstrates a semicircle in the lower frequency region for each electrode. The small semicircle of the MWCNTs is equivalent to a charge transfer resistance (Rct) equal to 230 ohms. The addition of H2bpabza to the MWCNT electrode reduced the semicircle diameter and decreased Rct to 133 ohms. In the case of the PGE, the Rct was estimated as 7250 ohms. This can be justified by the change in the electrochemical characteristics of the surface electrodes, which positively affected the electron transfer process. These results prove that the charge transfer of H2bpabza/MWCNT is faster than that of the MWCNT electrode. Hence, CV and EIS support the successful fabrication and excellent performance of H2bpabza/MWCNT.
3.4 Voltammetry analysis of Pb2+and Hg2+
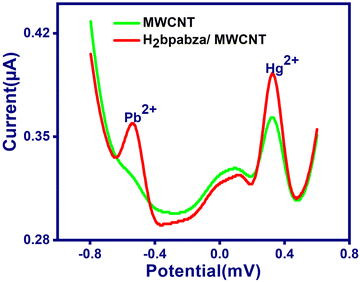
Although a number of reports on the determination of Pb2+and Hg2+ by anodic stripping voltammetry using different mercury-based electrodes can be found in the literature, the toxicity and associated health hazards surrounding mercury can be avoided by developing mercury-free electrodes for anodic stripping analysis. Preliminary investigation with the H2bpabza/MWCNT-modified electrode showed that the Schiff base ligand is a potential complexing agent and can be used for the preconcentration of Pb2+ and Hg2+. Hence, various parameters that can influence the anodic stripping voltammetric determination of Pb2+ and Hg2+ such as medium for complexation, pH, preconcentration time, and reduction potential have been optimized.Step 1: Pb2+ and Hg2+ ions were first preconcentrated by immersing the modified electrode into 0.1 M acetate buffer (pH 4.5) containing Pb2+ and Hg2+ under open circuit conditions (OCC) for 180 s to form complexes with the H2bpabza ligand.
| [M2+]solution + (H2bpabza/MWCNT)surface → (M2+ − H2bpabza/MWCNT)surface |
Step 2: a potential of −0.8 V in 0.1 M acetate buffer was applied for the reduction of (M2+ to M0).
| (M2+ − H2bpabza/MWCNT)surface + 2e− → (M0 − H2bpabza/MWCNT)surface |
Step 3: the metal present on the electrode surface was anodically stripped by scanning the potential from −0.8 V to 0.6 V using square wave anodic stripping with optimized parameters (frequency 25 Hz; amplitude 25 mV; increment potential 4 mV).
| (M0 − H2bpabza/MWCNT)surface → H2bpabza/MWCNTsurface + [M2+]solution + 2e− |
The metal ions on the electrode surface were removed by dipping in 0.1 M EDTA solution for 60 s with stirring. The metal ions from the electrode surface are introduced into solution via EDTA, and then form a complex with EDTA-M2+ at pH 4.5. Then, the H2bpabza/MWCNT-modified electrode was completely washed with distilled water to regenerate it for further experiments. The results obtained are shown in Fig. 6. The above procedure was applied for the MWCNT and H2bpabza/MWCNT electrodes.
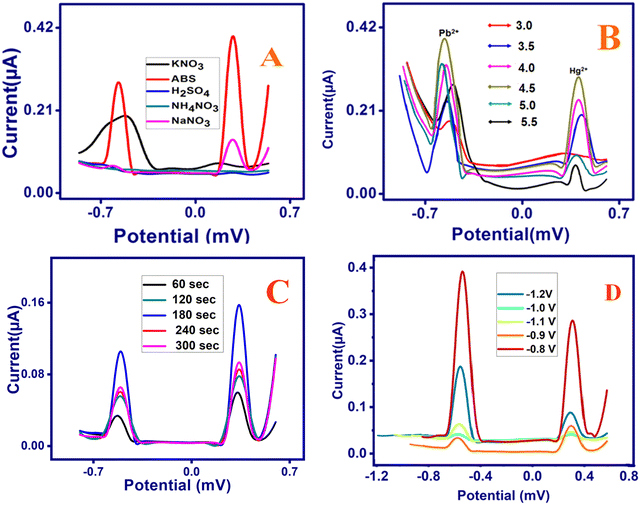
3.5. Studies on experimental variables for the anodic stripping voltammetry of Pb2+ and Hg2+
To increase the sensitivity for the determination of Pb2+ and Hg2+ with the H2bpabza/MWCNT electrode, different experimental conditions for SWASV were optimized such as the supporting electrolyte, pH, and preconcentration time.3.6. Calibration data
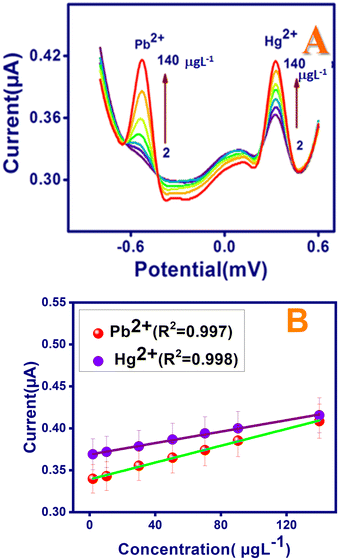
Under optimal conditions, the H2bpabza/MWCNT-modified electrode was applied for the determination of Pb2+ and Hg2+ in 0.1 M acetate buffer solution. The SWASV response towards Pb2+ and Hg2+ at different concentrations was measured by preconcentrating the metal ions for 180 s on the electrode surface in 0.1 M acetate buffer at pH 4.5. The electrode was then removed from the metal ion solution, washed, stored in fresh background electrolyte of acetate buffer, subsequently reduced at −0.8 V, and then, SWASV was recorded. The SWASV responses of Pb2+ and Hg2+ at different concentrations ranging from 2 to 140 μg L−1 are shown in Fig. 8(A). The stripping peak current was linear with increasing concentration of the metal ion. The correlation equations were defined as Y = 0.005x + 3.31 μg L−1, R2 = 0.98; Y = 0.005x + 3.221 μg L−1, R2 = 0.99 for Pb2+ and Hg2+, respectively (Y: current/μA, x: concentration/μg L−1), and the calibration curves are shown in Fig. 8(B). The limit of detection (LOD) was found to be 0.1 and 0.3 μg L−1 for Pb2+ and Hg2+, respectively. The results indicate that the proposed method shows high sensitivity for the detection of heavy metals. Furthermore, Table 1 shows that the H2bpabza/MWCNT-modified electrode exceeded previously reported modified electrodes in terms of linear range and detection limits. The table clearly shows that the proposed technique performs at or is comparable to the previously described methods in terms of detection limit and linearity range.| Modified electrode | LOD Pb2+ μg L−1 | LOD Hg2+ μg L−1 | Linear range of Pb2+ μg L−1 | Linear range of Hg2+ μg L−1 | Ref. |
|---|---|---|---|---|---|
| Hg-Bi/PDAAQ/GC | 3.8 | — | 10.0–120.0 | — | 38 |
| 3D printed graphene/poly(lactic acid) (PLA) | 4.1 | 6.1 | 16–10 | 0–120 | 39 |
| Hg/GCE, Au & dual-channel | 0.05 | — | 0.10–80 | — | 40 |
| Ca-MOF/3D printer | — | 0.6 | — | 2–40 | 41 |
| MMT-Ca/CPE | 105 | 540 | 3500–15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
42 |
| AuNP/0.75% APTES-ITO | 0.9 | — | 5–120 | — | 43 |
| Bi-BDC-NH2@NMCS | 0.36 | — | 1.0–1500 | — | 44 |
| rGO/MoS2/CS | 1.6 | 5–50 | — | 45 | |
| Eu3+ doped NiO/CPE | 0.1 | — | 0.8–165 | — | 46 |
| Fe3O4@MPC-1 | 34.2 | 19.3 | 0.2–1.0 | 1.0–4.0 | 47 |
| CuONPs/PANI–CPE | 0.40 | 0.66 | 0.2–7.8 | 0.2–7.8 | 48 |
| COFS-CH3-modified CPE | 0.01 | — | 0.1–1.0 | — | 49 |
| SNW1/GCE | 7.2 | 1.2 | 10–300 | 50–300 | 50 |
| H2bpabza/MWCNT | 0.1 | 0.3 | 2–140 | 2–140 | This work |
| Technique | Term | Modified electrode | ||
|---|---|---|---|---|
| PGE | MWCNT | H2bpabza/MWCNT | ||
| CV | I pa (μA) | 13.8 | 38.5 | 51.5 |
| ΔEp | 90 mV | 174 mV | 253 mV | |
| σ | 163 × 10−5 S cm−1 | 400 × 10−5 S cm−1 | 515 × 10−5 S cm−1 | |
| A (cm2) | 0.14 | 0.2 | 0.6 | |
| EIS | R S (Ω) | 18 | 25 | 40 |
| R CT (Ω) | 7250 | 230 | 133 | |
3.7. Reproducibility, stability, and reusability
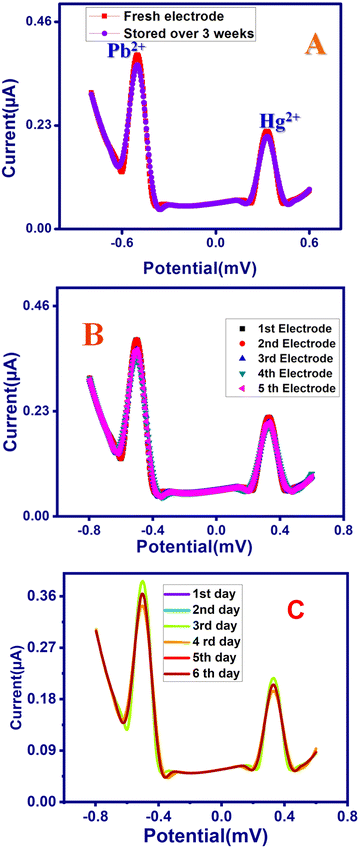
The H2bpabza/MWCNT electrode exhibited long-term stability with no systematic differences in performance between the freshly prepared electrodes and electrodes stored for 3 weeks after preparation, in SWASV experiments with 40 μg L−1 for Pb2+ and Hg2+, as Fig. 9(A) shows no significant change in the current response. Fig. 9(B) shows the reproducibility of five H2bpabza/MWCNT electrodes produced in 0.1 M acetate buffer with 40 μg L−1 of Pb2+ and Hg2+. There was satisfactory reproducibility, with a relative standard deviation (RSD) of 3.1% and 2.8% for Pb2+ and Hg2+ ions, respectively. The reusability of H2bpabza/MWCNT was analysed each day, up to six days, to detect the stripping peak current of Pb2+ and Hg2+, as shown in Fig. 9(C). Excellent reusability of the H2bpabza/MWCNT electrode was noted, with an RSD value of 1.5% for Pb2+ and 1.4% for Hg2+ ions. Therefore, the prepared electrode exhibits excellent stability, reproducibility, and reusability for the determination of Pb2+ and Hg2+ ions.3.8. Anti-interference studies
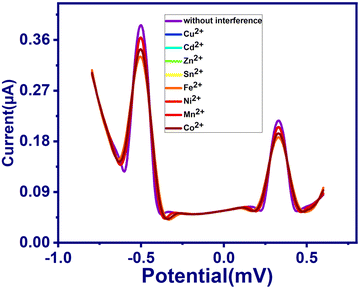
Anti-interference was performed to analyze the selectivity and feasible interference of the H2bpabza/MWCNT-modified electrode for the detection of Pb2+ and Hg2+ ions. The measurement was examined using SWASV analysis in 0.1 M acetate buffer solution (pH 4.5) containing 90 μg L−1 Pb2+ and Hg2+ with the addition of 50 μg L−1 of feasible interference ions of Cu2+, Cd2+, Zn2+, Sn2+, Fe2+, Ni2+, Mn2+, and Co2+ (Fig. 10). According to eqn (1), the stripping peak currents of Pb2+ and Hg2+ in the absence (I0) and presence (Ii) of interfering metal ions, and the relative signal changes, were calculated in Table 3. Using the equation, the RSD values were evaluated from Table 3. The results obtained from Table 3 show that the peak currents of Pb2+ in the presence of interfering metals Cu2+, Cd2+, Zn2+, Sn2+, Fe2+, Ni2+, Mn2+, and Co2+ endured a minimal decrease, with RSD% values of 1.05% (Co2+, Cu2+, Zn2+), 1.03% (Mn2+), and 1.31% (Cd2+, Sn2+, Fe2+, Ni2+). For the Hg2+ ion, interfering with Cu2+, Cd2+, Zn2+, Sn2+, Fe2+, Ni2+, Mn2+, and Co2+ resulted in RSD% value decreases of 0.9% (Co2+, Cu2+, Zn2+), 0.81% (Cd2+, Sn2+, Ni2+), 0.81% (Fe2+), and 0.95% (Mn2+). This can be explained by the presence of several metal ions between the analytes Pb2+ and Hg2+, which interfered with Cu2+, Cd2+, Zn2+, Sn2+, Fe2+, Ni2+, Mn2+, and Co2+ at the active sites on the H2bpabza/MWCNT electrode surface. This might be qualified as the result of different metals between the analytes Pb2+ and Cd2+, and these interfering Cu2+, Cd2+, Zn2+, Sn2+, Fe2+, Ni2+, Mn2+, and Co2+ ions on the active sites are present on the H2bpabza/MWCNT electrode surface 48.| RSD = (Ii/I0) − 1 | (1) |
| Interference | Peak current (μA) | Relative signals changes (%) | ||
|---|---|---|---|---|
| Pb2+ | Hg2+ | Pb2+ | Hg2+ | |
| No interference | 0.38 | 0.22 | — | — |
| Cu2+ | 0.34 | 0.20 | −1.05 | −0.9 |
| Cd2+ | 0.33 | 0.19 | −1.31 | −0.81 |
| Zn2+ | 0.34 | 0.20 | −1.05 | −0.9 |
| Sn2+ | 0.33 | 0.187 | −1.31 | −0.81 |
| Fe2+ | 0.33 | 0.185 | −1.31 | −0.812 |
| Ni2+ | 0.33 | 0.185 | −1.31 | −0.81 |
| Mn2+ | 0.35 | 0.21 | −1.03 | −0.95 |
| Co2+ | 0.34 | 0.20 | −1.05 | −0.9 |
3.9. Real samples
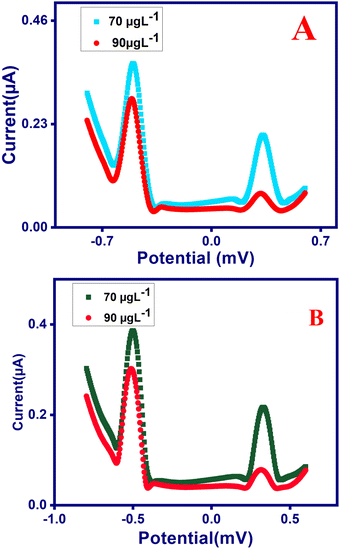
The H2bpabza/MWCNT was evaluated by measuring Pb2+ and Hg2+ in sample A (deveined shrimp) and sample B (eggshells) by SWASV. First, 1 g each of deveined shrimp and eggshells was diluted three times in 0.1 M acetate buffer at pH 4.5. The samples were also diluted three times for analysis of 70 to 90 μg L−1 of Pb2+ and Hg2+. The SWASV for determination of Pb2+ and Hg2+ in deveined shrimp (Fig. 11(A)) and eggshells (Fig. 11(B)), and the recovery % of the diluted (deveined shrimp and eggshell) samples are demonstrated in Table 4. The samples showed a superior recovery value of 99% to 101% for Pb2+ and 101% to 103% for Hg2+. The prepared H2bpabza/MWCNT electrode exhibited satisfactory accuracy (recovery) for the detection of Pb2+ and Hg2+ in the samples of deveined shrimp and eggshells.| Sample | Added (μg L−1) | Found (μg L−1) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| Pb2+ | Hg2+ | Pb2+ | Hg2+ | Pb2+ | Hg2+ | |
| Blank | 0 | 0 | 0.100 | 0.101 | 100 | 101 |
| Deveined shrimp | 70 | 70 | 70.1 ± 0.01 | 71.0 ± 0.04 | 100 | 101 |
| 90 | 90 | 90.5 ± 0.02 | 91 ± 0.01 | 101 | 101 | |
| Blank | 0 | 0 | 0.100 | 0.101 | 100 | 101 |
| Egg shell | 70 | 70 | 69.8 ± 0.03 | 71.8 ± 0.01 | 100 | 103 |
| 90 | 90 | 89.5 ± 0.01 | 91.5 ± 0.02 | 99 | 102 | |
4. Conclusions
In this work, a simple, reproducible, and sensitive procedure was developed for the simultaneous determination of Pb2+ and Hg2+ ions using an H2bpabza/MWCNT ligand-modified electrode. The results suggest that Schiff base ligand is a promising material that possesses advantages for stripping analysis in comparison with other suggested electrodes for detecting Pb2+ and Hg2+ ions with detection limits in the range of 0.1 μg L−1 and 0.6 μg L−1, with high sensitivity and long-term usage. It was found that operational parameters such as pH, deposition time, and supporting electrolyte systematically affected the stripping current. The H2bpabza/MWCNT-modified electrode showed satisfactory selectivity, stability, and reproducibility.Data availability
Data files can be shared on reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Thanks for providing laboratory facilities to carry our research work. There is no fund provided any of the our institutions for this research work. We have spent our own money for all the characterization studies.References
- K. Raj and A. P. Das, Lead pollution: Impact on environment and human health and approach for a sustainable solution, Environ. Chem. Ecotoxicol., 2023, 5, 79–85 CrossRef CAS.
- G. de Paula Arrifano, et al., Neurotoxicity and the global worst pollutants: astroglial involvement in arsenic, lead, and mercury intoxication, Neurochem. Res., 2023, 48(4), 1047–1065 CrossRef CAS PubMed.
- A. I. Gomez-Delgado, et al., Seasonal variations in mercury, cadmium, lead and arsenic species in Norwegian blue mussels (Mytilus edulis L.)–Assessing the influence of biological and environmental factors, J. Trace Elem. Med. Biol., 2023, 76, 127110 CrossRef CAS PubMed.
- A. Cuschieri, A. Joseph Ignatius and B. Renald, Effect of Non-essential Heavy Metals on Human Health. Heavy Metals in the Environment: Management Strategies for Global Pollution, Am. Chem. Soc., 2023, 117–133 CAS.
- Mi Ye, et al., Monitoring Hg2+ and MeHg+ poisoning in living body with an activatable near-infrared II fluorescence probe, J. Hazard. Mater., 2023, 445, 130612 CrossRef CAS PubMed.
- S. L. C. Ferreira, et al., Application of human health risk indices in assessing contamination from chemical elements in food samples, TrAC, Trends Anal. Chem., 2023, 117281 CrossRef CAS.
- C. Cao, et al., Zeolites synthesized from industrial and agricultural solid waste and their applications: A review, J. Environ. Chem. Eng., 2023, 110898 CrossRef CAS.
- S. Nawaz, et al., Effective assessment of biopolymer-based multifunctional sorbents for the remediation of environmentally hazardous contaminants from aqueous solutions, Chemosphere, 2023, 138552 CrossRef CAS PubMed.
- Z. M. Karazan and M. Roushani, Selective determination of cadmium and lead ions in different food samples by poly(riboflavin)/carbon black-modified glassy carbon electrode, Food Chem., 2023, 423, 136283 CrossRef CAS PubMed.
- Z. M. Karazan, M. Roushani and S. J. Hoseini, Simultaneous electrochemical sensing of heavy metal ions (Zn2+, Cd2+, Pb2+, and Hg2+) in food samples using a covalent organic framework/carbon black modified glassy carbon electrode, Food Chem., 2024,(442), 138500 CrossRef PubMed.
- Z. M. Karazan and M. Roushani, Electrochemical determination of mercury ions in different food samples using glassy carbon electrode modified with poly(crocin), Microchem. J., 2023, 195, 109402 CrossRef.
- M. Winter, F. Lessmann and V. Harth, A method for reliable quantification of mercury in occupational and environmental medical urine samples by inductively coupled plasma mass spectrometry, Anal. Methods, 2023, 15(16), 2030–2038 RSC.
- M. S. Shushtarian, et al., Speciation of inorganic arsenic by μ-thin-layer chromatography coupled with laser ablation inductively coupled plasma mass spectrometry based on an ion imprinted polymer, Anal. Methods, 2024, 16, 205–213 RSC.
- L. He, et al., Cross double point discharge as enhanced excitation source for highly sensitive determination of arsenic, mercury and lead by optical emission spectrometry., J. Anal. At. Spectrom., 2021, 36(6), 1193–1200 RSC.
- J.-Y. Cai, et al., One-Pot Pretreatment Coupled to Microplasma Optical Emission Spectrometry for Field and Sensitive Determination of Inorganic Mercury and Methylmercury in Fish, Anal. Chem., 2023, 95(26), 9813–9821 CrossRef CAS PubMed.
- J. C. García-Mesa, et al., Sensitive determination of mercury by magnetic dispersive solid-phase extraction combined with flow-injection-cold vapour-graphite furnace atomic absorption spectrometry, J. Anal. At. Spectrom., 2021, 36(5), 892–899 RSC.
- M. Atasoy, Development of a New Sensitive Method for Lead Determination by Platinum-Coated Tungsten-Coil Hydride Generation Atomic Absorption Spectrometry, ACS Omega, 2023, 8(25), 22866–22875 CrossRef CAS PubMed.
- X. Liu, et al., Electrothermal desolvation-enhanced dielectric barrier discharge plasma-induced vapor generation for sensitive determination of antimony by atomic fluorescence spectrometry., Anal. Chem., 2022, 94((10)), 4455–4462 CrossRef CAS PubMed.
- P. Srinivasan, et al., Chromoionophoric probe imbued porous polymer monolith as a Three-in-One Solid-state Naked-eye sensor for the selective sensing and recovery of Ultra-trace Lead, Mercury, and cadmium ions from Industrial/Environmental samples, Chem. Eng. J., 2023, 471, 144627 CrossRef CAS.
- J. Kaur, et al., Fabrication of novel copper MOF nanoparticles for nanozymatic detection of mercury ions, J. Mater. Res. Technol., 2023, 22, 278–291 CrossRef.
- D. B. C. Leslee, et al., Synthesis of a quinoxaline–hydrazinobenzothiazole based probe—single point detection of Cu2+, Co2+, Ni2+ and Hg2+ ions in real water samples, Org. Biomol. Chem., 2023, 21(19), 4130–4143 RSC.
- Y. Zhang, et al., Xylan derived carbon dots composite ZIF-8 and its immobilized carbon fibers membrane for fluorescence selective detection Cu2+ in real samples, Chem. Eng. J., 2023, 474, 145804 CrossRef CAS.
- E. Erdemir, et al., Smartphone-assisted dual-channel discriminative detection of Hg(II) and Cu(II) ions with a simple, unique, readily available probe., Sens. Actuators, B, 2023, 382, 133487 CrossRef CAS.
- J. Gayathri., S. Sivakumar. and S. S. Narayanan, Synthesis and characterization of Schiff base ligand-mutliwalled carbon nanotubes as mercury-free electrochemical sensor for detecting toxic metals in aquatic treatment, Diamond Relat. Mater., 2023, 136, 109984 CrossRef CAS.
- S. M. Ghalebi, H. Parham and A. Shirmardi, Hg2+ determination by DPASV by using poly(methylene disulfide)/Au nanoparticle/MWCNT modified glassy carbon electrode by differential pulse anodic stripping voltammetry (DPASV) technique, J. Porous Mater., 2023, 1–16 Search PubMed.
- J. Liu, et al., Efficient cobalt hydroxide nanosheets for enhanced electrochemical sensing of Hg(II) ion., Chemosphere, 2023, 334, 139015 CrossRef CAS PubMed.
- Y. Gadel Hak, et al., Nanomaterials-modified disposable electrodes and portable electrochemical systems for heavy metals detection in wastewater streams: A review, Microchem. J., 2023, 109043 CrossRef CAS.
- J. Zhang, et al., Bioamide-Decorated Polyfluoreneisocyanide: Preparation from Benzoxazine-Isocyanide Mechanochemistry Postmodification and Application as an Active Modifier for Pb2+/NO2− Electrochemical Probing, ACS Appl. Polym. Mater., 2023, 5(7), 5454–5465 CrossRef CAS.
- C. Ji, et al., Aptamer–Protein Interactions: From Regulation to Biomolecular Detection, Chem. Rev., 2023, 123(22), 12471–12506 CrossRef CAS PubMed.
- F. Torres-Rojas, et al., Synergistic effect of electrotrophic perchlorate reducing microorganisms and chemically modified electrodes for enhancing bioelectrochemical perchlorate removal, Environ. Res., 2023, 116442 CrossRef CAS PubMed.
- X. Liang, et al., A novel electrochemical acetaminophen sensor based on multiwalled carbon nanotube and poly(neutral red) modified electrodes with electropolymerization in ternary deep eutectic solvents., J. Electroanal. Chem., 2023, 936, 117366 CrossRef CAS.
- N. A. A. Elkanzi, et al., Synthesis, physicochemical properties, biological, molecular docking and DFT investigation of Fe(III), Co(II), Ni(II), Cu(II) and Zn(II) complexes of the 4-[{(5-oxo-4, 5-dihydro-1,3-thiazol-2-yl) hydrazono] methyl} phenyl 4-methylbenzenesulfonate Schiff-base ligand, Polyhedron, 2023, 230, 116219 CrossRef CAS.
- S. Jos and N. R. Suja, Chiral Schiff base ligands of salicylaldehyde: A versatile tool for medical applications and organic synthesis-A review, Inorg. Chim. Acta, 2023, 547, 121323 CrossRef CAS.
- S. Arulmozhi, et al., Chemical, Pharmacological, and Theoretical Aspects of Some Transition Metal(II) Complexes Derived from Pyrrole Azine Schiff Base., ACS Omega, 2023, 8(38), 34458–34470 CrossRef CAS PubMed.
- R. Kumar, et al., Recent advances in synthesis of heterocyclic Schiff base transition metal complexes and their antimicrobial activities especially antibacterial and antifungal, J. Mol. Struct., 2023, 1294, 136346 CrossRef CAS.
- S. Meghdadi, et al., Direct electrochemical synthesis of copper(II) and zinc(II) complexes of the tetradentate ligand N,N′-bis (2-pyridinecarboxamide)-2-aminobenzylamine (H2bpabza). The crystal structures of the ligand and its Cu(II) complex., Polyhedron, 2015, 85, 519–524 CrossRef CAS.
- J. Gayathri and S. Sivalingam, Determination of Pb2+ and Cd2+ ions in raw milk, honey and groundnut shell using TSAB/MWCNT, Mater. Adv., 2023, 4, 2502–2511 RSC.
- K. M. Hassan, et al., Single and simultaneous voltammetric sensing of lead(II), cadmium(II) and zinc(II) using a bimetallic Hg-Bi supported on poly(1, 2-diaminoanthraquinone)/glassy carbon modified electrode, Sens. Bio-Sens. Res., 2020, 29, 100369 CrossRef.
- J. G. Walters, et al., Trace analysis of heavy metals (Cd, Pb, Hg) using native and modified 3D printed graphene/poly(lactic acid) composite electrodes, Electroanalysis, 2020, 32(4), 859–866 CrossRef CAS.
- L. Bu, X. Qingji and M. Hai, Simultaneous sensitive analysis of Cd(II), Pb(II) and As(III) using a dual-channel anodic stripping voltammetry approach, New J. Chem., 2020, 44(15), 5739–5745 RSC.
- C. Kokkinos, et al., 3D-printed lab-in-a-syringe voltammetric cell based on a working electrode modified with a highly efficient Ca–MOF sorbent for the determination of Hg(II), Sens. Actuators, B, 2020, 321, 128508 CrossRef CAS.
- A. Moutcine, et al., A novel carbon paste electrode modified by NP-Al2O3 for the electrochemical simultaneous detection of Pb(II) and Hg(II), Diamond Relat. Mater., 2020, 104, 107747 CrossRef CAS.
- N. Mohamad Nor, et al., Simultaneous Sensing of Cd(II), Pb(II), and Cu(II) Using Gold Nanoparticle-Modified APTES-Functionalized Indium Tin Oxide Electrode: Effect of APTES Concentration, ACS Omega, 2023, 8(19), 16587–16599 CrossRef CAS PubMed.
- Q. Yang, et al., Bismuth Metal–Organic Framework/Carbon Nanosphere Composites for Ultrasensitive Simultaneous Electrochemical Detection of Lead and Cadmium, ACS Appl. Nano Mater., 2023, 6((9)), 7901–7909 CrossRef CAS.
- C. Guo, et al., A simple electrochemical sensor based on rGO/MoS2/CS modified GCE for highly sensitive detection of Pb(II) in tobacco leaves., RSC Adv., 2021, 11(47), 29590–29597 RSC.
- M. Malakootian, A. Hesam and H. Mahmoudi-Moghaddam, A novel electrochemical sensor based on the modified carbon paste using Eu3+ − doped NiO for simultaneous determination of Pb(II) and Cd(II) in food samples, J. Electroanal. Chem., 2020, 876, 114474 CrossRef CAS.
- Y. Liu, et al., Interface Co-Assembly Synthesis of Magnetic Fe3O4@ mesoporous Carbon for Efficient Electrochemical Detection of Hg(II) and Pb(II), Adv. Mater. Interfaces, 2023, 10(5), 2201631 CrossRef CAS.
- D. S. B. Ali, et al., Green synthesis of copper oxide nanoparticles using Ficus elastica extract for the electrochemical simultaneous detection of Cd2+, Pb2+, and Hg2+, RSC Adv., 2023, 13(27), 18734–18747 RSC.
- X. Tang, et al., Thiol-grafted covalent organic framework-based electrochemical platforms for sensitive detection of Hg(II) ions, Chem. Commun., 2023, 59, 8731–8734 RSC.
- M. R. J. Sarvestani, M. Tayyebeh and A. Abbas, Simultaneous determination of Pb2+ and Hg2+ at food specimens by a Melamine-based covalent organic framework modified glassy carbon electrode, Food Chem., 2023, 402, 134246 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |