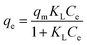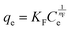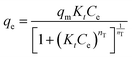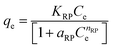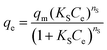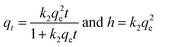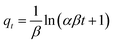Open Access Article
Open Access ArticleFunctional porous protein nanofibrils/polysaccharides aerogel beads for efficient dyes removal from water†
Mandana
Dilamian
a,
Majid
Montazer
b,
Hossein
Yousefi
cd,
Daniel E.
Otzen
 e and
Dina
Morshedi
e and
Dina
Morshedi
 *a
*a
aBioprocess Engineering Department, Institute of Industrial and Environmental Biotechnology, National Institute of Genetic Engineering and Biotechnology (NIGEB), P.O. Box 14965/161, Tehran, Iran. E-mail: morshedi@nigeb.ac.ir
bDepartment of Textile Engineering, Amirkabir University of Technology (Tehran Polytechnic), 15875-4413, Tehran, Iran
cLaboratory of Renewable Nanomaterials, Department of Wood Engineering and Technology, Gorgan University of Agricultural Sciences and Natural Resources, 4913815739, Gorgan, Iran
dNanonovin Polymer Co., Gorgan University of Agricultural Sciences and Natural Resources, 4913815482, Gorgan, Iran
eInterdisciplinary Nanoscience Centre (iNANO), Department of Molecular Biology and Genetics, Aarhus University, 8000 Aarhus C, Denmark
First published on 24th June 2024
Abstract
The rational design and fabrication of functional and feasible adsorbents with enhanced adsorption properties for pollutant removal remain challenging. Here, to achieve efficient adsorption of dyes, a radial-freezing technique was employed to develop freeze-dried bio-nanocomposites in the form of aerogel beads composed of cellulose nanofibers, protein nanofibers, and chitosan (CPCs). This strategy led to the formation of a spherical aerogel with a dandelion-like structure in the radial cross-section. FE-SEM micrographs of the aerogel beads revealed a highly porous morphology with a network of interconnected pores, allowing for the effective adsorption of liquids. The characterization results of the functional aerogel beads showed a remarkable ability to adsorb various cationic and anionic azo dyes. The maximum adsorption capacity of 1349.7 ± 34.36 mg g−1 and removal efficiency of nearly 100% in the initial 1000 mg L−1 Congo Red (CR) solution were obtained for CPCs aerogel beads. The resulting adsorption experimental data were fit by the sip isotherm and pseudo-second-order models. The porous structure of the CPCs aerogel bead enhanced the diffusion of dye molecules into the pores and inner surface. Furthermore, combined with the analysis results of FT-IR spectroscopy and XPS, multiple adsorption mechanisms (strong electrostatic interactions, hydrogen bonds, CH–π and π–π bonds) were ascribed between the CPCs composite and dye cations. It is believed that our CPCs aerogel beads can be regarded as a sustainable green bio-adsorbent for water remediation.
Introduction
Freshwater contamination caused by dyes is becoming a significant environmental problem. Dyes have extensive applications in various modern industries, including textiles, leather, food additives, paper printing, and cosmetics.1,2 Due to their complex chemical structures, most dyes are biologically non-degradable and threaten aquatic ecosystems and human health.3 The release of these intensely colored waste effluents without appropriate treatment also creates visual disturbances and hinders the penetration of light, thus impacting various biological processes within the watercourse. More than 60% of the annual production of dyes consists of azo dyes, which are soluble in water, exhibit high reactivity, and offer the most comprehensive range of colors.4 Several water remediation approaches, including membrane separation,5 photocatalytic degradation,6 ion exchange,7 electrochemical oxidation,8 ozonation, coagulation,4 and adsorption,9,10 have been utilized to remove harmful dyes.11–13Adsorption is an up-and-coming and straightforward technology for removing hazardous and detrimental contaminants from liquid waste, offering a high ratio of removal efficiency to cost.14 In particular, porous materials such as aerogels are widely used absorbents for wastewater treatment.14 Aerogels are solid materials with a highly interconnected porous structure. They are known for their exceptional properties such as extremely low density, high porosity, significantly high specific surface area, and excellent adsorption capacity.15 The effectiveness of wastewater treatment processes is significantly influenced by the structural characteristics of aerogel composites, such as pore geometry (lamellar, dendritic, and cellular) and the type of structural framework (organic or inorganic).16–19 The pore size and its fine distribution in porous aerogels are essential for flow dynamics, mechanical stability, and the ability to adsorb liquids or gases.20 The potential use of ice-templating for developing porous aerogels with various pore morphologies has been reported in previous literature.16 This is known as directional freezing or freeze-casting, which can provide a variety of pore morphologies with extensive potential to eliminate contaminants from water. The freezing direction refers to the nucleation and growth of ice crystals, which are highly dependent on the solidification rate. The freezing-direction technique is classified into four groups: non-directional, unidirectional, bidirectional, and radial freezing.16,18 Integrating radial freezing with spraying can facilitate the development of colloidal particles with microporous 3D networks, providing a radial diffusion channel for mass transfer. In a recent study, Liao et al. prepared graphene oxide aerogel microspheres with dandelion-like structures through a radial-directional freezing–thawing process. The obtained aerogel microspheres with a highly porous and hydrophobic structure revealed excellent absorptivity for oils and organic solvents (60–214 g g−1).21 In another report, graphene/chitosan composite aerogel microspheres with microchannel structures were developed via electrospraying and freeze-drying methods. The fabricated microspheres demonstrated a remarkable Young's modulus of 197 kPa. Results revealed that the oil adsorption kinetics of the microspheres followed the pseudo-second-order kinetic equation, while the isotherms aligned with the Langmuir model.22
Porous biocompatibility, cost-effectiveness, renewability, non-toxicity, and biodegradability of the bio-adsorbents have generated interest in using them in wastewater treatment. A variety of micro/nano-biopolymer-based aerogels have been designed and developed for the removal of contaminants from water.23–26 Polysaccharides, including cellulose nanofibers (CNFs) and chitosan (Cs), are produced from abundant and cheap raw materials, offering promising alternatives in biomaterials. CNFs generally provide advantages such as exceptional strength, stiffness, safety, and the ability to tailor their surface chemistry, making them highly appropriate for fabricating structural materials such as aerogels.27 Hence, cellulose-derived materials are more likely to be utilized as cost-effective adsorbents for removing contaminants such as heavy metals, pesticides, dyes, and nitroarenes in water/wastewater.27–29 However, the limited functionality of CNFs hinders their effectiveness as a dye adsorbent. This can be improved by attaching functional moieties such as carbonyl, carboxyl, and amino groups to the surface of CNFs.30 Cs demonstrates significant promise within sewage disposal due to its hydroxyl and amine functional groups, which exhibit a strong capacity for the adsorption of anionic organic pollutants.22,31,32 However, pure Cs is limited by restricted surface area and inadequate mechanical properties. Incorporating Cs into CNFs matrix composite aerogels is a practical solution to enhance adsorption efficiency, improve stability, and increase surface area.33 In this regard, various reports considered the preparation of hybrid aerogels made from Cs and CNFs using the freeze-casting method. For instance, Kim et al. reported that cellulose–chitosan foam exhibited a higher adsorption capacity (1170.2 mg g−1) of CR compared to cellulose (623.2 mg g−1).34 In addition, Wang et al. prepared cellulose/chitosan porous aerogel to eliminate CR, for which the adsorption capacity of the dye reached 381.7 mg g−1 at pH 7.0.35
Incorporating functionalized active fillers such as whey protein fibrils into the composite materials can further enhance adsorption capacities.36,37 Proteins with an abundance of amino and carboxyl groups can actively participate in the adsorption process.38,39 Proteins can form polyelectrolyte complex (PEC) hydrogels with chitosan, eliminating the requirement for additional auxiliaries.40 For instance, Tan et al. fabricated the chitosan-quinoa bran aerogel using the ice-templated assembly method that efficiently adsorbed CR and Cu2+ with a maximum adsorption capacity of 182.48 and 96.25 mg g−1, respectively.41 In this study quinoa bran was used as the by-product of quinoa processing, which was directly mixed with the chitosan–acetic acid solution.
Under appropriate denaturation and hydrolysis conditions, whey protein, a by-product generated from the cheese-making process, can undergo self-assembly to form protein nanofibrils (PNFs).38,42–44 The environmentally friendly characteristics of the process contribute to developing a cost-efficient technology with a minimal ecological footprint. PNFs are bundles of elongated supramolecular filaments that exhibit a well-organized structure, primarily stabilized by β-sheet secondary structures. The structure of PNFs (several μm lengths and ∼10 nm width) provides an exceptionally high surface-to-volume ratio, ensuring that most amino acids are exposed on the surface.45 Consequently, PNFs exhibit exceptional efficacy in removing heavy metals, dyes, and radioactive pollutants.46–48 Notably, the flexible structure of PNFs promotes the formation of entangled networks even at low concentrations, making them exceptionally suitable for developing low-density and three-dimensional porous aerogels.43 Nonetheless, PNFs-based adsorbents suffer from weak mechanical properties.49–53 Hence, it is imperative to investigate effective modification techniques to enhance the characteristics of PNFs aerogel. We need to enhance the structural integrity of fibrillar networks of functional protein fibrils while maintaining functional surfaces.
In this study, we focused on creating a ternary bead composite using CNFs, Cs, and PNFs through the radial freeze-casting technique. Our work emphasizes the importance of integrating functional biomaterials (PNFs and Cs) into the fibrous structure of CNF. We used a simple chemical process with a homogenizer to prepare a Cs hydrogel with a neutral pH. Additionally, we formed spherical aerogels to increase the surface area and achieve the best adsorption efficiency.
We examined the impact of the CNFs/Cs/PNFs aerogel beads composite (CPCs) on the adsorption capabilities of various dyes. Specifically, we investigated the adsorption efficiency of CR, a commonly used azo dye, by analyzing dosage, dye concentrations, contact time, pH value, adsorption time, and initial concentrations. We measured CR adsorption isotherms and removal kinetics in the presence of CPCs aerogel beads. Meanwhile, the physical and chemical characteristics of the CPCs, including surface morphology, pore distribution, surface area, chemical composition, crystalline phase, and zero-charge point, were evaluated and analyzed. Finally, the adsorption mechanism was analyzed by using field emission scanning electron microscopy- energy dispersive X-ray spectroscopy (FE-SEM-EDX), Fourier transform infrared spectroscopy (FTIR), and X-ray photoelectron spectroscopy (XPS).
Experimental
Materials
Whey protein isolate (WPI) was provided by a dairy company, (Iran). Cellulose nanofibers with 3 wt% solid mass were mechanically prepared from wood pulp in Nanonovin Polymer Co. (Iran) (Fig. 1A). The polyamide-amine-epichlorohydrin (PAE) resin (20 wt%) as a wet strength agent for crosslinking was kindly provided from Solenis (Kymene GHP20, Solenis Denmark ApS). Chitosan (Mw: 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–300
000–300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, DD >85%) was purchased from Sigma-Aldrich Acros-Organics. Citric acid (CAS Number 77-92-9) was obtained from Sigma-Aldrich. Thioflavin-T (CAS Number 2390–54-7), Crystal Violet (CV, CAS Number 548-62-9, C.I. 42555), malachite Green (MG, CAS Number 569-64-2, C.I. 42000), Bismarck Brown-R (BB, 40%, CAS Number 5421–66-9, C.I. 21010), chrysoidine-G (CG, dye content: 90%, CAS Number 532–82-1, C.I. 11270), acid red-88 (AR, 75%, CAS Number 1658–56-6, C.I. 16250), reactive black-5 (RB, 50%, CAS Number 17095-24-8, C.I. 20505), Congo Red (CR, ≥37%, CAS Number 573–58-0, C.I. 22120), direct violet-51 (DV, 50%, CAS number 5489–77-0, C.I. 42520), and reactive orange-16 (RO, ≥70%, CAS Number 12225-83-1, CI 21260), sodium hydroxide (NaOH), acetic acid (CH3COOH), and hydrochloric acid (HCl, 37%w/v) were from Merck (Germany). All solutions were prepared using deionized water.
000 g mol−1, DD >85%) was purchased from Sigma-Aldrich Acros-Organics. Citric acid (CAS Number 77-92-9) was obtained from Sigma-Aldrich. Thioflavin-T (CAS Number 2390–54-7), Crystal Violet (CV, CAS Number 548-62-9, C.I. 42555), malachite Green (MG, CAS Number 569-64-2, C.I. 42000), Bismarck Brown-R (BB, 40%, CAS Number 5421–66-9, C.I. 21010), chrysoidine-G (CG, dye content: 90%, CAS Number 532–82-1, C.I. 11270), acid red-88 (AR, 75%, CAS Number 1658–56-6, C.I. 16250), reactive black-5 (RB, 50%, CAS Number 17095-24-8, C.I. 20505), Congo Red (CR, ≥37%, CAS Number 573–58-0, C.I. 22120), direct violet-51 (DV, 50%, CAS number 5489–77-0, C.I. 42520), and reactive orange-16 (RO, ≥70%, CAS Number 12225-83-1, CI 21260), sodium hydroxide (NaOH), acetic acid (CH3COOH), and hydrochloric acid (HCl, 37%w/v) were from Merck (Germany). All solutions were prepared using deionized water.
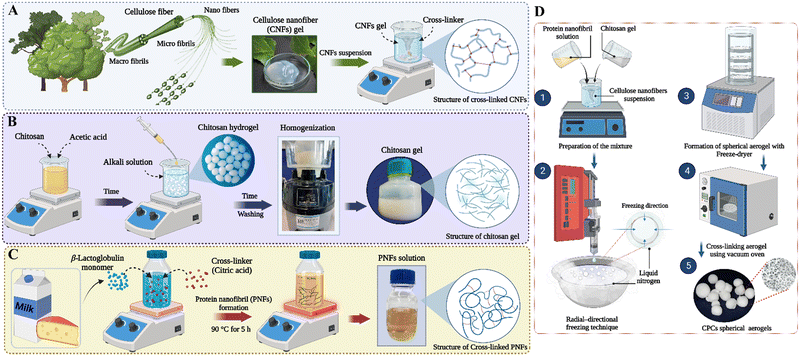 | ||
Fig. 1 Schematic illustration of fabricating composite aerogel beads. The preparation process of: (A) CNFs suspension, (B) chitosan hydrogel, and (C) protein nanofibrils from βLG monomers. (D) The schematic processes of producing the CPCs aerogels: The dispersion of CNF, PNF, and Cs at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 (CNFs 1.25 (CNFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PNFs PNFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cs) were prepared with agitating for 15 min.1 Next, the dispersion was frozen through the radial-directional freezing technique.2 Afterwards, the frozen aerogel beads were freeze-dried to obtain CPCs aerogel beads.3 The CPCs aerogel beads were dry-crosslinked in a vacuum oven (120 °C for 3 h).4,5 Cs) were prepared with agitating for 15 min.1 Next, the dispersion was frozen through the radial-directional freezing technique.2 Afterwards, the frozen aerogel beads were freeze-dried to obtain CPCs aerogel beads.3 The CPCs aerogel beads were dry-crosslinked in a vacuum oven (120 °C for 3 h).4,5 | ||
Preparation of chitosan hydrogel
Fig. 1B illustrates the procedure for preparing Cs beads. Initially, Cs powder (3% w/v) was dissolved in acetic acid (1% v/v) and stirred overnight to ensure complete dissolution. To create spherical Cs beads, the viscous chitosan–acetic acid solution was added drop-wise through a needle into a 200 mL bath containing NaOH 4M. The distance between the needle and the surface of the NaOH solution was 5 cm. This process consisted of two primary stages. First, droplets of the initial solution were dispersed and then underwent gelation when they encountered the liquid containing the cross-linking agent. Gelation occurred as sodium hydroxide diffused into the droplets, resulting in the formation of polymer cross-links. Thus, gel particles with a spherical shape were generated. These gel particles were subsequently filtered and rinsed with deionized water to eliminate any remaining alkalinity and form neutral Cs beads. Afterward, the resulting beads were added to 100 mL deionized water, and the dispersion was mixed vigorously using a mechanical mixer (Bead-Beater; Hamilton Beach Commercial) for 15 min to ensure homogenous hydrogel particles throughout the suspension. The weight fraction of the Cs hydrogel suspension was determined using gravimetric analysis.β-Lactoglobulin monomer purification
β-Lactoglobulin (βLG) monomers were purified from WPI according to the Usuelliet et al. protocol.53 Briefly, 10 g of WPI was dissolved in 90 g of Milli-Q water, and the pH was adjusted to 4.2 with HCl. Subsequently, the WPI solution was incubated in a shaking bath at 60 °C for 3 h. During this process, the WPI solution became cloudy as a sign of α-lactalbumin agglomeration. The turbid solution was then transferred to plastic Falcon tubes and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 r for 20 minutes to separate the α-lactalbumin fraction. The resulting solution was transferred into a Schott bottle.
000 r for 20 minutes to separate the α-lactalbumin fraction. The resulting solution was transferred into a Schott bottle.
Preparation of protein nanofibrils
The fibrillation of βLG (Fig. 1C) was followed according to the previously described procedures.49,54 Initially, the βLG monomer was dispersed in Milli-Q water at a concentration of 2 wt%. The pH of the solution was then lowered to pH 2 using HCl 2M. The βLG solution was incubated at 90 °C for 5 h, during which the protein solution underwent magnetic stirring at 150 rpm (PNFs). Subsequently, the protein solution was quenched in mixtures of ice and water to stop fibrillation. To enhance the interactions between protein fibrils, PNF-C1 and PNF-C1.5 samples were prepared by incorporating CA at 1 and 1.5wt% into the βLG dispersions. The same procedure was employed for their preparation. The as-prepared fibril solutions were kept at 4 °C for future usage. Notably, the βLG monomer underwent unfolding, hydrolysis, and self-assembly throughout the incubation process, ultimately forming PNFs.55 CA acts as a cross-linking agent in protein fibril formation. This contains carboxyl groups that can form covalent bonds with amino groups in the amyloidogenic proteins. These crosslinking interactions can stabilize the protein aggregates and promote the formation of PNFs.Fabrication of cellulose nanofibers/chitosan/protein nanofibrils aerogel beads
The schematic production of the CPCs aerogels is shown in Fig. 1D. Initially, a certain amount of CNFs suspension was dispersed in deionized water and stirred for 10 min at 300 rpm in an ice bath. Next, a weighed amount of PAE as a cross-linking agent (CNFs to the cross-linker ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was injected drop-wise into the CNFs dispersion. Then, the mixture was sonicated with an ultrasonic bath (at 250 Watt) for 10 min. In our previous study, the optimum component ratio for preparing CPCs composite aerogels was determined.56 We discovered that the best ratio of components, specifically 1
1) was injected drop-wise into the CNFs dispersion. Then, the mixture was sonicated with an ultrasonic bath (at 250 Watt) for 10 min. In our previous study, the optimum component ratio for preparing CPCs composite aerogels was determined.56 We discovered that the best ratio of components, specifically 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25
1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 (CNFs
1.25 (CNFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PNFs
PNFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cs), exhibited exceptional mechanical stress resistance in wet conditions. Furthermore, this optimal ratio not only showcased excellent performance in wet conditions but also led to the creation of a cylindrical composite aerogel. Herein, the resultant CNFs/PAE dispersion was progressively mixed with an equal amount of PNFs and Cs solutions (1.6 wt%) at a ratio of 1
Cs), exhibited exceptional mechanical stress resistance in wet conditions. Furthermore, this optimal ratio not only showcased excellent performance in wet conditions but also led to the creation of a cylindrical composite aerogel. Herein, the resultant CNFs/PAE dispersion was progressively mixed with an equal amount of PNFs and Cs solutions (1.6 wt%) at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25
1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25 (CNFs
1.25 (CNFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PNFs
PNFs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cs), and the mixture was stirred for 15 minutes in an ice bath (Fig. 1D-1). Afterward, the mixture was frozen via radial-directional freezing technique using liquid nitrogen (Fig. 1D-2) and further lyophilized in a freeze-dryer (Christ Freeze Dryer, Alpha 1–2 LD plus) at −80 °C for 24 h to obtain CPCs aerogel beads (Fig. 1D-3). Finally, the spherical aerogel beads were dry-crosslinked in a vacuum oven at 120 °C for 3 h to ensure covalent cross-linking (Fig. 1D-4,5). The CNFs and CNFs/Cs (CCs) aerogel beads were also formed as control samples following a similar protocol. The cross-linked pure CNFs aerogel beads were prepared from the aqueous solution of CNFs/PAE (1.6 wt%, 10
Cs), and the mixture was stirred for 15 minutes in an ice bath (Fig. 1D-1). Afterward, the mixture was frozen via radial-directional freezing technique using liquid nitrogen (Fig. 1D-2) and further lyophilized in a freeze-dryer (Christ Freeze Dryer, Alpha 1–2 LD plus) at −80 °C for 24 h to obtain CPCs aerogel beads (Fig. 1D-3). Finally, the spherical aerogel beads were dry-crosslinked in a vacuum oven at 120 °C for 3 h to ensure covalent cross-linking (Fig. 1D-4,5). The CNFs and CNFs/Cs (CCs) aerogel beads were also formed as control samples following a similar protocol. The cross-linked pure CNFs aerogel beads were prepared from the aqueous solution of CNFs/PAE (1.6 wt%, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). CCs aerogel beads were formed from the uniform mixture of CNFs and Cs suspensions (1.6 wt%) at a mass ratio of 3
1). CCs aerogel beads were formed from the uniform mixture of CNFs and Cs suspensions (1.6 wt%) at a mass ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.
7.
Material characterization
Fourier transform infrared spectroscopy (FTIR)
The chemical structure and functional groups of the freeze-dried PNFs, CNFs, Cs, and composite aerogel beads were investigated by FTIR (Bruker Tensor 27 spectrometer, Karlsruhe) in the range of 4000 to 400 cm−1. Each dry sample was grounded, blended with KBr (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, w/w), and then pressed to prepare transparent pellets.
100, w/w), and then pressed to prepare transparent pellets.
Wide-angle X-ray diffraction (XRD)
The XRD patterns of the freeze-dried PNFs, CNFs, Cs, and composite aerogel beads were recorded using an X-ray diffractometer (Equinox 3000 INEL) equipped with CuKα (λ = 1.54056 nm) radiation (operating at 40 kV and 40 mA). Measurements were performed in the 2θ range 5–40° with a step size of 0.026° at 25 °C.Zeta potential
The surface area and the surface charges of the composite aerogel beads (CPCs) were calculated using zeta potential (Malvern, UK) at 25 °C. The dry CPCs aerogel beads (15 mg) were added into 20 mL of deionized water and the pH of the suspension was adjusted with NaOH 0.1 N and hydrochloric acid to 7.4. After 24 h, the supernatant was collected for zeta potential measurements.X-ray photon spectroscopy (XPS)
A high-resolution X-ray photoelectron spectrometer (UIVAC-PHI) (XPS, Bes Tek instrument, Germany) was utilized to record the surface composition of CPCs aerogel for C 1s, N 1s, S 2p, and O 1s elements. The instrument was equipped with an Al-Kα source (1486.6 eV, 45 W), and the CPCs aerogel beads were conditioned and investigated at 10−10 mbar. The XPS data were analyzed using CasaXPS v2.3.2PR1.0 software.Adsorption experiments
The developed aerogel composite beads (0.0025–0.075 mg) were added to 25 mL−1 of dye solution (50–2000 mg L−1), while the pH medium was adjusted in the range of 4–10 using NaOH 0.1 M and HCl. The mixture of adsorbent and dye was agitated using a rotator shaker at a speed of 100 rpm and a temperature of 28 °C. At specific time intervals (1–180 min), the residual dye concentration was estimated using an EPOCH12 plate reader (BioTek, Winooski, Vermont, USA) at their maximum absorbance wavelength (λMax: at 497 nm for CR, 590 nm for CV, 620 nm for MG, 580 nm for BB, 470 nm for Chr, 500 nm for AR, 590 nm for DV, 554 nm for RB, and 493 nm for RO). The dye adsorption capacity at equilibrium (qe, mg g−1, eqn (1)) and the removal efficiency (η, %, eqn (2)) onto aerogel beads were calculated according to the following equations: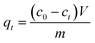 | (1) |
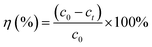 | (2) |
Results and discussion
Preparation of spherical composite aerogel
Tunable physicochemical properties, such as porosity, pore size, surface area, surface roughness, permeability, and function ability, directly influence the performance of an adsorbent to remove various contaminants efficiently. Hydrophilic composite sorbents, such as Cs and CNF-based aerogels, demonstrate strong molecular interactions with water, enabling efficient molecular transport in wastewater treatment processes. Furthermore, the polysaccharide polymer chains contain a large number of hydroxyl and amine groups, which enables them to be easily modified for various applications. Incorporating functional PNFs within the surface pores of polysaccharide-based aerogels enhances accessibility and affinity toward the targeted pollutants,20,53 further suggesting that the functional PNFs can be blended with polycationic moieties such as Cs to form polyelectrolyte complexed hydrogels.40 In this study, porous cross-linked CNFs aerogel beads with the ability to support functional biopolymers such as Cs and PNFs were developed. The covalent cross-linking between native nanofibrils and cross-linkers (PAE) led to a cross-linked fibrous CNFs aerogel with high stability in aqueous solution.17,26The schematic diagram in Fig. 1A–D shows the preparation procedure of the functional composite spherical aerogel using an ice-templating technique (Fig. 1D) from as-prepared precursors, namely, a natural CNFs suspension (Fig. 1A), a Cs hydrogel (Fig. 1B), and a PNFs solution (Fig. 1D). In the hydrogel state (Fig. 1B), Cs polymeric chains are physically interconnected by interactions such as hydrogen bonding and electrostatic attractions, which are the dominant interactions for forming the 3D network of Cs beads. In fact, at low pH (diluted acidic solution), Cs is positively charged, and through neutralizing the cationic amine sites (–NH3+) are deprotonated to –NH2, resulting in a significant reduction in ionic repulsion within polymer chains, enabling the emergence of hydrogen bonding between polymeric chains.40 The PNFs of βLG are semi-flexible colloids obtained from the protein aggregates. At specific fibril concentrations, these fibrils are self-assembled and create physical hydrogels. However, the elastic moduli of PNFs gels are often low (10–50 Pa), resulting in a weak fibril network59 that restricts further applications. One approach to boost the strength and mechanical properties of PNFs gels is using low molecular weight ions to form an ionic cross-linked matrix. Nyström et al. prepared the first cross-linked PNFs aerogel with high water stability only by adding butane tetracarboxylic acid to the fibril dispersion. However, in this investigation, citric acid (CA, cheap non-toxic polycarboxylic acid) was added to the protein solution to strengthen the PNFs network (Fig. 1C). As a hypothesis, CA is assumed to be uniformly distributed within the PNFs gel network, enhancing the electrostatic interaction between the amino group of the PNFs polymeric chain and the carboxylic group of citrate, resulting in a cross-linked PNFs gel (Fig. 1C). This hypothesis was further validated using analytical approaches (Fig. S1A and B, ESI†).
FE-SEM analysis
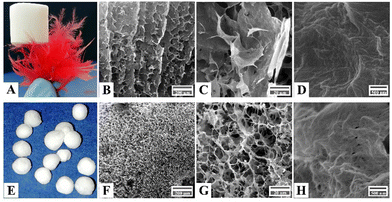
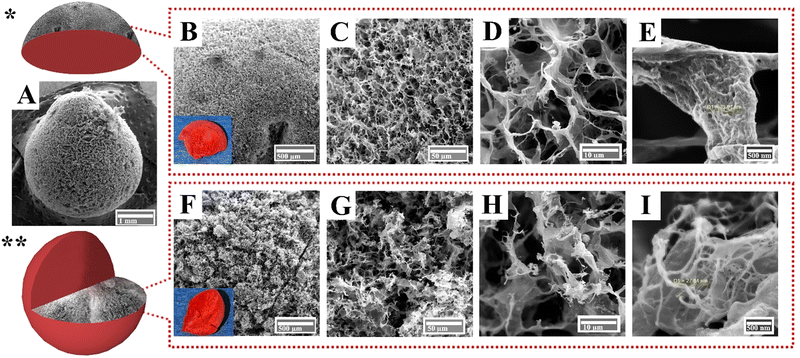
The surface morphological features of pristine CNFs, Cs, and PNFs, as well as an aerogel bead composite (CPCs), were investigated by FE-SEM. The CNFs aerogel bead with a concentration of 1.6 wt% (Fig. S2A, ESI†) exhibited a porous structure with a wide range of pore sizes classified into small and large pores (Fig. S2B–D, ESI†). The tiny pores were formed due to nanofiber network entanglement in cellulose platelets, whereas the large pores were those created as a result of ice crystal sublimation during the freeze-drying.17 The surface morphology of the freeze-dried Cs gel, on the other hand, revealed an uneven porous structure (Fig. S2E, ESI†). The crack in Fig. S2F (ESI†) resulted from the growth of ice lamella within the Cs gel and the formation of interstitial space between accumulated Cs during the freeze-drying. However, high-magnification FE-SEM images (Fig. S2G and H, ESI†) revealed the high porosity and the fibrillar interconnected structure of freeze-dried Cs gel. These features are highly beneficial for developing a bio-based composite aerogel. The surface morphology analysis of pristine PNFs derived from βLG indicated smooth, compact layered sheets (Fig. S2I and J, ESI†). The surface of PNFs contained multiple cracks extending several micrometers, as illustrated in Fig. S2J and K (ESI†). However, high magnification FE-SEM micrographs (Fig. S2L, ESI†) revealed smooth, homogeneous, and paper-like accumulating layers of the PNFs.To obtain a porous aerogel with superior pore geometry and rational pore size distribution for waste-water applications, two distinct freezing mechanisms of unidirectional freeze-casting and radial freezing were employed. In unidirectional freeze-casting, the CPCs dispersion was cast into a cylindrical mold placed directly with liquid nitrogen from the bottom for a defined period. The ice nucleated and grew unidirectionally from the bottom to the top of the mold along the imposed thermal gradient. Subsequently, the ice crystals were sublimated using a freeze-drying process and formed a 3D monolith aerogel (Fig. 2A).
Unlike unidirectional freezing, radial freezing consists of two steps: spraying and ice-templating.19,60 In radial freezing, the CPCs dispersion was dropped into liquid nitrogen via an electric nozzle tube. Ice crystals immediately started to nucleate and grow from the surface of droplets into the interior along radial directions. Finally, the frozen spherical samples were freeze-dried to sublimate the ice crystals inside the structure and translated to porous aerogels in 3D spherical shapes (Fig. 2E).
Fig. 2A shows an ultra-light cylindrical composite aerogel (supported by a dandelion) generated by an initial gel with a concentration of 1.6 wt%. FE-SEM images in Fig. 2B and C revealed a hierarchical porous structure of the cylindrical composite aerogel prepared by the unidirectional freezing technique. The lamellar walls observed in Fig. 2B resulted from the pushing forces applied by the growing ice crystals, which constrained the solid polymers into the space between the ice.17,60 Specifically, fractions of the solid polymer were trapped in ice crystals, causing the formation of inter-layer bridges formation between the lamellar walls (Fig. 2C). While the cylindrical composite aerogel was macro-porous, mesoporous structures (diameters between 2 and 50 nm) were difficult to detect on the walls of aerogel at higher magnification (Fig. 2D).
The technique of radial-freezing successfully created an open porous aerogel with uniform pore size distribution (Fig. 2F–H). Unlike the cylindrical composite aerogel (Fig. 2B), the surface morphology of CPCs spherical aerogel displayed a hierarchical porous structure with cellular interconnected pores (Fig. 2G). Interestingly, the composite spherical aerogel presented superior pore geometry, in which the interlinked pores provide pathways for rapid diffusion of fluids from the surface to the interior fractions (Fig. 2G). Considerably, fine-scaled pores were observed inside the thin walls of the channel (Fig. 2H). As shown in Fig. 2H, these pores facilitate superior mass transport and uniform fluid dispersion inside the porous structure. As a result, the radial freezing approach was chosen for creating spherical composite aerogels due to the enhanced pore morphology, as well as the simplicity, accessibility, and reproducibility of the process.16,19,61
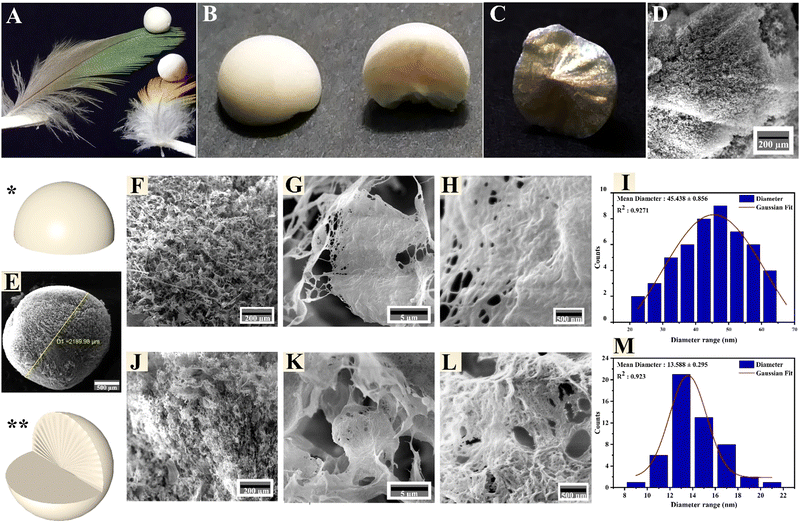
The CPCs aerogel beads had a well-preserved spherical shape that can sit firmly on top of a feather without disrupting its barbs (Fig. 3A). The surface (left) and the cross-section (right) of a perfectly spherical aerogel prepared through a radial-directional freezing mechanism are depicted in Fig. 3B. The inner structure of the CPCs aerogel sphere revealed a radial-patterned-like shape, indicating that the CPCs spherical droplets uniformly cooled from the surface to the interior; hence, the ice crystals nucleate and grow along the radial directions and form dandelion-like structure (Fig. 3C).19,21,62 The FE-SEM image of the lyophilized spherical aerogel revealed a radial-center-diverging microchannel structure (Fig. 3D) and a highly porous morphology.63Fig. 3E shows the external surface of the porous CPCs aerogel bead, which had an average diameter of 2.2 mm. The surface morphology of the CPCs aerogel (Fig. 3F) revealed a network of interconnected pores with ultra-thin and uniform film-like walls of assembled composite. Smaller pores were also observed in Fig. 3G and H. The obtained aerogel bead exhibited a dandelion-like structure in the radial cross-section (Fig. 3J), in which the channels/pores of the aerogel were radially aligned using a radial freezing technique. The pore channels of CPCs aerogel are essential factors affecting the structure and physicochemical properties of the CPCs aerogel.
These cellular interconnected pore channels of CPCs aerogel provide abundant transport pathways between multiple porous layers, capable of taking up, separating and removing dye molecules which can furthermore interact with functional groups (–COOH, –OH, and –NH2) within the pore channels. As shown in Fig. 3K, the thin layers of open cells in the aerogel contained a multitude of pores, creating a highly porous structure that allows for effective diffusion and adsorption of liquids. Additionally, the interconnected network of pores contributes to the mechanical stability of the aerogel, preventing collapse or deformation. High magnification FE-SEM images of CPCs aerogel in Fig. 3H and L revealed that the porous platelets of the aerogel were composed of interconnected fibrils with different size distributions. According to the statistics obtained from Digimizer software, the individual fibrils on the surface of the CPCs composite aerogel had a mean diameter of ∼45 nm (Fig. 3I), while fibrils with a mean diameter of ∼14 nm were observed within the interior walls of the porous aerogel (Fig. 3M).
Specific surface and pore structure of CPC
Brunauer–Emmett–Teller (BET) analysis was used to investigate the surface area of as-prepared aerogel beads. The results shown in Fig. 4, demonstrated accurate information about the specific surface area and porosity characteristic (pore volume and pore size) of CPCs aerogel. According to the isotherm classification proposed by IUPAC,64 the BET isotherm of CPCs aerogel revealed a typical class IV hysteresis loop with a sharp capillary condensation step at a relative pressure (P/P0) of 0.5–1, indicating the presence of mesopores and macropores of aerogel beads.65 Based on the nitrogen adsorption–desorption isotherms, the calculated specific surface area and the total pore volume were 29.61 m2 g−1 and 0.2 cm3 g−1, respectively. The Barrett–Joyner–Halender (BJH) pore-size distribution curve of CPCs aerogel in the inset to Fig. 4 showed a bimodal distribution with a sharp peak at 5 nm followed by a broader peak centered at 12 nm, demonstrating that the CPCs aerogel beads were mostly mesoporous.64 Expectedly, the results of BET were consistent with the high-resolution FE-SEM observations, implying that the CPCs aerogel has a porous structure consisting of mesoporous and flexible thin walls, which could endow the CPCs aerogel with enhanced adsorption capacity and rapid mass transportation of liquid.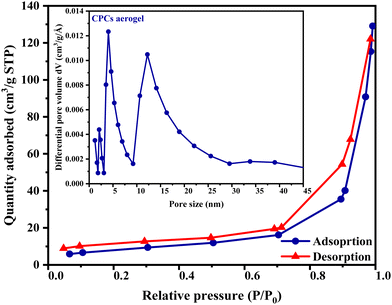 | ||
| Fig. 4 Surface and pore characteristics of CPCs aerogel: N2 adsorption/desorption isotherms (inset shows the pore size distribution for CPCs aerogel). | ||
Chemical structure analysis
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O backbone stretching vibrations of the amide I group of polypeptide chains. In addition, the bands at 1540 cm−1 and 1217 cm−1 are associated with a combination of C–N stretching and N–H in-plane bending (amid II) and amid III regions in protein, respectively.12,43,54 The wide amide band at 3286 cm−1 (N–H stretching) represented the strength of intermolecular hydrogen bonding in PNFs.66 No specific band attributed to a backbone C–C stretching vibration in the 850–960 cm−1 range was detected. In conjunction with the absorption peak of the amide II region, this result indicated that the protein might have the structure of random-coil.66 Upon cross-linking with CA (Fig. S3 and Fig. 5A, PNF-C1, ESI†), a new intense peak arose at 1720 cm−1, attributing to the carbonyl ester groups, resulting from chemical cross-linking interactions within the –COO− group of CA and –N–H group of PNFs.3 The amide I and amid II peak positions remained unchanged for cross-linked protein fibrils (PNFs-C), while the peak intensities were moderately reduced. After cross-linking, the peak position of the amide III at 1235 cm−1 shifted to a lower wavelength (1217 cm−1) and merged with the C–C and C–O stretching vibrations peaks in CA. These findings confirm the occurrence of the cross-linking between PNFs and are consistent with previous results that investigated the FTIR spectra of amyloid fibrils after cross-linking with 1,2,3,4 butanetetracarboxylic acid (BTCA).59,67 The spectrum of CNFs aerogel beads exhibited a strong peak in the 898–1160 cm−1 region, referred to as C–O stretching in the glycoside linkage,17,68 which represents the prominent feature peak of polysaccharides (Fig. 5A, CNFs). Notably, the detected peaks at 1640 cm−1 (carbonyl group of amide I) and 1550 cm−1 (N–H bending of amide II) confirmed the presence of PAE as a crosslinker and suggested the formation of self-crosslinking through its epichlorohydrin groups and the external covalent crosslinking between CNFs chains after heat treatment.17
O backbone stretching vibrations of the amide I group of polypeptide chains. In addition, the bands at 1540 cm−1 and 1217 cm−1 are associated with a combination of C–N stretching and N–H in-plane bending (amid II) and amid III regions in protein, respectively.12,43,54 The wide amide band at 3286 cm−1 (N–H stretching) represented the strength of intermolecular hydrogen bonding in PNFs.66 No specific band attributed to a backbone C–C stretching vibration in the 850–960 cm−1 range was detected. In conjunction with the absorption peak of the amide II region, this result indicated that the protein might have the structure of random-coil.66 Upon cross-linking with CA (Fig. S3 and Fig. 5A, PNF-C1, ESI†), a new intense peak arose at 1720 cm−1, attributing to the carbonyl ester groups, resulting from chemical cross-linking interactions within the –COO− group of CA and –N–H group of PNFs.3 The amide I and amid II peak positions remained unchanged for cross-linked protein fibrils (PNFs-C), while the peak intensities were moderately reduced. After cross-linking, the peak position of the amide III at 1235 cm−1 shifted to a lower wavelength (1217 cm−1) and merged with the C–C and C–O stretching vibrations peaks in CA. These findings confirm the occurrence of the cross-linking between PNFs and are consistent with previous results that investigated the FTIR spectra of amyloid fibrils after cross-linking with 1,2,3,4 butanetetracarboxylic acid (BTCA).59,67 The spectrum of CNFs aerogel beads exhibited a strong peak in the 898–1160 cm−1 region, referred to as C–O stretching in the glycoside linkage,17,68 which represents the prominent feature peak of polysaccharides (Fig. 5A, CNFs). Notably, the detected peaks at 1640 cm−1 (carbonyl group of amide I) and 1550 cm−1 (N–H bending of amide II) confirmed the presence of PAE as a crosslinker and suggested the formation of self-crosslinking through its epichlorohydrin groups and the external covalent crosslinking between CNFs chains after heat treatment.17
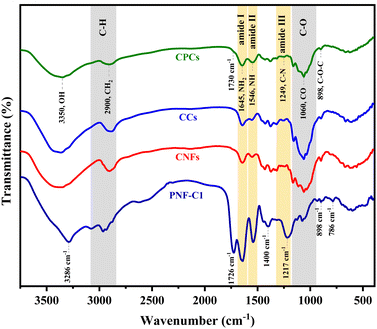 | ||
| Fig. 5 FTIR spectra of CCs and CPCs composite aerogel beads and their counterparts (CNFs, Cs, and PNFs). | ||
The characteristic peaks of CCs aerogel beads (Fig. 5A, CCs) were entirely similar to that of the CNFs (Fig. 1B), even though the peaks at 3367 cm−1 and 1060 cm−1 became sharper.69,70 Moreover, the adsorption peaks of carbonyl (amide I) and N–H groups in Cs merged with the functional groups of the bonded PAE, and thus, a broader peak in the 1520–1690 cm−1 range appeared. These results also indicated that the CCs aerobead structure was effectively crosslinked via intermolecular interactions between CNFs, PAE, and Cs. Compared with CNFs, CCs, and PNFs-C1 aerogel beads, the FTIR spectrum of CPCs composite aerogel revealed the functional groups of its original components (Fig. 5A, CPCs). However, the characteristic bands of Cs and PNFs-C1 were merged with that of the CNFs (the major component of the composite aerogel), making them challenging to recognize individually. Nevertheless, after the incorporation of PNFs into the CCs hybrid composite, the peak intensities of amide I (1645 cm−1) and amide II (1546 cm−1) increased significantly, suggesting an interaction of PNFs with the CNFs and Cs in CPCs aerogels.71 Moreover, in comparison with the spectrum of PNFs (crosslinked with CA), a weak shoulder at 1730 cm−1 was observed, which was attributed to the formation of an intermolecular carbonyl band as a result of the cross-linking reaction between PNFs and CA59 (Fig. S3, ESI†).
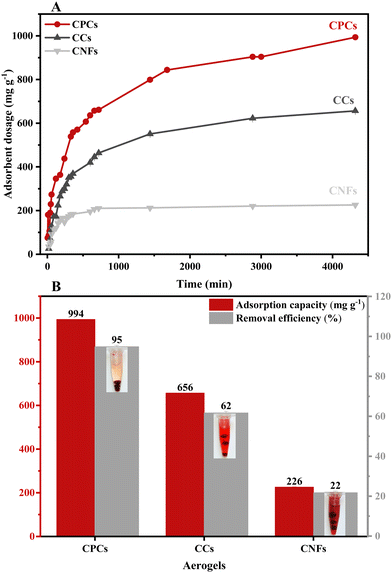 | ||
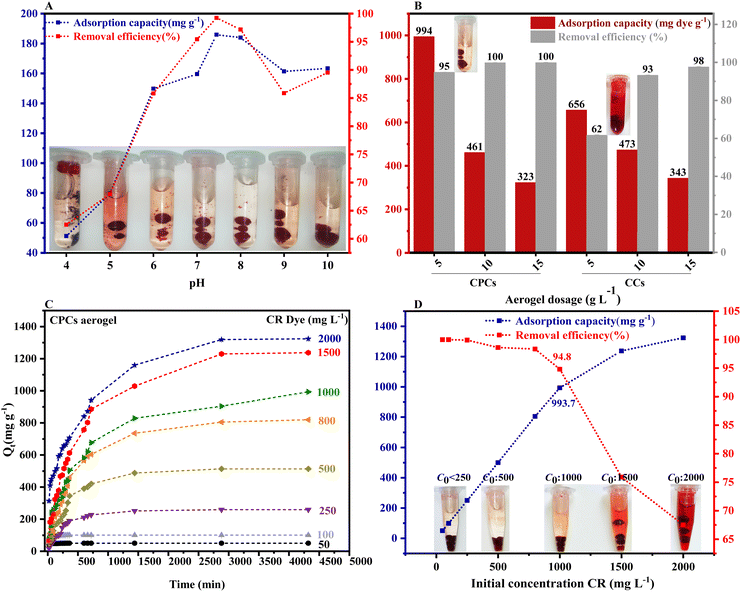
| Fig. 7 (A) The effect of contact time on the removal of CR by various aerogel beads, including CNFs, CCs, and CPCs. (B) The equilibrium adsorption and removal efficiency of CNFs, CCs, and CPCs aerogel beads (CR concentration:1000 mg L−1, adsorbent dosage:1 g L−1, pH:7.4). In Fig. 7B, the inset images show the successful removal of CR dye by CPCs aerogel. | ||
The Langmuir model hypothesizes that the adsorbent is homogenous, and the adsorption of a solute from solution occurs at energetically equivalent sites.88 As presented in Table 1, the Langmuir equation contains two parameters: qm (mg g−1), the maximum adsorption capacity, and KL (L mg−1), the Langmuir constant attributed to the apparent energy of sorption. The qe (mg g−1) and Ce are the equilibrium adsorption capacities and adsorbate concentration.
The Freundlich isotherm model describes multilayer adsorption onto heterogeneous surfaces.89 The two parameters of the Freundlich equation are KF (Freundlich constant) and nF (surface heterogeneity). The 1/nF factor represents the adsorption intensity, indicating the site energy distribution and heterogeneity of the adsorbate. Notably, the adsorption is favorable when 1/nF values are between 0 and 1 (0 < 1/nF < 1).
The Toth isotherm is a modified variant of the Langmuir equation, aiming to improve the fitting by minimizing the error between the experimental data and estimated value.90 The KT (L mg−1), nT, and qm (mg g−1) are the Toth constants and maximum adsorption capacity, respectively.
As a hybrid isotherm model, the Redlich–Peterson contains three parameters (KRP (L g−1), nRP, and aRP (L mg−1)), which comprise the feature of both Langmuir and Freundlich isotherms.91,92 The nRP is an exponent which lies between zero and one. When nRP is close to 1, the Redlich–Peterson approaches the Langmuir condition.
Sip isotherm is the combined form of the Langmuir and Freundlich isotherm models, developed to overcome the limitations of increasing adsorbate concentration primarily experienced in the Freundlich isotherm model.93 The parameters qm (mg g−1), KS (L g−1), and nS are the Sips maximum adsorption capacity and the Sip isotherm constant and exponent, respectively.
The nonlinear fitting of adsorption isotherm models to the equilibrium experimental data, derived from the adsorption of CR onto CPCs composite aerogel, is shown in Fig. 8. The adsorption profile of all isotherm models demonstrated a similar pattern. The predicted isotherm parameters for non-linear models (i.e., two and three-parameter models) are all fully presented in Table 1. Overall, comparison of the value of the correlation coefficient (R2) (Table 1) that three-parameter models estimated a higher correlation coefficient (>0.992) than two-parameter models.92 Furthermore, according to the presented data, the non-linearized Sip, Redlich–Peterson, and Langmuir isotherms provided a much better fit to the equilibrium experimental data than Toth and Freundlich isotherms. Among two-parameter models, the correlation coefficient (R2) value of the Langmuir model (0.982) was higher than that of the Freundlich isotherm (0.955), revealing that the Langmuir isotherm model yielded a better fit. In addition, the maximum adsorption capacity (qm) obtained by applying the Langmuir model equation was 1286.61 ± 52.07 mg g−1, which was far from the value predicted by Freundlich isotherm (347.24 ± 53.9). Notably, the constant parameter of 1/nF from the Freundlich equation is less than one (0.23), demonstrating that the CR dye is favorably adsorbed by porous CPCs aerogel bead. According to the R2 value of three-parameter models (Table 1), the correlation coefficient of Sip isotherm was higher (0.996) than the Redlich–Peterson and Toth models, which had equal values (0.9926), suggesting that the adsorption phenomena of CPCs composite bead aerogel are well described by Sip isotherm assumption.94–96 As shown in Fig. 8, the Sip isotherm curve acceptably fit the experimental data, and the shape of the curve was highly correlated with the characteristic of the Langmuir isotherm model.
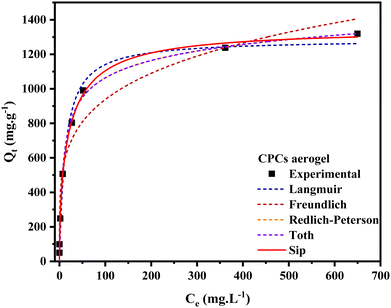 | ||
| Fig. 8 Nonlinear fitting of adsorption isotherm models to the equilibrium experimental data obtained from the adsorption of the CR onto the CPCs aerogel bead. | ||
Moreover, the Sip isotherm is identified by the heterogeneity factor, nS, and when nS = 1, the Sip model reduces to Langmuir isotherm.75,85,97 Herein, the nS value was 0.9, approximately, demonstrating that the adsorption process of CR dye onto CPCs aerogel bead was homogeneous and referred explicitly to monolayer adsorption. The maximum adsorption capacity calculated from Sip isotherm was 1349.77 ± 34.36 mg g−1. This high adsorption capacity is resulted from the impregnation of functional biomaterials (PNFs and Cs) into the CPCs composite aerogel, indicating that CPCs have the best capability to remove anionic dye from an aqueous solution. The extent of adsorption obtained from the two parameters of qm (794.73 ± 15.13) and the ratio of KRP/aRP (754.8 ± 10.8) related to Toth and Redlich–Peterson equations, respectively, were comparable.92,98 Consequently, adsorption mechanism follows the monolayer adsorption of CR dye on a homogeneous surface of CPCs composite aerogel beads, in which all active sites have a similar affinity for the adsorbate.
| qt = qe(1 − e−k1t) | (3) |
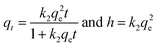 | (4) |
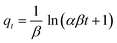 | (5) |
| qt = kidt1/2 + Ci | (6) |
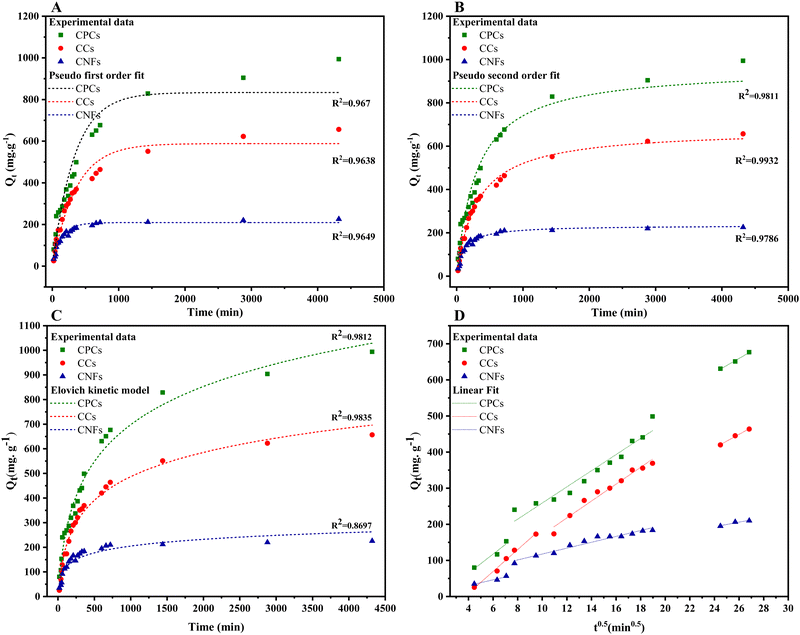
Fig. 9 presented the non-linear fitting of PFO, PSO, and the Elovich kinetic models for CR adsorption at the initial concentration of 1000 mg L−1 onto aerogels with various compositions, i.e., single-component (CNFs), hybrid (CCs), and three-component (CPCs) aerogel composite beads. Rate constants and other parameters of these kinetic models are summarized in Table 2. The coefficient of determination (R2) was used to assess the validity of adsorption kinetic models. Among nonlinear kinetic models exhibited in Fig. 9A–C, the PSO kinetic model (Fig. 9B) was fitted well to the experimental data. Moreover, the R2 value from the non-linear form of the PSO model (R2 ≥ 0.978) (Table 2) was higher than those of the PFO (R2 ≥ 0.965) and the Elovich models (R2 ≥ 0.869), suggesting that CR adsorption onto the aerogel beads (CNFs, CCs, and CPCs) was well expounded by PSO model which means that chemisorption might be the dominating mechanism of adsorption98,103,104 Furthermore, the theoretical adsorption capacity (qe,cal) of all types of aerogels calculated from PSO equation correlated strongly with the experimental adsorption capacity (qe,exp, 994 mg g−1) (Table 1). In the case of CPCs aerogel composite bead, for instance, qe,exp was 994 mg g−1; however, the qe,cal value obtained from PFO and PSO equation were 833.74 mg g−1 and 962.41 mg g−1, respectively, revealing an insignificant difference between qe,exp and qe,cal in PSO kinetic model. Similarly, the qe,cal values for CCs (679.36 mg g−1) and CNFs (234.41 mg g−1) aerogel beads from PSO were close to their values of qe,exp, which were 656 mg g−1 and 226 mg g−1, respectively. Notably, the plot of Qtvs. time for all aerogel beads (Fig. 9A–C) exhibited the rapid adsorption of CR on the outer surface of aerogel beads for the first 60 min. Concerning the PSO initial adsorption rate constant, h, the values reported in Table 2 increased as biomaterials such as Cs and PNFs with various functional groups were impregnated in the porous matrix of CNFs aerogel beads (CPCs).105 In the case of CNFs aerogel beads, for instance, the adsorption rate was around 0.021 (mg g−1 min−1); however, the mass transfer rate increased incredibly by almost 313.42% by the addition of Cs and PNFs to the aerogel structure (CPCs). The presence of accessible active sites on the surface of aerogels and the highly porous structure of CPCs aerogel beads provided an excellent affinity for adsorbing higher amounts of CR molecules. Consequently, the adsorption capacity of composite aerogel is enhanced to a greater extent. Thus, these results uncovered the outstanding performance of functionalized CNFs composite aerogel beads complexed with Cs and PNFs.
| Kinetic model | Pseudo-first order | Pseudo-second order | Elovich | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| q t = qe × (1 − eK1t) | |||||||||||
| q e,cal [mg g−1] | K 1 [10−1 min−1] | R 2 | q e,cal [mg g−1] | K 2 [10−6g mg−1 min−1] | h mg [g min−1] | R 2 | α [mg g−1 min−1] | β [g mg−1] | R 2 | ||
| Adsorbate | CNFs | 209 | 0.07 | 0.965 | 234.41 | 0.4 | 0.021 | 0.978 | 6.89 | 0.025 | 0.869 |
| CCs | 588.5 | 0.03 | 0.964 | 679.36 | 4.81 | 2.21 | 0.993 | 3.944 | 0.006 | 0.983 | |
| CPCs | 833.74 | 0.03 | 0.967 | 962.41 | 3.46 | 3.2 | 0.981 | 4.44 | 0.004 | 0.971 | |
Overall, adsorption kinetics are governed by multiple subsequent steps, i.e., film diffusion, surface diffusion, and intra-particle diffusion, of which intraparticle diffusion is considered the most rate-limiting step.87,106 The intraparticle diffusion model (eqn (4)) was used to study the diffusion mechanism, and the calculated results are presented in Fig. 9 and Table 2. The diffusion mechanism includes an initial portion attributed to boundary layer diffusion in which the dye molecules migrate from the bulk solution to the adsorbent and pass a boundary layer, rapid adsorption on the exterior surface of the adsorbent called surface adsorption, followed by a steady adsorption stage where dye molecules enter the pores of the sorbent and are known as the intra-particle diffusion. Lastly, a plateau to equilibrium was attained at the final stage, where the interparticle diffusion tended to reduce as the adsorbate concentration in the solution and accessible adsorption sites decreased.85,86,107 As shown in Fig. 9, the intra-particle diffusion curve of Qtversus t0.5 exhibited three linear regions for all aerogel beads (i.e., CNFs, CCs, and CPCs) that did not pass through the origin, expounding three steps governed the adsorption of CR onto the aerogels.
Furthermore, the negative intercept value (C) of the initial line implied minimum boundary-layer resistance. Overall, the ki value of all aerogels in each step followed the order of k1d > k2d > k3d, in which the initial sharp slope (k1d) corresponded to the accelerated migration of CR molecules from the aqueous solution to the exterior surface of aerogels (film or surface diffusion), the second moderate slope (k2d) demonstrated the steady adsorption of CR into the interior surface of porous aerogel beads which was also the rate-controlling step, and k3d represented the final step where the slow diffusion of CR molecules from larger pores into the macro and micro-pores caused a reduction in adsorption rate. According to Table 3, the highest and lowest values of k1d were observed for the CCs (30.3 mg g−1min−0.5) and CNFs (8.14 mg g−1min−0.5) aerogel beads, respectively. Surprisingly, the value of k1d for CCs hybrid aerogel beads was higher than that (26.5 mg g−1min−0.5) of the CPCs composite aerogel beads, indicating that the mass transfer of CR molecule from the solution to the outer surface of hybrid aerogel bead was faster than surface adsorption process of CPCs composite aerogel. In addition, the kid values of CCs aerogel in the second and third stages were about 23 mg g−1min−0.5 and 19 mg g−1min−0.5, respectively, which were approximately close to the k2d and k3d values obtained for CPCs composite aerogels. Comparing the value of R2 in the second and third steps, we propose that the mixture of pore diffusion and intra-particle diffusion is the rate-limiting step of the CR adsorption onto the composite aerogel beads108.
| Aerogel | First stage | Second stage | Third stage | ||||
|---|---|---|---|---|---|---|---|
| C | k 1d (mg g−1min−0.5) | R 2 | k 2d (mg g−1min−0.5) | R 2 | k 3d (mg g−1min−0.5) | R 2 | |
| CNFs | 8.14 ± 1.88 | 0.946 | 7.89 ± 0.59 | 0.954 | 6.4 ± 1.7 | 0.937 | |
| CCs | 30.27 ± 1.79 | 0.989 | 23.38 ± 1.6 | 0.97 | 18.814 ± 1.47 | 0.9939 | |
| CPCs | 26.49 ± 6.49 | 0.943 | 22.34 ± 1.84 | 0.942 | 19.59 ± 1.67 | 0.9927 | |
Upon comparing the outcomes of this study to the results of previous research on the adsorption capacities of different adsorbents for CR dye in aqueous solutions, it can be concluded that our results are highly favorable (Table S2, ESI†). All of the data collected were successfully fitted to the pseudo-second-order kinetic model.
The FTIR spectra of CPCs before and after adsorption of 1000 mg L−1 CR (Fig. 11A, red line), reveal new peaks at 1040, 1175, 1228, and 1373 cm−1, ascribed to S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching of the –SO3 group, while other peaks at 835 and 1459 cm−1 are attributed to aromatic backbone vibration of CR.69,70,109,110 A weak shoulder was observed at 1586 cm−1, attributed to the –N = N- stretching vibration, and the adsorption band of amide I was shifted to a lower wavelength (1618 cm−1).111 These findings indicated that CR was strongly adsorbed by CPCs composite aerogel.
O stretching of the –SO3 group, while other peaks at 835 and 1459 cm−1 are attributed to aromatic backbone vibration of CR.69,70,109,110 A weak shoulder was observed at 1586 cm−1, attributed to the –N = N- stretching vibration, and the adsorption band of amide I was shifted to a lower wavelength (1618 cm−1).111 These findings indicated that CR was strongly adsorbed by CPCs composite aerogel.
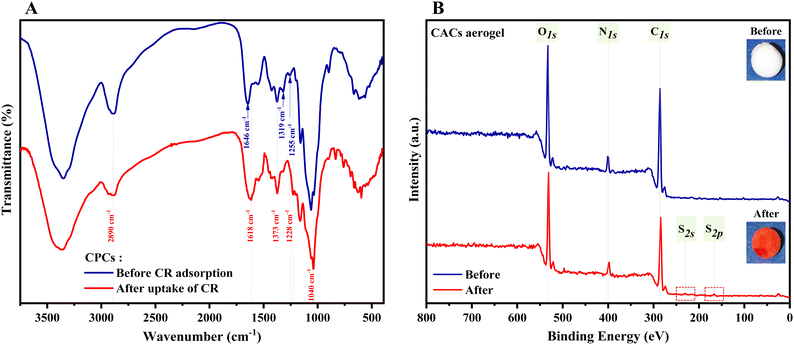 | ||
| Fig. 11 (A) FTIR and, (B) XPS survey spectra of CPCs aerogel before (blue line) and after (red line) CR adsorption (initial concentration of 1000 mg L−1) at pH = 7.4. | ||
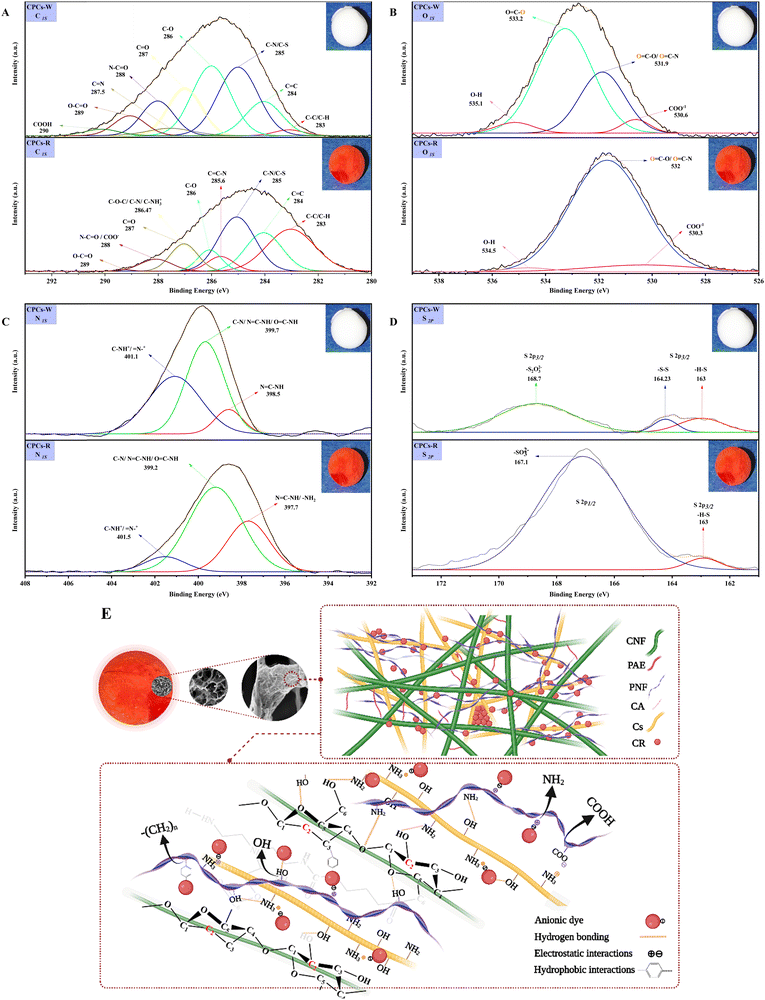
To verify the above-obtained results, the elemental composition of CPCs aerogel beads was characterized by XPS. As shown in Fig. 11B, the XPS survey spectra of CPCs aerogels before (CPCs-W, Inset image of white aerogel bead) and after adsorption of CR (CPCs-R, Inset image of red aerogel bead) showed similar characteristic peaks of C 1s (285 eV), O 1s (532 eV), and N 1s (399 eV), while the additional peaks at 230 eV (S 2s)112–115 and 167 eV (S 2p)116 were only detected for CPCs-R, implying that CR dye molecule was successfully adsorbed on the surface of CPCs aerogel. To obtain detailed information on the elemental composition of the aerogel composite and to compare the changes of the peaks before and after the adsorption of dye, the core peaks of the contained elements (C 1s, O 1s, N 1s, and S p) were separately deconvoluted and shown in Fig. 11B.
The high-resolution C 1s XPS spectrum of CPCs aerogel from Fig. 11B was deconvoluted (Fig. 12A) into five main peak components at 284 eV, 285 eV, 286 eV, 287 eV, and 288 eV, corresponding to the C–C/C–H/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, C–N/C–S, C–O, C
C, C–N/C–S, C–O, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and N–C
O, and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.82,117 The other weak peaks observed in the spectrum of CPCs-W aerogel at 287.5 eV (C
O, respectively.82,117 The other weak peaks observed in the spectrum of CPCs-W aerogel at 287.5 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) and 290 eV (COOH) disappeared after the adsorption of CR, and instead, new small peaks at 285.6 eV (C
N) and 290 eV (COOH) disappeared after the adsorption of CR, and instead, new small peaks at 285.6 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N) and 286.47 eV (C–NH3+, C–N) arose in the spectrum of CPCs-R. The strong peak at 283 eV in the CPCs-R spectra (Fig. 12A) can be related to the C–C/C–H/C
C–N) and 286.47 eV (C–NH3+, C–N) arose in the spectrum of CPCs-R. The strong peak at 283 eV in the CPCs-R spectra (Fig. 12A) can be related to the C–C/C–H/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C linkages of adsorbed CR dye. Furthermore, owing to the chemical structure of CR, the dye-adsorbed CPCs composite aerogel had a higher content of the C–N/C–NH3+ linkages, which might result in overlapping with the C–O functional groups of CPCs-W aerogel and thus weakening of the peak intensity at 286 eV.118 In Fig. 12A (CPCs-W), the binding energy at ∼289 eV was assigned to the side-chains protonated carboxylic acid groups in glutamic acid and aspartic acid, referring to the incorporation of PNFs into the CNFs/Cs composite aerogel.119 However, this peak disappeared in the spectrum of CPCs-R, suggesting the formation of hydrogen-bonding interaction between the carboxylic groups of CPCs aerogel and the amine group of CR dye.
C linkages of adsorbed CR dye. Furthermore, owing to the chemical structure of CR, the dye-adsorbed CPCs composite aerogel had a higher content of the C–N/C–NH3+ linkages, which might result in overlapping with the C–O functional groups of CPCs-W aerogel and thus weakening of the peak intensity at 286 eV.118 In Fig. 12A (CPCs-W), the binding energy at ∼289 eV was assigned to the side-chains protonated carboxylic acid groups in glutamic acid and aspartic acid, referring to the incorporation of PNFs into the CNFs/Cs composite aerogel.119 However, this peak disappeared in the spectrum of CPCs-R, suggesting the formation of hydrogen-bonding interaction between the carboxylic groups of CPCs aerogel and the amine group of CR dye.
A deconvoluted O 1s spectrum of CPCs-W aerogel (Fig. 12B) revealed four peaks at 530.6, 531.9, 533.2, and 535.1 eV, attributed to the COO−1, ![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif)
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–O/O
C–O/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N, O
C–N, O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–
C–![[O with combining low line]](https://www.rsc.org/images/entities/char_004f_0332.gif) , and O–H (H2O), respectively.117 Notably, after the adsorption of dye by CPCs aerogel, the spectrum of CPCs-R changed distinctly. In detail, the intensity of characteristic peaks at 530.6 and 535.1 eV decreased, and their peak position shifted to the lower binding energies by 0.3 eV and 0.6 eV. Besides, the carboxylic peak of CPCs-W at 533.2 eV disappeared or might overlap with the critical energy peak of the carbonyl group at 532 eV. These results might be related to the significant contribution of aliphatic carbon, aromatic, and amine groups of CR dye molecules in forming new chemical bonds with active components of CPCs composite aerogel.
, and O–H (H2O), respectively.117 Notably, after the adsorption of dye by CPCs aerogel, the spectrum of CPCs-R changed distinctly. In detail, the intensity of characteristic peaks at 530.6 and 535.1 eV decreased, and their peak position shifted to the lower binding energies by 0.3 eV and 0.6 eV. Besides, the carboxylic peak of CPCs-W at 533.2 eV disappeared or might overlap with the critical energy peak of the carbonyl group at 532 eV. These results might be related to the significant contribution of aliphatic carbon, aromatic, and amine groups of CR dye molecules in forming new chemical bonds with active components of CPCs composite aerogel.
The N 1s narrow region spectra of CPCs aerogels (Fig. 12C) were fitted into three sub-peaks at 398.5, 399.7, and 401.1 eV, which were attributed to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH, C–N/N
C–NH, C–N/N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH/O
C–NH/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH, and C–NH+/
C–NH, and C–NH+/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+–, respectively.47,96 The high-resolution spectrum of N 1s verified the amino acid sequences of the nanofibril protein (including His, Glu, Asp, Ala, Tyr, and Cys). His’ imidazole group was verified by lower N 1s binding energies at 398.5 eV for C
N+–, respectively.47,96 The high-resolution spectrum of N 1s verified the amino acid sequences of the nanofibril protein (including His, Glu, Asp, Ala, Tyr, and Cys). His’ imidazole group was verified by lower N 1s binding energies at 398.5 eV for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif)
![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) and at 399.7 eV for C–
and at 399.7 eV for C–![[N with combining low line]](https://www.rsc.org/images/entities/char_004e_0332.gif) H.120 In addition, His revealed a third N 1s signal at 401.4 eV, which could be related to the α-amine nitrogen, in line with the data of protonated α-nitrogen for other amino acids sequences such as Gly, Glu and Asp.
H.120 In addition, His revealed a third N 1s signal at 401.4 eV, which could be related to the α-amine nitrogen, in line with the data of protonated α-nitrogen for other amino acids sequences such as Gly, Glu and Asp.
A comparison of the changes in N 1s spectrum before and after CR dye adsorption (Fig. 12C) reveals that the intensity of the peak component belonging to C–NH+ (amine) and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–NH (amide) decreased, and the oxidation state (–N
C–NH (amide) decreased, and the oxidation state (–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ) at 397.7 eV increased. These results proved the presence of active functional groups in the structure of CPCs composite aerogel. They verified the formation of a chemical bond between the sulfite (–SO3−2) groups of dye molecule and protonated amine (–NH3+/
) at 397.7 eV increased. These results proved the presence of active functional groups in the structure of CPCs composite aerogel. They verified the formation of a chemical bond between the sulfite (–SO3−2) groups of dye molecule and protonated amine (–NH3+/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N+–) groups of CPCs composite aerogel47.
N+–) groups of CPCs composite aerogel47.
The deconvolution of the high-resolution S 2P spectra of CPCs composite aerogel bead before adsorption of CR (Fig. 12D) gave rise to peaks at 163 eV and 164.23 eV, accounting for the unbound thiols (S–H bonds), and disulfides (–S–S–), respectively.121,122 Protein nanofibrils have cysteine residues containing thiol groups at the C-terminus, which can either interact to form intra- and inter-molecular disulfide linkages (sulfur-sulfur) or may remain as free thiols.121 Moreover, owing to the oxidation phenomenon of the surface, thiosulfate (–S2O32−) species with an S 2p2/3 binding energy of 168.7 eV was also observed in the spectrum of CPCs-W. Interestingly, after the dye adsorption, a new strengthened peak with an S 2p1/2 binding energy at 167 eV arose in the CPCs-R aerogel spectrum (Fig. 12D). This characteristic peak was assigned to the sulfonate moieties (–SO3−) of the CR dye molecule. These results suggested that the sulfur element with the S 2p1/2 photoemission signal at 167 eV was the predominant element involved in the solid electrostatic interactions with the protonated amine (–NH3+) groups of CPCs aerogel96.
Conclusions
We have developed a green porous aerogel composed of whey protein nanofibers/cellulose nanofibers/chitosan through the facile procedure of ice-templating. Zeta potential analysis revealed a positively charged surface at pH 7.4, nearly 47.3 ± 1.34, which may be attributed to protonated amine groups (–NH3+) on the surface of Cs molecules and whey PNFs. Morphological studies revealed that the spherical CPCs aerogel had a specified center-diverging microchannel structure. The results of BET were consistent with the FE-SEM observations, implying that the CPCs aerogel has a porous structure consisting of mesoporous and flexible thin walls, which endowed the CPCs aerogel with enhanced adsorption capacity and rapid mass transportation of liquid. The maximum adsorption capacity calculated from Sip isotherm was 1349.77 ± 34.36 mg g−1, demonstrating that the adsorption process of CR dye onto CPCs aerogel bead was homogeneous and referred explicitly to monolayer adsorption. The interconnected pore channels found in CPCs aerogel provided abundant transport pathways, allowing for efficient separation and elimination of dye molecules. In contrast, the inclusion of functional groups within these channels facilitates their interaction with specific moieties. Our adsorption studies suggest that the mixture of pore diffusion and intra-particle diffusion might be the rate-limiting step of the CR adsorption onto the composite aerogel beads. Consequently, CPCs composite aerogel beads with homogeneous surface and active sites have a high potential to remove pollutants from wastewater.Abbreviation
| βLG | β-Lactoglobulin |
| CA | Citric acid |
| CCs | CNFs/Cs aerogel beads |
| CNFs | Cellulose nanofibers |
| CR | Congo red |
| Cs | Chitosan |
| CPCs | CNFs/Cs/PNFs aerogel beads |
| PAE | Polyamideamine-epichlorohydrin |
| PNFs | Protein nanofibrils |
| PNFs-C | Cross-linked protein fibrils |
| ThT | thioflavin T |
| WPI | Whey protein isolate |
Author contributions
Mandana Dilamian: conceptualization, methodology, investigation, writing – review & editing. Majid Montazer: validation, writing – review & editing. Hossein Yousefi: Validation, writing – review & editing. Daniel E. Otzen: validation, writing – review & editing, funding acquisition. Dina Morshedi: conceptualization, methodology, supervision, writing – review & editing, funding acquisition.Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to commercial reasons but the basic description of data types are available at: cellulose nanofibers/protein nanofibrils nanocomposite aerogels: preparation and characterization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Iran National Science Foundation (INSF). The authors would like to thank the Bioprocess Engineering Department, Institute of Industrial and Environmental Biotechnology, National Institute of Genetic Engineering and Biotechnology (NIGEB).References
- J. Wang and S. Zhuang, Removal of various pollutants from water and wastewater by modified chitosan adsorbents, Crit. Rev. Environ. Sci. Technol., 2017, 47(23), 2331 CrossRef CAS.
- M. N. Faiz Norrrahim, N. A. Mohd Kasim, V. F. Knight, M. S. Mohamad Misenan, N. Janudin and N. A. Ahmad Shah, et al., Nanocellulose: a bioadsorbent for chemical contaminant remediation, RSC Adv., 2021, 11(13), 7347 RSC.
- F. Jiang, D. M. Dinh and Y. L. Hsieh, Adsorption and desorption of cationic malachite green dye on cellulose nanofibril aerogels, Carbohydr. Polym., 2017, 173, 286 CrossRef CAS PubMed.
- D. Morshedi, Z. Mohammadi, M. M. A. Boojar and F. Aliakbari, Using protein nanofibrils to remove azo dyes from aqueous solution by the coagulation process, Colloids Surf., B, 2013, 112, 245 CrossRef CAS PubMed.
- T. Hou, K. Guo, Z. Wang, X. F. Zhang, Y. Feng and M. He, et al., Glutaraldehyde and polyvinyl alcohol crosslinked cellulose membranes for efficient methyl orange and Congo red removal, Cellulose, 2019, 26, 5065 CrossRef CAS.
- N. Ali, A. Said, F. Ali, F. Raziq, Z. Ali and M. Bilal, et al., Photocatalytic degradation of congo red dye from aqueous environment using cobalt ferrite nanostructures: development, characterization, and photocatalytic performance, Water, Air, Soil Pollut., 2020, 231, 1–16 CrossRef.
- D. N. Ahmed, L. A. Naji, A. A. H. Faisal, N. Al-Ansari and M. Naushad, Waste foundry sand/MgFe-layered double hydroxides composite material for efficient removal of Congo red dye from aqueous solution, Sci. Rep., 2020, 10(1), 2042 CrossRef CAS PubMed.
- N. P. Shetti, S. J. Malode, R. S. Malladi, S. L. Nargund, S. S. Shukla and T. M. Aminabhavi, Electrochemical detection and degradation of textile dye Congo red at graphene oxide modified electrode, Microchem. J., 2019, 146, 387 CrossRef CAS.
- U. J. Kim, S. Kimura and M. Wada, Highly enhanced adsorption of Congo red onto dialdehyde cellulose-crosslinked cellulose-chitosan foam, Carbohydr. Polym., 2019, 214, 294–302 CrossRef CAS PubMed.
- J. You, C. Liu, X. Feng, B. Lu, L. Xia and X. Zhuang, In situ synthesis of ZnS nanoparticles onto cellulose/chitosan sponge for adsorption–photocatalytic removal of Congo red, Carbohydr. Polym., 2022, 288, 119332 CrossRef CAS PubMed.
- L. E. Nita, A. Ghilan, A. G. Rusu, I. Neamtu and A. P. Chiriac, New Trends in Bio-Based Aerogels, Pharmaceutics, 2020, 12(5), 449 CrossRef CAS PubMed.
- M. Peydayesh, J. Vogt, X. Chen, J. Zhou, F. Donat and M. Bagnani, et al., Amyloid-based carbon aerogels for water purification, Chem. Eng. J., 2022, 449, 137703 CrossRef CAS.
- D. M. Guo, Q. D. An, Z. Y. Xiao, S. R. Zhai and D. J. Yang, Efficient removal of Pb (II), Cr (VI) and organic dyes by polydopamine modified chitosan aerogels, Carbohydr. Polym., 2018, 202, 306 CrossRef CAS PubMed.
- D. P. Stephen and S. B. Palanisamy, Advances in biopolymer composites and biomaterials for the removal of emerging contaminants, Phys. Sci. Rev., 2023, 8(8), 1789 Search PubMed.
- K. Ganesan, T. Budtova, L. Ratke, P. Gurikov, V. Baudron and I. Preibisch, et al., Review on the production of polysaccharide aerogel particles, Materials, 2018, 11(11), 1–37 CrossRef PubMed.
- M. A. Shahbazi, M. Ghalkhani and H. Maleki, Directional Freeze-Casting: A Bioinspired Method to Assemble Multifunctional Aligned Porous Structures for Advanced Applications, Adv. Eng. Mater., 2020, 22(7), 2000033 CrossRef CAS.
- M. Dilamian and B. Noroozi, Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel, Carbohydr. Polym., 2021, 251, 117016 CrossRef CAS PubMed.
- M. Zhang, M. Li, Q. Xu, W. Jiang, M. Hou and L. Guo, et al., Nanocellulose-based aerogels with devisable structure and tunable properties via ice-template induced self-assembly, Ind. Crops Prod., 2022, 179, 114701 CrossRef CAS.
- F. Zhao, L. Lin, J. Zhang, J. Liu, J. Shi and Y. L. Godec, et al., Ice-Templating: Integrative Ice Frozen Assembly to Tailor Pore Morphology of Energy Storage and Conversion Devices, Adv. Mater. Technol., 2023, 2201968, 1–25 Search PubMed.
- S. Ahmad, W. A. Siddiqi and S. Ahmad, Sustainable nanocomposite porous absorbent and membrane sieves: Definition, classification, history, properties, synthesis, applications, and future prospects, J. Environ. Chem. Eng., 2023, 11(2), 109367 CrossRef CAS.
- S. Liao, T. Zhai and H. Xia, Highly adsorptive graphene aerogel microspheres with center-diverging microchannel structures, J. Mater. Chem. A, 2016, 4(3), 1068 RSC.
- Q. Guo, E. Amendola, M. Lavorgna, Z. Li, H. Feng and Y. Wu, et al., Robust and recyclable graphene/chitosan composite aerogel microspheres for adsorption of oil pollutants from water, Carbohydr. Polym., 2022, 290, 119416 CrossRef CAS PubMed.
- H. Qin, Y. Zhang, J. Jiang, L. Wang, M. Song and R. Bi, et al., Multifunctional Superelastic Cellulose Nanofibrils Aerogel by Dual Ice-Templating Assembly, Adv. Funct. Mater., 2021, 31(46), 2106269 CrossRef CAS.
- N. Rong, C. Chen, K. Ouyang, K. Zhang, X. Wang and Z. Xu, Adsorption characteristics of directional cellulose nanofiber/chitosan/montmorillonite aerogel as adsorbent for wastewater treatment, Sep. Purif. Technol., 2021, 274, 119120 CrossRef CAS.
- J. Wang, Q. Yao, C. Sheng, C. Jin and Q. Sun, One-Step Preparation of Graphene Oxide/Cellulose Nanofibril Hybrid Aerogel for Adsorptive Removal of Four Kinds of Antibiotics, J. Nanomater., 2017, 2017, 5150613 Search PubMed.
- H. Cai, S. Sharma, W. Liu, W. Mu, W. Liu and X. Zhang, et al., Aerogel Microspheres from Natural Cellulose Nanofibrils and Their Application as Cell Culture Scaffold, Biomacromolecules, 2014, 15(7), 2540 CrossRef CAS PubMed.
- H. S. H. Nguyen, K. Phuong and T. Nguyen, Insights into sustainable aerogels from lignocellulosic materials, J. Mater. Chem. A, 2022, 10(44), 23467 RSC.
- Y. Lv, Z. Liang, Y. Li, Y. Chen, K. Liu and G. Yang, et al., Efficient adsorption of diclofenac sodium in water by a novel functionalized cellulose aerogel, Environ. Res., 2021, 194, 110652 CrossRef CAS PubMed.
- Q. Wang, W. Zuo, Y. Tian, L. Kong, G. Cai and H. Zhang, et al., Chemosphere An ultralight and flexible nanofibrillated cellulose/chitosan aerogel for efficient chromium removal: Adsorption-reduction process and mechanism, Chemosphere, 2023, 329, 138622 CrossRef CAS PubMed.
- D. M. Guo, Q. D. An, R. Li, Z. Y. Xiao and S. R. Zhai, Ultrahigh selective and efficient removal of anionic dyes by recyclable polyethylenimine-modified cellulose aerogels in batch and fixed-bed systems, Colloids Surf., A, 2018, 555, 150 CrossRef CAS.
- N. P. Raval, P. U. Shah, D. G. Ladha, P. M. Wadhwani and N. K. Shah, Comparative study of chitin and chitosan beads for the adsorption of hazardous anionic azo dye Congo Red from wastewater, Desalin. Water Treat., 2016, 57(20), 9247 CrossRef CAS.
- A. Sowmya and S. Meenakshi, An efficient and regenerable quaternary amine modified chitosan beads for the removal of nitrate and phosphate anions, J. Environ. Chem. Eng., 2013, 1(4), 906 CrossRef CAS.
- A. Pei, N. Butchosa, L. A. Berglund and Q. Zhou, Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes, Soft Matter, 2013, 9(6), 2047 RSC.
- U. J. Kim, D. Kim, J. You, J. W. Choi, S. Kimura and M. Wada, Preparation of cellulose-chitosan foams using an aqueous lithium bromide solution and their adsorption ability for Congo red, Cellulose, 2018, 25(4), 2615 CrossRef CAS.
- Y. Wang, H. Wang, H. Peng, Z. Wang, J. Wu and Z. Liu, Dye Adsorption from Aqueous Solution by Cellulose/Chitosan Composite: Equilibrium, Kinetics, and Thermodynamics, Fibers Polym., 2018, 19(2), 340 CrossRef CAS.
- S. Rahimi Aqdam, D. E. Otzen, N. M. Mahmoodi and D. Morshedi, Adsorption of azo dyes by a novel bio-nanocomposite based on whey protein nanofibrils and nano-clay: Equilibrium isotherm and kinetic modeling, J. Colloid Interface Sci., 2021, 602, 490–503 CrossRef CAS PubMed.
- Q. Zhang, S. Zhang, Z. Zhao, M. Liu, X. Yin and Y. Zhou, et al., Highly effective lead (II) removal by sustainable alkaline activated β-lactoglobulin nanofibrils from whey protein, J. Cleaner Prod., 2020, 255, 120297 CrossRef CAS.
- C. Lendel and N. Solin, Protein nanofibrils and their use as building blocks of sustainable materials, RSC Adv., 2021, 11(62), 39188 RSC.
- M. Peydayesh, M. K. Suter, S. Bolisetty, S. Boulos, S. Handschin and L. Nyström, et al., Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water, Adv. Mater., 2020, 32(12), 1–6 Search PubMed.
- J. Fu, F. Yang and Z. Guo, The chitosan hydrogels: From structure to function, New J. Chem., 2018, 42(21), 17162 RSC.
- M. Tan, H. Lv, Q. Zhao, B. Wang, S. Zheng and K. Li, Chitosan–Quinoa Bran Aerogel: A Low-Cost, Highly-Efficient, and Recyclable Adsorbents for Wastewater Treatment, Environ. Eng. Sci., 2023, 40(6), 233 CrossRef CAS.
- Y. Mansourpanah, Nanofiltration membranes, J. Membr. Sci. Res., 2017, 3(1), 1 Search PubMed.
- L. Severini, K. J. De France, D. Sivaraman, N. Kummer and G. Nyström, Biohybrid Nanocellulose–Lysozyme Amyloid Aerogels via Electrostatic Complexation, ACS Omega, 2022, 7(1), 578 CrossRef CAS PubMed.
- X. Ye, K. Junel, M. Gällstedt, M. Langton, X. F. Wei and C. Lendel, et al., Protein/Protein Nanocomposite Based on Whey Protein Nanofibrils in a Whey Protein Matrix, ACS Sustainable Chem. Eng., 2018, 6(4), 5462 CrossRef CAS.
- Y. Zhang, J. Hua, M. Zhao, H. Wu, Y. Shao and M. Zhou, et al., 1-(2-pyridylazo) 2-naphthol-modified bacterial cellulose/multiwall carbon nanotube/silicon dioxide membrane for selectively adsorbed Er(III), Fullerenes, Nanotubes Carbon Nanostruct., 2023, 31(3), 288 CrossRef CAS.
- S. Bolisetty, N. M. Coray, A. Palika, G. A. Prenosil and R. Mezzenga, Amyloid hybrid membranes for removal of clinical and nuclear radioactive wastewater, Environ. Sci., 2020, 6(12), 3249 CAS.
- Y. Zhang, J. Wen, Y. Zhou, J. Wang and W. Cheng, Novel efficient capture of hexavalent chromium by polyethyleneimine/amyloid fibrils/polyvinyl alcohol aerogel beads: Functional design, applicability, and mechanisms, J. Hazard. Mater., 2023, 458, 132017 CrossRef CAS PubMed.
- S. V. Kamath, M. H. Mruthunjayappa, D. Mondal and N. Sanna Kotrappanavar, Nanocomposite-based high-performance adsorptive water filters: recent advances, limitations, nanotoxicity and environmental implications, Environ. Sci.: Nano, 2022, 9(7), 2320 RSC.
- G. Nyström, W. K. Fong and R. Mezzenga, Ice-Templated and Cross-Linked Amyloid Fibril Aerogel Scaffolds for Cell Growth, Biomacromolecules, 2017, 18(9), 2858 CrossRef PubMed.
- M. Peydayesh, T. Greber, I. Haechler, A. Armanious, X. Jia and M. Usuelli, et al., Renewable Water Harvesting by Amyloid Aerogels and Sun, Adv. Sustainable Syst., 2022, 6(1), 2100309 CrossRef CAS.
- M. Peydayesh, M. K. Suter, S. Bolisetty, S. Boulos, S. Handschin and L. Nyström, et al., Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water, Adv. Mater., 2020, 32(12), 1–6 Search PubMed.
- L. Severini, K. J. De France, D. Sivaraman, N. Kummer and G. Nyström, Biohybrid Nanocellulose–Lysozyme Amyloid Aerogels via Electrostatic Complexation, ACS Omega, 2022, 7(1), 578 CrossRef CAS PubMed.
- M. Usuelli, T. Germerdonk, Y. Cao, M. Peydayesh, M. Bagnani and S. Handschin, et al., Polysaccharide-reinforced amyloid fibril hydrogels and aerogels, Nanoscale, 2021, 13(29), 12534 RSC.
- M. Peydayesh, X. Chen, J. Vogt, F. Donat, C. R. Müller and R. Mezzenga, Amyloid fibril-UiO-66-NH 2 aerogels for environmental remediation, Chem. Commun., 2022, 58(33), 5104 RSC.
- G. Nyström, L. Roder, M. P. Fernández-Ronco and R. Mezzenga, Amyloid Templated Organic–Inorganic Hybrid Aerogels, Adv. Funct. Mater., 2018, 28(27), 1–11 CrossRef.
- M. Dilamian and D. Morshedi, Cellulose nanofibers/protein nanofibrils nanocomposite aerogels: Preparation and characterization, J. Wood Forest Sci. Technol., 2022, 29(4), 113 Search PubMed.
- B. Ma, X. You and F. Lu, Inhibitory effects of β-ionone on amyloid fibril formation of β-lactoglobulin, Int. J. Biol. Macromol., 2014, 64, 162 CrossRef CAS PubMed.
- S. Brunauer, P. H. Emmett and E. Teller, Adsorption of Gases in Multimolecular Layers, J. Am. Chem. Soc., 1938, 60(2), 309 CrossRef CAS.
- G. Nyström, W. K. Fong and R. Mezzenga, Ice-Templated and Cross-Linked Amyloid Fibril Aerogel Scaffolds for Cell Growth, Biomacromolecules, 2017, 18(9), 2858 CrossRef PubMed.
- H. Zhang, I. Hussain, M. Brust, M. F. Butler, S. P. Rannard and A. I. Cooper, Aligned two- and three-dimensional structures by directional freezing of polymers and nanoparticles, Nat. Mater., 2005, 4(10), 787 CrossRef CAS PubMed.
- W. Shi, Y. C. Ching and C. H. Chuah, Preparation of aerogel beads and microspheres based on chitosan and cellulose for drug delivery: A review, Int. J. Biol. Macromol., 2021, 170, 751 CrossRef CAS PubMed.
- M. He, G. Fei, Z. Zheng, Z. Cheng, Z. Wang and H. Xia, Pt Nanoparticle-Loaded Graphene Aerogel Microspheres with Excellent Methanol Electro-Oxidation Performance, Langmuir, 2019, 35(10), 3694 CrossRef CAS PubMed.
- R. Yu, Y. Shi, D. Yang, Y. Liu, J. Qu and Z. Z. Yu, Graphene Oxide/Chitosan Aerogel Microspheres with Honeycomb-Cobweb and Radially Oriented Microchannel Structures for Broad-Spectrum and Rapid Adsorption of Water Contaminants, ACS Appl. Mater. Interfaces, 2017, 9(26), 21809 CrossRef CAS PubMed.
- K. S. W. Sing, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984), Pure Appl. Chem., 1985, 57(4), 603 CrossRef CAS.
- J. Rouquerol, D. Avnir, C. W. Fairbridge, D. H. Everett, J. M. Haynes and N. Pernicone, et al., Recommendations for the characterization of porous solids (Technical Report), Pure Appl. Chem., 1994, 66(8), 1739 CrossRef CAS.
- M. Di Foggia, P. Taddei, A. Torreggiani, M. Dettin and A. Tinti, Self-assembling peptides for biomedical applications: IR and Raman spectroscopies for the study of secondary structure, Proteomics Res. J., 2011, 2(3), 231 CAS.
- P. Singh and R. Kaur, One pot synthesis of bio-based porous isocyanate-free polyurethane materials, Mater. Lett., 2023, 331, 133433 CrossRef CAS.
- Y. Wang, H. Wang, H. Peng, Z. Wang, J. Wu and Z. Liu, Dye Adsorption from Aqueous Solution by Cellulose/Chitosan Composite: Equilibrium, Kinetics, and Thermodynamics, Fibers Polym., 2018, 19(2), 340 CrossRef CAS.
- U. J. Kim, D. Kim, J. You, J. W. Choi, S. Kimura and M. Wada, Preparation of cellulose-chitosan foams using an aqueous lithium bromide solution and their adsorption ability for Congo red, Cellulose, 2018, 25(4), 2615 CrossRef CAS.
- Y. Xinxin, Y. Li, H. Gao, C. Wang, X. Zhang and H. Zhou, One-step fabrication of chitosan-Fe(OH)3 beads for efficient adsorption of anionic dyes, Int. J. Biol. Macromol., 2018, 117, 30–41 CrossRef PubMed.
- M. Peydayesh, M. K. Suter, S. Bolisetty, S. Boulos, S. Handschin and L. Nyström, et al., Amyloid Fibrils Aerogel for Sustainable Removal of Organic Contaminants from Water, Adv. Mater., 2020, 32(12), 1–6 Search PubMed.
- M. Dilamian and B. Noroozi, Rice straw agri-waste for water pollutant adsorption: Relevant mesoporous super hydrophobic cellulose aerogel, Carbohydr. Polym., 2021, 251, 117016 CrossRef CAS PubMed.
- H. Tu, Y. Yu, J. Chen, X. Shi, J. Zhou and H. Deng, et al., Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: chitosan and cellulose, Polym. Chem., 2017, 8(19), 2913 RSC.
- M. Peydayesh and R. Mezzenga, Protein nanofibrils for next generation sustainable water purification, Nat. Commun., 2021, 12(1), 3248 CrossRef CAS PubMed.
- Y. Liu, Y. Ke, Q. Shang, X. Yang, D. Wang and G. Liao, Fabrication of multifunctional biomass-based aerogel with 3D hierarchical porous structure from waste reed for the synergetic adsorption of dyes and heavy metal ions, Chem. Eng. J., 2023, 451, 138934 CrossRef CAS.
- U. J. Kim, H. J. Kim, J. W. Choi, S. Kimura and M. Wada, Cellulose-chitosan beads crosslinked by dialdehyde cellulose, Cellulose, 2017, 24(12), 5517 CrossRef CAS.
- C. Wu, J. Scott and J. E. Shea, Binding of Congo red to amyloid protofibrils of the Alzheimer Aβ(9-40) peptide probed by molecular dynamics simulations, Biophys. J., 2012, 103(3), 550 CrossRef CAS PubMed.
- C. Q. Ruan, M. Stromme and J. Lindh, Preparation of porous 2,3-dialdehyde cellulose beads crosslinked with chitosan and their application in adsorption of Congo red dye, Carbohydr. Polym., 2018, 181, 200 CrossRef CAS PubMed.
- N. Du, L. Y. Huang, Y. S. Xiong, R. Tian, J. Y. Yin and D. Y. Cao, et al., Micro-mechanism insights into the adsorption of anionic dyes using quaternary ammonium-functionalised chitosan aerogels, Carbohydr. Polym., 2023, 313, 120855 CrossRef CAS PubMed.
- S. He, J. Li, X. Cao, F. Xie, H. Yang and C. Wang, et al., Regenerated cellulose/chitosan composite aerogel with highly efficient adsorption for anionic dyes, Int. J. Biol. Macromol., 2023, 244, 125067 CrossRef CAS PubMed.
- Z. L. Yaneva and N. V. Georgieva, Insights into Congo Red Adsorption on Agro-Industrial Materials - Spectral, Equilibrium, Kinetic, Thermodynamic, Dynamic and Desorption Studies, Review, 2012, 4, 127 Search PubMed.
- M. M. Abou Alsoaud, M. A. Taher, A. M. Hamed, M. S. Elnouby and A. M. Omer, Reusable kaolin impregnated aminated chitosan composite beads for efficient removal of Congo red dye: isotherms, kinetics and thermodynamics studies, Sci. Rep., 2022, 12(1), 1–19 CrossRef PubMed.
- L. Liu, Z. Y. Gao, X. P. Su, X. Chen, L. Jiang and J. M. Yao, Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbent, ACS Sustainable Chem. Eng., 2015, 3(3), 432 CrossRef CAS.
- S. Chatterjee, S. Chatterjee, B. P. Chatterjee and A. K. Guha, Adsorptive removal of congo red, a carcinogenic textile dye by chitosan hydrobeads: Binding mechanism, equilibrium and kinetics, Colloids Surf., A, 2007, 299(1), 146 CrossRef CAS.
- H. Y. Zhu, Y. Q. Fu, R. Jiang, J. H. Jiang, L. Xiao and G. M. Zeng, et al., Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies, Chem. Eng. J., 2011, 173(2), 494–502 CrossRef CAS.
- X. Huang, P. Hadi, R. Joshi, A. G. Alhamzani and B. S. Hsiao, A Comparative Study of Mechanism and Performance of Anionic and Cationic Dialdehyde Nanocelluloses for Dye Adsorption and Separation, ACS Omega, 2023, 8(9), 8634 CrossRef CAS PubMed.
- Li. Y. XinxinYang, H. Gao, C. Wang, X. Zhang and H. Zhou, One-step fabrication of chitosan-Fe(OH)3 beads for efficient adsorption of anionic dyes, Int. J. Biol. Macromol., 2018, 117, 30–41 CrossRef PubMed.
- I. Langmuir, The adsorption of gases on plane surfaces of glass, mica and platinum, J. Am. Chem. Soc., 1918, 40(9), 1361 CrossRef CAS.
- H. Freundlich, Über die adsorption in lösungen, Z. Phys. Chem., 1907, 57(1), 385–470 CrossRef CAS.
- K. Vijayaraghavan, T. V. N. Padmesh, K. Palanivelu and M. Velan, Biosorption of nickel (II) ions onto Sargassum wightii: application of two-parameter and three-parameter isotherm models, J. Hazard. Mater., 2006, 133(1–3), 304 CrossRef CAS PubMed.
- Y. S. Ho, Selection of optimum sorption isotherm, Carbon, 2004, 42(10), 2115 CrossRef CAS.
- M. Brdar, M. Šćiban, A. Takači and T. Došenović, Comparison of two and three parameters adsorption isotherm for Cr(VI) onto Kraft lignin, Chem. Eng. J., 2012, 183, 108 CrossRef CAS.
- R. Sips, On the structure of a catalyst surface, J. Chem. Phys., 1948, 16(5), 490 CrossRef CAS.
- L. B. L. Lim, N. Priyantha, S. A. A. Latip and Y. Lu, Sequestration of toxic congo red dye through the utilization of red dragon fruit peel: Linear versus nonlinear regression analyses of isotherm and kinetics, Desalin. Water Treat., 2021, 218, 409 CrossRef CAS.
- M. Usman, A. Ahmed, B. Yu, S. Wang, Y. Shen and H. Cong, Simultaneous adsorption of heavy metals and organic dyes by β-Cyclodextrin-Chitosan based cross-linked adsorbent, Carbohydr. Polym., 2021, 255, 117486 CrossRef CAS PubMed.
- Y. Zeng, X. Liu, Y. Zhang, Y. Qin, X. Tang and W. Zhang, et al., Excellent regeneration, easy separation and high capacity of 3D chitosan–melamine sponge composites for anionic dye removal, New J. Chem., 2023, 13, 6342 RSC.
- S. Chatterjee, M. W. Lee and S. H. Wooa, Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes, Bioresour. Technol., 2010, 101(6), 1800 CrossRef CAS PubMed.
- S. Chatterjee, H. N. Tran, O. B. Godfred and S. H. Woo, Supersorption Capacity of Anionic Dye by Newer Chitosan Hydrogel Capsules via Green Surfactant Exchange Method, ACS Sustainable Chem. Eng., 2018, 6(3), 3604 CrossRef CAS.
- U. J. Kim, S. Kimura and M. Wada, Highly enhanced adsorption of Congo red onto dialdehyde cellulose-crosslinked cellulose-chitosan foam, Carbohydr. Polym., 2019, 214, 294–302 CrossRef CAS PubMed.
- Y. S. Ho and G. McKay, Pseudo-second order model for sorption processes, Process Biochem., 1999, 34(5), 451 CrossRef CAS.
- K. Y. Chong, C. H. Chia, S. Zakaria, M. S. Sajab, S. W. Chook and P. S. Khiew, CaCO3-decorated cellulose aerogel for removal of Congo Red from aqueous solution, Cellulose, 2015, 22(4), 2683 CrossRef CAS.
- W. J. Weber Jr and J. C. Morris, Kinetics of adsorption on carbon from solution, J. Sanit. Eng. Div., 1963, 89(2), 31–59 CrossRef.
- P. Wang, T. Yan and L. Wang, Removal of Congo Red from Aqueous Solution Using Magnetic Chitosan Composite Microparticles, BioResources, 2013, 8(4), 6026 Search PubMed.
- Li. Y. XinxinYang, H. Gao, C. Wang, X. Zhang and H. Zhou, One-step fabrication of chitosan-Fe(OH)3 beads for efficient adsorption of anionic dyes, Int. J. Biol. Macromol., 2018, 117, 30–41 CrossRef PubMed.
- S. Chatterjee, M. W. Lee and S. H. Wooa, Adsorption of congo red by chitosan hydrogel beads impregnated with carbon nanotubes, Bioresour. Technol., 2010, 101(6), 1800 CrossRef CAS PubMed.
- Z. Jing, Y. Li, Y. Zhang, K. Chen, Y. Sun and M. Wang, et al., Simple synthesis of chitosan/alginate/graphene oxide/UiO-67 amphoteric aerogels: Characterization, adsorption mechanism and application for removal of cationic and anionic dyes from complex dye media, Int. J. Biol. Macromol., 2023, 242(P1), 124683 CrossRef CAS PubMed.
- C. Tang, P. Brodie, Y. Li, N. J. Grishkewich, M. Brunsting and K. C. Tam, Shape recoverable and mechanically robust cellulose aerogel beads for efficient removal of copper ions, Chem. Eng. J., 2020, 392, 124821 CrossRef CAS.
- C. Gerente, V. K. C. Lee, P. L. Cloirec and G. McKay, Application of Chitosan for the Removal of Metals From Wastewaters by Adsorption—Mechanisms and Models Review, Crit. Rev. Environ. Sci. Technol., 2007, 37(1), 41–127 CrossRef CAS.
- L. Wang and A. Wang, Adsorption properties of Congo Red from aqueous solution onto surfactant-modified montmorillonite, J. Hazard. Mater., 2008, 160(1), 173 CrossRef CAS PubMed.
- L. Zhang, P. Hu, J. Wang, Q. Liu and R. Huang, Adsorption of methyl orange (MO) by Zr (IV)-immobilized cross-linked chitosan/bentonite composite, Int. J. Biol. Macromol., 2015, 81, 818 CrossRef CAS PubMed.
- K. Litefti, M. S. Freire, M. Stitou and J. González-Álvarez, Adsorption of an anionic dye (Congo red) from aqueous solutions by pine bark, Sci. Rep., 2019, 9(1), 1–11 CrossRef CAS PubMed.
- S. Das, M. K. Kumawat, S. Ranganathan, R. Kumar, J. Adamcik and P. Kadu, et al., Cell Alignment on Graphene – Amyloid Composites, Adv. Mater. Interfaces, 2018, 5(18), 1800621 CrossRef.
- K. Fan, T. Zhang, S. Xiao, H. He, J. Yang and Z. Qin, Preparation and adsorption performance of functionalization cellulose-based composite aerogel, Int. J. Biol. Macromol., 2022, 211, 1–14 CrossRef CAS PubMed.
- A. Munir, T. Haq, A. Qurashi, H. Rehman and A. Ul-hamid, Ultrasmall Ni/NiO Nanoclusters on Thiol Functionalized and Exfoliated Ultrasmall Ni/NiO Nanoclusters on Thiol-Functionalized and -Exfoliated Graphene Oxide Nanosheets for Durable Oxygen Evolution Reaction, ACS Appl. Energy Mater., 2019, 2(1), 363–371 CrossRef CAS.
- F. Yang, Z. Yan, J. Zhao, S. Miao, D. Wang and P. Yang, Rapid capture of trace precious metals by amyloid- like protein membrane with high adsorption capacity and selectivity, J. Mater. Chem. A, 2020, 8(6), 3438 RSC.
- M. Mahapatra, M. Karmakar, A. Dutta, H. Mondal, J. Sankar and D. Roy, Journal of Environmental Chemical Engineering Microstructural analyses of loaded and/or unloaded semisynthetic porous material for understanding of superadsorption and optimization by response surface methodology, J. Environ. Chem. Eng., 2018, 6(1), 289–310 CrossRef CAS.
- M. Tan, S. Zheng, H. Lv, B. Wang, Q. Zhao and B. Zhao, Rational design and synthesis of chitosan-quinoa polysaccharide composite aerogel and its adsorption properties for Congo red and methylene blue, New J. Chem., 2021, 45(22), 9829 RSC.
- P. Joshi, A. Raturi, M. Srivastava and O. P. Khatri, Graphene oxide, kaolinite clay and PVA-derived nanocomposite aerogel as a regenerative adsorbent for wastewater treatment applications, J. Environ. Chem. Eng., 2022, 10(6), 108597 CrossRef CAS.
- J. S. Stevens, A. C. De Luca, M. Pelendritis, G. Terenghi, S. Downes and S. L. M. Schroeder, Quantitative analysis of complex amino acids and RGD peptides by X-ray photoelectron spectroscopy (XPS), Surf. Interface Anal., 2013, 45(8), 1238 CrossRef CAS.
- S. S. S. Wang, P. L. Hsieh, P. S. Chen, Y. T. Chen and J. S. Jan, Genipin-cross-linked poly(l-lysine)-based hydrogels: Synthesis, characterization, and drug encapsulation, Colloids Surf., B, 2013, 111, 423 CrossRef CAS PubMed.
- M. Peydayesh, S. Bolisetty, T. Mohammadi and R. Mezzenga, Assessing the Binding Performance of Amyloid – Carbon Membranes, Langmuir, 2019, 35(11), 4161–4170 CrossRef CAS PubMed.
- F. Yang, Z. Yan, J. Zhao, S. Miao, D. Wang and P. Yang, Rapid capture of trace precious metals by amyloid-like protein membrane with high adsorption capacity and selectivity, J. Mater. Chem. A, 2020, 8(6), 3438 RSC.
- X. Zheng, X. Li, J. Li, L. Wang, W. Jin and J. Liu, et al., Efficient removal of anionic dye (Congo red) by dialdehyde microfibrillated cellulose/chitosan composite film with significantly improved stability in dye solution, Int. J. Biol. Macromol., 2018, 107, 283 CrossRef CAS PubMed.
- Z. Jing, Y. Li, Y. Zhang, M. Wang, B. Chen and Y. Sun, et al., Effective Removal of Congo Red from Water using Chitosan@UiO-67 Hydrogel Spheres: Synthesis, Characterization and Adsorption Mechanisms, ChemistrySelect, 2023, 8(20), 202204367 CrossRef.
- K. L. Hudson, G. J. Bartlett, R. C. Diehl, J. Agirre, T. Gallagher and L. L. Kiessling, et al., Carbohydrate-Aromatic Interactions in Proteins, J. Am. Chem. Soc., 2015, 137(48), 15152 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma00380b |
| This journal is © The Royal Society of Chemistry 2024 |