Open Access Article
Open Access ArticleA non-invasive approach for H2S gas sensing under stimulated breathing conditions: a kag-MOF based gas sensor as a case study†
Mostafa
Zeama
a,
Jiangtao
Jia
a,
Sheng
Zhou
 a,
Murilo Calil
Faleiros
b,
Usman
Yaqoob
b,
Osama
Shekhah
a,
Murilo Calil
Faleiros
b,
Usman
Yaqoob
b,
Osama
Shekhah
 a,
Khaled N.
Salama
a,
Khaled N.
Salama
 *b and
Mohamed
Eddaoudi
*b and
Mohamed
Eddaoudi
 *a
*a
aFunctional Materials Design, Discovery, and Development research group (FMD3), Advanced Membranes & Porous Materials Center, Division of Physical Sciences and Engineering King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia. E-mail: mohamed.eddaoudi@kaust.edu.sa
bSensors Lab, Electrical Engineering Program, Division of Computer, Electrical and Mathematical Sciences and Engineering King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia. E-mail: khaled.salama@kaust.edu.sa
First published on 11th July 2024
Abstract
Herein we report the deployment of kag-MOF as a sensing layer on a capacitive IDE sensor for detecting H2S at room temperature. Compared to other gases such as nitrogen dioxide, ammonia, carbon dioxide, and carbon monoxide, kag-MOF exhibited a unique and selective response to H2S, making it a promising candidate for H2S sensing applications. In particular, the kag-MOF layer demonstrated a particularly good response and selectivity to H2S in stimulated human breath and could detect concentrations as low as 500 ppb, even under humid conditions. These results clearly demonstrate that kag-MOF has immense potential as a candidate for H2S sensing, and its sensing behaviour opens possibilities for developing gas/vapor sensors that could potentially diagnose uremia at an early stage by monitoring H2S levels in the breath, giving it a potential application in the field of healthcare.
Introduction
Uremia is a medical condition that occurs when the kidneys are unable to efficiently filter out metabolic waste products from the bloodstream. The buildup of toxins in the body can cause serious health complications if not detected and treated early. This condition can be caused by a variety of factors, including viral infections, kidney damage, or certain medications.1–4 When left untreated, uremia can cause fatigue, nausea, confusion, changes in urine properties, and in severe cases, the condition can lead to coma or even death.5–7 As such, early detection of uremia is crucial for the effective management and prevention of further complications.Fortunately, there are several methods available to detect uremia at an early stage. Medical tests such as blood, urine, and imaging tests, besides physical examination, can all be used to detect the presence of uremia. Interestingly, recent studies have shown that elevated levels of hydrogen sulfide (H2S) in the breath may be an indicator of uremia.8–10 The presence of any low concentration of H2S (as low as 100 ppb)11 in human breath means that there is a degree of malfunction of the kidneys, which indicates a degree of uremia. When kidneys fail to filter waste products from the blood, the levels of H2S build up in the body as certain sulfur-containing compounds are broken down. This leads to a condition called halitosis of uremic origin, or “uremic breath”.12–14
Monitoring H2S levels in breath using gas/vapor sensors is a non-invasive technique that could help in the early diagnosis of uremia. These sensors detect physical changes caused by the diffusion of various gases in the sensing layer.15–19 The interdigitated electrode (IDE) technology has emerged as a promising method for gas/vapor detection due to its high sensitivity, low cost, small size, and simplicity.20–23 Sensors are prepared by depositing a sensing layer over the electrode surface, and this layer is a critical component of any chemical sensor as it captures analyte gases.24,25 Among these electrodes, capacitive IDEs are particularly suitable for gas sensing because they consume less power.26–30
Porous materials like metal–organic frameworks (MOFs) and covalent organic frameworks (COFs) have garnered considerable attention in recent years for their potential applications in many fields like separation, catalysis and gas sensing.31–37 The development of MOF-based gas sensors has several advantages over traditional sensors due to their high selectivity, sensitivity, stability, and ability to detect low concentrations of gases.38–40 In addition, MOFs are easily tunable, allowing for the optimization of their properties for specific applications.41–43 Therefore, the use of MOFs in gas sensing applications, such as the detection of H2S in human breath, has the potential to revolutionize the field of chemical sensing.
One of the good MOF candidates is kag-MOF-1 which is constructed by a single metal ion building block approach. It is formed by connecting Zn+2 metal ions with a tetrazole organic linker forming a 2-D structure with one dimensional channel and a small pore size. The presence of the tetrazole linker forms a high localized charge density, which affords access to a unique multifunctional MOF adsorbent suitable and ideal for the removal of acid gases (CO2 and H2S), dehydration, and BTX sieving. This MOF has emerged as a promising candidate for H2S sensing, owing to its ability to uptake H2S at low concentration and ambient pressure.44
In this study, we developed a sensing device for detecting low levels of H2S in human breath using kag-MOF as the sensitive layer on the capacitive IDE.
Experimental section
Interdigitated electrode fabrication
The IDEs were fabricated using standard complementary metal oxide semiconductor (CMOS) processes. Firstly, a highly doped n-type silicon wafer underwent thermal growth of a thick (2 micrometers) SiO2 layer. The Si/SiO2 substrate was then patterned by spin-coating AZ5214 photoresist onto its surface. Afterward, the coated substrate was prebaked at a temperature of 110 °C for 2 minutes. The photoresist was subsequently exposed to UV light with a dose of 80 mJ cm−2 using a mask plate. Following the exposure, the photoresist was developed using AZ 726 developer for 1 minute before being cleaned in deionized (DI) water. Ti/Au electrodes were then sputtered, with thicknesses of 10 nm and 100 nm, respectively. The substrate underwent cleaning in an ultrasonic bath with acetone, followed by rinsing with isopropyl alcohol (IPA) and DI water.kag-MOF synthesis
The kag-MOF was prepared according to our previous report with a modified procedure to get a nano-sheet morphology structure.44 In general, tetrazole-5-ethylester (328 mg, 2 mmol) and 20 mL of H2O were heated in a Teflon-lined autoclave to 120 °C for 24 h. After cooling to room temperature, a solution of Zn(NO3)3·6H2O (297 mg, 1 mmol) in 20 mL water was added to the previous solution. The mixture was shaken for several minutes and held for 24 hours to get a white milk-like solution. The particles were centrifuged and washed with water and methanol.
To fabricate the sensing device, an ink was prepared by suspending 8 mg of kag-MOF in 8 ml of DMF and sonicated for 30 minutes. The resulting ink was then deposited on the IDE at 65 °C to allow for particle rearrangement. After the preparation, the electrode was found to be loaded with approximately 1 mg of the MOF material.
Activating MOF films is crucial to obtain a guest-free film prior to sensing signal measurements.38,40 To achieve this, the MOF adsorbent was fully reactivated by heating it at 150 °C in an oven for 12 hours to remove any residual solvent or water present in the pores.
Material characterization
Powder X-ray diffraction (PXRD) measurements were carried out using a Bruker D8 ADVANCE X-ray diffractometer with Cu Kα radiation (λ = 1.54178 Å). TGA measurements were performed with TA Instruments Q500 apparatus; the samples were heated under an air atmosphere (flow, 25 cm3 min−1; heating rate, 5 °C min−1). Low-pressure gas adsorption measurements were performed at relative pressures of up to 1 atm with a fully automated 3Flex high-resolution gas adsorption analyzer (Micromeritics). The bath temperature for the CO2 adsorption measurements was controlled with an ethylene glycol/H2O recirculating bath. Field emission scanning electron microscope (FE-SEM) images were taken on a Quattro Dual Beam microscope at an acceleration voltage of 10 kV.Sensing experiment
The sensing experiment utilized a custom cell with IDE-loaded material connected to a KEYSIGHT E4980AL LCR meter. The cell was sealed, and gas flow (regulated by an Alicat mass flow controller) was set to 200 SCCM. The schematic of the overall sensing system setup is shown elsewhere.24 System temperature was maintained at 24 °C with a Julabo EH-F33 water chiller. The experiment began by introducing dry compressed air to establish the reference capacitance (C0) until stabilization (material activation). Subsequently, the tested gas was introduced until capacitance saturation. After each experiment, the IDE was heated to 120 °C to fully recover the H2S molecules and to ensure the complete activation of the sensing layer before the next gas sensing trial. All the measurements were performed in dry air and at 24 °C except when mentioned otherwise.Testing gas preparation
The tested gases, purchased with specific concentrations (H2S: 100 ppm, NH3: 100 ppm, NO2: 100 ppm, CO: 1000 ppm, CO2: 1000 ppm), were dry and balanced with N2. Gas concentrations were regulated by mixing them with dry air using a mass flow controller set at a fixed total flow rate of 200 SCCM. For relative humidity measurement, compressed air was introduced into a water bubbler with temperature control via a water chiller, then mixed with dry air to adjust the relative humidity ratio.Results and discussion
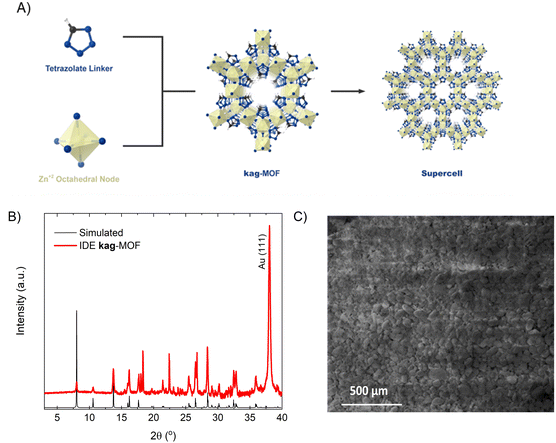
The coating layer exhibited a homogenous coverage and strong adhesion to the substrate. The MOF film's purity and crystallinity were validated by comparing its powder X-ray diffraction (PXRD) pattern with the simulated pattern (Fig. 1B).44 The film displayed excellent crystalline quality, featuring miniature two-dimensional sheets approximately 150 nm in size and well-mixed microcrystals with slight gaps in between, producing a consistent and dense MOF coating (Fig. 1C). The successful deposition of the MOF thin film onto the IDE substrate enabled us to use it for sensing permittivity alterations triggered by gas/vapor sorption.Gas sensing application in dry conditions
We examined the sensing properties of the kag-MOF film on capacitive IDEs for a range of gases, including H2S, NH3, NO2, CO2, and CO. Gas sensing experiments were conducted using a fully automated measurement system with LabVIEW.21 The coated sensor was placed inside a custom-built detection chamber and connected to an LCR meter to measure changes in capacitance.To prepare for each cycle of analyte exposure, the sensor was heated up to 120 °C using an in situ heating system in a homemade gas chamber. The temperature of the heater output was calibrated using a Micro radiometric thermal imaging microscope, which measures and displays the temperature distribution over the surface of small devices.45 This calibration is crucial to maintain the integrity of the MOF thin film and ensure that we have a guest-free activated kag-MOF before conducting sensing experiments.44 The sensing performance and characteristics of kag-MOF were evaluated at various H2S concentrations in both dry and humid conditions to assess the potential application of the newly developed sensor.
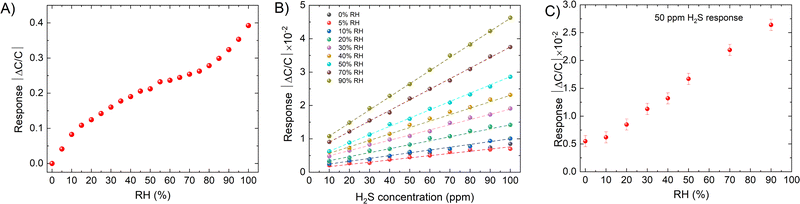
The sensor shows different responses across a wide range of H2S concentrations (from 0.5 to 100 ppm), it was observed that at higher concentration, the device reveals a linear response while at low concentration (<1 ppm) its slope changes. The capacitance can be determined using the standard capacitance equation.46 The change in capacitance is attributed to the dielectric constant dependency of the IDE's capacitive properties on the concentration of H2S. This is because H2S interaction with kag-MOF affects the local dielectric properties of the film. Therefore, exposure of the sensor to varying dry H2S concentrations results in an increase in capacitance. As depicted in Fig. 2, the sensor exhibits a good response at a low concentration of H2S (0.5 ppm), which makes it a suitable candidate for trace-level H2S detection. The sensor's response was assessed in both N2 and air atmospheres, revealing comparable responses in both environments (within the measurement's error range). This suggests that the sensor's reaction to various gases in the air mixture is negligible. Furthermore, the material demonstrates notable stability under both air and humid conditions.44
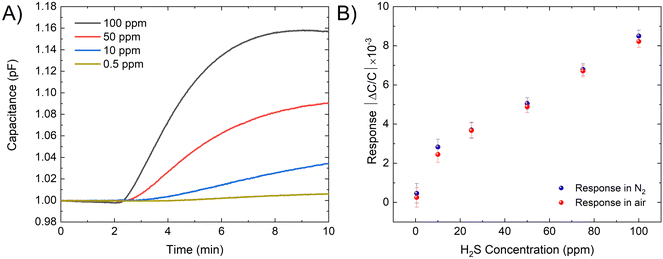 | ||
| Fig. 2 (A) Capacitive sensor response for kag-MOF in the presence of different concentration of H2S. (B) The response of the sensor for different H2S concentrations. | ||
As the stability and repeatability of a sensing device are crucial for its effectiveness. The stability of the device was assessed by exposing it to 100 ppm of H2S for 7 consecutive cycles. The device was activated in situ by heating it to 120 °C for five minutes and then allowing it to cool down under an air environment for 20 minutes. The detection levels were shown to be stable and uniform across all cycles. To assess the long-term stability of the sensor, cyclic exposure to 100 ppm of H2S was performed at room temperature every 24 hours for a period of 14 days. As depicted in Fig. 3, the results revealed that the sensor response remained consistent over time, indicating its long-term stability. The crystallinity of the sensing layer was further confirmed by comparing the PXRD patterns before and after the sensing experiment [Fig. SI, ESI†]. The results showed that the MOF retained its crystallinity, which confirms the stability of the film under the sensing conditions.
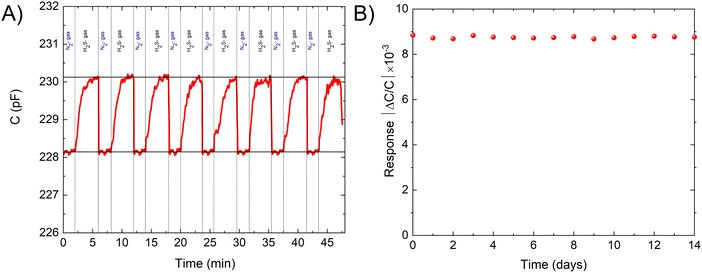 | ||
| Fig. 3 (A) The recyclability of the sensor in the presence of 100 ppm of H2S. (B) The response of the sensor for 100 ppm H2S measured daily for 2 weeks. | ||
Based on our sensing data and IDE stability analysis, we conducted further investigations to determine the selectivity of the sensor for H2S. To assess the sensor's selectivity, we evaluated its response to other gases at different concentrations. The results showed that the kag-MOF sensor had exceptional selectivity for H2S compared to other tested gases. Although the sensor displayed some response to gases such as ammonia (NH3), nitrogen dioxide (NO2), carbon dioxide (CO2), and carbon monoxide (CO), the signals produced were minor. Notably, NH3 and NO2 generated the most significant signals, whereas CO2 and CO produced nearly negligible signals, as illustrated in Fig. 4. Furthermore, the H2S signal was approximately nine times higher than that of NH3, indicating the sensor's high selectivity for H2S over other tested gases with distinct physical and chemical properties.
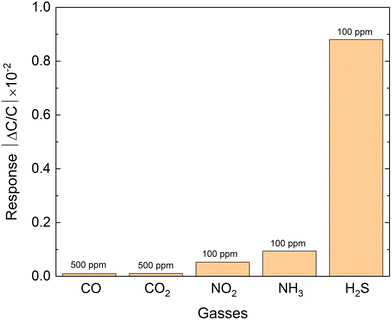 | ||
| Fig. 4 Selectivity of the kag-MOF sensor to hydrogen sulfide (H2S), ammonia (NH3), nitrogen dioxide (NO2), carbon dioxide (CO2), and carbon monoxide (CO) at different concentrations. | ||
Sensing mechanisms
The gas sensing response is driven by dipole interaction between physiosorbed gas and the MOF. Material characterization revealed a strong affinity of the MOF for H2S.44 DFT calculations were employed to understand the sensing mechanism and gas response disparity between H2S and NH3. Both gases were found to be adsorbed on the tetrazole ring in the pore, with calculated adsorption energy of 50 kJ mol−1 for H2S and 40 kJ mol−1 for NH3. Charge analysis showed a partial negative charge on S and N in H2S and NH3, respectively. The discrepancy in adsorption energy is attributed to a partial positive charge on the adsorbed MOF region, with charges of −2.4e and −1.3e on S and N, respectively. As system capacitance is charge-dependent, it varies with different gases, affecting the gas response. The greater affinity of kag-MOF for H2S, indicated by the difference in binding energy, explains the difference in response. This sensing mechanism is generalized for various tested gases physiosorbed on the framework.Relative humidity response
Environmental moisture can significantly affect the gas sensor response, as capacitive sensor technology is based on the film's dielectric changes caused by gas/vapor sorption, it is often criticized for being sensitive to humidity.46 In practice, water vapor and condensable vapours can significantly affect IDE-based sensors since water has a much larger dielectric constant (εr = 80) than H2S (εr = 5.8), which can interfere with the IDE sensory signal of H2S.47,48 Consequently, the presence of moisture can hinder the qualitative identification and quantification of H2S.We therefore investigated the concentration-dependent response of the IDE device coated with kag-MOF under humid conditions (Fig. 5). Our findings revealed that the presence of moisture had a substantial impact on the kag-MOF coated IDE sensors. In order to gain a better understanding of the sensing performance of water vapor adsorption, a detailed analysis of the response toward H2O was conducted as a function of relative humidity (RH), ranging from 5% to 90% RH. The sensor was activated at 120 °C and then exposed to varying levels of RH using dry compressed air as a carrier gas. The RH level was adjusted by changing the carrier flow (0–200 mL min−1) through water bubbling. The kag-MOF coated IDE sensor demonstrated a good response to humidity levels in a nonlinear fashion, as shown in Fig. 5A. These results are consistent with the dehydration ability of kag-MOF, which has a high affinity for water molecules due to its contracted pore system and localized high charge density and supported by its hydrolytic stability.44
Effect of humidity on H2S sensing
To evaluate the influence of humidity on the sensor's response toward H2S, we conducted a test to measure the sensor's response to H2S in a humid environment with a different relative humidity (5–90%). The introduction of H2S gas resulted in a reduction in the sensor's capacitance. However, the sensor's response for H2S was improved by a factor of 10, as shown in Fig. 5B. The decrease in capacitance suggests that kag-MOF experiences a partial desorption of non-coordinated water molecules due to their competition with H2S molecules.44,49The impact of relative humidity (RH) on the kag-MOF sensor's performance was investigated. The sensor was activated at 120 °C and exposed to varying RH levels using dry compressed air as a carrier gas. The RH was adjusted by modifying the carrier flow (0–200 mL min−1) through water bubbling. The chamber's humidity was set to the desired level (from dry air up to 90% RH), and the capacitance response was considered as the new baseline (Fig. S9, ESI†). At each RH level, a capacitance change was recorded in the presence of 50 ppm H2S (Fig. 5C). It was observed that the response is nearly similar to that for dry H2S up to 30% RH (Fig. 2B). Then, an increase in the sensor response with an increase in RH up to 50% RH is attributed to the dissolution of H2S molecules into the physiosorbed water on the kag-MOF layer. This is due to the fact that kag-MOF is known based on water sorption studies to become saturated with water above 30% RH.44
H2S response in simulated human exhale
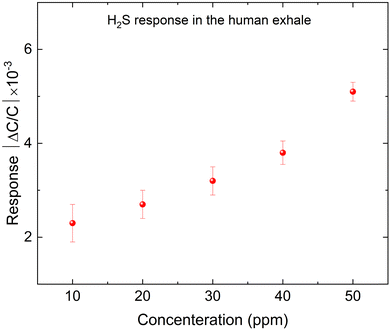
Based on the previous results, we proposed that the kag-MOF has potential for detecting H2S in human exhaled breath based on our studies. Since the humidity level in human breath falls within the range of 5% to 6.3%, we conducted tests to evaluate the sensor's response to human breath by exposing the IDE electrode to a gas mixture of 78% N2, 13% O2, and 4% CO2 with a humidity of 5% to mimic the conditions of human exhalation.50 Subsequently, various concentrations of H2S (10–40 ppm) were introduced, and the outcomes are presented in Fig. 6. The capacitance of the electrode increased non-linearly with increasing H2S concentration, indicating good response to H2S in human breath. The sensor's response to H2S in air was found to be comparable to its response in simulated human breath due to the low water concentration, and the negligible response to CO2 and O2 with respect to H2S.Volatile organic compounds (VOCs) such as formaldehyde usually coexist with other gases in human breath in the case of uremia. The selectivity of the sensor to formaldehyde (as one of the VOCs) is studied. Human exhaled air contains formaldehyde in concentrations ranging from 0.001 to 0.01 mg m−3, with an average value of about 0.005 mg m−3, equivalent to approximately 0.00774017 ppm.51–53 However, due to system limitations, we could not achieve this concentration in our experiment. Therefore, we tested the sensor at a 1 ppm concentration of formaldehyde (FA) under different humid conditions, as human breath is humid. Additionally, we tested 1 ppm FA at 5% RH with varying concentrations of H2S. The results (Fig. S10 and S11, ESI†) show that the sensor material has almost no sensitivity to FA in both tests. Therefore, there is no cross-sensitivity that could affect the sensor's performance.
Conclusion
In conclusion, this study showed the successful deployment of kag-MOF as a sensing layer on a capacitive IDE sensor for H2S detection at room temperature. The MOF layer could detect H2S concentrations as low as 500 ppb, even under humid conditions. The MOF sensor demonstrated good response and selectivity to H2S in human breath, and its stability was confirmed by various methods, demonstrating its greater chemical stability. Moreover, kag-MOF exhibited a unique and selective response to H2S compared to other gases such as nitrogen dioxide, ammonia, carbon dioxide, and carbon monoxide. This sensing behaviour of kag-MOF opens possibilities for developing gas/vapor sensors for the early detection of uremia. By monitoring H2S levels in the breath, healthcare professionals could potentially diagnose uremia at an early stage, which is crucial for the effective management and prevention of complications. However, it is essential to note that while gas/vapor sensors are a promising tool, they should not be used in isolation and should be used in conjunction with other diagnostic tests, such as blood and urine tests, to ensure accurate diagnosis.Data availability
Data supporting this study are included within the article and/or supporting materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the King Abdullah University of Science and Technology for funding this project.References
- T. W. Meyer and T. H. Hostetter, N. Engl. J. Med., 2007, 357, 1316–1325 CrossRef CAS PubMed.
- G. A. Kaysen, J. Am. Soc. Nephrol., 2001, 12, 1549–1557 CrossRef CAS.
- M. Schömig, A. Eisenhardt and E. Ritz, Blood Purif., 2000, 18, 327–332 CrossRef PubMed.
- D. Ormrod and T. Miller, Nephron, 2008, 26, 249–254 CrossRef.
- C. K. Yeung, D. D. Shen, K. E. Thummel and J. Himmelfarb, Kidney Int., 2014, 85, 522–528 CrossRef CAS PubMed.
- A. I. Arieff, S. G. Massry, A. Barrientos and C. R. Kleeman, Kidney Int., 1973, 4, 177–187 CrossRef CAS.
- D. J. Prezant, Lung, 1990, 168, 1–14 CrossRef CAS PubMed.
- E. Levine, Urol. Radiol., 1991, 13, 203–210 CrossRef.
- H. Uchida, N. Kiyokawa, H. Horie, J. Fujimoto and T. Takeda, Pediatr. Res., 1999, 45, 133–137 CrossRef CAS PubMed.
- T. W. Meyer and T. H. Hostetter, J. Am. Soc. Nephrol., 2014, 25, 2151–2158 CrossRef.
- H. E. Bass and E. Singer, J. Am. Med. Assoc., 1950, 144, 819–823 CrossRef CAS PubMed.
- A. Manolis, Clin. Chem., 1983, 29, 5–15 CrossRef CAS.
- M. L. Simenhoff, J. F. Burke, J. J. Saukkonen, A. T. Ordinario, R. Doty and S. Dunn, N. Engl. J. Med., 1977, 297, 132–135 CrossRef CAS PubMed.
- N. Pagonas, W. Vautz, L. Seifert, R. Slodzinski, J. Jankowski, W. Zidek and T. H. Westhoff, PLoS One, 2012, 7, e46258 CrossRef CAS PubMed.
- P. Bindra and A. Hazra, J. Mater. Sci.: Mater. Electron., 2018, 29, 6129–6148 CrossRef CAS.
- D. J. Wales, J. Grand, V. P. Ting, R. D. Burke, K. J. Edler, C. R. Bowen, S. Mintova and A. D. Burrows, Chem. Soc. Rev., 2015, 44, 4290–4321 RSC.
- J. R. Stetter, W. R. Penrose and S. Yao, J. Electrochem. Soc., 2003, 150, S11 CrossRef CAS.
- L. Zhang, P. De Schryver, B. De Gusseme, W. De Muynck, N. Boon and W. Verstraete, Water Res., 2008, 42, 1–12 CrossRef CAS.
- O. Yassine, O. Shekhah, A. H. Assen, Y. Belmabkhout, K. N. Salama and M. Eddaoudi, Angew. Chem., Int. Ed., 2016, 55, 15879–15883 CrossRef CAS PubMed.
- F. J. Pavinatto, C. W. Paschoal and A. C. Arias, Biosens. Bioelectron., 2015, 67, 553–559 CrossRef CAS.
- V. Tsouti, C. Boutopoulos, I. Zergioti and S. Chatzandroulis, Biosens. Bioelectron., 2011, 27, 1–11 CrossRef CAS PubMed.
- F. J. Pavinatto, C. W. A. Paschoal and A. C. Arias, Biosens. Bioelectron., 2015, 67, 553–559 CrossRef CAS PubMed.
- U. Yaqoob and M. I. Younis, Journal, 2021, 21 Search PubMed.
- C. Sapsanis, H. Omran, V. Chernikova, O. Shekhah, Y. Belmabkhout, U. Buttner, M. Eddaoudi and K. N. Salama, Journal, 2015, 15, 18153–18166 CAS.
- E. Llobet, Sens. Actuators, B, 2013, 179, 32–45 CrossRef CAS.
- M. Kitsara, D. Goustouridis, S. Chatzandroulis, M. Chatzichristidi, I. Raptis, T. Ganetsos, R. Igreja and C. J. Dias, Sens. Actuators, B, 2007, 127, 186–192 CrossRef CAS.
- C. Berggren, B. Bjarnason and G. Johansson, Electroanalysis, 2001, 13, 173–180 CrossRef CAS.
- A. M. Kummer and A. Hierlemann, IEEE Sens. J., 2006, 6, 3–10 CAS.
- U. Yaqoob, W. B. Lenz, N. Alcheikh, N. Jaber and M. I. Younis, IEEE Sens. J., 2022, 22, 19858–19866 CAS.
- U. Yaqoob, N. Jaber, N. Alcheikh and M. I. Younis, Sci. Rep., 2022, 12, 5297 CrossRef CAS.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Journal, 2012, 112, 673–674 CAS.
- R. J. Kuppler, D. J. Timmons, Q.-R. Fang, J.-R. Li, T. A. Makal, M. D. Young, D. Yuan, D. Zhao, W. Zhuang and H.-C. Zhou, Coord. Chem. Rev., 2009, 253, 3042–3066 CrossRef CAS.
- H. Furukawa, K. E. Cordova, M. O’Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef.
- V. Chernikova, O. Shekhah, Y. Belmabkhout, M. Karunakaran and M. Eddaoudi, Angew. Chem., 2023, 135, e202218842 CrossRef.
- P. T. Parvatkar, S. Kandambeth, A. C. Shaikh, I. Nadinov, J. Yin, V. S. Kale, G. Healing, A.-H. Emwas, O. Shekhah, H. N. Alshareef, O. F. Mohammed and M. Eddaoudi, J. Am. Chem. Soc., 2023, 145, 5074–5082 CrossRef CAS PubMed.
- M. Zeama, M. A. Morsy, M. Abdelnaby, L. Gutiérrez-Arzaluz, O. F. Mohammed and Z. H. Yamani, Chem. – Asian J., 2021, 16, 2520–2528 CrossRef CAS.
- M. Zeama, M. Morsy, S. Abdel-Azeim, M. Abdelnaby, A. Alloush and Z. Yamani, Inorg. Chim. Acta, 2020, 501, 119287 CrossRef CAS.
- S. Achmann, G. Hagen, J. Kita, I. M. Malkowsky, C. Kiener and R. Moos, Journal, 2009, 9, 1574–1589 CAS.
- M. Drobek, J.-H. Kim, M. Bechelany, C. Vallicari, A. Julbe and S. S. Kim, ACS Appl. Mater. Interfaces, 2016, 8, 8323–8328 CrossRef CAS.
- C. Arul, K. Moulaee, N. Donato, D. Iannazzo, N. Lavanya, G. Neri and C. Sekar, Sens. Actuators, B, 2021, 329, 129053 CrossRef CAS.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS.
- D.-X. Xue, Y. Belmabkhout, O. Shekhah, H. Jiang, K. Adil, A. J. Cairns and M. Eddaoudi, J. Am. Chem. Soc., 2015, 137, 5034–5040 CrossRef CAS.
- J. H. Choi, Y. J. Choi, J. W. Lee, W. H. Shin and J. K. Kang, Phys. Chem. Chem. Phys., 2009, 11, 628–631 RSC.
- M. I. H. Mohideen, R. S. Pillai, K. Adil, P. M. Bhatt, Y. Belmabkhout, A. Shkurenko, G. Maurin and M. Eddaoudi, Chem, 2017, 3, 822–833 CAS.
- G. Bieszczad and M. Kastek, Metrol. Meas. Syst., 2011, 18, 679–690 Search PubMed.
- W. C. Heerens, J. Phys. E: Sci. Instrum., 1986, 19, 897 CrossRef.
- S. Havriliak, R. Swenson and R. Cole, J. Chem. Phys., 1955, 23, 134–135 CrossRef CAS.
- G. Oster and J. G. Kirkwood, J. Chem. Phys., 1943, 11, 175–178 CrossRef CAS.
- A. Cadiau, Y. Belmabkhout, K. Adil, P. M. Bhatt, R. S. Pillai, A. Shkurenko, C. Martineau-Corcos, G. Maurin and M. Eddaoudi, Science, 2017, 356, 731–735 CrossRef CAS PubMed.
- G. Ejaimi and S. Saeed, Ann. Int. Med. Den. Res., 2016, 3, 1 Search PubMed.
- I. Kushch, K. Schwarz, L. Schwentner, B. Baumann, A. Dzien, A. Schmid, K. Unterkofler, G. Gastl, P. Spaněl, D. Smith and A. Amann, J. Breath Res., 2008, 2, 026002 CrossRef.
- B. Moser, F. Bodrogi, G. Eibl, M. Lechner, J. Rieder and P. Lirk, Respir. Physiol. Neurobiol., 2005, 145, 295–300 CrossRef CAS.
- A. Wehinger, A. Schmid, S. Mechtcheriakov, M. Ledochowski, C. Grabmer, G. A. Gastl and A. Amann, Int. J. Mass Spectrom., 2007, 265, 49–59 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ma00417e |
| This journal is © The Royal Society of Chemistry 2024 |