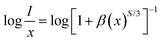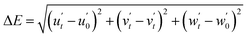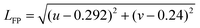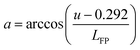DOI:
10.1039/D4MA00937A
(Paper)
Mater. Adv., 2024,
5, 9851-9861
White light emission and superior color stability in a single-component host with exceptional eminent color rendering and theoretical calculations on Duv for color quality†
Received
18th September 2024
, Accepted 11th November 2024
First published on 11th November 2024
Abstract
Correlated color temperature (CCT) is widely used to describe the chromaticity of white light sources, although chromaticity is only two-dimensional, and the distance from the Planckian locus is typically absent. Herein, a novel single-phase Ca3YAl3B4O15:Tm3+,Dy3+,Eu3+, an emerging white-emitting phosphor with good optical properties and thermal-stability, is produced, and the practical calculation methods to calculate the chromaticity-shift (ΔE) and Duv value for color-quality are also demonstrated, making it a good contender for possible use in LEDs. The incorporation of Eu3+ into Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+ resulted in attractive warm-white light with CCT declining from 4635 K to 3065 K. The Ca3YAl3B4O15:Tm3+,Dy3+,Eu3+ exhibited excellent thermal stability (I@400 K = ∼93%). The Ca3YAl3B4O15:Tm3+,Dy3+,Eu3+-based WLED exhibits satisfactory parameters of high Ra (89.9) and low-CCT (3065 K). Additionally, this article offers useful mathematical strategies for calculating Duv over a wide-range of chromaticity, from 2000 to 6000 K in CCT and from −0.002 to 0.014, which strongly matches the range in an American National Standards Institute (ANSI) standard. For the first time, white light with minimized thermal-quenching, improved CRI, and color quality has been used in near-UV chip-excited WLEDs.
1. Introduction
One of the most important characteristics of light sources for general illumination is chromaticity, which is typically defined using CIE coordinates (x, y) or (u, v). But these two numbers don't convey the color information.1,2 The chromaticity information of general light sources is frequently provided via correlated color temperature (CCT) for practical applications. However, CCT only offers one dimension of chromaticity; the other dimension is the position of chromaticity concerning the Planckian locus and is typically absent. Some areas of the industry have been using the term “Duv” (Deviation of Uniform Visual perception) or concepts akin to it, such as the distance from the Planckian locus for this purpose, although these terms have not been formally defined in any standards. An American National Standards Institute standard recently defined Duv.3 White LEDs, which are anticipated to be the next-generation light source,4,5 have gained popularity as a hot topic in lighting applications.6–9 YAG:Ce3+ and InGaN-based blue chips are usually used to create commercially available WLEDs. RBG light emission could be obtained by either single-phase or multi-color phosphors.6,10–12 The serious flaw in these systems is that they fall short of ideal standards. The challenge of producing white light in a single-component host has generated a significant deal of attention due to the substantial reabsorption of blue light by green and red. By adding a red phosphor to the system, warm white light with a high Ra is produced, producing the desired qualities.13 Meanwhile, the single-component phosphors play a vital role compared with the multi-phase photoluminescence materials, offering a higher color-rending index (CRI), better stability, better reproducibility, low manufacturing costs, no phase separation, and an informal fabrication method.14–16 Fortunately, it is possible to introduce a sensitizer to enhance the activator's emission, as was done previously.14,15 Consequently, using energy transfer (ET) to produce white light is still a hot topic.17
Of all the rare-earth ions, Dy3+ seems to be a noble candidate for white emission in hosts with a single phase. Dy3+ activated phosphors have been the subject of practical investigations because it is simple to produce white emission by altering the proportion of blue and yellow emissions from Dy3+, which correspond to the transitions of 4F5/2 → 6H15/2 and 4F5/2 →6H13/2.18,19 However, the Dy3+-activated phosphors exhibit a significant CCT issue that prevents their use in more vibrant applications.20
As mentioned above, they do not meet the prime desires of WLEDs. They still have the drawbacks of a low CRI and cold white light with a CCT > 6000 K because there is no red light in the spectrum, which prevents them from being used in applications that require more vibrant colors.9,21–24 Implementing more emissions in the red spectral regions is vital to resolving these issues and enhancing luminous performances. These can be effectively resolved by co-doping Tm3+ and Dy3+ into a host and another red-emitting center. Trivalent Eu3+ can produce red light originating from its ground state of 7F0 to excited states (e.g.5D4 ∼ 362 nm, 5L8 ∼ 366 nm, 5G4 ∼ 375 nm, 5G2 ∼ 380 nm, and 5L6 ∼ 393 nm). It can be an effective activator for presentations and unquestionably is a fantastic choice when used as a red-component center for WLEDs.25,26 It is possible to compensate for the emission of phosphors in the red area and increase the optical performance by co-doping Tm3+–Dy3+ and Eu3+. Consequently, multiple techniques need to be employed to broaden the emission of co-doped phosphors containing Tm3+, Dy3+, and Eu3+. The structure of Ca3YAl3B4O15 belongs to the space group P63/m, which is isostructural to gaudefroyite Ca4(MnO)3(BO3)3CO3. There are two available sites (Ca1 & Ca2) for the substitution of dopant ions. These sites are separated by BO3 and AlO6 groups, and yttrium ions occupy the Ca1 site.27,28
This work presents the development of a single-component Ca3YAl3B4O15:Tm3+,Dy3+,Eu3+ warm white phosphor with exceptional optical properties. XRD, crystal-refinements, TEM, DRS, and detailed optical properties characterized the resulting samples, and Duv is suggested as a simple way to express the chromaticity of white light sources for general illumination, together with color stability or chromaticity-shift (ΔE). Quantitative analysis was done on the crystal structure and the impact of Dy3+ doping on the electrical structure of CYAB, which has a prominent effect. Interestingly, the incorporation of Eu3+ into Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+ displayed attractive warm-white-light with CCT declining from 4635 K to 3065 K. Furthermore, a WLED device with a CRI of 89.9% was achieved by Eu3+ codoping. Additionally, this article provides useful calculation techniques for calculating Duv over a wide range of chromaticity, from −0.002 to 0.014 in Duv and 2000 to 6000 K in CCT.
2. Experimental section
2.1 Sample preparation
All reagents were obtained in pure form from commercial sources and were used directly. H3BO3 (A.R.), Y2O3, Eu2O3, Tm2O3, CaCO3 (A.R.), Al(OH)3 (A.R.), and Dy2O3 were sourced from Aladdin. The reagents were carefully combined in a stoichiometric ratio and crushed. After being placed in the crucible, the mixture was heated there for 10 hours at 900 °C. Then, for a further ten hours, it was heated at 1100 °C in a CO-reduction environment. The obtained phosphors are as follows; Ca3Y1−xAl3B4O15:xEu3+ (x = 0.1, 0.3, 0.5, 0.8,), Ca3Y1−xAl3B4O15:xTm (x = 0.005, 0.01, 0.015, 0.02, 0.025), Ca3Y1−xAl3B4O15:xDy (x = 0.01, 0.04, 0.08, 0.12, 0.16), and Ca3Y0.985−xAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ (x = 0.00, 0.001, 0.007, 0.01, 0.02). The produced phosphors were then employed for additional characterization (see the ESI† for more information).
3. Results and discussion
3.1 Structural characterization, micromorphology, and DFT calculations
Powder X-ray diffraction patterns of Ca3YAl3B4O15:xDy3+ (x = 0.01 to 0.16) samples are presented in Fig. 1a. The JCPDS dataset was used to index the prepared phosphor diffraction peaks (172![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 154). The X-ray diffraction study results showed that doping of Dy3+ does not have any other impurities or cause any notable change. As shown in Fig. S1 (ESI†), the Rietveld-refinement results of Ca3YAl3B4O15:Dy3+ confirmed that the phosphor crystalizes into a single garnet phase. The cell variables were a = 10.3867(3), c = 5.8146(2), and V = 534.94 Å3. The unit cell contains four distinct types of cationic sites, where the metal ions exhibit nine-fold and seven-fold coordination polyhedra at the Ca2+/Y3+(1) and Ca2+/Y3+(2) sites, respectively. Additionally, six coordinated aluminum sites and three coordinated boron sites were discovered. The Ca2+/Y3+(1) sites were separated from the Ca2+/Y3+(2) sites by the BO3 and AlO6 units in Fig. 1b and c. The AlO6 octahedra share edges to form chains, which are connected by triangular BO3 groups in the ab plane, creating a Kagome-type lattice characteristic of the gaudefroyite structure. The P63/m space group accurately predicts all points in the X-ray diffraction pattern. However, the Ln3+ rare-earth ions in Ca3YAl3B4O15 have somewhat different distributions in these two sites, as Ln3+ ions can occupy both cation sites in the structure, with the Ca2+/Y3+(1) sites being occupied by 79% Ln3+ ions and the Ca2+/Y3+(2) sites being occupied by 21% Ln3+.29 As for our system, the dopant's behavior is opposite to what has been previously reported.30 Our modifications showed that the Ca2+/Y3+(2) site in Ca3YAl3B4O15:Dy3+ should be occupied by a very heavy-ion, rather than the Ca2+/Y3+(1) site. Thus, we assume that in Ca3YAl3B4O15:Dy3+ ions fully occupy the Ca2+/Y3+(2) site. As shown in Fig. 1c (coordinating conditions of Ca2+/Y3+(1) and Ca2+/Y3+(2)), the distance between the sites of Ca2+/Y3+(1) is shorter than the Ca2+/Y3+(2) sites, which may be the reason for the preferences of occupancy (Table S1, ESI†). All the other elements containing Ca, Y, Dy, Al, and O in the matrix were detected except boron (Fig. 1d and e). The (1 1 2) and (−1 2 1) planes are well represented by a lattice fringe in the HRTEM picture with interplanar lattice spacings of 0.52 and 0.35 nm of Ca3YAl3B4O15:0.08Dy3+ shown in Fig. S2 (ESI†). The XPS results gave additional proof for the elemental makeup (Fig. 2). XPS spectral analysis showed peaks at 284.5 eV and 530.7 eV attributed to C 1s and O 1s, respectively (details are given in the ESI†).31 The Kubelka–Munk function was used to calculate the phosphors' energy bandgap (Eg), which was found to be 5.13 eV from the diffuse reflection spectroscopy (DRS) analysis (Fig. 3a and b).32
154). The X-ray diffraction study results showed that doping of Dy3+ does not have any other impurities or cause any notable change. As shown in Fig. S1 (ESI†), the Rietveld-refinement results of Ca3YAl3B4O15:Dy3+ confirmed that the phosphor crystalizes into a single garnet phase. The cell variables were a = 10.3867(3), c = 5.8146(2), and V = 534.94 Å3. The unit cell contains four distinct types of cationic sites, where the metal ions exhibit nine-fold and seven-fold coordination polyhedra at the Ca2+/Y3+(1) and Ca2+/Y3+(2) sites, respectively. Additionally, six coordinated aluminum sites and three coordinated boron sites were discovered. The Ca2+/Y3+(1) sites were separated from the Ca2+/Y3+(2) sites by the BO3 and AlO6 units in Fig. 1b and c. The AlO6 octahedra share edges to form chains, which are connected by triangular BO3 groups in the ab plane, creating a Kagome-type lattice characteristic of the gaudefroyite structure. The P63/m space group accurately predicts all points in the X-ray diffraction pattern. However, the Ln3+ rare-earth ions in Ca3YAl3B4O15 have somewhat different distributions in these two sites, as Ln3+ ions can occupy both cation sites in the structure, with the Ca2+/Y3+(1) sites being occupied by 79% Ln3+ ions and the Ca2+/Y3+(2) sites being occupied by 21% Ln3+.29 As for our system, the dopant's behavior is opposite to what has been previously reported.30 Our modifications showed that the Ca2+/Y3+(2) site in Ca3YAl3B4O15:Dy3+ should be occupied by a very heavy-ion, rather than the Ca2+/Y3+(1) site. Thus, we assume that in Ca3YAl3B4O15:Dy3+ ions fully occupy the Ca2+/Y3+(2) site. As shown in Fig. 1c (coordinating conditions of Ca2+/Y3+(1) and Ca2+/Y3+(2)), the distance between the sites of Ca2+/Y3+(1) is shorter than the Ca2+/Y3+(2) sites, which may be the reason for the preferences of occupancy (Table S1, ESI†). All the other elements containing Ca, Y, Dy, Al, and O in the matrix were detected except boron (Fig. 1d and e). The (1 1 2) and (−1 2 1) planes are well represented by a lattice fringe in the HRTEM picture with interplanar lattice spacings of 0.52 and 0.35 nm of Ca3YAl3B4O15:0.08Dy3+ shown in Fig. S2 (ESI†). The XPS results gave additional proof for the elemental makeup (Fig. 2). XPS spectral analysis showed peaks at 284.5 eV and 530.7 eV attributed to C 1s and O 1s, respectively (details are given in the ESI†).31 The Kubelka–Munk function was used to calculate the phosphors' energy bandgap (Eg), which was found to be 5.13 eV from the diffuse reflection spectroscopy (DRS) analysis (Fig. 3a and b).32
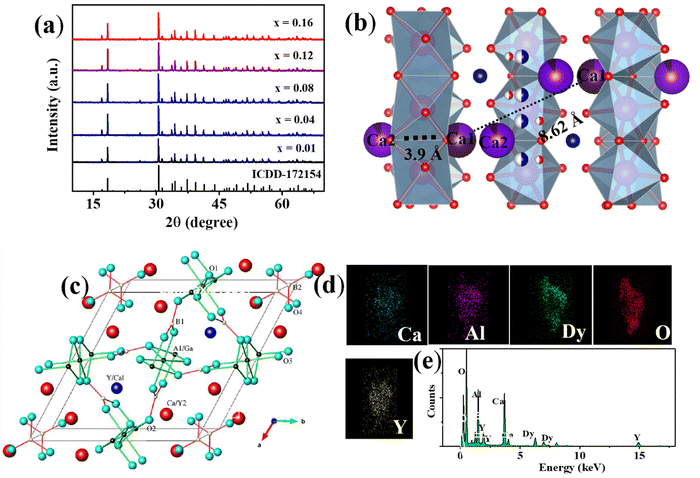 |
| | Fig. 1 (a) XRD pattern of Ca3YAl3B4O15:xDy3+ (y = 0.01 to 0.16). (b) and (c) coordination environments of CYAB and (d) and (e) EDS elemental distribution. | |
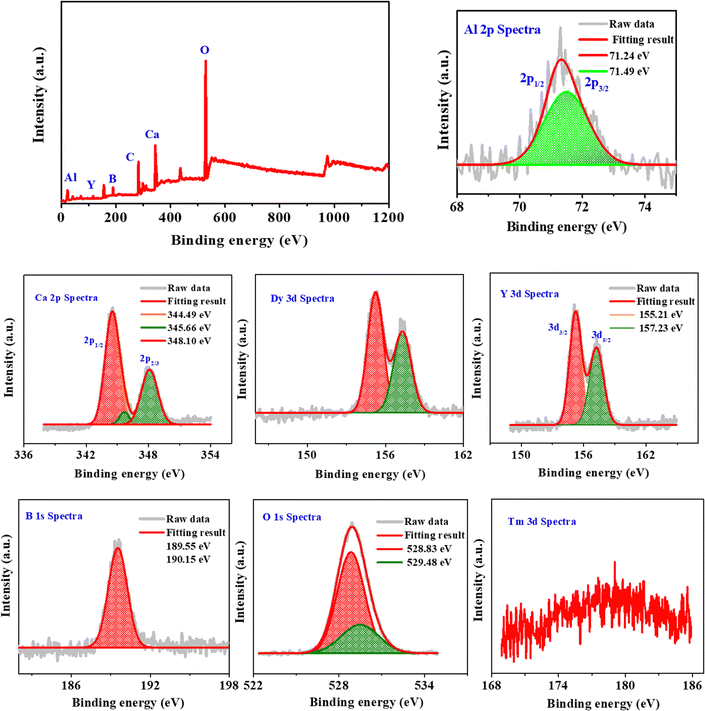 |
| | Fig. 2 XPS spectra of CYAB:Tm3+,Dy3+ and XPS spectral analysis of Al, Ca, Dy, Y, B, O, and Tm. | |
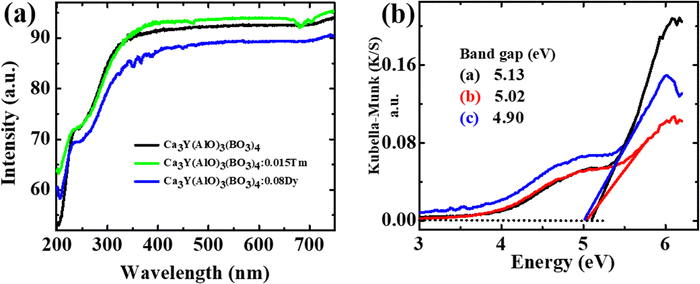 |
| | Fig. 3 (a) and (b) DR spectra, and the bandgap DRS spectra of the CYAB, CYAB:0.015Tm3+ CYAB:0.08Dy3+ phosphors. | |
3.2 Photoluminescence properties of Tm3+/Dy3+/Eu3+ single doped Ca3YAl3B4O15
Fig. 4a and b show the PLE and PL spectra of Ca3YAl3B4O15:Eu3+. The wide band between 250 and 300 nm, peaking at about 265 nm, and numerous brilliant, crisp lines between 300 and 480 nm are present in the PLE spectra recorded at 618 nm. The CTB transition from the filled 2p orbitals of O2+ ions to the partially filled 4f orbitals of Eu3+ was first thought to be responsible for the broadband emission (Fig. 4a).14 Ca3YAl3B4O15:xEu3+ phosphors were found to emit strong red light at 394 nm excitation. The PL spectra of Ca3YAl3B4O15:xEu3+ (x = 0.1, 0.3, 0.5, 0.8) phosphors with various Eu3+ doping concentrations are shown in Fig. 4b under excitation at 397 nm. Each sample showed typical red Eu3+ ion emissions, and the intensity of the 5D0 → 7F2 emission was altered. There was no concentration quenching effect seen when the doping level increased as suggested by the concentration-dependent luminescence intensity of Ca3YAl3B4O15:Eu3+. The Ca3YAl3B4O15:Eu3+ sample's CIE coordinates were found to be (0.653, 0.342), which was relatively near to the NTSC standard value for red phosphor (0.670, 0.330). Notably, the CIE value of Ca₃YAl₃B₄O₁₅:Eu3+ was superior to that of the commercial phosphor Y₂O₂S:Eu3+ (0.622, 0.351), as shown in Fig. S3a (ESI†).33 Color purity is regarded as a crucial component of a material's efficiency for WLED applications. The following equation was used to obtain the color purity for all the samples that were doped with Eu3+.33–35| |
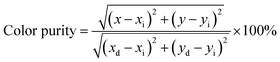 | (1) |
where (x, y), (xi, yi), and (xd, yd) correspond to the CIE diagrams of the phosphor, white illumination, and dominant wavelength. Ca3YAl3B4O15:xEu3+ was found to have a color purity of 93.8% in Fig. S3a (ESI†), which exceeds the value of the previously known red-emitting phosphors.23,36–43 The decay curves of Ca3YAl3B4O15:xEu3+ are explored in Fig. S3b (ESI†). Eqn (2) provides a good match to the appropriate results for all of the analyses.33–35| |
I = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ) exp(−t/τ)
| (2) |
where I refers to the photoluminescence intensity at time t, B is a constant, and τ is the luminescence lifetime, respectively. The value of τ is adjusted to 1.22 ms so that the second-order exponential may more easily fit decay curves. For a deeper look at the luminescence performance, we assessed the IQE of the Ca3YAl3B4O15:Eu3+ sample. Under 394 nm excitation, the IQE of Ca3YAl3B4O15:xEu3+ reached 82.5%, which is higher than that of the commercial phosphor Y2O2S xEu3+ (IQE: 35%).33 Thermal stability is a highly significant parameter that can affect the phosphor's color output and brightness. Consequently, Fig. S3c (ESI†) displays the temperature-dependent spectra of Ca3YAl3B4O15:xEu3+. Unexpectedly, the PL intensity at 400 K was around 85.2% higher than at 303 K. This shows that the Ca3YAl3B4O15:xEu3+ phosphor has better heat stability and is suitable for making WLEDs. After that, the activation energy (E) was determined using the Arrhenius equation and the thermal quenching data are shown in Fig. S3d (ESI†). In contrast to the literature, the heat stability of phosphors is typically significantly healthier (Table 1).
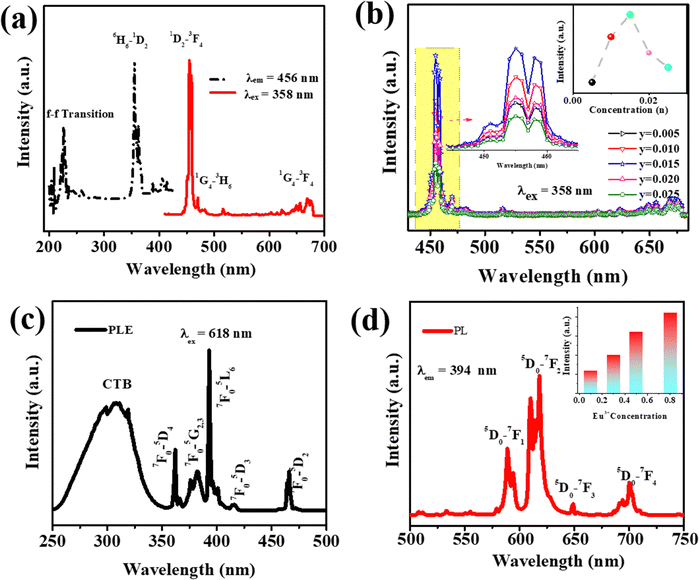 |
| | Fig. 4 (a) PLE and PL spectra, and (b) concentration-dependent luminescence intensity of the Ca3YAl3B4O15:xTm3+. (c) PLE and (d) PL spectra (with the inset showing the concentration-dependent luminescence intensity) of the Ca3YAl3B4O15:xEu3+. | |
Table 1 Assessment of the recent and earlier literature based on thermal stability, correlated color temperature (CCT), color rendering (Ra), color stability or chromaticity shift (ΔE) and Duv value for color stability
| |
Materials |
Thermal stability |
CCT |
R a |
D uv |
ΔE |
Ref. |
| 1 |
K2BaCa(PO4)2:Eu2+ |
96% |
5326 |
85.0 |
None |
None |
44 |
| 2 |
(Sr,Ca)AlSiN3:Eu2+ |
78% |
6119 |
92.2 |
None |
None |
45 |
| 3 |
SrLiAl3N4:Eu2+ |
81% |
3738 |
85.0 |
None |
None |
46 |
| 4 |
NaBiF4:Eu3+ |
67% |
6851 |
88.2 |
None |
None |
23 |
| 5 |
Ca3YAl3B4O15:Eu3+ |
85.2% |
None |
None |
None |
None |
This work |
| 6 |
Ca3YAl3B4O15:Tm3+ |
91.3% |
None |
None |
None |
None |
This work |
| 7 |
Ca3YAl3B4O15:Dy3+ |
89.5% |
None |
None |
None |
None |
This work |
| 8 |
CaSc2O4:0.15Eu3+,0.03Sm3+ |
96.1% |
5348 |
81.0 |
None |
None |
47 |
| 9 |
Na2Y2Ti3O10:Eu3+,Sm3+ |
84% |
5556 |
83.0 |
None |
None |
48 |
| 10 |
Cs2NaYCl6:Sb3+,Mn2+ |
None |
5410 |
81.2 |
None |
None |
49 |
| 11 |
Rb0.5K1.5Ca0.995PO4(F, Cl):Eu2+ |
87% |
4163 |
93.3 |
None |
None |
50 |
| 12 |
Sn2+/Mn2+ glass |
56% |
3811 |
95.3 |
None |
None |
9 |
| 13 |
SLGO:Dy3+,Sm3+ |
50% |
5284 |
91.1 |
None |
None |
26 |
| 14 |
Ba3GdNa(PO4)3F Eu2+ |
∼60% |
5402 |
81.0 |
None |
None |
51 |
| 15 |
Zn(SCN)2 |
None |
6046 |
86.0 |
None |
None |
52 |
| 16 |
CsPbX3 |
None |
8335 |
93.0 |
None |
None |
53 |
| 17 |
Cs2AgInCl6 |
None |
3878 |
85.0 |
None |
None |
54 |
| 18 |
Ca3YAl3B4O15:Tm3+,Dy3+ Eu3 |
93% |
3065 |
89.9 |
−0.006 |
12.31 × 10−3 |
This work |
The excitation and emission spectra of Ca3YAl3B4O15:Tm3+ and concentration-dependent photoluminescence intensity of the Ca3Y1−xAl3B4O15:xTm3+ (x = 0.005–0.025) phosphor are presented in Fig. 4c and d. The PLE achieved by placing the detector at 456 nm contains a sharp absorption band at 358 nm attributed to the 3H6 → 1D2 transition of Tm3+. The phosphor emits characteristic light associated with 1D2 → 3F4 transitions of Tm3+ peaking at 456 nm, under 360 nm excitation. The Ca3Y1−xAl3B4O15:xTm3+ emission spectrum displayed an increase in emission intensity under stimulation at 356 nm. The quenching concentration of Ca3Y1−xAl3B4O15:xTm3+ is depicted in Fig. 4d. The cross-relaxation between 1D2 and 3F4 energy levels is what causes quenching. The energy level's schematic picture shows how the transition of Tm3+ in CYAB is likely to occur. The chromaticity diagram of Ca3YAl3B4O15:Tm3+ was obtained using the PL spectrum in Fig. S5a (ESI†), and it was found to be (0.154, 0.042), which was extremely near to the BAM:Eu2+ blue light (0.170, 0.045). The temperature-dependent PL spectra of Ca3YAl3B4O15:Tm3+ from 300 K to 500 K are displayed in Fig. S4b (ESI†). Unexpectedly, the PL intensity at 400 K was approximately 91% higher than at 303 K. This shows that the Ca3YAl3B4O15:Tm3+ phosphor has better heat stability and is suitable for making WLEDs. After that, the activation energy was determined using the Arrhenius equation and the thermal quenching data in Fig. S4c (details are given in the ESI†). The thermal stability of Ca3YAl3B4O15:Eu3+ phosphor is superior to that reported in the literature (Table 1).
Besides, the PLE and PL spectra of the Dy3+-activated Ca3YAl3B4O15 are illustrated in Fig. S5a and b (ESI†). The excitation spectrum was obtained by placing a detector at 570 nm (4F9/2 → 6H13/2). The f–f transitions of Dy3+ are responsible for several peaks in the excitation. A series of peaks observed at 295, 324, 340, 351, 365, 389, 426, 452, and 475 nm match the 6H15/2 to ΔJ = 2 transition.55 The Ca3Y1−xAl3B4O15:xDy3+ emission spectrum under 350 nm excitation (y = 0.01, 0.04, 0.08, 0.12, and 0.16) showed increasing emission intensity (Fig. S5b, ESI†). The magnetic dipole (4F9/2 → 6H15/2) transition of Dy3+ is thought to be responsible for the emission peaks at 474 and 489 nm, which seldom change with the chemical environment or the crystal field nearby Dy3+. Another key emission peak at 569 nm is from the 4F9/2 → 6H13/2 (electric dipole) transition. Dy3+ ions undergo the forced electric dipole transition via a 4F9/2 → 6H13/2 hypersensitive transition (ΔJ = 2), which is largely controlled by the chemical atmosphere close to the Dy3+. Fig. S5c (ESI†) demonstrates the temperature-dependent PL spectra of Ca3YAl3B4O15:Dy3+ from 300 K to 500 K. It is evident that when the temperature increased, the PL of Dy3+ decreased. The emission intensities dropped until they reached 89.6% at 400 K (Fig. S5d, ESI†). The primary aspect governing the potential usage of phosphor is the temperature-dependent properties of materials. The decay curves for Dy3+ in CYAB at various doping concentrations (x = 0.01, 0.04, 0.08, 0.12, and 0.16) are presented in Fig. S6a (ESI†). By adjusting the value of τ to be between 0.38 and 0.26 ms, the first-order exponential (eqn (2)) may suit decay curves effectively, as shown in Fig. S6a (ESI†).
The critical distance (RC) between Dy3+ ions must be determined to study the CQ process, and it was determined that the RC value was 9.98 Å (ESI†). Therefore, in the CQ process of the Dy3+ ion, the multipolar–multipolar interaction dominated. The following equation was utilized to comprehend the link between PL intensity (I) and Dy3+ concentration (x);33–35
| |
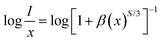 | (3) |
where
S stands for multipolar interaction and
β is a constant. When a similar activator is doped, CQ causes EM to occur as depicted in Fig. S6b (ESI
†). And
s = 6, 8, and 10 correspond to the interactions between D–D (dipole–dipole), D–Q (dipole–quadrupole), and Q–Q (quadrupole–quadrupole), respectively. As a result,
S = 6.27, which is closer to 6 showing that in Ca
3YAl
3B
4O
15:Dy
3+, the electric D–D mechanism predominates.
3.3. Single-component white light emission
The CIE coordinates of Tm3+ and Dy3+-activated Ca3YAl3B4O15 were observed to be (0.160, 0.062) and (0.358, 0.386) respectively, matching the colors of blue and yellow, for the co-doped phosphors and were based on their corresponding emission spectra as (0.321, 0.335) and (0.338, 0.364) for the Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+ phosphor. Under excitation at 358 nm, the Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+ phosphor displayed strong white light emissions that were simply tuned from blue (Tm3+) to yellow (Dy3+). The CIE coordinates of the phosphor (0.321, 0.335) are very close to those of daylight (0.333, 0.333), corresponding to a color temperature of 5742 K. Furthermore, it is important to highlight that warm white light with a correlated color temperature (CCT) below 5000 K is particularly desirable in solid-state lighting applications. Therefore, Eu3+ ions are used in the Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ phosphor to enhance the red-emission. The presence of ET between Tm3+ and Eu3+ ions is suggested by the fact that the excitation bands of Eu3+ and Tm3+ overlap to some degree in the 450–465 nm region (Fig. S7, ESI†). The emission spectra of Ca3Y0.905−xAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ (x = 0.001, 0.007, 0.01, 0.02) phosphors (λex = 358 nm, λex = 361 nm) are given in Fig. 5a and b. The CIE diagram of Ca3Y0.905−xAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ is shown in Fig. 5c and d and the data are given in Table S2 (ESI†). By appropriately adjusting the dopant concentration of Tm3+, Dy3+, and Eu3+, it is possible to realize nearly the entire white light area upon excitation of 361 nm. The synthesized Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ (x = 0.007, 0.01, 0.02) phosphors exhibited exceptionally alluring warm-white-light emissions with CCT of 4635 K, 3406 K, and 3065 K as given in the insets of Fig. 5. Because 358 nm light is not highly efficient for excitation of Eu3+, additional Eu3+ ions are required to generate warm white-light emission. At the same time, a minor amount of Eu3+ is sufficient for a 361 nm excitation. The Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ phosphor luminescence decay times are depicted in Fig. S7 and Table S3 (ESI†). It is evident from Fig. S7 (ESI†) that raising the Eu3+ concentration reduces the decay durations for Tm3+ ions. Furthermore, to evaluate the ET phenomenon from Tm3+ into the activator, the decay plots of the sensitizer Eu3+ in CYAB:Tm3+,Dy3+ were considered. The mechanism often relies on dipole–dipole or dipole–quadrupole interactions, where energy can transfer effectively from one dopant ion to another based on the Dexter ET model described elsewhere.24,25 As presented in Fig. S7b (ESI†), the best linear relationship was detected when n = 6 for the lifetime (τ/τ0) versus the xn/3, where x is the concentration of the sensitizer and activator. The rate of ET and ET efficiency were calculated using the equation kET = 1/τ − 1/τ0, and η = 100(1 − τ/τ0), where τ and τ0 define the Tm3+ sensitizer lifetime with and without an activator. The calculated value of kET rate and ET efficiency (η) as a function of Eu3+ concentration in Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ were as high as 97.58 μs−1 and 72.92%, as seen in Fig. S7b and Table S3 (ESI†). The Tm3+ ions will probably sensitize the Eu3+ emissions. The phosphors with ET adjustment are obviously more appropriate for WLEDs.
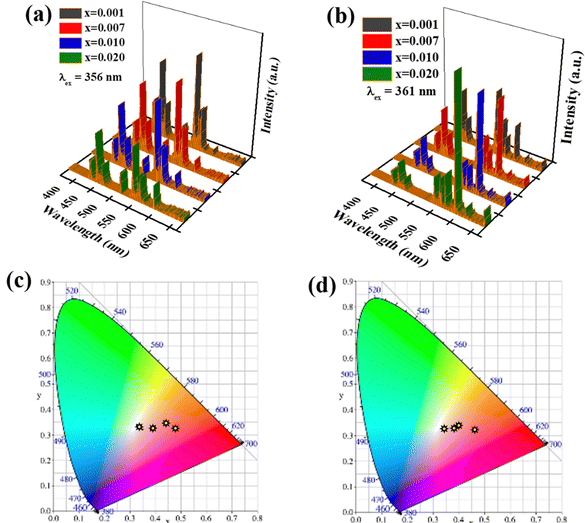 |
| | Fig. 5 (a) and (b) Photoluminescence properties and (c) and (d) CIE diagram of CYAB:0.015Tm3+,0.08Dy3+,xEu3+ with (λex = 358 nm, λex = 361 nm) excitation. | |
3.4 Thermal and color stability in a single-component host
Thermal stability is a critical factor affecting the performance of white light-emitting diodes (WLEDs), particularly in maintaining their efficiency and longevity under operational conditions. Typical operating temperatures for WLEDs range from 50 °C to 100 °C, influenced by factors such as power levels, heat dissipation design, and environmental conditions. Lower-power WLEDs generally operate at around 50–70 °C, while high-power variants can reach 80–100 °C or higher, especially in demanding environments. Thermal stability plays a crucial role in maintaining WLED performance under these conditions, as materials with high thermal stability help mitigate thermal quenching, ensuring consistent brightness and color accuracy. This stability prolongs device lifespan and improves energy efficiency by allowing more electrical energy to be converted into visible light rather than being lost as heat. Therefore, thermally stable phosphors and LEDs are essential for applications ranging from residential lighting to industrial and outdoor settings.6,56Fig. 6a shows the emission spectra of Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+ at varying temperatures from 300 K to 550 K at 361 nm. The steady loss of emission intensity was primarily attributed to the increased non-radiative emission at higher temperatures. The inset in Fig. 6b displays the integrated variation trend of the emission intensity of the Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+ sample at different temperatures. The total luminescence at 400 K was found to preserve 93% of its level at ambient temperature. Even at 475 K, the light intensity was maintained at 83.34% of room temperature, demonstrating the phosphor's high thermal stability. It is important to note that the thermal stability of the Eu3+ emission in the tridoped system was greatly improved when compared to the single-doped examples, whereas the thermal stability of the Tm3+ luminescence continues to decline. According to Fig. 6c, there appears to be a “temperature enhancement effect” that balances the thermal quenching of Eu3+ and Dy3+ and enhances Eu3+ emission. High color stability can finally be realized. The color stability can be quantitatively explained by the chromaticity shift (ΔE) using the following equation.6| |
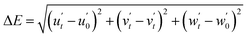 | (5) |
where u′ = 4x/(3 − 2x + 12y), v′ = 9y/(3 − 2x + 12y), and w′ = 1 − u′ − v′. x and y are the chromaticity coordinates. The chromaticity shift values are listed in Table S4 (ESI†). At 400 K, the conventional white light-emitting phosphor chromaticity shift is around 7.61 × 10−3. It is notable that at 400 K, the value of ΔEs is 7.61 × 10−3, which is significantly lower than that of industrial phosphors, such as BaMgAl10O17 with ΔEs of 15.2 × 10−3, and CaAlSiN3+ with ΔEs of 44 × 10−3. This demonstrates that the studied phosphor shows strong resistance to color shift, which is essential for reliable performance in mini and micro-LED applications.
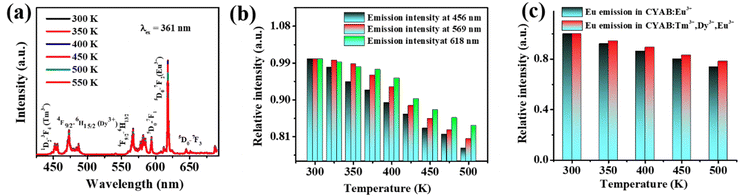 |
| | Fig. 6 (a) The temperature-dependent PL spectra of Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+ and (b) Tm3+, Dy3+ and Eu3+ emission intensities that are dependent on temperature as a function of temperature and (c) comparison with the PL intensity of a singly doped Eu3+ activator. | |
3.5. Application to white LEDs and Duv color quality of light sources for lighting
In practical applications, thermally stable phosphors play a crucial role in enhancing energy efficiency, lowering maintenance costs, and ensuring a reliable and consistent light source. This stability is particularly important in settings where color accuracy and stable light output are essential, such as in commercial displays, healthcare environments, and residential lighting, where sustained color fidelity is desired. WLEDs that are environmentally friendly, exhibit high luminance, and possess a long lifespan are valuable across various applications. The single-phase white emission and good thermal stability make the Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+ phosphor an attractive candidate for solid-state lighting. The construction of the LED lamp involved utilizing a 360 nm UV LED chip, along with the Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+ emitting sample, to showcase the vivid application of the synthesized phosphor (Fig. 7a). The electroluminescence spectrum displayed numerous emission peaks at about 455, 473, 573, 613, 650, and 700 nm, which originated from the phosphor shown in Fig. 7b. Most importantly, the corresponding Ra value up to 89.9 was better than that of other stated phosphors, as listed in Table 1. These findings identified that Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.01Eu3+ is appropriate for generating intense white emission upon UV light excitation.
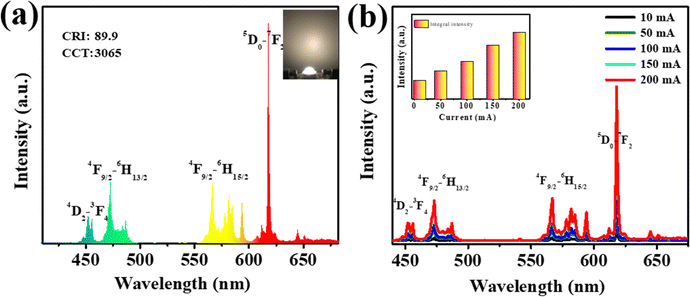 |
| | Fig. 7 (a) WLED device fabricated using the optimized Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+. (b) EL spectra of the WLED derived by the different currents. | |
When talking about color-sensitive lighting applications, the Duv (Duv) computation is a crucial measure.1,2Duv is crucial for the color accuracy of light sources. Duv is frequently overlooked in specifications. When describing the chromaticity of white light sources, correlated color temperature (CCT) is frequently utilized. However, as chromaticity is only two-dimensional, the distance from the Planckian locus is frequently absent. Though not yet extensively used, Duv is specified in ANSI C78.377 for this purpose. This is because CCT and CRI do not fully explain color quality. Duv explains how far the light coordinate point is from the blackbody curve. The chromaticity of light sources can be specified using CCT and Duv exactly like (x, y). Contrary to (x, y), the two integers (CCT, & Duv) intuitively provide color information. CIE must specify what Duv is. The formula below converts the CIE 1931 x and y values to their equivalent Duv values.3
(1) Convert (x, y) or (u′, v′) to (u, v)
| u = 4x/(−2x + 12y + 3) u = u′ |
| v = 6y/(−2x + 12y + 3) or v = 2v′/3 |
(2) Duv is obtained by
| LBB = k6a6 + k5a5 + k4a4 + k3a3 + k2a2 + k1a + k0 |
where,
k0 = −0.471106,
k1 = 1.925865,
k2 = −2.4243787,
k3 = 1.5317403,
k4 = −0.5179722,
k5 = −0.08939440, and
k6 = −0.00616793. The equation above can easily be used to compute
Duv. The calculated value of
Duv of the typical white emission is about −0.002 to 0.014 (
Fig. 8 and
Table 1). Using the above equation, the calculated value of
Duv for Ca
3Y
0.985−xAl
3B
4O
15:0.015Tm
3+,0.08Dy
3+,
xEu
3+ is listed in Table S4 (ESI
†). The recommended
Duv range is −0.006 to +0.006 according to ANSI and Energy Star. A
Duv range between −0.003 and +0.003 is ideal for practical uses that are more demanding (Waveform Lighting's FilmGrade series of products have a
Duv tolerance of ±0.003). The computed value of
Duv in this study is between −0.002 to +0.014, which strongly matches the range in the ANSI standard.
3 The term
Duv represents the deviation from the blackbody locus on a chromaticity diagram, indicating the color difference between the light source and natural daylight or blackbody radiation at a similar CCT. In lighting,
Duv is critical because even small deviations can mark the perception of warmth, neutrality, or coolness, making it a key parameter in human-centric lighting applications.
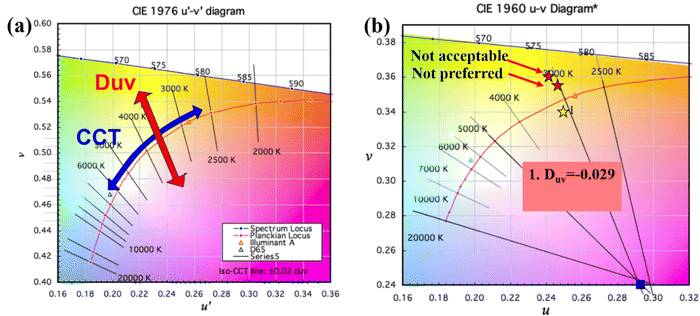 |
| | Fig. 8 (a) and (b) CCT-Duv chart based on the CIE 1976 (u, v) diagram and CCT-Duv chart of Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+ on the CIE 1976 diagram. | |
4. Conclusion
Novel Ca3YAl3B4O15 phosphors with Tm3+, Dy3+, and Eu3+ doping were studied to determine their possible applications, chromaticity shift, or color stability, and Duv is recommended as a simple way to understand the chromaticity of white light sources used for general lighting. The Ca3YAl3B4O15:Tm3+, Ca3YAl3B4O15:Dy3+, and Ca3YAl3B4O15:Eu3+ samples exhibited the characteristic transitions of Tm3+ (3H6 → 1D2), Dy3+ (6H15/2 → J3,17,5,13), and Eu3+ (5D0,1,2 → 7F0,1,2,3,4). The incorporation of Eu3+ into Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+ displayed attractive warm-white light with CCT declining from 4635 K to 3065 K. Importantly, the temperature-dependent PL of Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,0.02Eu3+ at 400 K was 93% of that at 300 K, showing excellent thermal stability. With the incorporation of Eu3+ as a coactivator into Ca3YAl3B4O15:0.015Tm3+,0.08Dy3+,xEu3+ (x = 0.007, 0.01, and 0.02), the CIE coordinates can be adjusted from (0.3279, 0.3259) to (0.4559, 0.3376), with CCT dropping from 4535 K to 3065 K. The Ca3YAl3B4O15:Tm3+,Dy3+,Eu3+-based WLED exhibits satisfactory parameters of high Ra (89.9) and low-CCT (3065 K). Furthermore, this article provides useful calculation techniques for calculating Duv over a wide range of chromaticity, from −0.002 to 0.014 in Duv and 2000 to 6000 K in CCT. The computed value of Duv is ±0.002 to ±0.014, which strongly matches the range in an American National Standards Institute (ANSI) standard.
Data availability
Data will be made available on request. The datasets supporting this article have been uploaded as part of the ESI.†
Conflicts of interest
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of Guangdong Province (2414050004479), and the Science and Technology Planning Project of Shenzhen Municipality under grant no. 20241063010062. The Chinese Scholarship Council, China postdoc, and the Guangdong Government are acknowledged for the support to Dr Wasim Ullah Khan during their PhD and postdoc fellowship.
References
- D. Baxter, M. Royer and K. Smet, Leukos, 2024, 20, 55–66 CrossRef.
- M. Royer, M. J. Murdoch, K. Smet, L. Whitehead, A. David, K. Houser, T. Esposito, J. Livingston and Y. Ohno, Leukos, 2023, 19, 35–52 CrossRef.
- B. Das, S. Bardhan, T. Maity and S. Mazumdar, Results Optics, 2020, 1, 100013 CrossRef.
- P. Pust, V. Weiler, C. Hecht, A. Tücks, A. S. Wochnik, A.-K. Henß, D. Wiechert, C. Scheu, P. J. Schmidt and W. Schnick, Nat. Mater., 2014, 13, 891 CrossRef CAS PubMed.
- A. Layek, P. C. Stanish, V. Chirmanov and P. V. Radovanovic, Chem. Mater., 2015, 27, 1021–1030 CrossRef CAS.
- J. Li, Q. Liang, J.-Y. Hong, J. Yan, L. Dolgov, Y. Meng, Y. Xu, J. Shi and M. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 18066–18072 CrossRef CAS PubMed.
- S. Liang, L. Song, W. Nie, Z. Wang, D. Chen, F. Lin and H. Zhu, Adv. Opt. Mater., 2024, 12, 2301502 CrossRef CAS.
- W. U. Khan, L. Qin, W. U. Khan, S. U. Khan, M. M. Hussain, F. Ahmed, S. Kamal and P. Zhou, ACS Appl. Nano Mater., 2023, 6, 17838–17847 CrossRef CAS.
- Y. Zhang, B. Chen, X. Zhang, Y. Cao, J. Zhang, S. Xu, X. Li, H. Yu, D. Gao, X. Sha, L. Wang, X. Chen and H. Lin, Chem. Eng. J., 2023, 467, 143467 CrossRef CAS.
- Y. Wei, L. Cao, L. Lv, G. Li, J. Hao, J. Gao, C. Su, C. C. Lin, H. S. Jang and P. Dang, Chem. Mater., 2018, 30, 2389–2399 CrossRef CAS.
- P. Zhu, Q. Zhu, H. Zhu, H. Zhao, B. Chen, Y. Zhang, X. Wang and W. Di, Opt. Mater., 2008, 30, 930–934 CrossRef CAS.
- W. U. Khan, L. Qin, A. Alam, P. Zhou, Y. Peng and Y. Wang, Nanoscale, 2021, 13, 4301–4307 RSC.
- D. Wen, J. Shi, M. Wu and Q. Su, ACS Appl. Mater. Interfaces, 2014, 6, 10792–10801 CrossRef CAS PubMed.
- W. U. Khan, J. Li, X. Li, Q. Wu, J. Yan, Y. Xu, F. Xie, J. Shi and M. Wu, Dalton Trans., 2017, 46, 1885–1891 RSC.
- W. U. Khan, L. Zhou, Q. Liang, X. Li, J. Yan, N. U. Rahman, L. Dolgov, S. U. Khan, J. Shi and M. Wu, J. Mater. Chem. C, 2018, 6, 7612–7618 RSC.
- W. U. Khan, L. Qin, P. Zhou, A. Alam, Z. Ge and Y. Wang, ACS Appl. Mater. Interfaces, 2023, 15, 45616–45625 CrossRef CAS PubMed.
- F. Jahanbazi, N. Dimakis and Y. Mao, Adv. Opt. Mater., 2024, 12, 2301219 CrossRef CAS.
- T. Nakajima and T. Tsuchiya, ACS Appl. Mater. Interfaces, 2015, 7, 21398–21407 CrossRef CAS PubMed.
- J. Long, F. Chu, Y. Wang, C. Zhao, W. Dong, X. Yuan, C. Ma, Z. Wen, R. Ma and M. Du, Inorg. Chem., 2017, 56, 10381–10386 CrossRef CAS PubMed.
- Y. Liu, G. Liu, J. Wang, X. Dong and W. Yu, Inorg. Chem., 2014, 53, 11457–11466 CrossRef CAS PubMed.
- J. Qiao, L. Ning, M. S. Molokeev, Y.-C. Chuang, Q. Liu and Z. Xia, J. Am. Chem. Soc., 2018, 140, 9730–9736 CrossRef CAS PubMed.
- Z. Wang, J. Ha, Y. H. Kim, W. B. Im, J. McKittrick and S. P. Ong, Joule, 2018, 2, 914–926 CrossRef CAS.
- P. Du, X. Huang and J. S. Yu, Chem. Eng. J., 2018, 337, 91–100 CrossRef CAS.
- W. U. Khan, P. Zhou, L. Qin, A. Alam, Z. Ge and Y. Wang, Mater. Today Nano, 2022, 18, 100205 CrossRef CAS.
- R. Shi, J. Xu, G. Liu, X. Zhang, W. Zhou, F. Pan, Y. Huang, Y. Tao and H. Liang, J. Phys. Chem. C, 2016, 120, 4529–4537 CrossRef CAS.
- Z. Zhang, J. Li, N. Yang, Q. Liang, Y. Xu, S. Fu, J. Yan, J. Zhou, J. Shi and M. Wu, Chem. Eng. J., 2020, 390, 124601 CrossRef CAS.
- C.-H. Huang and T.-M. Chen, J. Phys. Chem. C, 2011, 115, 2349–2355 CrossRef CAS.
- Y. Yu, Q. Wu and R. Li, J. Solid State Chem., 2006, 179, 429–432 CrossRef CAS.
- W. Ullah Khan, S. B. Mane, S. Ullah Khan, D. Zhou, D. Khan, Q. Yu, W. Zhou, L. Zhou, J. Shi and M. Wu, RSC Adv., 2018, 8, 40693–40700 RSC.
- W. U. Khan, W. U. Khan, H. Lin, Z. Cheng, M. W. Shah and Y. Zhang, ACS Appl. Electron. Mater., 2021, 3, 4218–4227 CrossRef CAS.
- N. U. Rahman, W. U. Khan, S. Khan, X. Chen, J. Khan, J. Zhao, Z. Yang, M. Wu and Z. Chi, J. Mater. Chem. A, 2019, 7, 6467–6474 RSC.
- Z. Xu, Z. Xia, B. Lei and Q. Liu, J. Mater. Chem. C, 2016, 4, 9711–9716 RSC.
- W. U. Khan, W. U. Khan, Y. Peng, Z. Cheng, T. A. Saleh and Y. Zhang, J. Colloid Interface Sci., 2021, 600, 219–228 CrossRef PubMed.
- W. U. Khan, Z. Ye, M. Boubeche, T. Liu, Z. Guo and Y. Zhang, J. Alloys Compd., 2021, 888, 161538 CrossRef.
- W. U. Khan, W. U. Khan, Z. Ye, M. Boubeche, T. Shi, D. Khan and Y. Zhang, Ceram. Int., 2022, 48, 5689–5697 CrossRef CAS.
- N. Yang, J. Li, Z. Zhang, D. Wen, Q. Liang, J. Zhou, J. Yan and J. Shi, Chem. Mater., 2020, 32, 6958–6967 CrossRef CAS.
- O. A. Lipina, L. L. Surat, Y. V. Baklanova, L. Y. Mironov, A. N. Enyashin, A. Y. Chufarov, A. P. Tyutyunnik and V. G. Zubkov, New J. Chem., 2020, 44, 16400–16411 RSC.
- P. Du and J. S. Yu, J. Alloys Compd., 2019, 785, 789–797 CrossRef CAS.
- J. Li, Q. Liang, Y. Cao, J. Yan, J. Zhou, Y. Xu, L. Dolgov, Y. Meng, J. Shi and M. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 41479–41486 CrossRef CAS PubMed.
- G.-H. Li, N. Yang, J. Zhang, J.-Y. Si, Z.-L. Wang, G.-M. Cai and X.-J. Wang, Inorg. Chem., 2020, 59, 3894–3904 CrossRef CAS PubMed.
- P. Dang, G. Li, X. Yun, Q. Zhang, D. Liu, H. Lian, M. Shang and J. Lin, Light: Sci. Appl., 2021, 10, 1–13 CrossRef PubMed.
- C. Guo, L. Luan, C. Chen, D. Huang and Q. Su, Mater. Lett., 2008, 62, 600–602 CrossRef CAS.
- Q. Zhang, X. Wang and Y. Wang, Inorg. Chem. Front., 2020, 7, 1034–1045 RSC.
- J. Qiao, L. Ning, M. S. Molokeev, Y.-C. Chuang, Q. Liu and Z. Xia, J. Am. Chem. Soc., 2018, 140, 9730–9736 CrossRef CAS PubMed.
- M. Zhao, Z. Xia, X. Huang, L. Ning, R. Gautier, M. S. Molokeev, Y. Zhou, Y. C. Chuang, Q. Zhang, Q. Liu and K. R. Poeppelmeier, Sci. Adv., 2019, 5, eaav0363 CrossRef PubMed.
- X. Zhang, Y.-T. Tsai, S.-M. Wu, Y.-C. Lin, J.-F. Lee, H.-S. Sheu, B.-M. Cheng and R.-S. Liu, ACS Appl. Mater. Interfaces, 2016, 8, 19612–19617 CrossRef CAS PubMed.
- S. U. Khan, W. U. Khan, W. U. Khan, D. Khan, S. Saeed, S. Badshah, M. Ikram and T. A. Saleh, Small, 2020, 16, 2001551 CrossRef CAS PubMed.
- D. Kang, H. S. Yoo, S. H. Jung, H. Kim and D. Y. Jeon, J. Phys. Chem. C, 2011, 115, 24334–24340 CrossRef CAS.
- S. Bai, H. Liang, C. Li, C. Tang, G. Yang, X. Xu, X. Yang, G. Pan and Y. Zhu, Ceram. Int., 2023, 49, 1102–1107 CrossRef CAS.
- M. Liao, F. Wu, D. Zhu, X. Zhang, H. Dong, Z. Lin, M. Wen and Z. Mu, Chem. Eng. J., 2022, 449, 137801 CrossRef CAS.
- J. Chen, N. Zhang, C. Guo, F. Pan, X. Zhou, H. Suo, X. Zhao and E. M. Goldys, ACS Appl. Mater. Interfaces, 2016, 8, 20856–20864 CrossRef CAS PubMed.
- C. Dzorkpata, S. Thapa, H. Zhu, A. Grigoriev, D. Babaian, S. Guha and P. Zhu, J. Alloys Compd., 2024, 1005, 176064 CrossRef CAS.
- S. Thapa, G. C. Adhikari, H. Zhu, A. Grigoriev and P. Zhu, Sci. Rep., 2019, 9, 18636 CrossRef CAS PubMed.
- P. Zhu, S. Thapa, H. Zhu, S. Wheat, Y. Yue and D. Venugopal, J. Alloys Compd., 2023, 960, 170836 CrossRef CAS.
- Z. Yu, X. Li, J. Wang and S. Zhang, Mater. Today Chem., 2023, 33, 101739 CrossRef CAS.
- W. U. Khan, L. Zhou, X. Li, W. Zhou, D. Khan, S.-I. Niaz and M. Wu, Chem. Eng. J., 2021, 410, 128455 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article ab,
Waheed Ullah Khan
ab,
Waheed Ullah Khan c,
Haris Zamand,
Ayaz Mahsude,
Dilfaraz Khan*e,
Salim Ullah Khanf,
Shuakat Khang and
Yueli Zhang
c,
Haris Zamand,
Ayaz Mahsude,
Dilfaraz Khan*e,
Salim Ullah Khanf,
Shuakat Khang and
Yueli Zhang *ab
*ab
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 154). The X-ray diffraction study results showed that doping of Dy3+ does not have any other impurities or cause any notable change. As shown in Fig. S1 (ESI†), the Rietveld-refinement results of Ca3YAl3B4O15:Dy3+ confirmed that the phosphor crystalizes into a single garnet phase. The cell variables were a = 10.3867(3), c = 5.8146(2), and V = 534.94 Å3. The unit cell contains four distinct types of cationic sites, where the metal ions exhibit nine-fold and seven-fold coordination polyhedra at the Ca2+/Y3+(1) and Ca2+/Y3+(2) sites, respectively. Additionally, six coordinated aluminum sites and three coordinated boron sites were discovered. The Ca2+/Y3+(1) sites were separated from the Ca2+/Y3+(2) sites by the BO3 and AlO6 units in Fig. 1b and c. The AlO6 octahedra share edges to form chains, which are connected by triangular BO3 groups in the ab plane, creating a Kagome-type lattice characteristic of the gaudefroyite structure. The P63/m space group accurately predicts all points in the X-ray diffraction pattern. However, the Ln3+ rare-earth ions in Ca3YAl3B4O15 have somewhat different distributions in these two sites, as Ln3+ ions can occupy both cation sites in the structure, with the Ca2+/Y3+(1) sites being occupied by 79% Ln3+ ions and the Ca2+/Y3+(2) sites being occupied by 21% Ln3+.29 As for our system, the dopant's behavior is opposite to what has been previously reported.30 Our modifications showed that the Ca2+/Y3+(2) site in Ca3YAl3B4O15:Dy3+ should be occupied by a very heavy-ion, rather than the Ca2+/Y3+(1) site. Thus, we assume that in Ca3YAl3B4O15:Dy3+ ions fully occupy the Ca2+/Y3+(2) site. As shown in Fig. 1c (coordinating conditions of Ca2+/Y3+(1) and Ca2+/Y3+(2)), the distance between the sites of Ca2+/Y3+(1) is shorter than the Ca2+/Y3+(2) sites, which may be the reason for the preferences of occupancy (Table S1, ESI†). All the other elements containing Ca, Y, Dy, Al, and O in the matrix were detected except boron (Fig. 1d and e). The (1 1 2) and (−1 2 1) planes are well represented by a lattice fringe in the HRTEM picture with interplanar lattice spacings of 0.52 and 0.35 nm of Ca3YAl3B4O15:0.08Dy3+ shown in Fig. S2 (ESI†). The XPS results gave additional proof for the elemental makeup (Fig. 2). XPS spectral analysis showed peaks at 284.5 eV and 530.7 eV attributed to C 1s and O 1s, respectively (details are given in the ESI†).31 The Kubelka–Munk function was used to calculate the phosphors' energy bandgap (Eg), which was found to be 5.13 eV from the diffuse reflection spectroscopy (DRS) analysis (Fig. 3a and b).32
154). The X-ray diffraction study results showed that doping of Dy3+ does not have any other impurities or cause any notable change. As shown in Fig. S1 (ESI†), the Rietveld-refinement results of Ca3YAl3B4O15:Dy3+ confirmed that the phosphor crystalizes into a single garnet phase. The cell variables were a = 10.3867(3), c = 5.8146(2), and V = 534.94 Å3. The unit cell contains four distinct types of cationic sites, where the metal ions exhibit nine-fold and seven-fold coordination polyhedra at the Ca2+/Y3+(1) and Ca2+/Y3+(2) sites, respectively. Additionally, six coordinated aluminum sites and three coordinated boron sites were discovered. The Ca2+/Y3+(1) sites were separated from the Ca2+/Y3+(2) sites by the BO3 and AlO6 units in Fig. 1b and c. The AlO6 octahedra share edges to form chains, which are connected by triangular BO3 groups in the ab plane, creating a Kagome-type lattice characteristic of the gaudefroyite structure. The P63/m space group accurately predicts all points in the X-ray diffraction pattern. However, the Ln3+ rare-earth ions in Ca3YAl3B4O15 have somewhat different distributions in these two sites, as Ln3+ ions can occupy both cation sites in the structure, with the Ca2+/Y3+(1) sites being occupied by 79% Ln3+ ions and the Ca2+/Y3+(2) sites being occupied by 21% Ln3+.29 As for our system, the dopant's behavior is opposite to what has been previously reported.30 Our modifications showed that the Ca2+/Y3+(2) site in Ca3YAl3B4O15:Dy3+ should be occupied by a very heavy-ion, rather than the Ca2+/Y3+(1) site. Thus, we assume that in Ca3YAl3B4O15:Dy3+ ions fully occupy the Ca2+/Y3+(2) site. As shown in Fig. 1c (coordinating conditions of Ca2+/Y3+(1) and Ca2+/Y3+(2)), the distance between the sites of Ca2+/Y3+(1) is shorter than the Ca2+/Y3+(2) sites, which may be the reason for the preferences of occupancy (Table S1, ESI†). All the other elements containing Ca, Y, Dy, Al, and O in the matrix were detected except boron (Fig. 1d and e). The (1 1 2) and (−1 2 1) planes are well represented by a lattice fringe in the HRTEM picture with interplanar lattice spacings of 0.52 and 0.35 nm of Ca3YAl3B4O15:0.08Dy3+ shown in Fig. S2 (ESI†). The XPS results gave additional proof for the elemental makeup (Fig. 2). XPS spectral analysis showed peaks at 284.5 eV and 530.7 eV attributed to C 1s and O 1s, respectively (details are given in the ESI†).31 The Kubelka–Munk function was used to calculate the phosphors' energy bandgap (Eg), which was found to be 5.13 eV from the diffuse reflection spectroscopy (DRS) analysis (Fig. 3a and b).32


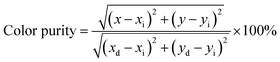
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ)
exp(−t/τ)