Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A concept of dual-responsive prodrugs based on oligomerization-controlled reactivity of ester groups: an improvement of cancer cells versus neutrophils selectivity of camptothecin†
Insa
Klemt‡
 a,
Viktor
Reshetnikov‡
a,
Subrata
Dutta
a,
Galyna
Bila
b,
Rostyslav
Bilyy
a,
Viktor
Reshetnikov‡
a,
Subrata
Dutta
a,
Galyna
Bila
b,
Rostyslav
Bilyy
 b,
Itziar Cossío
Cuartero
c,
Andrés
Hidalgo
c,
Adrian
Wünsche
a,
Maximilian
Böhm
a,
Marit
Wondrak
d,
Leoni A.
Kunz-Schughart
b,
Itziar Cossío
Cuartero
c,
Andrés
Hidalgo
c,
Adrian
Wünsche
a,
Maximilian
Böhm
a,
Marit
Wondrak
d,
Leoni A.
Kunz-Schughart
 de,
Rainer
Tietze
de,
Rainer
Tietze
 f,
Frank
Beierlein
gh,
Petra
Imhof
f,
Frank
Beierlein
gh,
Petra
Imhof
 gh,
Sabrina
Gensberger-Reigl
gh,
Sabrina
Gensberger-Reigl
 i,
Monika
Pischetsrieder
i,
Monika
Pischetsrieder
 i,
Marlies
Körber
a,
Tina
Jost
j and
Andriy
Mokhir
i,
Marlies
Körber
a,
Tina
Jost
j and
Andriy
Mokhir
 *a
*a
aDepartment of Chemistry and Pharmacy, Organic Chemistry II, Friedrich-Alexander-University of Erlangen-Nürnberg (FAU), 91058 Erlangen, Germany. E-mail: Andriy.Mokhir@fau.de
bDepartment of Histology, Cytology and Embryology, Danylo Halytsky Lviv National Medical University, 79010 Lviv, Ukraine
cProgram of Cardiovascular Regeneration, Centro Nacional de Investigaciones Cardiovasculares Carlos III (CNIC), C. Melchor Fernández Almagro, 3, 28029 Madrid, Spain
dOncoRay, National Center for Radiation Research in Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, TU Dresden and Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany
eNational Center for Tumor Diseases (NCT), Partner Site Dresden, Germany
fDepartment of Otorhinolaryngology, Head and Neck Surgery, Section of Experimental Oncology and Nanomedicine (SEON), FAU, University Hospital, 91054 Erlangen, Germany
gErlangen National High Performance Computing Center (NHR@FAU), FAU, 91058 Erlangen, Germany
hComputer-Chemistry-Center, Department of Chemistry and Pharmacy, FAU, Germany
iDepartment of Chemistry and Pharmacy, Food Chemistry, FAU, 91058 Erlangen, Germany
jDepartment of Radiation Oncology, FAU, University Hospital, 91054 Erlangen, Germany
First published on 31st January 2024
Abstract
Many known chemotherapeutic anticancer agents exhibit neutropenia as a dose-limiting side effect. In this paper we suggest a prodrug concept solving this problem for camptothecin (HO-cpt). The prodrug is programmed according to Boolean “AND” logic. In the absence of H2O2 (trigger T1), e.g. in the majority of normal cells, it exists as an inactive oligomer. In cancer cells and in primed neutrophils (high H2O2), the oligomer is disrupted forming intermediate (inactive) lipophilic cationic species. These are accumulated in mitochondria (Mit) of cancer cells, where they are activated by hydrolysis at mitochondrial pH 8 (trigger T2) with formation of camptothecin. In contrast, the intermediates remain stable in neutrophils lacking Mit and therefore a source of T2. In this paper we demonstrated a proof-of-concept. Our prodrug exhibits antitumor activity both in vitro and in vivo, but is not toxic to normal cell and neutrophils in contrast to known single trigger prodrugs and the parent drug HO-cpt.
Introduction
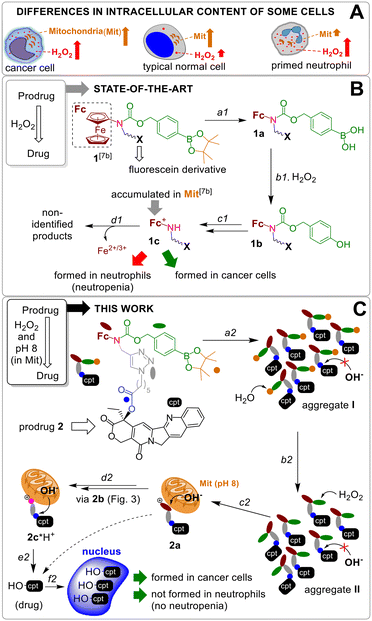
Chemotherapy is one of the three major concepts in current cancer treatment alone, or in combination with surgery and radiotherapy. Clinically used chemotherapeutics include both natural and synthetic compounds, e.g., camptothecins,1 bleomycin,2 doxorubicin,3 paclitaxel4 and Pt(II) complexes (cisplatin, oxaliplatin).5 Their common side effect is neutropenia,1–5 a condition characterized by the low number of neutrophils. Since neutrophils belong to the first line of defence against infections, neutropenia causes strong suppression of the immune system in patients and, as a consequence, even minor infections can become life threatening.Side effects of drugs can be eliminated by converting them to prodrugs, which are activated under cancer specific conditions, but remain inactive in other cells.6 For example, we7 and others8 have used differences in the amount of H2O2 in cancer and normal cells9 to design H2O2-responsive anticancer prodrugs. However, neutrophils also produce large amounts of H2O2 that can activate the prodrugs causing neutropenia. Representative examples include H2O2-responsive prodrugs of (a) gemcitabine exhibiting residual neutrophil toxicity compared to the vehicle10 and (b) N-alkylaminoferrocene (AF) causing death of neutrophils via formation of neutrophil extracellular traps (NETs).11 These literature data indicate that improved concepts for H2O2-responsive prodrugs are warranted.
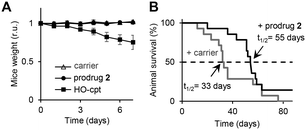
Since the number of mitochondria (Mit) in cancer cells is substantially higher than that in neutrophils (Fig. 1A),12 we hypothesized that a combination of the H2O2-mediated activation with the Mit-driven chemistry can be used to improve cancer cells versus neutrophils specificity of H2O2-responsive prodrugs. Herein, we report on the data confirming the feasibility of this hypothesis. We developed AFPG2/PG1-L-E-cpt (where AF: aminoferrocene, PG2/PG1: a H2O2-responsive protecting group, L: a linker, E: an ester moiety, cpt: a camptothecin residue, Fig. 1 and 2), which is a prodrug of an inhibitor of topoisomerase I (TOPI) camptothecin (HO-cpt). HO-cpt was selected, since along with the strong anticancer activity it exhibits severe neutropenia.1 Two HO-cpt derivatives (irinotecan and topotecan) are already used in clinics.1 Single trigger H2O2 – activated prodrugs of HO-cpt13 as well as single trigger mitochondrial OH− (OH−Mit, pH 8)14 – activated prodrugs have been previously described.15 In contrast, the reported here AFPG2/PG1-L-E-cpt is a dual trigger prodrug activated only when H2O2 (T1) and a OH−Mit (T2) are present (Fig. 1B and C). It exhibits low μM anticancer activity in vitro towards a variety of human cancer cell lines representing blood, ovarian, prostate, pharynx and tongue, but is not toxic towards normal cells including PMA-primed neutrophils. Its anticancer activity is potentiated by ionizing radiation. The prodrug retains its activity in vivo in Nemeth–Kellner lymphoma model of murine cancer. In contrast to HO-cpt, it does not induce neutropenia in vivo.
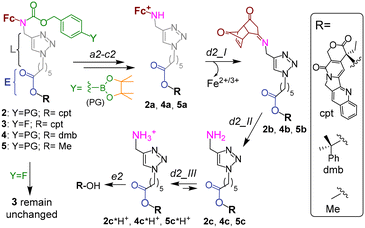 | ||
| Fig. 2 The mechanism of activation of prodrug 2, its analogues 4 and 6 as well as unreactive controls 3 and 5. Steps a2–c2 and e2 are described in the caption to Fig. 1C. Steps d2_I and d2_II occur spontaneously. | ||
Results and discussion
A previously reported prodrug 1 is hydrolyzed within <2 h in aqueous solution with formation of the boronic acid 1a (step a1, Fig. 1B).7b The 1a is taken up by both normal and cancer cells, but is activated only in H2O2-rich, cancer cells via steps b1–c1 with formation of a lipophilic cation ferrocenium 1c (via intermediate 1b), which is found to be accumulated in Mit.7b The 1c is decomposed forming not identified polar products. The T1 (H2O2)-responsive moiety of the 1 was applied to design the prodrug reported in this paper.Design of a T1/T2-responsive prodrug 2
The proposed activation mechanism of prodrug 2 and its chemical structure are shown in Fig. 1C and 2.It includes three elements: moieties responsive to T1 (arylboronic acid pinacol ester, coloured green in Fig. 2 (ref. 7b)) and T2 (L-E) as well as a drug fragment (cpt), deactivated by acylation of the critical for the activity HO group with formation of an ester E.16 Ideally, the E is cleaved in the presence of both H2O2 (T1) and OH−Mit (T2) releasing the active drug HO-cpt (cancer cell: T1+/T2+), but remains intact when one of the triggers is absent (normal cell: T1−/T2+; neutrophil: T1+/T2−). This goal is achieved in the following way. The prodrug is designed to be lipophilic enough to exist in the oligomer-form in aqueous solution (a mixture of aggregates I and II, Fig. 1C). In the aggregates the hydrophobic L-E moiety is buried in the interior and, therefore, not accessible to the hydrolysis by hydrophilic T2. A polar boronic acid moiety is located at the exterior of the aggregate and accessible to T1. Assuming that the T1-responsive moiety7b retains its properties in the prodrug, its activation induced by T1 will occur as outlined in Fig. 2. In steps b2 and c2, the prodrug will be converted to ferrocenium 2a in the reaction with T1. A positive charge of this intermediate should destabilize the aggregate, thereby favouring the monomeric species. In cells, the de-aggregation will be further supported by loading of the 2a into Mit (Fig. 1C). In this state the ester E will be accessible for T2 and can therefore be hydrolysed forming HO-cpt as indicated with a dashed arrow in Fig. 1C. As it is described in the experimental part, the latter reaction occurs stepwise (d2 + e2) via intermediates 2b, 2c and 2c*H+ (Fig. 2). In step f2, the drug is accumulated in the nucleus, where it inhibits TOPI.
Activation of the prodrug by T1 and T2 in cell free settings
Synthesis of the prodrug and controls is described in the ESI.† Their purity was confirmed by C, H, N elemental analysis and was found to be >95%. The characterization and confirmation of prodrug stability in solid state and solution are provided in Fig. S1–S24.†Aggregation of the prodrug
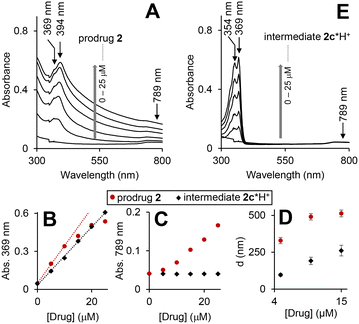
The prodrug 2 is lipophilic as evidenced by its high n-octanol/water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) of 4.27 ± 0.04 (Table S1, ESI†). However, it is still moderately soluble at ≤30 μM in Dulbecco's phosphate-buffered saline (DPBS) (Table S2, ESI†). To investigate the aggregation phenomena in the aqueous solutions, we recorded UV-visible spectra of the prodrug at concentrations up to 25 μM (Fig. 3A).
P) of 4.27 ± 0.04 (Table S1, ESI†). However, it is still moderately soluble at ≤30 μM in Dulbecco's phosphate-buffered saline (DPBS) (Table S2, ESI†). To investigate the aggregation phenomena in the aqueous solutions, we recorded UV-visible spectra of the prodrug at concentrations up to 25 μM (Fig. 3A).
As expected, the spectra are dominated by the cpt chromophore (λmax = 369 and 394 nm), whereas the ferrocenyl moiety is not visible due to its low extinction coefficient. Absorbance at these maxima does not obey Beer–Lambert law (Fig. 3B: representative data for λmax = 369 nm) indicating the presence of more than one cpt-chromophore-containing species in solution. Interestingly, the significant absorbance at >500 nm is also observed (Fig. 3A and D). Since the prodrug has no chromophores absorbing >500 nm, this feature can be interpreted by Rayleigh light scattering on an aggregate. By using dynamic light scattering (DLS) we confirmed the formation of aggregates with sizes ranging from 350 ± 21 to 535 ± 65 nm. The λmax values of the prodrug (369/394 nm) are close to those observed for the solution of HO-cpt dissolved in unpolar solvent toluene (369/386 nm), but do not match those of HO-cpt dissolved in polar water (355/368 nm).17 These data indicate that the cpt moiety is located in the hydrophobic interior of the aggregates. Since the E-L is directly linked to the cpt, it will also be located in the hydrophobic interior that will protect it from the attack by T2 (Fig. 1C).
Reaction of the prodrug with H2O2
The prodrug 2 as well as the 3, where the H2O2-responsive boronic acid moiety was replaced with a fluorine, are practically not fluorescent in aqueous solutions (Fig. 4A). The possible reasons are (a) photo-induced electron transfer (PET) from the Fc moiety to the excited state of the cpt and (b) aggregation. We observed the H2O2-dose dependent increase of the fluorescence characteristic for the cpt fluorophore (λex = 365 nm, λem = 460 nm) in the mixtures of prodrug (20 μM) and H2O2 (10 μM to 10 mM, Fig. 4A and B). Further data obtained at pH 6, 7, 8 and 10 are shown in Fig. S26A, ESI.† This reaction is facilitated at higher pH (1.5- and 1.9-fold faster at pH 8 and 10 than at pH 7) and substantially slowed down at the acidic pH (Table S3, ESI†). Importantly, the prodrug activation by H2O2 is not affected by the physiological concentration of glutathione (GSH, 5 mM) (Fig. 4C) and is ∼2-fold activated by fetal bovine serum (FBS) (5%) (Fig. S26C, ESI†) indicating that the reaction is possible within living cells.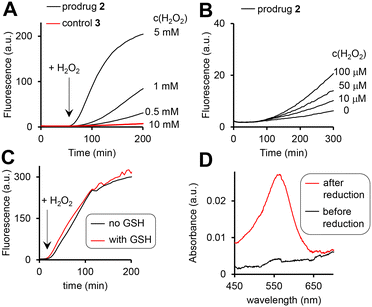 | ||
| Fig. 4 The fluorescence increase (λex = 365 nm, λem = 400 nm) in aqueous solutions of the prodrug 2 (10 μM, pH 8) in the presence of H2O2 (A: 0.5–10 mM; B: 0–100 μM). C: Data indicating that GSH (5 mM) does not affect the activation of the prodrug by H2O2 (10 mM). D: Detection of Fe2+ (“before reduction”) and the sum of Fe3+ and Fe2+ (“after reduction”) in the solution of the prodrug (20 μM, pH 8) incubated with H2O2 (10 mM) for 2 h. Other experimental details are provided in the ESI.† | ||
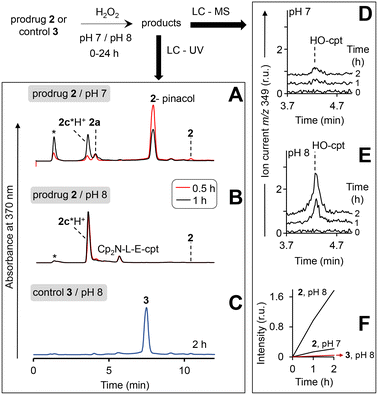
Furthermore, the activation relies on the presence of the arylboronic acid group, since the control 3 lacking this group remains non-emissive even in the presence of the highest H2O2 concentration tested (10 mM, Fig. 4A, red colored trace). Importantly, both the prodrug 2 and the control 3 are stable at pH 7 and 8 in the absence of H2O2, indicating that the direct hydrolysis of the ester E does not take place. Compared to pH 7, the direct hydrolysis is facilitated at pH 10 by 6-fold for the prodrug and 2.5-fold for the control (Table S3, ESI†). The fluorescent product generated upon the prodrug activation can be both HO-cpt and other fluorescent intermediates as will be discussed later. To identify these products, we first examined the release of Fe ions in the mixture of the prodrug (20 μM) and H2O2 (10 mM) by making use of the formation of red colored complex of Fe2+ with ferrozine.18 We detected no Fe2+ both at pH 7 and 8 after 2 h incubation (ESI†). However, when the mixtures were first reduced by hydroxylamine to convert Fe3+ to Fe2+, followed by addition of ferrozine, the characteristic red solution was obtained at both pH 7 and 8: representative data obtained at pH 8 are shown in Fig. 4D. This indicates the H2O2-mediated release of Fe3+, occurring in the result of the decomposition of the intermediate 2a (Fig. 2). Other intermediates and HO-cpt were identified by using HPLC coupled to UV-light (LC-UV) and electrospray ionization MS detectors (LC-MS, Fig. 5).
In particular, at pH 7 traces of the prodrug are observed at the retention time Rt = 10.5 min (m/z 986 [M–e−]+) after 30 min incubation with H2O2 (10 mM) (red line, Fig. 5A). After 1 h incubation it is not detectable anymore (black line). The major peak at both 30 min and 1 h incubation at pH 7 is the hydrolyzed prodrug, which has lost a pinacol moiety – 2-pinacol: Rt = 7.8 min (m/z 904 [M–e−]+). Further detected intermediates include a 2a (Rt = 4.1 min, m/z 726 [M]+) and its decomposition product 2c*H+ (Rt = 3.7 min, m/z 543 [M]+). The formation of the latter intermediate is in agreement with the release of Fe3+ (Fig. 4D). At 30 min incubation ferrocenium/amine ratio is equal to 3/4, whereas at 1 h incubation it is 1/9. At pH 8 the same intermediates are formed. However, the reaction is substantially faster. Already after 30 min, neither prodrug nor its hydrolyzed form are detectable, whereas the ferrocenium intermediate is present only as traces. The major product is 2c*H+. Additionally, a weak peak is observed at Rt = 5.6 min (m/z 701 [M]+), which corresponds to 2b formed in the result of decomposition of 2a (Fig. S27, ESI†).
We observed that at the separation conditions selected for LC-UV (Fig. 5A and B) both HO-cpt and 2c*H+ (Fig. S28, ESI†) elute at the same time (3.7 min) that did not allow for the accurate quantification of HO-cpt. We solved this problem by using more efficient chromatographic separation with high-resolution mass spectrometry (Fig. S29, ESI†). The data obtained are provided in Fig. 5D and E. In Fig. 5F, areas of the peaks corresponding to HO-cpt are plotted as a function of time. In the prodrug/H2O2 mixture at pH 7 a HO-cpt peak is observed only after 2 h incubation (Fig. 5D). In contrast, at pH 8 this peak is detectable already after 1 h and the peak intensity is increased after 2 h. Importantly, practically no HO-cpt could be detected in the mixture of the control 3/H2O2 at pH 8 (red trace, Fig. 5F) indicating that the direct hydrolysis of E does not take place.
To investigate whether the latter reaction sequence is specific for cpt derivatives, we prepared a prodrug 4 containing 1,1-dimethylbenzyl in place of the cpt moiety. By using LC-UV-MS we confirmed that this compound forms related intermediates as the prodrug 2 in the presence of H2O2 (Fig. S30A, ESI†). The reaction was also found to be facilitated at pH 8 as compared to pH 7. By using GC-MS we confirmed the formation of HO-dmb in the 4/H2O2/pH 8 mixture (Fig. S30B, ESI†). Thus, these data indicate that the reaction sequence reported in this paper is not restricted to the cpt-prodrugs. It can potentially be applied to design prodrugs of other anticancer drugs, which possess a tertiary OH group critical for their activity. Possible examples of such drugs are clinically used irinotecan and topotecan.1
The mechanism of the reaction of the prodrug with H2O2 at pH 8
According to the data discussed above the prodrug is aggregated in aqueous solution that blocks the reactivity of the L-E (Fig. 3A and D). In the presence of H2O2 the prodrug is converted to the intermediate 2c*H+ (Fig. 5A and B). This compound is monomeric according to the following experimental evidences. First, its UV-visible spectra at 0 to 25 μM are characteristic for a monomeric cpt derivative. In particular, the absorbance maxima (354/369 nm) are close to those observed for HO-cpt dissolved water (355/368 nm).17 Absorbance at these maxima obey Beer–Lambert law (Fig. 3B, black trace). No absorbance is observed at >500 nm, indicating the absence of aggregates able to scatter light. Finally, DLS data indicate the absence of aggregates larger than 100 nm at the compound concentration <5 μM. This indicates that the cpt in 2c*H+ is exposed to the solvent.To find out whether the hydrolysis is facilitated by the intramolecular interactions, we analysed possible conformations of 2c*H+ in aqueous solution by molecular dynamics (MD) simulations (Fig. S31A–F, ESI†). We observed only low probabilities for conformations where hydrogen bonding between the ammonia and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (E) is possible (Fig. S31A–C, ESI†). Combined quantum mechanical and molecular mechanical (QM/MM) simulations of the hydrolysis reaction by an OH− ion on 2c*H+ In a stretched conformation, i.e. with the ammonium group far from the E, show a step-wise reaction, in which the rate-determining step is the OH− attack with formation of a tetrahedral intermediate with a feasible free energy barrier of 34.0 ± 0.7 kcal mol−1 (Fig. S31E and F, ESI†). In simulations, in which the ammonium group has been constrained to be close to the C
O (E) is possible (Fig. S31A–C, ESI†). Combined quantum mechanical and molecular mechanical (QM/MM) simulations of the hydrolysis reaction by an OH− ion on 2c*H+ In a stretched conformation, i.e. with the ammonium group far from the E, show a step-wise reaction, in which the rate-determining step is the OH− attack with formation of a tetrahedral intermediate with a feasible free energy barrier of 34.0 ± 0.7 kcal mol−1 (Fig. S31E and F, ESI†). In simulations, in which the ammonium group has been constrained to be close to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (E), the free energy barrier for the nucleophilic attack does not change significantly (36.1 ± 0.6 kcal mol−1) compared to the simulations without this distance constraint (Fig. S31F†). This indicates that hydrolysis of E does not rely on intramolecular interactions involving the ammonium group. It is therefore conceivable that the hydrolysis occurs by direct OH− attack of the E of 2c*H+. Further experimental data are necessary to confirm or reject this suggestion.
O (E), the free energy barrier for the nucleophilic attack does not change significantly (36.1 ± 0.6 kcal mol−1) compared to the simulations without this distance constraint (Fig. S31F†). This indicates that hydrolysis of E does not rely on intramolecular interactions involving the ammonium group. It is therefore conceivable that the hydrolysis occurs by direct OH− attack of the E of 2c*H+. Further experimental data are necessary to confirm or reject this suggestion.
Effect of the prodrug on viability of cancer and normal cells
We selected several representative human cancer cell lines from different organs, including Burkitt's lymphoma BL-2 (blood), A2780 (ovary), DU-145 (prostate), SAS (tongue) and FaDu (pharynx), known to produce elevated ROS amounts.7,19,20 Incubation of these cells with the prodrug for 96 h affects strongly their viability. In particular, half inhibitory concentration (IC50) ranges from 0.3 ± 0.1 μM (for most sensitive BL-2 cells) to 1.1 ± 0.5 μM (for least sensitive DU-145 cells) (Table 1).Experiments with controls revealed the importance of the individual components of the prodrug. In particular, the control 5 (ref. 7b) lacking the cpt fragment is from 34- to 93-fold and the control 3 lacking the H2O2-responsive fragment is from 4.3 to 27.5-fold less active than the prodrug (Table 1). The trend is retained at the shorter incubation times of 24 and 48 h (Tables S5 and S6, ESI†). Importantly, the prodrug does not affect the viability of representative normal cell lines, producing low amounts of intracellular ROS: normal human dermal fibroblasts (NHDF), IC50 > 20 μM; retinal pigment epithelia (ARPE-19) cells, IC50 > 10 μM and primary human fibroblasts (HF), IC50 > 10 μM (Table S6, Fig. S32, ESI†). The differences in the anticancer effects of the prodrug and the controls can be caused by the different uptake efficacy. To evaluate this possibility, we studied the uptake of the latter compounds in representative cancer cells – BL-2 (Tables S8 and S9 ESI†). We found that the uptake of control 3 is more efficient than that of the prodrug 2, whereas control 5 (ref. 7b) and the prodrug 2 are taken up with almost the same efficacy. Thus, the stronger anticancer effect of the prodrug than that of the controls is not caused by the differences in their uptake efficacy.
Enhancement of the prodrug efficacy
The anticancer effect of the prodrug 2 is 13 to 37-fold weaker than that of HO-cpt (Table 1) suggesting that its intracellular activation is not complete. Since ionizing irradiation (IR) increases ROS in cells,21 we assumed that it could also facilitate the prodrug activation.22 We tested this hypothesis in two representative cell lines, SAS and FaDu, which are cancer models established in our laboratory for exploring synergistic effects between IR and new drugs.22First, we confirmed that the prodrug inhibits growth of both SAS (IC50 = 4 ± 2 μM) and FaDu cells (IC50 = 8 ± 2 μM) at 48 h incubation time. At the concentration of 0.5 μM (but not 0.2 μM) it also inhibits the capacity of these cells to form colonies (p < 0.01). Next, we investigated inhibition of colony formation capacity of SAS and FaDu cells by the combination of the prodrug and IR. The prodrug at non-toxic (0.2 μM) and moderately toxic (0.5 μM) concentrations and IR at doses ranging from 0 to 10 Gy were used. For the highest IR dose of 10 Gy and one representative cell line (FaDu) the IR-induced increase of the intracellular ROS level was confirmed (Fig. S33, ESI†). We were pleased to observe that the effect of the prodrug at both tested concentrations on both cell lines is strongly enhanced by IR (p < 10−3) (Fig. S34, ESI†). In contrast, the H2O2-resistant control FcNF-L-E-cpt exhibits practically no synergy with IR (Fig. S34, ESI†).
A mechanism of anticancer activity of the prodrug
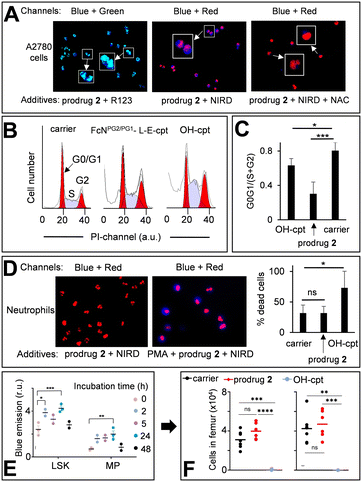
The prodrug 2 is not fluorescent (Fig. 4A). When it reacts with H2O2 two long- (2c*H+ and HO-cpt) and one short-living (2a) fluorescent products are formed, each exhibiting a broad emission in the range of 420–470 nm (Fig. 4A and 5A, B, D and E). Further, we will call a mixture of these compounds prodrug-derived fluorescent products (PDFPs). Intermediates 2a and 2c*H+ are inactive cpt esters unable to bind TOPI, whereas HO-cpt is a TOPI inhibitor and a highly potent anticancer drug. Thus, the fluorescence increase in the reaction of the prodrug 2 with H2O2 will not necessarily correlate with the high anticancer activity, but is rather an indication of the first step of the activation. We investigated formation and intracellular localization of PDFPs (blue channel) in representative cancer A2780 cells. Mit was labelled with rhodamine 123 (R123: green channel) and nuclei – with NUCLEAR-ID® Red dye (NIRD: red channel). As shown in Fig. 6A, the blue signal is clustered in particular regions. This signal is co-localized with both R123 (green, Pearson's coefficient r = 0.68) and NIRD (red, r = 0.81) confirming that the PDFPs are present in Mit and in nuclei of the cells. We observed that the signal of PDFPs is practically absent in A2780 cells pretreated with ROS scavenger N-acetylcysteine (NAC, Fig. 6A, right image) indicating that PDFPs are formed in cells in the ROS-dependent reaction. This data are in agreement with the suggested mechanism (Fig. 1C) including formation of 2a in the cytoplasm, its accumulation in Mit, where it is transformed via the sequence of the OH−Mit-mediated reactions to HO-cpt. The drug then migrates to the nucleus, where it is trapped by binding to its target TOPI.23Next, we investigated whether the prodrug acts as a cpt-derivative releasing HO-cpt in cells or as a typical AF-prodrug. HO-cpt affects intracellular ROS levels weakly, but is known to induce a strong cell cycle arrest in S and G2 phases as reflected, amongst others, in altered static DNA histograms (Fig. 6B and C). In contrast, the typical effect of AF prodrugs is amplification of intracellular ROS (e.g. control 5,7b Fig. S37 and S38, ESI†), whereas no major effect on the cell cycle distribution – rather a slight increase in the G1 phase cell fraction – is observed.7,19 The prodrug practically does not facilitate ROS production (Fig. S37–S39, ESI†), but causes changes of cell cycle distribution characteristic for HO-cpt (Fig. 6B and C). It is therefore likely that HO-cpt is intracellularly released from the prodrug.
Effects of the prodrug on neutrophils
We investigated the activation of the prodrug with formation of PDFPs in human neutrophils from healthy individuals. Nuclei of the neutrophils were counter-stained with NIRD (red channel). As shown in Fig. 6D (left image), the blue signal is absent in the cells incubated with the prodrug 2 for 2 h indicating that the latter compound remains intact. In contrast, neutrophils primed with phorbol myristate acetate (PMA, 50 nM), inducing H2O2 generation, feature the evenly distributed, intense blue signal indicating PDFPs formation (Fig. 6D, right image). However, HO-cpt does not seem to be formed under these conditions, since the prodrug 2 does not enhance the PMA toxicity of neutrophils (Fig. 6D, right plot). In contrast, HO-cpt is toxic under the same conditions.Next, we assessed the characteristic for PDFPs fluorescence in neutrophils of blood and bone marrow (BM) as well as in immature BM cells (LineageNEG Sca-1+ c-Kit+ (LSK) and myeloid progenitor (MP) cells) from mice treated with the prodrug. At the early incubation time of 2 h, we observed some minor (p < 0.05) increase of the fluorescence of blood neutrophils (Fig. S40A, ESI†). The more pronounced fluorescence increase was observed in LSK (2/24 h incubation) and MP cells (24 h) (Fig. 6E). These data indicate formation of PDFPs in vivo. However, this does not correlate with a decrease of the number of the corresponding cells in blood and BM (Fig. 6F, S40B and C, ESI†), thereby allowing concluding that toxic drug HO-cpt is not formed at these conditions. Thus, the in vivo data are also in agreement with the suggested mechanism of action of the prodrug (Fig. 1C).
Antitumor activity of the prodrug in vivo
Encouraged by the excellent in vitro properties of the prodrug 2, we studied its toxicity and antitumor activity in vivo. We observed that at doses of 3 to 24 mg kg−1 (interperitoneal (i.p.) injections on days 0, 2 and 4), it does not affect weight of Balb/c mice in a 7-day experiment (Fig. S41, Table S10, ESI†). On day 7, the animals were sacrificed and their internal organs inspected. We detected no apparent damage (Fig. S42, ESI†). Further, we extracted the prodrug 2 and PDFPs from the organs and measured the fluorescence of the extracts at λex = 365 nm, λem = 450 nm (parameters characteristic for PDFPs). We observed no fluorescence increase between untreated and prodrug 2-treated groups (Fig. S43, ESI†). After the extracts were treated with H2O2 to activate the prodrug 2, significant signals could be detected in extracts from blood (p < 0.001), spleen (p < 0.001), adipose tissue (p < 0.01) and peritoneum (p < 0.01) (Fig. S44, ESI†). Thus, the prodrug 2 is accumulated in the mentioned organs, but remains there in the intact state. All these data indicate that at doses ≤24 mg kg−1, the prodrug 2 does not exhibit acute toxicity. In contrast, at 6 mg kg−1, HO-cpt exhibits acute toxicity reflected in its negative effect on weight of mice (Fig. 7A). At the dose of 24 mg kg−1 a precipitate of the prodrug 2 was detected on the surface of internal organs of peritoneal cavity and, locally, near the i.p. injection site. All further experiments were conducted at the highest tested dose, at which no precipitates of the prodrug were detected in vivo: 12 mg kg−1 (Fig. S42, ESI†). We studied the antitumor effect of the prodrug 2 in the Nemeth–Kellner (NK) lymphoma (Ly) NK/Ly model. The prodrug was injected i.p. on days 1, 3, 6, 8, 10, 13, 15, 17 and 20 at the dose of 12 mg kg−1. The control group received only the carrier (DMSO). We observed statistically significant (p < 0.05) prolongation of mice survival (t1/2) in the treated group to 55 days versus 33 days in the control group (Fig. 7B).Experimental
All experimental data are provided in the ESI.†Ethical statement
Black C57/BL6N mice and white mice Balb/c were bred at Animal house of Danylo Halytsky Lviv National Medical University (Lviv, Ukraine). The animal studies were approved by the local ethical committee (Permission to R. Bilyy; 20180226/P2 and 2014-2018/P6) and conducted according to the guidelines of the Federation of European Laboratory Animal Science Associations (FELASA).Conclusions
We developed the dual camptothecin prodrug, which is activated in cancer cells in the presence of H2O2 and OH−Mit. This mode of action relies on aggregation-derived blocking of the OH−Mit-sensitive ester E in the prodrug, which is released by de-aggregation in the presence of H2O2. The prodrug discriminates cancer cells from primed (H2O2-rich) neutrophils, since the latter cells have the low number of mitochondria and correspondingly lack the OH−Mit-trigger. We confirmed the effects in cell free settings. The prodrug exhibits high nM to low μM anticancer activity towards a variety of cancer cell lines derived from blood, ovary, prostate, tongue and pharynx in vitro and towards Nemeth–Kellner lymphoma in vivo, but does not induce neutropenia usual for camptothecins both in vitro and in vivo. We demonstrated that the prodrug concept reported in this paper is not restricted to the derivatives of cpt and can potentially be applied to other drugs containing the tertiary HO group critical for their activity.In summary, our approach allows cancer cell-specific delivery of anticancer drugs. Other known approaches for cell specific drug delivery based on nanosized materials have been revewed.24–26
Author contributions
Insa Klemt, Viktor Reshetnikov, Subrata Dutta, Galyna Bila, Itziar Cossío Cuartero, Adrian Wünsche, Maximilian Böhm, Marit Wondrak, Rainer Tietze, Frank Beierlein, Sabrina Gensberger-Reigl, Marlies Körber, Tina Jost: investigation, formal analysis, data curation, visualization, writing – original draft. Rostyslav Bilyy, Andrés Hidalgo, Leoni A. Kunz-Schughart, Rainer Tietze, Petra Imhof, Monika Pischetsrieder, Andriy Mokhir: conceptualization, project administration, funding acquisition, resources, supervisions, writing – original draft, review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank German Research Council (DFG) (AM 1418/7-2) and European Commission (861878 – NeutroCure) for funding this study. The CNIC is supported by the MCIN and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (CEX2020-001041-S). The contribution of DFG to the mass spectrometry unit (timsTOF Pro) is highly appreciated (INST 90/950-1). The authors acknowledge the scientific support and HPC resources provided by the Erlangen National High Performance Computing Center (NHR@FAU) of the Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU). The hardware is funded by the DFG. Open access funding enabled and organized by Projekt DEAL.Notes and references
- (a) Y. Pommier, Nat. Rev. Cancer, 2006, 6, 789 CrossRef CAS PubMed; (b) W. J. Slichenmyer, E. K. Rowinsky, R. C. Donehower and S. H. Kaufmann, J. Natl. Cancer Inst., 1993, 85, 271 CrossRef CAS PubMed.
- J. Chen and J. Stubbe, Nat. Rev. Cancer, 2005, 5, 102 CrossRef CAS PubMed.
- D. C. Dale, G. M. McCarter, J. Crawford and G. H. Lyman, J. Natl. Compr. Cancer Network, 2003, 1(3), 440–454 CAS.
- (a) M. A. Jordan and L. Wilson, Nat. Rev. Cancer, 2004, 4, 253 CrossRef CAS PubMed; (b) E. K. Rowinsky and R. C. Donehower, N. Engl. J. Med., 1995, 332, 1004 CrossRef CAS PubMed.
- (a) L. Kelland, Nat. Rev. Cancer, 2007, 7, 573 CrossRef CAS PubMed; (b) R. Oun, Y. Moussa and N. J. Wheate, Dalton Trans., 2018, 47, 6645 RSC.
- J. Rautio, H. Kumpulainen, T. Heimbach, R. Oliyai, D. Oh, T. Jaervinen and J. Savolainen, Nat. Rev. Drug Discovery, 2008, 7, 255 CrossRef CAS PubMed.
- (a) H.-G. Xu, M. Schikora, M. Sisa, S. Daum, I. Klemt, C. Janko, C. Alexiou, G. Bila, R. Bilyy, W. Gong, M. Schmitt, L. Sellner and A. Mokhir, Angew. Chem., Int. Ed., 2021, 60, 11158 CrossRef CAS PubMed; (b) V. Reshetnikov, S. Daum, C. Janko, W. Karawacka, R. Tietze, C. Alexiou, S. Paryzhak, T. Dumych, R. Bilyy, P. Tripal, B. Schmid, R. Palmisano and A. Mokhir, Angew. Chem., Int. Ed., 2018, 57, 11943 CrossRef CAS PubMed; (c) S. Daum, V. Reshetnikov, M. Sisa, T. Dumych, M. D. Lootsik, R. Bilyy, E. Bila, C. Janko, C. Alexiou, M. Herrmann, L. Sellner and A. Mokhir, Angew. Chem., Int. Ed., 2017, 56, 15545 CrossRef CAS PubMed.
- (a) G. Jaouen, A. Vessières and S. Top, Chem. Soc. Rev., 2015, 44, 8802 RSC; (b) M. F. Renschler, Eur. J. Cancer, 2004, 40, 1934 CrossRef CAS PubMed; (c) M. Doering, L. A. Ba, N. Lilienthal, C. Nicco, C. Scherer, M. Abbas, A. A. Peer Zada, R. Coriat, T. Burkholz, L. Wessjohann, M. Diederich, F. Batteux, M. Herling and C. Jacob, J. Med. Chem., 2010, 53, 6954 CrossRef CAS PubMed.
- (a) D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646 CrossRef CAS PubMed; (b) L. M. Coussens and Z. Werb, Nature, 2002, 420, 860 CrossRef CAS PubMed; (c) M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2006, 39, 44 CrossRef PubMed; (d) F. Antunes and R. Cadenas, FEBS Lett., 2000, 475, 121 CrossRef CAS PubMed; (e) T. P. Szatrowski and C. F. Nathan, Cancer Res., 1991, 51, 794 CAS.
- K. Matsushita, T. Okuda, S. Mori, M. Konno, H. Eguchi, A. Asai, J. Koseki, Y. Iwagami, D. Yamada, H. Akita, T. Asaoka, T. Noda, K. Kawamoto, K. Gotoh, S. Kobayashi, Y. Kasahara, K. Morihiro, T. Satoh, Y. Doki, M. Mori, H. Ishii and S. Obika, ChemMedChem, 2019, 14, 1384 CrossRef CAS PubMed.
- M. Euler and M. H. Hoffmann, Biochem. Soc. Trans., 2019, 47, 1921 CrossRef CAS PubMed.
- N. A. Maianski, J. Geissler, S. M. Srinivasula, E. S. Alnemri, D. Roos and T. W. Kuijpers, Cell Death Differ., 2004, 11, 143 CrossRef CAS PubMed.
- (a) L. Wang, S. Xie, L. Ma, Y. Chen and W. Lu, Eur. J. Med. Chem., 2016, 116, 84 CrossRef CAS PubMed; (b) S. Ayyanaar, M. P. Kesavan, G. Sivaraman, R. P. Raja, V. Vijayakumar, J. Rajesh and G. Rajagopal, J. Drug Delivery Sci. Technol., 2019, 52, 722 CrossRef CAS; (c) T. Zhang, X. Ma, S. Bai, Y. Wang, X. Zhang, Y. Lu, F. Wen, P. Xue, Y. Kang and Z. Xu, J. Mater. Chem. B, 2020, 8, 1245 RSC; (d) B. Chu, Y. Qu, X. He, Y. Hao, C. Yang, Y. Yang, D. Hu, F. Wang and Zh. Qian, Adv. Funct. Mater., 2020, 30, 2005918 CrossRef CAS; (e) X. Luo, X. Chi, Y. Lin, Zh. Yang, H. Lin and J. Gao, Chem. Commun., 2021, 57, 11033 RSC.
- H. Cho, Y.-Y. Cho, M. S. Shim, J. Y. Lee, H. S. Lee and H. C. Kang, Biochim. Biophys. Acta, Mol. Basis Dis., 2020, 1866, 165808 CrossRef CAS PubMed.
- (a) Y. Tan, Y. Zhu, Y. Zhao, L. Wen, T. Meng, X. Liu, X. Yang, S. Dai, H. Yuan and F. Hu, Biomaterials, 2018, 154, 169 CrossRef CAS PubMed; (b) B.-Y. Liu, X.-L. Yang, X. Xing, J. Li, Y.-H. Liu, N. Wang and X.-Q. Yu, ACS Macro Lett., 2019, 8, 719 CrossRef CAS PubMed.
- M. R. Redinbo, L. Stewart, P. Kuhn, J. J. Champoux and W. G. J. Hol, Science, 1998, 279, 1504 CrossRef CAS PubMed.
- D. Dvoranová, M. Bobeničová, S. Čoralová and M. Breza, Chem. Phys. Lett., 2013, 580, 141 CrossRef.
- E. Kinski, P. Marzenell, W. Hofer, H. Hagen, J. A. Raskatov, K. X. Knaup, E. M. Zolnhofer, K. Meyer and A. Mokhir, J. Inorg. Biochem., 2016, 160, 218 CrossRef CAS PubMed.
- P. Marzenell, H. Hagen, L. Sellner, T. Zenz, R. Grinyte, V. Pavlov, S. Daum and A. Mokhir, J. Med. Chem., 2013, 56, 6935 CrossRef CAS PubMed.
- S. Daum, S. Babiy, H. Konovalova, W. Hofer, A. Shtemenko, N. Shtemenko, C. Janko, C. Alexiou and A. Mokhir, J. Inorg. Biochem., 2017, 178, 9 CrossRef PubMed.
- E. I. Azzam, J.-P. Jay-Gerin and D. Pain, Cancer Lett., 2012, 327, 48 CrossRef CAS PubMed.
- H.-G. Xu, V. Reshetnikov, M. Wondrak, L. Eckardt, L. A. Kunz-Schughart, C. Janko, R. Tietze, C. Alexiou, H. Borchardt, A. Aigner, W. Gong, M. Schmitt, L. Sellner, S. Daum, H. G. Özkan and A. Mokhir, Cancers, 2022, 14, 208 CrossRef CAS PubMed.
- Y. Mao, I. R. Mehl and M. T. Muller, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 1235 CrossRef CAS PubMed.
- R. C. P. A. Remmers and K. Neumann, Biomater. Sci., 2023, 11, 1607 RSC.
- W. Zhao, Y. Zhao, Q. Wang, T. Liu, J. Sun and R. Zhang, Small, 2019, 15, 1903060 CrossRef CAS PubMed.
- A. M. Diez-Pascual and A. Rahdar, ChemMedChem, 2022, 17, e202200142 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3md00609c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |