Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dynamic mechanochemistry: accelerated self-sorting of two imine-based metal complexes under solvent-free mechanochemical conditions†
Thomas E.
Shaw
a,
Justin
Arami
 a,
Jean-François
Ayme
a,
Jean-François
Ayme
 b,
Jean-Marie
Lehn
b,
Jean-Marie
Lehn
 b and
Titel
Jurca
b and
Titel
Jurca
 *ac
*ac
aDepartment of Chemistry, University of Central Florida, Orlando, FL 32816, USA. E-mail: titel.jurca@ucf.edu
bLaboratoire de Chimie Supramoleculaire, Institut de Science et d'Ingenierie Supramoleculaires, Universite de Strasbourg, Strasbourg, France
cRenewable Energy and Chemical Transformations (REACT) Cluster, University of Central Florida, Orlando, FL 32816, USA
First published on 14th February 2024
Abstract
Self-sorting of two imine-based Cu(I) and Fe(II) coordination complexes from a six-component reagent library has been achieved through solvent-free mechanochemistry. The reaction proceeds rapidly, yielding the thermodynamically favored products in less than 24 hours. The results point to the potential of mechanochemistry to achieve increasingly complex multi-metallic systems through one-pot protocols.
Introduction
Constitutional dynamic chemistry (CDC), based on molecules which are capable of altering their constitution by reversible bond formation and exchange of their components, provides a promising avenue to access complex dynamic systems.1–13 One of our groups previously reported the simultaneous formation of bidentate and tridentate imine-containing ligands bound to two different metal centers, from a library comprised of two imines, two aldehydes, and the respective BF4− salts of Cu(I) and Fe(II) (Fig. 1).14 The sorting behaviour is driven by two factors (a) the difference in the preferred coordination geometries of the metals (Cu(I) – tetrahedral, Fe(II) – octahedral),15 and (b) the minimization of steric repulsion around the metal center, nominally the preference for a less crowded binding pocket around Fe(II).16 Thus, a cocktail of 2 equivalents each of 4-methylaniline (1), aminoquinoline (2), 5-methylpyridine-2-carboxaldehyde (3), and 6-methylpyridine-2-carboxaldehyde (4), with 1 equivalent each of Cu(CH3CN)4(BF4) and Fe(BF4)2·6H2O, in a 20 mM solution of CD3CN at 60 °C yielded a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of complexes [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2 after 20 days (Fig. 1); the product mixture under thermodynamic equilibrium. Shorter reaction time, or lower reagent concentration (3.6 mM) resulted in incomplete sorting, with notable presence of mixed ligand species [Fe(2,3)(2,4)][BF4]2 . The details and rationale of this self-sorting behaviour has been described in detail in prior works.14
1 ratio of complexes [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2 after 20 days (Fig. 1); the product mixture under thermodynamic equilibrium. Shorter reaction time, or lower reagent concentration (3.6 mM) resulted in incomplete sorting, with notable presence of mixed ligand species [Fe(2,3)(2,4)][BF4]2 . The details and rationale of this self-sorting behaviour has been described in detail in prior works.14
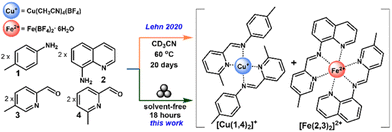 | ||
| Fig. 1 Schematic for the simultaneous formation of complexes [Cu(1,4)2]+ and [Fe(2,3)2]2+ through previously reported solution and current mechanochemical routes. | ||
These solution state results inspire exploration of self-sorting phenomena in the solid state, via mechanochemistry (vibratory ball milling).17,18 Therein, self-sorting behaviour can be studied under a solvent-free process that eliminates concentration effects noted in solution, but may introduce crystal packing effects.17 Mechanochemical reactions have been shown to proceed at accelerated time scales compared to solution.19 This raises the question, will self-sorting of transition metal complexes occur rapidly under these solvent-free milling conditions? Herein, the application of solvent-free mechanochemical synthesis, via vibratory ball-milling, that can replicate the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 thermodynamic equilibrium product ratio of [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2 in 18 hours is demonstrated (Fig. 1). Notably, while the system studied begins as solvent-free, as the reaction progresses 6 equivalents of H2O are released from Fe(BF4)2·6H2O, and another 4 equivalents are released from the imine formation reactions. This can be understood to create a liquid assisted grinding (LAG) process, which itself has been shown to enhance milling reaction rates, and potentially influence selectivity.20–23
1 thermodynamic equilibrium product ratio of [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2 in 18 hours is demonstrated (Fig. 1). Notably, while the system studied begins as solvent-free, as the reaction progresses 6 equivalents of H2O are released from Fe(BF4)2·6H2O, and another 4 equivalents are released from the imine formation reactions. This can be understood to create a liquid assisted grinding (LAG) process, which itself has been shown to enhance milling reaction rates, and potentially influence selectivity.20–23
Over the past decade, mechanochemical synthesis of transition and main group metal coordination complexes,24–35 and germane to this work, Schiff base species, has gained increasing attention.36–46 This work highlights the synthesis and stability of four transition metal complexes, and the application of mechanochemistry towards the dynamic assembly of a complex multi-ligand, multi-metal system. The former adds to this growing body of mechanochemically prepared coordination complexes, and the latter demonstrates the feasibility of increasingly complex one-pot reaction systems via mechanochemistry, with notable prior examples from Friščić, Lamaty, James, Hanusa, Garcia, and others.24–48
Results and discussion
First, a series of reactions were conducted to assess that the individual homoleptic complexes that could arise from the six-component system in Fig. 1 are feasible under solvent-free mechanochemical conditions. Notably, the molecules targeted were: [Cu(1,3)2][BF4], [Cu(1,4)2][BF4] [Fe(2,3)2][BF4]2, and [Fe(2,4)2][BF4]2 (Fig. 2). The experiments were conducted with a SPEX 8000M mill (18 Hz) in 5 mL stainless steel (ss) Smartsnap™ grinding jars from Form-Tech Scientific and four 3.175 mm ss (440c) balls. An aluminum holder assembly was used to facilitate three concurrent trials per run.40 Individual complexes were synthesized using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of metal salts to ligand components. The total reagent mass in each vial was approximately 200 mg. All reactions were conducted for 2, 8, 12, and 18 h. Upon completion, 0.7 mL of CD3CN was introduced to the grinding jar and passed through a 22 μm nylon filter, with a syringe, directly into an NMR tube. The 1H NMR was recorded immediately after sample preparation (Fig. 2).
2 molar ratio of metal salts to ligand components. The total reagent mass in each vial was approximately 200 mg. All reactions were conducted for 2, 8, 12, and 18 h. Upon completion, 0.7 mL of CD3CN was introduced to the grinding jar and passed through a 22 μm nylon filter, with a syringe, directly into an NMR tube. The 1H NMR was recorded immediately after sample preparation (Fig. 2).
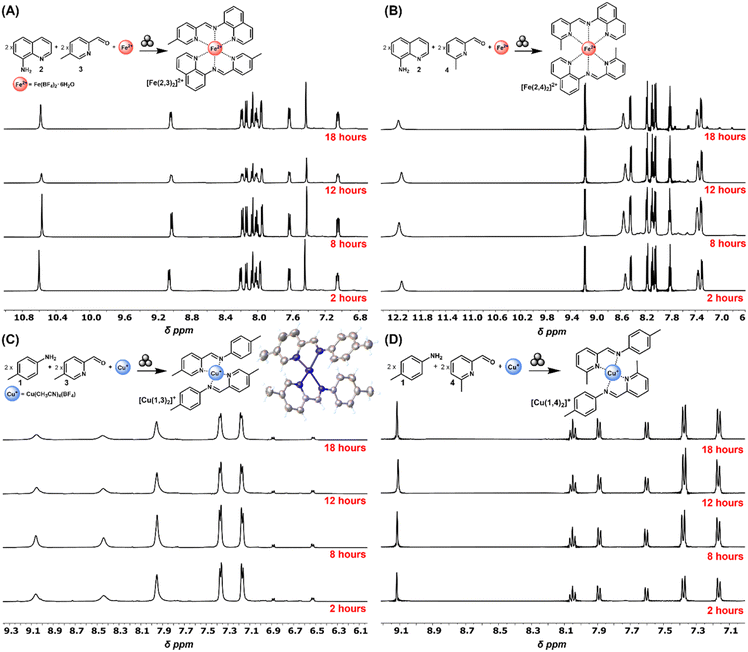 | ||
| Fig. 2 Partial 1H NMR (CD3CN) for the one-pot formation of complexes (A) [Fe(2,3)2]2+, (B) [Fe(2,4)2]2+, (C) [Cu(1,3)2]+, (inset single crystal X-ray structure with thermal ellipsoids at 50% and BF4− anion omitted for clarity) and (D) [Cu(1,4)2]+, through vibratory ball-milling. Each timepoint represents a separate reaction. Full 1H NMR spectra reported in the ESI, Fig. S1.† | ||
[Cu(1,4)2][BF4], [Fe(2,3)2][BF4]2, and [Fe(2,4)2][BF4]2 were characterized in detail in one of our groups' prior reports and served as the guideline for validating the formation of the products via1H NMR (Fig. 2A, B and D).14 [Cu(1,3)2][BF4], although not reported in the original manuscript,14 was also readily accessible, but displayed lower solubility resulting in broadened 1H NMR signals (Fig. 2C). A single crystal X-ray structure confirmed the identity of [Cu(1,3)2][BF4] as a distorted tetrahedral Cu(I) complex (Fig. 2C inset). The material could readily be synthesized on a 1 g scale and isolated in near quantitative yield over 1 h of grinding (see ESI†). Notably, all four complexes could be cleanly accessed in a one-pot, one-step, solvent-free mechanochemical process ([Fe(2,3)2][BF4]2, and [Fe(2,4)2][BF4]2 can be considered LAG as 6 equivalents of H2O are released per Fe) starting with the unreacted ligand components, and with no evidence of other product formation. Significantly, these reactions occur quickly, reaching completion at 2 hours (or less), and also showing no signs of degradation after 18 h of continuous milling. While the nature and utility of these complexes is beyond the scope of this work, it should be noted that homoleptic Schiff base Cu(I) have garnered significant attention as photoactive molecules49 and as catalysts for copper-catalysed azide–alkyne cycloadditions (CuAAC).50 Analogously, Fe(II) bis tridentate Schiff base species have received attention as spin crossover complexes.51–53
With the knowledge that the individual homoleptic products from the multicomponent system could be readily accessed, and remain stable over prolonged mechanochemical grinding, self-sorting trials were targeted. Individual self-sorting trials were conducted in a similar fashion, with a bulk reagent mass of approximately 250 mg with components present in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
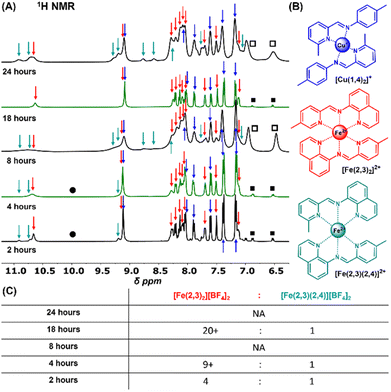
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio of metal salts to ligand precursors (Fig. 1), and at times of 2, 4, 8, 18, and 24 hours. 1H NMR sample preparation and analysis was conducted as noted prior. The 2 h trial displayed predominantly homoleptic [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2, with a significant amount of heteroleptic [Fe(2,3)(2,4)][BF4]2 (ca. 4
2 molar ratio of metal salts to ligand precursors (Fig. 1), and at times of 2, 4, 8, 18, and 24 hours. 1H NMR sample preparation and analysis was conducted as noted prior. The 2 h trial displayed predominantly homoleptic [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2, with a significant amount of heteroleptic [Fe(2,3)(2,4)][BF4]2 (ca. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [Fe(2,3)2][BF4]2
1 [Fe(2,3)2][BF4]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fe(2,3)(2,4)][BF4]2 based on integration of imine C–H). The 4 h trial displayed near complete conversion to [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2, the expected thermodynamic products, with <10% heteroleptic [Fe(2,3)(2,4)][BF4]2, as indicated by very low intensity signals at δ 9.2 (broad shoulder), δ 10.7 (broad shoulder), and δ 10.9 (broad singlet) ppm (Fig. 3). The 18 h sample further diminished the level of [Fe(2,3)(2,4)][BF4]2 (<5%). Trace signals at δ 6.3 and δ 6.9 ppm are diagnostic of free amine. Clear signals for [Cu(1,3)2][BF4] were not observed under mechanochemical conditions herein, or in the solution-based system previously reported,14 likely due to lower solubility (vide supra).
[Fe(2,3)(2,4)][BF4]2 based on integration of imine C–H). The 4 h trial displayed near complete conversion to [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2, the expected thermodynamic products, with <10% heteroleptic [Fe(2,3)(2,4)][BF4]2, as indicated by very low intensity signals at δ 9.2 (broad shoulder), δ 10.7 (broad shoulder), and δ 10.9 (broad singlet) ppm (Fig. 3). The 18 h sample further diminished the level of [Fe(2,3)(2,4)][BF4]2 (<5%). Trace signals at δ 6.3 and δ 6.9 ppm are diagnostic of free amine. Clear signals for [Cu(1,3)2][BF4] were not observed under mechanochemical conditions herein, or in the solution-based system previously reported,14 likely due to lower solubility (vide supra).
These results validate that the solution self-sorting behaviour previously reported for this system can be replicated under solvent-free/LAG mechanochemical conditions. The dramatically accelerated reaction time for reaching the thermodynamic equilibrium product mixture, compared to the solution-based system originally reported can be explained in part due to concentration effects, and additionally to other characteristics associated with the mechanochemical process which have been extensively detailed in the literature.50,51 With regards to concentration, in solution, self-sorting was achieved in 20 days at 20 mM (at 60 °C), whereas 3.6 mM retained significant amounts of [Fe(2,3)(2,4)][BF4]2. The mechanochemical approach reported herein begins as “neat”, gradually releasing up to 10 equivalents of H2O (per every Fe and Cu), and is thus not limited by concentration-based mass transport limitations of a dilute solution. Similar effects have been noted prior in comparing solvent-free/LAG mechanochemical grinding to standard solution-based protocols.18,19,36
Interestingly, for trials at 8 h and 24 h, beyond the expected combination of [Cu(1,4)2][BF4], [Fe(2,3)2][BF4]2, and [Fe(2,3)(2,4)][BF4]2, significant line broadening is observed, in addition to high intensity broad peaks at ca. δ 6.5 and δ 7.0 ppm. Notably, these signals were not observed in the solution-based study. Tentatively, it is postulated that they may represent paramagnetic trigonal bipyramidal [Fe(1,4)(2,3)][BF4]2 or [Fe(1,3)(2,4)][BF4]2 species (Fig. S7†). Further proof of paramagnetic species is found in very broad signals at δ 44, δ 40, δ 32, δ 30, δ 17, δ 15, δ −5, and δ −34 ppm for the 8 h and 24 h trials, (Fig. S6†). It is not surprising that the different reaction environment49 of solvent-free mechanochemical grinding may favour formation of different, or longer-lived metastable intermediates than the nominally dilute (20 mM) solution-based system. However, the transient nature of the formation of the thermodynamic equilibrium product mixture at 4 h, and what appears to be a scrambling at 8 h, subsequent reconstitution of the product mixture at 18 h, and scrambling at 24 h was unexpected based on the original solution studies under steady-state conditions.
Vibratory ball-milling is a complex, non-equilibrium process,54,55 and with the system utilized herein, there is no temperature control under continuous grinding. Typically, the system warms rapidly over the first few hours to reach a steady temperature that only changes gradually as a function of time.56 However, it should be noted that heated mechanochemistry has been successfully demonstrated for multiple reaction systems, with many significant contributions from Užarević and coworkers.57–59 For the system utilized herein, to ascertain any potential temperature fluctuation effects, the temperature of the room (1 m from the ball mill), the motor, and the outside of the reactor vials was measured over 24 h (Fig. S8†). While the inside of the vials could not be measured, observations in literature and from one of our groups point to a close relationship between interior and exterior with a ΔT < 5 °C; though higher variances up to 25 °C between milling balls and vial exterior have been reported.60 Interestingly, the observed formation of the thermodynamic equilibrium product mixture coincided with periods of slightly lower temperature, or regions where the temperature remained steady for prolonged periods; e.g. at 4 hours as the system was warming up, and at 18 hours as the system was cooling steadily overnight. Sampling times coincident with a positive temperature change/gradient led to a mixture of products. Thus it is postulated that even under dynamic conditions, the reaction environment achieves a pseudo-equilibrium for enough time to allow for the isolation of the thermodynamic equilibrium product mixture, congruent with the solution state observations. Shifts in temperature and concomitant changes to local reaction environment appear to trigger further reaction and a scrambling of the ideal product distribution of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2, which can be expected for a dynamic system.
1 [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2, which can be expected for a dynamic system.
The self-sorting reactions were repeated at 0.5 × concentration using Celite as a solid diluent (∼250 mg total weight including 125 mg Celite). We have previously demonstrated the potential of Celite to be an unreactive additive for mechanochemical preparation of imines.35 The 4 h trial displayed predominantly homoleptic [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2, with a significant amount of heteroleptic [Fe(2,3)(2,4)][BF4]2 (ca. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [Fe(2,3)2][BF4]2
1 [Fe(2,3)2][BF4]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fe(2,3)(2,4)][BF4]2 based on integration of imine C–H). The 18 h sample further diminished [Fe(2,3)(2,4)][BF4]2 to trace levels (Fig. S9†). Paramagnetic broadening and evolution of new signals is again evident at 8 h and 24 h; corroborating the reproducibility of this phenomenon under slightly altered reaction environment (see ESI† for more details). Repeating the 18 h reaction with Celite starting at a different time of day (subject to different environmental temperatures) yielded a 1
[Fe(2,3)(2,4)][BF4]2 based on integration of imine C–H). The 18 h sample further diminished [Fe(2,3)(2,4)][BF4]2 to trace levels (Fig. S9†). Paramagnetic broadening and evolution of new signals is again evident at 8 h and 24 h; corroborating the reproducibility of this phenomenon under slightly altered reaction environment (see ESI† for more details). Repeating the 18 h reaction with Celite starting at a different time of day (subject to different environmental temperatures) yielded a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [Fe(2,3)2][BF4]2
2 [Fe(2,3)2][BF4]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fe(2,3)(2,4)][BF4]2 ratio. Similarly, repeating the 18 h reaction and replacing Celite with MgSO4, a desiccant, yielded a ca. 5
[Fe(2,3)(2,4)][BF4]2 ratio. Similarly, repeating the 18 h reaction and replacing Celite with MgSO4, a desiccant, yielded a ca. 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 [Fe(2,3)2][BF4]2
3 [Fe(2,3)2][BF4]2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Fe(2,3)(2,4)][BF4]2 ratio (Fig. S10†). These observations point to a dynamic system, where changes in temperature, and the nature of additive can have a significant impact on product distribution as a function of time.
[Fe(2,3)(2,4)][BF4]2 ratio (Fig. S10†). These observations point to a dynamic system, where changes in temperature, and the nature of additive can have a significant impact on product distribution as a function of time.
Reversible behaviour and equilibration under mechanochemical conditions was first reported by Belenguer and Friščić for organic aromatic disulfides.17 They noted that mechanochemical equilibration led to different outcomes from conventional solution state reactions, and changes of mechanochemical reaction environment could also yield different equilibrium products, driven by crystal packing effects. Herein, both mechanochemical reaction systems can yield similar thermodynamic equilibrium product mixtures as the solution based system.14 Even though the individual homoleptic species (Fig. 2) are readily accessed and stable under vibratory ball milling conditions, the system equilibrates to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2. This is tentatively attributed to a combination of the favourability of [Fe(2,3)2][BF4]2, the unfavorability of the transient kinetic products, and what appears to be the overall ease of reversible imine bond formations for these molecules.
1 [Cu(1,4)2][BF4] and [Fe(2,3)2][BF4]2. This is tentatively attributed to a combination of the favourability of [Fe(2,3)2][BF4]2, the unfavorability of the transient kinetic products, and what appears to be the overall ease of reversible imine bond formations for these molecules.
Conclusion
In summary, the self-sorting of two imine-based Cu(I) and Fe(II) complexes has been successfully demonstrated under solvent-free/LAG mechanochemical conditions. The system achieves a transient equilibrium, enabling access exclusively to the thermodynamic equilibrium product mixture of homoleptic tetrahedral Cu(I) and octahedral Fe(II) species, replicating a multi-week solution process in less than one day. Additionally, the synthesis and validation of stability of four transition metal complexes under vibratory milling conditions has been achieved. These results add to a growing body of mechanochemical syntheses of transition metal coordination complexes, and provide compelling evidence for the potential of mechanochemistry as an avenue towards sorting complex mixtures of different metallic species.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by NSF grant CHE-2154061. The authors thank J. Furst and A. Altomare, University of Central Florida, for assistance with mass spectrometry.Notes and references
- J.-M. Lehn, Angew. Chem., Int. Ed., 2013, 52, 2836–2850 CrossRef CAS PubMed.
- J.-M. Lehn, Angew. Chem., Int. Ed., 2015, 54, 3276–3289 CrossRef CAS PubMed.
- J.-F. Ayme and J.-M. Lehn, Adv. Inorg. Chem., 2018, 71, 3–78 CrossRef CAS.
- J.-M. Lehn, Top. Curr. Chem., 2012, 322, 1–32 CAS.
- Constitutional Dynamic Chemistry, Topics in Current Chemistry, ed. M. Barboiu, Springer, Berlin, 2012, vol. 322 Search PubMed.
- G. Men and J.-M. Lehn, J. Am. Chem. Soc., 2017, 139, 2474–2483 CrossRef CAS PubMed.
- P. Kovaricek, A. C. Meister, K. Flidrova, R. Cabot, K. Kovarickova and J.-M. Lehn, Chem. Sci., 2016, 7, 3215–3226 RSC.
- O. Shyshov, R.-C. Brachvogel, T. Bachmann, R. Srikantharajah, D. Segets, F. Hampel, R. Puchta and M. von Delius, Angew. Chem., Int. Ed., 2017, 56, 776–781 CrossRef CAS PubMed.
- H. Löw, E. Mena-Osteritz and M. von Delius, Chem. Sci., 2018, 9, 4785–4793 RSC.
- N. Hafezi and J.-M. Lehn, J. Am. Chem. Soc., 2012, 134, 12861–12868 CrossRef CAS PubMed.
- G. Vantomme, S. Jiang and J.-M. Lehn, J. Am. Chem. Soc., 2014, 136, 9509–9518 CrossRef CAS PubMed.
- J. Holub, G. Vantomme and J.-M. Lehn, J. Am. Chem. Soc., 2016, 138, 11783–11791 CrossRef CAS PubMed.
- S. Dhers, J. Holub and J.-M. Lehn, Chem. Sci., 2017, 8, 2125–2130 RSC.
- J.-F. Ayme and J.-M. Lehn, Chem. Sci., 2020, 11, 1114–1121 RSC.
- Comprehensive Coordination Chemistry II, ed. J. A. McCleverty and T. J. Meyer, Elsevier Ltd, Amsterdam, 2nd edn, 2004 Search PubMed.
- J.-F. Ayme, J. Lux, J.-P. Sauvage and A. Sour, Chem–Eur. J., 2012, 18, 5565–5573 CrossRef CAS PubMed.
- A. M. Belenguer, T. Friščić, G. M. Day and J. K. M. Sanders, Chem. Sci., 2011, 2, 696–700 RSC.
- A. M. Belenguer, A. A. L. Michalchuk, G. I. Lampronti and J. K. M. Sanders, ChemSusChem, 2022, 15, e202102416 CrossRef CAS PubMed.
- J.-L. Do and T. Friščić, ACS Cent. Sci., 2017, 3, 13–19 CrossRef CAS PubMed.
- G. Bowmaker, Chem. Commun., 2013, 49, 334–348 RSC.
- P. Ying, J. Yu and W. Su, Adv. Synth. Catal., 2021, 363, 1246–1271 CrossRef CAS.
- N. Shan, F. Toda and W. Jones, Chem. Commun., 2002, 2372–2373 RSC.
- T. Friščić, A. V. Trask, W. Jones and W. D. S. Motherwell, Angew. Chem., Int. Ed., 2006, 45, 7546–7550 CrossRef PubMed.
- K. Kubota, R. Takahashi and H. Ito, Chem. Sci., 2019, 10, 5837–5842 RSC.
- J. G. Hernández, I. S. Butler and T. Friščić, Chem. Sci., 2014, 5, 3576–3582 RSC.
- D. V. Aleksanyan, S. G. Churusova, R. R. Aysin, Z. S. Klemenkova, Y. V. Nelyubina and V. A. Kozlov, Inorg. Chem. Commun., 2017, 76, 33–35 CrossRef CAS.
- P. Liang, A. Kobayashi, T. Hasegawa, M. Yoshida and M. Kato, Eur. J. Inorg. Chem., 2017, 44, 5134–5142 CrossRef.
- J.-L. Do, D. Tan and T. Friščić, Angew. Chem., Int. Ed., 2018, 57, 2667–2671 CrossRef CAS PubMed.
- N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352–2362 RSC.
- D. V. Aleksanyan and V. A. Kozlov, Mendeleev Commun., 2023, 33, 287–301 CrossRef CAS.
- D. Tan and F. Garcia, Chem. Soc. Rev., 2019, 48, 2274–2292 RSC.
- I. R. Speight, S. C. Chmely, T. P. Hanusa and A. L. Rheingold, Chem. Commun., 2019, 55, 2202–2205 RSC.
- A. Beillard, X. Bantreil, T.-X. Métro, J. Martinez and F. Lamaty, Dalton Trans., 2016, 45, 17859–17866 RSC.
- A. Beillard, X. Bantreil, T.-X. Métro, J. Martinez and F. Lamaty, Green Chem., 2018, 20, 964–968 RSC.
- F. Leon and F. Garcia, 19—Metal Complexes in Mechanochemistry, Comprehensive Coordination Chemistry III, 9, ed. E. C. Constable, G. Parkin and L. Que Jr., Elsevier, Amsterdam, 2021, pp. 620–679, ISBN 9780081026892 Search PubMed.
- T. E. Shaw, L. Mathivathanan and T. Jurca, Organometallics, 2019, 38, 4066–4070 CrossRef CAS.
- J. Wang, R. Ganguly, L. Yongxin, J. Díaz, H. S. Soo and F. García, Dalton Trans., 2016, 45, 7941–7946 RSC.
- S. Zuo, S. Zheng, J. Liu and A. Zuo, Beilstein J. Org. Chem., 2022, 18, 1416–1423 CrossRef CAS PubMed.
- T. E. Shaw, L. R. Shultz, L. R. Garayeva, R. G. Blair, B. C. Noll and T. Jurca, Dalton Trans., 2018, 47, 16876–16884 RSC.
- M. Ferguson, N. Giri, X. Huang, D. Apperley and S. James, Green Chem., 2014, 16, 1374–1382 RSC.
- L. Leoni, A. Carletta, L. Fusaro, J. Dubois, N. A. Tumanov, C. Aprile, J. Wouters and A. Dalla Cort, Molecules, 2019, 24, 2314 CrossRef CAS PubMed.
- D. Crawford, J. Casaban, R. Haydon, N. Giri, T. McNally and S. L. James, Chem. Sci., 2015, 6, 1645–1649 RSC.
- D. E. Crawford, C. K. Miskimmin, J. Cahir and S. L. James, Chem. Commun., 2017, 53, 13067–13070 RSC.
- P. Milbeo, F. Quintin, L. Moulat, C. Didierjean, J. Martinez, X. Bantreil, M. Calmès and F. Lamaty, Tetrahedron Lett., 2021, 63, 152706 CrossRef CAS.
- V. K. Singh, A. Chamberlain-Clay, H. C. Ong, F. León, G. Hum, M. Y. Par, P. Daley-Dee and F. García, ACS Sustainable Chem. Eng., 2021, 9, 1152–1160 CrossRef CAS.
- F. León, C. Li, J. F. Reynes, V. K. Singh, X. Lian, H. C. Ong, G. Hum, H. Sun and F. García, Faraday Discuss., 2023, 241, 63–78 RSC.
- R. Tedjini, R. Viveiros, T. Casimiro and V. D. B. Bonifácio, React. Chem. Eng., 2021, 6, 2140–2145 RSC.
- G. Pisanò and C. S. J. Cazin, Green Chem., 2020, 22, 5253–5256 RSC.
- J. Beaudelot, S. Oger, S. Peruško, T.-A. Phan, T. Teunens, C. Moucheron and G. Evano, Chem. Rev., 2022, 122, 16365–16609 CrossRef CAS PubMed.
- E. Haldón, M. C. Nicasio and P. J. Pérez, Org. Biomol. Chem., 2015, 13, 9528–9550 RSC.
- R. Saiki, H. Miyamoto, H. Sagayama, R. Kumai, G. N. Newton, T. Shiga and H. Oshio, Dalton Trans., 2019, 48, 3231–3236 RSC.
- S. Choi and M. Shatruk, Chem. Sci., 2021, 12, 10765–10779 RSC.
- T. K. Ekanayaka, Ö. Üngör, Y. Hu, E. Mishra, J. P. Phillips, A. S. Dale, S. Yazdani, P. Wang, K. A. McElveen, M. Z. Zaz, J. Zhang, A. T. N'Diaye, C. Klewe, P. Shafer, R. Y. Lai, R. Streubel, R. Cheng, M. Shatruk and P. A. Dowben, Mater. Chem. Phys., 2023, 296, 127276 CrossRef CAS.
- K. S. McKissic, J. T. Caruso, R. G. Blair and J. Mack, Green Chem., 2014, 16, 1628–1632 RSC.
- R. T. O'Neill and R. Boulatov, Nat. Rev. Chem., 2021, 5, 148–167 CrossRef PubMed.
- R. Schmidt, H. M. Scholze and A. Stolle, Int. J. Ind. Chem., 2016, 7, 181–186 CrossRef.
- G. Báti, D. Csókás, T. Yong, S. M. Tam, R. R. S. Shi, R. D. Webster, I. Pápai, F. García and M. C. Stuparu, Angew. Chem., Int. Ed., 2020, 59, 21620–21626 CrossRef PubMed.
- N. Cindro, M. Tireli, B. Karadeniz, T. Mrla and K. Užarević, ACS Sustainable Chem. Eng., 2019, 7, 16301–16309 CrossRef CAS.
- V. Martinez, T. Stolar, B. Karadeniz, I. Brekalo and K. Užarević, Nat. Rev. Chem., 2023, 7, 51–65 CrossRef CAS PubMed.
- L. Takacs and J. S. McHenry, J. Mater. Sci., 2006, 41, 5246–5249 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR for reactions, ESI MS, 1H and 13C NMR and crystallographic details of [Cu(1,3)2][BF4]. CCDC 2285502. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3mr00021d |
| This journal is © The Royal Society of Chemistry 2024 |