Open Access Article
Open Access Article“What makes every work perfect is cooking and grinding”: the ancient roots of mechanochemistry†
Marianna
Marchini
 *a,
Giacomo
Montanari
b,
Lucia
Casali
*a,
Giacomo
Montanari
b,
Lucia
Casali
 ac,
Matteo
Martelli
b,
Lucia
Raggetti
b,
Matej
Baláž
ac,
Matteo
Martelli
b,
Lucia
Raggetti
b,
Matej
Baláž
 d,
Peter
Baláž
d,
Peter
Baláž
 d and
Lucia
Maini
d and
Lucia
Maini
 *a
*a
aDepartment of Chemistry “Giacomo Ciamician”, University of Bologna, 40126, Bologna, Italy. E-mail: marianna.marchini2@unibo.it; l.maini@unibo.it
bDepartment of Philosophy and Communication Studies, University of Bologna, 40126, Bologna, Italy
cBAM Federal Institute for Materials Research and Testing, Richard-Willstätter-Strasse 11, 12489 Berlin, Germany
dInstitute of Geotechnics, Slovak Academy of Sciences, Watsonova 45, 04001 Kosice, Slovakia
First published on 14th February 2024
Abstract
This paper explores the historical significance of milling in various technological areas from ancient times, emphasizing its role beyond the simple ingredient reduction. The study focuses on sources from the 1st to the 10th centuries: philologists selected, studied, and translated ancient sources, while chemists provided chemical interpretations by replicating the recipes in the laboratory. The study delves into the synthesis of cinnabar from mercury and sulphur, or mineral ores such as orpiment, realgar, and stibnite. While the mercury–sulphur reaction is known, the synthesis from sulphide ores is not reported in the literature. Chemical replication assessed the reactions' feasibility and confirmed the fundamental role of grinding for the yield of the reaction, which was already recognized by the alchemist Zosimus of Panopolis (3rd–4th cent. CE) who claimed “what makes every work perfect is cooking and griding”.
Introduction
Milling has accompanied human beings through all the steps of technological progress since the dawn of time, from the milling of grains to make flour or the discovery of flint and its use to kindle fires. However, milling is not just the simple reduction of ingredients into minute particles, but it can be regarded as a fundamental process in transforming substances.Theophrastus' On Stones (3rd cent. BCE) describes the extraction of mercury by grinding cinnabar in a copper mortar in the presence of vinegar. Takacs1,2 rightly identified this procedure as the first documented mechanochemical reaction, long before the birth of chemistry itself. The importance of extracting mercury from ores by mechanical means in ancient metallurgy was further evidenced by Baláž.3 However, no other mechanochemical reaction was detected in the historical record before the 19th century, with the work of Faraday,4 Ostwald5 and Lea.6
The apparent lack of other references to the importance of grinding for transformation processes is rather suspicious, given the relevance that this procedure had in the early days of alchemy. A possible explanation probably lies in the difficulty of accessing the sources, which are few, especially before mediaeval time, and often not yet edited and translated into modern languages. The difficulties in the interpretation of alchemical texts are renown: the style can be obscure as meant for acolytes, and the texts are composed in languages that are not in the usual domain of the average chemist, such as Latin, Greek, Arabic, or Syriac. Hence, the modern chemist who would like to navigate through historical works has to face several difficulties that can turn the intriguing prospect into a daunting experience.7–9
In this paper, we will focus on a number of sources composed between the 1st and the 10th century, which testify to the importance of grinding according to ancient scholars and practitioners. The selection of the sources has been made by the philologists, who prepared editions and translations of the texts, while the chemists proposed chemical interpretations and tested these hypotheses in the laboratory. By replicating the recipes, we explored the viability and feasibility of the reactions, as well as the technical underpinnings of the texts, in order to disclose the material and practical dimensions of ancient alchemy. Textual and chemical interpretations constitute a hermeneutical cycle, in a sort of ouroboros, the emblematic serpent of ancient Egypt and Greece which was represented with its tail in its mouth, continually devouring and regenerating itself at the same time.
We collected several recipes dealing with the synthesis of cinnabar, as follow up of our previous work on the cold extraction of mercury from cinnabar.10 This close interest in mercury comes from the peculiar physical and chemical properties of this element, which captured the attention of early practitioners. Its ability to form amalgams with several other metals and to react with many compounds led to its conceptualization as the common constituent of all metals.
Ancient recipes were transmitted in manuscript form and copied, time after time, on papyrus or parchment. If today we think of copying as the act of creating an exact reproduction, the approach to the transmission was very different in premodern times, particularly in the case of technical procedures. When copying, copyists could add words and ingredients or, alternatively, summarise passages and change ingredients, in the measure of their knowledge and experience. When it comes to the study of the chemical reality behind the text, a single variant of the recipe randomly chosen among those that came down to us, may not be enough. The close comparison with different occurrences of the same procedure offers a larger perspective for the chemical interpretation. The goal of the recipe is a pivotal point on which we establish a parallel between different descriptions of the same procedure.
The importance of grinding in the cold extraction of mercury from cinnabar has already been described, therefore here we focus our attention on the synthesis of cinnabar starting from mercury and sulphur or mineral ores such as orpiment (As2S3), realgar (As4S4), and stibnite (Sb2S3). While the reaction between mercury and sulphur is known,11 the synthesis from sulphide ores has not been reported in contemporary literature yet.
By means of chemical replication, we wanted to establish the feasibility of the reactions and the role of the grinding, to determine whether it was merely a matter of size reduction and homogenization of the powder or it was a first fundamental step of the process.
Results and discussion
Synthesis of cinnabar from sulphur and mercury
The synthesis of mercury sulphide from sulphur and mercury is a known process, reported in modern literature as a common procedure for the production of vermilion in mediaeval Europe, though the reaction has deeper roots in the past.12 Indeed, there is a rich corpus of sources dating from the first to the tenth century which describe the synthesis of cinnabar. Two detailed accounts come from late Byzantine sources.The making of cinnabar . You must put unburnt sulphur, one ounce, and mercury, two ounces, in a mortar. After grinding them both in the mortar for a day, put them in a glass flask and seal its opening with a three-fingers thick fireclay made of mud and coal. Put them on a fire for the spontaneous digestion for 6 to 9 hours, then take it out and you will find an iron-coloured mass. Grind it several times in the sun with water. In fact, the more you grind, the more it turns yellow. Indeed, unburnt sulphur makes volatile substances fixed.
Preparation of cinnabar . Take mercury, two parts, naturally occurring sulphur (sulphur vivum) that has been ground, (a part), pure urine, a part. Take a clean, hard flask, which is resistant to a smokeless fire, and put the mixture in it. Do not fill the flask, but rather let it remain empty for two or three fingers. Mix all the ingredients together and set up an oven similar to the one used by glassmakers. The flask must be large. Leave enough room (in the oven) to fit the flask, split the reeds, and light the oven. There must be a little window where the flame can escape all around. This is the sign that the mixture is cooked: look at the empty part of the flask, and if you see a rising smoke that looks purple and has the colour of cinnabar, you must know that it is done. Do not allow the glass to be heated any longer. In fact, glass will break if you continue to heat it up too much.
The first recipe describes a two-step procedure: a first step in which a mechanochemical reaction is involved, and a second step in which the ground mixture is heated in a vessel. The second recipe cursorily refers to the grinding of sulphur, and it does not provide detailed description of the procedure, but it only mentions the heating at high temperature and the smokes that rise from the vessel.
Upon grinding sulphur and mercury with mass ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
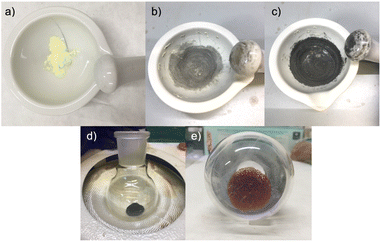
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which correspond to large excess of sulphur (see ESI† for more details), as describes in the first recipe (Fig. 1a), the formation of a greyish powder is observed after several minutes of grinding (Fig. 1b); going on with the grinding, all mercury and sulphur are converted into a black powder (Fig. 1c) and drops of mercury are no longer visible in the mortar. This is something that probably caught the attention of the ancients, as the formulation “sulphur makes volatile substances fixed” suggests.
2, which correspond to large excess of sulphur (see ESI† for more details), as describes in the first recipe (Fig. 1a), the formation of a greyish powder is observed after several minutes of grinding (Fig. 1b); going on with the grinding, all mercury and sulphur are converted into a black powder (Fig. 1c) and drops of mercury are no longer visible in the mortar. This is something that probably caught the attention of the ancients, as the formulation “sulphur makes volatile substances fixed” suggests.
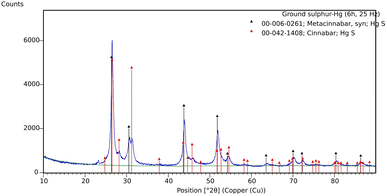
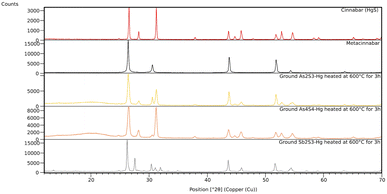
The black powder obtained after three hours of ball milling was identify as unreacted sulphur and metacinnabar, a well-known polymorph of mercury sulphide, metastable at room temperature but thermodynamically stable at high temperatures, above about 370 °C.15 Interestingly, when the reaction is carried for six hours, the black powder is a mixture of the two polymorphs of mercury sulphide (Fig. 2). The possibility of obtaining cinnabar and metacinnabar by grinding has been recently reported by Fukuda et al. but despite the time of grinding they never observed the total conversion of the black pigment into the red one.16
Since the mechanochemical reactions between sulphur and mercury may occur in several steps, we decided to monitor its progress in order to get a better understanding of the chemistry involved. As shown in the graph in Fig. 3, the appearance of the metacinnabar was already detectable in the first minutes and lasted for all the three hours of the reaction, without the appearance of cinnabar phase, and with the peaks of unreacted sulphur still detectable.
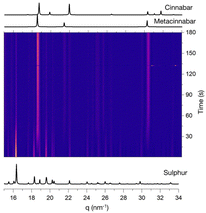 | ||
| Fig. 3 Time evolution of the milling reaction between sulphur and mercury. Milling conditions: 50 Hz for three hours with three 4 mm steel milling balls. | ||
Moving on to the second step of the procedure, which involved the heating of the ground powders, it is possible to convert the metacinnabar into the red form. When metacinnabar is annealed, it allows the spontaneous conversion from metacinnabar to cinnabar, as the powder is cooled down (Fig. 1e). Sometimes, however, the conversion does not occur, probably due to the presence of some impurities. In that case, the formation of red cinnabar can be trigged by grinding the powder. Indeed, in the recipe one can read “the more you grind, the more it turns yellow”. The word for “yellow” in the Greek original text (ξανθóς, xanthós) covers a whole spectrum of hues, from blond hair to the reddish blaze of fire; therefore, it can well be interpreted as a kind of golden red, and it is sometimes associated with cinnabar. In the second recipe (Text 2), the grinding step of the synthesis is not mentioned. However, there is a precise description of the heating conditions, in which “an oven similar to the one used by glassmakers” is used, which means that the recipes require a high temperature (about 600–800 °C). In order to replicate similar heating conditions, we have used a custom-made furnace with charcoal as fuel. The furnace has a hole from which air can be blown inside to increase the temperature (see ESI† for more details).
Mercury and sulphur, without being ground together beforehand, were placed in a home-made crucible and heated at high temperature (about 600 °C) for three hours: at the end of the reaction, a black powder was present inside the crucible, and this converted into a red powder (cinnabar) upon grinding.
The importance of grinding, as well as that of the heating process was clearly emphasized by the Graeco-Egyptian alchemist Zosimus of Panopolis (3rd–4th cent. CE).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 17.
I taught you that what makes every work perfect is cooking and grinding. If you seek the truth, know that mercury is what transforms natures (that) are confined/fixed in it and through it.
17.
I taught you that what makes every work perfect is cooking and grinding. If you seek the truth, know that mercury is what transforms natures (that) are confined/fixed in it and through it.
Although the reaction can happen by simple heating, grinding the reagents is a crucial element of the procedure. The milling of mercury and sulphur is an important step to avoid the need for high temperature, and increases the yield of the reaction too. In fact, by heating mercury and sulphur at 350 °C without grinding, the reaction occurs only partially and metacinnabar is found condensed on the lid, mixed with sulphur. On the other hand, using a higher temperature increases the sublimation of the reagents, as well as their loss, which leads to a lower yield.11
Synthesis of cinnabar from mineral sulphides and mercury
Interestingly, sulphur was not the only substance that the ancients used to «fix» mercury. The alchemist pseudo-Democritus (1st cent. CE) in the section on the making of gold of his books on dyeing wrote.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 18.
Take mercury and make it solid (let. ‘fix/freeze it’) with the body of “3agnesia”,‡ or the body of Italian stibnite, or with unburnt sulphur, or with moon foam, or with roasted lime, or with alum from Milos, or with orpiment, or according to your knowledge.
18.
Take mercury and make it solid (let. ‘fix/freeze it’) with the body of “3agnesia”,‡ or the body of Italian stibnite, or with unburnt sulphur, or with moon foam, or with roasted lime, or with alum from Milos, or with orpiment, or according to your knowledge.
Among the ingredients listed in the recipe, some of which are not easy to identify, the presence of two mineral sulphides, namely orpiment (As2S3) and stibnite (Sb2S3), both well-known by the ancients, caught our attention. The use of both minerals to treat mercury is well attested in the Syriac and Arabic traditions of Zosimus' works.
Likewise, in the Arabic dialogue entitled Tome of Images attributed to Zosimus, we find the following description of the ‘reaction’ between mercury and orpiment (here called ‘the male’, from its Greek name arsenikón, which literally means ‘male’)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 21.
Do you not see how the sage said: ‘if you put the mercury from cinnabar with it (i.e. ‘the male’), then a great secret belongs to them’? she said: ‘And what is that secret?’. He said: ‘Take the two and mix them. After mixing them until they thicken, you will find that the mercury becomes thick and the male turns into ashes, hidden in the mercury’.
21.
Do you not see how the sage said: ‘if you put the mercury from cinnabar with it (i.e. ‘the male’), then a great secret belongs to them’? she said: ‘And what is that secret?’. He said: ‘Take the two and mix them. After mixing them until they thicken, you will find that the mercury becomes thick and the male turns into ashes, hidden in the mercury’.
Zosimus claimed that, if orpiment is mixed with mercury, the metal “becomes thick”, again, conveying the idea that this arsenic sulphide could be used for “fixing” mercury. Moreover, in various writings (Texts 4 and 5) Zosimus also mentions realgar along with orpiment. All those recipes suggest that ancient practitioners included orpiment, stibnite, and realgar under the same group of substances as sulphur, recognizing a common nature in them, long time before the chemical composition of the minerals became part of the scientific discourse. Indeed, in De materia medica, Dioscorides (1st cent. CE) claimed that realgar smells like sulphur, and that it can be treated in the same way as orpiment.§
In light of all these sources, we decided to set up experiments to investigate whether mineral sulphides, such as orpiment, stibnite and realgar, can be used in place of sulphur in the synthesis of cinnabar, or in other word, to make mercury solid.
We followed the steps reported in the Byzantine anonymous recipe mentioned above (Text 1) as well as Zosimus' belief (Text 3): “what makes all the work perfect is cooking and grinding”. First, we ground mercury with each of mineral sulphides mentioned in the sources. The grinding was done with a ball miller, not only to avoid inhaling the toxic powders, but also to reduce the reaction time. The mass ratio between mineral sulphides and mercury was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as suggested by the sources, which corresponds to molar ratio around Hg
2 as suggested by the sources, which corresponds to molar ratio around Hg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S 1
S 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (see ESI† for more details).
1 (see ESI† for more details).
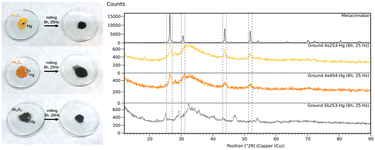
In all the reactions, mercury drops are no longer visible after grinding and, in the case of orpiment and realgar, a black powder is formed, while the stibnite is already black at the beginning (Fig. 4, left). It is worth noting that all the powders obtained after grinding look alike in colour and no mercury droplets are detected.
The ground powders were analysed with XRPD. In the reactions of orpiment and realgar with mercury, the characteristic peaks of metacinnabar along with peaks of unreacted reagents are present in the patterns (Fig. 4, right and Fig. S11 and S16†), while the formation of metacinnabar is not observed using stibnite, even after prolonged grinding; yet mercury drops as no longer visible. In all three cases the high background in the pattern suggests the presence of amorphous phases due to unreacted mercury, amorphous sulphide, and/or amorphous products.
The mechanochemical reactions between mercury and mineral sulphides were followed at the μSpot beamline BESSY II, which showed the formation of metacinnabar after a couple of minutes in the case of orpiment, and after one hour in the case of realgar (Fig. 5). We were not able to gain more information about the chemistry of the reactions, because no transient species or metallic arsenic were detected while milling. When it comes to the milling of mercury with stibnite, no reaction occurs (Fig. S20†), as already observed in the previous experiment.
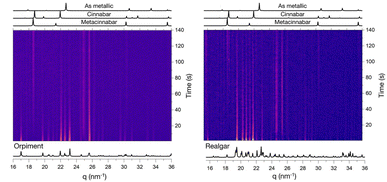 | ||
| Fig. 5 In situ XRPD of the milling reaction between mercury and orpiment (left), mercury and realgar (right). Milling conditions: 50 Hz for three hours with three 4 mm steel milling balls. | ||
While the oxidation of mercury and the production of metacinnabar are evident, determining which species undergoes reduction was challenging. The simplest explanation is the reduction of arsenic to As(0), which is known to have different allotropes: grey arsenic (the stable form at ambient condition), yellow arsenic (most unstable; it decomposes quite readily, especially when exposed to light) and black arsenic; this last can be amorphous.22 Amorphous black arsenic can be hidden in the broad background of the diffraction patterns, always present at the end of milling, and by the black colour of metacinnabar in the samples themselves.
In order to obtain the desired cinnabar, we heated the powders at 350 °C for three hours in separate Kjeldahl flasks, the same condition used in the first reaction.
Several substances condensed on the wall of the Kjeldahl flask which were analyzed separately from the powder left on the bottom of the flask (Fig. 6).
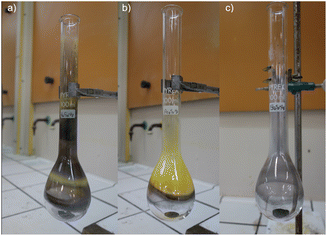 | ||
| Fig. 6 Kjeldahl flasks at the end of the heating step of (a) Hg and orpiment, (b) Hg and realgar, (c) Hg and stibnite. | ||
In the case of the reaction with the arsenic sulphides, the residual powder at the bottom of the flask contains only metacinnabar with a very flat background; whereas the powder collected from the glassware is mainly arsenic oxide and metacinnabar.
In the case of mercury ground with stibnite, upon heating the formation of metacinnabar occurs which is detected along with antimony oxide in the residual powder at the bottom of the flask (Fig. S24†), while on the glassware only droplets of mercury are recovered.
The presence of arsenic oxide and antimony oxide can be due to the decomposition of the relative sulphide or the oxidation of the metal (both reactions happen at high temperature in presence of oxygen).23 The second case would support the reduction of the arsenic suggested before. Because the as-received mixtures were heated in air in the second step, this scenario is highly probable. It is worth noting that, in the case of the reaction with arsenic sulphides, no unreacted mercury was observed hence we can consider that the reactions (1) and (2) reached the completeness.
| 4Hg(l) + As4S4(s) → 4HgS(s) + 4As (amorphous) | (1) |
| 3Hg(l) + As2S3(s) → 3HgS(s) + 2As (amorphous) | (2) |
Upon heating the As(0) oxidizes into As2O3 which is volatile and it is recovered only on the glassware.
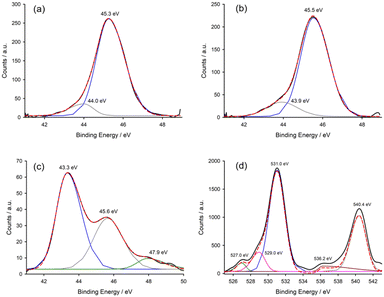
To grasp more information, the powders at the end of the heating step were analysed with X-ray photoelectron spectroscopy (XPS) to determine the oxidative state of the present elements. The powders at the bottom of the flask (As2S3-bottom, As4S4-bottom and Sb2S3-bottom) were analysed separately from the ones on the glassware (As2S3-neck and As4S4-neck). The As 3d XPS spectra for the arsenic sulphides–mercury mixtures are provided in Fig. 7a–c. For orpiment–mercury mixture, no arsenic was detected in the powder at the bottom of the flask.
The powders of the reactions with both arsenic sulphides collected from the flask neck (Fig. 7a and b) clearly show the signal of the arsenic peak with a binding energy above 45 eV confirming the presence of 3d5/2 peak of arsenic in oxidation state (III), which is in accordance with the As2O3 compound in literature, and consistent with the presence of As2O3 observed in the XRPD for these samples. The fitting of the As 3d spectra showed the presence of another small peak located at 44 eV. This most probably belongs to the As(III) of a small amount of non-reacted arsenic sulphides.24 Upon analysing the powders collected from the bottom of the flask, the presence of arsenic was detected only in the case of realgar–mercury reaction (Fig. 7c) and it was absent in the one with orpiment. The reason for this most probably a more complete reaction in As2S3 + Hg system. More interestingly, the binding energy of the arsenic in As4S4-bottom sample is significantly different from the ones collected from the neck (As4S4-neck), thus pointing to the presence of arsenic in a different compound. The main peak is located at 43.3 eV, which could be a proof of the non-reacted realgar As4S4. The binding energy of elemental arsenic As(0) is even lower, i.e. around 41.5 eV and thus, its presence cannot be confirmed. This sample also contains As2O3 as documented by the peak at 45.6 eV, so even it is not visible in the XRD pattern (see Fig. S18†), it might be present on the surface.
The Sb 3d/O 1s XPS spectra of the sample collected after heating the ground stibnite–mercury mixture exhibits four peaks. The two most intensive ones (3d5/2 and 3d3/2) are located at 531.0 and 540.4 eV, respectively. They correspond to antimony in oxidation state (III)25 and this is in agreement with the presence of Sb2O3 observed in the XRPD pattern. The doublet separation is 9.4 eV, which is in perfect agreement with literature. For the peak at 531 eV, a shoulder peak at 529.0 was found, and this belongs to oxygen (O 1s) in oxidation state (II) in Sb2O3. Most importantly, two other peaks at 527.0 and 536.2 eV belonging to elemental antimony Sb(0) were identified, thus are a proof of the antimony reduction from sulphide to its elemental form during mechanochemical reaction and/or subsequent heating. Interestingly, under these conditions, the conversion of metacinnabar to cinnabar was not trigged, even upon grinding. It could be partially observed, though, when the temperature was increased at 600 °C by mean of the furnace (Fig. 8). We suppose that the presence of impurities could have prevented the conversion.
Conclusion
This work presents the fruitful collaboration between chemists and philologists who apply their textual research to the study of the history of science. This allowed the analysis and replication of ancient recipes for the synthesis of cinnabar. Although these recipes may not have immediate technological significance for the modern world, they provide a glimpse into how our ancestors conceptualized the nature around them and allow us to widen our understanding of the earliest phases of the history of chemistry. While the work of philologists is essential for reading and contextualizing the sources, the laboratory replication by chemists explores the feasibility and materiality of the recipes.The recipes discussed here deal with the reaction of mercury with sulphur (which has been previously reported) as well as with the much less investigated reaction of mercury with mineral sulphides.
The replications proved the formation of HgS in all cases, mainly metacinnabar from grinding which converts to cinnabar upon heating, except when using stibnite, that requires heating to trigger the reaction. From the sources, it emerges that the ancient practitioners identified the close relation between sulphur and realgar, orpiment, and stibnite. Indeed, they recognized that sulphur and arsenic/antimony sulphides can have a similar role in the synthesis of cinnabar.
In the cases of realgar, orpiment, and stibnite, we did not clearly detect metallic arsenic or antimony. It is plausible, however, that the oxidation of mercury to mercury sulphide is due to the reduction of the arsenic or antimony into As/Sb(0) which then reacts with the oxygen to yield the relative oxides.
The absence of visible secondary products and the observation that liquid mercury disappears during the grinding step are possibly to be linked to Zosimus' idea: “that mercury is what transforms natures (that) are confined/fixed in it and through it” (Text 3).
Our replications confirm the importance of grinding in the procedures. The milling process shows a dramatic change in the initial compounds. This is visible already to the naked eye, in the form of colour change and the disappearance of mercury drops. Nowadays, we are able to detect the formation of the metacinnabar with XRPD. Furthermore, the black powder obtained in this way, that is metacinnabar, can be easily converted by heating into the red form, which is the desired goal of the procedure, namely cinnabar.
Although cinnabar can be obtained just by heating, the milling step has several advantages. Without grinding the reagents, higher temperatures are needed (as reported in the primary source), which implies the use of a more sophisticate oven and, eventually, the sublimation and decomposition causes loss of reagents and lower yields.
When the reagents are ground to form metacinnabar by a mechanochemical reaction, mild heating is enough for the subsequent annealing process to convert it to cinnabar, reducing the loss of reagents by sublimation and increasing the yield of the reaction.
In this context, it is easy to understand why Zosimus, who finds in grinding and heating the perfect sequence for the transformation of matter, concludes with the statement: “I taught you that what makes every work perfect is cooking and grinding”.
Materials and methods
Milling condition
A Retsch MM200 ball mill was used for the mechanochemical reactions, using 1 mL steel jars that contained three steel spheres (Ø = 3 mm, 0.545 g) at 25 Hz; in order to obtained more homogenous milling condition, the jars were opened each 90 minutes and the content was removed from the jars walls and manually mixed.Heating condition
A heating mantle was used to reach 350 °C; the mixture of sulphur and mercury was heated in a round-bottom flask with a watch glass lean on top, in order to avoid the loss of toxic fumes and the increase of pressure. The mixture of mineral sulphides and mercury were heated in a Kjeldahl flask with a septum on top of the neck. For reaching higher temperature a custom-made furnace with a hairdryer was used (Fig. S8†); the reagents were inserted in home-made clay crucibles; the temperature was controlled with a thermocouple.X-ray powder diffraction
For the purpose of identifying the individual phases, XRPD patterns were collected using a PANalytical X'Pert Pro Automated diffractometer equipped with an X'celerator detector conforming to Bragg–Brentano geometry; Cu-Kα radiation (γ = 1.5418 Å) without a monochromator at 2θ ranged between 7° and 90° (step size: 0.033°; time per step: 20 s; Soller slit: 0.04 rad; antiscatter slit: 1; divergence slit: 1/2; 40 mA × 40 kV).PDF2 release 2004 was used for phase identification.
In situ powder X-ray diffraction
In situ X-ray diffraction data were collected at 30 s intervals at the μSpot beamline (BESSY II, Helmholtz Centre Berlin for Materials and Energy) in a vibration vertical ball mill (Pulverisette 23, Fritsch, Germany). The reactions were performed at 50 Hz with three 4 mm steel milling balls using a custom-made Perspex grinding jar. The experiments were conducted with a wavelength of 0.7314 Å using a double crystal monochromator (Si 111). The obtained scattering images were integrated with the Dpdak-software and background corrected with a Python script.X-ray photoelectron spectroscopy
The X-ray source is a not-monochromatized Mg anode that generates a MgKα photon at 1253.6 eV. The electron energy analyzer (a VSW HA100 hemispherical electron energy analyzer with a PSP power supply and control) can work at different resolutions depending on the Pass Energy (PE) and the slit aperture (1 × 10 or 5 × 10). We performed the following analysis on each sample: a long energy range spectrum at low resolution (PE = 50 eV, Δ = 1 eV) to identify the chemical species and high resolution (PE = 20 eV, Δ = 0.025 eV) spectra for every core level detected. Slits have been fixed at 1 × 10. Powders were supported on carbon tape fixed on a silicon wafer (12 mm × 12 mm).Synthesis condition
The amount of reagents used in the mechanochemical reactions was based on the recipe 1. The mass ration indicated the recipe was one ounce of sulphur (S) and two ounces of mercury (Hg). We used 350 mg of sulphur and 700 mg of mercury which corresponds to a molar ratio 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 S
1 S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg.
Hg.
The same mass was used when the sulphur was replaced by mineral sulphides. Under this condition, the molar ratio, estimated considering the mineral sulphide pure, is different for each sulphide, in particular the ratio S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg is 0.9
Hg is 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for realgar
1 for realgar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for orpiment
1 for orpiment![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) and 0.9
and 0.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for stibnite. Sulphur was purchased from Merck and mercury was recovered from old electrodes; the sulphides were obtained by grinding the corresponding mineral.
1 for stibnite. Sulphur was purchased from Merck and mercury was recovered from old electrodes; the sulphides were obtained by grinding the corresponding mineral.
Author contributions
M. Marchini writing – original draft; M. Marchini, L. M., G. M., L. R., M. Martelli, L. C., M. B. and P. B. writing – review & editing; M. Marchini, L. M., L. R. and M. Martelli conceptualization; M. Marchini, G. M., L. M., L. R., and M. Martelli methodology; M. Marchini and L. M. formal analysis; M. B. analysis XPS; L. C. in situ XRPD measurements; M. Martelli funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This publication is part of the research project AlchemEast (G. A. 724914) and the FARE project “The Western AlchemEast” (R18W2STNE2). This work benefited from networking activities carried out within the EU-funded COST Action CA18112-Mechanochemistry for Sustainable Industry. The financial support of the Scientific Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic (project 2/0112/22) is also gratefully acknowledged. We wish to acknowledge the collaboration with the UseFool project (G. A. 101043939); we would like to thank Christian Heinekamp for his help with the in situ measurements.Notes and references
- L. Takacs, J. Met., 2000, 12–13 CAS.
- L. Takacs, Chem. Rev., 2013, 42, 7649–7659 CAS.
- P. Baláž, Acta Metall. Slovaca, 2001, 4, 23–28 Search PubMed.
- (a) M. Faraday, Q. J. Lit., Sci. Arts, 1820, 8, 374 Search PubMed; (b) L. Takacs, J. Therm. Anal. Calorim., 2007, 90(1), 81–84 CrossRef CAS.
- (a) W. Ostwald, Lehrbuch der Allgemeinen Chemie, Vol. 2, Part 1: Chemische Energie, Verlag von Wilhelm Engelmann, Leipzig, 2nd edn, 2nd print, 1903, p. 1079 Search PubMed; (b) W. Ostwald, Die chemische Literatur und die Organisation der Wissenschaft, in Handbuch der allgemeinen Chemie, ed. W. Ostwald and C. Drucker, Akademische Verlagsgesellschaft mbH, Leipzig, 1919, pp. 70 and 77 Search PubMed.
- M. C. Lea, Am. J. Sci., 1892, 43, 527–531 CrossRef.
- L. M. Principe, Alchemy and Chemistry: Breaking up and Making up (Again and Again), Dibner Library Publications, Smithsonian Institution, 2017 Search PubMed.
- H. Fors, L. M. Principe and H. O. Sibum, Ambix, 2016, 63(2), 85–97 CrossRef PubMed.
- L. M. Principe, Texts and Practices: The Promises and Problems of Laboratory Replication and the Chemical Explanation of Early Alchemical Processes, in Greek Alchemy from Late Antiquity to Early Modernity, ed. E. Nicolaidis, 2018, pp. 159–69 Search PubMed.
- M. Marchini, M. Gandolfi, M. Maini, L. Raggetti and M. Martelli, Proc. Natl. Acad. Sci. U. S. A., 2022, 119, e2123171119 CrossRef CAS PubMed.
- M. J. Melo and C. Miguel, Fatto D'Archimia – History and Identification of Artificial Pigments, 2010, pp. 181–195 Search PubMed.
- C. Miguel, J. V. Pinto, M. Clarke and M. J. Melo, Dyes Pigm., 2014, 102, 210–217 CrossRef CAS.
- R. Halleux, Traités des arts et métiers, Paris, 2021, p. 118 Search PubMed.
- A. Colinet, Recettes alchimiques (Par. Gr. 2419; Holkhamicus 109) Cosmas le Hiéromoine, Chrysopée, Paris, 2010, pp. lxxxviii–lxxxix Search PubMed.
- P. Ballirano, M. Botticelli and A. Maras, Eur. J. Mineral., 2013, 25, 957–965 CrossRef CAS.
- N. Fukuda, M. Takaoka, K. Oshita and T. Mizuno, J. Hazard. Mater., 2014, 276, 433–441 CrossRef CAS PubMed.
- Arabic text in Dār al-Kutub, MS Kīmiyā' 23, fol. 51r5–r6 Search PubMed.
- M. Martelli, The Four Books of Pseudo-Democritus, Leeds, 2013, p. 86 Search PubMed.
- Syriac text in Cambridge University Library, MS Mm. 6.29, fol. 57r20–r57v3 Search PubMed.
- M. Martelli, AION. Annali dell'Università degli Studi di Napoli «L'Orientale», 2014, vol. 36, 17–47, p. 37 Search PubMed.
- Arabic text in Istanbul Archaeology Museum Library, MS 1574, fol. 50r15–50v1 Search PubMed.
- M. Hart, J. Chen, A. Michaelides, A. Sella, M. S. P. Shaffer and C. G. Salzmann, Inorg. Chem., 2019, 58(22), 15216–15224 CrossRef CAS PubMed.
- K. Castro, E. Balladares, O. Jerez, M. Pérez-Tello and Á. Aracena, Metals, 2022, 12, 457 CrossRef CAS.
- C. Corkhill, P. L. Wincott and D. J. Vaughan, Surf. Sci. Spectra, 2006, 13, 100–108 CrossRef CAS.
- F. Montilla, E. Morallón, A. De Battisti, S. Barison, S. Daolio and J. L. Vázquez, J. Phys. Chem. B, 2004, 108, 15976–15981 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mr00035d |
| ‡ The term 3agnesia is a transliteration of the Greek word present in the ancient manuscript. Up to now there is no certain identification of this ingredient |
| § “You must select the realgar that is deep red, [friable], easily triturated and clean, having the colour of cinnabar, and also smelling like sulphur. It has the same properties and it is baked the same way as orpiment” (Dioscorides, De materia medica, V 105, 1st century CE, transl. Beck, 2011, 385). |
| This journal is © The Royal Society of Chemistry 2024 |