Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nanogold-coated stent facilitated non-invasive photothermal ablation of stent thrombosis and restoration of blood flow†
Nitesh
Singh
a,
Paresh P.
Kulkarni
a,
Prashant
Tripathi
b,
Vikas
Agarwal
c and
Debabrata
Dash
 *a
*a
aCentre for Advanced Research on Platelet Signaling and Thrombosis Biology, Department of Biochemistry, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India. E-mail: ddash.biochem@gmail.com; niteshsingh264@gmail.com; pareshkulbmc@gmail.com
bSchool of Physical Sciences, Jawaharlal Nehru University, New Mehrauli Road, New Delhi, Delhi-110067, India. E-mail: pra.jest01@gmail.com
cDepartment of Cardiology, Institute of Medical Sciences, Banaras Hindu University, Varanasi-221005, India. E-mail: vikky25@yahoo.com
First published on 30th January 2024
Abstract
In-stent restenosis (ISR) and stent thrombosis (ST) are the most serious complications of coronary angioplasty and stenting. Although the evolution of drug-eluting stents (DES) has significantly restricted the incidence of ISR, they are associated with an enhanced risk of ST. In the present study, we explore the photothermal ablation of a thrombus using a nano-enhanced thermogenic stent (NETS) as a modality for revascularization following ST. The photothermal activity of NETS, fabricated by coating bare metal stents with gold nanorods generating a thin plasmonic film of gold, was found to be effective in rarefying clots formed within the stent lumen in various in vitro assays including those under conditions mimicking blood flow. NETS implanted in the rat common carotid artery generated heat following exposure to a NIR-laser that led to effective restoration of blood flow within the occluded vessel in a model of ferric chloride-induced thrombosis. Our results present a proof-of-concept for a novel photothermal ablation approach by employing coated stents in the non-invasive management of ST.
Introduction
Coronary artery disease (CAD) is the leading cause of mortality worldwide with an estimated 17.9 million people succumbing to it each year.1,2 Ever since the first successful coronary artery bypass procedure was performed by Rene Favaloro in 1968, it has become a standard of care in patients with significant coronary atherosclerosis. However, due to this being a major surgery and a highly invasive procedure, angioplasty was developed as a relatively non-invasive substitute. Although balloon angioplasty was effective in the management of coronary stenosis due to atherosclerotic plaque, its use was associated with serious complications. Stents were developed in an attempt to provide a temporary scaffold to tide over limitations associated with balloon angioplasty. Stent implantation eliminated acute vessel recoil and chronic constrictive remodelling, thereby reducing restenosis rates. Use of adjuvant dual antiplatelet therapy also reduced the rates of thrombosis to acceptable levels. In sum, current treatment options for CAD include anti-platelet medications, percutaneous coronary intervention (PCI) and coronary artery bypass grafting (CABG). PCI with stenting has become the preferred mode of treatment, especially when the extent of stenosis is more than 50%. Although highly effective, the procedure is plagued with two serious limitations.1–3 First, there could be thrombosis in the peri-stent region (stent thrombosis or ST), which can occur early (less than 30 days), late (30 days to 1 year) or very late (more than 1 year) following the procedure. The common etiological mechanisms of ST include: (i) exposure of blood to the prothrombotic subendothelial matrix, stent struts and/or polymer before they are covered by re-endothelialization, (ii) persistent slow coronary blood flow, (iii) inadequate suppression of platelet activation by antiplatelet drugs and (iv) systemic prothrombotic states.4 The rates of ST vary from as high as 20% without antiplatelet/anticoagulant drugs5 to as low as 2% with aggressive dual anti-platelet therapy.6 Second, there could be re-narrowing of the vessel lumen (in-stent restenosis or ISR) in 10–30% of the patients7–9 characterized by excessive smooth muscle cell proliferation and migration into the intimal layer. This complication of neointimal hyperplasia is addressed with drug-eluting stents (DES), which release anti-proliferative medications like sirolimus and paclitaxel. Although DES remarkably restricted the incidence of ISR to less than 10%, they are ironically associated with a higher risk of late or very late ST than bare metal stents (BMS) due to several reasons.4 First, re-endothelialization is significantly delayed with DES compared to BMS, thus providing a substrate of injured vessel wall for eventual stent thrombosis.10 Second, late stent malapposition as a result of positive vessel wall remodelling away from the stent is more common with DES than BMS.11 Lastly, appearance of new atherosclerotic plaques in the peri-stent area, whose rupture can lead to ST, occurs earlier in DES than BMS.12,13 ST is life-threatening with mortality rates ranging from 11% to 42% and warrants immediate repeat angioplasty or CABG surgery. However, it is important here to emphasize that there has been no development in coronary stent technologies to directly address this complication.Advantages of minimal invasive photothermal therapy have widely been recognized as an effective, safer and sound approach in wound healing,14–17 treatment of bacterial infection,18–21 pain relief,22,23 drug delivery24–27 and cancer management.27–29 For example, an attempt has been made to manage obstructive rectal cancer30 or minimize rat esophageal granulation tissue31 with heat generated from photothermally active stents. By employing both in vitro assays and a murine model of thrombosis in vivo, we have for the first time demonstrated that fibrin clots/thrombi could be destabilized by heat due to dissociation of non-covalently assembled fibrin monomers, and provided a proof-of-concept to harness photothermal therapy as an effective tool towards targeted thrombolysis.32 Our study was succeeded by several follow-up reports supporting the hypothesis.33–35 The concept introduced a safe and smart approach to revascularization, which minimizes the off-target life-threatening complications associated with the currently employed fibrinolytic drug regime. In the present study, we have extended this concept to fabricate novel thermogenic stents employing GNRs, which have the ability to generate heat upon irradiation with a NIR-laser from outside. Thermogenic stents implanted in situ can effectively bring about photothermal lysis of in-stent/peri-stent clots that can be washed off from lesion sites by arterial hydrodynamic shear, leading to partial or complete restoration of blood flow. This being the first study would need further investigation towards clinical validation.
Materials and methods
Alexa Fluor 488 – conjugated fibrinogen was purchased from Invitrogen. Human fibrinogen, thrombin and gold nanorods (GNRs) (axial diameter: 9.0–11.0 nm; longitudinal diameter: 36.9–45.1 nm), methyl methacrylate (MMA), butylmethacrylate (BMA), methylbenzoate, polyethylene glycol 400, benzoyl peroxide and N,N-dimethyl-p-toluidine were procured from Sigma, while methylene blue, benzoyl peroxide and Drabkin's solution were products of Merck, Thomas baker and Linear Chemicals S.L.U., respectively. The rest of the chemicals were either from Sigma or Merck. All reagents were of analytical grade. Type I deionized water (18.2 MΩ cm, Millipore) has been used throughout the experiment.Stents
For in vitro studies bare metal cobalt–chromium alloy stents (Flexinnium, length, 32 mm; diameter, 2 mm; strut thickness, 60 μm) were procured from M/S Sahajanad Medical Technology, India. For implantation in the rat carotid artery stainless steel stents (length, 2.5 mm; diameter, 635 μm; strut thickness, 70 μm) were purchased from M/S Olexander R&D Inc., USA.Thin-layer coating of NIR-active nanomaterials on the stent surface
Thin layer coating of gold on the bare metal stent surface by employing photothermally active gold nanorods was achieved in three steps: (a) activation step: prior to coating, the stent surface was activated by treatment with HCl (0.1 M) solution for 1–2 h. This was followed by washing with type I deionized water and short bath sonication (Branson 2510 Ultrasonic Bath, 40 Hz) to remove excess acid. The washing was repeated 3 times and the stent was dried in a vacuum oven (Anil Scientico) at 80 °C for 20 min. (b) Coating step: next, assembly of the nanomaterial on the stent surface was accomplished by bath sonicating the stent in the presence of gold nanorods (30 μg ml−1) for 10 min each time with 10 min intervals for up to 3 h, which was followed by stirring in GNR solution with an Eppendorf Thermomixer (model 5350) for 4 h. (c) Annealing step: finally, the thin plasmonic film of gold on the stent was achieved by placing the stent in a vacuum oven at 350 °C under an inert (nitrogen gas) environment for 3 h. The coating and annealing steps were repeated for 4 cycles to achieve a dense layer of gold coating on the stent surface.Characterization of nano-enhanced thermogenic stents (NETS)
Surface morphologies of stents following coating were characterized by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS or EDX) coupled with SEM (M/S FEI quanta 200). The bare metal and coated stents were mounted on carbon tape adhered on a sample holder (stub) and SEM and EDX images were captured.36 The stent underwent additional characterization through a comprehensive analytical approach utilizing Raman spectroscopy and Fourier-transform infrared spectroscopy (FTIR) in attenuated total reflection (ATR) mode (detailed information in the ESI section†).The photothermal efficiency of NETS was evaluated by irradiating the stents with a 3 W solid-state continuous diode laser (808 nm) (M/S Changchun New Industries, China) at 1.05 W cm−2 and the rise in temperature was recorded with an infra-red thermal camera (M/S FLIR, USA) (ESI Fig. 1†). In order to investigate the stability of the coating on the stent surface when exposed to hydrodynamic shear of perpetual blood flow as may occur within the coronary artery, a buffer was allowed to flow over the coated stents employing a high-resolution peristaltic pump (Bio-Rad). The superfusion of the buffer upon the stent was maintained consistently for 6 to 8 h each day for 30 days. The stability of the coating was then evaluated from the uniformity in heat generated on different days when the stent was irradiated with a laser.
Characterization of thrombolysis elicited in and around NIR laser-irradiated NETS in vitro
Clot formation (fibrin polymerization) was induced around the thermogenic stent by addition of 2–5 mM CaCl2 and 1 U ml−1 thrombin to purified human fibrinogen as described.32 Whenever required, a fluorescent fibrin clot was generated by adding 45 μl purified fibrinogen (1 mg ml−1) and 5 μl Alexa Fluor 488 – conjugated fibrinogen. Next, the stent was irradiated with an NIR laser and the extent of clot lysis due to the heat generated from the fabricated stents was determined by various assays as already reported.32 (a) Fluorescence-based assay: NETS was irradiated with a NIR laser for 30 min and the supernatant was collected. The presence of fluorescent fibrin in the supernatant was analysed with a fluorescence multi-mode microplate reader (BioTek model Synergy H1) (excitation, 488 nm; emission, 519 nm) as a measure of fibrin released due to thrombolysis. (b) Drabkin's assay: a clot containing trapped RBCs was generated around the stent by mixing 2.5 μl RBCs and 47.5 μl fibrinogen solution followed by thrombin and CaCl2 as mentioned above. Thermal disruption of the thrombus released RBCs, which was quantified using Drabkin's reagent. The supernatant (containing released RBCs) was added to an equal volume of Drabkin's solution (K2HPO4, 50 mM; KCN, 38 mM; [K3Fe(CN)6], 30 mM; surfactant, 2–5% w/v), and kept at RT for 20 min. Absorbance was recorded at 540 nm as a measure of the cyanoderivative of hemoglobin. (c) Methylene blue assay: ablation of the clot was determined from the extent of release of methylene blue from fibrin mesh around the stent. The clot was induced to form as described above where 0–1 mg ml−1 dye replaced the RBCs. Following laser irradiation of the NETS the supernatant was collected, and the extent of methylene blue released was recorded from the absorbance at 540 nm. (d) Scanning electron microscopy (SEM): the change in surface morphology of the fibrin clot and thrombus-depleted areas, as well as diameter of fibrin fibres was recorded using an analytical SEM37,38 as mentioned in the ESI section.† (e) Confocal laser scanning microscopy (CLSM): the fiber density and thrombus-depleted areas in the fluorescently labelled clots were analysed employing confocal microscopy37,38 (details in the ESI section†).Fluorescence recovery after photobleaching (FRAP) analysis
The altered molecular dynamism within the structure of the in-stent/peri-stent thrombus following exposure to the NIR laser, reflective of fibrin depolymerization/repolymerization kinetics, was evaluated employing the FRAP analysis tool. A fluorescent clot was generated around the stent as stated above. The clot-moulded stent was carefully transferred to a fabricated microscopic slide (bordered with adherent tape as a spacer; depth: 450 μm) as already described.32,39 The in-stent clot was exposed to the NIR laser (808 nm) in a humid chamber for 15 min. FRAP was performed on a confocal microscope (Zeiss, model LSM 700) equipped with a plan-apochromat 1.4 NA oil immersion objective (63×), 488 nm argon ion laser, and LSM acquisition software. The detector gain and offset were set before collecting FRAP images. The detector gain was set to a level such that very few or no pixels were saturated. Selected areas on the fluorescent thrombus were photobleached using the 488 nm laser at 100% output at a rate of 20 ms per pixel. Five pre-bleached images were captured as a reference for the steady-state distribution of fluorescent molecules. Images of fluorescence recovery were collected at 2% excitation laser power using Zen Imaging software and analysed using ImageJ software (National Institutes of Health). Data were analysed with GraphPad Prism software.NETS-mediated thrombolysis under arterial shear
In order to evaluate stent thrombolysis against arterial fluid shear resembling the physiological milieu, a fluorescent thrombus was generated around the stent as described above. The stent carrying the thrombus was placed within a fabricated silicon tube, and fluid was allowed to perfuse over it at arteriolar hydrodynamic shear (1500 s−1) precisely regulated with a high-resolution peristaltic pump (Bio-Rad) (ESI Fig. 2†).32 The stent was exposed to the NIR laser for 30 min and the extent of thrombolysis was quantified from the release of the fluorescent fibrin in the fluid of the reservoir as described above.In-stent/peri-stent thrombolysis by the heat generated from a NIR laser-irradiated thermogenic stent grafted in the rat common carotid artery in situ
After polymerization, plastic blocks were carefully removed from the glass vials and were trimmed to the desired size for sectioning. The 3–5 mm thick sections were cut using a rotary microtome (Leica RM2255) by keeping the block and blade moist with 30–40% methanol. Sections were transferred to slides coated with poly-L-lysine and carefully stretched or flattened using a rubber roller. Next, the slides were covered with polyethylene and clamped to a slide press, which was followed by incubation at 42 °C for 2 days. The de-plasticization of the sections was performed by employing two changes of dichloromethane and acetone for 20 and 10 min, respectively. Finally, sections were subjected to hematoxylin–eosin staining and analyzed under a light microscope (Nikon, model Eclipse Ti-E) at 4× magnification to evaluate the clot lysis efficacy of implanted NETS following NIR laser irradiation.
Statistical methods
Data are presented as mean ± SEM of at least 3 independent experiments. Two-tailed Student's t-tests were performed using GraphPad Prism software to evaluate the significance in difference between groups. Values were considered significant when p < 0.05.Ethical statement
All animal procedures were carried out in strict adherence to the Guidelines for Care and Use of Laboratory Animals of Banaras Hindu University. The experimental protocols were approved by the Animal Ethics Committee of Banaras Hindu University (IAEC-3258).Results
Characterization of coated thermogenic stents
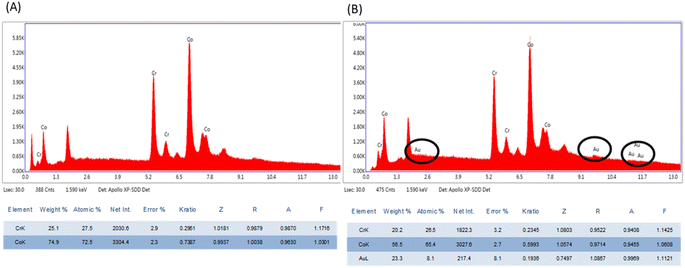
The thin plasmonic film of gold on the stent surface achieved by employing GNRs was characterized by SEM and EDX. The SEM images signified the rough surface associated with coated stents as compared to bare metal stents (Fig. 1), which was further examined by EDX analysis (Fig. 2). Chemical microanalysis of the coated stent confirmed the presence of an adequate quantity of the thin plasmonic film of gold on the thermogenic stent surface (Fig. 2). Raman spectra were used to examine the coating efficacy on the stent by assessing the structural and vibrational characteristics of gold. The image captured using a 50× magnification objective lens (Zeiss) of a Raman instrument showed a dense layer of coating on the stent surface (ESI Fig. 3A†). The observed prominent peak in the Raman spectrum clearly indicated the presence of gold.51 FTIR spectroscopy was performed to examine the coating on the stent surface. The spectrum of the coated stent exhibited characteristic peaks at ∼2930, 2850 and 1430 cm−1 that correspond to the presence of a thin plasmonic film of gold52,53 (ESI Fig. 3B†). It may be noted here that annealing at temperatures below 100 °C also yielded satisfactory results.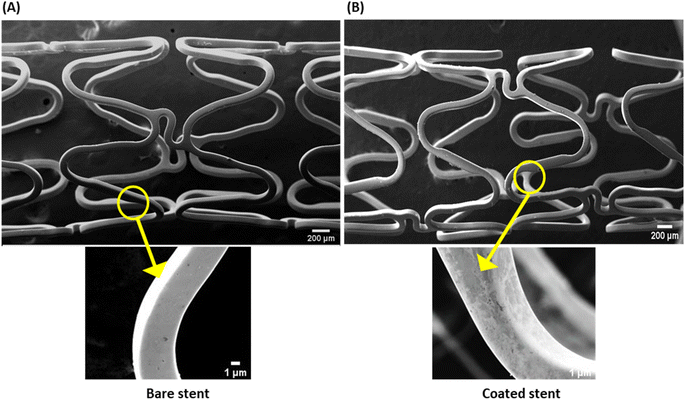 | ||
| Fig. 1 Scanning electron micrographs of surface morphologies of (A) bare (uncoated) and (B) coated stents. Magnifications: upper panels, 25× (scale, 200 μm); lower panels, 400× (scale, 1 μm). | ||
To investigate the stability of the glossy coating on the stent surface against hydrodynamic shear of perpetual intramural blood flow, the buffer was allowed to perfuse over the coated stents in vitro at the arterial shear rate (1500 s−1), which closely simulated fluidics in vivo.32 The buffer was allowed to flow over the stent for 6 to 8 h continuously each day for 30 days. The stability of the glossy coating was then verified from the uniformity in heat generated upon irradiation with the NIR laser on different days up to the 30th day. Temperature was recorded to be 60 °C, 61 °C, 58 °C and 59 °C, respectively, on days 0, 10, 20 and 30 (ESI Fig. 4†), which underscored the lack of erosion of the gold coating by hydrodynamic shear over time.
Characterization of thrombolysis induced by the heat generated from NIR laser-irradiated thermogenic stents in vitro
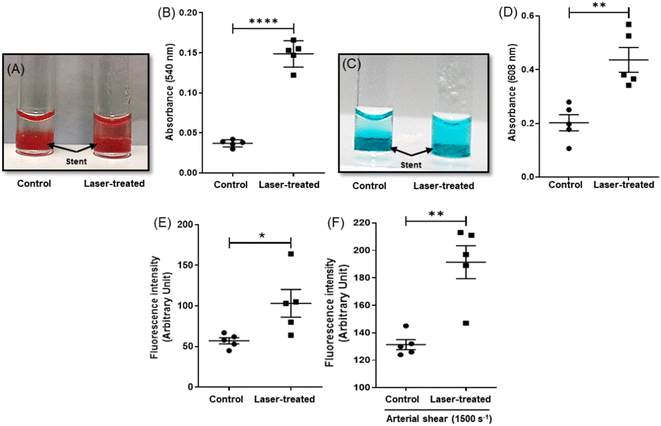
In order to study the efficiency of NETS in inducing photothermal ablation of clots, a thrombus was allowed to form around the stent placed within a glass cuvette followed by irradiation with the NIR laser for 30 min, which raised the temperature up to 50 °C. The extent of thrombolysis was evaluated by three standard assays as described earlier.32 Photothermal fibrinolysis was found to be 57% higher in the fluorescence-based assay whereas the rise was 73% each in Drabkin's and methylene blue assays, respectively (Fig. 3A–E). In order to evaluate stent thrombolysis under arterial hydrodynamic shear resembling the physiological milieu, the fluid was allowed to perfuse over the stent carrying a fluorescent thrombus at a shear rate of 1500 s−1 (ESI Fig. 2†). The extent of thrombolysis as quantified from the release of Alexa 488-labeled fibrin was found to be 35% (Fig. 3F), which thus confirmed photothermal ablation of stent thrombosis.The heat-induced alteration in fibrin clot structural morphology following 30 min NIR laser irradiation was evaluated using orthogonal tools like SEM and CLSM. The SEM images were consistent with a significant 12-fold increase in thrombus-depleted areas (ESI Fig. 5A–C†) associated with a drop (by 5%) in fibrin diameter (ESI Fig. 5D and E†). In agreement, confocal images too demonstrated a nearly 5 times expansion in areas of thrombus depletion examined at different depths in Z-stacking mode (ESI Fig. 6A–C†) and 56% reduction in fiber density (ESI Fig. 6D†) in laser-irradiated samples.
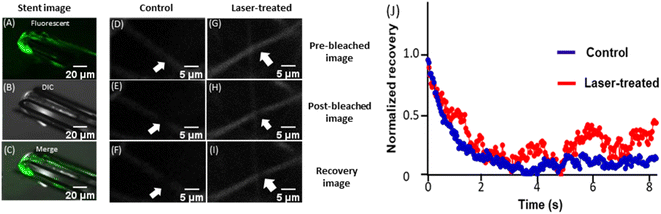
Thermal destabilization of in-stent/peri-stent thrombi following 15 min NIR-laser exposure was further validated employing FRAP analysis. FRAP characterizes the dynamism of fibrin monomers/oligomers within the clot by quantifying fibrin mobility and turnover kinetics.32 The in-stent clot was focused on and observed under a microscope (Fig. 4A–C). A narrow region within the fluorescent thrombus was photobleached using a 488 nm laser (at 100% power) and fluorescence recovery in that region was recorded over time at 2% excitation laser power in both control (Fig. 4D–F), as well as NIR laser-irradiated (Fig. 4G–I) thrombi. The speed of fluorescence recovery in thermally loosened fibrin strands was reflective of the extent of fibrin mobility within destabilized thrombi, which amounted to 13.4% mobile fibrin fractions in NIR laser-irradiated stents as compared to 5.3% in non-irradiated counterparts (ESI Fig. 7†). Thus, results from this study underscore enhanced fibrin dynamism within the in-stent thrombi following photothermal heat generation that could lead to thrombus rarefication.
Photothermal lysis of in-stent thrombi and partial restoration of blood flow upon NIR laser irradiation of implanted NETS
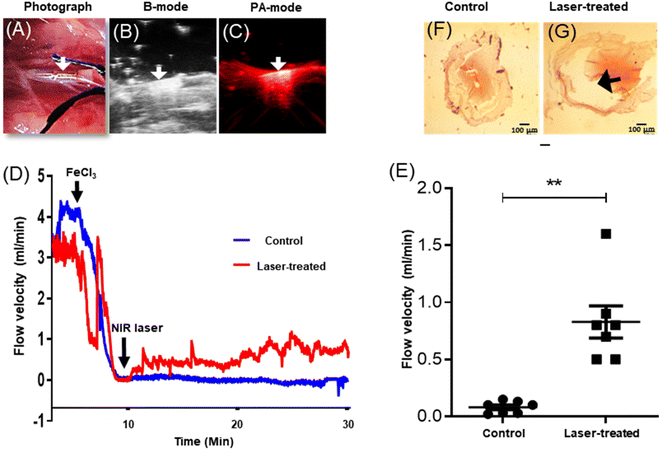
Rats medicated with oral aspirin (10 mg kg−1 d−1) for 5 days42 were anesthetised. The coated thermogenic stents (length, 2.5 mm; diameter, 635 μm; strut thickness, 70 μm) were carefully guided into and deployed in the murine common carotid artery (Fig. 5 and 6A), as described. The anatomical and optoacoustic imaging of the implanted stent was carried out in B (ultrasound)-mode and PA (photoacoustic)-mode by employing a photoacoustic imaging platform (Fig. 6B and C). The thrombus was induced locally by topical application of 10% FeCl3, which was followed by irradiation with a 3 W solid-state continuous diode laser (808 nm) for 15 min. Temperature around the stent was found to be elevated up to 47 °C following laser exposure as captured by a thermal camera. Blood flow was assessed by placing a Doppler flow probe on the exposed artery, the signal from which was collected through a transonic flow probe and further analysed. Restriction in blood flow due to the growing thrombus was found to be 98%, which was partially restored (by 30%) upon laser irradiation (Fig. 6D and E), underscoring the applicability of the novel NETS as an essential tool against stent-thrombosis. The hematoxylin–eosin-stained sections of stented carotid segments revealed a significant reduction in size of the in-stent thrombus in laser-treated rats as compared to control animals (Fig. 6F and G; ESI Fig. 8†), thus validating the therapeutic efficacy of NETS.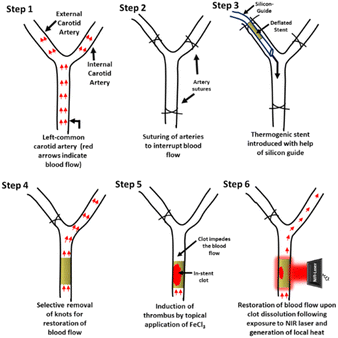 | ||
| Fig. 5 Scheme for grafting a thermogenic stent in the common carotid artery of a rat, induction of a thrombus and photothermal thrombolysis. | ||
Discussion
In this report we demonstrate the potential of ‘thermogenic’ stents (NETS) in bringing about relief from ‘stent thrombosis’, a life-threatening complication that warrants urgent interventions like repeat angioplasty and/or CABG surgery. Notably, none of the existing stent technologies permits lysis of the in-stent thrombus, thus underlining the need for novel approaches to counter the threat of scaffold thrombosis. Subjects carrying coated NETS can be irradiated frontally from an external NIR laser source upon diagnosis of stent thrombosis. The heat generated from the deployed stent would result in ablation/rarefication of the thrombus in and around the implant leading to restoration of blood flow that can be monitored with color Doppler. Although we have observed ∼30% restoration in blood flow following 15 min irradiation with the laser, the duration of laser exposure can be expanded to achieve greater relief from occlusion. The occluded stent can thus become functional again in the absence of any painful invasive procedure or expensive setup and the patient can be discharged early. A coating of a NIR-active material on the stent surface was found to be stable against fluid shear and can coexist with the drug-eluting polymer.Photothermal therapy (PTT) is a well-established but burgeoning field of medical research that has been widely employed in wound healing,14–17 treatment of bacterial infection,18–21 pain relief,22,23 drug delivery24–27 and for selective thermal ablation of malignant tissues54–58 at temperatures above 40 °C, which ensues protein denaturation and disruption of target cell membranes. Subsequent studies from our group and others established the anti-thrombotic attributes of PTT as it leads to disintegration of fibrin-rich clots by segregation of component fibrin strands, which are then washed off from the lesion site under arterial hydrodynamic shear.32–35 These studies opened newer areas of application for thermal therapy against mural thrombosis in addition to its well reported application against tumors.
The effect of temperature on tissues surrounding the deployed stent is the subject of future investigation. Temperature at the ‘heat focus’ area (maximum ∼47 °C) is significantly quenched by the ongoing blood flow (2 mm s−1), thus restricting the temperature in the surrounding blood from exceeding ∼43 °C.59 Temperatures in the intimal layer and sub-endothelium in direct contact with the stent drop nonlinearly with depth that is further compensated by the blood flow.59 Considering the thickness of intima to be ∼600 μm,60 conduction of heat to cardiac tissues is tolerated without seriously compromising cell viability and primarily localized to the intima.
Although ours is the first study directed at photothermal management of stent thrombosis, hyperthermia has earlier been employed for control of in-stent restenosis as mild heat (between 45 and 50 °C) effectively destroys intimal cells.61–63 Hyperthermia, too, has successfully been used for controlled site-specific drug delivery around the implanted magnetizable stent when applied under a uniform magnetic field.64 There have been several approaches in the past to expose the stent in situ to local heat though each procedure has its share of limitations. These include invasive procedures like insertion of a special thermal catheter into the deployed stent,65 or guiding the NIR beam through the optical fiber to endovascular locations,59,66,67 as well as non-invasive methods like induction (eddy-current) heating of stents using electromagnetic fields.62,68,69 Non-invasive attempts have been made like induction (eddy-current) heating of stents using electromagnetic fields,62 or forming a radiofrequency resonant circuit.70 PTT and photothermal effect-induced drug release have also been explored previously as a modality to prevent in-stent restenosis by employing gold nanoparticle-coated multifunctional stents.59
The penetration depth of the NIR laser at 808 nm is about 2 cm,36,69 which can be further enhanced by switching to longer wavelength NIR (1000–1350 nm; bio window II),57,71 thus allowing non-invasive photothermal ablation of stent thrombosis when irradiated from an external source. Alternatively, the occluded stent can be irradiated with an optical fiber-guided NIR laser72,73 though guiding the NIR beam to endovascular locations for PTT still remains challenging. In conclusion, this study for the first time presents a proof-of-concept in favour of deployment of thermogenic coronary scaffolds as a guard against stent thrombosis and requires further investigation towards clinical validation.
Data availability
All relevant data that support the findings of this study are provided within the paper. Raw data incorporated in Microsoft excel and/or GraphPad Prism files are available on request from the corresponding authors.Author contributions
D. D. supervised the entire work; N. S., P. P. K. and P. T. performed various experiments; D. D., V. A., N. S. and P. P. K. analyzed the results and wrote the manuscript.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This research was supported by a J. C. Bose National Fellowship (JBR/2023/000004), and grants received by D. Dash from the Science and Engineering Research Board (SERB) (CRG/2021/000305), Government of India, the Indian Council of Medical Research (ICMR) under CAR/Task Force (71/4/2018-BMS/CAR), Institute of Eminence (IoE) Faculty Incentive Grant (OH-31-IOE/6031), Banaras Hindu University, and the Department of Science & Technology (DST) (DST/NM/NT/2018/134), Ministry of Science & Technology, Government of India. N. S. is a recipient of Scientist-C position from ICMR under CAR. We gratefully acknowledge useful discussions with Prof. S. K. Dwivedi, Department of Cardiology, King George's Medical University, Lucknow, for this study. The authors dedicate this work to late Prof. O. N. Srivastava, Department of Physics, Institute of Science, Banaras Hindu University, who has pioneered nanotechnology research in the university.References
- WHO, Cardiovascular diseases, https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1).
- CDC, Heart Disease Deaths Vary by Sex, Race, and Ethnicity, https://www.cdc.gov/heartdisease/facts.htm#:%7E:text=HeartDiseaseintheUnitedStates%26text=Onepersondiesevery2034,UnitedStatesfromcardiovasculardisease.%26text=About697%2C000peopleinthe,1inevery5deaths.%26text=HeartdiseasecosttheUnited,yearfrom2017to2018.
- T. Simard, B. Hibbert, F. D. Ramirez, M. Froeschl, Y. X. Chen and E. R. O'Brien, Can. J. Cardiol., 2014, 30, 35–45 CrossRef PubMed
.
- B. E. Claessen, J. P. Henriques, F. A. Jaffer, R. Mehran, J. J. Piek and G. D. Dangas, JACC Cardiovasc. Interv., 2014, 7, 1081–1092 CrossRef PubMed
.
- P. W. Serruys, B. H. Strauss, K. J. Beatt, M. E. Bertrand, J. Puel, A. F. Rickards, B. Meier, J. J. Goy, P. Vogt and L. Kappenberger,
et al.
, N. Engl. J. Med., 1991, 324, 13–17 CrossRef CAS PubMed
.
- A. Schomig, F. J. Neumann, A. Kastrati, H. Schuhlen, R. Blasini, M. Hadamitzky, H. Walter, E. M. Zitzmann-Roth, G. Richardt, E. Alt, C. Schmitt and K. Ulm, N. Engl. J. Med., 1996, 334, 1084–1089 CrossRef CAS PubMed
.
- D. L. Fischman, M. B. Leon, D. S. Baim, R. A. Schatz, M. P. Savage, I. Penn, K. Detre, L. Veltri, D. Ricci and M. Nobuyoshi,
et al.
, N. Engl. J. Med., 1994, 331, 496–501 CrossRef CAS PubMed
.
- M. Li, J. Hou, X. Gu, R. Weng, Z. Zhong and S. Liu, Eur. J. Med.Res., 2022, 27, 12 CrossRef CAS PubMed
.
- I. D. Moussa, D. Mohananey, J. Saucedo, G. W. Stone, R. W. Yeh, K. F. Kennedy, R. Waksman, P. Teirstein, J. W. Moses and C. Simonton, J. Am. Coll. Cardiol., 2020, 76, 1521–1531 CrossRef PubMed
.
- M. Joner, A. V. Finn, A. Farb, E. K. Mont, F. D. Kolodgie, E. Ladich, R. Kutys, K. Skorija, H. K. Gold and R. Virmani, J. Am. Coll. Cardiol., 2006, 48, 193–202 CrossRef PubMed
.
- S. Cook, P. Wenaweser, M. Togni, M. Billinger, C. Morger, C. Seiler, R. Vogel, O. Hess, B. Meier and S. Windecker, Circulation, 2007, 115, 2426–2434 CrossRef CAS PubMed
.
- K. Hasegawa, H. Tamai, E. Kyo, K. Kosuga, S. Ikeguchi, T. Hata, M. Okada, S. Fujita, T. Tsuji, S. Takeda, R. Fukuhara, Y. Kikuta, S. Motohara, K. Ono and E. Takeuchi, Catheter. Cardiovasc. Interv., 2006, 68, 554–558 CrossRef
.
- G. Nakazawa, M. Vorpahl, A. V. Finn, J. Narula and R. Virmani, JACC Cardiovasc. Imaging, 2009, 2, 625–628 CrossRef
.
- H. Ma and M. Xue, J. Mater. Chem. A, 2021, 9, 17569–17591 RSC
.
- L. He, D. Di, X. Chu, X. Liu, Z. Wang, J. Lu, S. Wang and Q. Zhao, J. Controlled Release, 2023, 363, 180–200 CrossRef CAS
.
- Y. Li, M. Han, Y. Cai, B. Jiang, Y. Zhang, B. Yuan, F. Zhou and C. Cao, Biomater. Sci., 2022, 10, 1068–1082 RSC
.
- B. Tao, C. Lin, Y. Deng, Z. Yuan, X. Shen, M. Chen, Y. He, Z. Peng, Y. Hu and K. Cai, J. Mater. Chem. B, 2019, 7, 2534–2548 RSC
.
- G. Wei, G. Yang, Y. Wang, H. Jiang, Y. Fu, G. Yue and R. Ju, Theranostics, 2020, 10, 12241–12262 CrossRef CAS
.
- H. Wei, L. Yang, C. Pang, L. Lian and L. Hong, Biomater. Sci., 2023, 11, 5634–5640 RSC
.
- Z. Yan, D. Wang and Y. Gao, Front. Bioeng. Biotechnol., 2023, 11, 1192960 CrossRef PubMed
.
- J. Shi, J. Li, Y. Wang, J. Cheng and C. Y. Zhang, J. Mater. Chem. B, 2020, 8, 5793–5807 RSC
.
- M. Salimi, S. Mosca, B. Gardner, F. Palombo, P. Matousek and N. Stone, Nanomaterials, 2022, 12, 922 CrossRef CAS PubMed
.
- A. R. Rastinehad, H. Anastos, E. Wajswol, J. S. Winoker, J. P. Sfakianos, S. K. Doppalapudi, M. R. Carrick, C. J. Knauer, B. Taouli, S. C. Lewis, A. K. Tewari, J. A. Schwartz, S. E. Canfield, A. K. George, J. L. West and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 18590–18596 CrossRef CAS
.
- R. Li, Q. Zheng, Q. Deng, Y. Wang, H. Yang, J. Shen, Y. Liu and J. Zhou, Mol. Pharm., 2022, 19, 1449–1457 CrossRef CAS
.
- P. Manivasagan, S. W. Jun, V. T. Nguyen, N. T. P. Truong, G. Hoang, S. Mondal, M. Santha Moorthy, H. Kim, T. T. Vy Phan, V. H. M. Doan, C.-S. Kim and J. Oh, J. Mater. Chem. B, 2019, 7, 3811–3825 RSC
.
- J. Wang, Y. Wu, K. Liu, W. Yang, W. Zeng, X. Gao, S. Liu and B. Zhang, APL Bioeng., 2023, 7, 016115 CrossRef CAS PubMed
.
- X. Wu, J. Liu, L. Yang and F. Wang, Colloids Surf. B: Biointerfaces., 2019, 175, 239–247 CrossRef CAS PubMed
.
- T. J. Vogl, R. Straub, K. Eichler, O. Sollner and M. G. Mack, Radiology, 2004, 230, 450–458 CrossRef PubMed
.
- V. Arienti, S. Pretolani, C. M. Pacella, F. Magnolfi, B. Caspani, G. Francica, A. S. Megna, R. Regine, M. Sponza, E. Antico and F. M. Di Lascio, Radiology, 2008, 246, 947–955 CrossRef PubMed
.
- H. T. Hu, J. H. Park, Z. Wang, N. Bakheet, S. J. Xu, E. J. Lee, D. H. Kim, S. H. Kim, H. Y. Song, J. Y. Jeon and S. Chang, ACS Biomater. Sci. Eng., 2021, 7, 5890–5898 CrossRef CAS
.
- Y. C. Cho, J. M. Kang, W. Park, D. H. Kim, J. H. Shin, D. H. Kim and J. H. Park, Sci. Rep., 2021, 11, 10558 CrossRef CAS PubMed
.
- N. Singh, A. Varma, A. Verma, B. N. Maurya and D. Dash, Nano Res., 2016, 9, 2327–2337 CrossRef CAS
.
- T. Burnouf, C. H. Chen, S. J. Tan, C. L. Tseng, K. Y. Lu, L. H. Chang, B. Nyambat, S. C. Huang, P. R. Jheng, R. N. Aditya, F. L. Mi and E. Y. Chuang, Acta Biomater., 2019, 96, 468–479 CrossRef CAS
.
- C. Fang, Z. Zhong, T. Zhang, S. Jia, X. Ding, W. Zhou and X. Wang, J. Mater. Chem. B, 2020, 8, 10854–10866 RSC
.
- T. Y. Lu, C. Y. Chiang, Y. J. Fan, P. R. Jheng, E. D. Quinones, K. T. Liu, S. H. Kuo, H. Y. Hsieh, C. L. Tseng, J. Yu and E. Y. Chuang, ACS Appl. Mater. Interfaces, 2021, 13, 10287–10300 CrossRef CAS
.
- Y. Koo, T. Tiasha, V. N. Shanov and Y. Yun, Sci. Rep., 2017, 7, 1173 CrossRef PubMed
.
- V. G. Matveeva, E. A. Senokosova, V. V. Sevostianova, M. Y. Khanova, T. V. Glushkova, T. N. Akentieva, L. V. Antonova and L. S. Barbarash, Biomedicines, 2022, 10, 789 CrossRef CAS PubMed
.
- I. Varju, E. Toth, A. Z. Farkas, V. J. Farkas, E. Komorowicz, T. Feller, B. Kiss, M. Z. Kellermayer, L. Szabo, A. Wacha, A. Bota, C. Longstaff and K. Kolev, J. Thromb. Haemostasis, 2022, 20, 2862–2872 CrossRef CAS
.
- K. C. Gersh, S. Zaitsev, D. B. Cines, V. Muzykantov and J. W. Weisel, Blood, 2011, 117, 4964–4967 CrossRef CAS
.
- S. Simsekyilmaz, F. Schreiber, S. Weinandy, F. Gremse, T. T. Sonmez and E. A. Liehn, J. Visualized Exp., 2013, e50233 Search PubMed
.
- B. Polyak, M. Medved, N. Lazareva, L. Steele, T. Patel, A. Rai, M. Y. Rotenberg, K. Wasko, A. R. Kohut, R. Sensenig and G. Friedman, ACS Nano, 2016, 10, 9559–9569 CrossRef CAS PubMed
.
- M. K. Nayak, A. Dash, N. Singh and D. Dash, PLoS One, 2014, 9, e105049 CrossRef
.
- X. Bai, X. Gong, W. Hau, R. Lin, J. Zheng, C. Liu, C. Zeng, X. Zou, H. Zheng and L. Song, PLoS One, 2014, 9, e92463 CrossRef
.
- T. Bonnard and C. E. Hagemeyer, J. Visualized Exp., 2015, e52838 Search PubMed
.
- W. Li, M. Nieman and A. Sen Gupta, J. Visualized Exp., 2016, 115, 54479 Search PubMed
.
- Y. Shim, I. Kwon, Y. Park, H. W. Lee, J. Kim, Y. D. Kim, H. S. Nam, S. Park and J. H. Heo, Yonsei Med. J., 2021, 62, 1032–1041 CrossRef CAS
.
- R. J. Westrick, M. E. Winn and D. T. Eitzman, Arterioscler., Thromb., Vasc. Biol., 2007, 27, 2079–2093 CrossRef CAS PubMed
.
- W. Li, T. M. McIntyre and R. L. Silverstein, Redox Biol., 2013, 1, 50–55 CrossRef CAS PubMed
.
- T. A. White, T. Johnson, N. Zarzhevsky, C. Tom, S. Delacroix, E. W. Holroyd, S. A. Maroney, R. Singh, S. Pan, W. P. Fay, J. van Deursen, A. E. Mast, G. S. Sandhu and R. D. Simari, Blood, 2010, 116, 1787–1794 CrossRef CAS PubMed
.
- R. G. Erben, J. Histochem. Cytochem., 1997, 45, 307–313 CrossRef CAS PubMed
.
- C.-H. Lin, P.-H. Wang, T.-H. Wang, L.-J. Yang and T.-C. Wen, Nanoscale, 2020, 12, 1075–1082 RSC
.
- G. Su, C. Yang and J.-J. Zhu, Langmuir, 2015, 31, 817–823 CrossRef CAS PubMed
.
- H. R. de Barros, L. Piovan, G. L. Sassaki, D. de Araujo Sabry, N. Mattoso, Á. M. Nunes, M. R. Meneghetti and I. C. Riegel-Vidotti, Carbohydr. Polym., 2016, 152, 479–486 CrossRef CAS PubMed
.
- E. B. Dickerson, E. C. Dreaden, X. Huang, I. H. El-Sayed, H. Chu, S. Pushpanketh, J. F. McDonald and M. A. El-Sayed, Cancer Lett., 2008, 269, 57–66 CrossRef CAS
.
- Z. M. Markovic, L. M. Harhaji-Trajkovic, B. M. Todorovic-Markovic, D. P. Kepic, K. M. Arsikin, S. P. Jovanovic, A. C. Pantovic, M. D. Dramicanin and V. S. Trajkovic, Biomaterials, 2011, 32, 1121–1129 CrossRef CAS
.
- A. Espinosa, R. Di Corato, J. Kolosnjaj-Tabi, P. Flaud, T. Pellegrino and C. Wilhelm, ACS Nano, 2016, 10, 2436–2446 CrossRef CAS PubMed
.
- X. Han, J. Huang, X. Jing, D. Yang, H. Lin, Z. Wang, P. Li and Y. Chen, ACS Nano, 2018, 12, 4545–4555 CrossRef CAS PubMed
.
- T. I. Liu, T. Y. Lu, Y. C. Yang, S. H. Chang, H. H. Chen, I. L. Lu, A. Sabu and H. C. Chiu, Biomaterials, 2020, 257, 120229 CrossRef CAS PubMed
.
- D. Son, J. Lee, D. J. Lee, R. Ghaffari, S. Yun, S. J. Kim, J. E. Lee, H. R. Cho, S. Yoon, S. Yang, S. Lee, S. Qiao, D. Ling, S. Shin, J. K. Song, J. Kim, T. Kim, H. Lee, J. Kim, M. Soh, N. Lee, C. S. Hwang, S. Nam, N. Lu, T. Hyeon, S. H. Choi and D. H. Kim, ACS Nano, 2015, 9, 5937–5946 CrossRef CAS
.
- E. de Groot, G. K. Hovingh, A. Wiegman, P. Duriez, A. J. Smit, J. C. Fruchart and J. J. Kastelein, Circulation, 2004, 109, III33–38 CrossRef PubMed
.
- K. Orihara, S. Biro, S. Hamasaki, H. Eto, M. Miyata, Y. Ikeda and C. Tei, J. Mol. Cell. Cardiol., 2002, 34, 1205–1215 CrossRef CAS PubMed
.
- L. Li, R. Wang, H. H. Shi, L. Xie, J. D. Li, W. C. Kong, J. T. Tang, D. N. Ke and L. Y. Zhao, Exp. Ther. Med., 2013, 6, 347–354 CrossRef PubMed
.
- Y. Yi, J. Chen, M. Selvaraj, Y. Hsiang and K. Takahata, IEEE Trans. Biomed. Eng., 2020, 67, 1097–1104 Search PubMed
.
- M. Chorny, I. Fishbein, B. B. Yellen, I. S. Alferiev, M. Bakay, S. Ganta, R. Adamo, M. Amiji, G. Friedman and R. J. Levy, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 8346–8351 CrossRef CAS PubMed
.
- C. Brasselet, E. Durand, F. Addad, F. Vitry, G. Chatellier, C. Demerens, M. Lemitre, R. Garnotel, D. Urbain, P. Bruneval and A. Lafont, Eur. Heart J., 2008, 29, 402–412 CrossRef
.
- H. Yoo, J. W. Kim, M. Shishkov, E. Namati, T. Morse, R. Shubochkin, J. R. McCarthy, V. Ntziachristos, B. E. Bouma, F. A. Jaffer and G. J. Tearney, Nat. Med., 2011, 17, 1680–1684 CrossRef CAS
.
- D. Yeager, Y. S. Chen, S. Litovsky and S. Emelianov, Theranostics, 2013, 4, 36–46 CrossRef PubMed
.
- A. B. Levitt, K. Robinson, N. A. Chronos and W. Daum, Cardiovasc. Radiat. Med., 2003, 4, 133–138 CrossRef PubMed
.
- M. G. Floren, R. W. Gunther and T. Schmitz-Rode, Invest. Radiol., 2004, 39, 264–270 CrossRef PubMed
.
- Y. Yi, J. Chen, Y. Hsiang and K. Takahata, Adv. Healthcare Mater., 2019, 8, e1900708 CrossRef PubMed
.
- X. Deng, S. Liang, X. Cai, S. Huang, Z. Cheng, Y. Shi, M. Pang, P. Ma and J. Lin, Nano Lett., 2019, 19, 6772–6780 CrossRef CAS PubMed
.
- J. Stallard, Light-Activated Drug Destroys Small Prostate Cancers with Fewer Side Effects, https://www.mskcc.org/news/light-activated-drug-destroys-small-prostate-cancers-fewer-side-effects).
- M. D. Stringasci, T. C. Fortunato, L. T. Moriyama, J. D. V. Filho, V. S. Bagnato and C. Kurachi, Lasers Med. Sci., 2017, 32, 1009–1016 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na00751k |
| This journal is © The Royal Society of Chemistry 2024 |