Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Thermal properties of nanofluids using hydrophilic and hydrophobic LiYF4:Yb/Er upconverting nanoparticles†
João M.
Gonçalves
 ab,
Ana R. N.
Bastos
ab,
Ana R. N.
Bastos
 a,
Sidney J. L.
Ribeiro
a,
Sidney J. L.
Ribeiro
 c,
L. D.
Carlos
c,
L. D.
Carlos
 a,
Ricardo L.
Longo
a,
Ricardo L.
Longo
 *d,
José Maurício A.
Caiut
*d,
José Maurício A.
Caiut
 *b and
Rute A. S.
Ferreira
*b and
Rute A. S.
Ferreira
 *a
*a
aDepartment of Physics, CICECO – Aveiro Institute of Materials, University of Aveiro, Aveiro, 3810-193, Portugal. E-mail: rferreira@ua.pt
bDepartment of Chemistry, Faculdade de Filosofia, Ciências e Letras, University of São Paulo, Ribeirão Preto, 14040-900, Brazil. E-mail: caiut@ffclrp.usp.br
cInstitute of Chemistry, Universidade Estadual Paulista «Júlio de Mesquisa Filho», Araraquara, 14800-060, Brazil
dDepartamento de Química Fundamental, Universidade Federal de Pernambuco, Recife, PE 50740-540, Brazil. E-mail: ricardo.longo@ufpe.br
First published on 14th February 2024
Abstract
Luminescent nanoparticles have shown great potential for thermal sensing in bio-applications. Nonetheless, these materials lack water dispersibility that can be overcome by modifying their surface properties with water dispersible molecules such as cysteine. Herein, we employ LiYF4:Er3+/Yb3+ upconverting nanoparticles (UCNPs) capped with oleate or modified with cysteine dispersed in cyclohexane or in water, respectively, as thermal probes. Upconversion emission was used to sense temperature with a relative thermal sensitivity of ∼1.24% K−1 (at 300 K) and a temperature uncertainty of 0.8 K for the oleate capped and of 0.5 K for cysteine modified NPs. To study the effect of the cysteine modification in the heat transfer processes, the thermal conductivity of the nanofluids was determined, yielding 0.123(6) W m−1 K−1 for the oleate capped UCNPs dispersed in cyclohexane and 0.50(7) W m−1 K−1 for the cysteine modified UCNPs dispersed in water. Moreover, through the heating curves, the nanofluids' thermal resistances were estimated, showing that the cysteine modification partially prevents heat transfer.
1. Introduction
Temperature is a key quantity for describing either natural or engineered systems, regardless of their scale.1 Recent technologies involving, e.g., microelectronics,2–4 the Internet of Thing,5 e-Health,6 and intracellular processes7,8 strongly rely on nanoscale, which imposes a challenge in measuring temperature, because conventional thermometry cannot be used at the submicron scale, like intracellular temperature fluctuations and microfluidics.3,8,9 For this reason, thermometers working at the nanoscale have been investigated in recent years,9 with special attention to luminescent nanothermometers.3,8–12Luminescence nanothermometers exploit the temperature-dependent luminescence of a thermally imaged object, yielding high relative thermal sensitivity (>1% K−1) and spatial resolution (<10 μm).3 Several types of luminescent nanothermometers have been recently developed for this specific purpose such as green fluorescent protein,13,14 small organic molecules,15 quantum dots,16 polymers,17,18 polymer dots19 and lanthanide-doped nanoparticles.8,20,21 Amongst these promising materials, lanthanide ion (Ln3+) based nanomaterials show several unique features and advantages, such as absence of photodegradation, high thermal and chemical stability, and due to the shielding of 4f-electrons and forbidden nature of their 4f–4f transitions,10,22,23 Ln3+ show very narrow emission bands (∼100 cm−1) and long luminescent lifetimes (in the order of ms).24 Such materials are also widely employed in energy conversion processes, particularly, upconversion (UC) emission, which converts photons in the near infrared region into visible radiation.25 These and other remarkable features make Ln3+ based materials specially suitable for biological applications, because one can avoid background fluorescence using time-resolved emission spectroscopy, and use the UC to modulate absorption into biological windows.26,27 For instance, Yb3+-doped nanoparticles are excited at 980 nm, which is located in the first biological window, attracting the attention for several biological applications.28–31 Usually, Er3+ is employed as a dopant for thermal sensing, in these Yb3+-doped nanoparticles, because it presents two thermally coupled levels, 2H11/2 and 4S3/2, which follows the Boltzmann distribution. This temperature dependence is due to a suitable energy separation (∼700 cm−1) between the barycenter of these two levels. The rate of equilibration of these two states is on the order of 1012 s−1, which then dominates over the radiative, non-radiative, and energy transfer rates.1,3,32 Thus, these nanothermometers can be characterized by a well-establish state equation (Boltzmann statistics), which relates the intensity ratio between the 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions and the absolute temperature, providing a primary thermometer.33
Several materials have been explored for their UC properties, such as oxides34,35 and glasses,36,37 but special attention has been given to lanthanide-doped fluoride upconverting nanoparticles (UCNPs), due to their low phonon energy and high UC efficiency.23 In fact, different fluoride UCNPs have been explored in the literature such as NaYF4,38–40 LiYF4 (ref. 41–44) and NaGdF4.45,46 However, one of the main disadvantages of these materials is their lack of water dispersibility in some synthetic routes. To overcome this limitation, the UCNPs can be encapsulated in silica28,47 or by performing ligand exchange.48,49 Alternatively, it is also possible to modify their surface by coating with an organic layer (e.g., oleic acid), which can then be modified using a variety of chemical reactions. For instance, the functional groups (e.g., carboxylate, alkene) of the oleate layer in capped LiYF4 nanocrystals can be used for reactions with modifying agents to achieve water dispersibility, biocompatibility, targeting, etc. Indeed, cysteine is an amino acid with three different functional groups (–NH2, –COOH, and –SH) that provides a wide range of reactions, from the formation of disulphide bonds50 to click chemistry.51 Cysteine is also known to render water dispersibility to otherwise hydrophobic nanoparticles.52 Hence, we propose to modify oleate capped LiYF4 nanocrystals by reacting with cysteine, because all reagents are promptly available and highly versatile.
An important issue is whether the addition of an organic coating, to functionalize the nanoparticle for biocompatibility, water dispersibility, and targeting, impacts its ability to accurately sense the local temperature, and if heat transfer from the external environment reaches the nanoparticle to establish thermal equilibrium. To date, the thermal conductivity and the thermal resistance are often measured using experimental electric methods (e.g. 3ω-method and null-point scanning thermal microscopy), which are complex, expensive and had limitations for nanomaterials.53 UC thermometry has been recently used to overcome this issue by irradiating the sample with a laser beam leading to a temperature increase. With the dependence of the maximum temperature increase with the laser power density, it is possible to estimate the thermal conductivity, as shown in phospholipid capped LiYF4 nanocrystals where the thermal conductivity of a lipid bilayer was calculated.32 By analysing the temporal evolution of the heat dissipation (transient regime), the transient time can be determined to provide estimates of other thermal properties such as thermal resistance.54 This approach has been used to estimate the thermal resistances of a powder KLu0.94Ho0.01Tm0.05(WO4)2 (ref. 53) in contact with air, and free-standing films of Er3+/Yb3+ co-doped GeO2–Ta2O5 particles dispersed in poly(methyl methacrylate).54 Both steady-state and transient regimes provided an easy access to thermal parameters (e.g., thermal conductivity, heat transfer coefficient, thermal resistance, and thermal capacity) with the advantage of being independent of the sample electrical conductivity.
Herein, we synthesised oleate capped LiYF4:Er3+/Yb3+ UCNPs and performed epoxidation of oleic acid followed by reaction with cysteine to provide water dispersibility for the cysteine modified UCNPs. Thus, two different nanofluids were investigated as luminescent thermal probes: a cyclohexane dispersed-oleate capped LiYF4:Er3+/Yb3+ UCNPs and water dispersed-cysteine modified LiYF4:Er3+/Yb3+ UCNPs. Both nanofluids function as primary nanothermometers within the temperature range of 290 to 320 K, displaying a notable relative thermal sensitivity and temperature uncertainty. To precisely determine the effect of the cysteine modification on thermal properties of the UCNPs, the thermal conductivities of the nanofluids were obtained from the steady-state regime of the heating curves, and their thermal resistances were estimated using the transient regime. The optical heating and temperature sensing properties of these nanofluids can potentially provide information regarding the thermophoresis (Soret effect) of the nanoparticles.
2. Experimental section
2.1. Materials
Y2O3, Yb2O3, Er2O3, trifluoroacetic acid, lithium trifluoroacetate (LiTFA), oleic acid, 1-octadecene, cysteine, HAuCl4, sodium citrate and solvents were all purchased from Sigma Aldrich and were used without further purification.2.2. Synthesis of LiYF4:Er3+/Yb3+ UCNPs
LiYF4 nanoparticles doped with Er3+ (0.025%) and Yb3+ (3%), LiYF4:Er3+/Yb3+ UCNPs, were synthesized via thermal decomposition as described in the literature.42 The procedure was performed in two steps. First, the lanthanide trifluoroacetate salts, Ln(TFA)3, were obtained. Diluted trifluoroacetic acid (50% v/v in MilliQ water) was added to the Ln2O3 in a round-bottom flask and heated to 80 °C under reflux until a transparent solution was obtained, then it was dried at the same temperature. In the second step, 4 mmol of LiTFA, 3.879 mmol of Y(TFA)3, 0.120 mmol of Yb(TFA)3, 0.001 mmol of Er(TFA)3, 40 mL of oleic acid and 40 mL of 1-octadecene were mixed in a three-neck round-bottom flask and heated to 110 °C in Ar atmosphere for 30 minutes in order to remove residual water and oxygen. After this time, the mixture was heated to 300 °C for 1 hour under Ar atmosphere. After reaching room temperature, the particles were precipitated using a mixture of hexane![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetone (1
acetone (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v) and the precipitate was centrifugated at 3600 rpm for 10 minutes. The resultant powder was washed 3 times using the same hexane:acetone mixture. The LiYF4:Er3+/Yb3+ UCNPs were dispersed in cyclohexane with a concentration of 4.0 mg mL−1.
4 v/v) and the precipitate was centrifugated at 3600 rpm for 10 minutes. The resultant powder was washed 3 times using the same hexane:acetone mixture. The LiYF4:Er3+/Yb3+ UCNPs were dispersed in cyclohexane with a concentration of 4.0 mg mL−1.
2.3. Cysteine modification
The cysteine modification was performed by epoxidation of oleic acid followed by reaction with cysteine.52 First, approximately 18 mg of UCNP and 0.01 mmol of 3-chloroperbenzoic acid were dispersed in 4 mL of cyclohexane and 2 mL of dichloromethane and heated to 40 °C under reflux for 3 h. Then it was cooled down to room temperature, 0.2 mmol of L-cysteine were added, heated again to 40 °C and let it react for 5 h. The resulting nanoparticles were isolated via centrifugation at 3600 rpm for 10 minutes, washed 2 times with ethanol, and twice more with distilled water to remove impurities. The cysteine modified LiYF4:Er3+/Yb3+ UCNPs were dispersed in distilled water with a concentration of 4.0 mg mL−1.2.4. Transmission electron microscopy
The images of LiYF4:Yb3+/Er3+ UCNPs were collected using a JEOL JEM-2100 microscope operating at 200 keV. The oleate capped UCNPs were prepared by dispersing the powder sample in dichloromethane, whereas the cysteine modified UCNPs were dispersed in ethanol. Then, one drop of the dispersions was placed onto a grid followed by evaporation of the solvent. The average sizes were calculated over 300 particles.2.5. Visible-NIR absorption spectroscopy
Visible and NIR absorption spectra were recorded at room temperature, using a dual-beam spectrometer Lambda 950 (PerkinElmer) with a 150 mm diameter Spectralon integrating sphere over the range 400–1100 nm with a resolution of 1.0 nm. The baseline was recorded with two 10 mm path-length quartz cuvettes (2 polished windows) containing the reference solvent (water or cyclohexane). The molar extinction coefficient was estimated from the Lambert–Beer law.2.6. Photoluminescence
A Hellma Analytics quartz cuvette (101-40-10) was used to perform the photoluminescence measurements. It was placed into a cuvette holder with a temperature controller from Quantum Northwest. For the irradiation of the UCNPs, a BrixX 980-1000 HD laser peaking at 980 nm was focused through an optical lens (7.5 cm focal distance). The laser power density was quantified as reported in Bastos et. al.32 using a power meter (FieldMaxII-TOP OP-2 Vis, Coherent), a CCD camera (BC106N-VIS/M, Thorlabs) and neutral density filters (NE10B-B, NE13B-B and NE20B-B, Thorlabs).The spectrum acquisition was performed using a MAYA Pro 2000 (Ocean Optics) portable spectrometer connected to an optical fibre with an integration time of 2 s. Temperature-dependent fluorescence emission spectra were obtained in a temperature range between 290 and 320 K. For the calibration measurements, the temperature was increased using a Peltier-based temperature-controlled cuvette holder (TLC 50, Quantum Northwest) with accuracy of ±0.2 °C, and recorded using an immersed thermocouple (0.1 °C accuracy, K-type, Pico Technology TC-08). To ensure that the solutions reached the steady-state temperature, a time interval of 300 s was allowed between consecutive temperature measurements.
2.7. Dynamic temperature measurements
The nanofluids were irradiated by a pulsed laser (BrixX 980-1000 HD) at 980 nm with power densities ranging from ca. 65 to 250 W cm−2. During the heating regime, the nanofluids were irradiated for 600 s with a high-pulsed frequency of 1.5 MHz equivalent to a continuous irradiation. The resulting temperature increase was measured over time with an immersed thermocouple (K-type, 0.1 K accuracy, Pico Technology TC-08) placed at a fixed position (0.5 cm) from the excitation beam. The emission spectra of the nanofluids were recorded with the above-mentioned portable spectrometer using an integration time of 4 s.3. Results and discussion
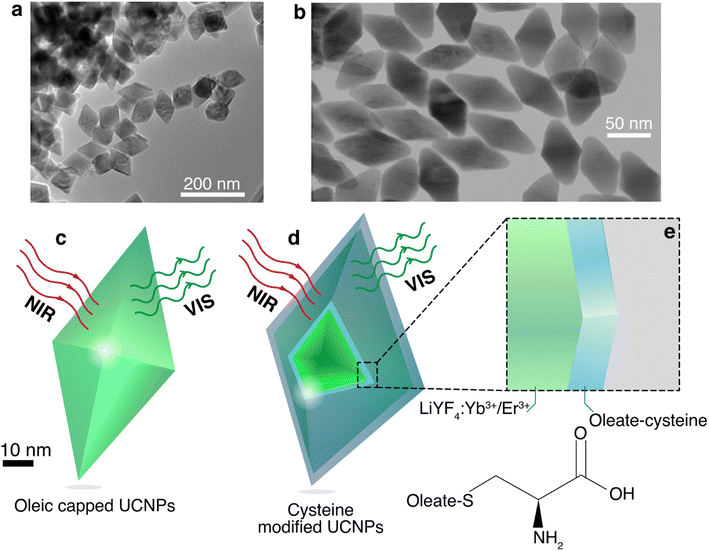
Oleate capped Yb3+/Er3+ doped LiYF4 nanoparticles were synthesized using the thermal decomposition method,42 and dispersed in cyclohexane. These UCNPs presented narrow peaks in X-ray diffraction pattern (Fig. S1, ESI†) consistent with the LiYF4 tetragonal phase,42 and presented a diamond-shaped with an average size of 94 ± 15 nm (longer diagonal) and 65 ± 6 nm (shorter diagonal) with an aspect ratio of 1.4 (Fig. 1a), determined from transmission electron microscopy (TEM). To provide water dispersibility for the UCNPs, the oleate was modified via epoxidation followed by reaction with cysteine. The modified UCNPs maintained the same morphology with an average size of 85 ± 12 nm (longer diagonal) and 46 ± 5 nm (shorter diagonal) with an aspect ratio of 1.8 (Fig. 1b). This decrease of the average size of the nanoparticles upon modification could be due to the chemical reaction at oleate layer that might induce its tightening and compression. To ascertain the successful cysteine modification, Fourier Transform Infrared spectra (Fig. S2, ESI†) were obtained, showing new bands related with the functional groups of the cysteine amino acid, such as –NH2 or –NH3 rock at 1146 cm−1, N–C stretch at 1208 cm−1, and –CO2 asymmetric stretch at 1680 cm−1.52,55The absorption spectra of the nanofluids (Fig. S3, ESI†) were measured and the corresponding molar extinction coefficients at 980 nm were estimated to be 5.7 ± 0.2 and 14.3 ± 0.1 L mol−1 cm−1 for the oleate capped and cysteine modified nanoparticles, respectively (eqn (S2), ESI†). The absorption cross section was also determined at 980 nm, yielding values of (9.3 ± 0.4) × 10−15 and (2.3 ± 0.1) × 10−14 cm2 for the oleate capped and cysteine modified nanoparticles, respectively, which are in same order of magnitude of similar UCNPs.32,33 This increase of ca. 2.5-fold in the absorption may be explained by the increase of the number of high energy oscillators such as N–H and O–H from the amino acid as well as the increase of the hydration shell due to the surface becoming more hydrophilic upon modification.
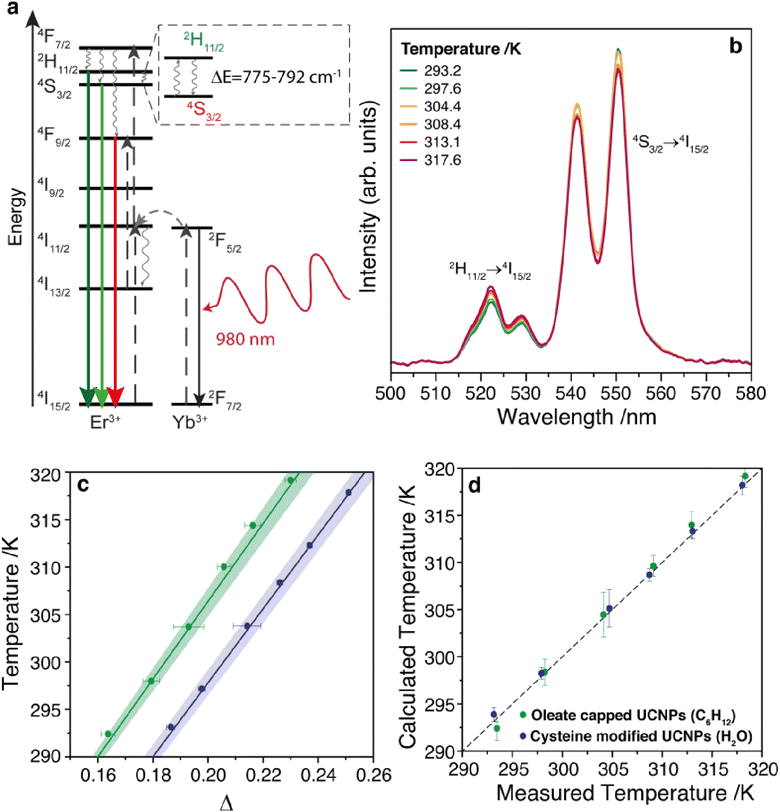
The typical Er3+ UC emission spectrum was observed upon excitation at 980 nm, as depicted in Fig. 2a and b and S5, ESI.† Two transitions were observed in the green region (between 500 and 575 nm) assigned to the 2H11/2 → 4I15/2 and 4S13/2 → 4I15/2 transitions, Fig. 2a, and one 4F9/2 → 4I15/2 transition in the red region (between 675 and 700 nm),38,44,56 Fig. S5, ESI.† The ratio between the integrated intensity between the green and red emissions (Fig. S5, ESI†) is 1.44 for the oleate capped particles dispersed in cyclohexane and 1.07 for the cysteine modified particles dispersed in water. This slight enhancement of the red emission relative intensity in water suspensions was also observed in other nanoparticulated systems,57 and it was attributed to an increase of the nonradiative transitions from the upper green emitting levels 2H11/2 and 4S3/2 to the lower red emitting level 4F9/2 due to the high energy oscillators present in aqueous environment.57,58
The dependence of the UC spectra with temperature was investigated from 293 to 317 K (Fig. 2b and S6, ESI†). The thermometric parameter Δ, defined as the intensity ratio between 2H11/2 → 4I15/2 and 4S13/2 → 4I15/2 transitions, was determined and shows a clear linear dependence with the temperature, Fig. 2c. This linear behavior, with a positive slope, can be obtained from a Boltzmann-based thermometer when considering a small temperature range compared to the central temperature (24/305 = 0.079).59 Adopting the primary thermometer strategy reported previously,1,3 the temperature of the nanofluids was determined using Δ and eqn (S9) (ESI),† with the values for ΔE obtained from the emission spectra (Fig. S7, ESI†), the intensity ratio in the limit of low excitation power, and the corresponding temperature (Section IV and Table S1, ESI†). Fig. 2d shows a very good agreement between the predicted temperature using the Δ values and the measured temperature using a thermocouple immersed into the nanofluid, yielding a thermal sensitivity at 300 K of 1.24% K−1 for both nanofluids, and a thermal uncertainty of 0.8 and 0.5 K for the oleate capped particles dispersed in cyclohexane and cysteine modified particles dispersed in water, respectively (Fig. S8, ESI†), which is in agreement with values found in the literature for Yb3+ and Er3+ doped UCNPs.1,32
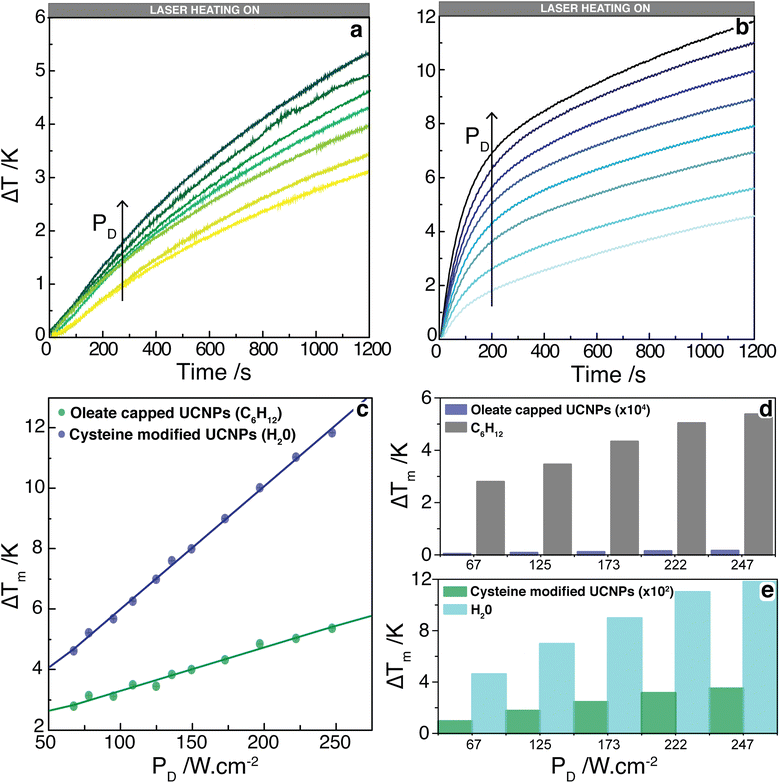
Upon irradiation at 980 nm, it can be observed a transient heating curve of the nanofluids that was recorded with a thermocouple as the temperature increase with time, Fig. 3a and b. Simultaneously, during the heating regime, the emission spectra were recorded to compare the temperature increase profiles recorded with the immersed thermocouple and the one calculated using the emission spectra. Within the experimental uncertainty of both measurements, the temperature values were similar for both nanofluids (Fig. S9, ESI†). It can also be observed that, for the same laser power density, the temperature increase is significantly higher on the cysteine modified UCNPs dispersed in water in comparison to the oleate capped UCNPs dispersed in cyclohexane. This can be explained by the ∼2.5 times increase of the absorption cross section of the UCNPs upon modification with cysteine as well as the stronger absorption of water compared to cyclohexane. To better understand this effect, the dependence of ΔT with the laser power density (PD), on the presence or absence of a cysteine, and on the solvent used was analyzed. From the maximum temperature increase (ΔTm) recorded at steady-state, it is possible to determine the thermal conductivities of the nanofluids.32 If both the solvent and the UCNPs contribute to the temperature increase in the nanofluid independently, eqn (1) can be used to model ΔTm as function of PD, where the first term refers to the heating by the solvent, and the second term represents the heating promoted by the UCNPs,
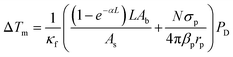 | (1) |
The separate contribution of the solvent and the nanoparticles on the total heating of the system was calculated using eqn (1) and is presented in Fig. 3d. It can be observed that the solvents, water, or cyclohexane, were responsible to almost the totality of the thermal properties of the system. Moreover, the cysteine modified UCNPs was responsible for a higher increase of temperature than the oleate capped UCNPs, which is related to the ca. 2.5-fold larger absorption cross section of the former. It is noteworthy that the temperature increase has not reached steady-state for any power density employed. However, the analysis performed regarding the linear relationship between ΔTm and PD in eqn (1) is still valid because by determining ΔT at different times (e.g., 200, 600, 800, and 1200 s), this linear relationship is maintained with the same slopes (Fig. S10, ESI†).
The temporal dependence of the temperature increase, also known as a transient regime before reaching steady-state, can provide additional thermal properties of the nanofluid. The temperature increase with time can described by:64
| ΔT(t) = ΔTm(1 − e−t/τ), τ = mc/(hA) | (2) |
It is noteworthy that the transient behavior of these nanofluids is quantitatively very distinct from the nanofluids composed of coated (lipid bilayers) and uncoated UCNPs (LiYF4:Er3+/Yb3+) suspended in water (H2O and D2O).64 The main difference between these nanofluids is the dynamics of their thermal properties. For instance, the typical transient times (initial temperature increase until constant temperature) for the cysteine-modified UCNPs dispersed in water were ca. 350-fold larger than for the UCNPs suspended in water. These different behaviors can be attributed to concentration effects and dispersion versus suspension of similar amounts of UCNPs. In the present case, the cysteine-modified UCNPs are soluble in water, hence forming a dispersion with very low concentration. As described, the thermal properties of the nanofluid are dominated by the solvent and the UCNPs have negligible effects. On the other hand, the unmodified UCNPs (either capped or uncapped) were insoluble in water and the nanofluids were investigated as suspensions. Notice that despite the amounts of UCNPs employed in all these experiments being practically the same, the concentrations of the NPs in the suspensions are much higher than those in dispersions, so the thermal behaviors of these nanofluid should be quite distinct. In addition, the motions (diffusion and thermal-diffusion) of the UCNPs in the suspensions are much more restricted than those in diluted dispersions.
Then, it could be inferred that by increasing the concentration of UCNPs dispersed in water, the thermal properties of the nanofluid would become dependent on these UCNPs and novel experiments could be designed to quantify new properties. In this case, as a UCNP leaves the excitation beam, a large amount of solvent replaces it and increases the absorption cross-section, thus increasing the power absorbed from the laser beam, which increases the optical heating and the time to the temperature reaching a steady-state regime. A reverse effect can be devised by a UCNP entering the excitation cylinder. In fact, if the temporal dependence of the temperature increase for the dispersions is affected by the migration of the UCNPs in and out of the excitation cylinder, this could lead to a novel approach to measure the Soret coefficient, ST, of NPs, which is relevant to opto-thermophoresis, particle separation, and related effects.72,73 For instance, at steady-state conditions, the concentration profile of the NPs is described as74–76
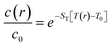 | (3) |
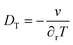 | (4) |
Thus, these optical techniques provide easy access to the thermal properties of nanoparticles and nanofluids (e.g., thermal resistance and conductivity), with the advantage of being independent of the sample electrical conductivity. Potentially, by probing the dispersed UCNPs the thermophoretic properties of the NPs can also be promptly accessed.
4. Conclusions
The ability of LiYF4:Yb3+/Er3+ nanoparticles to be a luminescent thermometer was assessed in two different media. Since oleate capped UCNPs are hydrophobic, cyclohexane was chosen as the solvent. To make the UCNPs water dispersible, a cysteine modification was used by epoxidation of the carbon double bond and opening of the epoxide ring using cysteine. In both nanofluids it was possible to observe an intense Er3+ emission when excited at 980 nm. In water, there was a slight increased red emission attributed to a decay from green emitting levels to the red emitting level. Both nanofluids worked as primary thermometers in the temperature range of 290–320 K with a good thermal sensitivity of 1.24% K−1, and a thermal uncertainty of 0.8 and 0.5 K for the oleate capped particles dispersed in cyclohexane and cysteine modified particles dispersed in water, respectively. Transient heating curves were obtained, where the water dispersion heated more than the cyclohexane one. Through the steady-state regime of the heating curves, the thermal conductivity of the nanofluids was estimated. The estimated thermal conductivities were 0.123 ± 0.006 W m−1 K−1 for the oleate capped UCNPs dispersed in cyclohexane, and 0.50 ± 0.07 W m−1 K−1 for the cysteine modified UCNPs dispersed in water, remarkably close the values of pure solvents, since the nanoparticles had a minor role in the total heating of the system, because of their low concentrations. Using the transient regime of the heating curves, the thermal resistances were calculated, with their values being attributed to the pure solvents because of the diluted dispersions used and could be considered as the first reported values for cyclohexane and water. These tools to determine the nanofluids thermal properties, enlarge the application of luminescence thermometry, allowing the exploitation of the heat transfer processes occurring at the micro and nanoscale.Conflicts of interest
There are no conflits to declare.Acknowledgements
This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020 (DOI 10.54499/UIDB/50011/2020), UIDP/50011/2020 (DOI 10.54499/UIDP/50011/2020) & LA/P/0006/2020 (DOI 10.54499/LA/P/0006/2020), financed by national funds through the FCT/MCTES (PIDDAC) and the project PHEASANT (2022.15735.CMU) under the Carnegie Mellon Portugal Program. Also, this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 and Capes PrInt program. The authors acknowledge the Brazilian funding agency FAPESP (Proc. 2016/11670-5, Proc. 2019/18828-1), as well as the University of São Paulo.References
- S. Balabhadra, M. L. Debasu, C. D. S. Brites, R. A. S. Ferreira and L. D. Carlos, Upconverting Nanoparticles Working As Primary Thermometers In Different Media, J. Phys. Chem. C, 2017, 121(25), 13962–13968, DOI:10.1021/acs.jpcc.7b04827.
- L. Aigouy, G. Tessier, M. Mortier and B. Charlot, Scanning Thermal Imaging of Microelectronic Circuits with a Fluorescent Nanoprobe, Appl. Phys. Lett., 2005, 87, 184105, DOI:10.1063/1.2123384.
- C. D. S. Brites, A. Millán and L. D. Carlos, Lanthanides in Luminescent Thermometry, in Handbook on the Physics and Chemistry of Rare Earths, 2016, pp. 339–427, DOI:10.1016/bs.hpcre.2016.03.005.
- RAS Rute, Photonic-on-a-chip: a thermal actuated Mach-Zehnder interferometer and a molecular thermometer based on a single di-ureasil organic-inorganic hybrid, Laser Photonics Rev., 2013, 7(6), 1027–1035, DOI:10.1002/lpor.201300080.
- J. F. C. B. Ramalho, S. F. H. Correia, L. Fu, L. F. António, C. Breites, R.A.S. Ferreira and L. D. Carlos, Luminescence Thermometry on the Route of the Mobile-Based Internet of Things (IoT): How Smart QR Codes Make It Real, Adv. Sci., 2019, 1900950, DOI:10.1002/advs.201900950.
- J. F. C. B. Ramalho, L. M. S. Dias, L. Fu, A. M. P. Botas, L. D. Carlos, P. S. André and R. A. S. Ferreira, Customized Luminescent Multiplexed Quick-Response Codes as Reliable Temperature Mobile Optical Sensors for eHealth and Internet of Things, Adv. Photonics Res., 2021, 3, 2100206, DOI:10.1002/adpr.202100206.
- S. Uchiyama, T. Tsuji, K. Ikado, A. Yoshida, K. Kawamoto, T. Hayashi and N. Inada, A Cationic Fluorescent Polymeric Thermometer for the Ratiometric Sensing of Intracellular Temperature, Analyst, 2015, 140(13), 4498–4506, 10.1039/c5an00420a.
- F. Vetrone, R. Naccache, A. Zamarron, A. Juarranz de la Fuente, F. Sanz-Rodriguez, L. Martinez Maestro, E. Martin Rodriguez, D. Jaque, J. Garcia Sole and J. A. Capobianco, Temperature Sensing Using Fluorescent Nanothermometers, ACS Nano, 2010, 4(6), 3254–3258, DOI:10.1021/nn100244a.
- C. D. Brites, P. P. Lima, N. J. Silva, A. Millan, V. S. Amaral, F. Palacio and L. D. Carlos, Thermometry at the Nanoscale, Nanoscale, 2012, 4(16), 4799–4829, 10.1039/c2nr30663h.
- T. J. S. Ramos, R. L. Longo, C. D. S. Brites, R. A. S. Ferreira and O. L. LD, Exploring the Intra-4f and the Bright White Light Upconversion Emissions of Gd2O3:Yb3+,Er3+-Based Materials for Thermometry, Nanoscale, 2023, 15, 9993–10003, 10.1039/d3nr01764h.
- A. M. P. Botas, C. D. S. Brites, J. Wu, U. Kortshagen, R. N. Pereira, L. D. Carlos and R. A. S. Ferreira, A New Generation of Primary Luminescent Thermometers Based on Silicon Nanoparticles and Operating in Different Media, Part. Part. Syst. Charact., 2016, 33(10), 740–748, DOI:10.1002/ppsc.201600198.
- D. Jaque and F. Vetrone, Luminescence Nanothermometry, Nanoscale, 2012, 4(15), 4301–4326, 10.1039/c2nr30764b.
- O. A. Savchuk, O. F. Silvestre, R. M. R. Adao and J. B. Nieder, GFP Fluorescence Peak Fraction Analysis Based Nanothermometer for the Assessment of Exothermal Mitochondria Activity in Live Cells, Sci. Rep., 2019, 9(1), 7535, DOI:10.1038/s41598-019-44023-7.
- R.N. Bastos Ana, Martins Margarida, P.E. Macário Inês, Veloso Telma, L. Pereira Joana, A.P. Coutinho João, P.M. Ventura Sónia, S. André Paulo and A.S. Ferreira Rute, Bio-Based Solar Energy Harvesting for Onsite Mobile Optical Temperature Sensing in Smart Cities, Adv. Sci., 2022, 9, 2104801, DOI:10.1002/advs.202104801.
- D. Chretien, P. Benit, H. H. Ha, S. Keipert, R. El-Khoury, Y. T. Chang, M. Jastroch, H. T. Jacobs, P. Rustin and M. Rak, Mitochondria Are Physiologically Maintained at Close to 50 Degrees C, PLoS Biol., 2018, 16(1), e2003992, DOI:10.1371/journal.pbio.2003992.
- R. Tanimoto, T. Hiraiwa, Y. Nakai, Y. Shindo, K. Oka, N. Hiroi and A. Funahashi, Detection of Temperature Difference in Neuronal Cells, Sci. Rep., 2016, 6, 22071, DOI:10.1038/srep22071.
- X. Chen, Q. Xia, Y. Cao, Q. Min, J. Zhang, Z. Chen, H. Y. Chen and J. J. Zhu, Imaging the Transient Heat Generation of Individual Nanostructures with a Mechanoresponsive Polymer, Nat. Commun., 2017, 8(1), 1498, DOI:10.1038/s41467-017-01614-0.
- K. Okabe, N. Inada, C. Gota, Y. Harada, T. Funatsu and S. Uchiyama, Intracellular Temperature Mapping with a Fluorescent Polymeric Thermometer and Fluorescence Lifetime Imaging Microscopy, Nat. Commun., 2012, 3, 705, DOI:10.1038/ncomms1714.
- F. Ye, C. Wu, Y. Jin, Y. H. Chan, X. Zhang and D. T. Chiu, Ratiometric Temperature Sensing with Semiconducting Polymer Dots, J. Am. Chem. Soc., 2011, 133(21), 8146–8149, DOI:10.1021/ja202945g.
- M. Suzuki, V. Tseeb, K. Oyama and S. Ishiwata, Microscopic Detection of Thermogenesis in a Single HeLa Cell, Biophys. J., 2007, 92(6), L46–L48, DOI:10.1529/biophysj.106.098673.
- R. Pinol, C. D. Brites, R. Bustamante, A. Martinez, N. J. Silva, J. L. Murillo, R. Cases, J. Carrey, C. Estepa, C. Sosa, F. Palacio, L. D. Carlos and A. Millan, Joining Time-Resolved Thermometry and Magnetic-Induced Heating in a Single Nanoparticle Unveils Intriguing Thermal Properties, ACS Nano, 2015, 9(3), 3134–3142, DOI:10.1021/acsnano.5b00059.
- D. Tu, W. Zheng, P. Huang and X. Chen, Europium-Activated Luminescent Nanoprobes: From Fundamentals to Bioapplications, Coord. Chem. Rev., 2019, 378, 104–120, DOI:10.1016/j.ccr.2017.10.027.
- R. Naccache, Q. Yu and J. A. Capobianco, The Fluoride Host: Nucleation, Growth, and Upconversion of Lanthanide-Doped Nanoparticles, Adv. Opt. Mater., 2015, 3(4), 482–509, DOI:10.1002/adom.201400628.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer, 1997 Search PubMed.
- F. Auzel, Upconversion and Anti-Stokes Processes with f and d Ions in Solids, Chem. Rev., 2004, 104(1), 139–173, DOI:10.1021/cr020357g.
- F. Wang, D. Banerjee, Y. Liu, X. Chen and X. Liu, Upconversion Nanoparticles in Biological Labeling, Imaging, and Therapy, Analyst, 2010, 135(8), 1839–1854, 10.1039/c0an00144a.
- E. Hemmer, A. Benayas, F. Légaré and F. Vetrone, Exploiting the Biological Windows: Current Perspectives on Fluorescent Bioprobes Emitting above 1000 Nm, Nanoscale Horiz., 2016, 1(3), 168–184, 10.1039/c5nh00073d.
- R. Abdul Jalil and Y. Zhang, Biocompatibility of Silica Coated NaYF(4) Upconversion Fluorescent Nanocrystals, Biomaterials, 2008, 29(30), 4122–4128, DOI:10.1016/j.biomaterials.2008.07.012.
- D. Yang, P. Ma, Z. Hou, Z. Cheng, C. Li and J. Lin, Current Advances in Lanthanide Ion (Ln(3+))-Based Upconversion Nanomaterials for Drug Delivery, Chem. Soc. Rev., 2015, 44(6), 1416–1448, 10.1039/c4cs00155a.
- A. Skripka, V. Karabanovas, G. Jarockyte, R. Marin, V. Tam, M. Cerruti, R. Rotomskis and F. Vetrone, Decoupling Theranostics with Rare Earth Doped Nanoparticles, Adv. Funct. Mater., 2019, 29(12), 1807105, DOI:10.1002/adfm.201807105.
- L. P. Qian, L. H. Zhou, H.-P. Too and G.-M. Chow, Gold Decorated NaYF4:Yb,Er/NaYF4/Silica (Core/Shell/Shell) Upconversion Nanoparticles for Photothermal Destruction of BE(2)-C Neuroblastoma Cells, J. Nanopart. Res., 2010, 13(2), 499–510, DOI:10.1007/s11051-010-0080-6.
- A. R. N. Bastos, C. D. S. Brites, P. A. Rojas-Gutierrez, C. DeWolf, R. A. S. Ferreira, J. A. Capobianco and L. D. Carlos, Thermal Properties of Lipid Bilayers Determined Using Upconversion Nanothermometry, Adv. Funct. Mater., 2019, 29(48), 1905474, DOI:10.1002/adfm.201905474.
- J. C. Martins, A. R. N. Bastos, R. A. S. Ferreira, X. Wang, G. Chen and L. D. Carlos, Primary Luminescent Nanothermometers for Temperature Measurements Reliability Assessment, Adv. Photonics Res., 2021, 2(5), 2000169, DOI:10.1002/adpr.202000169.
- F. Vetrone, J.-C. Boyer, J. A. Capobianco, A. Speghini and M. Bettinelli, Significance of Yb3+ Concentration on the Upconversion Mechanisms in Codoped Y2O3:Er3+, Yb3+ Nanocrystals, J. Appl. Phys., 2004, 96(1), 661–667, DOI:10.1063/1.1739523.
- D. Li, Y. Wang, X. Zhang, K. Yang, L. Liu and Y. Song, Optical Temperature Sensor through Infrared Excited Blue Upconversion Emission in Tm3+/Yb3+ Codoped Y2O3, Opt. Commun., 2012, 285(7), 1925–1928, DOI:10.1016/j.optcom.2011.12.075.
- C. Basavapoornima, K. Linganna, C. R. Kesavulu, S. Ju, B. H. Kim, W. T. Han and C. K. Jayasankar, Spectroscopic and Pump Power Dependent Upconversion Studies of Er3+-Doped Lead Phosphate Glasses for Photonic Applications, J. Alloys Compd., 2017, 699, 959–968, DOI:10.1016/j.jallcom.2016.12.199.
- D. Manzani, J. F. Petruci, K. Nigoghossian, A. A. Cardoso and S. J. Ribeiro, A Portable Luminescent Thermometer Based on Green Up-Conversion Emission of Er(3+)/Yb(3+) Co-Doped Tellurite Glass, Sci. Rep., 2017, 7, 41596, DOI:10.1038/srep41596.
- J. C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, Synthesis of Colloidal Upconverting NaYF4 Nanocrystals Doped with Er3+, Yb3+ and Tm3+, Yb3+via Thermal Decomposition of Lanthanide Trifluoroacetate Precursors, J. Am. Chem. Soc., 2006, 128(23), 7444–7445, DOI:10.1021/ja061848b.
- B. Dong, S. Xu, J. Sun, S. Bi, D. Li, X. Bai, Y. Wang, L. Wang and H. Song, Multifunctional NaYF4: Yb3+,Er3+@Ag Core/Shell Nanocomposites: Integration of Upconversion Imaging and Photothermal Therapy, J. Mater. Chem., 2011, 21(17), 6193–6200, 10.1039/c0jm04498a.
- C. D. Brites, X. Xie, M. L. Debasu, X. Qin, R. Chen, W. Huang, J. Rocha, X. Liu and L. D. Carlos, Instantaneous Ballistic Velocity of Suspended Brownian Nanocrystals Measured by Upconversion Nanothermometry, Nat. Nanotechnol., 2016, 11(10), 851–856, DOI:10.1038/nnano.2016.111.
- Y. P. Du, Y. W. Zhang, L. D. Sun and C. H. Yan, Optically Active Uniform Potassium and Lithium Rare Earth Fluoride Nanocrystals Derived from Metal Trifluroacetate Precursors, Dalton Trans., 2009, 40, 8574–8581, 10.1039/b909145a.
- V. Mahalingam, F. Vetrone, R. Naccache, A. Speghini and J. A. Capobianco, Colloidal Tm3+/Yb3+-Doped LiYF4 Nanocrystals: Multiple Luminescence Spanning the UV to NIR Regions via Low-Energy Excitation, Adv. Mater., 2009, 21(40), 4025–4028, DOI:10.1002/adma.200901174.
- D. Yang, Y. Dai, P. Ma, X. Kang, M. Shang, Z. Cheng, C. Li and J. Lin, Synthesis of Li1−xNaxYF4:Yb3+/Ln3+ (0 ≤ x ≤ 0.3, Ln = Er, Tm, Ho) Nanocrystals with Multicolor up-Conversion Luminescence Properties for in Vitro Cell Imaging, J. Mater. Chem., 2012, 22(38), 20618–20625, 10.1039/c2jm33910b.
- L. Zhang, Z. Wang, Z. Lu, K. Xia, Y. Deng, S. Li, C. Zhang, Y. Huang and N. He, Synthesis of LiYF4:Yb, Er Upconversion Nanoparticles and Its Fluorescence Properties, J. Nanosci. Nanotechnol., 2014, 14(6), 4710–4713, DOI:10.1166/jnn.2014.8641.
- K. Nigoghossian, Y. Messaddeq, D. Boudreau and S. J. L. Ribeiro, UV and Temperature-Sensing Based on NaGdF4:Yb3+:Er3+@SiO2-Eu(tta)3, ACS Omega, 2017, 2(5), 2065–2071, DOI:10.1021/acsomega.7b00056.
- Y. Hou, R. Qiao, F. Fang, X. Wang, C. Dong, K. Liu, C. Liu, Z. Liu, H. Lei, F. Wang and M. Gao, NaGdF4 Nanoparticle-Based Molecular Probes for Magnetic Resonance Imaging of Intraperitoneal Tumor Xenografts in Vivo, ACS Nano, 2013, 7(1), 330–338, DOI:10.1021/nn304837c.
- C. Li, D. Yang, P. Ma, Y. Chen, Y. Wu, Z. Hou, Y. Dai, J. Zhao, C. Sui and J. Lin, Multifunctional Upconversion Mesoporous Silica Nanostructures for Dual Modal Imaging and in Vivo Drug Delivery, Small, 2013, 9(24), 4150–4159, DOI:10.1002/smll.201301093.
- W. Kong, T. Sun, B. Chen, X. Chen, F. Ai, X. Zhu, M. Li, W. Zhang, G. Zhu and F. Wang, A General Strategy for Ligand Exchange on Upconversion Nanoparticles, Inorg. Chem., 2017, 56(2), 872–877, DOI:10.1021/acs.inorgchem.6b02479.
- N. Bogdan, F. Vetrone, G. A. Ozin and J. A. Capobianco, Synthesis of Ligand-Free Colloidally Stable Water Dispersible Brightly Luminescent Lanthanide-Doped Upconverting Nanoparticles, Nano Lett., 2011, 11(2), 835–840, DOI:10.1021/nl1041929.
- L. B. Poole, The Basics of Thiols and Cysteines in Redox Biology and Chemistry, Free Radical Biol. Med., 2015, 80, 148–157, DOI:10.1016/j.freeradbiomed.2014.11.013.
- C. E. Hoyle, A. B. Lowe and C. N. Bowman, Thiol-Click Chemistry: A Multifaceted Toolbox for Small Molecule and Polymer Synthesis, Chem. Soc. Rev., 2010, 39(4), 1355–1387, 10.1039/B901979K.
- Z. Wei, L. Sun, J. Liu, J. Z. Zhang, H. Yang, Y. Yang and L. Shi, Cysteine Modified Rare-Earth Up-Converting Nanoparticles for in Vitro and in Vivo Bioimaging, Biomaterials, 2014, 35(1), 387–392, DOI:10.1016/j.biomaterials.2013.09.110.
- O. A. Savchuk, J. J. Carvajal, C. D. S. Brites, L. D. Carlos, M. Aguilo and F. Diaz, Upconversion Thermometry: A New Tool to Measure the Thermal Resistance of Nanoparticles, Nanoscale, 2018, 10(14), 6602–6610, 10.1039/c7nr08758f.
- F. J. Caixeta, A. R. N. Bastos, A. M. P. Botas, L. S. Rosa, V. S. Souza, F. H. Borges, A. N. C. Neto, A. Ferrier, P. Goldner, L. D. Carlos, R. R. Gonçalves and R. A. S. Ferreira, High-Quantum-Yield Upconverting Er3+/Yb3+-Organic–Inorganic Hybrid Dual Coatings for Real-Time Temperature Sensing and Photothermal Conversion, J. Phys. Chem. C, 2020, 124(37), 19892–19903, DOI:10.1021/acs.jpcc.0c03874.
- S. F. Parker, Assignment of the Vibrational Spectrum of L-Cysteine, Chem. Phys., 2013, 424, 75–79, DOI:10.1016/j.chemphys.2013.04.020.
- X. Xue, S. Uechi, R. N. Tiwari, Z. Duan, M. Liao, M. Yoshimura, T. Suzuki and Y. Ohishi, Size-Dependent Upconversion Luminescence and Quenching Mechanism of LiYF4: Er3+/Yb3+ Nanocrystals with Oleate Ligand Adsorbed, Opt. Mater. Express, 2013, 3(7), 989–999, DOI:10.1364/ome.3.000989.
- R. Kumar, M. Nyk, T. Y. Ohulchanskyy, C. A. Flask and P. N. Prasad, Combined Optical and MR Bioimaging Using Rare Earth Ion Doped NaYF4 Nanocrystals, Adv. Funct. Mater., 2009, 19(6), 853–859, DOI:10.1002/adfm.200800765.
- H. Schäfer, P. Ptacek, K. Kömpe and M. Haase, Lanthanide-Doped NaYF4Nanocrystals in Aqueous Solution Displaying Strong Up-Conversion Emission, Chem. Mater., 2007, 19(6), 1396–1400, DOI:10.1021/cm062385b.
- M. Suta and A. A. Meijerink, Theoretical Framework for Ratiometric Single Ion Luminescent Thermometers—Thermodynamic and Kinetic Guidelines for Optimized Performance, Adv. Theory Simul., 2020, 3, 2000176, DOI:10.1002/adts.202000176.
- G. Baffou, R. Quidant and F. J. Garcia de Abajo, Nanoscale Control of Optical Heating in Complex Plasmonic Systems, ACS Nano, 2010, 4(2), 709–716, DOI:10.1021/nn901144d.
- A. Koutian, M. J. Assael, M. L. Huber and R. A. Perkins, Reference Correlation of the Thermal Conductivity of Cyclohexane from the Triple Point to 640 K and up to 175 MPa, J. Phys. Chem. Ref. Data, 2017, 46(1), 013102, DOI:10.1063/1.4974325.
- S. A. Angayarkanni and J. Philip, Review on Thermal Properties of Nanofluids: Recent Developments, Adv. Colloid Interface Sci., 2015, 225, 146–176, DOI:10.1016/j.cis.2015.08.014.
- M. L. V. Ramires, C. A. Nieto de Castro, Y. Nagasaka, A. Nagashima, M. J. Assael and W. A. Wakeham, Standard Reference Data for the Thermal Conductivity of Water, J. Phys. Chem. Ref. Data, 1995, 24(3), 1377–1381, DOI:10.1063/1.555963.
- A. R. N. Bastos, C. D. S. Brites, P. A. Rojas-Gutierrez, R. A. S. Ferreira, R. L. Longo, C. DeWolf, J. A. Capobianco and L. D. Carlos, Thermal properties of lipid bilayers derived from the transient heating regime of upconverting nanoparticles, Nanoscale, 2020, 12, 24169–24176, 10.1039/D0NR06989B.
- T. L. Bergman, A. S. LavineF. P. Incropera and D. P. DeWitt, Introduction to Heat Transfer, John Wiley & Sons, New York, 6th edn, 2011 Search PubMed.
- D. Liu, R. Xie, N. Yang, B. Li and J. T. L. Thong, Profiling Nanowire Thermal Resistance with a Spatial Resolution of Nanometers, Nano Lett., 2014, 14(2), 806–812, DOI:10.1021/nl4041516.
- R. Prasher, Predicting the Thermal Resistance of Nanosized Constrictions, Nano Lett., 2005, 5(11), 2155–2159, DOI:10.1021/nl051710b.
- M. Liu, L. Qiu, X. H. Zheng, J. Zhu and D. W. Tang, Study on the Thermal Resistance in Secondary Particles Chain of Silica Aerogel by Molecular Dynamics Simulation, J. Appl. Phys., 2014, 116(9), 93503, DOI:10.1063/1.4894511.
- F. Meng, M. Elsahati, J. Liu and R. F. Richards, Thermal Resistance between Amorphous Silica Nanoparticles, J. Appl. Phys., 2017, 121(19), 194302, DOI:10.1063/1.4983753.
- S. Grauby, E. Puyoo, J.-M. Rampnoux, E. Rouvière and S. Dilhaire, Si and SiGe Nanowires: Fabrication Process and Thermal Conductivity Measurement by 3ω-Scanning Thermal Microscopy, J. Phys. Chem. C, 2013, 117(17), 9025–9034, DOI:10.1021/jp4018822.
- W. Chen, Y. Feng, L. Qiu and X. Zhang, Scanning Thermal Microscopy Method for Thermal Conductivity Measurement of a Single SiO2 Nanoparticle, Int. J. Heat Mass Transf., 2020, 154, 119750, DOI:10.1016/j.ijheatmasstransfer.2020.119750.
- K. Lee, R. Mishra and T. Kim, Review of micro/nanofluidic particle separation mechanisms: Toward combined multiple physical fields for nanoparticles, Sens. Actuators, A, 2023, 363, 114688, DOI:10.1016/j.sna.2023.114688.
- R. T. Schermer, C. C. Olson, J. P. Coleman and F. Bucholtz, Laser-induced thermophoresis of individual particles in a viscous liquid, Opt. Express, 2011, 19, 10571–10586, DOI:10.1364/OE.19.010571.
- S. Duhr and D. Braun, Why molecules move along a temperature gradient, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 19678–19682, DOI:10.1073/pnas.0603873103.
- S. Duhr and D. Braun, Thermophoretic Depletion Follows Boltzmann Distribution, Phys. Rev. Lett., 2006, 96, 168301, DOI:10.1103/PhysRevLett.96.168301.
- R. Piazza and A. Parola, Thermophoresis in colloidal suspensions, J. Phys.: Condens. Matter, 2008, 20, 153102, DOI:10.1088/0953-8984/20/15/153102.
- C. D. S. Brites, B. Zhuang, M. L. Debasu, D. Ding, X. Qin, F. E. Maturi, W. W. Y. Lim, D. W. Soh, J. Rocha, Z. Yi, X. Liu and L. D. Carlos, Decoding a percolation phase transition of water at ∼330 K with a nanoparticle ruler, J. Phys. Chem. Lett., 2020, 11, 6704–6711, DOI:10.1021/acs.jpclett.0c02147.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3na01114c |
| This journal is © The Royal Society of Chemistry 2024 |