Open Access Article
Open Access ArticleSynthesis of a novel nanomagnetic N4 bis schiff base complex of copper(II) as an efficient catalyst for click synthesis of tetrazoles
Chou-Yi
Hsu
 a,
Ahmed Rafiq
AlBajalan
a,
Ahmed Rafiq
AlBajalan
 *b,
Sameer A.
Awad
c,
Muath
Suliman
d,
Nizomiddin
Juraev
ef,
Carlos
Rodriguez-Benites
g,
Hamad
AlMohamadi
h and
Abed J.
Kadhim
i
*b,
Sameer A.
Awad
c,
Muath
Suliman
d,
Nizomiddin
Juraev
ef,
Carlos
Rodriguez-Benites
g,
Hamad
AlMohamadi
h and
Abed J.
Kadhim
i
aThunderbird School of Global Management, Arizona State University Tempe Campus, Phoenix, Arizona 85004, USA
bPetroleum Technology Department, Erbil Polytechnic University, Erbil, Kurdistan Region, Iraq. E-mail: ahmedrafiqalbajalan@gmail.com; ahmed.al_bajalan@epu.edu.iq
cDepartment of Medical Laboratories Techniques, College of Health and Medical Technology, University of Al Maarif, Al Anbar, 31001, Iraq
dDepartment of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
eResearcher, Faculty of Chemical Engineering, New Uzbekistan University, Tashkent, Uzbekistan
fScientific and Innovation Department, Tashkent State Pedagogical University, Tashkent, Uzbekistan
gUniversidad Nacional de Trujillo, Trujillo, Peru
hDepartment of Chemical Engineering, Faculty of Engineering, Islamic University of Madinah, Madinah, Saudi Arabia
iDepartment of Medical Engineering, Al-Nisour University College, Baghdad, Iraq
First published on 25th October 2024
Abstract
In this study, we have prepared a novel bis-Schiff-base copper(II) complex by modifying Fe3O4 with acetylacetone functionalities and subsequently forming a Schiff base with 2-picolylamine and CuCl2 through a template method. Immobilization of 2,4-pentanedione and its reaction with 2-picolylamine enabled the synthesis of 1,3-diketimines (HNacNac) as an anionic ligand. This unique design resulted in a tetradentate N4 coordination sphere for copper(II) ion complexation. The resulting heterogeneous catalyst, [Fe3O4@Sil-Schiff-base-Cu(II)], efficiently catalyzed the click condensation of diverse aryl nitriles with sodium azide to produce 5-substituted 1H-tetrazoles in high yields and selectivity. The catalyst demonstrated remarkable stability and recyclability without appreciable loss of catalytic activity, as confirmed by hot filtration and reusability studies.
1. Introduction
Tetrazoles are indispensable nitrogen rich aromatic heterocyclic scaffolds that offer a broad spectrum of applications in various domains such as medicinal chemistry, high energy material science, biochemistry, pharmacology etc. They do not exist in nature and their preparation often involves artificial methods.1,2 Tetrazoles can be synthesized through various methods, including the Schmidt reaction, 1,3-dipolar cycloaddition, and click chemistry. Catalysts like copper(I) salts, gold(I) complexes, and organic catalysts play a crucial role in enhancing reaction efficiency and selectivity.3–8 In this sense, click chemistry is indeed a particularly valuable approach in tetrazole synthesis.9–11 Its high efficiency, selectivity, and mild reaction conditions make it an ideal tool for constructing complex tetrazole-containing molecules.12 The transition metals catalyzed azide–alkyne cycloaddition is the most prominent click reaction in this context.13,14 By employing this strategy, researchers can rapidly build diverse tetrazole libraries for drug discovery, materials science, and other applications.15Creating organic compounds through chemical reactions while protecting the environment is a major challenge in organic chemistry.16 The increasing concern for the planet has made it essential to find eco-friendly ways to perform these reactions. Therefore, scientists are actively searching for new catalysts that can produce desired compounds without harming the environment.17 Recent advancements have seen heterogeneous materials emerge as efficient catalysts for tetrazole synthesis, offering advantages like high surface area and ease of modification.8,18 These developments have expanded the possibilities for creating diverse tetrazole derivatives with potential applications in medicine, agriculture, and materials science.19–21 These studies have investigated a variety of materials as foundations for catalysts, such as graphene oxide,22 MCM-41,23 SBA-15, charcoal,24 boehmite13 and magnetic nanoparticles.25,26 Among these, magnetic nanoparticles composed of magnetite have garnered significant attention. Their magnetic properties enable rapid and convenient separation, while their biocompatibility, cost-effectiveness, and durability make them ideal for industrial processes.27,28 As a result, these nanoparticles have been employed across numerous applications, including catalysis and biomedical technologies.29
A variety of metal-based catalyst complexes have been immobilized on magnetic supports for diverse applications.30–32 Among these, copper complexes derived from Schiff bases have attracted considerable attention due to their facile synthesis and potential catalytic activity.33,34 Conventional approaches for immobilizing Schiff base ligands on magnetic nanoparticles typically involve the nucleophilic reaction of amine-functionalized supports with aldehydes or ketones.34–36 However, the heterogeneous distribution of amine groups on the nanoparticle surface often hinders the formation of multidentate ligands, which are crucial for creating efficient catalytic sites. This limitation, coupled with the complex and costly synthesis of pre-functionalized ligands, underscores the need for alternative support materials.
To address this challenge, acetylacetone (acac), a versatile β-diketone, emerges as a promising candidate. Acac can function as a bidentate ligand, forming stable complexes with metal ions.37 Its two carbonyl groups provide opportunities for further functionalization through reactions with amines to generate Schiff base ligands.38 The proximity of these carbonyl groups within the acac structure facilitates the formation of multidentate chelating systems.39 Additionally, the active methylene group in acac allows for alkylation reactions,40–42 enabling covalent attachment to various support and linker materials.
In this study, we aim to immobilize acetylacetone onto magnetic nanoparticles and subsequently synthesize a novel tetradentate salen-type Schiff base ligand and related complex through condensation with 2-picolylamine in the presence of CuCl2. The resulting copper complex will be evaluated as a catalyst for 5-Substituted 1H-tetrazoles synthesis under green conditions.
2. Experimental
2.1. Typical procedure for synthesis of [Fe3O4@Sil-Schiff-base-Cu(II)] complex
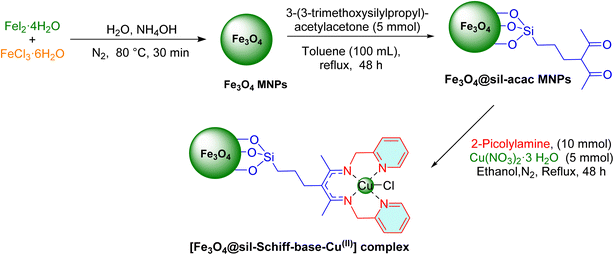
The synthesis began with the preparation of Fe3O4 MNPs using a previously established method.43 Concurrently, 2 g of Fe3O4 MNPs were dispersed in 100 milliliters of toluene for 30 minutes. Subsequently, 3-(3-trimethoxysilylpropyl)–acetylacetone (5 mmol) was introduced into the reaction mixture, which was then refluxed under vigorous stirring and a nitrogen (N2) atmosphere for 12 hours. Following this, the resulting Fe3O4@Sil–acac MNPs product was magnetically isolated, washed ethanol (3 × 25 mL), and subsequently dried at 80 °C for 6 hours. Subsequently, 3 grams of Fe3O4@Sil–acac were dispersed in 100 mL of degassed ethanol through sonication for 30 minutes. Then, 2-picolylamine (10 mmol) and copper(II) nitrate trihydrate (5 mmol) were sequentially added to the prepared suspension, followed by refluxing under vigorous stirring and a N2 atmosphere for 48 hours. Upon completion of the reaction, the [Fe3O4@Sil-Schiff-base-Cu(II)] complex was magnetically separated, washed multiple times with hot water and ethanol, and finally dried at 80 °C for 6 hours (Scheme 1).2.2. General procedure for synthesis of 5-substituted 1H-tetrazoles catalyzed by [Fe3O4@Sil-Schiff-base-Cu(II)] complex
To a 2 mL solution of aryl nitrile (1 mmol) and sodium azide (1.3 mmol) in PEG-400, [Fe3O4@Sil-Schiff-base-Cu(II)] complex (5 mol%) was added. The mixture was stirred at 120 °C until reaction completion. After cooling to room temperature, the reaction mixture was diluted with hot water, and the catalyst was removed by magnetic separation. The aqueous phase was acidified to pH 1 with 1 M hydrochloric acid and extracted with ethyl acetate (45 mL). The organic phase was washed repeatedly with water, dried over magnesium sulfate, and concentrated under reduced pressure. The crude product was purified by preparative thin-layer chromatography (TLC).3. Results and discussions
In this research, a novel Schiff base copper complex was synthesized via a three-step procedure. A template Hugo Schiff reaction of nanomagnetized acetyl acetone backbone with two equivalents of 2-picolylamine in the presence of one equivalent of copper salt, acting as both catalyst and coordination partner, yielded the desired five-coordinate copper(II) complex as the stabilized catalytic site (Scheme 1). The structure of the prepared nanoparticles was comprehensively characterized using FT-IR, XRD, TGA, EDAX, ICP-OES, EDS mapping, FE-SEM, TEM, and VSM analyses.3.1. Catalyst characterization
Fig. 1 presents the FT-IR spectra of Fe3O4, Fe3O4@Sil–acac, and the [Fe3O4@Sil-Schiff-base-Cu(II)] complex. Broad absorption band centered at around 3400 cm−1 is observed in the curve a, indicative of hydroxyl groups, likely attributable to adsorbed water or surface hydroxyl functionalities. The characteristic peaks at 568 and 634 cm−1 correspond to Fe–O vibrations within the iron oxide lattice, confirming the formation of the Fe3O4 MNPs.44 The spectrum of Fe3O4@Sil-acac exhibits additional bands at around 2900 cm−1, 1715 cm−1, and 1063 cm−1. These features can be assigned to C–H stretching, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and Si–O–Si stretching vibrations, respectively. The emergence of the Si–O–Si band confirms the successful immobilization of Sil–acac onto the Fe3O4 nanoparticles. In the spectrum of the [Fe3O4@Sil-c-Cu(II)] complex, the disappearance of the C
O stretching, and Si–O–Si stretching vibrations, respectively. The emergence of the Si–O–Si band confirms the successful immobilization of Sil–acac onto the Fe3O4 nanoparticles. In the spectrum of the [Fe3O4@Sil-c-Cu(II)] complex, the disappearance of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band and the concomitant appearance of new peaks around 1637 cm−1 strongly suggest the formation of the Schiff base and subsequent complexation with Cu(II) ions. These spectral alterations collectively provide evidence for the successful introduction of organic functionalities onto the Fe3O4 surface and the formation of the desired complex.
O band and the concomitant appearance of new peaks around 1637 cm−1 strongly suggest the formation of the Schiff base and subsequent complexation with Cu(II) ions. These spectral alterations collectively provide evidence for the successful introduction of organic functionalities onto the Fe3O4 surface and the formation of the desired complex.
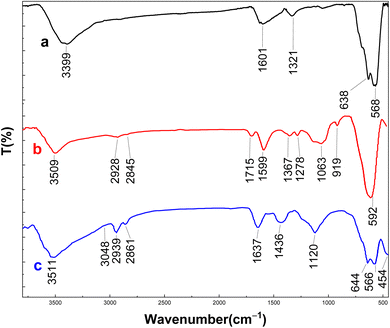 | ||
| Fig. 1 FT-IR analysis of (a) Fe3O4, (b) Fe3O4@Sil–acac and (c) [Fe3O4@Sil-Schiff-base-Cu(II)] complex. | ||
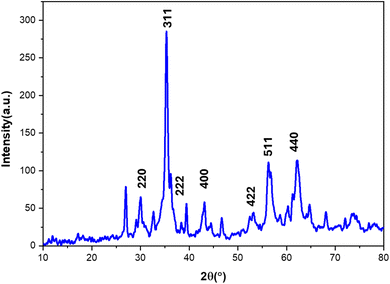
Structural characterization of the [Fe3O4@Schiff-base-Cu(II)] complex was performed using X-ray diffraction (XRD) in the 10–80° 2θ range (Fig. 2). Characteristic peaks corresponding to magnetite (Fe3O4) were observed at 2θ values of 30.02°, 35.31°, 43.22°, 53.19°, 56.89°, and 62.89° which are corresponded to (220), (311), (222), (400), (422), (511), (440), lattice planes of Fe3O4 and confirming the successful incorporation of magnetic nanoparticles. Additional peaks at 2θ values of 32.67°, 36.21°, 39.43°, 49°, 54°, and 60.29° suggest the presence of copper and the formation of a Schiff base–Cu complex on the Fe3O4 surface.
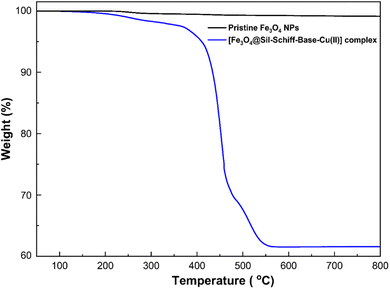
Thermogravimetric analysis (TGA) was conducted to assess the thermal stability of pristine Fe3O4 nanoparticles in comparison to their corresponding [Fe3O4@Sil-Schiff-base-Cu(II)] complex (Fig. 3). The pristine Fe3O4 nanoparticles exhibited negligible weight loss across the entire temperature range, indicative of their high thermal stability. In contrast, the [Fe3O4@Schiff-base-Cu(II)] complex demonstrated a pronounced two-step weight loss profile. The weight loss occurring between 200 and 600 °C is attributed to the decomposition of the organic silane linker and Schiff base ligand situated on the Fe3O4 surface. These findings collectively highlight the substantial influence of the organic coating on the thermal properties of the composite material and corroborate the successful formation of the [Schiff-base-Cu(II)] complex on the Fe3O4 surface.
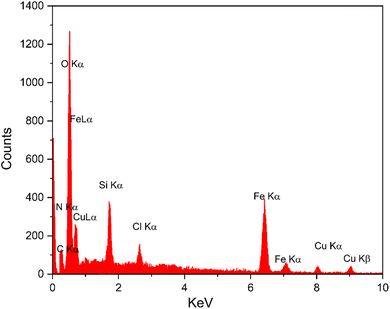
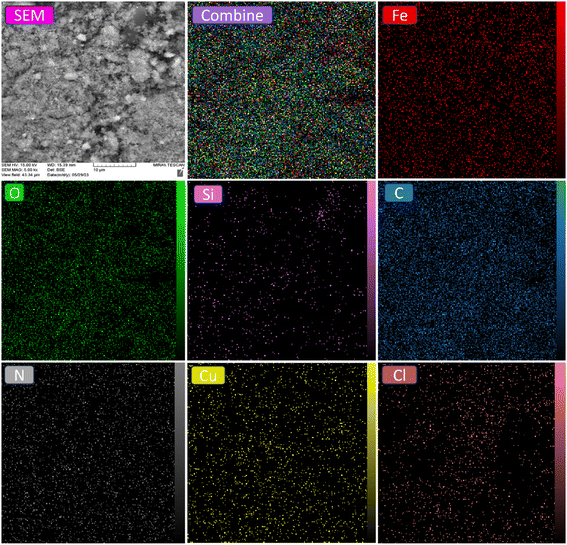
EDAX analysis confirms the successful synthesis of the [Fe3O4@Sil-Schiff-base-Cu(II)] complex. The detected elements, including Fe, O, Si, C, N, Cu, and Cl, are consistent with the theoretical composition of the material (Fig. 4). The sharp peaks associated with Fe and O suggest the presence of Fe3O4 nanoparticles, while the detection of Si confirms the integration of the organosilica linker. The presence of C and N is indicative of the Schiff base ligand, and the detection of Cu verifies the coordination of the copper ion to the ligand. The identified chlorine supports its role in the formation of a five-coordinate copper(II) complex via coordination to the copper precursor. Additionally, ICP-OES analysis revealed a copper content of approximately 1.73 × 10−3 mol g−1 in the sample.
EDAX mapping indicates a widespread distribution of Fe and O, suggesting the formation of Fe3O4. The mapping also revealed a homogeneous distribution of Si, C, and N elements throughout the Fe3O4 support, implying the presence of nitrogen-containing functional groups (Fig. 5). However, the silicon content appears lower than that of carbon and nitrogen, aligning with its approximate proportion in the stabilized complex composition. Conversely, copper and chlorine elements show a uniform distribution across the surface, facilitating optimal accessibility for guest reactant species.
SEM analysis of the [Fe3O4@Sil-Schiff-base-Cu(II)] complex revealed a heterogeneous population of agglomerated spherical particles with rough surface textures (Fig. 6). The particles exhibited a broad size distribution, averaging approximately 47.46 nm in diameter. These morphological features suggest a complex microstructure composed of both agglomerated and discrete nanoparticles.
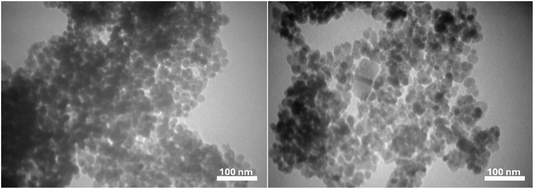
TEM analysis of [Fe3O4@Sil-Schiff-base-Cu(II)] complexes revealed well-defined, spherical nanoparticles with a smooth surface and a narrow size distribution centered around 20–25 nm (Fig. 7). These nanoparticles were evenly dispersed without aggregation, indicating high-quality synthesis. These characteristics, including uniform shape and size, suggest promising potential for various applications due to the nanoparticles' consistent behavior and reactivity.
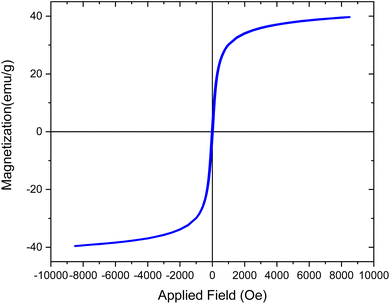
VSM analysis corroborated ferromagnetic behavior for the [Fe3O4@Sil-Schiff-base-Cu(II)] complex, as evidenced by a hysteresis loop (Fig. 8). This finding indicates the retention of Fe3O4 intrinsic magnetic properties. Nevertheless, the complex exhibited a reduced saturation magnetization (Ms = 39.87 emu g−1) compared to pristine Fe3O4 (Ms ≈ 63 emu g−1),45 indicating a lower magnetic moment. Conversely, an augmented coercivity was observed, implying enhanced resistance to magnetic field reversal. These magnetic property modifications are likely attributable to surface interactions, potential spin canting, and magnetic dilution arising from the organic ligand, which confirm the successful stabilization of [Sil-Schiff-base-Cu(II)] complex on Fe3O4 surface.
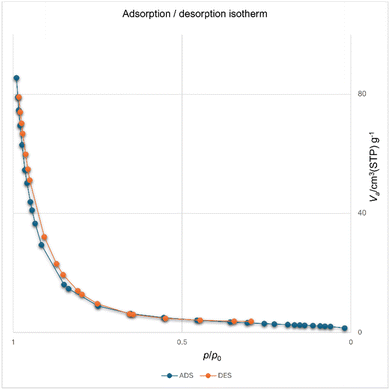
The [Fe3O4@Sil-Schiff-base-Cu(II)] complex exhibits a moderate BET surface area of 145.17 m2 g−1, indicating a sufficient surface area for adsorption (Fig. 9). The total pore volume of 0.1375 cm3 g−1 and mean pore diameter of 17.369 nm reveal a relatively narrow pore size distribution. t-plot and BJH analyses further confirm the micropore volume and pore size distribution characteristics of the catalyst. These combined properties make the catalyst well-suited for a range of applications in heterogeneous catalysis processes.
3.2. Catalytic study
The catalytic efficacy of the [Fe3O4@Sil-Schiff-base-Cu(II)] complex was evaluated in the click reaction of benzonitrile and sodium azide for the synthesis of 5-substituted 1H-tetrazoles, serving as a model reaction for the optimization of effective reaction parameters (Table 1). Blank test demonstrated that the reaction did not proceed in the absence of a catalyst, even after a long reaction time of 10 hours (Table 1, entry 1). Upon introduction of the [Fe3O4@Sil-Schiff-base-Cu(II)] complex to the reaction mixture, the conversion initiated, with a concomitant increase in yield as the catalyst loading was augmented. Optimal results were achieved with a 5 mg catalyst loading, affording complete conversion within 10 minutes. Further increasing of the catalyst amount to 7 mg did not result in enhanced reaction kinetics or product yield. Given the established utility of polyethylene glycols as solvents in analogous processes, the influence of average molecular weight on the current reaction was examined. While comparable results were obtained across different polyethylene glycol variants, PEG-400 exhibited marginally superior performance and was consequently selected as the optimal solvent. A reduction in reaction temperature led to diminished product yields, thereby establishing 120 °C as the preferred reaction temperature.| Entry | Amount of catalyst (mol%) | Solvent | Temperature (°C) | Time (min) | Yielda,b (%) |
|---|---|---|---|---|---|
| a Isolated yield. b Conditions: benzonitrile (1 mmol), sodium azide (1.3 mmol), [Fe3O4@Sil-Schiff-base-Cu(II)] complex (mol%) and solvent (2 mL). | |||||
| 1 | — | PEG-400 | 120 | 600 | NR |
| 2 | 1 | PEG-400 | 120 | 10 | 64 |
| 3 | 2 | PEG-400 | 120 | 10 | 72 |
| 4 | 4 | PEG-400 | 120 | 10 | 93 |
| 5 | 5 | PEG-400 | 120 | 10 | 99 |
| 6 | 7 | PEG-400 | 120 | 10 | 99 |
| 7 | 5 | Ethanol | 120 | 10 | 43 |
| 8 | 5 | Ethylene glycol | 120 | 10 | 71 |
| 9 | 5 | PEG-200 | 120 | 10 | 98 |
| 10 | 5 | PEG-600 | 120 | 10 | 98 |
| 11 | 5 | PEG-1000 | 120 | 10 | 95 |
| 12 | 5 | PEG-400 | 90 | 10 | 88 |
| 13 | 5 | PEG-400 | 60 | 10 | 53 |
| 14 | 5 | PEG-400 | 40 | 10 | 24 |
| 15 | 5 | PEG-400 | r. t. | 10 | NR |
The optimized method was applied to a diverse array of aryl nitriles to assess the method scope and the influence of electronic and steric factors on reaction efficiency (Table 2). Electron-withdrawing substituents on aryl nitriles exhibited enhanced reactivity compared to electron-donating groups due to increased electrophilicity of the nitrile carbon. Furthermore, the substituent's position significantly impacted reactivity, with ortho substituents generally affording lower yields and requiring longer reaction times than their para counterparts. This behavior can be attributed to both steric hindrance and electronic effects. Despite these variations, the majority of investigated aryl nitriles produced the corresponding products in satisfactory to excellent yields. To assess the selectivity of our method, we conducted comparative experiments with aliphatic nitriles. Under identical reaction conditions, no product formation was observed after 4 hours, suggesting a high degree of selectivity toward aromatic nitriles. This selectivity allows for the selective conversion of aromatic nitriles to tetrazole derivatives in the presence of aliphatic nitrile functionalities without interference.
| Entry | Aryl nitrile | Product | Time (min) | Yielda,b (%) | TON | TOF (min−1) | M. P. | Ref. |
|---|---|---|---|---|---|---|---|---|
| a Isolated yield. b Conditions: aryl nitrile (1.0 mmol), sodium azide (1.3 mmol) and [Fe3O4@Sil-Schiff-base-Cu(II)] complex (5 mg) in PEG-400 (1 mL) at 120 °C. | ||||||||
| 1 |
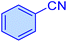
|
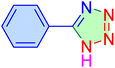
|
10 | 98 | 1960 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 172 172 |
219–221 | 46 |
| 2 |
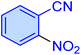
|
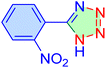
|
25 | 91 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 248 248 |
158–161 | 47 |
| 3 |
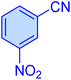
|
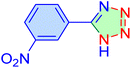
|
12 | 97 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213 213 |
56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 069 069 |
155–157 | 46 |
| 4 |
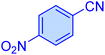
|
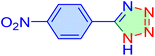
|
10 | 99 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 445 445 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670 |
220–22 | 46 |
| 5 |
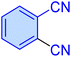
|
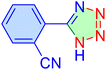
|
25 | 89 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289 289 |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 693 |
209–211 | 48 |
| 6 |
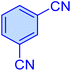
|
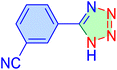
|
15 | 96 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 098 098 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 393 393 |
210–212 | 48 |
| 7 |
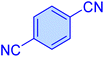
|
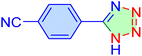
|
8 | 98 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 329 329 |
84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 971 971 |
254–256 | 48 |
| 8 |
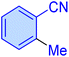
|
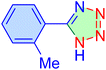
|
20 | 88 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 173 173 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
153–155 | 49 |
| 9 |
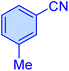
|
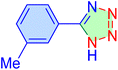
|
15 | 95 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
151–152 | 50 |
| 10 |
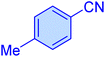
|
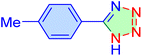
|
15 | 95 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 930 930 |
248–25 | 46 |
| 11 |
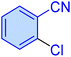
|
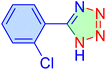
|
20 | 93 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 751 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 254 254 |
180–181 | 51 |
| 12 |
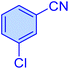
|
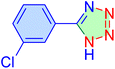
|
15 | 97 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 213 213 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 855 855 |
139–141 | 52 |
| 13 |
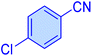
|
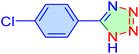
|
10 | 98 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 329 329 |
67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 976 976 |
155–156 | 46 |
| 14 |
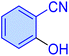
|
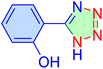
|
45 | 89 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 289 289 |
13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 718 718 |
224–225 | 53 |
| 15 |
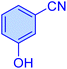
|
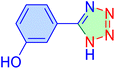
|
20 | 95 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 947 947 |
245–246 | 54 |
| 16 |
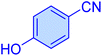
|
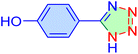
|
30 | 93 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 751 751 |
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 502 502 |
233–23 | 46 |
| 17 |
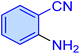
|
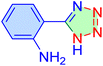
|
65 | 86 | 9942 | 9177 | 220–222 | 55 |
| 18 |
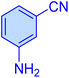
|
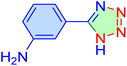
|
20 | 95 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 946 946 |
199–200 | 56 |
| 19 |
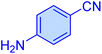
|
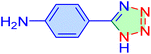
|
40 | 91 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 520 520 |
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 780 780 |
266–268 | 46 |
| 20 |
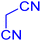
|
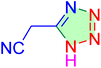
|
240 | N. R. | — | — | — | — |
| 21 |
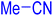
|
|
240 | N. R. | — | — | — | — |
The proposed mechanism for the synthesis of 5-aryl-1H-tetrazoles catalyzed by [Fe3O4@Sil-Schiff-base-Cu(II)] is outlined in Scheme 2.6,57,58 The catalytic cycle is initiated by the coordination of the nitrile substrate to the copper center within the catalyst, thereby activating the nitrile group. Subsequently, a nucleophilic attack of the azide ion on the activated nitrile species triggers a [2 + 3] cycloaddition cascade, resulting in the formation of the tetrazole ring. The reaction is finalized during the workup stage, where acidification protonates the intermediate sodium salt to afford the desired 5-aryl-1H-tetrazole product.
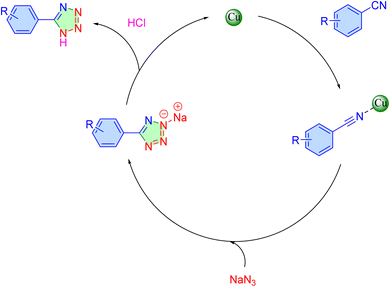 | ||
| Scheme 2 Possible mechanism for the click condensation of aryl nitriles and sodium azide over the catalysis of [Fe3O4@Sil-Schiff-base-Cu(II)] complex. | ||
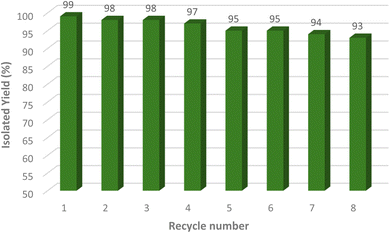
3.3. Reusability of catalyst
To evaluate the [Fe3O4@Sil-Schiff-base-Cu(II)] complex recyclability, it was magnetically separated from the reaction mixture after each cycle using an external magnet, followed by washing with hot ethanol and water. The recovered catalyst was reused in subsequent reaction cycles without significant loss of activity, demonstrating its robustness and potential for multiple applications. The catalyst exhibited consistent catalytic performance over eight consecutive cycles, highlighting its promising recyclability and economic viability (Fig. 10). To assess the structural integrity and functional group composition of the recovered catalyst, FT-IR analysis was conducted. As depicted in Fig. 11, the spectrum closely resembles that of the pristine catalyst, indicating its structural stability.3.4. Hot filtration test
To investigate the heterogeneous nature of the [Fe3O4@Sil-Schiff-base-Cu(II)] catalyst, a hot filtration test was performed. At the midpoint of the reaction, the catalyst was magnetically separated from the reaction mixture, which was subsequently allowed to proceed for an additional eight hours. The negligible product formation observed post-filtration confirmed the catalyst's predominantly heterogeneous character and indicated minimal metal leaching.3.5. Comparison study of catalytic activity
A comparative analysis of the catalytic efficiency of the herein reported [Fe3O4@Sil-Schiff-base-Cu(II)] catalyst with those previously documented in the literature was conducted, with particular attention to reaction conditions and isolated product yields (Table 3). While prior studies exhibit commendable attributes, they often necessitate extended reaction times, high catalyst loadings, or the employment of precious metal catalysts. In contrast, the Cu complexes presented herein demonstrate competitive product yields, coupled with advantages such as reduced catalyst loading, enhanced turnover frequencies, facile separation protocols, the use of environmentally conditions, and the absence of hazardous byproducts.| Entry | Catalyst | Time (min) | Yield (%) | Reference |
|---|---|---|---|---|
| 1 | CoFe2O4/MCM-41/PA/Cu | 20 | 91 | 59 |
| 2 | Amberlyst-15 | 720 | 91 | 60 |
| 3 | ZnFe2O4@SiO2–SO3H | 60 | 100 | 61 |
| 4 | CuFe2O4@SiO2–MnCl | 60 | 97.8 | 62 |
| 5 | Cu–Amd–RGO | 30 | 96 | 63 |
| 6 | CAES | 60 | 95 | 64 |
| 7 | f Cu–DPMI@biochar | 60 | 98 | 65 |
| 8 | MCM-41@Gln@Cu–Fe | 25 | 73 | 66 |
| 9 | [Fe3O4@Sil-schiff-base-Cu(II)] | 10 | 99 | This work |
Conclusions
A novel copper(II) bis-Schiff base complex was synthesized through post-synthetic modification of nanomagnetic Fe3O4 using HNacNac and pyridine building blocks. The complex features a tetradentate N4 coordination sphere, resulting in a five-coordinate copper center upon reaction with CuCl2. The resulting complex exhibited high catalytic activity in the synthesis of 5-substituted 1H-tetrazoles from diverse aromatic nitriles and sodium azide under mild conditions. The catalyst performance was influenced by electronic and steric factors of the substrates. Remarkably, the catalyst demonstrated exceptional stability and reusability, maintaining its catalytic activity over eight consecutive cycles without significant loss of efficiency. This work introduces a promising and sustainable catalytic system for the efficient production of tetrazoles.Data availability
The authors declare that all the data in this manuscript are available upon request.Author contributions
Chou-Yi Hsu: characterization of catalyst. Ahmed Rafiq AlBajalan: conceptualization, analysis, review draft, acquiring research funding and supervision. Sameer A. Awad: writing original draft, laboratory works. Muath Suliman: laboratory works. Nizomiddin Juraev: catalysis studies and analysis. Carlos Rodriguez-Benites: software and review/editing. Hamad AlMohamadi: conceptualization, laboratory works, analysis, review/editing. Abed J. Kadhim: software and review/editing.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
The authors express their gratitude to the Deanship of Scientific Research at King Khalid University for funding this work through the Large Research Group Project under grant number RGP.02/375/44.References
- C. G. Neochoritis, T. Zhao and A. Dömling, Tetrazoles via Multicomponent Reactions, Chem. Rev., 2019, 119, 1970–2042 CrossRef CAS PubMed.
- N. Dhiman, K. Kaur and V. Jaitak, Tetrazoles as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies, Bioorg. Med. Chem., 2020, 28, 115599 CrossRef CAS PubMed.
- A. Maleki and A. Sarvary, Synthesis of tetrazoles via isocyanide-based reactions, RSC Adv., 2015, 5, 60938–60955 RSC.
- M. M. Maseer, T. Kikhavani and B. Tahmasbi, A multidentate copper complex on magnetic biochar nanoparticles as a practical and recoverable nanocatalyst for the selective synthesis of tetrazole derivatives, Nanoscale Adv., 2024, 6, 3948–3960 RSC.
- G. Baskaya, İ. Esirden and E. Erken, et al., Synthesis of 5-Substituted-1H-Tetrazole Derivatives Using Monodisperse Carbon Black Decorated Pt Nanoparticles as Heterogeneous Nanocatalysts, J. Nanosci. Nanotechnol., 2017, 17, 1992–1999 CrossRef CAS.
- S. M. Joshi, R. B. Mane and K. R. Pulagam, et al., The microwave-assisted synthesis of 5-substituted 1: H -tetrazoles via [3+2] cycloaddition over a heterogeneous Cu-based catalyst: Application to the preparation of 13N-labelled tetrazoles, New J. Chem., 2017, 41, 8084–8091 RSC.
- H. C. Du, M. M. Matzuk and Y. C. Chen, Synthesis of 5-substituted tetrazoles: via DNA-conjugated nitrile, Org. Biomol. Chem., 2020, 18, 9221–9226 RSC.
- S. Swami, S. N. Sahu and R. Shrivastava, Nanomaterial catalyzed green synthesis of tetrazoles and its derivatives: a review on recent advancements, RSC Adv., 2021, 11, 39058–39086 RSC.
- M. Aldhoun, A. Massi and A. Dondoni, Click Azide−Nitrile Cycloaddition as a New Ligation Tool for the Synthesis of Tetrazole-Tethered C -Glycosyl α-Amino Acids, J. Org. Chem., 2008, 73, 9565–9575 CrossRef CAS PubMed.
- L. V. Myznikov, U. N. Dmitrieva and T. V. Artamonova, et al., Tetrazoles: LVII. Preparation and chemical properties of 1-substituted 5-arylsulfonyltetrazoles, Russ. J. Org. Chem., 2013, 49, 754–757 CrossRef CAS.
- S. Krištafor, A. Bistrović and J. Plavec, et al., One-pot click synthesis of 1,2,3-triazole-embedded unsaturated uracil derivatives and hybrids of 1,5- and 2,5-disubstituted tetrazoles and pyrimidines, Tetrahedron Lett., 2015, 56, 1222–1228 CrossRef.
- S. M. Kondengadan, S. Bansal and C. Yang, et al., Click chemistry and drug delivery: A bird’s-eye view, Acta Pharm. Sin. B, 2023, 13, 1990–2016 CrossRef CAS PubMed.
- M. Mohammadi, M. Khodamorady and B. Tahmasbi, et al., Boehmite nanoparticles as versatile support for organic–inorganic hybrid materials: Synthesis, functionalization, and applications in eco-friendly catalysis, J. Ind. Eng. Chem., 2021, 97, 1–78 CrossRef CAS.
- R. Bikas, N. Heydari and T. Lis, Catalytic synthesis of tetrazoles by a silica supported Zn(II) coordination compound containing azide ligand, J. Mol. Struct., 2023, 1281, 135120 CrossRef CAS.
- S. V. Voitekhovich, O. A. Ivashkevich and P. N. Gaponik, Synthesis, properties, and structure of tetrazoles: Certain achievements and prospects, Russ. J. Org. Chem., 2013, 49, 635–654 CrossRef CAS.
- M. C. Bryan, P. J. Dunn and D. Entwistle, et al., Key Green Chemistry research areas from a pharmaceutical manufacturers' perspective revisited, Green Chem., 2018, 20, 5082–5103 RSC.
- S. G. Koenig, D. K. Leahy and A. S. Wells, Evaluating the Impact of a Decade of Funding from the Green Chemistry Institute Pharmaceutical Roundtable, Org. Process Res. Dev., 2018, 22, 1344–1359 CrossRef CAS.
- M. Kazemi, Copper Catalysts Immobilized on Magnetic Nanoparticles: Catalysis in Synthesis of Tetrazoles, Nanomater. Chem., 2023, 1, 1–11 Search PubMed.
- S. Leyva-Ramos, Recent Developments in the Synthesis of Tetrazoles and their Pharmacological Relevance, Curr. Org. Chem., 2022, 25, 388–403 CrossRef.
- E. A. Popova, A. V. Protas and R. E. Trifonov, Tetrazole Derivatives as Promising Anticancer Agents, Anti-Cancer Agents Med. Chem., 2018, 17, 1856–1868 CrossRef.
- B. Chen, H. Lu and J. Chen, et al., Recent Progress on Nitrogen-Rich Energetic Materials Based on Tetrazole Skeleton, Top. Curr. Chem., 2023, 381, 25 CrossRef CAS PubMed.
- A. Singh and A. Agarwal, Anchoring CuO nanoparticle on nitrogen-doped reduced graphene oxide as nanocatalyst for the synthesis of 5-substituted-1H-tetrazole and 1,2,3- triazole derivatives, Mol. Catal., 2023, 547, 113377 CrossRef CAS.
- M. Ghadermazi, S. Molaei and S. Khorami, Synthesis, characterization and catalytic activity of copper deposited on MCM-41 in the synthesis of 5-substituted 1H-tetrazoles, J. Porous Mater., 2023, 30, 949–963 CrossRef CAS.
- A. Singh and A. Agarwal, Anchoring CuO nanoparticle on nitrogen-doped reduced graphene oxide as nanocatalyst for the synthesis of 5-substituted-1H-tetrazole and 1,2,3- triazole derivatives, Mol. Catal., 2023, 547, 113377 CrossRef CAS.
- A. Alexis Ramírez-Coronel, R. Sivaraman and Y. M. Ahmed, et al., A Green and Ecofriendly Catalytic System for One-Pot Three-Component Synthesis of 5-Substituted 1H-Tetrazoles Under Microwave Irradiation, Polycyclic Aromat. Compd., 2024, 44, 577–590 CrossRef.
- Y. S. Priyanka and P. Rana, et al., Unexplored catalytic potency of a magnetic CoFe2O4/Ni-BDC MOF composite for the one-pot sustainable synthesis of 5-substituted 1-H tetrazoles, Chem. Eng. J., 2024, 496, 153995 CrossRef.
- S. Khizar, N. M. Ahmad and N. Zine, et al., Magnetic Nanoparticles: From Synthesis to Theranostic Applications, ACS Appl. Nano Mater., 2021, 4, 4284–4306 CrossRef CAS.
- Z. Ma, J. Mohapatra and K. Wei, et al., Magnetic Nanoparticles: Synthesis, Anisotropy, and Applications, Chem. Rev., 2023, 123, 3904–3943 CrossRef CAS.
- Q. Zhang, X. Yang and J. Guan, Applications of Magnetic Nanomaterials in Heterogeneous Catalysis, ACS Appl. Nano Mater., 2019, 2, 4681–4697 CrossRef CAS.
- M. Nasrollahzadeh, Advances in Magnetic Nanoparticles-Supported Palladium Complexes for Coupling Reactions, Molecules, 2018, 23, 2532 CrossRef.
- R. Dalpozzo, Magnetic nanoparticle supports for asymmetric catalysts, Green Chem., 2015, 17, 3671–3686 RSC.
- J. Rakhtshah, A comprehensive review on the synthesis, characterization, and catalytic application of transition-metal Schiff-base complexes immobilized on magnetic Fe3O4 nanoparticles, Coord. Chem. Rev., 2022, 467, 214614 CrossRef CAS.
- C. Boulechfar, H. Ferkous and A. Delimi, et al., Schiff bases and their metal Complexes: A review on the history, synthesis, and applications, Inorg. Chem. Commun., 2023, 150, 110451 CrossRef CAS.
- J. Rakhtshah, A comprehensive review on the synthesis, characterization, and catalytic application of transition-metal Schiff-base complexes immobilized on magnetic Fe3O4 nanoparticles, Coord. Chem. Rev., 2022, 467, 214614 CrossRef CAS.
- A. Ghorbani-Choghamarani, Z. Darvishnejad and M. Norouzi, Synthesis and characterization of copper(II) Schiff base complex supported on Fe 3 O 4 magnetic nanoparticles: a recyclable catalyst for the one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-ones, Appl. Organomet. Chem., 2015, 29, 707–711 CrossRef CAS.
- M. Pawlaczyk, R. Frański and M. Cegłowski, et al., Mass Spectrometric Investigation of Organo-Functionalized Magnetic Nanoparticles Binding Properties toward Chalcones, Materials, 2021, 14, 4705 CrossRef CAS PubMed.
- M. Kremer and U. Englert, N Donor substituted acetylacetones – versatile ditopic ligands, Z. Kristallogr.–Cryst. Mater., 2018, 233, 437–452 CrossRef CAS.
- S. K. Gupta, P. B. Hitchcock and Y. S. Kushwah, Synthesis, Characterization and Crystal Structure of a Nickel(II) Schiff Base Complex Derived from Acetylacetone and Ethylenediamine, J. Coord. Chem., 2002, 55, 1401–1407 CrossRef CAS.
- G. O. Dudek and R. H. Holm, A Proton Resonance Study of Bis-(acetylacetone)-ethylenediimine and Related Schiff Bases, J. Am. Chem. Soc., 1961, 83, 2099–2104 CrossRef CAS.
- B. Bisek and W. Chaładaj, Access to 2-Alkenyl-furans via a Cascade of Pd-Catalyzed Cyclization/Coupling Followed by Oxidative Aromatization with DDQ, J. Org. Chem., 2024, 89, 7275–7279 CrossRef CAS PubMed.
- A. Shrinidhi and C. L. Perrin, Nucleophilic Addition of Enolates to 1,4-Dehydrobenzene Diradicals Derived from Enediynes: Synthesis of Functionalized Aromatics, ACS Omega, 2022, 7, 22930–22937 CrossRef CAS PubMed.
- F.-X. Wang, J.-L. Yan and Z. Liu, et al., Assembly of multicyclic isoquinoline scaffolds from pyridines: formal total synthesis of fredericamycin A, Chem. Sci., 2021, 12, 10259–10265 RSC.
- Y. Wei, B. Han and X. Hu, et al., Synthesis of Fe3O4 Nanoparticles and their Magnetic Properties, Procedia Eng., 2012, 27, 632–637 CrossRef CAS.
- A. E. Ferenj, D. M. Kabtamu and A. H. Assen, et al., Hagenia abyssinica-Biomediated Synthesis of a Magnetic Fe3O4/NiO Nanoadsorbent for Adsorption of Lead from Wastewater, ACS Omega, 2024, 9, 6803–6814 CrossRef CAS PubMed.
- F. Alemi-Tameh, J. Safaei-Ghomi and M. Mahmoudi-Hashemi, et al., A comparative study on the catalytic activity of Fe3O4@SiO2–SO3H and Fe3O4@SiO2–NH2 nanoparticles for the synthesis of spiro [chromeno [2, 3-c] pyrazole-4, 3′-indoline]-diones under mild conditions, Res. Chem. Intermed., 2016, 42, 6391–6406 CrossRef CAS.
- Z. He, L. Feng and P. Wu, et al., A Top-Down Approach to Synthesis of pH-Controlled Cu NPs: Their Catalytic Activity toward the One-Pot Preparation of α-Aminonitriles and 5-Substituted 1H-Tetrazoles from Aldehydes, ChemistrySelect, 2020, 5, 7753–7767 CrossRef CAS.
- J. Bonnamour and C. Bolm, Iron salts in the catalyzed synthesis of 5-substituted 1H-tetrazoles, Chem.–Eur. J., 2009, 15, 4543–4545 CrossRef CAS PubMed.
- M. Esmaeilpour, J. Javidi and S. Zahmatkesh, One-pot synthesis of 1- and 5-substituted 1 H -tetrazoles using 1,4-dihydroxyanthraquinone–copper(II) supported on superparamagnetic Fe 3 O 4 @SiO 2 magnetic porous nanospheres as a recyclable catalyst, Appl. Organomet. Chem., 2016, 30, 897–904 CrossRef CAS.
- A. M. Liao, T. Wang and B. Cai, et al., Design, synthesis and evaluation of 5-substituted 1-H-tetrazoles as potent anticonvulsant agents, Arch. Pharmacal Res., 2017, 40, 435–443 CrossRef CAS PubMed.
- Y. Zhu, Y. Ren and C. Cai, One-pot synthesis of 5-substituted lH-tetrazoles from aryl bromides with potassium hexakis(cyano-κC)ferrate(4 -)(K4[Fe(CN)6]) as cyanide source, Helv. Chim. Acta, 2009, 92, 171–175 CrossRef CAS.
- S. Rostamizadeh, H. Ghaieni and R. Aryan, et al., Zinc chloride catalyzed synthesis of 5-substituted 1H-tetrazoles under solvent free condition, Chin. Chem. Lett., 2009, 20, 1311–1314 CrossRef CAS.
- N. Nowrouzi, S. Farahi and M. Irajzadeh, 4-(N,N-Dimethylamino)pyridinium acetate as a recyclable catalyst for the synthesis of 5-substituted-1H-tetrazoles, Tetrahedron Lett., 2015, 56, 739–742 CrossRef CAS.
- M. Abdollahi-Alibeik and A. Moaddeli, Multi-component one-pot reaction of aldehyde, hydroxylamine and sodium azide catalyzed by Cu-MCM-41 nanoparticles: a novel method for the synthesis of 5-substituted 1H-tetrazole derivatives, New J. Chem., 2015, 39, 2116–2122 RSC.
- P. Akbarzadeh, N. Koukabi and E. Kolvari, Anchoring of triethanolamine–Cu(II) complex on magnetic carbon nanotube as a promising recyclable catalyst for the synthesis of 5-substituted 1H-tetrazoles from aldehydes, Mol. Diversity, 2020, 24, 319–333 CrossRef CAS.
- S. A. Padvi and D. S. Dalal, Choline chloride–ZnCl 2 : Recyclable and efficient deep eutectic solvent for the [2+3] cycloaddition reaction of organic nitriles with sodium azide, Synth. Commun., 2017, 47, 779–787 CrossRef CAS.
- J. M. McManus and R. M. Herbst, Tetrazole Analogs of Aminobenzoic Acid Derivatives, J. Org. Chem., 1959, 24, 1044–1046 CrossRef CAS.
- R. S. B. Gonçalves and e M. L. S. de Mariz, Copper catalysis in the synthesis of 1,2,3-triazoles and tetrazoles, in Copper in N-Heterocyclic Chemistry, Elsevier, pp. , pp. 75–113 Search PubMed.
- M. Norouzi and S. Beiranvand, Fe3O4@SiO2@BHA-Cu(II) as a new, effective, and magnetically recoverable catalyst for the synthesis of polyhydroquinoline and tetrazole derivatives, J. Chem. Sci., 2023, 135, 86 CrossRef CAS.
- S. Molaei and M. Ghadermazi, Copper-decorated core–shell structured ordered mesoporous containing cobalt ferrite nanoparticles as high-performance heterogeneous catalyst toward synthesis of tetrazole, Sci. Rep., 2023, 13, 15146 CrossRef CAS PubMed.
- R. Shelkar, A. Singh and J. Nagarkar, Amberlyst-15 catalyzed synthesis of 5-substituted 1-H-tetrazole via [3+2] cycloaddition of nitriles and sodium azide, Tetrahedron Lett., 2013, 54, 106–109 CrossRef CAS.
- A. Nozari, H. Hassani and A. Karimian, ZnFe2O4@SiO2–SO3H Magnetic Nanoparticles: A New, Efficient, and Recyclable Heterogeneous Nanocatalyst for Successful Synthesis of 5-Substituted-1H-tetrazoles, Russ. J. Org. Chem., 2023, 59, 1370–1381 CrossRef CAS.
- M. Yousefizadeh, S. Saeednia and A. M. Hatefi, et al., CuFe2O4@SiO2-LMnCl: an efficient, highly recyclable magnetic nanoparticle for synergic catalyzing of tetrazoles, J. Iran. Chem. Soc., 2023, 20, 1569–1578 CrossRef CAS.
- P. A. Kulkarni, A. K. Satpati and M. Thandavarayan, et al., An efficient Cu/functionalized graphene oxide catalyst for synthesis of 5-substituted 1H-tetrazoles, Chem. Pap., 2021, 75, 2891–2899 CrossRef CAS.
- N. Razavi and B. Akhlaghinia, Cu(ii) immobilized on aminated epichlorohydrin activated silica (CAES): As a new, green and efficient nanocatalyst for preparation of 5-substituted-1H-tetrazoles, RSC Adv., 2015, 5, 12372–12381 RSC.
- M. Alekasir, S. Heydarian and B. Tahmasbi, The synthesis of biochar from biomass waste recycling and its surface modification for immobilization of a new Cu complex as a reusable nanocatalyst in the homoselective synthesis of tetrazoles, Res. Chem. Intermed., 2024, 50, 2031–2049 CrossRef CAS.
- M. Ghadermazi, S. Molaei and S. M. Mousavipour, Nano Architectonics of Fe–Cu Bimetallic Particles Confined in a Mesoporous Silica Network for the Synthesis of 5-Substituted 1H-tetrazoles, J. Inorg. Organomet. Polym. Mater., 2023, 33, 3128–3145 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |