Epitaxial strain manipulation of the cluster glass state in LaMnO3 films
Yongmei
Liang
a,
Haonan
Lian
 a,
Yaqiang
Li
c,
Dan
Liu
a,
Baochen
Han
*a,
Jian
Qi
a,
Yaqiang
Li
c,
Dan
Liu
a,
Baochen
Han
*a,
Jian
Qi
 *b and
Dongxiao
Ma
*c
*b and
Dongxiao
Ma
*c
aHebei Key Laboratory of Flexible Functional Materials, School of Materials Science and Engineering, Hebei University of Science and Technology, Shijiazhuang 050018, China. E-mail: hbchebust@163.com
bState Key Laboratory of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100049, China. E-mail: jqi@ipe.ac.cn
cSchool of Automotive Engineering, Hebei Vocational University of Technology and Engineering, Xingtai, 054000, China. E-mail: madongxiao1258@163.com
First published on 1st August 2024
Abstract
As a new-type magnetic state, the cluster glass state in manganite is arousing considerable attention due to its important theoretical value and extensive application prospects in condensed matter physics and spintronics. Due to the complex magnetic interactions, the cluster glass state is difficult to form and regulate in single films. Studies report a new phenomenon that epitaxial strain can regulate the formation of the cluster glass state in LaMnO3 (LMO) films. Comparing LMO thin films with different thicknesses grown on a (001)-oriented LaAlO3 (LAO) single crystal substrate, we found that the 20-nm-thick LMO film is more likely to form the cluster glass state than the 60-nm-thick and 120-nm-thick films. This can be attributed to the uneven distribution of strain and Mn ions in the depth profile. Our work demonstrates that thickness is an important method for regulating the formation of the cluster glass state in LMO films.
New conceptsAs a novel magnetic state, the cluster glass state in manganite has garnered significant interest owing to its critical theoretical implications and broad potential applications in condensed matter physics and spintronics. Originating from the competition between ferromagnetic and antiferromagnetic interactions, the cluster glass state is usually found to exist in heterostructures or multilayer films. The formation and regulation of the cluster glass state in single films poses challenges due to the intricate magnetic interactions involved. The epitaxial strain arising from the lattice mismatch between the substrate and film plays a pivotal role in influencing the ferromagnetic and antiferromagnetic interactions between Mn ions in manganite films. Manipulating the thickness of the film serves as a potent method for controlling epitaxial strain. This paper introduces a novel concept and methodology for achieving the regulation of the cluster glass state by influencing strain through the manipulation of film thickness. Our study delves deeper into the regulation mechanism underlying the cluster glass state, offering valuable insights that contribute to the enhanced comprehension of this novel magnetic state. This research holds significant importance in advancing our understanding of the cluster glass state and its potential applications in the field of condensed matter physics and spintronics. |
Introduction
LaMnO3 (LMO) films have received widespread attention due to the unique interfacial phenomena, such as the exchange bias (EB) effect in the paramagnetic LaNiO3 and ferromagnetic (FM) LaMnO3 superlattices,1 charge transfer in LMO/BiFeO3 heterostructures,2 and half-metallicity in LMO/SrTiO3 (STO) heterostructures.3 Unlike the A-type antiferromagnetic insulator in bulk LMO, there are some unexpected physical phenomena in LMO thin films due to the unexpected ferromagnetic behavior caused by cation deficiency or strain.4–7The cluster glass state is widely recognized due to its close relationship with some unique physical properties,8–10 including the exchange bias effect in compound Mn2PtIn,11 magneto-dielectric effect in giant dielectric double perovskite La1.8Pr0.2CoFeO6,12 photoinduced magnetization in (Ni,Zn,Fe,Ti)3O4 thin films,13 giant vertical magnetization in SrFe0.25Co0.75O2.63,14 and so on.15 Originating from the competition between ferromagnetic and antiferromagnetic interactions, the cluster glass state was found to exist in the LMO films, which is unique in the manganite single films.16,17 The epitaxial strain induced by the lattice mismatch between the substrate and film is a critical factor affecting a wide range of physical properties such as electron mobility, magnetic ordering, superconductivity and ferroelectricity by manipulating the antiferromagnetic interactions between Mn ions in manganite films.18,19 Li et al. found that biaxial strain can effectively modulate the interface magnetoelectric coupling, orbital occupancy, and half-metallicity of La2/3Sr1/3MnO3/BiFeO3 heterostructures.20 Moreover, it is a powerful tool for manipulating epitaxial strain by controlling the thickness of the film.21 Prasad et al. presented that film thickness, which can control the degree of compressive strain, has a significant influence on the magnetic transport properties of Nd0.51Sr0.49MnO3, and the increasing thickness will lead to strain relaxation, which is conducive to the ferromagnetic metallic phase.22 That is, the thickness of the thin film is closely related to magnetic interaction. Therefore, the thickness of the film may realize the control of the formation of the cluster glass state by influencing the strain as well as the magnetic interactions of LMO films.
In this study, we deposit epitaxial 20-, 60- and 120-nm-thick LMO thin films on a (001)-oriented LaAlO3 (LAO) single-crystal substrate by pulsed laser deposition (PLD), and we have found that the cluster glass state is most likely to be formed in the 20-nm-thick LMO film, while it is the most difficult to form the cluster glass state in the 120-nm-thick LMO film. The formation of cluster glass states in LMO films is the result of the combined effect of strain and Mn ion valence states. Comparing the different thicknesses of LMO films, we can find that the strain of the LMO films varies as the thickness increases, and at the same time, the percentage of Mn2+, Mn3+, and Mn4+ ions also changes.
Experimental section
The 20-, 60- and 120-nm-thick LMO films were deposited on a (001)-oriented LAO substrate (2.5 mm × 10 mm) by sputtering the homemade LMO target using a KrF excimer laser (wavelength λ = 248 nm, frequency f = 5 Hz) in PLD. The samples were grown at 700 °C under an oxygen pressure of 30 Pa and then cooled down to room temperature in 5 × 104 Pa of oxygen at a rate of 2 °C min−1. The 20-, 60- and 120-nm-thick LMO films were prepared by controlling the growth time as 5, 15 and 30 min, respectively. The crystalline structure and strain state of the films were analyzed by X-ray diffraction (XRD) (Rigaku, D/max2000, Cu Kα radiation) and high-resolution XRD (Bruker D8 DISCOVER), respectively. The crystalline quality of the films was analyzed by transmission electron microscopy (TEM, Tecnai, F20, Netherlands). The magnetic properties of the LMO films were determined by using a superconducting quantum interference device magnetometer (SQUID) applying an in-plane magnetic field. X-ray photoelectron spectroscopy (XPS; Thermo ESCALAB 250; Al Kα source, 1486.6 eV, energy step: 0.1 eV, and resolution: 400 meV) was chosen to analyze the chemical valence states and oxygen vacancy concentration of Mn ions in the LMO films.Results and discussion
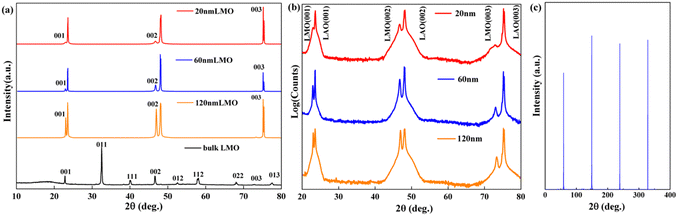
Fig. 1(a) and (b) show X-ray patterns of the 20-, 60- and 120-nm-thick LMO thin films grown on a (001)-oriented LAO substrate and bulk LMO. Only the (00l) diffraction peaks can be detected in the LMO films, indicating that all LMO films have epitaxially grown on the LAO substrates. Transmission electron microscopy (TEM) was utilized to further study the epitaxial nature of the LMO films grown on the (001)-oriented LAO substrate. Fig. 1(c) shows XRD ϕ scans taken on the (101) reflections of 20-nm-thick LMO films. Fourfold symmetry is clearly seen for the LMO films, indicating the cubic-on-cubic epitaxial growth of LMO films on LAO substrates.Fig. 2(a) and (b) show the cross-sectional TEM images of the 20-nm-thick LMO film grown on the (001)-oriented LAO substrate and the high-resolution TEM image (HRTEM). The pictures show that the interface between the LMO film and LAO substrate is clear and well defined. Fig. 2(c) shows the fast Fourier transform (FFT) pattern of the LMO/LAO interface, indicating that the epitaxial relationship between the LMO film and LAO substrate is (100)LMO‖(100)LAO and (001)LMO‖(001)LAO. The above XRD and TEM results show that high-quality LMO films grown on (001)-oriented LAO substrates have been produced to study the magnetic properties.
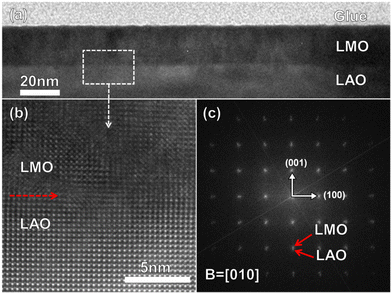 | ||
| Fig. 2 (a) Cross-sectional TEM images of the 20-nm-thick LMO film. (b) HRTEM image of the LMO/LAO interface. (c) FFT pattern of the LMO/LAO interface transformed from (b). | ||
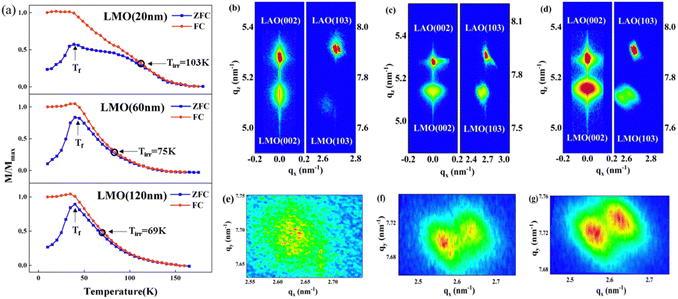
As shown in Fig. 3(a), the magnetization versus temperature (M–T) curves of the LMO films were measured after field-cooling (FC) and zero field-cooling (ZFC) processes. Both the FC and ZFC magnetization of all LMO films increases first with the decrease of temperature. The difference is that the ZFC magnetization reaches the maximum at the freezing temperature (Tf = 40 K) and then rapidly decreases below Tf, while the FC magnetization does not significantly change when the temperature is below Tf. It is clear that the FC and ZFC M–T curves bifurcate at the irreversibility temperature (Tirr), and the Tirr of the 20-, 60- and 120-nm-thick LMO films is 103 K, 75 K and 69 K, respectively. The criterion for judging Tirr is that the difference in the Y-axis coordinates of the ZFC and FC curves is greater than 0.05. Furthermore, the Tirr is far away from Tf, which is one of the typical characteristics of the cluster glass state.23 Combining the above phenomena, it is not difficult to draw a conclusion that the cluster glass state is formed in all samples. Moreover, it is believed that the higher the irreversible temperature, the easier the formation of the cluster glass state.17 Hence, the cluster glass state is most likely to form in the 20-nm-thick LMO film, while it is the most difficult to form in the 120-nm-thick LMO film.
In order to investigate why different thicknesses of LMO films lead to varying degrees of difficulty in forming cluster glass states, the X-ray diffraction reciprocal space maps (RSMs) were analyzed to study the strain state of the LMO films, as shown in Fig. 3(b)–(d). Reciprocal space maps around the (103) reflections of the 20-, 60- and 120-nm-thick LMO films show that the LMO layer gradually divides into two parts as the thickness of the film increases. An enlarged view of the RSM around the (103) reflections of the LMO layer (Fig. 3(e)–(g)) shows that the trend of layering that appears in the 60-nm-thick LMO film, and the 120-nm-thick film has obvious delamination. Moreover, the in-plane and out-of-plane lattice constants of all LMO films were calculated according to the RSM around (002) reflections and (103) reflections. The calculated values of lattice constants (a, b, and c) and strain values are listed in Table 1. The biaxial strain (ε) of the LMO films can be calculated according to the following formula24,25
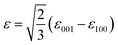 | (1) |
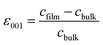 and
and 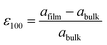 . The positive (negative) value of ε indicates that the film is in the tensile (compressive) strain state. The data show that the strain states of films with different thicknesses are different. The 20-nm-thick LMO film is in the biaxial tensile strain state (+0.94%). For the 60-nm-thick and 120-nm-thick LMO films, the lower layer (near the substrate) of the films is in the biaxial tensile strain state (+2.30% and +1.70%), while the upper layers (slightly away from LAO substrate) of the films are both in the biaxial compressive stress state (−1.28% and −1.97%). The presence of both compressive and tensile strain in a single thin film can be explained by the interplay of factors such as film thickness and lattice mismatch. In thinner films (20 nm), the biaxial tensile strain state is dominant due to the lattice mismatch between the LAO substrate and LMO film. However, in thicker LMO films (60 nm and above), the lower layer remains in a biaxial tensile strain state influenced by the substrate, while the upper layer experiences a different strain due to its distance from the substrate and proximity to the lower layer with different lattice constants. This leads to strain relaxation in the upper layer of thicker films, resulting in the coexistence of compressive and tensile strain within the same film. Then, combined with the analysis of M–T curves, it is obvious that the larger biaxial tensile strain (+0.94%) is in favor of the formation of the cluster glass state in the 20-nm-thick LMO film. And then strain relaxation occurs as the thickness increases and is detrimental to the formation of the cluster glass state.
. The positive (negative) value of ε indicates that the film is in the tensile (compressive) strain state. The data show that the strain states of films with different thicknesses are different. The 20-nm-thick LMO film is in the biaxial tensile strain state (+0.94%). For the 60-nm-thick and 120-nm-thick LMO films, the lower layer (near the substrate) of the films is in the biaxial tensile strain state (+2.30% and +1.70%), while the upper layers (slightly away from LAO substrate) of the films are both in the biaxial compressive stress state (−1.28% and −1.97%). The presence of both compressive and tensile strain in a single thin film can be explained by the interplay of factors such as film thickness and lattice mismatch. In thinner films (20 nm), the biaxial tensile strain state is dominant due to the lattice mismatch between the LAO substrate and LMO film. However, in thicker LMO films (60 nm and above), the lower layer remains in a biaxial tensile strain state influenced by the substrate, while the upper layer experiences a different strain due to its distance from the substrate and proximity to the lower layer with different lattice constants. This leads to strain relaxation in the upper layer of thicker films, resulting in the coexistence of compressive and tensile strain within the same film. Then, combined with the analysis of M–T curves, it is obvious that the larger biaxial tensile strain (+0.94%) is in favor of the formation of the cluster glass state in the 20-nm-thick LMO film. And then strain relaxation occurs as the thickness increases and is detrimental to the formation of the cluster glass state.
| In-plane lattice (a = b) (Å) | ε 100 (%) | Out-of-plane lattice (c) (Å) | ε 001 (%) | ε (%) | |
|---|---|---|---|---|---|
| LMO (20 nm) | 3.850 | −1.05 | 3.895 | +0.10 | +0.94 |
| LMO (60 nm) | 3.780, 3.951 | −2.85, +1.54 | 3.890 | +0.03 | +2.30, −1.28 |
| LMO (120 nm) | 3.795, 3.970 | −2.46, +2.03 | 3.876 | −0.39 | +1.70, −1.97 |
| Bulk LMO | 3.891 | 0 | 3.891 | 0 | 0 |
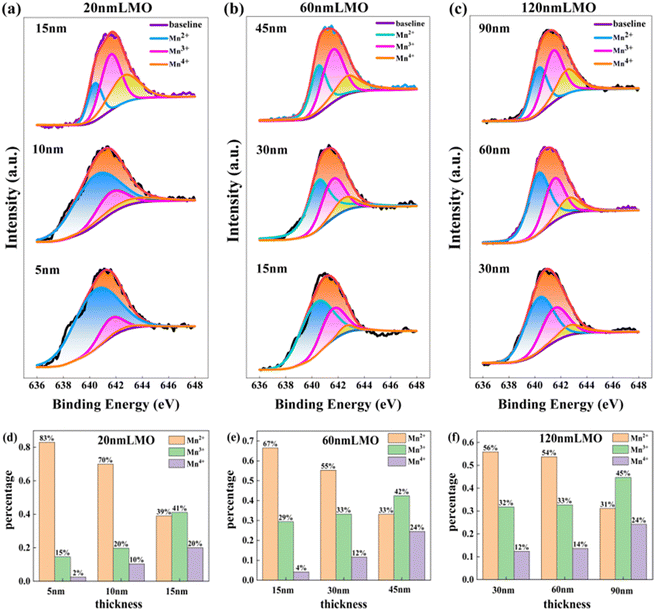
To investigate why the strain state can affect the degrees of difficulty in forming cluster glass states, the Mn ion valence states at different depths of LMO films were analyzed by X-ray photoelectron spectroscopy (XPS). Fig. 4(a)–(c) show the detailed core-level spectra of Mn 2p3/2, which are fitted very well with the peaks at the binding energies of 640.8, 641.6 and 642.6 eV, corresponding to the peak position of Mn2+, Mn3+, and Mn4+ ions,26–28 respectively. The percentage content of Mn2+, Mn3+, and Mn4+ of the 20-, 60- and 120-nm-thick LMO films are shown in Fig. 4(d)–(f). The content of Mn2+ ions is much larger than that of Mn3+ and Mn4+ ions at the lower layer for all LMO films. Furthermore, for the 20-nm-thick film, the proportion of Mn2+ ions is higher than that of the 60-nm-thick and 120-nm-thick film at each layer. That is, the proportion of Mn2+ ions decreases when the strain relaxation occurs. It is widely believed that the interaction between Mn2+ ions is super-exchange antiferromagnetic interaction,29 while the Mn2+–O–Mn3+ and Mn3+–O–Mn4+ are double-exchange ferromagnetic interaction.21,30 Therefore, the antiferromagnetic interaction of the 20-nm-thick film is strongest due to the highest proportion of Mn2+ ions. Given that the competitive relationship between ferromagnetic interactions and antiferromagnetic interactions mainly depends on the degree of antiferromagnetic interactions, it can be concluded that the competition of the 20-nm-thick film is fiercest, which leads to the easiest formation of the cluster glass state. In brief, the various strain states of LMO films with different thickness causes changed proportions of Mn2+, Mn3+ and Mn4+, which affect the competitive relation between ferromagnetic and antiferromagnetic interactions and then the formation of the cluster glass state.
The oxygen vacancy concentrations are estimated by XPS spectra of O 1s. Fig. 5 shows that the O 1s spectra are fitted very well with the peaks at the binding energies of 529.9 and 532.0 eV, which are associated with lattice oxygen (Oa) and oxygen vacancies (Ob),31 respectively. The oxygen vacancy concentration in all films does not show significant differences with depth variation. That is, the change of Mn valence state is independent of oxygen vacancy concentration. The reason may be that the strain causes the distortion of the MnO6 octahedron, which changes the length of the Mn–O bond and angle of the Mn–O–Mn bond.32
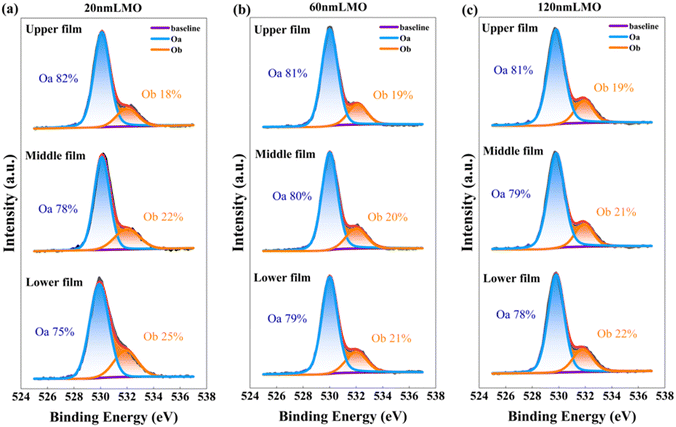 | ||
| Fig. 5 XPS spectra of the O 1s peaks for the (a) 20-nm-thick, (b) 60-nm-thick and (c) 120-nm-thick LMO films at different depths. | ||
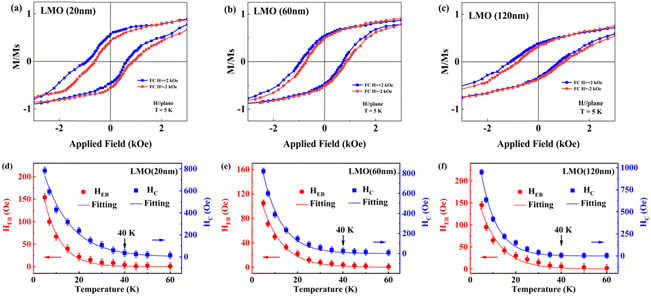
Fig. 6(a)–(c) shows the magnetic hysteresis (M–H) loops of the 20-, 60- and 120-nm-thick LMO films measured at 5 K after field cooling at ±2 kOe. The coercivity (HC) and the exchange bias field (HEB) with temperature are shown in Fig. 6(d)–(f). The HC and the HEB of all LMO films decrease with the increase of temperature and finally disappear. The temperature dependences of the HC and HEB can be fitted by the exponential formula:33HC = H1 exp (−T/T1) and HEB = H2 exp (−T/T2), where H1 and H2 represent the extrapolations of HC and HEB at 0 K and T1 and T2 are constants. Obviously, the disappearing temperature of HEB for all LMO films is the same (about at 40 K), which matches well with the Tf shown in the ZFC M–T curve of Fig. 3(a). This demonstrates that the exchange bias effect mainly originates from the cluster glass state in the LMO films,11,19 which is due to the spins in the cluster glass state being frozen along the direction of the applied magnetic field below Tf. The coercivity (HC) of the 20-, 60- and 120-nm-thick LMO films is 782, 822 and 950 Oe, respectively. Moreover, the HEB of the 20-nm-thick LMO film (HEB = 154 Oe) is higher than that of the 60-nm-thick (HEB = 105 Oe) and 120-nm-thick LMO films (HEB = 145 Oe). The highest HEB in the 20-nm-thick LMO film is due to the strong pinning effect of the easiest formation of the cluster glass state. The abnormal increase of HEB in the 120-nm-thick LMO film may be due to the increased ferromagnetic interactions caused by inconsistent strain on the upper and lower layers of the film.
Conclusions
In this study, 20-, 60- and 120-nm-thick LMO films have been grown on a (001)-oriented LAO substrate by PLD, and the formation of the cluster glass state in the LMO films was studied. The results show that the cluster glass state is most likely to be formed in the 20-nm-thick LMO film, while it is the most difficult to form in the 120-nm-thick LMO film. Moreover, we demonstrate that the biaxial strain and Mn ion valence states will induce competition between ferromagnetic and antiferromagnetic interactions in the LMO films of different thicknesses, which leads to varying degrees of difficulty in the formation of the cluster glass state.Author contributions
Yongmei Liang: carried out the experiments and wrote the original draft. Haonan Lian and Yaqiang Li: assisted with resources, software, and validation. Dan Liu: analyzed data. Baochen Han: funding acquisition and revised the manuscript. Jian Qi: writing-reviewing and editing. Dongxiao Ma: coordinated the study and revised the manuscript.Data availability
The data that support the findings of this study are available within the article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 52002112, 52204342, 52201073), Natural Science Foundation of Hebei Province (E2022208056) and Natural Science Foundation of Hebei Education Department (BJK2024194).Notes and references
- M. Gibert, P. Zubko, R. Scherwitzl, J. Iniguez and J. M. Triscone, Nat. Mater., 2012, 11, 195–198 CrossRef CAS PubMed.
- Y. M. Liang, X. K. Ning, Z. J. Wang, B. He, Y. Bai, X. G. Zhao, W. Liu and Z. D. Zhang, J. Phys. Chem. C, 2017, 121, 16810–16818 CrossRef CAS.
- H. Nakao, C. Tabata, Y. Murakami, Y. Yamasaki, H. Yamada, S. Ishihara and M. Kawasaki, Phys. Rev. B: Condens. Matter Mater. Phys., 2018, 98, 245146 CrossRef CAS.
- A. S. Moskvin, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 115102 CrossRef.
- I. Loa, P. Adler, A. Grzechnik, K. Syassen, U. Schwarz, M. Hanfland, G. K. Rozenberg, P. Gorodetsky and M. P. Pasternak, Phys. Rev. Lett., 2001, 87, 125501 CrossRef CAS PubMed.
- T. Mertelj, D. Kuščer, M. Kosec and D. Mihailovic, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, 15102–15107 CrossRef CAS.
- S. Satpathy, Z. S. Popović and F. R. Vukajlović, J. Appl. Phys., 1996, 79, 4555–4557 CrossRef CAS.
- K. Vijayanandhini, C. Simon, V. Pralong, V. Caignaert and B. Raveau, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 224407 CrossRef.
- Y.-k Tang, Y. Sun and Z.-h Cheng, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 174419 CrossRef.
- X. G. Li, X. J. Fan, G. Ji, W. B. Wu, K. H. Wong, C. L. Choy and H. C. Ku, J. Appl. Phys., 1999, 85, 1663–1666 CrossRef CAS.
- A. K. Nayak, C. Shekhar, J. Winterlik, A. Gupta and C. Felser, Appl. Phys. Lett., 2012, 100, 152404 CrossRef.
- P. Singh, M. Alam, S. Kumar, K. Anand, V. K. Gangwar, S. Ghosh, M. Sawada, K. Shimada, R. Singh and A. Ghosh, Appl. Phys. A: Mater. Sci. Process., 2020, 32, 445801 CAS.
- M. Seki, A. K. M. A. Hossain, T. Kawai and H. Tabata, J. Appl. Phys., 2005, 97, 083541 CrossRef.
- M. Chennabasappa, A. Pautrat and O. Toulemonde, J. Alloys Compd., 2022, 892, 162095 CrossRef CAS.
- R. Kumar and A. Sundaresan, Phys. Rev. B: Condens. Matter Mater. Phys., 2022, 106, 134423 CrossRef CAS.
- T. Yu, X. K. Ning, W. Liu, J. N. Feng, X. G. Zhao and Z. D. Zhang, J. Appl. Phys., 2014, 116, 083908 CrossRef.
- Y. M. Liang, Z. J. Wang, Y. J. Wu, J. L. Lin and Z. D. Zhang, J. Alloys Compd., 2020, 847, 156227 CrossRef CAS.
- J. Roqueta, A. Pomar, L. Balcells, C. Frontera, S. Valencia, R. Abrudan, B. Bozzo, Z. Konstantinović, J. Santiso and B. Martínez, Cryst. Growth Des., 2015, 15, 5332–5337 CrossRef CAS.
- Y. M. Liang, Z. J. Wang, Y. Bai, Y. J. Wu, X. K. Ning, X. G. Zhao, W. Liu and Z. D. Zhang, J. Phys. Chem. C, 2019, 123, 14842–14848 CrossRef CAS.
- L. Yin, Q. Zhang, W. Mi and X. Wang, J. Appl. Phys., 2016, 120, 165303 CrossRef.
- J. J. Peng, C. Song, B. Cui, F. Li, H. J. Mao, Y. Y. Wang, G. Y. Wang and F. Pan, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 89, 165129 CrossRef.
- R. Prasad, M. P. Singh, P. K. Siwach, A. Kaur, P. Fournier and H. K. Singh, Appl. Phys. A: Mater. Sci. Process., 2010, 99, 823–829 CrossRef CAS.
- X. H. Huang, J. F. Ding, Z. L. Jiang, Y. W. Yin, Q. X. Yu and X. G. Li, J. Appl. Phys., 2009, 106, 083904 CrossRef.
- F. Tsui, M. C. Smoak, T. K. Nath and C. B. Eom, Appl. Phys. Lett., 2000, 76, 2421–2423 CrossRef CAS.
- P. Dey, T. K. Nath and A. Taraphder, Appl. Phys. Lett., 2007, 91, 012511 CrossRef.
- Y. Wu, X. Ning, Z. Wang, Q. Wang and Z. Zhang, J. Alloys Compd., 2016, 667, 317–322 CrossRef CAS.
- X. Ning, Z. Wang and Z. Zhang, Sci. Rep., 2015, 5, 8460 CrossRef CAS PubMed.
- E. S. Ilton, J. E. Post, P. J. Heaney, F. T. Ling and S. N. Kerisit, Appl. Surf. Sci., 2016, 366, 475–485 CrossRef CAS.
- W. Niu, W. Liu, M. Gu, Y. Chen, X. Zhang, M. Zhang, Y. Chen, J. Wang, J. Du, F. Song, X. Pan, N. Pryds, X. Wang, P. Wang, Y. Xu, Y. Chen and R. Zhang, Adv. Electron. Mater., 2018, 4, 1800055 CrossRef.
- G. Tan, Z. Chen and X. Zhang, Sci. China, Ser. G: Phys., Mech. Astron., 2009, 52, 987–992 CrossRef CAS.
- Q. Gao, Y. Dai, C. Li, L. Yang, X. Li and C. Cui, J. Alloys Compd., 2016, 684, 669–676 CrossRef CAS.
- R. K. Zheng, Y. Wang, H. L. W. Chan, C. L. Choy and H. S. Luo, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 212102 CrossRef.
- X. H. Huang, J. F. Ding, G. Q. Zhang, Y. Hou, Y. P. Yao and X. G. Li, Phys. Rev. B: Condens. Matter Mater. Phys., 2008, 78, 224406 CrossRef.
| This journal is © The Royal Society of Chemistry 2024 |