Low-resistivity Ohmic contacts of Ti/Al on few-layered 1T′-MoTe2/2H-MoTe2 heterojunctions grown by chemical vapor deposition†
Ping-Feng
Chi
a,
Jing-Jie
Wang
b,
Jing-Wen
Zhang
b,
Yung-Lan
Chuang
a,
Ming-Lun
Lee
*c and
Jinn-Kong
Sheu
 *ab
*ab
aDepartment of Photonics, National Cheng Kung University, Tainan, Taiwan. E-mail: jksheu@ncku.edu.tw
bAcademy of Innovative Semiconductor and Sustainable Manufacturing, National Cheng Kung University, Tainan City, 70101, Taiwan
cDepartment of Electro-Optical Engineering, Southern Taiwan University of Science and Technology, Tainan City, 71001, Taiwan. E-mail: minglun@stust.edu.tw
First published on 11th September 2024
Abstract
This study explores the phase-controlled growth of few-layered 2H-MoTe2, 1T′-MoTe2, and 2H-/1T′-MoTe2 heterostructures and their impacts on metal contact properties. Cold-wall chemical vapor deposition (CW-CVD) with varying growth rates of MoOx and reaction temperatures with Te vapors enabled the growth of continuous thin films of either 1T′-MoTe2 or 2H-MoTe2 phases on two-inch sapphire substrates. This methodology facilitates the meticulous optimization of chemical vapor deposition (CVD) parameters, enabling the realization of phase-controlled growth of few-layered MoTe2 thin films and their subsequent heterostructures. The study further investigates the influence of a 1T′-MoTe2 intermediate layer on the electrical properties of metal contacts on few-layered 2H-MoTe2. Bi-layer Ti/Al contacts directly deposited on 2H-MoTe2 exhibited Schottky behavior, indicating inefficient carrier transport. However, introducing a few-layered 1T′-MoTe2 intermediate layer between the metal and 2H-MoTe2 layers improved the contact characteristics significantly. The resulting Al/Ti/1T′-MoTe2/2H-MoTe2 contact scheme demonstrates Ohmic behavior with a specific contact resistance of around 1.7 × 10−4 Ω cm2. This substantial improvement is attributed to the high carrier concentration of the 1T′-MoTe2 intermediate layer which could be attributed tentatively to the increased tunneling events across the van der Waals gap and enhancing carrier transport between the metal and 2H-MoTe2.
New conceptsThis study demonstrates a novel approach to engineering Ohmic contacts for few-layer 2H-MoTe2 devices by incorporating a strategic 1T′-MoTe2 intermediate layer. Unlike existing methods, this research introduces phase-controlled growth of MoTe2 using a cold-wall CVD technique, enabling precise control over the crystalline phase, whether 2H- or 1T′-MoTe2, across wafer-scale thin films. This phase control is crucial for optimizing device functionality. Furthermore, the study leverages the high carrier concentration of 1T′-MoTe2 as an interlayer, effectively reducing contact resistance by promoting efficient carrier transporting across the van der Waals gap, a significant improvement over traditional Schottky barrier-limited contacts. This work expands the toolbox for 2D material-based device engineering, offering a versatile platform for customizing material properties and presenting a viable solution for minimizing contact resistance in TMDC-based devices. The insights gained from this research pave the way for developing next-generation electronics and photonics by overcoming the limitations of traditional contact engineering, thus contributing significantly to the fields of nanoscience and nanotechnology. |
Introduction
Two-dimensional (2D) materials, comprised of single atomic layers bound by van der Waals forces, exhibit unique structural and electronic properties due to their extreme anisotropy. Graphene, the archetypal 2D material, possesses remarkable characteristics such as high electrical conductivity, exceptional mechanical strength, and excellent optical transparency.1–4 However, its semimetallic character poses challenges for conventional digital circuits and optoelectronic device applications.Recently, significant interest has emerged in transition metal dichalcogenides (TMDCs) as a promising class of 2D materials for electronic and photonic devices. These MX2-type compounds, where M represents transition metals (groups IV–VI), and X denotes chalcogen elements (S, Se, Te), typically exhibit semiconducting bandgaps, paving the way for transistor and light-emitting device applications. However, achieving low contact resistance between 3D metal electrodes and 2D TMDC active layers remains a critical challenge. The formation of energy barriers at these interfaces impedes carrier transport and limits current injection efficiency.5 Several approaches have been proposed to tackle the crucial issue of high contact resistance in 2D TMDC-based devices. These approaches include heavy local doping of the TMDC channels.6,7 This method involves introducing a high concentration of dopants in specific regions of the TMDC material to improve conductivity. In addition, the Fermi level can be manipulated through phase transitions8 utilizing phase changes in the material to alter the energy level of its electrons, thereby affecting contact resistance. Integration with graphene within van der Waals heterostructures onto the TMDC to create a more efficient contact was also demonstrated.9 Overcoming these interfacial barriers is crucial for unlocking the full potential of TMDCs and realizing their transformative impact on future electronic and photonic devices. Molybdenum ditelluride (MoTe2) exhibits remarkable structural polymorphism, existing in three primary crystalline phases: the hexagonal α-phase (2H-MoTe2), the monoclinic β-phase (1T′-MoTe2), and the orthorhombic β′-phase (1T-MoTe2). Only the 2H-MoTe2 and 1T′-MoTe2 exhibit ambient stability and have been extensively investigated due to their unique electronic properties.10 Band structure calculations and experimental data reveal a small bandgap of approximately 60 meV in thin-film 1T′-MoTe2, indicative of its semimetallic character,11 rendering it an attractive candidate for Ohmic contact engineering in TMDC-based devices. It can be an effective interlayer between conventional metals and TMDCs, minimizing interfacial barriers and facilitating carrier transport. In contrast, bulk 2H-MoTe2 behaves as a semiconductor with an indirect bandgap of ∼0.88 eV. Interestingly, dimensionality reduction to few-layered or monolayer thin films causes a transition to a direct bandgap exceeding 1.0 eV.11 This fascinating behavior manifests as intense near-infrared fluorescence, propelling 2H-MoTe2 into the spotlight for next-generation electronic and photonic applications.12 Furthermore, few-layered MoTe2 exhibits remarkable environmental sensitivity, readily undergoing phase transitions and reacting with atmospheric oxygen. This susceptibility to tellurium–oxygen substitution alters its electrical conductivity, enabling switching between n-type and p-type behavior.13,14 This unique property positions few-layered MoTe2 as a promising candidate for diversifying and modulating the functionalities of electronic and photonic devices.15 Qu et al.13 observed that tellurium vacancies prevalent in CVD-grown MoTe2 induce defect states close to the conduction band. These states pin the Fermi level, hindering the formation of Ohmic contacts in field-effect transistors (FETs). Consequently, a Schottky barrier forms, compromising device performance via reduced switching speed and output current. Two primary strategies have been proposed to address this challenge. The general approach involves heavy doping of the 2D semiconductors to shrink the Schottky barrier width, facilitating carrier transport through tunneling. However, implementing this strategy in 2D materials poses significant technical challenges. Alternatively, introducing a thin dielectric layer at the interface between the 3D metal and 2D semiconductors can decouple the metal-induced gap states (MIGS) and alleviate Fermi-level pinning. Nevertheless, this method also expands the Schottky barrier width due to the increased metal–semiconductor separation.16,17
Several studies have explored using chemical vapor deposition (CVD) to grow large-area MoTe2.18–22 Additionally, creating a 1T′-MoTe2/2H-MoTe2 heterostructure has been shown to reduce contact resistance between metal electrodes and few-layer 2H-MoTe2.23–26 Xu et al.25 proposed that single-crystalline 2H-MoTe2 nanosheets exfoliated from bulk crystals could be used as seed crystals by transferring them via a dry transfer method to the center of a 1T′-MoTe2 wafer, thereby triggering the phase transition and recrystallization process. However, achieving precise control over the positioning of the coplanar 1T′-MoTe2/2H-MoTe2 heterostructure remains challenging. The random placement of junctions significantly complicates device fabrication. To tackle this issue, our study introduces a new growth method using cold-wall chemical vapor deposition (CW-CVD), which allows for precise control of the junction position in vertically stacked 1T′-MoTe2/2H-MoTe2 heterostructures over the whole wafer. This study also proposes leveraging few-layered 1T′-MoTe2 as an interfacial layer between conventional metal electrodes and few-layered 2H-MoTe2 to achieve a marked band bending of 2H-MoTe2 layers, thereby leading to a tunneling junction. By facilitating carrier transport through tunneling across the Schottky barrier, this strategy aims to minimize contact resistance. Achieving this relies on precise control of stoichiometry and thickness during crystal growth to selectively grow either the 2H or 1T' phase of few-layered MoTe2.22,23
Experimental
This study used the cold-wall chemical vapor deposition (CW-CVD) method to grow few-layered MoTe2 continuous thin films on wafer-level substrates. In the CW-CVD system, a top showerhead is employed to ensure an even distribution of precursor gases composed of hydrogen (H2) and reactive source gas. The gas passes through the showerhead and flows toward the SiC susceptor, where the growth substrates are located. Two small furnaces housing the precursor powders are connected to the reactor chamber. They serve to vaporize solid sources like MoO3 or tellurium (Te) powders. The temperatures of the small furnaces are independently elevated to evaporate the precursor powders. Following this, the resulting precursor vapors are introduced by carrier gases into the mixing zone of the reactor chamber. The flow rates of carrier gases, which include argon (Ar) and H2, are accurately regulated using mass flow controllers. The growth zone in the CW-CVD system is a susceptor equipped with a heater that can be rotated and moved vertically so that a laminar flow can be built rightly on the growth substrate to grow the few-layer MoTe2 films. The growth parameters, which include temperatures, gas flow rates, pressures, time duration of each step, and rotating speed of the susceptor for growing the few-layer MoTe2 thin films, are all controlled by programmed recipes. Fig. S1 (ESI†) shows a schematic diagram of the CW-CVD system used in this study. The SiC susceptor used in this system can accommodate up to 4-inch substrates (Al2O3 or Si). This study grows the few-layer MoTe2 thin films with different phases by regulating the growth temperatures and pressures to control the growth rates of MoOx thin films. The subsequent reaction process of MoOx in an ambient of Te vapors, known as the tellurization process, leads to the reaction of MoOx with Te precursor vapors to form the few-layer MoTe2 thin films on the substrates. In particular, a relatively high growth rate of MoOx thin films leads to the synthesis of few-layer 1T′-MoTe2 thin films, whereas the few-layer 2H-MoTe2 thin films are produced when the growth rate of MoOx is comparatively lower, For detailed information, please refer to ESI† and Fig. S2. The few-layered 1T′-MoTe2 thin films can be synthesized by first growing MoOx thin films at a high growth rate (H.G.R) of 5 Å min−1 and then subjecting them to a tellurization process for 2 hours at a substrate temperature of 400 °C. On the other hand, the few-layered 2H-MoTe2 thin films can be synthesized by growing MoOx thin films at a low growth rate (L.G.R) of 1 Å min−1 and then setting the substrate temperature to 500 °C followed by a tellurization process for 2 hours. To create a 2H/1T′-MoTe2 vertically stacked structure, two-step grown MoOx thin films with different growth rates are first grown on the substrate and then subjected to a tellurization process for 2 hours. The detailed growth procedures are illustrated in Fig. S3 of the ESI.† Different vibrational modes of Micro-Raman spectroscopy were used to identify the crystal phases of the few-layered MoTe2 thin films. The few-layered 2H-MoTe2, 1T′-MoTe2, and 2H/1T′-MoTe2 heterostructures were also inspected using high-resolution transmission electron microscopy (HR-TEM). The electrical properties of the thin films of the few-layered 2H-MoTe2 and 1T′-MoTe2 were determined using Hall-effect measurements. Ultraviolet photoelectron spectroscopy (UPS) and low energy inverse photoelectron spectroscopy (LEIPS, Model: PHI Versa Probe 4) were employed to resolve the valence bands, conduction bands, and work functions of the few-layered 2H-MoTe2 and 1T′-MoTe2 thin films. The standard photolithography and wet chemical etching process were performed to pattern the few-layered MoTe2 films, followed by metal deposition to form the Ohmic contacts with a transfer length model (TLM) structure for evaluating the specific contact resistance (ρc).Results and discussion
The photographs in Fig. 1 exhibit the exteriors of the few-layered MoTe2 wafers grown on sapphire substrates. The reference wafer is a sapphire substrate, which is labeled as Wafer A. The other wafers, B, C, and D, represent few-layered 2H-MoTe2, 1T′-MoTe2, and stacked 2H/1T′-MoTe2 thin films grown on sapphire substrates, respectively. Fig. S4 (ESI†) shows the typical inspection of cross-section HR-TEM performed on the wafers B, C, and D to determine the thicknesses of the few-layered MoTe2 continuous thin films grown on sapphire substrates, the typical thicknesses of the few-layered 2H-MoTe2 (Wafer B), 1T′-MoTe2 (Wafer C) and 2H/1T′-MoTe2 (Wafer D) thin films were 3.1 nm, 2.3 nm, and 7.6 nm, respectively, which correspond to 4, 3, and 10 layers.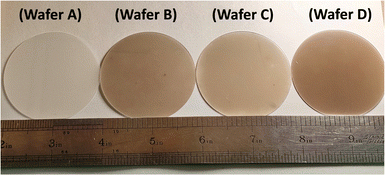 | ||
| Fig. 1 Photographs taken from a 2-inch sapphire substrate (Wafer A), a 4-layer 2H-MoTe2 (Wafer B), a 3-layer 1T′-MoTe2 (Wafer C) and a 2H/1T′-MoTe2 (3/7 layers) heterostructure (Wafer D). | ||
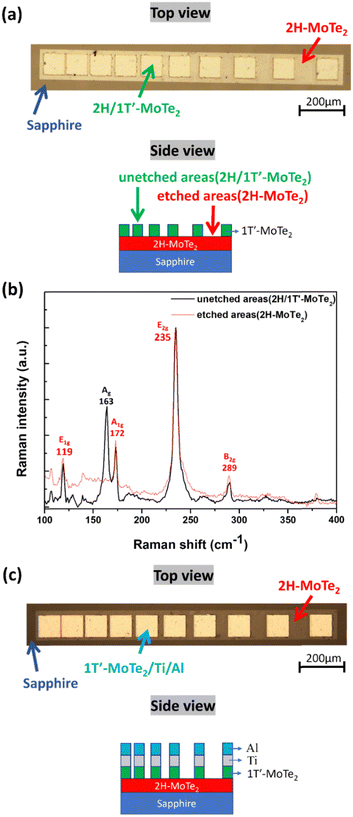
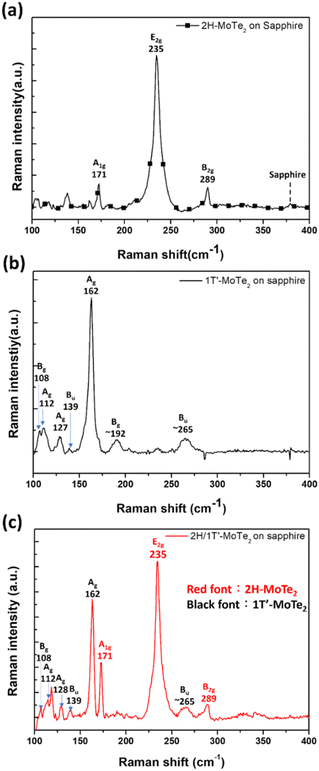
Fig. 2(a) shows a typical Raman spectrum of the 4-layer 2H-MoTe2 thin films (Wafer B) when excited by a 532 nm laser. The 235, 171, and 289 cm−1 peaks correspond to the in-plane E2g, out-of-plane A1g, and out-of-plane B2g mode peaks, respectively. Fig. 2(b) shows a typical Raman spectrum taken from the 3-layer 1T′-MoTe2 thin films (Wafer C) when excited by a 532 nm laser. This spectrum exhibits a dominant peak at 162 cm−1, corresponding to the Ag vibration mode of 1T′-MoTe2. Additionally, six peaks around 108, 112, 127, 139, 192, and 265 cm−1 are assigned to the Bg, Ag, Ag, Bu, Bg, and Bu vibrational modes, respectively.27,28 Compared to hot-wall CVD, CW-CVD directly heats the substrate, minimizing unwanted chamber wall coatings that can contaminate wafers and degrade film quality. In addition, as per our CW-CVD reactor, the combination of substrate rotation and the ability to adjust the distance between the substrate and the gas showerhead allows us to achieve excellent uniformity in both film composition and thickness across wafer-level substrates. Fig. S5 (ESI†) shows Raman spectra of few-layered 1T′-MoTe2 and 2H-MoTe2 from five points on the 2-inch wafer, indicating uniform thickness and composition for the large-area and continuous MoTe2 thin films on sapphire substrates. Fig. 2(c) displays a typical Raman spectrum taken from the vertically stacked 2H/1T′-MoTe2 heterostructure. The spectrum has all the characteristic peaks of 2H and 1T′-MoTe2, indicating that this wafer has 2H and 1T′-MoTe2 crystal phases. In order to further verify that wafer D consists of a 2H-/1T′-MoTe2 stacked heterostructure in a vertical manner and confirm that the lower layers belong to 2H-MoTe2 while the upper layers belong to 1T′-MoTe2, this study employed a chemical solution for the etching of 2H-/1T′-MoTe2 heterostructure from the surface in sequence. Fig. S6 (ESI†) displays the intensity-normalized Raman spectra of 2H/1T′-MoTe2 heterostructures etched at different times. The intensities of characteristic peaks belonging to the 1T′-MoTe2 phase decreased with an increase in etching times, and they disappeared eventually. The Raman spectra confirm that the 1T′-MoTe2 layers stack right on the 2H-MoTe2 layers. The few-layered MoTe2 thin films were patterned into the TLM structures to evaluate the rc of metal contacts on the MoTe2 thin films. These TLM structures were obtained by performing an etching process on the stacked 2H-/1T′-MoTe2 structure, using an etching solution (volume ratio is H2SO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O2
H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DI water = 5
DI water = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), which removed the 1T′-MoTe2 top layer through control etching time, as shown in Fig. 3(a). The etching process was also performed on the few-layered 2H/1T′-MoTe2 thin films for comparison. Raman spectroscopy was performed on the etched area to confirm whether the 1T′-MoTe2 top layer was completely removed and the 2H-MoTe2 layer remained in the etched area. Fig. 3(b) displays typical Raman spectra obtained from both the etched and unetched areas of the 2H/1T′-MoTe2 vertically stacked heterostructures. The etched area did not show peaks corresponding to the 1T′-MoTe2 layers, whereas the unetched area showed both few-layered 2H-MoTe2 and 1T′-MoTe2-related peaks. In order to prove whether the 1T′-MoTe2 top layers can assist conventional metals, such as titanium and nickel, in creating Ohmic contacts on the few-layered 2H-MoTe2 thin films, bilayer metal (Ti/Al) contacts were formed with TLM patterns using a photolithography technique. Fig. 3(c) displays the Ti/Al contacts on the few-layered MoTe2 thin films. The process flow for the TLM contact patterns on both few-layered 2H-MoTe2 and 2H/1T′-MoTe2 stacked heterostructures can be seen in Fig. S7 (ESI†). Current–voltage (I–V) characteristics measured from the Ti/Al contacts on the few-layered MoTe2 thin films using a semiconductor parameter analyzer (Keysight B1500) were performed to evaluate the ρc. Table 1 displays the electrical parameters obtained from Hall-effect measurements for the few-layered 2H-MoTe2 and 1T′-MoTe2 thin films. Compared to the few-layered 2H-MoTe2, the 1T′-MoTe2 thin films exhibit a lower sheet resistivity and a relatively higher carrier concentration.29 Forming Ohmic contacts between metals and semiconductors relies on efficient carrier transport across the interface.
1), which removed the 1T′-MoTe2 top layer through control etching time, as shown in Fig. 3(a). The etching process was also performed on the few-layered 2H/1T′-MoTe2 thin films for comparison. Raman spectroscopy was performed on the etched area to confirm whether the 1T′-MoTe2 top layer was completely removed and the 2H-MoTe2 layer remained in the etched area. Fig. 3(b) displays typical Raman spectra obtained from both the etched and unetched areas of the 2H/1T′-MoTe2 vertically stacked heterostructures. The etched area did not show peaks corresponding to the 1T′-MoTe2 layers, whereas the unetched area showed both few-layered 2H-MoTe2 and 1T′-MoTe2-related peaks. In order to prove whether the 1T′-MoTe2 top layers can assist conventional metals, such as titanium and nickel, in creating Ohmic contacts on the few-layered 2H-MoTe2 thin films, bilayer metal (Ti/Al) contacts were formed with TLM patterns using a photolithography technique. Fig. 3(c) displays the Ti/Al contacts on the few-layered MoTe2 thin films. The process flow for the TLM contact patterns on both few-layered 2H-MoTe2 and 2H/1T′-MoTe2 stacked heterostructures can be seen in Fig. S7 (ESI†). Current–voltage (I–V) characteristics measured from the Ti/Al contacts on the few-layered MoTe2 thin films using a semiconductor parameter analyzer (Keysight B1500) were performed to evaluate the ρc. Table 1 displays the electrical parameters obtained from Hall-effect measurements for the few-layered 2H-MoTe2 and 1T′-MoTe2 thin films. Compared to the few-layered 2H-MoTe2, the 1T′-MoTe2 thin films exhibit a lower sheet resistivity and a relatively higher carrier concentration.29 Forming Ohmic contacts between metals and semiconductors relies on efficient carrier transport across the interface.
 | ||
| Fig. 2 Typical Raman spectra of (a) 2H-MoTe2, (b) 1T′-MoTe2, and (c) 2H/1T′-MoTe2 vertically stacked heterostructure. | ||
| Sheet resistivity (Ohm sq−1) | Mobility (cm2 V s−1) | Sheet concentration (cm−2) | Bulk concentration (cm−3) | |
|---|---|---|---|---|
| 2H-MoTe2 | 6.94 × 104 | 4.54 | +1.97 × 1013 | +6.59 × 1019 |
| 1T′-MoTe2 | 8734 | 0.79 | −9.0 × 1014 | −2.25 × 1021 |
Although a lack of direct evidence for how the carriers transport via tunneling or thermionic emission, due to the high carrier concentration in few-layered 1T′-MoTe2, the probability of carrier transport via tunneling through a barrier is expected to be increased over other mechanisms, for example, via the thermionic emission over the barrier between the 1T′-MoTe2 and 2H-MoTe2 layers. However, more experimental tasks, such as the evaluation of temperature-dependent I–V characteristics, are necessary to clarify this contention. Ultraviolet photoelectron spectroscopy (U.P.S.) and low energy inverse photoelectron spectroscopy (LEIPS) were conducted to examine the work functions (WF) and electron affinities (EA) of the 2H-MoTe2 and 1T′-MoTe2 thin films. The WF and EA values, 4.43 eV and 4.83 eV, respectively, measured for the 1T′-MoTe2 thin films are in agreement with published results30,31 indicating the degenerate nature of the few-layered 1T′-MoTe2 thin films. For few-layered 2H-MoTe2 films, measurements show WF of 4.87 eV,14EA of 4.32 eV, and energy from the valence band maximum to the Fermi energy level (EVBM–EF) of 0.53 eV. These values reveal an energy bandgap (Eg) of around 1.08 eV for the few-layered 2H-MoTe2 films, suggesting they are suitable for use in FETs and photonic devices such as solar cells and LEDs.
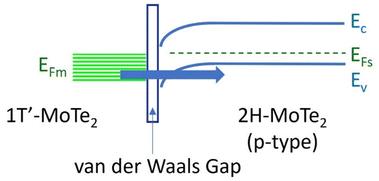
Building upon the determined electronic properties of the constituent layers, a band diagram was constructed for the few-layered 2H/1T′-MoTe2 vertical heterojunction.32 Informed by the extracted work functions and electron affinities, the schematic band diagram of the vertically stacked 2H/1T′-MoTe2 heterostructure is presented in Fig. 4. Notably, the band diagram reveals the emergence of a van der Waals gap at the interface upon the 2H/1T′-MoTe2 heterostructures. This band diagram suggests that carrier transport across the junction may occur via tunneling mechanisms.
Fig. 5 shows the typical current–voltage (I–V) curves for bi-layer metal contacts (Ti/Al) deposited on both few-layered 2H-MoTe2 films and 2H/1T′-MoTe2 heterostructures. The Ti/Al contacts directly deposited on the few-layered 2H-MoTe2 exhibit non-linear I–V curves, demonstrating the formation of Schottky contacts as shown in Fig. 5(a). This phenomenon arises from the large van der Waals gap between the metal and the few-layered 2H-MoTe2, which hinders carrier transport in both directions. Additionally, structural defects like atomic vacancies in the MoTe2 films, caused by their growth process, could lead to Fermi-level pinning. As a result, the Schottky barrier height might be higher than the ideal value, which is simply the difference between the work functions of Ti and 2H-MoTe2.8 Nevertheless, this scenario can be altered by introducing a 1T′-MoTe2 intermediate layer. Fig. 5(b) shows the linear I–V characteristics for bi-layer metal contacts (Ti/Al) on few-layered 2H-/1T′-MoTe2 heterostructures, indicating Ohmic contacts, Fig. 5(b) inset includes a plot of the contact resistance corresponding to different spacing lengths between metal contacts.
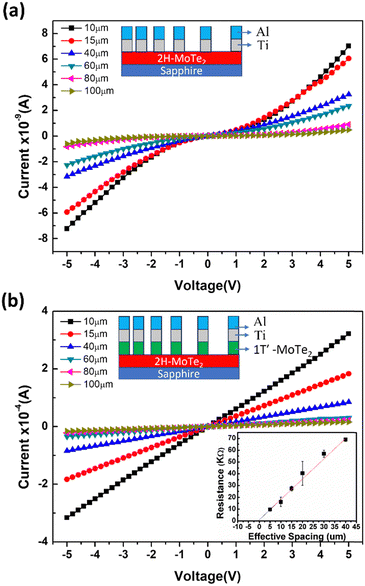 | ||
| Fig. 5 Typical I–V characteristics taken from the bi-layer Ti/Al contacts with different spacings onto the few-layered (a) 2H-MoTe2 and (b) 2H/1T′-MoTe2 heterostructures. The insets of Fig. 5 depict the schematic layer structures of Ti/Al metal pads. | ||
This transformation is thanks to the 1T′-MoTe2's high carrier concentration (∼1021 atoms cm−3, as shown in Table 1). This abundance of carriers may enable tunneling junctions at both the Ti/1T′-MoTe2 and 1T′-MoTe2/2H-MoTe2 interfaces, leading to the formation of Ohmic contacts for the entire few-layered 2H/1T′-MoTe2/Ti/Al stack. As a result, the specific contact resistance (ρc) drops to 1.7 × 10−4 Ω cm2. While still not quite reaching the ultra-low ρc one dreams of for future high-performance 2D-semiconductor FETs, these preliminary results hold significant promise. This research suggests that a vertically stacked 2H/1T′-MoTe2 heterostructure could mitigate the adverse effects of Fermi-level pinning at the interface between metals and 2D materials, opening up possibilities for more efficient and high-performance devices.
Table 2 presents a comparison of contact resistance values obtained using different metals on few-layer MoTe2, highlighting the current state of the art in this field.
| Metal contact scheme | Growth method | Specific contact resistance (Ω cm2) | Contact resistance (Ω μm) | Ref. |
|---|---|---|---|---|
| a The contact resistance was characterized by a TLM method.38 | ||||
| Au/Ti/2H-MoTe2 | MOCVD | NA | 10.2 MΩ μm | 33 |
| Au/1T′-MoTe2/2H-MoTe2 | CVD | NA | 14 kΩ μm | 34 |
| Te NW/2H-MoTe2 | MOCVD | NA | 12.3 kΩ μm | 35 |
| Pd/1T′-Mo6Te6/2H-MoTe2 | MOCVD | NA | 29 MΩ μm | 36 |
| Au/2H-MoTe2 | Mechanically exfoliated | 1.2 × 10−2 | NA | 37 |
| Au/1T′-MoTe2/2H-MoTe2/1T′-MoTe2 | Mechanically exfoliated | 6.1 × 10−3 | NA | 37 |
| Au/1T′-MoTe2 | Mechanically exfoliated | 7.0 × 10−4 | NA | 37 |
| Al/Ti/1T′-MoTe2/2H-MoTe2 | CW-CVD | 1.7 × 10−4 | 27.5a kΩ μm | This work |
Conclusions
Using the CW-CVD technique, this study demonstrated the feasibility of growing continuous and few-layered MoTe2 thin films and their heterostructures on wafer-level sapphire substrates. The obtained results indicated a promising degree of control over the crystal phase of these films, achieved through variations in the growth rate of the MoOx layer. Raman spectroscopy corroborated the successful phase transition between 2H and 1T′, while spatial-dependent Raman spectra confirmed acceptable thickness uniformity across the entire wafer. Further analysis employing Hall-effect measurements revealed a noteworthy 1T′ phase characteristic – a substantially high carrier concentration. This property exhibits similarities to those of semimetals with degenerate behavior. Building upon this finding, the potential of such films for improved contact properties on 2H MoTe2 was explored. The investigation into vertically stacked 1T′/2H MoTe2 heterostructures to facilitate the formation of low-resistivity Ohmic contacts yielded encouraging preliminary results. This novel heterostructure demonstrates a potential solution to the challenge posed by Fermi-level pinning at the metal-2D material interface, potentially paving the way for future devices made of 2D semiconductors.Author contributions
Ping-Feng Chi: experiment execution, data analysis, writing – original draft. Jing-Jie Wang: experiment execution data analysis, Jing-Wen Zhang: experiment execution data analysis, Yung-Lan Chuang: experiment execution data analysis, Ming-Lun Lee: conceptualization, supervision, project administration, funding acquisition. Jinn-Kong Sheu: conceptualization, writing – review & editing, supervision, project administration, funding acquisition.Data availability
The data that support the findings of this study are available from the corresponding author, J. K. Sheu, upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science and Technology Council, Taiwan, for financial support under contract No. 113-2112-M-006-021-MY3, 111-2221-E-006 -058 -MY3, 113-2112-M-006-009-MY2, 110-2112-M-006-021-MY3, 111-2221-E-218-004-MY3, 111-2221-E-006-030-MY3 and 111-2221-E-006-058-MY3. The authors would like to thank Prof. Chin-Shan Lue, Prof. Tzung-Fang Guo and Prof. Ya-Ju Lee, from National Cheng Kung University for their support in the experiment facility. The authors would also like to thank Professor Ming-Wen Chu of the National Taiwan University for providing discussions and insights related to TEM images.Notes and references
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D.-E. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385–388 CrossRef CAS PubMed.
- R. R. Nair, P. Blake, A. N. Grigorenko, K. S. Novoselov, T. J. Booth, T. Stauber, N. M. Peres and A. K. Geim, Science, 2008, 320, 1308 CrossRef CAS.
- D. Nikal, E. Pokatilov, A. Askerov and A. Balandin, Phys. Rev. B, 2009, 79(15), 155413 CrossRef.
- K. Sotthewes, R. Van Bremen, E. Dollekamp, T. Boulogne, K. Nowakowski, D. Kas, H. J. Zandvliet and P. Bampoulis, J. Phys. Chem. C, 2019, 123, 5411–5420 CrossRef CAS.
- A. Allain and A. Kis, ACS Nano, 2014, 8, 7180–7185 CrossRef CAS.
- J. Suh, T.-E. Park, D.-Y. Lin, D. Fu, J. Park, H. J. Jung, Y. Chen, C. Ko, C. Jang and Y. Sun, Nano Lett., 2014, 14, 6976–6982 CrossRef CAS PubMed.
- S. Song, A. Yoon, S. Jang, J. Lynch, J. Yang, J. Han, M. Choe, Y. H. Jin, C. Y. Chen and Y. Cheon, Nat. Commun., 2023, 14, 4747 CrossRef CAS PubMed.
- F. Wang, L. Yin, Z. Wang, K. Xu, F. Wang, T. A. Shifa, Y. Huang, Y. Wen, C. Jiang and J. He, Appl. Phys. Lett., 2016, 109(19), 19311 Search PubMed.
- T. A. Empante, Y. Zhou, V. Klee, A. E. Nguyen, I.-H. Lu, M. D. Valentin, S. A. Naghibi Alvillar, E. Preciado, A. J. Berges and C. S. Merida, ACS Nano, 2017, 11, 900–905 CrossRef CAS PubMed.
- D. H. Keum, S. Cho, J. H. Kim, D.-H. Choe, H.-J. Sung, M. Kan, H. Kang, J.-Y. Hwang, S. W. Kim and H. Yang, Nat. Phys., 2015, 11, 482–486 Search PubMed.
- I. G. Lezama, A. Arora, A. Ubaldini, C. Barreteau, E. Giannini, M. Potemski and A. F. Morpurgo, Nano Lett., 2015, 15, 2336–2342 CrossRef CAS PubMed.
- D. Qu, X. Liu, M. Huang, C. Lee, F. Ahmed, H. Kim, R. S. Ruoff, J. Hone and W. J. Yoo, Adv. Mater., 2017, 29, 1606433 CrossRef.
- Y. J. Park, A. K. Katiyar, A. T. Hoang and J. H. Ahn, Small, 2019, 15, 1901772 CrossRef PubMed.
- S. Aftab, M. F. Khan, P. Gautam, H. Noh and J. Eom, Nanoscale, 2019, 11, 9518–9525 RSC.
- P.-C. Shen, C. Su, Y. Lin, A.-S. Chou, C.-C. Cheng, J.-H. Park, M.-H. Chiu, A.-Y. Lu, H.-L. Tang and M. M. Tavakoli, Nature, 2021, 593, 211–217 CrossRef CAS PubMed.
- Y. Liu, P. Stradins and S.-H. Wei, Sci. Adv., 2016, 2, e1600069 CrossRef PubMed.
- T. Kim, H. Park, D. Joung, D. Kim, R. Lee, C. H. Shin, M. Diware, W. Chegal, S. H. Jeong and J. C. Shin, Adv. Mater. Interfaces, 2018, 5, 1800439 CrossRef.
- Q. He, P. Li, Z. Wu, B. Yuan, Z. Luo, W. Yang, J. Liu, G. Cao, W. Zhang and Y. Shen, Adv. Mater., 2019, 31, 1901578 CrossRef PubMed.
- L. Zhou, K. Xu, A. Zubair, A. D. Liao, W. Fang, F. Ouyang, Y.-H. Lee, K. Ueno, R. Saito and T. Palacios, J. Am. Chem. Soc., 2015, 137, 11892–11895 CrossRef CAS.
- T. Ma, H. Chen, K. Yananose, X. Zhou, L. Wang, R. Li, Z. Zhu, Z. Wu, Q.-H. Xu and J. Yu, Nat. Commun., 2022, 13, 5465 CrossRef CAS PubMed.
- L. Ma, J. Zhu, W. Li, R. Huang, X. Wang, J. Guo, J.-H. Choi, Y. Lou, D. Wang and G. Zou, J. Am. Chem. Soc., 2021, 143, 13314–13324 CrossRef CAS.
- D. Wu, C. Guo, L. Zeng, X. Ren, Z. Shi, L. Wen, Q. Chen, M. Zhang, X. J. Li and C.-X. Shan, Light: Sci. Appl., 2023, 12, 5 CrossRef CAS PubMed.
- Y. Pan, R. Guzman, S. Li, W. Xu, Y. Li, N. Tang, H. Yin, J. He, A. Wu and J. Chen, Nat. Synth., 2022, 1, 701–708 CrossRef.
- X. Xu, Y. Pan, S. Liu, B. Han, P. Gu, S. Li, W. Xu, Y. Peng, Z. Han and J. Chen, Science, 2021, 372, 195–200 CrossRef CAS.
- S. Cho, S. Kim, J. H. Kim, J. Zhao, J. Seok, D. H. Keum, J. Baik, D.-H. Choe, K. J. Chang and K. Suenaga, Science, 2015, 349, 625–628 CrossRef CAS PubMed.
- X. Ma, P. Guo, C. Yi, Q. Yu, A. Zhang, J. Ji, Y. Tian, F. Jin, Y. Wang and K. Liu, Phys. Rev. B, 2016, 94, 214105 CrossRef.
- J. Wang, X. Luo, S. Li, I. Verzhbitskiy, W. Zhao, S. Wang, S. Y. Quek and G. Eda, Adv. Funct. Mater., 2017, 27, 1604799 CrossRef.
- S. Cheng, L. Yang, J. Li, Z. Liu, W. Zhang and H. Chang, CrystEngComm, 2017, 19, 1045–1051 RSC.
- C. Zhang, W. Liu, F. Zhan, T. Zhang, L. Liu, M. Zhang, S. Xie, Z. Li, H. Sang and H. Ge, Adv. Funct. Mater., 2021, 31, 2103384 CrossRef CAS.
- C. Zhang, Z. Chen, H. Bai, W. Lin, M. Yang, M. Hong, F. Zhan, S. Xie, M. Zhang and Z. Li, Small, 2023, 19, 2370277 CrossRef.
- S. Yang, X. Xu, W. Xu, B. Han, Z. Ding, P. Gu, P. Gao and Y. Ye, ACS Appl. Nano Mater., 2020, 3, 10411–10417 CrossRef CAS.
- D. M. Kim, S.-i Kim and T. Kim, Electron. Mater. Lett., 2021, 17, 307–314 CrossRef CAS.
- J. H. Sung, H. Heo, S. Si, Y. H. Kim, H. R. Noh, K. Song, J. Kim, C.-S. Lee, S.-Y. Seo and D.-H. Kim, Nat. Nanotechnol., 2017, 12, 1064–1070 CrossRef CAS PubMed.
- D. Choi, D. Kim, Y. Jo, J. Kim, E. Yoon, H.-C. Lee and T. Kim, Appl. Surf. Sci., 2021, 565, 150521 CrossRef CAS.
- R. S. Lee, D. Kim, S. A. Pawar, T. Kim, J. C. Shin and S.-W. Kang, ACS Nano, 2019, 13, 642–648 CrossRef CAS PubMed.
- R. Qi, Y. Ding, L. Zhou, C. Wang, L. Lin, Z. Cai, S. Xiao, X. Gu and H. Nan, Mater. Sci. Semicond. Process., 2024, 169, 107889 CrossRef CAS.
- A. Allain, J. Kang, K. Banerjee and A. Kis, Nat. Mater., 2015, 14, 1195–1205 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4nh00347k |
| This journal is © The Royal Society of Chemistry 2024 |