Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Enhancing the chemotherapeutic efficacy of platinum prodrug nanoparticles and inhibiting cancer metastasis by targeting iron homeostasis
Fang
Ding
abc,
Lingpu
Zhang
bd,
Hao
Chen
bd,
Haiqin
Song
*ef,
Shiguo
Chen
*a and
Haihua
Xiao
*bc
aNanshan District Key Lab for Biopolymers and Safety Evaluation, Shenzhen Key Laboratory of Polymer Science and Technology, Guangdong Research Center for Interfacial Engineering of Functional Materials, College of Materials Science and Engineering, Shenzhen University, Shenzhen, 518060, P. R. China. E-mail: csg@szu.edu.cn
bBeijing National Laboratory for Molecular Sciences, State Key Laboratory of Polymer Physics and Chemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: hhxiao@iccas.ac.cn
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
dCollege of Life Science and Technology, Beijing University of Chemical Technology, Beijing 100029, P. R. China
eDepartment of General Surgery, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, P. R. China
fShanghai Minimally Invasive Surgery Center, Shanghai, 200025, P. R. China. E-mail: shq604shq@163.com
First published on 26th September 2024
Abstract
Correction for ‘Enhancing the chemotherapeutic efficacy of platinum prodrug nanoparticles and inhibiting cancer metastasis by targeting iron homeostasis’ by Fang Ding et al., Nanoscale Horiz., 2020, 5, 999–1015, https://doi.org/10.1039/D0NH00148A.
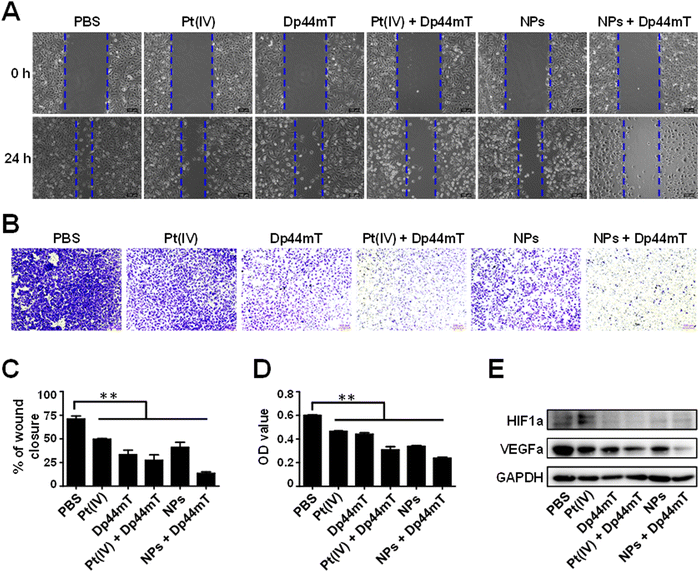
The authors regret errors in Fig. 5 and 7 in the original article. In Fig. 5A, the wound healing image of cells treated with NPs + Dp44mT at 0 h was identical to the NPs treatment group at 0 h due to an error when creating the figure, and the scale bars were not consistent in Fig. 5A or 5B. The new Fig. 5 provided here replaces the originally published figure and contains the correct images.
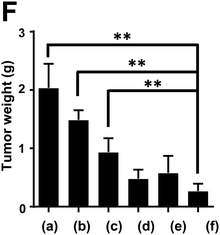
In Fig. 7F, the weight of tumors of mice at the end of the administration period with the treatment of (b) cisplatin (2.5 mg Pt per kg body weight) and (c) Dp44mT (3 mg Pt per kg body weight) were labelled conversely by accident. This is corrected in the new Fig. 7F here.
An independent expert has viewed the corrected figures and confirmed that they are consistent with the discussions and conclusions presented.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2024 |